Abstract
In this study, a multicomponent reaction involving carbohydrates, β-dicarbonyl compounds, and malononitrile was disclosed to synthesize a new class of polyhydroxy compounds incorporating pyrano[2,3-d]pyrimidine, pyrido[2,3-d]pyrimidine and chromene heterocycles under mild conditions. For the synthesis of this class of compounds, glucose, galactose, arabinose, maltose, and lactose were used as aldehyde component in the reaction with barbituric acid and malononitrile to produce pyrano[2,3-d]pyrimidine derivatives. By use of 1,3-cyclohexanedione instead of barbituric acid, chromene derivatives incorporating carbohydrate moieties were obtained. Also, the four-component condensation reaction between d-glucosamine, aldehyde, malononitrile, and barbituric acid was efficiently provided polyhydroxy-substituted pyrido[2,3-d]pyrimidine derivatives. This new combinatorial approach gave a range of carbohydrate-derived heterocycles in good to excellent yields with high potential biological applications. The antioxidant activities were evaluated using 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) antioxidant measuring system, and the data were expressed as Trolox equivalent antioxidant capacity. All of these compounds display significant antioxidant activity. The maximum and minimum antioxidant activities were observed for 4j and 6b, respectively. Our results indicated encouraging perspectives for the improvement and usage of this type of synthetic compounds, indicating significant levels of antioxidant activity.
Introduction
Carbohydrates are recognized as environmentally friendly starting materials with several key characteristics, including abundant availability, high water solubility, and optical activity.1 Synthesis of biologically active compounds, design of molecular recognition devices, and their use as chiral auxiliaries in asymmetric synthesis are discussed in most of the utilization reports on carbohydrates.2−4 The existence of a large number of free hydroxyl groups in the structure of carbohydrates caused their high solubility in an aqueous environment. Thus, the water solubility of a biologically active molecule can be tuned by incorporation of a carbohydrate moiety in its structure. Due to these facts, there are a lot of applications for carbohydrate derivatives in medicinal chemistry. Several drugs based on carbohydrates for antibiotics, antiviral, protein glycosylation, and glycosylation inhibitors have been developed.5−8
Moreover, carbohydrates have been used as reagent in organic synthesis to obtain new organic molecules containing carbohydrate fragments. Along this line, some of the heterocycles, including pyrazines, imidazoles, quinoxalines, pyrazolo[3,4-b]-quinoxalines, and benzimidazoles, have been synthesized using carbohydrates as reagent.9−15 For example, there are some reports on the use of carbohydrate-based heterocycles as corrosion inhibitor of mild steels.16,17
One of the most important strategies to provide structurally diverse natural productlike compounds is multicomponent reaction (MCR).18−20 MCR is a smart synthetic strategy, because it is possible to make a complex product in a single step, whereas molecular diversity can be simply achieved by the selection of desired components. In this situation, MCRs are widely used to generate new compounds based on carbohydrate derivatives. According to the functionality of carbohydrate, it is possible to consider it as a component in MCRs. For example, reducing sugars can be used as aldehyde and amino sugars as amine component. The presence of a carbohydrate as aldehyde or an amine component in MCRs opens our hands in the synthesis of new carbohydrate-based library molecules.21−25
Aminocarbonitrile derivatives are an important class of organic molecules that possess many applications in organic synthesis. In this regard, preparation of aminocarbonitriles is very attractive and MCRs are useful approaches in the synthesis of aminocarbonitrile derivatives.26−31 More importantly, aminocarbonitriles also showed interesting biological activities. For instance, there is a report on anticancer activity of some 2-amino-chromene-nitriles.32
Oxidative stress causes an imbalance between creation and accumulation of oxygen reactive species in cells and tissue, playing a major role in the progress of various chronic and degenerative illnesses, such as aging, cancer, cataract, autoimmune disorders, rheumatoid arthritis, cardiovascular, and neurodegenerative diseases. The human body has several mechanisms to offset oxidative stress by producing antioxidants, which are either naturally produced in human body or externally provided through foods and/or supplements.33 However, without exogenous antioxidant compounds, our endogenous antioxidant defense systems are incomplete. Hence, there are continuous requests for exogenous antioxidants to prevent oxidative stress.34
Previously, our research group reported on the use of natural small molecules, including carbohydrates, nucleosides, and curcumin, in MCRs for one-pot synthesis of some biologically active compounds.35−41 In continuation of our program on the synthesis of natural product-based compound libraries, in this study, we disclosed a new MCR between carbohydrates, β-dicarbonyl compounds, and malononitrile. This combinatorial reaction afforded synthesis of novel classes of pyrano[2,3-d]pyrimidine, pyrido[2,3-d]pyrimidine, and chromene derivatives, incorporating carbohydrate moieties. Also, the antioxidant activity of the synthetic compounds was investigated, demonstrating that all of them have reasonable antioxidant activity.
Results and Discussion
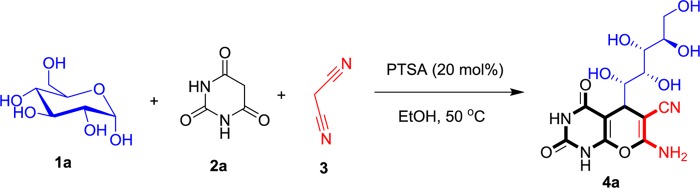
Initially, (+)-d-glucose (1a) was reacted with barbituric acid (2a) and malononitrile (3a) in EtOH in the presence of 20 mol % para-toluenesulfonic acid (PTSA) to give glucose analogue 4a as a white crystal in 85% isolated yield (Scheme 1).
Scheme 1. One-Pot Synthesis of a Pyrano[2,3-d]pyrimidine-6-carbonitrile Based on (+)-d-Glucose.
Reaction conditions: (+)-d-glucose (1 mmol), barbituric acid (1 mmol), malononitrile (1 mmol), EtOH (5 mL), and PTSA (20 mol %).
To find the optimum condition for this reaction, different experiments were done on the above reaction (Scheme 1) and results are shown in Table 1.
Table 1. Optimization of Reaction Conditiona.
| entry | catalyst (mol %) | solvent | temp (°C) | time (h) | yield 4a (%)b |
|---|---|---|---|---|---|
| 1 | none | EtOH | 50 | 24 | 15 |
| 2 | PTSA (20) | EtOH | 50 | 12 | 85 |
| 3 | PTSA (30) | EtOH | 50 | 12 | 89 |
| 4 | PTSA (35) | EtOH | 50 | 12 | 88 |
| 5 | l-proline (10) | EtOH | 50 | 18 | 68 |
| 6 | Et3N (50) | EtOH | 50 | 24 | 52 |
| 7 | PTSA (30) | H2O | 80 | 24 | 65 |
| 8 | PTSA (30) | CH3CN | 70 | 24 | 25 |
| 9 | PTSA (30) | EtOH | 35 | 24 | 65 |
| 10 | PTSA (30) | EtOH | rt | 24 | 35 |
| 11 | PTSA (30) | EtOH | 80 | 12 | 90 |
Reaction conditions: (+)-d-glucose (1 mmol), barbituric acid (1 mmol), malononitrile (1 mmol), EtOH (5 mL), and catalyst.
Isolated yield.
As shown in Table 1, low yield of product was obtained in the absence of catalyst in reflux ethanol condition after 24 h (Table 1, entry 1). The reaction yield was enhanced to 85% after addition of 20 mol % PTSA as catalyst (Table 1, entry 2). An improvement in yield was observed by use of 30 mol % of catalyst (Table 1, entry 3). There was no improvement in the reaction yield with increasing the amount of catalyst to 35 mol % (Table 1, entry 4). Thus 30 mol % of PTSA as catalyst was recognized as optimum catalyst loading for the reaction. For comparison, two other catalysts, including l-proline and Et3N, were also tested but no improvement in reaction yield was detected (Table 1, entries 5 and 6). Other solvents including water and acetonitrile were also checked, and no superiority in reaction yield was observed (Table 1, entries 7 and 8). Decreasing the reaction temperature reduces the reaction yield significantly so that at room temperature, only 35% of the product was isolated (Table 1, entries 9 and 10). Also, no improvement in the reaction yield was observed by increase of the reaction temperature to 80 °C (Table 1, entry 11).
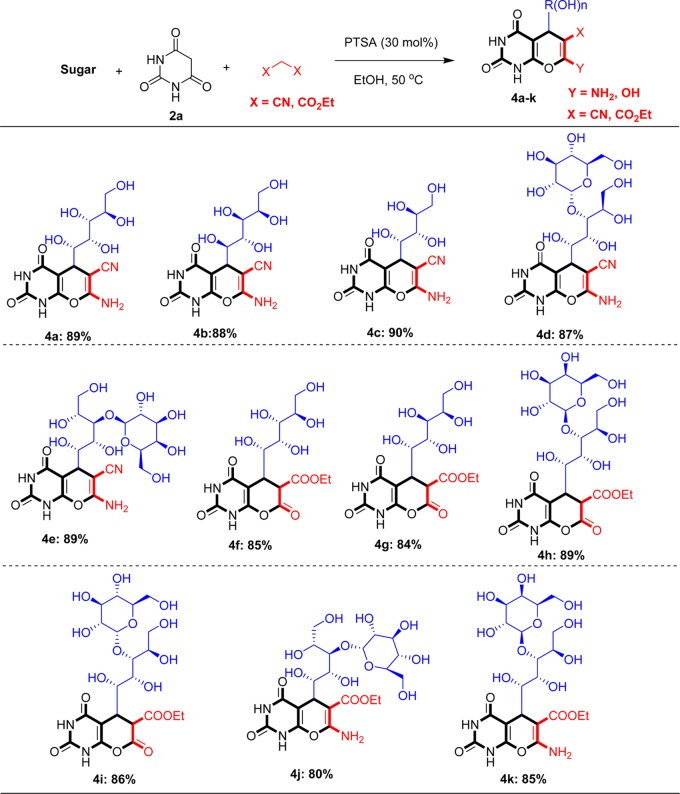
After optimization study, to show the generality and scope of the protocol, some new derivatives using different sugars were synthesized and results are summarized in Scheme 2.
Scheme 2. One-Pot Synthesis of a Pyrano[2,3-d]pyrimidine Derivatives Using MCR of Sugars, Barbituric Acid, and Malononitrile.
Reaction conditions: sugar (1 mmol), barbituric acid (1 mmol), malononitrile/diethyl malonate/ethyl 2-cyanoacetate (1 mmol), EtOH (5 mL), and PTSA (30 mol %).
As shown in Scheme 2, this methodology is efficient for the synthesis of diverse pyrano[2,3-d]pyrimidine derivatives using different sugars and malononitrile/diethyl malonate/ethyl 2-cyanoacetate. Since only the anomeric carbon engaged in the reaction, it is logical that the enantiomeric centers remain unchanged in the product.
By the use of galactose instead of glucose in the reaction, compound 4b was obtained in 88% isolated yield. Arabinose as a pentose was also used in the reaction and afforded compound 4c, incorporating a 4-hydroxy carbon chain. Lactose and maltose were also used as disaccharide carbohydrate to show further applicability of this method in the synthesis of other polyhydroxy-substituted pyrano[2,3-d]pyrimidines (4d and 4e).
With this procedure, diethyl malonate was also obtained and ester derivative of polyhydroxy-substituted pyrano[2,3-d]pyrimidine was synthesized in good to excellent yields (4f–i). As shown, both monosaccharides and disaccharides underwent the reaction to afford corresponding products under optimized conditions.
Ethyl 2-cyanoacetate is another reagent that was used in this protocol, and β-amino ester derivatives of pyrano[2,3-d]pyrimidines were obtained (4j and 4k).
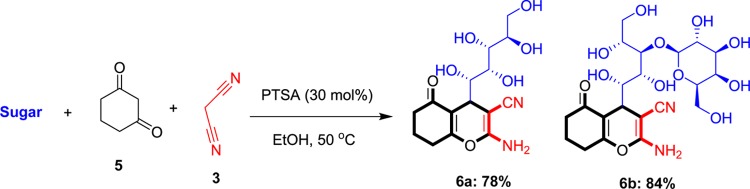
In another attempt, cyclohexane-1,3-dione was used instead of barbituric acid and this reaction resulted to polyhydroxy-functionalized chromene-3-carbonitrile derivatives (Scheme 3). These experiments resulted in the production of compounds 6a and 6b in 78 and 84% under optimized conditions, respectively. Compound 6a was obtained from glucose and 6b was obtained from lactose.
Scheme 3. One-Pot Synthesis of Chromene-3-carbonitrile Derivatives Using MCR of Sugars, Cyclohexane-1,3-dione, and Malononitrile.
Reaction conditions: sugar (1 mmol), cyclohexane-1,3-dione (1 mmol), malononitrile (1 mmol), EtOH (5 mL), and PTSA (30 mol %).
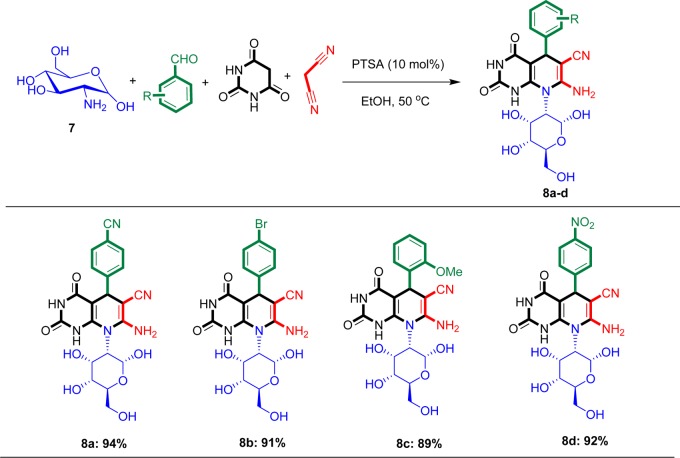
To show the generality and scope of the reaction further, a four-component coupling reaction was conducted between d-glucosamine, aldehyde, barbituric acid, and malononitrile. This MCR resulted in the generation of pyrido[2,3-d]pyrimidine derivatives in good to excellent yields (Scheme 4). In this reaction, it is possible to synthesize different derivatives using a selection of different aldehydes. Both electron-donating and electron-withdrawing aldehydes gave products under standard conditions. For aldehydes with electron-withdrawing group such as nitro and cyano, more than 92% of product was isolated (8a and 8d). As shown in this reaction, d-glucosamine (an amino sugar) acts as an amine component.
Scheme 4. One-Pot Synthesis of a Class of Pyrido[2,3-d]pyrimidine Derivatives Using a Four-Component Reaction of d-Glucosamine, Aldehyde, Barbituric Acid, and Malononitrile.
Reaction conditions: d-glucosamine (1 mmol), barbituric acid (1 mmol), malononitrile (1 mmol), aldehyde (1 mmol), EtOH (5 mL), and PTSA (10 mol %). All yields refer to isolated products.
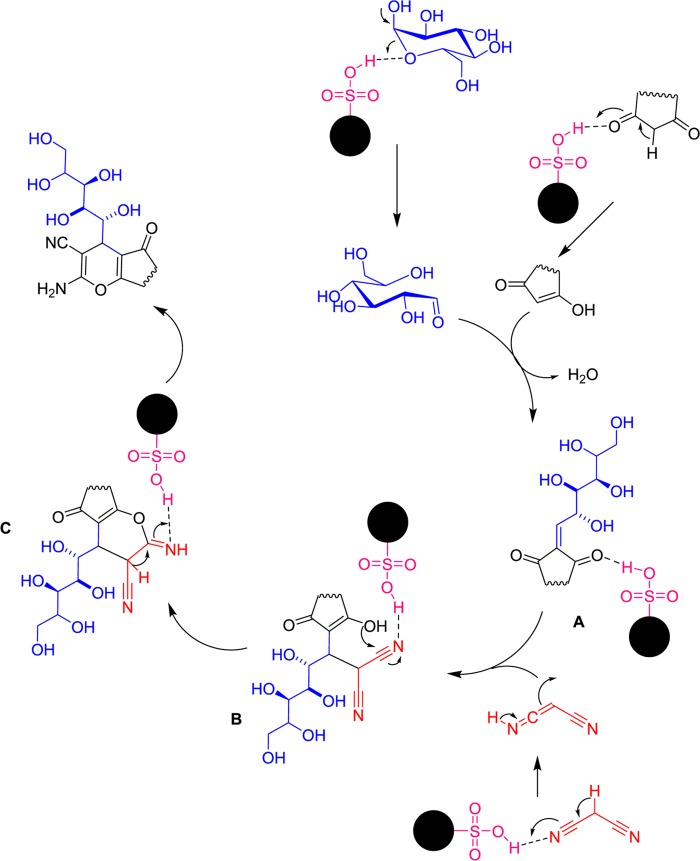
In view of the reaction mechanism (Scheme 5), according to the literature,42,43 it seems that the first activated dicarbonyl compound reacts with sugar as an aldehyde component to form a Knoevenagel intermediate (A). Reaction of malononitrile to the Knoevenagel intermediate resulted in the production of (B). Nucleophilic addition of hydroxy group in enol form with cyano group resulted in the formation of intermediate (C). The tautomerization in this intermediate affords pyrano[2,3-d]pyrimidine and chromene derivatives.
Scheme 5. Proposed Mechanism for Polyhydroxy-Functionalized Pyrano[2,3-d]pyrimidine and Chromene Derivatives.
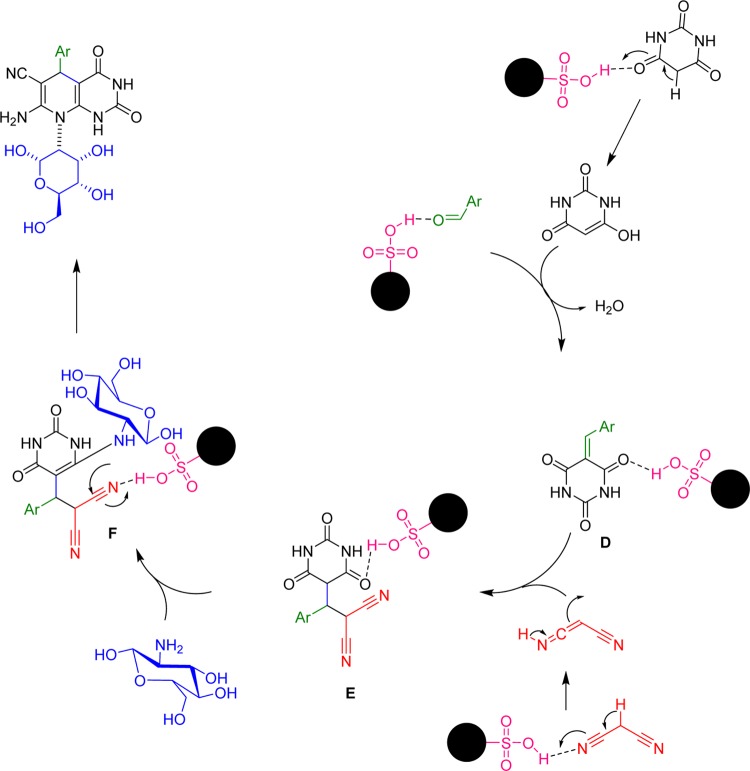
In the four-component reaction (Scheme 6),42,43 the reaction proceeds with the formation of Knoevenagel intermediate between barbituric acid and aldehyde (D). Michael addition of malononitrile with the Knoevenagel intermediate led to the production of an intermediate (E). It is proposed that in this stage, d-glucosamine as an amine component reacts with the intermediate (E) and forms enamine (F), which is highly disposed to undergo cyclization via reaction of amino group and cyano group.
Scheme 6. Proposed Reaction Mechanism for One-Pot Synthesis of Pyrido[2,3-d]pyrimidine Derivatives Using d-Glucose Amine.
After synthesis and characterization of the carbohydrate derivatives, their antioxidant activity was evaluated and results were expressed as Trolox equivalent antioxidant capacity (TEAC). The higher TEAC value shows the higher antioxidant power of the compound, as shown in Table 2. The results indicated that all of these synthetic compounds have significant antioxidant activity, and among them, compound 4j with the TEAC value of 5.237 ± 0.053 μM displays the most antioxidant activity than other synthetic compounds. Also, thereafter, 4k and 4c, respectively, stand in the second and third positions on the basis of their antioxidant activity.
Table 2. Comparison between Antioxidant Activities (As TEAC) of Different Synthesized Compounds at Specific Time Points.
| synthetic compounds | carbohydrate part | heterocycle fragments | TEAC ± SD (μM) |
|---|---|---|---|
| 4a | glucose | pyrano[2,3-d]pyrimidines | 3.208 ± 0.053 |
| aminocarbonitrile | |||
| 4b | galactose | pyrano[2,3-d]pyrimidines | 1.234 ± 0.014 |
| aminocarbonitrile | |||
| 4c | arabinose | pyrano[2,3-d]pyrimidines | 4.914 ± 0.196 |
| aminocarbonitrile | |||
| 4d | lactose | pyrano[2,3-d]pyrimidines | 3.909 ± 0.008 |
| aminocarbonitrile | |||
| 4e | maltose | pyrano[2,3-d]pyrimidines | 3.070 ± 0.070 |
| aminocarbonitrile | |||
| 4f | glucose | pyrano[2,3-d]pyrimidine | 3.959 ± 0.029 |
| β-keto ester | |||
| 4g | galactose | pyrano[2,3-d]pyrimidine | 3.744 ± 0.025 |
| β-keto ester | |||
| 4h | lactose | pyrano[2,3-d]pyrimidine | 3.995 ± 0.036 |
| β-keto ester | |||
| 4i | maltose | pyrano[2,3-d]pyrimidine | 2.216 ± 0.019 |
| β-keto ester | |||
| 4j | maltose | pyrano[2,3-d]pyrimidine | 5.237 ± 0.053 |
| β-amino ester | |||
| 4k | lactose | pyrano[2,3-d]pyrimidine | 4.978 ± 0.018 |
| β-amino ester | |||
| 6a | glucose | chromene-3-carbonitrile | 0.177 ± 0.020 |
| aminocarbonitrile | |||
| 6b | lactose | chromene-3-carbonitrile | 0.169 ± 0.019 |
| aminocarbonitrile | |||
| 8a | d-glucosamine | pyrido[2,3-d]pyrimidine | 1.177 ± 0.013 |
| –Ph–CN | aminocarbonitrile | ||
| 8b | d-glucosamine | pyrido[2,3-d]pyrimidine | 1.643 ± 0.026 |
| –Ph–Br | aminocarbonitrile | ||
| 8c | d-glucosamine | pyrido[2,3-d]pyrimidine | 2.385 ± 0.018 |
| Ph–OMe | aminocarbonitrile | ||
| 8d | d-glucosamine | pyrido[2,3-d]pyrimidine | 3.242 ± 0.031 |
| Ph–NO2 | aminocarbonitrile |
The experiments were performed in triplicate, and mean ± standard deviations (SDs) were determined.
As shown in Table 2, the significant antioxidant properties were observed for the synthetic compounds 4j, 4k, and 4c. The antioxidant activity of these compounds changed with change of substructures. When in a class of compounds a part is fixed, it is observable that the antioxidant activity is changed remarkably, which can be attributed to the reducing (electron-donating) power of that fragment. For compounds 4a–e (the heterocycle parts are pyrano[2,3-d]pyrimidine and aminocarbonitrile), results demonstrated that compound 4c with arabinose carbohydrate is a superior antioxidant.44 As shown in Table 2, the antioxidant activity of compounds containing galactose is less than that of others (4b and 4i). With pyrano[2,3-d]pyrimidine compounds containing β-keto ester, the antioxidant activity is to some extent more than that of pyrano[2,3-d]pyrimidines incorporating aminocarbonitrile fragment, representing that the role of β-keto ester moiety is effective than that of aminocarbonitrile in antioxidant activity of these compounds.45 The high antioxidant activity was obtained for the compounds containing aminocarbonitrile and β-amino ester moieties (4j and 4k).46 Also, the lowest antioxidant activity was observed for compound 6b with a TEAC value of 0.169 ± 0.019 μM, which contains a chromene-3-carbonitrile moiety.47 For compounds 8a–d, which are pyrido[2,3-d]pyrimidine derivatives containing d-glucosamine, the antioxidant activity depends on the type of aryl ring, and for rings bearing nitro group (8d), a relatively good activity was obtained.35
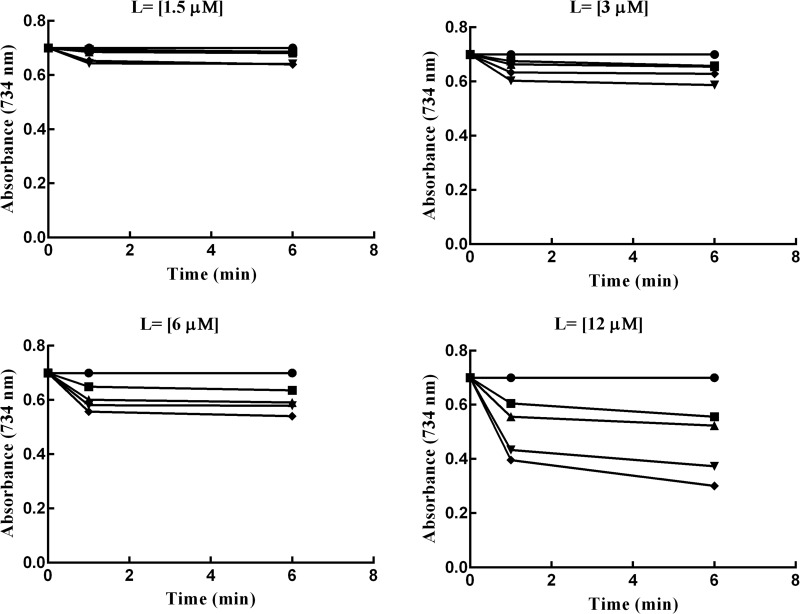
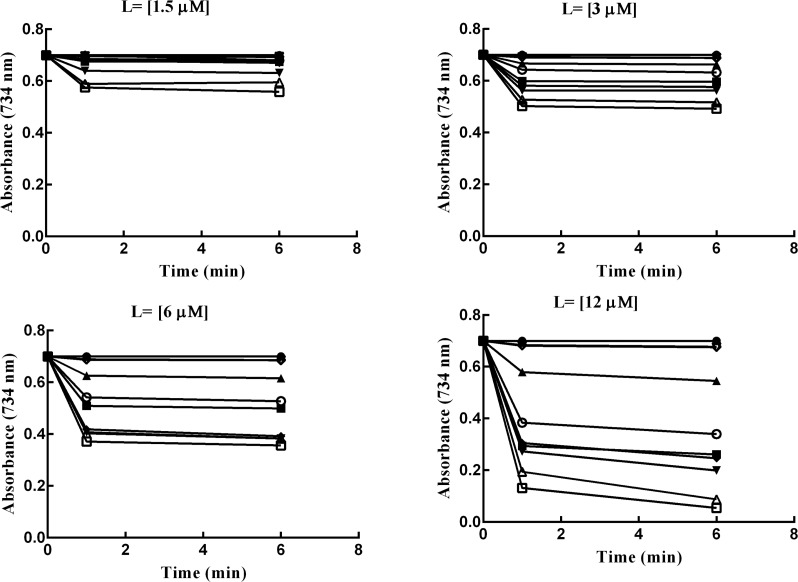
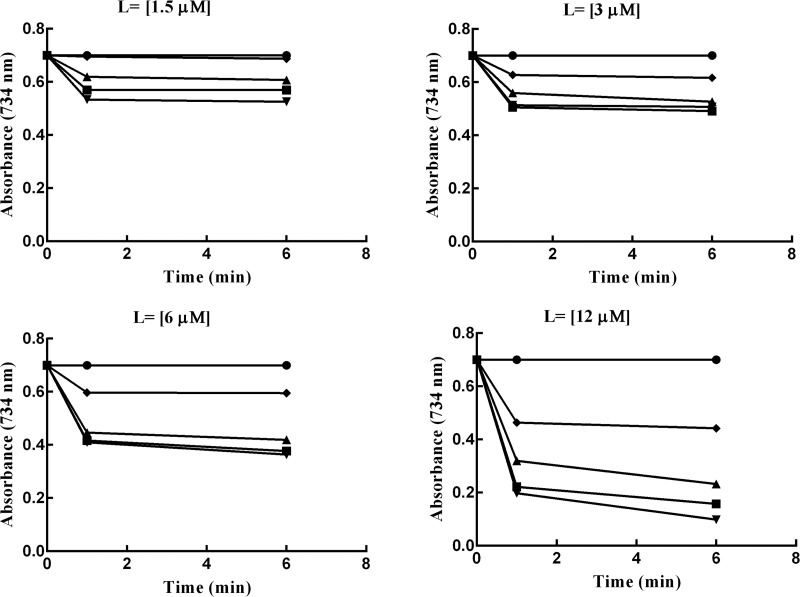
Also, data in Figures 1–3, are in complete agreement with TEACs values (Table 2). The results presented in these figures suggest that the higher antioxidant activity associates with more reduction in the absorbance value at 734 nm. Antioxidant capacity of the synthetic ligands was measured using the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) decolorization assay. To express antioxidant capacity, we used the water soluble vitamin E analogue as a standard compound. The reduction of the radical cation by the synthetic compounds was measured as the percentage inhibition of absorbance at 734 nm. The percentage inhibition curves were drawn as a function of concentration of the test compounds. The slope of these curves was obtained and divided with that of Trolox (4.87), and the final results were expressed as TEAC.48
Figure 1.
Effects of time on suppression of the absorbance of the ABTS•+. The experiments were performed in different concentrations of each ligand (1.5–12 μM). The curves represent the effects of the specific antioxidant on the suppression of the absorbance of the ABTS•+ at 734 nm. The symbols used are as follows: control ABTS•+ radical cation (solid circles), 8a (squares), 8b (upward triangles), 8c (downward triangles), and 8d (diamonds).
Figure 3.
Effects of time on suppression of the absorbance of the ABTS•+. The experiments were performed in different concentrations of each ligand (1.5–12 μM). The curves represent the effects of the specific antioxidant on the suppression of the absorbance of the ABTS•+ at 734 nm. The symbols used are as follows: control ABTS•+ radical cation (solid circles), 4a (squares), 4b (upward triangles), 4c (downward triangles), 4d (diamonds), 4e (hollow circles), 4j (hollow squares), 4k (hollow upward triangles), 6a (hollow downward triangles), and 6b (hollow diamonds).
Figure 2.
Effects of time on suppression of the absorbance of the ABTS•+. The experiments were performed in different concentrations of each ligand (1.5–12 μM). The curves represent the effects of the specific antioxidant on the suppression of the absorbance of the ABTS•+ at 734 nm. The symbols used are as follows: control ABTS•+ radical cation (solid circles), 4f (squares), 4g (upward triangles), 4h (downward triangles), and 4i (diamonds).
Conclusions
In summary, we have illustrated an efficient multicomponent approach for the synthesis of a new library of polyhydroxy-functionalized heterocyclic compounds using carbohydrates. In this protocol, reducing sugars act as aldehyde component and amino sugars act as amine component to participate in MCRs containing dicarbonyl compounds and malononitrile. These MCRs afforded a range of novel pyrano[2,3-d]pyrimidine, pyrido[2,3-d]pyrimidine, and chromene derivatives incorporating carbohydrate moieties and 3-aminocarbonitrile unite under environmental conditions. The method is simple, and gave the target products in good to excellent isolated yields and with high purity. The diversity of molecules in this study achieved by selection of carbohydrate (monosaccharide and disaccharide), dicarbonyl compound, and malononitrile, suggests an interesting scaffold containing both chiral polyhydroxy chain and biologically active heterocycles. The synthetic compounds revealed excellent antioxidant effectiveness in the ABTS system, which increases their potential therapeutic values.
Experimental Section
General
Chemicals were purchased from Fluka and Aldrich chemical companies and used without further purification. The known products were characterized by comparison of their spectra and physical data with those reported in the literature. Fourier transform infrared (FT-IR) spectroscopy (Shimadzu FT-IR 8300 spectrophotometer) was employed for characterization of the synthesized compounds. 1H NMR spectra were recorded at 250 and 400 MHz, and 13C NMR spectra were recorded at 62.5, 75, and 100 MHz in dimethyl sulfoxide-d6 using tetramethylsilane as an internal standard. Melting points were determined in open capillary tubes in a Barnstead Electrothermal 9100 BZ circulating oil melting point apparatus. Thin-layer chromatography (TLC) was carried out on silica gel 254 analytical sheets obtained from Fluka. Column chromatography was carried out on silica gel 60 Merck (230–240 mesh) in glass columns (2 or 3 cm diameter) using 15–30 g of silica gel per 1 g of the crude mixture.
Chemistry
General Procedure for Preparation of 4a–k
A mixture of barbituric acid (1.0 mmol), sugar (1.0 mmol), malononitrile/diethyl malonate/ethylacetoacetate (1.0 mmol), and para-toluenesulfonic acid (30.0 mol %) in EtOH (5.0 mL) at 50 °C was stirred for 20 h. Completion of the reaction was confirmed by TLC (eluent EtOAc–MeOH). The reaction mixture was cooled to room temperature. Then, the reaction mixture was filtered and the remaining mixture was washed with ethanol (3 × 5 mL). Removal of the solvent under reduced pressure gave almost pure products, which were further purified by recrystallization from EtOH.
Procedure To Prepare Compound 6a and 6b
A mixture of cyclohexane-1,3-dione (1 mmol), sugar (1 mmol), malononitrile (1 mmol), and PTSA (30 mol %) in EtOH (3 mL) at 50 °C was stirred for 15 h. Completion of the reaction was confirmed by TLC (eluent EtOAc–MeOH). The reaction mixture was cooled to room temperature. The extraction and purification of products were same as compound 4a–k.
General Procedure To Prepare Compound 8a–d
A mixture of barbituric acid (1 mmol), aldehyde (1 mmol), d-glucosamine (1 mmol), and PTSA (10 mol %) in ethanol (5 mL) at 50 °C was stirred for 12 h. Completion of the reaction was confirmed by TLC (eluent CH2Cl2/MeOH). The reaction mixture was cooled to room temperature. The extraction and purification of products were same as those for compound 4a–k.
Biology
The ABTS procedure assay followed the method originally suggested by Re et al.49 The ABTS radical cation (ABTS•+) was generated by reaction of ABTS solution (7 mM) and (2.45 mM final concentration) potassium persulfate solution at a molar ratio of 1:0.5. The mixture was held in dark at 25 °C for 12–16 h before being used, and then it was diluted with 5 mM phosphate buffer (pH 7.4) to obtain an absorbance of 0.7 ± 0.02 units at 734 nm using a T90+ UV–vis spectrophotometer. An accurate amount of sample was added to 1 mL of the reagent and incubated at 25 °C. Then, the decrease of absorbance at 734 nm was measured at the end point of 6 min. A solvent blank was run in each assay and a water soluble vitamin E analogue (Trolox) used as the standard. The results were expressed as Trolox equivalent antioxidant capacity (TEAC).
Acknowledgments
We acknowledge Shiraz University for partial support of this work.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01124.
Spectral data for synthesized compounds and copy of 1H NMR, 13C NMR, and FT-IR of products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hollingsworth R. I.; Wang G. Toward a carbohydrate-based chemistry: progress in the development of general-purpose chiral synthons from carbohydrates. Chem. Rev. 2000, 100, 4267–4282. 10.1021/cr990374e. [DOI] [PubMed] [Google Scholar]
- Shi Y. Organocatalytic asymmetric epoxidation of olefins by chiral ketones. Acc. Chem. Res. 2004, 37, 488–496. 10.1021/ar030063x. [DOI] [PubMed] [Google Scholar]
- Ferreira S. B.; Sodero A. C. R.; Cardoso M. F. C.; Lima E. S.; Kaiser C. R.; Silva F. P. Jr.; Ferreira V. F. Synthesis, biological activity, and molecular modeling studies of 1 h-1, 2, 3-triazole derivatives of carbohydrates as α-glucosidase inhibitors. J. Med. Chem. 2010, 53, 2364–2375. 10.1021/jm901265h. [DOI] [PubMed] [Google Scholar]
- Iglesias-Guerra F.; Romero I.; Alcudia F.; Vega-Pérez J. M. Alkylating agents from sugars. cyclophosphamides derived from 2-amino-2-deoxy-d-allose. Carbohydr. Res. 1998, 308, 57–62. 10.1016/S0008-6215(98)00056-1. [DOI] [Google Scholar]
- Rodrigues A. G.; Pina-Vaz C.; Costa-de-Oliveira S.; Tavares C. Expression of plasma coagulase among pathogenic Candida species. J. Clin. Microbiol. 2003, 41, 5792–5793. 10.1128/JCM.41.12.5792-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R. J.; Von Itzstein M.. Carbohydrate-Based Drug Discovery; Wong C. H., Ed.; Wiley-VCH: Weinheim, 2003; pp 831–886. [Google Scholar]
- Xie J.; Bogliotti N. Synthesis and applications of carbohydrate-derived macrocyclic compounds. Chem. Rev. 2014, 114, 7678–7739. 10.1021/cr400035j. [DOI] [PubMed] [Google Scholar]
- Jimenez-Barbero J.; Canada F. J.; Martin-Santamaria S.. Carbohydrates in Drug Design and Discovery; Royal Society of Chemistry: U.K., 2015. [Google Scholar]
- Brust A.; Cuny E. Reducing disaccharides and their 1, 2-dicarbonyl intermediates as building blocks for nitrogen heterocycles. RSC Adv. 2014, 4, 5759–5767. 10.1039/c3ra47349j. [DOI] [Google Scholar]
- Cai S.; Xiang S.; Zeng J.; Gorityala B. K.; Liu X.-W. Facile synthesis of carbohydrate-integrated isoxazolines through tandem [4+ 1] cycloaddition and rearrangement of 2-nitroglycals. Chem. Commun. 2011, 47, 8676–8678. 10.1039/c1cc12327k. [DOI] [PubMed] [Google Scholar]
- Brust A.; Cuny E. Conversion of reducing carbohydrates into hydrophilic substituted imidazoles. Green Chem. 2013, 15, 2993–2998. 10.1039/c3gc41203b. [DOI] [Google Scholar]
- Lin C.; Lai P. T.; Liao S. K. S.; Hung W. T.; Yang W. B.; Fang J. M. Using molecular iodine in direct oxidative condensation of aldoses with diamines: an improved synthesis of aldo-benzimidazoles and aldo-naphthimidazoles for carbohydrate analysis. J. Org. Chem. 2008, 73, 3848–3853. 10.1021/jo800234x. [DOI] [PubMed] [Google Scholar]
- Rohovec J.; Kotek J.; Peters J. A.; Maschmeyer T. A Clean conversion of D-glucosamine hydrochloride to a pyrazine in the presence of phenylboronate or borate. Eur. J. Org. Chem. 2001, 2001, 3899–3901. . [DOI] [Google Scholar]
- Mostafa M. A.; Aboulela S. L.; Sallam M. A. E.; Louis F. F.; Anthonsen T. Aspects on the mechanism of the 1-phenyl-1H-pyrazolo [3, 4-b] quinoxaline formation. Green Sustainable Chem. 2012, 2, 71–75. 10.4236/gsc.2012.22012. [DOI] [Google Scholar]
- Cabrele C.; Reiser O. The modern face of synthetic heterocyclic chemistry. J. Org. Chem. 2016, 81, 10109–10125. 10.1021/acs.joc.6b02034. [DOI] [PubMed] [Google Scholar]
- Verma C.; Quraishi M. A.; Kluza K.; Makowska-Janusik M.; Olasunkanmi L. O.; Ebenso E. E. Corrosion inhibition of mild steel in 1 M HCl by D-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci. Rep. 2017, 7, 44432 10.1038/srep44432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma C.; Olasunkanmi L. O.; Ebenso E. E.; Quraishi M. A.; Obot I. B. Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J. Phys. Chem. C 2016, 120, 11598–11611. 10.1021/acs.jpcc.6b04429. [DOI] [Google Scholar]
- Mironov M. A. Design of multi-component reactions: from libraries of compounds to libraries of reactions. QSAR Comb. Sci. 2006, 25, 423–431. 10.1002/qsar.200540190. [DOI] [Google Scholar]
- Sunderhaus J. D.; Martin S. F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. - Eur. J. 2009, 15, 1300–1308. 10.1002/chem.200802140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein B. H.; Zaretsky S.; Rai V.; Yudin A. K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. 10.1021/cr400615v. [DOI] [PubMed] [Google Scholar]
- Beck B.; Larbig G.; Mejat B.; Magnin-Lachaux M.; Anne P.; Herdtweck E.; Dömling A. Short and diverse route toward complex natural product-like macrocycles. Org. Lett. 2003, 5, 1047–1050. 10.1021/ol034077e. [DOI] [PubMed] [Google Scholar]
- Lockhoff O. An access to glycoconjugate libraries through multicomponent reactions. Angew. Chem., Int. Ed. 1998, 37, 3436–3439. . [DOI] [PubMed] [Google Scholar]
- Voigt B.; Mahrwald R.. Domino and Intramolecular Rearrangement Reactions as Advanced Synthetic Methods in Glycoscience; Witczak Z. J., Bielski R., Eds.; John Wiley & Sons, 2016. [Google Scholar]
- Khan M. M.; Yousuf R.; Khan S.; Shafiullah Recent advances in multicomponent reactions involving carbohydrates. RSC Adv. 2015, 5, 57883–57905. 10.1039/C5RA08059B. [DOI] [Google Scholar]
- Voigt B.; Linke M.; Mahrwald R. Multicomponent cascade reactions of unprotected carbohydrates and amino Acids. Org. Lett. 2015, 17, 2606–2609. 10.1021/acs.orglett.5b00887. [DOI] [PubMed] [Google Scholar]
- Elinson M. N.; llovaisky A. I.; Merkulova V. M.; Barba F.; Batanero B. General approach to spiroacenaphthylenepentacyclic systems: direct multicomponent assembling of acenaphthenequinone and cyclic carbonyl compounds with two molecules of malononitrile. Tetrahedron 2013, 69, 7125–7130. 10.1016/j.tet.2013.06.015. [DOI] [Google Scholar]
- Gajulapalli V. P. R.; Vinayagam P.; Kesavan V. Enantioselective assembly of functionalized carbocyclic spirooxindoles using an L-proline derived thiourea organocatalyst. RSC Adv. 2015, 5, 7370–7379. 10.1039/C4RA13711F. [DOI] [Google Scholar]
- Sawargave S. P.; Kudale A. S.; Deore J. V.; Bhosale D. S.; Divse J. M.; Chavan S. P.; Borate H. B. One-step synthesis of 4-alkyl-3-aryl-2, 6-dicyanoanilines and their use in the synthesis of highly functionalized 2, 3, 5, 6, 7-and 2, 3, 4, 5, 7-substituted indoles. Tetrahedron Lett. 2011, 52, 5491–5493. 10.1016/j.tetlet.2011.08.064. [DOI] [Google Scholar]
- Molla A.; Hussain S. Borax catalyzed domino reactions: synthesis of highly functionalised pyridines, dienes, anilines and dihydropyrano [3, 2-c] chromenes. RSC Adv. 2014, 4, 29750–29758. 10.1039/C4RA03627A. [DOI] [Google Scholar]
- Mojtahedi M. M.; Pourabdi L.; Abaee M. S.; Jami H.; Dini M.; Halvagar M. R. Facile one-pot synthesis of novel ortho-aminocarbonitriles and dicyanoanilines fused to heterocycles via pseudo four-component reactions. Tetrahedron 2016, 72, 1699–1705. 10.1016/j.tet.2016.02.023. [DOI] [Google Scholar]
- Zhang L. Z.; Wan Y.; Zhang X. X.; Cui H.; Zou H.; Zhou Q. J.; Wu H. Noncovalent catalysis of glucose-containing imidazolium salt in solvent-free one-pot synthesis of Ortho-aminocarbonitriles. Tetrahedron Lett. 2015, 56, 4934–4937. 10.1016/j.tetlet.2015.06.094. [DOI] [Google Scholar]
- Keerthy H. K.; Garg M.; Mohan C. D.; Madan V.; Kanojia D.; Shobith R.; Nanjundaswamy S.; Mason D. J.; Bender A.; Basappa; Rangappa K. S.; Koeffler H. P. Synthesis and characterization of novel 2-amino-chromene-nitriles that target Bcl-2 in acute myeloid leukemia cell lines. PLoS One 2014, 9, e107118 10.1371/journal.pone.0107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Huy L. A.; He H.; Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [PMC free article] [PubMed] [Google Scholar]
- Bouayed J.; Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longevity 2010, 3, 228–237. 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourisefat M.; Panahi F.; Khalafi-Nezhad A. Carbohydrates as a reagent in multicomponent reactions: one-pot access to a new library of hydrophilic substituted pyrimidine-fused heterocycles. Org. Biomol. Chem. 2014, 12, 9419–9426. 10.1039/C4OB01791A. [DOI] [PubMed] [Google Scholar]
- Khalafi-Nezhad A.; Divar M.; Panahi F. Nucleosides as reagents in multicomponent reactions: one-pot synthesis of heterocyclic nucleoside analogues incorporating pyrimidine-fused rings. Tetrahedron Lett. 2013, 54, 220–222. 10.1016/j.tetlet.2012.11.003. [DOI] [Google Scholar]
- Toobaei Z.; Yousefi R.; Panahi F.; Shahidpour S.; Nourisefat M.; Doroodmand M. M.; Khalafi-Nezhad A. Synthesis of novel poly-hydroxyl functionalized acridine derivatives as inhibitors of α-glucosidase and α-amylase. Carbohydr. Res. 2015, 411, 22–32. 10.1016/j.carres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Yousefi A.; Yousefi R.; Panahi F.; Sarikhani S.; Zolghadr A. R.; Bahaoddini A.; Khalafi-Nezhad A. Novel curcumin-based pyrano [2, 3-d] pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: Implications for their pleiotropic effects against diabetes complications. Int. J. Biol. Macromol. 2015, 78, 46–55. 10.1016/j.ijbiomac.2015.03.060. [DOI] [PubMed] [Google Scholar]
- Panahi F.; Yousefi R.; Mehraban M. H.; Khalafi-Nezhad A. Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr. Res. 2013, 380, 81–91. 10.1016/j.carres.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Yousefi R.; Alavian-Mehr M. M.; Mokhtari F.; Panahi F.; Mehraban M. H.; Khalafi-Nezhad A. Pyrimidine-fused heterocycle derivatives as a novel class of inhibitors for α-glucosidase. J. Enzyme Inhib. Med. Chem. 2013, 28, 1228–1235. 10.3109/14756366.2012.727812. [DOI] [PubMed] [Google Scholar]
- Shahidpour S.; Panahi F.; Yousefi R.; Nourisefat M.; Nabipoor M.; Khalafi-Nezhad A. Design and synthesis of new antidiabetic α-glucosidase and α-amylase inhibitors based on pyrimidine-fused heterocycles. Med. Chem. Res. 2015, 24, 3086–3096. 10.1007/s00044-015-1356-2. [DOI] [Google Scholar]
- Khalafi-Nezhad A.; Panahi F. Synthesis of new dihydropyrimido [4, 5-b] quinolinetrione derivatives using a four-component coupling reaction. Synthesis 2011, 2011, 984–992. 10.1055/s-0030-1258446. [DOI] [Google Scholar]
- Khalafi-Nezhad A.; Nourisefat M.; Panahi F. l-Cysteine functionalized magnetic nanoparticles (LCMNP): a novel magnetically separable organocatalyst for one-pot synthesis of 2-amino-4 H-chromene-3-carbonitriles in water. Org. Biomol. Chem. 2015, 13, 7772–7779. 10.1039/C5OB01030F. [DOI] [PubMed] [Google Scholar]
- Mahae N.; Chalat C.; Muhamud P. Antioxidant and antimicrobial properties of chitosan-sugar complex. Int. Food Res. J. 2011, 18, 1543–1551. [Google Scholar]
- Sadiq A.; Mahmood F.; Ullah F.; Ayaz M.; Ahmad S.; Haq F. U.; Khan G.; Jan M. S. Synthesis, anticholinesterase and antioxidant potentials of ketoesters derivatives of succinimides: a possible role in the management of Alzheimer’s. Chem. Cent. J. 2015, 9, 31–39. 10.1186/s13065-015-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattamwar P. P.; Biswal D.; Cochran D. B.; Lyvers A. C.; Eitel R. E.; Anderson K. W.; Hilt J. Z.; Dziubla T. D. Synthesis and characterization of poly (antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Fallah-Tafti A.; Tiwari R.; NasrolahiShirazi A.; Akbarzadeh T.; Mandal D.; Shafiee A.; Parang K.; Foroumadi A. 4-Aryl-4H-chromene-3-carbonitrile derivatives: evaluation of Src kinase inhibitory and anticancer activities. Med. Chem. 2011, 7, 466–472. 10.2174/157340611796799258. [DOI] [PubMed] [Google Scholar]
- Huang D.; Ou B.; Prior R. L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Riceevans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.