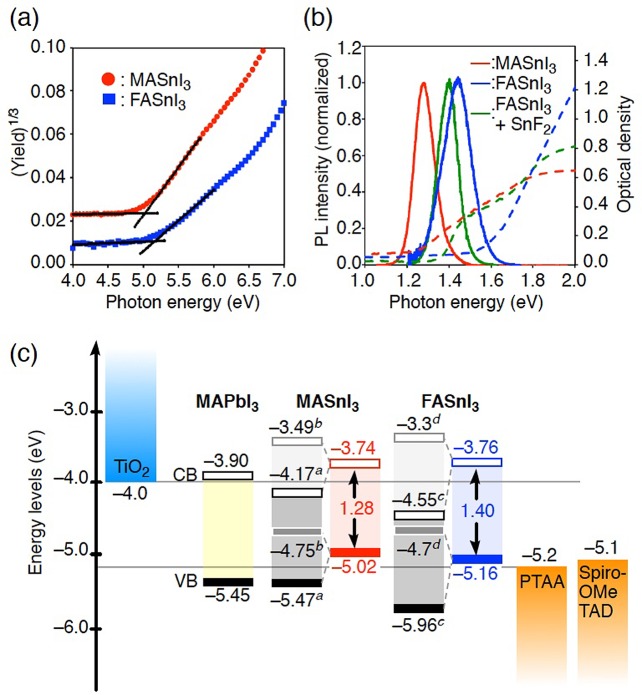
Abstract
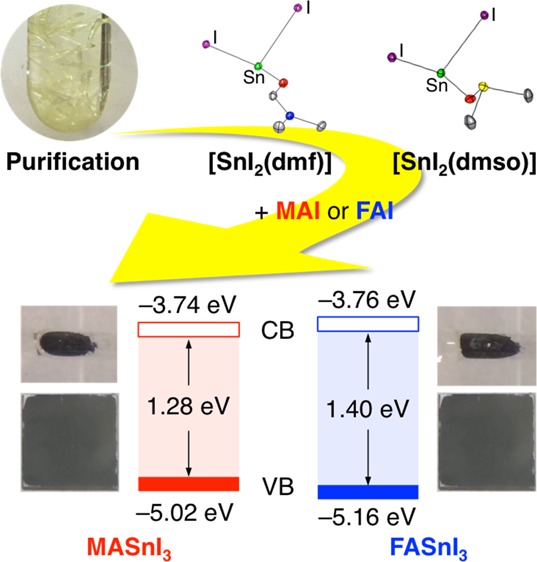
A series of solvent-coordinated tin halide complexes were prepared as impurity-free precursors for tin halide perovskites, and their structures were determined by single-crystal X-ray diffraction analysis. Using these precursors, the tin halide perovskites, MASnI3 and FASnI3, were prepared, and their electronic structures and photophysical properties were examined under inert conditions by means of photoelectron yield spectroscopy as well as absorption and fluorescence spectroscopies. Their valence bands (MASnI3: −5.02 eV; FASnI3: −5.16 eV) are significantly higher than those of MAPbI3 or the typical hole-transporting materials 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene and poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine). These results suggest that to develop the solar cells using these tin halide perovskites with efficient hole-collection properties, hole-transporting materials should be chosen that have the highest occupied molecular orbital levels higher than −5.0 eV.
Introduction
Perovskite solar cells have attracted much attention as the potential next generation of photovoltaics. Especially, solar cells that use lead halide perovskites as light-harvesting materials have been intensively studied.1−4 Even though their performances with respect to power-conversion efficiencies (PCEs) and durability1−4 have been improved substantially within only a few years, the inherent toxicity of lead compounds remains a concern and bottleneck for practical applications. As potential alternative light-harvesting materials, Sn-based perovskites have attracted attention, particularly owing to their lower toxicity.5−7 Although the highest reported PCEs of Sn-based perovskite solar cells are 5–8%,8−11 their performance in photovoltaic devices is still lower than that of Pb-based perovskite solar cells and moreover suffers from reproducibility issues. Different from Pb-based solar cells,12 the inferior performance of Sn-based perovskite solar cells should probably be attributed to the lower quality of the perovskite layer.13 The major difference between materials based on Pb and Sn is the stability of their divalent ions. In contrast to Pb2+, Sn2+ is easily oxidized to the more stable Sn4+. Indeed, Sn-based perovskite materials, such as MASnI3 (MA: methylammonium, CH3NH3+) and FASnI3 (FA: formamidinium, CH(NH2)2+), are sensitive to oxidation upon exposure to air. The resulting Sn4+ should subsequently affect the device performance by self-doping.14,15 For the fabrication of efficient Pb-based perovskite solar cells with high reproducibility, we have demonstrated that the purity of the starting materials, such as PbI2, is crucial.16 For Sn-based perovskite solar cells, the preparation and use of highly purified tin halide precursor materials should be even more important.
Upon complexation of SnX2 (X = F, Cl, Br, and I) with coordinating solvents such as N,N-dimethylformamide (DMF) or dimethyl sulfoxide (DMSO), we developed a series of purified tin halide materials [SnX2(S)n] (S = DMF and DMSO) in this study. Using the thus-obtained highly purified precursors, such as [SnI2(dmf)], [SnI2(dmso)], and [SnI2(dmso)2], the Sn-based perovskites MASnI3 and FASnI3 were prepared, and their electronic and optical properties were examined under inert conditions by means of photoelectron yield spectroscopy (PYS) and photophysical measurements.
Results and Discussion
Initially, we analyzed a commercially available sample of SnI2 (99.9%, trace metals basis, purchased from Kojundo Chemical Laboratory Co., Ltd.) by 119Sn magic-angle spinning (MAS) NMR spectroscopy, thermogravimetric analysis (TGA), and Karl Fischer titration. Surprisingly, the purchased sample of SnI2 contained up to 10 wt % SnI4, together with ∼10 000 ppm water. In the 119Sn MAS NMR spectrum of the commercial sample, a signal at −1743 ppm, which arises from the presence of SnI4, was observed in addition to the signals at −389 and −527 ppm corresponding to SnI2 (Figure 1a).17 TGA measurements revealed a significant weight loss (10.1%) at ∼150 °C, which corresponds to the sublimation of SnI4, before another substantial weight loss (63.9%) was observed at ∼330 °C, which should be attributed to SnI2 (Figure 1b). On the basis of these results, we purified SnI2 (10.7 g) by sublimation under reduced pressure (100 Pa). After removal of SnI4 (2.1 g, 3.4 mmol) as an orange crystalline powder at 150 °C, further heating to 330 °C afforded SnI2 (7.1 g, 19 mmol) as a red crystalline powder. According to 119Sn MAS NMR (−585 and −605 ppm)18,19 and atmospheric pressure chemical ionization mass spectrometry (MS) measurements (Figures S2 and S23), the residual dark brown solid (0.85 g) should consist mostly of SnO2.
Figure 1.
119Sn MAS NMR spectra and thermogravimetric curves of (a, b) commercially available SnI2 (99.9%, trace metals basis) and (c, d) sublimated SnI2.
Although SnI4 could be removed by sublimation (Figure 1c,d), the sublimed SnI2 powder afforded yellow solutions upon dissolution in DMF or DMSO that contained insoluble small brown particles of SnO2, which was confirmed by a mass spectroscopic analysis (Figure S3). This result indicated that one sublimation should not be sufficient to purify SnI2. As the higher homologue PbI2 forms complexes with DMF16 and DMSO,20 we tried to further purify SnI2 by recrystallization from these solvents. For that purpose, filtered solutions of SnI2 in DMF or DMSO were layered with toluene or dichloromethane (CH2Cl2). The slow diffusion of toluene into a DMF solution of SnI2 afforded colorless crystalline needles of [SnI2(dmf)], which was confirmed by single-crystal X-ray diffraction (XRD) analysis (Figure 2). In these crystals, one molecule of DMF coordinates to the tin center [Sn–O: 2.209(2) Å], which results in Sn–I bond lengths of 2.9744(3) and 3.0065(4) Å for Sn(1)–I(1) and Sn(1)–I(2), respectively. The packing structure of [SnI2(dmf)] is characterized by a linear alignment. During the recrystallizations, the choice of antisolvent (less soluble solvent) was found to determine the nature of the SnI2 complex. For example, diffusion of CH2Cl2 into a DMF solution of SnI2 afforded orange crystalline needles of [Sn3I6(dmf)2] and the 3:2 ratio between SnI2 and DMF was confirmed by a single-crystal X-ray diffraction analysis (Figure S5).
Figure 2.

Molecular structure of [SnI2(dmf)]: (a) Oak Ridge thermal ellipsoid plot (ORTEP) drawing with thermal ellipsoids at 50% probability; (b) perspective view along the a axis. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å): Sn–O 2.209(2); Sn(1)–I(1) 2.9744(3); Sn(1)–I(2) 3.0065(4); Sn(1)–Sn(1)#1 4.4957(3); Sn(1)#1–I(1) 3.3972(3); Sn(1)#1–I(2) 3.4354(3).
Slow diffusion of CH2Cl2 into a DMSO solution of SnI2 furnished colorless needles of [SnI2(dmso)], which were structurally characterized by single-crystal X-ray diffraction analysis (Figure 3). In the crystal structure of [SnI2(dmso)], one molecule of DMSO coordinates to the tin center [Sn–O: 2.167(3) Å] and the packing structure is defined by a linear alignment. Interestingly, when toluene was used as the antisolvent in the recrystallization from DMSO, colorless crystals of [SnI2(dmso)2] were obtained as the only product, in which two molecules of DMSO coordinate to the tin center (Figure S6). Kanatzidis and co-workers have reported that [SnI2(dmso)3] can be obtained by treating a DMSO solution of MASnI3 with CH2Cl2, which is regarded as an intermediate in the one-step tin perovskite spin-coating process21 and could also be potentially used for the purification of tin-based perovskite materials for solar cells. The advantage of using the [SnI2(dmf)], [SnI2(dmso)], and [SnI2(dmso)2] complexes obtained in the present study as precursor materials is that they are prepared with relative ease from SnI2 solutions. Although these complexes are also air sensitive, they can be stored in vial containers under inert condition (O2, H2O < 10 ppm). Although both of SnI2 and SnI4 are red solids (Figure S1), these SnI2 complexes are colorless crystalline solids. SnI2 complexes immediately turn reddish brown upon exposure to air, suggesting that the purity of these complexes can be checked by the naked eye.
Figure 3.
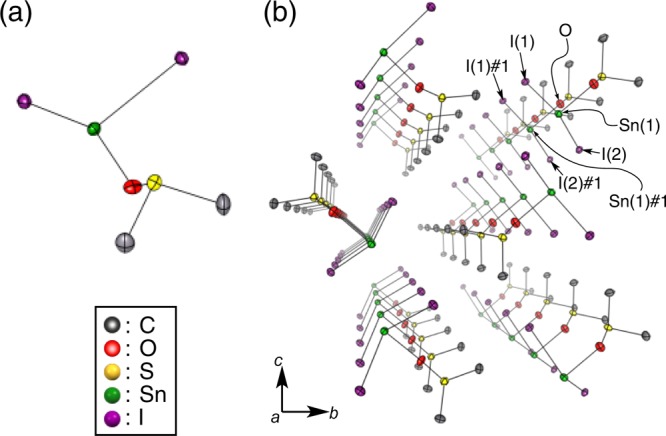
Molecular structure of [SnI2(dmso)]: (a) ORTEP drawing with thermal ellipsoids at 50% probability; (b) perspective view along the a axis. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å): Sn–O 2.167(3); Sn(1)–I(1) 2.9910(5); Sn(1)–I(2) 3.0587(5); Sn(1)#1–I(1) 3.3119(5); Sn(1)#1–I(2) 3.3965(5); Sn(1)–Sn(1)#1 4.4931(8).
In analogy to the SnI2 complexes, several solvent-coordinated tin complexes containing other halides, such as [SnBr2(dmf)], [SnBr2(dmso)2], [SnCl2(dmf)], and [Sn2F4(dmso)2]22 were obtained in a similar fashion. Their structures were unambiguously determined by single-crystal X-ray diffraction analyses (Figures S7–S10), and their purity was confirmed by elemental analysis and 119Sn MAS NMR spectroscopy (see SI). All of these tin halide complexes can be used as purified precursor materials for tin-based perovskites. In addition to their high purity, these tin halide complexes offer the advantage that their solutions can be prepared rapidly. For example, the preparation of a 1.5 M solution of sublimed SnI2 in DMF or DMSO requires more than 30 min stirring, whereas [SnI2(dmf)], [SnI2(dmso)], [SnI2(dmso)2], or other such complexes dissolve immediately (<10 min) in these solvents (Figure S11).
To demonstrate the utility of these complexes as purified precursors for tin halide perovskite materials, and to investigate their properties, crystalline powders and films of MASnI3 and FASnI3 were prepared using [SnI2(dmf)] or [SnI2(dmso)2] in an Ar-filled glove box (O2, H2O < 10 ppm). Crystalline powder samples of MASnI3 and FASnI3 were prepared from a 1.0 M EtOH solution of SnI2(dmf) and methylammonium iodide (MAI) or formamidinium iodide (FAI) using a temperature gradient from 80 °C to room temperature. The film samples were prepared by a one-step solution method, that is, a 1.5 M DMSO solution of [SnI2(dmso)2] and MAI or FAI in a 1:1 ratio was deposited on a quartz substrate by spin-coating. After thermal annealing at 100 °C, black films were obtained. The formation of MASnI3 and FASnI3 was confirmed by X-ray diffraction (XRD) analyses of the crystalline powders and films using synchrotron and Cu Kα radiation, respectively (Figure 4b,d). The XRD patterns of the crystalline powder samples, which were enclosed in glass capillaries after grinding under Ar, showed peaks quite similar to those of the simulated patterns for the corresponding perovskite crystals (Figure 4a,c).23,24 The film samples were covered with poly(methyl methacrylate) (PMMA) by spin-coating to protect the films from oxidation by air during the XRD measurements. The film samples of MASnI3 and FASnI3 also showed peaks similar to the simulations, whereas the XRD pattern for the film of MASnI3 showed strong preferred orientations along the (100) direction, which is consistent with the report in the literature.24
Figure 4.

X-ray diffraction patterns for (a, b) MASnI3 and (c, d) FASnI3. Samples of (a, c) crystalline powders and (b, d) the films were measured using synchrotron (λ = 0.61992 Å) and Cu Kα radiation (λ = 1.5406 Å), respectively. Simulated XRD patterns from cell parameters of tetragonal-MASnI3 and orthorhombic-FASnI323,24 are shown for comparison.
To find suitable applications for tin halide perovskite materials in the development of solar cells, it should be important to gain a better understanding of their inherent electronic structures. Using a powder sample of purified SnI2, we examined the valence band (VB) by means of PYS under vacuum (<10–2 Pa). The PYS spectra of MASnI3 and FASnI3 (Figure 5a) revealed onset positions, based on (yield)1/3, for the VB maxima of MASnI3 and FASnI3 at −5.02 and −5.16 eV, respectively. Interestingly, these values are intermediate relative to previously reported values (MASnI3: −4.7525 or −5.47 eV;26 FASnI3: −4.727 or −5.96 eV28). Compared to the VBs of the lead halide perovskite MAPbI3 (−5.45 eV)29 and typical hole-collection materials such as 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamino)-9,9′-spirobifluorene (Spiro-OMeTAD; −5.1 eV)29 and poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) (PTAA; −5.2 eV),26 the VBs of these tin halide perovskites are relatively high. These results suggest that in the corresponding tin-based perovskite solar cells, a pairing with hole-transporting materials (HTMs) that exhibit high highest occupied molecular orbital (HOMO) levels should be necessary to ensure efficient hole collection.
Figure 5.
(a) PYS spectra of MASnI3 (red) and FASnI3 (blue) measured under vacuum (<10–2 Pa).30 (b) Photoluminescence (PL) (solid line) and absorption spectra (dashed line) of MASnI3 (red), FASnI3 (blue), and FASnI3 with 10% SnF2 (green), measured under Ar (O2, H2O < 10 ppm). (c) Energy level diagram of TiO2, MAPbI3, MASnI3, FASnI3, PTAA, and Spiro-OMeTAD. aRef (26); bref (25); cref (28); dref (27).
Subsequently, we examined the absorption and photoluminescence (PL) spectra of films of MASnI3 and FASnI3. Whereas the PL measurements were carried out on PMMA-covered film samples under Ar, absorption spectra were recorded in air after the PL measurements. The MASnI3 film exhibited the PL peak position at 1.28 eV with a full width at half-maximum (fwhm) of 110 meV determined by Gaussian fitting and an absorption onset at 1.3 eV (Figure 5b; red line). These values are in good agreement with previously reported ones (1.30,8,26 1.26 eV25). On the other hand, the FASnI3 film showed a PL peak at 1.44 eV with a wider fwhm (160 meV), whereas the absorption onset was observed at ∼1.6 eV (Figure 5b; blue line). After several measurements, we found that the PL peak of FASnI3 is more sensitive to oxidation than that of MASnI3 (Figure S12 ).30 The large difference between the PL peak and the absorption edge should originate from the rapid oxidation upon exposure to ambient conditions, leading to a blue shift during the absorption measurement. To suppress the oxidation, we added 10 mol % SnF2, which is widely used as a stabilizer,10,11,31 to the Sn-based perovskite precursor solution. XRD measurements on the thus-obtained film confirmed that the crystal structure of FASnI3 was not affected by the presence of 10 mol % SnF2 (Figure S13). The FASnI3 film containing 10 mol % SnF2 exhibited a narrower PL (fwhm: 110 meV) with a peak at 1.40 eV and an absorption onset at ∼1.4 eV (Figure 5b; green line). These values are also in good agreement with previously reported values (1.41,28 1.4 eV27). On the basis of these results, we determined the band gaps of MASnI3 (1.28 eV) and FASnI3 (1.40 eV). Consequently, the conduction band (CB) minima of MASnI3 (−3.74 eV) and FASnI3 (−3.76 eV) were estimated on the basis of the VBs determined by PYS (Figure 5).
Conclusions
In summary, we prepared a series of tin halide complexes that contain DMF or DMSO. [SnI2(dmf)] and [SnI2(dmso)2] were used as soluble purified precursor materials to generate the Sn-based perovskites, MASnI3 and FASnI3. The electronic and photophysical properties, including the VBs and CBs, of MASnI3 and FASnI3 were examined using XRD analyses, as well as PYS and photophysical spectroscopy measurements. The CBs of MASnI3 and FASnI3 are slightly higher than that of MAPbI3, whereas the VBs (MASnI3: −5.02 eV; FASnI3: −5.16 eV) are significantly higher, even when compared to typical hole-transporting materials such as Spiro-OMeTAD and PTAA or to MAPbI3. These results suggest that in the interest of developing Sn-based perovskite solar cells with efficient hole-collection properties, hole-transporting materials should be chosen that exhibit the HOMO level higher than −5.0 eV. In line with this device design principle, the development of efficient Sn-based perovskite solar cells using such purified tin halide materials is currently in progress in our laboratory and the results will be reported in due course.
Acknowledgments
This study was supported by the JST ALCA and NEDO (Japan). The authors would like to thank Prof. H. Kaji, Dr. K. Suzuki (Kyoto University), and A. Maeno at the Joint Usage/Research Center (JURC; Kyoto University) for fruitful discussions on the 119Sn MAS NMR measurements, as well as T. Hirano (JURC; Kyoto University) for carrying out elemental analyses. The authors also thank Prof. S. Hayase and Dr. Y. Ogomi (Kyusyu Institute of Technology) for fruitful discussion on a complex of [SnI2(dmso)2]. Synchrotron radiation experiments were performed at the Taiwan Photon Source (TPS) 09A beamline at the National Synchrotron Radiation Research Center (NSRRC; proposal 2017-1-125-2). Preliminary experiments were carried out at the BL19B2 beamline of SPring-8 with the approval of Japan Synchrotron Radiation Research Institute (JASRI; proposal No. 2017A1703). We thank Drs. Y.-C. Chung, H.-S. Sheu, W.-T. Chen, K. Osaka, and H. Hirosawa for their help during the experiments. The sublimed tin halide samples were kindly gifted by Tokyo Chemical Industry Co., Ltd. (TCI).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01292.
Details on the experiment procedures, X-ray structural analysis, XRD, TGA, MS, and 119Sn MAS NMR (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Miyasaka T. Perovskite photovoltaics: Rare functions of organo lead halide in solar cells and optoelectronic devices. Chem. Lett. 2015, 44, 720–729. 10.1246/cl.150175. [DOI] [Google Scholar]
- Park N.-G.; Grätzel M.; Miyasaka T.; Zhu K.; Emery K. Towards stable and commercially available perovskite solar cells. Nat. Energy 2016, 1, 16152 10.1038/nenergy.2016.152. [DOI] [Google Scholar]
- Correa-Baena J.-P.; Abate A.; Saliba M.; Tress W.; Jacobsson T. J.; Grätzel M.; Hagfeldt A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. 10.1039/C6EE03397K. [DOI] [Google Scholar]
- Zhao Y.; Zhu K. Organic–inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. 10.1039/C4CS00458B. [DOI] [PubMed] [Google Scholar]
- Babayigit A.; Ethirajan A.; Muller M.; Conings B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. 10.1038/nmat4572. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Guo J.; Chen Y.; Li Q.; Pan Y.; Zhang H.; Xia Y.; Huang W. Lead-free organic–inorganic hybrid perovskites for photovoltaic applications: recent advances and perspectives. Adv. Mater. 2017, 29, 1605005. 10.1002/adma.201605005. [DOI] [PubMed] [Google Scholar]
- Hoefler S. F.; Trimmel G.; Rath T. Progress on lead-free metal halide perovskites for photovoltaic applications: A review. Monatsh. Chem. 2017, 148, 795. 10.1007/s00706-017-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F.; Stoumpos C. C.; Cao D. H.; Chang R. P. H.; Kanatzidis M. G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. 10.1038/nphoton.2014.82. [DOI] [Google Scholar]
- Noel N. K.; Stranks S. D.; Abate A.; Wehrenfennig C.; Guarnera S.; Haghighirad A.-A.; Sadhanala A.; Eperon G. E.; Pathak S. K.; Johnston M. B.; Petrozza A.; Herz L. M.; Snaith H. J. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. 10.1039/C4EE01076K. [DOI] [Google Scholar]
- Liao W.; Zhao D.; Yu Y.; Grice C. R.; Wang C.; Cimaroli A. J.; Schulz P.; Meng W.; Zhu K.; Xiong R.-G.; Yan Y. Lead-free inverted planar formamidinium tin triiodide perovskite solar cells achieving power conversion efficiencies up to 6.22%. Adv. Mater. 2016, 28, 9333. 10.1002/adma.201602992. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Gu F.; Li Y.; Sun W.; Ye S.; Rao H.; Liu Z.; Bian Z.; Huang C. Mixed-organic-cation tin iodide for lead-free perovskite solar cells with an efficiency of 8.12%. Adv. Sci. 2017, 1700204. 10.1002/advs.201700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa T.; Tex D. M.; Shimazaki A.; Wakamiya A.; Kanemitsu Y. Charge injection mechanism at heterointerfaces in CH3NH3PbI3 perovskite solar cells revealed by simultaneous time-resolved photoluminescence and photocurrent measurements. J. Phys. Chem. Lett. 2017, 8, 954–960. 10.1021/acs.jpclett.6b02847. [DOI] [PubMed] [Google Scholar]
- Handa T.; Yamada T.; Kubota H.; Ise S.; Miyamoto Y.; Kanemitsu Y. Photocarrier recombination and injection dynamics in long-term stable lead-free CH3NH3SnI3 perovskite thin films and solar cells. J. Phys. Chem. C 2017, 121, 16158–16165. 10.1021/acs.jpcc.7b06199. [DOI] [Google Scholar]
- Takahashi Y.; Obara R.; Lin Z.-Z.; Takahashi Y.; Naito T.; Inabe T.; Ishibashi S.; Terakura K. Charge-transport in tin-iodide perovskite CH3NH3SnI3: origin of high conductivity. Dalton Trans. 2011, 40, 5563–5688. 10.1039/c0dt01601b. [DOI] [PubMed] [Google Scholar]
- Wang F.; Ma J.; Xie F.; Li L.; Chen J.; Fan J.; Zhao N. Organic cation-dependent degradation mechanism of organotin halide perovskites. Adv. Funct. Mater. 2016, 26, 3417–3423. 10.1002/adfm.201505127. [DOI] [Google Scholar]
- Wakamiya A.; Endo M.; Sasamori T.; Tokitoh N.; Ogomi Y.; Hayase S.; Murata Y. Reproducible fabrication of efficient perovskite-based solar cells: x-ray crystallographic studies on the formation of CH3NH3PbI3 layers. Chem. Lett. 2014, 43, 711–713. 10.1246/cl.140074. [DOI] [Google Scholar]
- Schaeffer R. W.; Chan B.; Molinaro M.; Morissey S.; Yoder C. H.; Yoder C. S.; Shenk S. Synthesis, characterization, and Lewis acidity of SnI2 and SnI4. J. Chem. Educ. 1997, 74, 575–577. 10.1021/ed074p575. [DOI] [Google Scholar]
- Goward G. R.; Nazar L. F.; Power W. P. Electrochemical and multinuclear solid-state NMR studies of tin composite oxide glasses as anodes for Li ion batteries. J. Mater. Chem. 2000, 10, 1241–1249. 10.1039/b001352h. [DOI] [Google Scholar]
- Wang J.; Su Y.; Xu J.; Ye C.; Deng F. Acid sites and oxidation center in molybdena supported on tin oxide as studied by solid-state NMR spectroscopy and theoretical calculation. Phys. Chem. Chem. Phys. 2006, 8, 2378–2384. 10.1039/b516833c. [DOI] [PubMed] [Google Scholar]
- Miyamae H.; Numahata Y.; Nagata M. The crysatal structure of lead(II) iodide-dimethylsulphoxide(1/2), PbI2(dmso)2. Chem. Lett. 1980, 9, 663–664. 10.1246/cl.1980.663. [DOI] [Google Scholar]
- Hao F.; Stoumpos C. C.; Guo P.; Zhou N.; Marks T. J.; Chang R. P. H.; Kanatzidis M. G. Solvent-mediated crystallization of CH3NH3SnI3 films for heterojunction depleted perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 11445–11452. 10.1021/jacs.5b06658. [DOI] [PubMed] [Google Scholar]
- Gurnani C.; Hector A. L.; Jager E.; Levason W.; Pugh D.; Reid G. Tin(II) fluoride vs. tin(II) chloride – a comparison of their coordination chemistry with neutral ligands. Dalton Trans. 2013, 42, 8364–8374. 10.1039/c3dt50743b. [DOI] [PubMed] [Google Scholar]
- Stoumpos C. C.; Malliakas C. D.; Kanatzidis M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- Dang Y.; Zhou Y.; Liu X.; Ju D.; Xia S.; Xia H.; Tao X. Formation of hybrid perovskite tin iodide single crystals by top-seeded solution growth. Angew. Chem., Int. Ed. 2016, 55, 3447–3450. 10.1002/anie.201511792. [DOI] [PubMed] [Google Scholar]
- Yokoyama T.; Cao D. H.; Stoumpos C. C.; Song T.-B.; Sato Y.; Aramaki S.; Kanatzidis M. G. Overcoming short-circuit in lead-free CH3NH3SnI3 perovskite solar cells via kinetically controlled gas–solid reaction film fabrication process. J. Phys. Chem. Lett. 2016, 7, 776–782. 10.1021/acs.jpclett.6b00118. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Zhao D.; Grice C. R.; Meng W.; Wang C.; Liao W.; Cimaroli A. J.; Zhang H.; Zhu K.; Yan Y. Thermally evaporated methylammonium tin triiodide thin films for lead-free perovskite solar cell fabrication. RSC Adv. 2016, 6, 90248–90254. 10.1039/C6RA19476A. [DOI] [Google Scholar]
- Ke W.; Stoumpos C. C.; Logsdon J. L.; Wasielewski M. R.; Yan Y.; Fang G.; Kanatzidis M. G. TiO2–ZnS cascade electron transport layer for efficient formamidinium tin iodide perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 14998–15003. 10.1021/jacs.6b08790. [DOI] [PubMed] [Google Scholar]
- Koh T. M.; Krishnamoorthy T.; Yantara N.; Shi C.; Leong W. L.; Boix P. P.; Grimsdale A. C.; Mhaisalkar S. G.; Mathews N. Formamidinium tin-based perovskite with low Eg for photovoltaic applications. J. Mater. Chem. A 2015, 3, 14996–15000. 10.1039/C5TA00190K. [DOI] [Google Scholar]
- Nishimura H.; Ishida N.; Shimazaki A.; Wakamiya A.; Saeki A.; Scott L. T.; Murata Y. Hole-transporting materials with a two-dimensionally expanded π-system around an azulene core for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 15656–15659. 10.1021/jacs.5b11008. [DOI] [PubMed] [Google Scholar]
- Nishikubo R.; Ishida N.; Katsuki Y.; Wakamiya A.; Saeki A. Minutes-scale degradation and shift of valence band maxima of (CH3NH3)SnI3 and HC(NH2)2SnI3 perovskite upon air exposure. J. Phys. Chem. C 2017, 121, 19650–19656. 10.1021/acs.jpcc.7b06294. [DOI] [Google Scholar]
- Lee S. J.; Shin S. S.; Kim Y. C.; Kim D.; Ahn T. K.; Noh J. H.; Seo J.; Seok S. I. Fabrication of efficient formamidinium tin iodide perovskite solar cells through SnF2–pyrazine complex. J. Am. Chem. Soc. 2016, 138, 3974–3977. 10.1021/jacs.6b00142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.