Abstract
22q11.2 Deletion Syndrome (22q11.2DS) is a genetic condition associated with a high prevalence of neuropsychiatric conditions that include autism spectrum disorder (ASD). While evidence suggests that clinical phenotypes represent distinct neurodevelopmental outcomes, it remains unknown whether this translates to the level of neurobiology. To fractionate the 22q11.2DS phenotype on the level of neuroanatomy, we examined differences in vertex-wise estimates of cortical volume, surface area, and cortical thickness between 1) individuals with 22q11.2DS (n = 62) and neurotypical controls (n = 57) and 2) 22q11.2DS individuals with ASD symptomatology (n = 30) and those without (n = 25). We firstly observed significant differences in surface anatomy between 22q11.2DS individuals and controls for all 3 neuroanatomical features, predominantly in parietotemporal regions, cingulate and dorsolateral prefrontal cortices. We also established that 22q11.2DS individuals with ASD symptomatology were neuroanatomically distinct from 22q11.2DS individuals without ASD symptoms, particularly in brain regions that have previously been linked to ASD (e.g., dorsolateral prefrontal cortices and the entorhinal cortex). Our findings indicate that different clinical 22q11.2DS phenotypes, including those with ASD symptomatology, may represent different neurobiological subgroups. The spatially distributed patterns of neuroanatomical differences associated with ASD symptomatology in 22q11.2DS may thus provide useful information for patient stratification and the prediction of clinical outcomes.
Keywords: 22q11.2 Deletion Syndrome, autism spectrum disorder, brain anatomy, neurodevelopment, surface based morphometry
22q11.2 Deletion Syndrome (22q11.2DS) is a genetic condition resulting from a microdeletion at the q11.2 band of chromosome 22 (Scambler et al. 1992). The estimated prevalence of 22q11.2DS is about 1 in 4000 individuals (Tezenas Du Montcel et al. 1996), with an equal proportion of affected males and females (Swillen and McDonald-McGinn 2015). This makes 22q11.2DS the most common microdeletion syndrome in humans (Goodship et al. 1998; Scambler 2000). While all individuals with 22q11.2DS display a deletion within the same locus of chromosome 22, the phenotypic consequences of the deletion are, however, both complex and highly variable (McDonald-McGinn et al. 2015), making the neurobiology of 22q11.2DS inherently difficult to describe.
On the phenotypic level, 22q11.2DS is associated with a broad range of symptoms that encompass medical and neuropsychiatric conditions. For example, congenital and somatic features of 22q11.2DS typically include heart disease, palate abnormalities, as well as endocrine, (auto)immune, and gastrointestinal problems (Tezenas Du Montcel et al. 1996; Shprintzen 2000; McDonald-McGinn et al. 2015). In terms of psychiatric conditions, 22q11.2DS is associated with a high prevalence of attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), anxiety disorders, and obsessive-compulsive disorders (OCD) during childhood (Antshel et al. 2007; Habel et al. 2014); a high incidence of subthreshold psychotic symptoms during adolescence (Shapiro et al. 2011; Gothelf 2014); and psychotic spectrum disorders during adulthood (Murphy et al. 2000; Schreiner et al. 2013). Thus, the clinical phenotype of 22q11.2DS is highly heterogeneous and variable across the human lifespan, and poses a significant disease burden on affected individuals as well as their carers.
There is evidence to suggest that some of the neuropsychiatric disorders associated with 22q11.2DS represent distinct clinical outcomes. For example, a study by Fikinski et al. (2017) indicated that ASD and schizophrenia in individuals with the microdeletion should be regarded as 2 unrelated phenotypic manifestations (Fiksinski et al. 2017). This is consistent with the concept of neuropsychiatric “pleiotropy,” which refers to the notion that the same genetic variant has the potential to result in 2 or more distinct clinical phenotypes (Vorstman et al. 2013; Fiksinski et al. 2017). A similar principle might also apply to the level of neurobiology (i.e., the same genotype might result in different neurobiological phenotypes with distinct clinical symptoms). It remains, however, currently unknown whether the various neuropsychiatric phenotypes associated with 22q11.2DS are underpinned by different neurobiological mechanisms, and whether the neurobiology in 22q11.2DS individuals with a particular neuropsychiatric diagnosis significantly differs from those without. While there is some evidence to suggest that the neuropathology of the brain in 22q11.2DS is modulated by the existence and severity of positive psychotic symptoms (Jalbrzikowski et al. 2013), little is currently known about 22q11.2DS phenotypes with neurodevelopmental disorders, and ASD in particular.
Even though ASD is commonly observed in 22q11.2DS, with prevalence rates estimated to range from 18% to 58% (Antshel et al. 2007; Schneider et al. 2014; Fiksinski et al. 2017; Jalbrzikowski et al. 2017), the neuroanatomical underpinnings modulating ASD symptomatology in 22q11.2DS remain largely unknown. To date, several neuroimaging studies have been conducted to elucidate the neuroanatomy of 22q11.2DS. For example, it has been reported that 22q11.2DS individuals have reduced volume and surface area (SA) and an increase in cortical thickness (CT) compared with neurotypical controls (Jalbrzikowski et al. 2013; Schmitt et al. 2015; Lin et al. 2017). Further, 2 neuroimaging studies have examined the neuroanatomical correlates of ASD symptomatology in 22q11.2DS and taken together, these studies reported no significant difference in overall brain volume (Antshel et al. 2007; Jalbrzikowski et al. 2017), CT or SA (Jalbrzikowski et al. 2017) between 22q11.2DS individuals with and without ASD symptomatology. However, they report of significant differences in the volume of the right amygdala, with one study reporting an increase (Antshel et al. 2007) and the other a decrease (Jalbrzikowski et al. 2017). Further, a significant decrease was found in parahippocampal CT (Jalbrzikowski et al. 2017). However, these studies employed a region of interest rather than voxel- or vertex-wise approach, which may not be optimally suited to detect the subtle and spatially distributed neuroanatomical differences that are characteristic for ASD (Amaral et al. 2008; Ecker et al. 2010). To fractionate the highly heterogeneous 22q11.2DS phenotype, the present study thus aimed to establish differences in the surface anatomy of the cortex 1) between individuals with 22q11.2DS and neurotypical controls and 2) between 22q11.2DS individuals with ASD symptomatology and those without. Unlike previous investigations, we employed a spatially unbiased (i.e., vertex-wise) approach based on measures of cortical volume (CV), SA, and CT. It was hypothesized that individuals with 22q11.2DS would have globally decreased CV and SA compared with controls, and increased CT, as found in previous studies (Jalbrzikowski et al. 2013; Schmitt et al. 2015; Lin et al. 2017). Further, it was hypothesized that 22q11.2DS individuals with ASD symptomatology would significantly differ from those without, particularly in brain regions that have previously been linked to wider autistic symptoms and traits (e.g., frontotemporal and frontoparietal regions) (Ecker 2017).
Methods and Materials
Participants
A total of 62 individuals with a confirmed molecular diagnosis of 22q11.2DS (6–31 years, 30 males and 32 females) and 57 neurotypical controls (6–27 years, 27 males and 30 females), were recruited at 2 different sites: 1) The Institute of Psychiatry, Psychology and Neuroscience (IoPPN), London, UK; and 2) The Semel Institute for Neuroscience and Human Behaviour, University of California (UCLA), Los Angeles, USA. Approximately equal proportions of cases were recruited at each site (IoPPN N = 28, UCLA N = 34) (see Table 1 for participant demographics). The deletion was confirmed by in-situ hybridization (FISH) or microarray analysis test. Exclusion criteria for all participants included contraindications to magnetic resonance imaging (MRI), medical conditions or chromosomal anomalies other than 22q11.2DS, which may be associated with ASD or psychosis (e.g., tuberous sclerosis, fragile X syndrome, or Prader–Willi syndrome). Out of the 62 individuals with 22q11.2DS, 10 individuals were taking antidepressants, 4 were taking psychostimulants, and 5 were on antipsychotic medication. Out of the 5 individuals who were currently taking antipsychotic medication, 2 had a diagnosis of psychosis, 1 had a previous episode of psychosis but no current symptoms, and 2 did not have a diagnosis of psychosis. There were no other participants with a diagnosis of psychosis in our sample. Overall intellectual ability was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999). Participants with a full-scale IQ below 60 were excluded from the study.
Table 1.
Participant demographics and global brain measures for 22q11.2DS compared with controls
| 22q11.2DS | Control | Significance | ||||
|---|---|---|---|---|---|---|
| (n = 62 [30♂, 32♀]) | (n = 57 [27♂, 30♀]) | t | P | |||
| Age (years) | 16 ± 7 | (6–31) | 15 ± 6 | (6–27) | 0.71 | 0.480 |
| Full-scale IQ | 82 ± 13 | (60–116) | 114 ± 16 | (76–148) | −11.84 | <0.001 |
| Total gray vol (L) | 0.59 ± 0.23 | (0.41–0.89) | 0.64 ± 0.24 | (0.41–0.89) | −1.29 | 0.201 |
| Average CT (mm) | 2.72 ± 0.14 | (0.25–0.31) | 2.73 ± 0.12 | (2.46–2.99) | −0.64 | 0.522 |
| Total SA (m2) | 0.20 ± 0.20 | (0.13–0.25) | 0.23 ± 0.20 | (0.19–0.27) | −5.95 | <0.001 |
| Psychotropic medication (none/antipsych/antidep/stimul) | (43/5/10/4) | (49/0/6/2) | ||||
Data expressed as mean ± standard deviation (range). Medication: none, no medication; antipsych, antipsychotic medication; antidep, antidepressant medication; stimul, psychostimulant medication.
All participants with 22q11.2DS were assessed for ASD symptoms using the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000). We employed the same diagnostic criteria as outlined by Jalbrzikowski et al. (2017) and a consensus diagnosis of ASD symptomatology was given if participants scored above threshold on the reciprocal social interaction domain (cut-off = 10), as well as on either communication (cut-off = 8) or restricted, repetitive or stereotyped patterns domain (cut-off = 3) on the ADI-R, in addition to scoring above threshold on the combined score of social and communication in the ADOS (Jalbrzikowski et al. 2017). In our sample, this equated to all individuals with ASD symptomatology meeting diagnostic cut-offs in the social and communication domain of the ADI-R, but not in the repetitive domain. Of the 62 individuals with 22q11.2DS, 7 did not have ADI information, so were excluded from the comparisons within the 22q11.2DS group (see Table 2 for details). Out of the 55 randomly sampled 22q11.DS individuals with available ADI scores (i.e., we did not intentionally oversample for ASD), 30 met our ASD criteria (i.e., 54.5%). This group will subsequently be referred to as 22q11.ASD. A total of 25 individuals (i.e., 45.5%) did not meet our diagnostic criteria for ASD (subsequently referred to as 22q11.nonASD). For reasons of completeness, we also performed the analysis within the 22q11.2DS group employing “gold-standard” diagnostic criteria for ASD (i.e., meeting diagnostic thresholds on all 3 domains of the ADI-R). The results of this analysis are presented in Figure S1 in Supplementary Materials.
Table 2.
Participant demographics, clinical measures and global brain measures for 22q11.nonASD compared with 22q11.ASD
| 22q11.nonASD | 22q11.ASD | Significance | ||||
|---|---|---|---|---|---|---|
| (n = 25 [11♂, 14♀]) | (n = 30 [16♂, 14♀]) | t | P | |||
| Age (years) | 15 ± 6 | (6–25) | 16 ± 7 | (6–31) | −0.635 | 0.528 |
| Full-scale IQ | 86 ± 15 | (60–116) | 82 ± 12 | (61–112) | 1.066 | 0.291 |
| ADI-R Sociala | 5 ± 4 | (1–9) | 19 ± 5 | (10–28) | −11.00 | <0.001* |
| ADI-R Communicationa | 6 ± 4 | (0–16) | 14 ± 4 | (8–24) | −6.027 | <0.001* |
| ADI-R Repetitivea | 1 ± 1 | (0–4) | 3 ± 3 | (0–12) | −3.481 | 0.001* |
| ADOS Communicationb | 2 ± 1 | (0–6) | 3 ± 2 | (0–6) | −4.253 | <0.001* |
| ADOS Socialb | 3 ± 2 | (0–8) | 7 ± 3 | (1–14) | −5.036 | <0.001* |
| ADOS Repetitiveb | 1 ± 1 | (0–2) | 1 ± 1 | (0–1) | −1.494 | 0.141 |
| SIPS Positivec | 4 ± 4 | (0–10) | 4 ± 4 | (0–16) | −0.304 | 0.763 |
| SIPS Negativec | 5 ± 4 | (0–18) | 6 ± 5 | (0–15) | −1.365 | 0.180 |
| SIPS Disorganizedc | 2 ± 2 | (0–8) | 3 ± 3 | (0–9) | −0.834 | 0.409 |
| SIPS Generalc | 3 ± 3 | (0–10) | 5 ± 3 | (0–12) | −1.265 | 0.213 |
| Psychotropic medication (none/antipsych/antidep/stimul) | 21/1/1/2 | 20/4/6/1 | ||||
| Total gray vol (L) | 0.60 ± 0.22 | (0.41–0.88) | 0.60 ± 0.22 | (0.59–0.80) | 0.148 | 0.883 |
| Average CT (mm) | 2.71 ± 0.17 | (2.45–3.06) | 2.73 ± 0.12 | (2.53–2.92) | −0.239 | 0.812 |
| Total SA (m2) | 0.20 ± 0.25 | (0.13–0.25) | 0.21 ± 0.18 | (0.17–0.24) | −0.883 | 0.381 |
Data expressed as mean ± standard deviation (range). aData based on 55 individuals. bData based on 53 individuals. cData based on 43 individuals. Medication: none, no medication; antipsych, antipsychotic medication; antidep, antidepressant medication; stimul, psychostimulant medication. *P < 0.05.
Lastly, we administered the Structured Interview for Prodromal Syndromes (SIPS) (McGlashan 2001) to all participants. SIPS positive scores were used as a dimensional measure of positive psychotic symptoms (e.g., unusual thought content, suspiciousness, grandiose ideas, perceptual abnormalities, and/or disorganized communication), and negative scores were used as a dimensional measure of negative psychotic symptoms (e.g., social anhedonia, avolition, expression of emotion, experience of emotions and self, ideational richness, and occupational functioning). Items were rated on a scale from 0 to 6 (0=absent; 6=extreme severe level). There were no significant differences in positive or negative symptoms between the 22q11.ASD and 22q11.nonASD groups.
All participants, and parents accompanying those under 18, gave informed written consent/assent in accordance with ethics approval by National Research Ethics Service (NRES) Committee South Central (study reference: 12/SC/0576) and/or the UCLA Institutional Review Board (IRB).
MRI Data Acquisition
All participants were scanned with a contemporary MRI scanner operating at 3 T (Signa, GE Medical Systems at the IoPPN, London; Siemens “Tim Trio” at UCLA). High-resolution structural T1-weighted volumetric images were acquired with full head coverage, 166 contiguous slices (1.2-mm thickness, with 1.2 × 1.2-mm2 in-plane resolution), repetition time/echo time (TR/TE) of 7/2.8 ms (flip angle = 8 in, FOV = 26 cm). Consistent image quality was ensured by a semiautomated quality control procedure at both sites.
Cortical Surface Reconstruction Using FreeSurfer
FreeSurfer v6.0.0 software (http://surfer.nmr.mgh.harvard.edu/) was used to derive models of the cortical surface for each T1-weighted image. These well-validated and fully automated procedures have been extensively described elsewhere (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000; Segonne et al. 2004; Jovicich et al. 2006). In brief, a single filled white-matter volume was generated for each hemisphere after intensity normalization, extracerebral tissue was cropped, and image segmentation performed using a connected components algorithm. A triangular tessellated surface was then generated for each white-matter volume by fitting a deformable template, resulting in a cortical mesh for the pial (i.e., outer) and white-matter (i.e., inner) surface. The resulting surface models (n = 172) were visually inspected for reconstruction errors, and either 1) accepted “as is” (112 out of 172 or 65%), 2) rejected “as is” (34 out of 172 or 20%), mostly due to severe (motion) artefacts and/or the existence of extrabrain tissue such as dura that precluded a successful FreeSurfer reconstruction, or 3) referred for manual editing (26 out of 172 or 15%) in case of smaller (i.e., “local”) reconstruction errors. Following manual editing, the 26 images were (re-)preprocessed. Out of these, 19 surface reconstructions (i.e., 73%) did not improve significantly and were subsequently excluded from the statistical analysis. This meant that a total of 53 scans or 31% were excluded overall. The overall dropout was approximately equally distributed across sites, with 28 out of 91 scans excluded from UCLA (i.e., 30.77% including n = 15 controls, n = 4 22q11.ASD, and n = 9 22q11.nonASD individuals), and 25 out of 81 scans excluded from IoPPN (i.e., 30.86% including n = 12 controls, n = 6 22q11.ASD, and n = 7 22q11.nonASD individuals). In terms of diagnostic criteria, we excluded a total of 27 out of 84 controls (32%), and 26 out of 88 22q11.2DS individuals (29.5%). Dropout rates for cases and controls were thus very closely matched. Moreover, out of 81 individuals with available ADI-R data, we excluded 10 out of 40 (i.e., 25%) 22q11.ASD individuals, and 16 out of 41 (39%) 22q11.nonASD individuals. The difference in the proportion of excluded ASD versus non-ASD was, however, not statistically significant (χ2 = 1.2403, df = 1, P = 0.2654).
Measures of CT were computed as the closest distance from the gray-white matter boundary to the gray matter-cerebrospinal fluid boundary at each vertex on the tessellated surface (Fischl et al. 1999). For each participant, we also computed mean CT across the entire brain and total SA. Vertex-based estimates of SA were derived as outlined by (Winkler et al. 2012). To improve the ability to detect population changes, each parameter was smoothed using a 10-mm surface-based smoothing kernel.
Statistical Analyses
Statistical analyses were conducted using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat/) for Matlab (R2017a; MathWorks). Parameter estimates for vertex-based measures of CT, SA, and CV were estimated by regression of a general linear model (GLM) for each vertex i and subject j, with 1) group, gender and site as categorical fixed-effects factor; and 2) age and IQ as continuous covariates. As 22q11.2DS had decreased overall SA relative to controls, total SA was added as a covariate in the case–control analysis, so that:
where εi is the residual error at vertex i. To examine the influence of antipsychotic medication on the results, we also included medication status as a categorical covariate. The results of these analyses are presented in Figures S2 and S3 in Supplementary Materials. All between-group differences (22q11.2DS vs. controls and 22q11.ASD vs. 22q11.nonASD) were estimated from the corresponding coefficient β1, normalized by the corresponding standard error respectively. Corrections for multiple comparisons across the whole brain were performed using “random field theory” (RFT)-based cluster analysis for nonisotropic images using a cluster based significance threshold of P < 0.05 (2-tailed) (Worsley et al. 1999). Last, we examined pairwise Pearson correlation coefficients between morphometric features of individuals with 22q11.ASD (extracted as mean value across vertices within significant clusters), and measures of symptom severity (ADI-R and ADOS scores).
Results
Participant Demographics, Clinical Assessments, and Global Brain Measures
We found no significant differences in age, total CV, or mean CT between 22q11.2DS individuals and neurotypical controls (P < 0.05, 2-tailed; Table 1). However, individuals with 22q11.2DS had significantly lower full-scale IQ (t[117]=−11.838, P < 0.001), and had a significantly reduced total SA of the cortex (t[117]=−5.952, P < 0.001) (see Table 1 for details). There were no significant differences in age, full-scale IQ, total brain measures or SIPS scores between the 22q11.ASD and 22q11.nonASD groups (P < 0.05, 2-tailed; Table 2).
Vertex-wise Differences in CV, SA, and CT in 22q11.2DS Compared With Controls
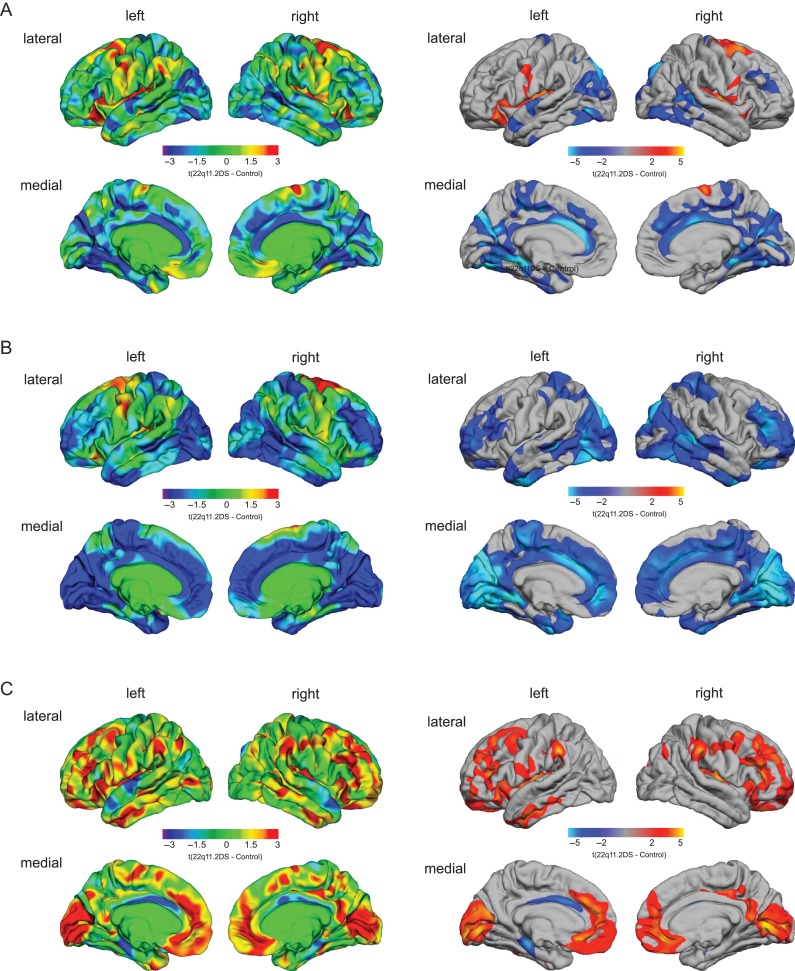
Following correction for multiple comparisons, we found significant neuroanatomical differences in 22q11.2DS compared with controls in several large clusters distributed across the cortex. More specifically, 22q11.2DS individuals had significant volumetric decreases in bilateral parietotemporal regions (approximate Brodmann area(s) [BA] 17–19) and in bilateral cingulate cortices (BA 23–24/30). Furthermore, we found increased volume in 22q11.2DS relative to controls in the bilateral precentral and postcentral gyrus, the insular cortex (BA 1–4), the right superior frontal gyrus (BA 6), and the left inferior frontal gyrus (BA 45/47) (Fig. 1A). Notably, the volumetric decreases we observed in the parietotemporal lobe and cingulate cortex in 22q11.2DS appeared to be largely driven by a significant decrease in SA, rather than differences in CT. In addition, 22q11.2DS individuals had significantly decreased SA in the bilateral dorsolateral–prefrontal cortices (DLPFC; BA 9–10/46–47), and medial–prefrontal regions (BA 9–11/32) (Fig. 1B).
Figure 1.
Significant differences in cortical volume (A), surface area (B), and cortical thickness (C) in individuals with 22q11.2DS compared with neurotypical controls. The left panel shows the unthresholded t-maps where increases in 22q11.2DS relative to controls are indicated in yellow-red (i.e., 22q11.2DS > controls), and decreases in cyan-blue (i.e., 22q11.2DS < controls). The right panel shows the random-field-theory (RFT)-based cluster-corrected (P < 0.05, 2-tailed) difference maps indicating significant increases (marked in red) and decreases (marked in blue) following correction for multiple comparisons.
In terms of CT, we observed that individuals with 22q11.2DS had significant increases in the bilateral dorsolateral–prefrontal cortices (DLPFC; BA 10–11/32) and in the medial regions of the occipital lobe (BA 17–19), as well as decreased CT in the left cingulate (BA 24) and parahippocampal gyrus (BA 34) (see Fig. S1C and Tables 1–3 in Supplementary Material for cluster summaries and statistical details). These results did not change significantly when covarying for the use of antipsychotic medication (see Figs S2 and S3 in Supplementary Materials).
Vertex-wise Differences in CV, SA, and CT in 22q11.ASD Compared With 22q11.nonASD
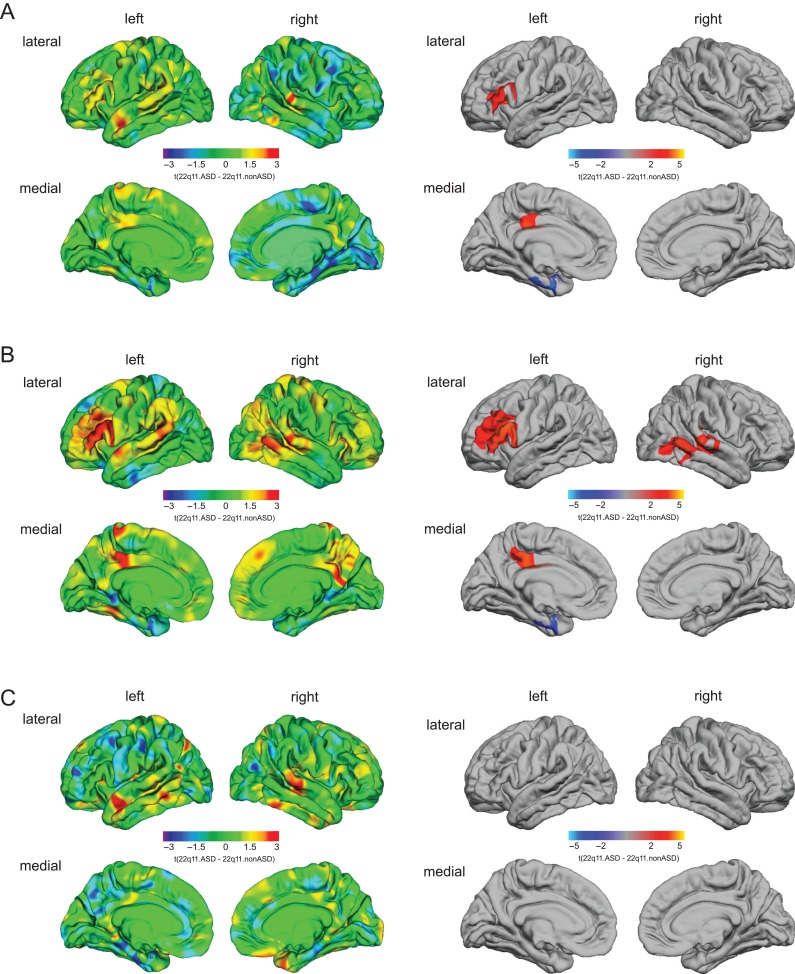
22q11.ASD compared with 22q11.nonASD individuals had significantly increased CV in left DLPFC (BA 45–46) and in left posterior cingulate cortex (PCC; BA 24) (Fig. 2A). In these clusters, the increased CV in 22q11.ASD individuals appeared to be driven by a significant increase in SA rather than an increase in CT (Fig. 2B). Furthermore, we also observed significantly increased SA in a large right-hemisphere cluster in 22q11.ASD relative to 22q11.nonASD individuals, which included temporoparietal junction (TPJ; BA 39/40), middle and superior temporal sulcus (STS) (BA 21–22/42), and the inferior temporal gyrus (BA 20/37) (see Table S4 in Supplementary Materials for cluster summaries and statistical details).
Figure 2.
Significant differences in cortical volume (A), surface area (B), and cortical thickness (C) in 22q11.ASD compared with 22q11.nonASD. The left panel shows the unthresholded t-maps where increases in 22q11.ASD relative to 22q11.nonASD are indicated in yellow-red (i.e., 22q11.ASD > 22q11.nonASD), and decreases in cyan-blue (i.e., 22q11.ASD < 22q11.nonASD). The right panel shows the random-field-theory (RFT)-based cluster-corrected (P < 0.05, 2-tailed) difference maps indicating significant increases (marked in red) and decreases (marked in blue) following correction for multiple comparisons.
Notably, individuals with 22q11.ASD had significantly decreased CV in the left entorhinal cortex (BA 28/34–35), which was accompanied by a commensurate decrease in SA. However, we did not observe any significant differences in CT in these clusters, nor anywhere else in the cortex (see Fig. 2C for details). Thus, individuals with 22q11.2DS who met ADI-R diagnostic cut-offs in the social and communication domain were neuroanatomically distinct from those 22q11.2DS individuals that did not meet cut-offs. Moreover, the volumetric differences associated with the occurrence of ASD symptomatology in 22q11.2DS appeared to be predominantly driven by differences in SA rather than CT. Comparable results were observed when covarying for the use of antipsychotic medication (see Fig. S4 in Supplementary Materials).
In the clusters with a significant difference in SA between 22q11.2DS individuals and controls, as well as between 22q11.2DS individuals with and without ASD symptomatology, we found that reductions in SA were more prominent in the 22q11.nonASD group than in the 22q11.ASD group, except for the cluster in the entorhinal cortex where the 22q11.2DS individuals with ASD symptomatology were more severely affected than the 22q11.2DS individuals without ASD symptomatology (see Fig. S4 in Supplementary Materials).
Relationship Between Neuroanatomical Differences in 22q11.ASD and the Severity of Autistic Symptoms
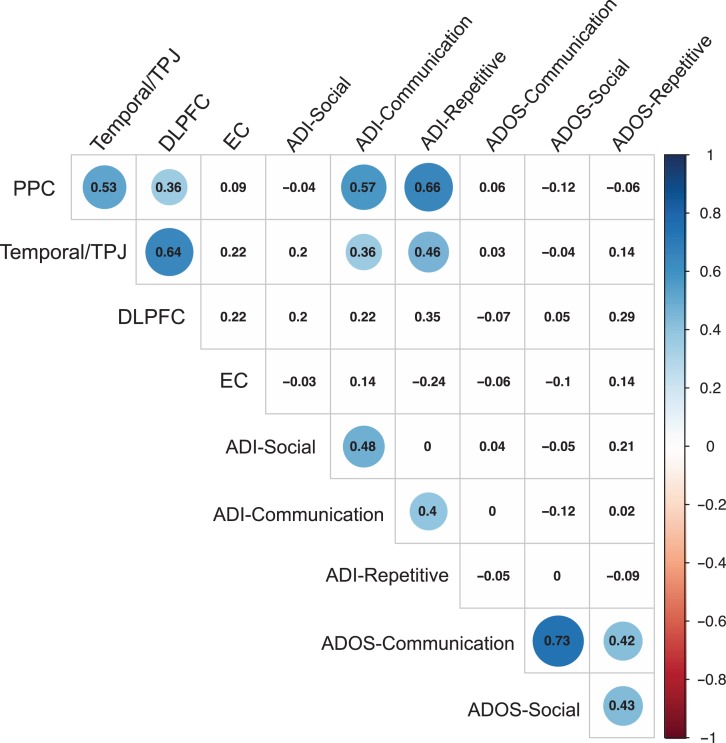
Within the 22q11.ASD group (n = 30), there were significant positive correlations between ADI-R communication scores and mean SA of the left PCC (r = 0.573, P < 0.001) and the right temporoparietal junction (temporal/TPJ; r = 0.363, P < 0.05). In addition, we found a significant positive correlation between the ADI-R repetitive behavior scores and SA of the left PCC (r = 0.658, P < 0.001) and right TPJ (r = 0.462, P < 0.05) (see Fig. 3 for details).
Figure 3.
Correlations between measures of ASD symptomatology and clusters with a significant difference in surface area between 22q11.ASD and 22q11.nonASD. Significant correlations (P < 0.05, uncorrected) are highlighted in blue.
Discussion
The present study aimed to establish differences in the surface anatomy of the cortex between 1) individuals with 22q11.2DS and neurotypical controls, and 2) between 22q11.2DS individuals with ASD symptomatology and 22q11.2DS individuals without. We report region-specific volumetric differences between 22q11.2DS individuals and neurotypical controls, particularly in parietotemporal and cingulate regions, which appeared to be largely driven by commensurate decreases in SA. More importantly, however, we also established that 22q11.2DS individuals with ASD symptomatology were neuroanatomically distinct from those without. Our findings suggest that the highly complex and heterogeneous clinical phenotype associated with 22q11.2DS may be further parsed out on the level of neurobiology, and that different neuroanatomical subgroups mediate the different clinical phenotypes commonly observed in 22q11.2DS.
We firstly compared individuals with 22q11.2DS with neurotypical controls and found neuroanatomical differences across all 3 measures of surface anatomy. For example, we observed significantly decreased volume in parietotemporal and cingulate regions in 22q11.2DS individuals, which were largely driven by a decrease in SA. We also observed increased volume in the bilateral insula. Further, 22q11.2DS individuals had significantly decreased SA in the bilateral dorsolateral–prefrontal cortices (DLPFC). In addition, we observed that individuals with 22q11.2DS had significantly increased CT in the DLPFC, medial regions of the occipital lobe and reduced CT in bilateral parahippocampal gyrus. Most of these regions have previously been highlighted by existing structural neuroimaging studies in 22q11.2DS. For example, Jalbrzikowski et al. (2013) and Schmitt et al. (2015) found increased volume in the bilateral insula, and decreased volume in parietotemporal regions and anterior cingulate cortices, which were accompanied by a commensurate decrease in SA (Jalbrzikowski et al. 2013; Schmitt et al. 2015). These studies also reported increased CT in the bilateral insula, paracentral, and medial orbitofrontal regions, as well as a cortical thinning in the bilateral parahippocampal gyrus (Jalbrzikowski et al. 2013; Schmitt et al. 2015). Further, in line with previous studies, we observed volume in the frontal lobes to be relatively preserved in 22q11.2DS (Eliez et al. 2000), suggesting the thickness of the cortex in this region might cancel out the significantly decreased SA (Schmitt et al. 2015). Our study thus complements previous findings, suggesting that the 22q11.2DS microdeletion is associated with structural abnormalities in the brain, which may impact on the various clinical phenotypes associated with the condition.
Secondly, we established that individuals with 22q11.2DS and ASD symptomatology significantly differ from 22q11.2DS individuals without ASD symptoms in terms of their neuroanatomy. For example, we observed significantly increased CV and SA in 22q11.ASD in left DLPFC, and posterior cingulate cortex (PCC), and an increase in SA at the right temporal lobe (inferior, middle, and superior). Furthermore, individuals with 22q11.ASD had significantly decreased volume and SA in the left entorhinal cortex. Notably, the increased SA we observed in the DLPFC, PCC and in the TPJ in 22q11.ASD relative to 22q11.nonASD corresponded to decreased SA resulting from the overall comparison between the 22q11.2DS and control group. It thus seems that the neuroanatomy in brain regions associated with ASD symptomatology—apart from the entorhinal cortex—does not represent a simple exacerbation of the 22q11.2DS phenotype per se, that is, more severe behavioral impairments are associated with more pronounced neuroanatomical differences. Rather, it seems that in these brain regions, ASD characteristics interact with 22q11.2DS to elicit neuroanatomical differences that cannot be explained by either the microdeletion or ASD symptomatology alone. For example, the SA of the dorsolateral prefrontal and cingulate cortices has recently been reported to be increased in ASD in a large sample of boys with idiopathic autism compared with neurotypical controls (Ohta et al. 2016). These brain regions also expand more rapidly during early brain development in ASD as has recently been demonstrated in a seminal study by Hazlett et al. (2017) (Hazlett et al. 2017). It is therefore likely that the set of brain regions we identified as being neuroanatomically distinct between 22q11.2DS individuals with and without ASD symptomatology highlights the set of brain regions where ASD interacts with—or modulates—the neuropathology of the brain in 22q11.2DS (or vise versa).
Although future studies are required to determine the extent, to which the pattern of neuroanatomical variability associated with ASD symptomatology in 22q11.2DS individuals overlap with the neuropathology of ASD in the general population (e.g., via the direct comparison of ASD individuals ± the 22q11.2 microdeletion), many of the brain regions highlighted in the present study are also brain regions that 1) are neuroanatomically different in idiopathic ASD (for a review see Ecker 2017), and 2) are functionally related to wider autistic symptoms and traits. For instance, the DLPFC, the STS, and the entorhinal cortex are integral parts of the so-called “social” and “emotional” brain, which encompasses a set of brain regions involved in wider aspects of social cognition and emotional processing (reviewed in Blakemore 2008; Pessoa 2008). ASD-related neuroanatomical variation in these regions has therefore also been linked to deficits in theory of mind (Castelli et al. 2002), face processing (Golarai et al. 2006), and various other aspects of impaired social cognition, for example, the perception of biological motion (Pelphrey et al. 2003), as well as self-referential cognition and empathy (Lombardo et al. 2010). Moreover, the PCC is an integral component of the so-called “default mode network” (DMN) that characterizes a wider network of brain regions that show decreased activity during cognitive tasks and that are active when the brain is “at rest” (Uddin et al. 2009). In ASD, the DMN has been reported to be among the most disrupted functional networks, and disrupted intrinsic DMN organization (e.g., in terms of functional connectivity patterns) has been associated with social deficits in children and adults with ASD (reviewed in Padmanabhan et al. 2017). Overall, there is a strong spatial correspondence between the neuroanatomical underpinnings of ASD symptomatology in 22q11.2DS, and the functional deficits and neuroanatomical hallmark of ASD in the general population.
Notably, we did not observe any significant differences in CT in relation to ASD symptomatology, despite the widespread differences observed when comparing the overall 22q11.2DS group to typically developing controls. This is perhaps not surprising as CT and SA represent independent (i.e., uncorrelated) sources of neuroanatomical variability, and hence contribute idiosyncratically to differences in CV. For example, it is known that CT and SA 1) are mediated by different sets of genes (Pontious et al. 2008; Fernández et al. 2016), 2) have separable neurodevelopmental trajectories (Ecker et al. 2014), and 3) are related to distinct aspects of the neural architecture (reviewed in (Geschwind and Rakic 2013). It is therefore important to examine the neuroanatomical variability of these features in isolation, to disentangle different molecular pathways underpinning the neuroanatomical differences in 22q11.2DS. Notably, differences in both CT and SA have previously been reported in individuals with 22q11.2DS relative to neurotypical controls, with most studies reporting significantly increased CT and decreased SA in 22q11.2DS (Jalbrzikowski et al. 2013; Schmitt et al. 2015). There is also evidence to suggest that measures of brain morphometry are susceptible to gene-dosage-effects in 22q11.2DS, with a positive relationship for SA (i.e., deletion < control < duplication), and negative variability for CT (i.e., deletion > control > duplication) (Lin et al. 2017). Thus, when investigating and interpreting the relationship between brain morphometry and different clinical subgroups of 22q11.2DS individuals, it will be important for future studies to establish whether this relationship is significantly modulated by genetic effects, such as gene-dosage-dependent variability in brain phenotypes.
While a thorough examination of the influence of psychotic symptoms on the neuroanatomy of 22q11.2DS would go beyond the initial scope of our study, we did assess the impact of psychotic symptoms and the use of antipsychotic medication on our results. We found that the neuroanatomical differences we observed between 22q11.ASD and 22q11.nonASD individuals were not driven by the co-occurrence of psychotic symptoms in our sample, as both groups were matched with regards to positive and negative psychotic symptoms as assessed by the SIPS, and 9 individuals (equally distributed across groups) had prodromal symptoms of psychosis, as measured by the SIPS. Further, most 22q11.2DS individuals (i.e., 97%), identified as having ASD symptomatology, did not have a comorbid diagnosis of psychosis. Naturally, the low degree of comorbidity between ASD and psychosis is to be expected in our sample of children and adolescence with 22q11.2DS (mean age = 16), given that the prevalence rate of psychosis is highest during adulthood in 22q11.2DS (Murphy et al. 2000; Schreiner et al. 2013). Our observation is also supported by previous clinical studies suggesting that psychosis and ASD in 22q11.2DS represent distinct clinical outcomes (Vorstman et al. 2013; Fiksinski et al. 2017). Further, when controlling for antipsychotic medication status, we found that our results remained stable in terms of effect sizes and the spatially distributed patterns of neuroanatomical differences observed (see Figs S2 and S3 in Supplementary Materials). Future research is, however, needed to directly compare 22q11.2DS individuals with ASD symptomatology and a diagnosis of psychosis to establish whether 1) the putative independence of these clinical outcomes is also mirrored on the level of neurobiology, and 2) whether neurobiological data can therefore successfully be utilized for their prediction.
Our results need to be interpreted in the light of several other methodological limitations. First, it is important to highlight that this study excluded a relatively high number of scans due to movement artefacts and reconstruction errors (i.e., 31%), compared with other studies employing similar techniques. Although this might affect the generalizability of the results, the excluded participants were very closely matched across both sites and groups, and we are confident that there was no systematic sampling bias affecting our results. Second, to ensure the comparability of our vertex-wise approach with a previously published region-of-interest analysis, we based the diagnostic criteria for ASD on a previously published study by Jalbrzikowski et al. (2017). In our sample, this resulted in all ASD individuals meeting ADI-R diagnostic cut-offs in the social and communication domain of the ADI-R. However, some individuals (n = 20) fell short of the cut-off in the repetitive domain, leading to an ASD prevalence rate of 54.4% in our sample. While this prevalence rate seems high, it compares well to other studies employing similar criteria for assessing ASD in 22q11.2DS populations (Antshel et al. 2007; Vorstman et al. 2013; Fiksinski et al. 2017; Jalbrzikowski et al. 2017). Our approach thus differs from the clinical “gold-standard” for diagnosing ASD in the research setting, where individuals are expected to meet cut-offs in all 3 domains of the ADI-R. To examine how the clinical characterization of ASD individuals affects our results, we have also performed a preliminary analysis of the neuroanatomy of 22q11.2DS when applying gold-standard diagnostic criteria for ASD. However, when comparing the individuals (n = 10) with a gold-standard diagnosis of ASD with the individuals who did not meet diagnostic cut-offs in all 3 ADI-R domains (n = 45), we observed no significant differences in brain anatomy (see Fig. S1 in Supplementary Materials). This lack of statistical significance might partially be explained by the small sample size and low statistical power; for example, the probability of detecting a significant effect within our sample on the vertex level is only 0.3 if one assumes a medium effect size of 0.5. Alternatively, our nonsignificant finding might be due to the fact that the 10 individuals meeting cut-offs in all 3 domains of the ADI-R are being compared with a group of 45 individuals out of which 20 individuals did meet cut-offs in the social and communication domain. This means that there is significant clinical overlap between both groups with regards to social and communication deficits, which could preclude the detection of a significant between-group difference. Our study was therefore only adequately powered to examine the neuroanatomical correlates of social and communication deficits in 22q11.2DS that are also a characteristic hallmark for ASD. This is not to say that repetitive/stereotyped behaviors may be less common in 22q11.2DS individuals, as has been noted previously (Kates et al. 2007). However, given our small sample size, we were unable to also examine repetitive symptoms directly. Future research in larger samples is thus necessary to also examine the neuroanatomical correlates of ASD-related repetitive symptoms in 22q11.2DS.
Third, a multicenter design was employed for MRI data acquisition to overcome single-site recruitment limitations. Although there are some disadvantages of using a multicenter approach, we carefully matched acquisition parameters across sites, and accounted for inter-site effects in the statistical model and our findings are therefore unlikely to be driven by inter-site effects alone. Further, due to difficulties scanning individuals with intellectual disability (IQ < 60), we only included those individuals who scored above 60 on the WASI. This resulted in both of our groups having a mean IQ around 10 points above average found in the general 22q11.2DS population (Antshel et al. 2005), which needs to be considered when interpreting our findings. Lastly, while we did correct for age-effects in the statistical model, our sample included individuals from a wide age range (6–31 years). Given the fact that the nature and severity of neuropsychiatric conditions associated with 22q11.2DS varies across different stages of development, it will be crucial to investigate the link between neuroanatomy and symptomatology within well-defined age groups in the future. The acquisition of large longitudinal samples will also be essential for the development of early biomarkers that are able to accurately predict the various clinical outcomes associated with 22q11.2DS, even before first symptoms manifest. Our findings indicating that different clinical manifestations of 22q11.2DS may be mediated by distinct neurobiological mechanisms is hence of importance for future studies designed to utilize such neurobiological information in the development of “preventative” treatment strategies based on an individual’s most likely clinical outcome(s).
Supplementary Material
Funding
National Institute of Mental Health (NIMH) RO129053 to C.E.B. Prof. Dr Ecker acknowledges support by grants EC480/1-1 and EC480/2-1 from the German Research Foundation under the Heisenberg Programme.
Notes
Furthermore, we would like to thank the National Institute for Health Research, Biomedical Research Centre for Mental Health, and the Dr Mortimer and Theresa Sackler Foundation. Conflict of Interest: None declared.
References
- Amaral DG, Schumann CM, Nordahl CW. 2008. Neuroanatomy of autism. Trends Neurosci. 31(3):137–145. [DOI] [PubMed] [Google Scholar]
- Antshel KM, AbdulSabur N, Roizen N, Fremont W, Kates WR. 2005. Sex differences in cognitive functioning in velocardiofacial syndrome (VCFS). Dev Neuropsychol. 28(3):849–869. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, AbdulSabur N, Higgins AM, Shprintzen RJ, Kates WR. 2007. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion). J Autism Dev Disord. 37(9):1776–1786. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. 2008. The social brain in adolescence. Nat Rev Neurosci. 9(4):267–277. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. 2002. Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 125:1839–1849. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis—i. Segmentation and surface reconstruction. Neuroimage. 9(2):179–194. [DOI] [PubMed] [Google Scholar]
- Ecker C. 2017. The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism. 21(1):18–28. [DOI] [PubMed] [Google Scholar]
- Ecker C, Rocha-Rego V, Johnston P, Mourao-Miranda J, Marquand A, Daly EM, Brammer MJ, Murphy C, Murphy DG, Consortium MA. 2010. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage. 49(1):44–56. [DOI] [PubMed] [Google Scholar]
- Ecker C, Shahidiani A, Feng Y, Daly E, Murphy C, D’Almeida V, Deoni S, Williams SC, Gillan N, Gudbrandsen M, et al. 2014. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. J Neural Transm. 121(9):1157–1170. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. 2000. Children and adolescents with velocardiofacial syndrome: a volumetric mri study. Am J Psychiat. 157(3):409–415. [DOI] [PubMed] [Google Scholar]
- Fernández V, Llinares‐Benadero C, Borrell V. 2016. Cerebral cortex expansion and folding: what have we learned? EMBO J. 35(10):1021–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, Vorstman JAS. 2017. Autism spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophr Res. 188:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis—ii: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 9(2):195–207. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. 2013. Cortical evolution: judge the brain by its cover. Neuron. 80(3):633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Grill-Spector K, Reiss AL. 2006. Autism and the development of face processing. Clin Neurosci Res. 6(3–4):145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. 1998. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 79(4):348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D. 2014. Measuring prodromal symptoms in youth with developmental disabilities: a lesson from 22q11 deletion syndrome. J Am Acad Child Adolesc Psychiatr. 53(9):945–947. [DOI] [PubMed] [Google Scholar]
- Habel A, Herriot R, Kumararatne D, Allgrove J, Baker K, Baxendale H, Bu’Lock F, Firth H, Gennery A, Holland A, et al. 2014. Towards a safety net for management of 22q11.2 deletion syndrome: guidelines for our times. Eur J Pediatr. 173(6):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Gu HB, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu HT, Otteron KNB, et al. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542(7641):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Hamzah Ahmed K, Patel A, Jonas R, Kushan L, Chow C, Bearden CE. 2017. Categorical versus dimensional approaches to autism-associated intermediate phenotypes in 22q11.2 microdeletion syndrome. Biol Psychiatry. 2(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, Bearden CE. 2013. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. NeuroImage Clin. 3:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J, et al. 2006. Reliability in multi-site structural mri studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 30(2):436–443. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM. 2007. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 ds) with and without autism. Am J Med Genet A. 143a(22):2642–2650. [DOI] [PubMed] [Google Scholar]
- Lin A, Ching CRK, Vajdi A, Sun D, Jonas RK, Jalbrzikowski M, Kushan-Wells L, Pacheco Hansen L, Krikorian E, Gutman B, et al. 2017. Mapping 22q11.2 gene dosage effects on brain morphometry. J Neurosci. 37:6183–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S, Consortium MA. 2010. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 22(7):1623–1635. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A. 1994. Autism diagnostic interview-revised—a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24(5):659–685. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, et al. 2015. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 1:15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH. 2001. Structured interview for prodromal syndromes (SIPS). New Haven: Yale University. [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. 2000. High rates of schizophrenia in adults with velo-cardio-facial syndrome (vcfs). Schizophr Res. 41(1):29. [DOI] [PubMed] [Google Scholar]
- Ohta H, Nordahl CW, Iosif AM, Lee A, Rogers S, Amaral DG. 2016. Increased surface area, but not cortical thickness, in a subset of young boys with autism spectrum disorder. Autism Res. 9(2):232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. 2017. The default mode network in autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2(6):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. 2003. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J Neurosci. 23(17):6819–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. 2008. On the relationship between emotion and cognition. Nat Rev Neurosci. 9(2):148–158. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. 2008. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci Basel. 30(1–3):24–32. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. 2000. The 22q11 deletion syndromes. Hum Mol Genet. 9(16):2421–2426. [DOI] [PubMed] [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. 1992. Velo-cardio-facial syndrome associated with chromosome-22 deletions encompassing the digeorge locus. Lancet. 339(8802):1138–1139. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, Whinna D, Souders MC, Satterwaite TD, Prabhakaran K, et al. 2015. Aberrant cortical morphometry in the 22q11.2 deletion syndrome. Biol Psychiatry. 78(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbane M, Bassett AS, Chow EWC, Fung WLA, van den Bree MBM, Owen M, Murphy KC, Niarchou M, Kates WR, et al. 2014. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry. 171(6):627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner MJ, Lazaro MT, Jalbrzikowski M, Bearden CE. 2013. Converging levels of analysis on a genomic hotspot for psychosis: insights from 22q11.2 deletion syndrome. Neuropharmacology. 68:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF. 2011. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophr Res. 129(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. 2000. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev Disabil Res Rev. 6(2):142–147. [DOI] [PubMed] [Google Scholar]
- Swillen A, McDonald-McGinn D. 2015. Developmental trajectories in 22q11.2 deletion syndrome. Am J Med Genet C. 169(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezenas Du Montcel S, Mendizabai H, Ayme S, Levy A, Philip N. 1996. Prevalence of 22q11 microdeletion. J Med Genet. 33(8):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. 2009. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 30(2):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JAS, Breetvelt EJ, Thode KI, Chow EWC, Bassett AS. 2013. Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophr Res. 143(1):55–59. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler abbreviated scale of intelligence (WASI) manual In: San Antonio. TX: Psychological Corporation. [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BTT, Fischl B, Greve DN, Kochunov P, Nichols TE, Blangero J, Glahn DC. 2012. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage. 61(4):1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Andermann M, Koulis T, MacDonald D, Evans A. 1999. Detecting changes in nonisotropic images. Hum Brain Mapp. 8(2–3):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.