Abstract
Intraventricular hemorrhage (IVH) is a common complication of prematurity in infants born at 23–28 weeks of gestation. Survivors exhibit impaired growth of the cerebral cortex and neurodevelopmental sequeale, but the underlying mechanism(s) are obscure. Previously, we have shown that neocortical neurogenesis continues until at least 28 gestational weeks. This renders the prematurely born infants vulnerable to impaired neurogenesis. Here, we hypothesized that neurogenesis is impaired by IVH, and that signaling through GSK3β, a critical intracellular kinase regulated by Wnt and other pathways, mediates this effect. These hypotheses were tested observationally in autopsy specimens from premature infants, and experimentally in a premature rabbit IVH model. Significantly, in premature infants with IVH, the number of neurogenic cortical progenitor cells was reduced compared with infants without IVH, indicating acutely decreased neurogenesis. This finding was corroborated in the rabbit IVH model, which further demonstrated reduction of upper layer cortical neurons after longer survival. Both the acute reduction of neurogenic progenitors, and the subsequent decrease of upper layer neurons, were rescued by treatment with AR-A014418, a specific inhibitor of GSK3β. Together, these results indicate that IVH impairs late stages of cortical neurogenesis, and suggest that treatment with GSK3β inhibitors may enhance neurodevelopment in premature infants with IVH.
Keywords: GSK3β, intermediate progenitors, intraventricular hemorrhage, neurogenesis, Pax6, Tbr2
Introduction
Intraventricular hemorrhage (IVH) remains a major problem in extremely premature infants. Survivors with IVH develop cognitive disabilities, mental retardation, learning disabilities, neurodevelopmental delay, and psychiatric disorders (Stephens and Vohr 2009; Indredavik et al. 2010). Developmental studies have reported that premature infants with even a low grade IVH are at a greater risk for impaired neurodevelopmental outcomes relative to preterm infants without IVH (Pinto-Martin et al. 1995; Whitaker et al. 1997; Vasileiadis et al. 2004). Consistent with neonatal follow-up data, volumetric MRI techniques have shown that premature infants with uncomplicated IVH display a major reduction in the cortical gray matter volume at near-term age (Vasileiadis et al. 2004). About 35% of preterm infants born between 23 and 28 weeks of gestation suffer from IVH (Adams-Chapman et al. 2008; Stoll et al. 2010). Since glutamatergic pyramidal neurons are produced until 28 weeks of gestation (Malik et al. 2013), the onset of IVH might adversely affect glutamatergic neurogenesis. Therefore, we asked whether occurrence of IVH would inhibit glutamatergic neurogenesis and reduce population of neurons in the cerebral cortex, and if so, how this could be ameliorated.
During early mammalian cortical development, radial glial cells in the ventricular zone (VZ) self-renew by symmetrical divisions, however, they switch later to asymmetric division to produce one daughter radial glia and one intermediate progenitor (IP) cell (Hansen et al. 2011). Differentiating IPs express transcription factor Tbr2 and migrate to the subventricular zone (SVZ), where they divide symmetrically to generate 2 neurons, or less frequently, self-amplify to generate 2 IPs (Englund et al. 2005). While some neurons are generated directly from radial glial progenitors, most cortical pyramidal-projection neurons develop from IPs (Kowalczyk et al. 2009). The neocortex in humans, in contrast to rodents, is larger and highly folded and exhibits a large outer SVZ. Cells in the outer SVZ express radial glia (nestin, Pax6, GFAP) and IP cell markers (Tbr2) and contribute to the neuronal production and development of gyrated human cerebral cortex (Lui et al. 2011). Glutamatergic neurogenesis in the dorsal telencephalon is orchestrated under the influence of transcription factors including Pax6, Neurogenin (Ngn) 1/2, Emx1/2, and Insm1; and the neuronal specification of upper cortical layers is regulated by Cux1, Brn2, and Satb2 genes (Englund et al. 2005; Bernardino et al. 2008; Lui et al. 2011). Neurogenesis is also regulated by effectors of Wnt and other signaling pathways, which control neuronal specification in the dorsal telencephalon (Lui et al. 2011). Together with transcription factors, Wnt signals are central regulators of proliferation as well as differentiation of neural progenitor cells (Pontious et al. 2008; Lui et al. 2011; Munji et al. 2011).
Glycogen synthase kinases (GSKs) are serine/threonine kinases that play key roles in neurogenesis (Hur and Zhou 2010). GSK3β, an isoform of GSK, is a dynamic enzyme that affect a broad range of transcription factors including neurogenin-2, β-catenin, SMAD1, and cyclic AMP response element-binding protein (CREB). In addition, Wnt signaling pathways inhibit GSK3β, which degrades β-catenin, a major effector of Wnt signaling. GSK3β is also regulated by sonic hedgehog (Shh), and plays a role in regulating Notch signaling, 2 additional pathways that are important in neuronal progenitor proliferation and maturation. Mice deficient in both GSK3α and GSK3β exhibit increased cortical surface area with a convoluted shape, but the cortex is thinner relative to control littermates, due to severely impaired neuronal differentiation (Kim et al. 2009). Consistent with these findings, inactivation of GSK3β by siRNA in E14.5 mice increased proliferation of radial glia cells, reduced Tbr2+ IPs, and diminished the population of Cux1+ upper cortical neurons (Ma et al. 2017). However, in vitro studies using adult neural stem cells suggest that GSK3β inhibition promotes neuronal differentiation (Maurer et al. 2007; Ahn et al. 2014). Accordingly, studies in animal models have revealed that GSK3β inhibition may promote neurogenesis (Guo et al. 2012; Aloni et al. 2015). Hence, the effect of GSK3β depends on the experimental context.
The occurrence of IVH in humans and rabbits reduces proliferation of all progenitors in the VZ and SVZ of both dorsal and ventral telencephalon (Del Bigio 2011; Dummula et al. 2011), but the effect of IVH specifically on neurogenesis has not been studied. Since differentiating neurons are recruited to cortical layers in an inside–out manner, and gestational ages 23–28 weeks correspond to late stages of neurogenesis, the upper cortical layers are most likely to suffer diminished growth due to IVH in preterm infants. Based on these considerations, we hypothesized that the occurrence of IVH would reduce glutamatergic neurogenesis in the dorsal SVZ thus decreasing neuronal populations in the upper cortical layers, and that GSK3β inhibition might restore neurogenesis and cortical development in survivors with IVH. To test these hypotheses, we studied autopsy samples from premature human infants, and we employed a rabbit model of prematurity with IVH. Our results highlight that IVH reduces neurogenesis and the population of upper cortical neurons in premature neonates, and that GSK3β inhibition ameliorates neurogenesis and the population of neurons in the upper cortical layers.
Materials and Methods
Human Subjects
The Institutional Review Board at the Albert Einstein College of Medicine in Bronx, NY, approved the use of autopsy brain samples from premature infants for the present study. The postmortem samples were forebrain tissue samples harvested from premature infants with and without IVH of 23–26 gestational weeks (gw). These postnatal age of infants was less or equal to 5 days (Supplementary Table S1), and the autopsy materials were obtained within 18 h of their demise. We excluded premature neonates with hypoxic-ischemic encephalopathy, meningitis, culture proven sepsis, major brain or spinal cord malformation, and chromosomal defects from the study. We included 6 preterm infants with IVH and 6 premature infants without IVH. The wall of the cerebral hemisphere in premature infants consists of VZ, SVZ, intermediate zone, subplate, cortical plate, and marginal zone, as described before (Nowakowski et al. 2016). In this article, we used the term intermediate-zone embryonic white matter interchangeably with white matter, ganglionic eminence with germinal matrix, and cerebral cortex with cortical plate.
Human Tissue Collection and Processing
We processed the human tissues as in our previous study (Ballabh et al. 2007). Coronal slices of 3–4 mm thickness were cut at the level of head of caudate nucleus from the frontoparietal lobe. The coronal blocks consisted of cortical plate, embryonic white matter, and ganglionic eminence. The samples were immersion-fixed in 4% paraformaldehyde in PBS overnight and were then cryoprotected by keeping them into a 15% sucrose solution in PBS, followed by 30% sucrose in PBS. The tissues were next frozen after embedding them into optimum cutting temperature compound (Sakura, Japan). Frozen coronal blocks were cut into sections of 18 μm thickness on a crytostat.
Animal Model of IVH
This study was approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine in Bronx, NY. We employed our preterm rabbit model of glycerol-induced IVH that has been extensively validated in prior studies (Chua et al. 2009; Vinukonda et al. 2010, 2016; Vose et al. 2013). We bought timed-pregnant New Zealand rabbits from Charles River Laboratories, Inc. (Wilmington, MA). C-section was carried out to deliver the premature kits at 28.5 days of gestational age (full-term = 32 days). Newborn kits were reared in an infant incubator at a temperature of 35 °C. We used rabbit milk replacer (Wombaroo, Glen Osmond, Australia) to gavage-feed the kits in a volume of 2 mL every 12 h (100 mL/kg/day) during the first 2 days, and feeds were then advanced to 125, 150, 200, 250, and 280 mL/kg at postnatal days 3, 5, 7, 10, and 14, respectively. To induce IVH, we treated rabbit kits of either sex with 50% glycerol (6.5 gm/kg) intraperitoneally at 4 h of age. Intraperitoneal glycerol produces IVH by causing intravascular dehydration, an increase in serum osmolality, consequent decline in intracranial pressure and rupture of fragile vessels in the ganglionic eminence. (Ballabh et al. 2007; Georgiadis et al. 2008). We quantified the severity of IVH by measuring ventricular volume (length, breadth, and depth in coronal and sagittal views) on head ultrasound at 24 h age using an Acuson X700 (Siemens) ultrasound machine. Kits with IVH were classified as moderate (70–150 mm3) and severe (151–250 mm3) IVH, based on ventricular volume (Fig. 3A). A ventricular volume <70 mm3 indicated either an absence of IVH or presence of small or microscopic hemorrhage. A ventricular volume of >200 mm3 indicate very severe IVH, which carried high risk of hydrocephalus and thinning of cerebral cortex at postnatal day (D)14. We thus excluded all kits with ventricular volume >200 mm3 noted at 24 h age and also kits with hydrocephalus at D14. Hydrocephalus was defined as a ventricular area that measures more than 3 SDs above the mean for age in kits without IVH. Thus, at 2-week age, a ventricular area of more than 9 mm2 at midseptal nucleus level or 12.4 mm2 (mean ± 3 standard deviation) at ventral posterolateral thalamus level was considered hydrocephalus. The rabbit kits with IVH were assigned to either treatment or control group at 24 h age, so that the severity of IVH was balanced between the comparison groups. In our model and in humans with IVH, hemorrhage in the ventricle is frequently associated with hemorrhage in the VZ, SVZ, and adjacent white matter which can be seen microscopically (Supplementary Fig. S1) and sometimes macroscopically.
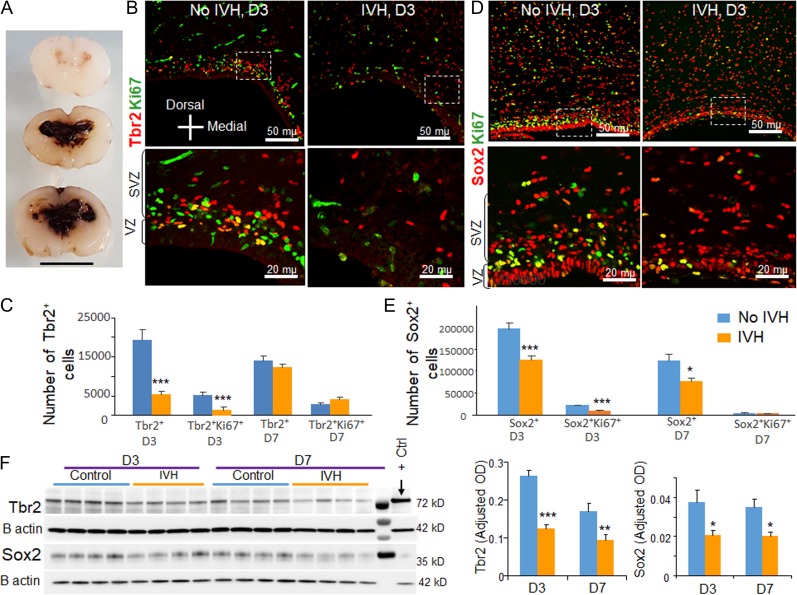
Figure 3.
Occurrence of IVH reduced Sox2 and Tbr2 in preterm rabbits. (A) Coronal brain slice from the frontoparietal lobe of E28.5 rabbit kits which show slit like ventricles in kits without IVH (upper panel) and moderate to severe IVH resulting in fusion of the lateral ventricles (middle and lower panel). Scale bar, 1 cm. (B–E) Representative immunofluorescence of cryosections from E28.5 rabbit kits with and without IVH at D3 (as indicated) labeled with ki67 and Tbr2/Sox2 specific antibodies. Upper panel is low power image and lower panel is high magnification images of the boxed area in the upper panel. Note diminished number of Tbr2+ and Sox2+ cells in rabbits with IVH relative to controls without IVH. The bar charts are mean ± s.e.m. (n = 5 each). The total and cycling Tbr2+ cells were reduced in rabbits with IVH compared with glycerol controls without IVH at D3, not at D7. All Sox2+ cells were reduced in rabbits with IVH compared with glycerol controls without IVH at both D3 and D7. Cycling Sox2+ cells were reduced in rabbits with IVH at D3, not at D7. (F) Representative Western blot analyses for Tbr2 and Sox2 on brain homogenates of preterm rabbits with and without IVH at D3 and D7. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Both Sox2 and Tbr2 levels were reduced in kits with IVH relative to controls without IVH at D3 and D7. ***P < 0.001, **P < 0.01, *P < 0.05 indicate comparison between rabbits with and without IVH at D3 and D7. Scale bar as indicated.
AR-A014418 Treatment
Rabbit kits with IVH were sequentially treated with either intramuscular AR-A014418 (10 μL, 20 mg/kg) or vehicle (DMSO) twice a day for 7 days, starting at day 1. The severity of IVH, evaluated by head ultrasound, was similar between the comparison groups—AR-A014418-treated and vehicle-treated kits with IVH. The dose of IM AR-A014418 was determined based on the prior studies (Kalinichev and Dawson 2011; Lee et al. 2016). Additionally, E28.5 kits (untreated with glycerol) were treated with either intramuscular AR-A014418 (20 mg/kg) or vehicle (DMSO) twice a day for 3 days, starting within 2 h age.
Rabbit Tissue Collection and Processing
We processed the tissues as described before (Ballabh et al. 2007). Briefly, the brain slices of 3 mm thickness at the level of midseptal nucleus were immersed into 4% paraformaldehyde in phosphate buffered saline (PBS; 0.1 M, pH 7.4) overnight and then were cryoprotected by submerging them into 15% sucrose in 0.1 M PBS buffer for 24 h followed by 30% sucrose for the next 24 h. We next froze the tissue slices after embedding them into an optimum cutting temperature compound (Sakura, Japan). We cut frozen coronal blocks into coronal sections of 18 μm thickness on a cryostat. For Western blot analyses, a 2 mm thick coronal slice was harvested at the level of the midseptal nucleus and snap-frozen on dry ice.
Stereological Assessment of Sox2+, Tbr2+, Satb2+, and Cux1+ Cells in the Dorsal Telencephalon
The techniques are described in Supplementary Methods.
Immunohistochemistry, Western Blot Analyses, Real Time Quantitative PCR, and Quantification of Pax6+ and Apoptotic Sox2+ Cells Under Confocal Microscope
The technical details are in Supplementary Methods.
Statistics and Analysis
Data are presented as means ± standard error of the mean (s.e.m.). To compare Tbr2+ and Sox2+ cells between rabbits with and without IVH and between AR-A014418 and vehicle controls at days 3 and 7, we used 2-way ANOVA. Presence of IVH (IVH vs. no IVH) and postnatal age (D3 or D7) were 2 independent variables. To compare Cux1+ and Satb2+ cells between 2 groups, we employed t-test. Similarly, we compared real time-qPCR and Western blot analyses data between AR-A014418 and vehicle treated kits at D3 and D7, using 2-way ANOVA. All post hoc comparisons between means were done by Tukey multiple comparison test at 0.05 significance.
Results
IVH Suppresses Neurogenesis in Human Premature Infants
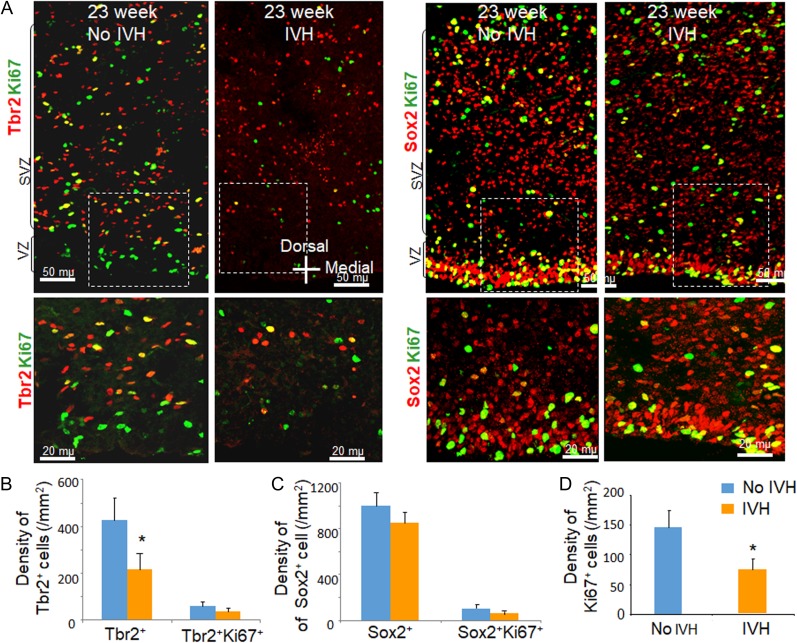
Neonatal hypoxia-ischemia increases hippocampal neurogenesis 1–2 weeks after injury in some studies (Plane et al. 2004; Ong et al. 2005; Spiegler et al. 2007). However, the effect of IVH or any other perinatal brain injury on glutamatergic neurogenesis in the neocortical SVZ has not been studied. We postulated that IVH might affect neocortical neurogenesis in premature human infants. To this end, we compared the density of total and proliferating Sox2+ and Tbr2+ cells (radial glial progenitors and IPs, respectively) between preterm infants with IVH and without IVH of 23–26 weeks gestation and 4–8 days postnatal age (Supplementary Table S1). The VZ and SVZ of dorsal telencephalon in autopsy brain samples were evaluated at level of the head of caudate nucleus. We found that the density of total Tbr2+ cells, as well as all proliferating cells (Ki67+), were reduced in infants with IVH compared with controls (P < 0.048 and 0.017 respectively; Fig. 1). However, the abundance of proliferating IPs (Ki67+/Tbr2+) was not reduced, suggesting that differentiating IPs were preferentially affected. Both total and cycling Sox2+ cells showed a trend toward decline in infants with IVH relative to infants without IVH, but the comparisons were not statistically significant. Tbr2+ cells appeared to be more abundant in the outer SVZ relative to the inner SVZ, consistent with our previous observation (Malik et al. 2013). Together with the significant reduction (~50%) in the density of Tbr2+ IPCs and all proliferating progenitors in the inner SVZ, we conclude that IVH in human infants reduces neocortical neurogenesis in extreme preterm infants.
Figure 1.
Occurrence of IVH reduced all cycling (Ki67+) and total Tbr2+ cells in human preterm infants. (A) Representative immunofluorescence of cryosections from preterm infants with and without IVH of 23 weeks gestation (as indicated) labeled with Ki67 and Tbr2/Sox2 specific antibodies. Upper panel is low power image and lower panel is high magnification images of the boxed area in the upper panel. Note diminished number of all cycling and total Tbr2+ cells in infants with IVH relative to controls without IVH. (B–D) The bar charts are mean ± s.e.m. (n = 5 each). The total Tbr2+ cells were reduced in infants with IVH compared with controls without IVH. Sox2+ cells were comparable between infants with IVH and without IVH. Total Ki67+ cells were reduced in infants with IVH compared with controls without IVH. *P < 0.05 indicate comparison between infants with and without IVH.
Progressive Decline in the Population of Radial Glia and IPCs as a Function of Postnatal Age in E28.5 Rabbits
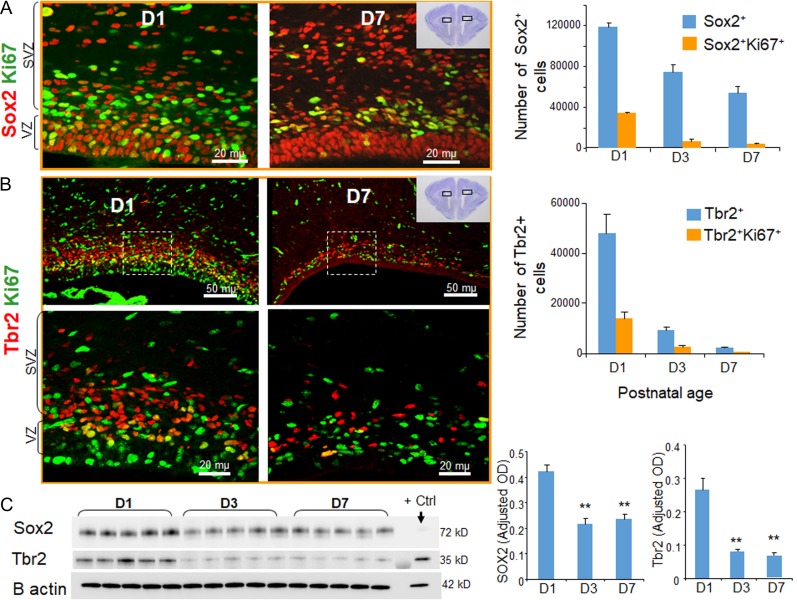
Since IVH suppresses neurogenesis in premature humans, we set out to evaluate whether IVH inhibits neurogenesis in premature rabbit kits as well. During development, glutamatergic neurogenesis terminates at about 28 weeks of gestation in humans, P10 in ferrets, and P5 in rats (Martinez-Cerdeno et al. 2012; Malik et al. 2013), however, the duration of pyramidal cell neurogenesis has not been previously evaluated in preterm rabbits (E28.5). We therefore evaluated the population of total and cycling radial glia and IPs in preterm rabbits at postnatal days (D) 1, 3, and 7, employing the same markers as used to evaluate human specimens, in coronal sections (midseptal nucleus level) from preterm rabbit forebrains. We found that Sox2+ radial glial cells and Tbr2+ IPs were abundantly present in the VZ and SVZ of rabbit dorsal telencephalon (Fig. 2A,B). Stereological quantification revealed that the number of total and cycling Sox2+ radial glia declined significantly as a function of postnatal age from D1 through D7 (P < 0.01, both; Fig. 2A). We also noted an abundance of Tbr2+ cells in the neocortical SVZ in E28.5 kits born prematurely. The total and cycling Tbr2+ IPs also declined with postnatal age from D1 to D7 (P < 0.01 both, Fig. 2B), and were scarce on D7. Both proliferating Tbr2+ and Sox2+ cells were more frequent on the inner SVZ compared with outer SVZ of premature rabbits.
Figure 2.
Reduction in the population of radial glia and IPCs as a function of postnatal age in E28.5 rabbits. (A) Representative immunofluorescence of cryosections from E28.5 rabbit kits labeled with Sox2 and Ki67 specific antibodies at D1 and D7 (as indicated). Note reduced expression of total and cycling Sox2+ cells in the VZ and SVZ of the dorsal telencephalon of rabbits at D7 compared with D1. The bar charts are mean ± s.e.m. (n = 5 each). The number of total and cycling Sox2+ radial glia were reduced as a function of postnatal age from D1 through D7. (B) The coronal cryosections from forebrain of E28.5 kits at D1 and 7 were labeled with Tbr2 and Ki67 specific antibodies. Upper panel is low power image and lower panel is high magnification images of the boxed area in the upper panel. Note abundance of Tbr2+ cells in the SVZ (as indicated) in E28.5 kits at D1 and scarcity of Tbr2 at D7. The bar charts are mean ± s.e.m. (n = 5 each). The number of total and cycling Tbr2+ IPC were reduced as a function of postnatal age from D1 through D7. (C) Representative Western blot analyses for Sox2 and Tbr2 on forebrain homogenates of preterm rabbits at D1, D3, and D7. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Both Sox2 and Tbr2 levels were reduced in kits at D3 and D7 relative to D1. **P < 0.01: indicate comparison between rabbits at D3 and D7 versus D1. Scale bar as indicated.
Consistent with these observations, Western blot analyses showed that both Tbr2 and Sox2 expression significantly declined from D1 through D7 (P = 0.01, both; Fig. 2C). Together, these results show that Sox2+ radial glia and Tbr2+ IPC were abundantly present in the dorsal telencephalon in E28.5 rabbits and diminished in number as a function of postnatal age.
IVH Suppresses Neurogenesis in Premature Rabbit Kits
As radial glial cells and IPs were abundantly present in the dorsal telencephalon of E28.5 rabbits during the first week of postnatal life, we employed a preterm rabbit model of IVH (Fig. 3A) to assess the effect of IVH on glutamatergic neurogenesis. In this model, IVH is induced by injecting intraperitoneal glycerol at 2 h of age, and rabbits get IVH within 6 h of administration. We evaluated double-labeled coronal sections (midseptal nucleus level) with Tbr2 and Ki67 specific antibodies. We found that both total and cycling Tbr2+ IPs were reduced in rabbits with IVH compared with glycerol controls without IVH at D3 (P < 0.001, Fig. 3B,C), but not at D7. We next evaluated the effect of IVH on radial glia cells. We observed that all Sox2+ radial glial cells were reduced in rabbits with IVH compared with glycerol controls without IVH at both D3 and D7 (P = 0.001 and 0.028, Fig. 3D,E). Proliferating Sox2+ cells were reduced in rabbits with IVH at D3 (P < 0.001), but not at D7.
To further confirm our stereological quantification of radial glia cells and IPCs, we performed western blot analyses. Consistent with our immunohistochemical findings, we found that both Tbr2 and Sox2 levels were reduced in rabbits with IVH compared with controls without IVH at both D3 (P = 0.001 and 0.013, respectively) and D7 (P = 0.008 and 0.026, respectively, Fig. 2F).
To determine the number of actively dividing progenitors in the dorsal SVZ in S phase and mitotic phase (G2/M), we quantified BrdU+ and Phospho-Histone H3+ (Ph3+) cells in the dorsal VZ and SVZ. To this end, we treated rabbits with Brdu (25 mg/kg twice a day for 3 days) and euthanized them at D1 (2 dose of BrdU) and D3 (6 doses of BrdU). Evaluation of coronal sections labeled with BrdU and Ph3 specific antibody revealed that BrdU+ cells were fewer in rabbits with IVH at both D1 and D3 (P < 0.01 both; Supplementary Fig. S2A,B). Accordingly, Ph3+ cells were reduced in rabbits with IVH at D3 (P = 0.04), but not at D1. However, Ki67+ cells were significantly reduced in rabbits with IVH at both D1 and D3 (P = 0.04 and 0.008, respectively; Supplementary Fig. S2C,D). Since Sox2+ and Tbr2+ cells are abundant in the dorsal SVZ, these data suggest that number of these progenitors in S-phase and M-phase are reduced in rabbits with IVH compared with controls.
These results suggest that IVH suppresses proliferation of both radial glia cells and IPs, as well as differentiation of Sox2+ radial glia into Tbr2+ IPCs in the VZ and SVZ of the dorsal telencephalon.
IVH Induces Apoptosis of Neuronal Progenitors
Our previous studies have shown that IVH induces apoptosis of neural cells in the periventricular germinal zones, peaking at 24 h after the occurrence of IVH (Georgiadis et al. 2008). We therefore postulated that IVH will result in apoptosis of radial glia cells and IP cells. To this end, we performed TUNEL staining of coronal sections and then immunostained the sections with Sox2 or Tbr2 specific antibodies. We found that TUNEL+ cells were about 4-fold higher in the VZ, SVZ, and the adjacent white matter of rabbit kits with IVH compared with glycerol controls without IVH (P < 0.001). Of all TUNEL+ cells, ~10% colabeled with Sox2+ antibodies. The density of TUNEL+Sox2+ cells were significantly higher in kits with IVH compared with controls without IVH (P < 0.001, Supplementary Fig. S3).
We next evaluated cells colabeled with TUNEL and Tbr2 specific antibody. They were rare to absent and thus were not quantified. This may be attributed to their reduced vulnerability to apoptosis and their relatively smaller number compared with Sox2+ cells in the VZ and SVZ in E28.5 rabbits at 24 h age.
IVH Reduces the Number of Pyramidal Neurons in Upper Cortical Layers
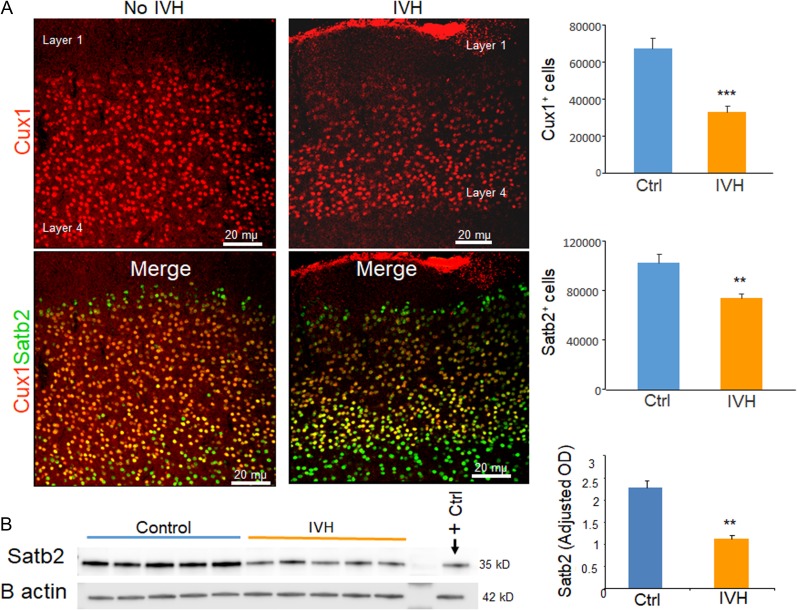
As IVH suppresses the number of neurogenic progenitors in dorsal VZ and SVZ, we reasoned that reduced glutamatergic neurogenesis might lead to diminished number of neurons in upper layers of the cerebral cortex. Transcription factors Cux1/2 have been identified as restricted molecular markers of upper layer (II–IV) neurons in murine and human cerebral cortex; likewise, Satb2 is also expressed predominantly in upper layer neurons (Ong et al. 2005; Arion et al. 2007; Britanova et al. 2008). We thus labeled coronal sections with Cux1 and Satb2 specific antibodies, and compared their abundance in rabbits with and without IVH at D14. Stereological quantification revealed that number of both Cux1+ and Satb2+ neurons were reduced in rabbits with IVH compared with controls without IVH at D14 (P = 0.001 and 0.01, respectively; Fig. 4A).
Figure 4.
IVH reduced number of neurons in the upper cortical layer. (A) Representative immunofluorescence of cryosections from preterm rabbits with and without IVH at D14 (as indicated) labeled with Cux1 and Satb2 specific antibodies. Upper panel is Cux1 and lower panel is merge image of Cux1 and Satb2 form upper cortical layer as indicated. Note reduced number of Cux1+ and Satb2+ neurons in rabbits with IVH compared with controls without IVH. The bar charts are mean ± s.e.m. (n = 5 each). Stereological quantification revealed that both Cux1+ and Satb2+ neurons were reduced in rabbits with IVH compared with controls. (B) Representative Western blot analyses for Satb2 on brain homogenates of preterm rabbits with and without IVH at D14. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Satb2 levels were reduced in rabbits with IVH relative to controls. **P < 0.01, ***P < 0.001 indicate comparison between infants with and without IVH.
To further confirm our finding, we performed Western blot analyses and found that Satb2 protein levels were diminished in rabbits with IVH compared with controls without IVH (P = 0.01, Fig. 4B). Together, the data suggest that IVH not only suppresses neurogenesis, but also reduces the number of cortical neurons in layers II–IV.
IVH Increases Pax6 Levels and Reduces Phosphorylation of Retinoblastoma Protein
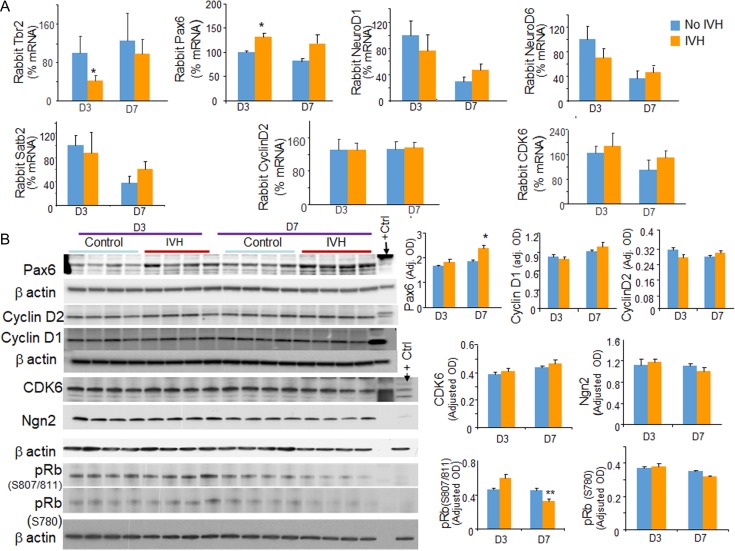
To determine the mechanisms underlying the effect of IVH on neurogenesis, we evaluated transcription factors regulating glutamatergic neurogenesis and corticogenesis, including Pax6, NeuroD1, NeuroD6, and Satb2, by real time qPCR using TaqMan probes (Englund et al. 2005; Lui et al. 2011). Neurogenin1/2 TaqMan probe could not be constructed as these gene sequences are unknown for rabbits. The mRNA expression of Tbr2 was reduced in rabbits with IVH at D3 (P = 0.04, Fig. 5A), but not at D7. In contrast, mRNA expression of Pax6 was elevated in rabbits with IVH compared with controls without IVH at D3 (P = 0.048), but not at D7. The expression of NeuroD1, NeuroD6, and Satb2 were comparable between rabbits with and without IVH at both D3 and D7. Accordingly, Western blot analyses also showed that Pax6 levels were elevated in rabbits with IVH compared with controls without IVH at D7 (Fig. 5B), not at D3. It is plausible that an early increase in Pax6 transcription (D3) was reflected as an elevated Pax6 protein level at D7. Ngn2 protein levels were comparable between kits with and without IVH. Together, these data suggest that a reduction in neurogenesis in rabbits with IVH can be attributed to a downregulation of Tbr2, and the increased expression of Pax6.
Figure 5.
IVH increases Pax6 levels and reduces phosphorylation of retinoblastoma protein. (A) The mRNA expressions of Tbr2, Pax6, NeuroD1, NeuroD6, Satb2, CYCLIN D2, and CDK6 were quantified by real time-qPCR using TaqMan probes in rabbits with and without IVH at D3 and D7. Note reduced expression of Tbr2 at D3 and elevated levels of Pax6 at D3 in rabbits with IVH relative to controls without IVH. The expression of NeuroD1, NeuroD6, and Satb2 were comparable between rabbits with and with IVH at both D3 and D7. (B) Representative Western blot analyses for Pax6, cyclin D1, cyclin D2, CDK6, Ngn2, p-Rb (S807/811), and p-Rb (S780) on brain homogenates of preterm rabbits with and without IVH at D3 and 7. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Pax6 levels were increased in rabbits with IVH relative to controls at D7; and p-Rb (S807/811) levels were reduced in rabbits with IVH at D7. *P < 0.05, **P < 0.01 indicate comparison between infants with and without IVH for D3 or D7.
Cell cycle is linked with neurogenesis and brain size is governed by cell cycle machinery (Spiegler et al. 2007). Therefore, we evaluated key molecules regulating cell cycle. Both mRNA and protein expression of cyclin D1, cyclin D2 and CDK6 were comparable between rabbits with and without IVH. Since, phosphorylation of the retinoblastoma (Rb) protein during the G1 phase of the mammalian division cycle is a major control element regulating passage of cells into S phase and through the division cycle, we quantified serine 780 and serine 807/811 phosphorylation of Rb. We found that pRb (serine 780) was reduced in rabbits with IVH compared with controls without IVH (P < 0.05), but not pRb (serine 807/811). Together, data suggest that an elevation in Pax6 as well as a reduction in Tbr2 transcription and phosphorylation of Rb protein contributes to reduced neurogenesis in rabbits with IVH. The data are consistent with the previous studies showing that the suppression of Pax6 promotes cell proliferation of human retinoblastoma cells (Seira and Del Rio 2014).
GSK3β Inhibition by AR-A014418 (ARA) Treatment Reduces Apoptosis of Neuronal Progenitors
Since activation of Wnt signaling promotes neurogenesis in E13.5 and 15.5 mice (Munji et al. 2011), we chose to upregulate Wnt signaling by GSK3β inhibition to restore neurogenesis in rabbits with IVH. To this end, we treated IVH-affected premature rabbits with intramuscular ARA or vehicle. To determine whether ARA treatment stimulated Wnt signaling, we performed Real time qPCR for Axin2. We found that the development of IVH reduced mRNA expression of Axin2 (P = 0.037) and ARA treatment increased Axin2 expression in kits with IVH at D7 (P = 0.04, Supplementary Fig. S4). To determine whether ARA treatment upregulates Wnt signaling specifically on Sox2+ progenitors, we labeled coronal sections with Axin2 and Sox2 or Tbr2 specific antibodies. We observed increased Axin2 immunoreactivity around Sox2+ cells in the dorsal SVZ of ARA-treated kits with IVH compared with vehicle controls at D7 (Supplementary Fig. S4). We could not combine immunolabeling of Axin2 and Tbr2 specific antibodies related to technical reasons. The data suggest that IVH downregulates Wnt signaling, which is reversed by ARA treatment (Supplementary Fig. S4).
GSK3β promotes apoptosis by inhibiting prosurvival factors such as CREB and heat shock protein, and facilitating proapoptotic factor p53 (Grimes and Jope 2001; Watcharasit et al. 2002). Accordingly, GSK3-β inhibition reduces apoptosis and offers neuroprotection in traumatic brain injury through activation of Wnt and RTK signaling pathways (Shim and Stutzmann 2016). Therefore we postulated that GSK3-β inhibition would reduce IVH-induced apoptosis of Sox2+ cells in the dorsal telencephalon. To this end, we compared the density of cells colabeled with caspase-3 and Sox2 antibodies between rabbits without IVH, vehicle- and ARA-treated rabbits with IVH.
Quantification of apoptotic cells in immunolabeled sections showed that caspase-3+Sox2+ cells were significantly higher in rabbits with IVH compared with controls without IVH at D3 (P < 0.001, Supplementary Fig. S5). More importantly, ARA treatment reduced the density of caspase-3 and Sox2 colabeled cells at D3 (P < 0.05). In addition, ARA treatment reduced the total number of capsase3+ cells (P < 0.01). Data show that ARA treatment offers neuroprotection by reducing apoptosis of Sox2+ radial glia cells in the dorsal SVZ.
GSK3β Inhibition by AR-A014418 (ARA) Treatment Enhances Neurogenesis
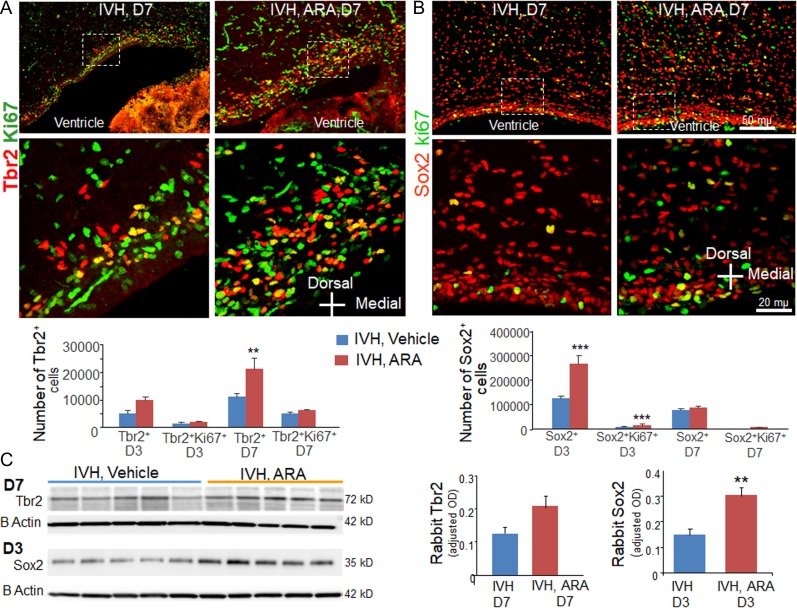
GSK3β inhibition stimulates Wnt signaling to increase β catenin levels and enhances neurogenesis in both culture studies and in vivo experiments (Hirsch et al. 2007; Munji et al. 2011; Seib et al. 2013). Therefore, we assessed the effect of GSK3β inhibition on neurogenesis in the dorsal SVZ. To this end, we immunolabeled coronal sections from ARA and vehicle treated kits with IVH using Ki67 and Tbr/Sox2 specific antibodies and quantified the total and cycling Tbr2+ IPCs and Sox2+ radial glia. Stereological quantification showed that all Tbr2+ cells were increased in ARA-treated kits compared with vehicle controls at D7 (P = 0.007, Fig. 6A), but not at D3. The cycling Tbr2+ cells showed a trend toward increase at both D3 and D7, but the difference was not significant. We next quantified Sox2+ cells and found that both total and cycling Sox2+ cell were higher in ARA-treated kits compared with vehicle controls at D3 (P < 0.001, both; Fig. 6B), but not at D7.
Figure 6.
AR-A014418 (ARA) treatment enhances neurogenesis. (A, B) Representative immunofluorescence of cryosections from preterm rabbits with vehicle and ARA treatment at D7 (as indicated) labeled with Ki67 and Tbr2/Sox2 specific antibodies. Upper panel is low power image and lower panel is high magnification images of the boxed area in the upper panel. Note abundance of Tbr2+ and Sox2+ cells in ARA-treated kits with IVH compared with vehicle controls with IVH. The bar charts are mean ± s.e.m. (n = 5 each).The total number of Tbr2+ IPCs were increased in ARA-treated kits compared with Vehicle controls at D7. The total and cycling Sox2+ cell were higher in ARA-treated kits compared with vehicle controls at D3. (C) Western blot analyses was performed on brain homogenates of preterm rabbits with and without IVH using Tbr2 and Sox2 specific antibodies. Sox2 expression was increased in ARA-treated rabbits compared with vehicle controls with IVH at D3. Tbr2 levels were comparable between groups. **P < 0.01, ***P < 0.01 indicate comparison between ARA and vehicle treated kits with IVH for D3 or D7.
To confirm the immunohistochemical data, we performed Western blot analyses. We found that Sox2 protein levels were increased in ARA-treated rabbits compared with vehicle controls at D3 (P < 0.01). Tbr2 protein levels also showed an insignificant trend toward elevation in ARA-treated kits compared with vehicle controls with IVH (P = 0.06, Fig. 6C). Together, ARA treatment increased the population of total and cycling Sox2+ radial glial progenitors at D3, and the number of Tbr2+ cells increased with decline in Sox2 cells at D7. This suggests that ARA treatment enhances both proliferation and differentiation of neuronal progenitors.
GSK3β Inhibition Does not Affect Neurogenesis in Healthy Kits Without IVH
Since GSK3β inhibition promotes adult neurogenesis (Morales-Garcia et al. 2012), we postulated that GSK3-β inhibition might enhance neurogenesis in the dorsal SVZ of healthy E28.5 kits (untreated with glycerol). To this end, we treated the preterm kits with either ARA or vehicle for 3 days and evaluated total and cycling Sox2 and Tbr2 cells in immunolabeled sections. Our stereological quantification showed that ARA treatment did not significantly affect the number of total and cycling Sox2+ in the dorsal telencephalon at D3 (P = 0.4 and 0.6, respectively; Supplementary Fig. S6). Moreover, total or cycling Tbr2+ cells were comparable between ARA and vehicle treated kits (P = 0.28 and 0.08, respectively). This suggests that ARA treatment does not affect neurogenesis in healthy kits, unlike kits with IVH.
GSK3β Inhibition Expands Upper Layer Cortical Neuronal Population
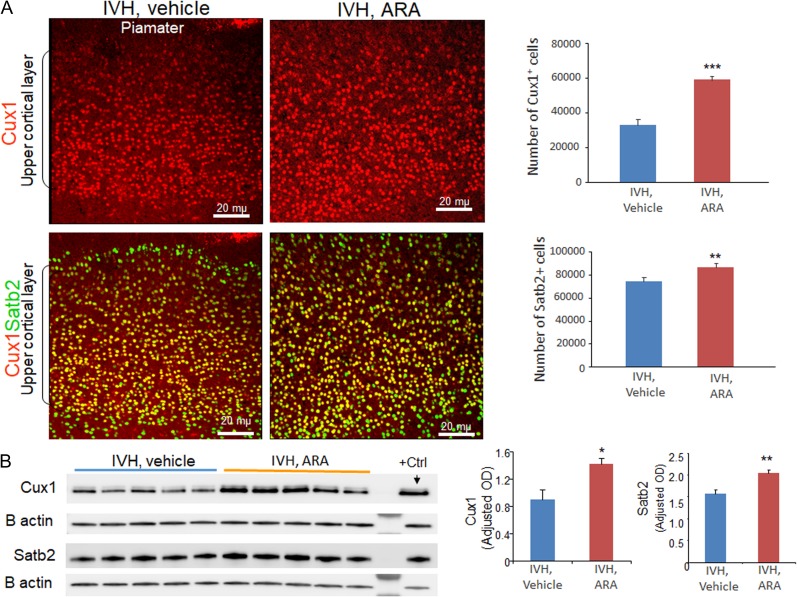
As ARA treatment increased production of IPs, we reasoned that this might also increase the number of cortical upper layer neurons. To this end, we labeled brain sections from ARA- and vehicle-treated kits with IVH at D14, using Cux1 and Satb2 specific antibodies. Stereological quantification of the cell number in the upper cortical layer revealed that the numbers of both Cux1+ and Satb2+ cells were increased in ARA-treated kits compared with vehicle controls at D14 (P = 0.001, 0.04 respectively; Fig. 7A).
Figure 7.
ARA treatment increases the number of neurons in the upper cortical layer. (A) Representative immunofluorescence of cryosections from preterm rabbits with IVH treated with ARA or vehicle at D14 (as indicated) labeled with Cux1 and Satb2 specific antibodies. Upper panel is Cux1 and lower panel is merge image of Cux1 and Satb2 form upper cortical layer as indicated. Note increased number of Cux1+ and Satb2+ neurons in ARA-treated rabbits with IVH compared with vehicle controls. The bar charts are mean ± s.e.m. (n = 5 each). Stereological quantification revealed that both Cux1+ and Satb2+ neurons were increased in ARA-treated rabbits with IVH compared with vehicle controls. (B) Representative Western blot analyses for Cux1 and Satb2 on brain homogenates of preterm rabbits with IVH treated with ARA or vehicle at D14. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Satb2 and Cux1 levels were increased in ARA-treated rabbits with IVH relative to vehicle controls. *P < 0.05, **P < 0.01, ***P < 0.001 indicate comparison between ARA and vehicle treated kits with IVH for D3 or D7.
Consistent with immunohistochemistry, Western blot analysis showed elevation of Cux1 and Satb2 protein levels at D14 in ARA-treated compared with vehicle-treated kits with IVH (P = 0.014 and 0.003, respectively; Fig. 7B). The data suggest that GSK3-β inhibition increased glutamatergic neurons in the upper cortical layers.
GSK3β Inhibition Increases Tbr2 and NeuroD1 Transcription, and Reduces Pax6 Expression
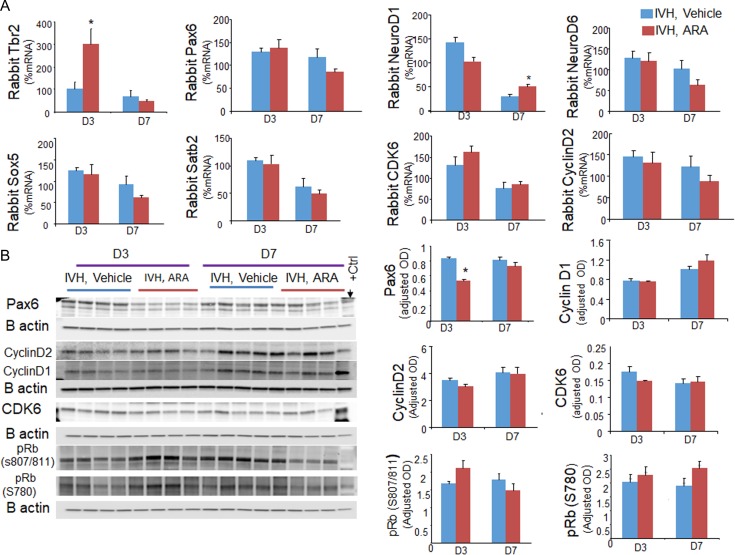
To understand the underlying mechanisms of GSK3-β inhibition promoting neurogenesis and corticogenesis, we quantified key transcription factors regulating glutamatergic neurogenesis and corticogenesis including Pax6, NeuroD1, NeuroD6, and Satb2 by real time qPCR using TaqMan probes (Englund et al. 2005; Lui et al. 2011). We found that mRNA expression of Tbr2 was elevated at D3 (P = 0.02) and NeuroD1 was increased at D7 in ARA-treated rabbits with IVH compared with vehicle controls (P = 0.002, Fig. 8A). Although, mRNA expression of Pax6 was similar between ARA and vehicle treated rabbits with IVH, the protein level of Pax6 was reduced in ARA-treated kits compared with controls at D3 (P < 0.001, Fig. 8B), but not at D7. This discrepancy between mRNA and protein level can be attributed to a rapid turnover of Pax6 mRNA. The expression of NeuroD6, Satb2 and Sox5 mRNA expression was comparable between rabbits with and without IVH at both D3 and D7.
Figure 8.
GSK3-β inhibition increases Tbr2 and NeuroD1 mRNA, and reduces Pax6 protein expression. (A) The mRNA expressions of Tbr2, Pax6, NeuroD1, NeuroD6, Satb2, Sox5, cyclin D2, and CDK6 were quantified by Real time-qPCR using TaqMan probes in ARA and vehicle treated rabbits with IVH at D3 and D7. Note increased expression of Tbr2 at D3 and NeuroD1 at D7 in ARA-treated rabbits with IVH relative to vehicle controls with IVH. The expression of Pax6, NeuroD1, NeuroD6, Satb2, Sox5, cyclin D2, and CDK6 were comparable between ARA and vehicle treated rabbits with IVH at both D3 and D7. (B) Representative Western blot analyses for Pax6, cyclin D1, cyclin D2, CDK6, pRB (S807/811), and pRB (S780) on brain homogenates of preterm rabbits with and without IVH at D3 and 7. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. Pax6 levels were reduced in ARA-treated rabbits with IVH relative to vehicle controls at D3. *P < 0.05, **P < 0.01 indicate comparison between ARA and vehicle treated kits with IVH for D3 or D7.
Since Pax6 protein levels were increased in Western blot analyses in rabbits with IVH compared with controls without IVH; and as ARA treatment reduced Pax6 levels, we chose to quantify Pax6+ cells in immunolabled sections of the VZ and SVZ of the dorsal telencephalon. We found that Pax6+ cells were increased in rabbits with IVH at both D3 and D7 (P = 0.04 and 0.03, respectively, Supplementary Fig. S7) and that ARA treatment reduced their density at both the days (P = 0.001 and 0.01, respectively).
We next evaluated important molecules regulating cell cycle. Real time-qPCR employing TaqMan probes showed that cyclin D2 and CDK6 levels were similar between ARA and vehicle treated kits with IVH. Accordingly, western blot analyses revealed that cyclin D1, cyclin D2, CDK6, p-Rb protein (serine 780) and p-Rb (serine 807/811) levels were comparable between ARA and vehicle treated rabbits at D3 and D7. Together, the data suggest that ARA treatment advances the transition of Pax6+ radial glia progenitors into Tbr2+ IPs and thereby enhances neurogenesis in ARA-treated rabbits with IVH.
Discussion
The production of pyramidal neurons in the dorsal telencephalon continues until 28 weeks of human gestation (Malik et al. 2013) and the integration of differentiating neurons into the cortical layers would continue during late pregnancy. Since IVH occurs most frequently in premature infants of 23–28 weeks of gestational age, these infants are at the risk of IVH-induced disruption in neurogenesis. Indeed, survivors of IVH have major reduction in gray matter volume and suffer from cognitive disabilities, intellectual disabilities, learning disabilities, and psychiatric disorders (Indredavik et al. 2010; Whitaker et al. 2011). Despite all this, the effect of IVH on neurogenesis and corticogenesis is obscure. Here, we demonstrated that IVH suppressed production of neuronal progenitors in the dorsal telencephalon and reduced the population of neurons in the upper cortical layers in the prematurely born rabbits This was attributed to impaired differentiation of radial glial cells as indicated by elevated Pax6 protein levels, reduced Tbr2 transcription, and diminished phosphorylation of Rb protein. Therapeutically, GSK3β inhibition restored both neurogenesis and neuronal population in the upper layer of cortex, which we ascribed to enhanced genesis of neurogenic IP cells (Tbr2+) from multipotent radial glial cells (Sox2+).
The major finding in the present study is that IVH suppresses cortical pyramidal cell (glutamatergic) neurogenesis in premature humans and rabbits with IVH. To our knowledge, this is the first demonstration of a reduction in total and proliferating radial glial cells and IP cells in autopsy samples from preterm infants with IVH, and in the animal model of IVH. Moreover, glutamatergic neurogenesis in the dorsal SVZ has not been studied previously in any perinatal brain injury paradigm, but the present study indicates the importance and feasibility of studying neurogenesis in premature newborns with brain bleeds. In agreement with the present study, a reduction in proliferation (Ki67 index) of cells in the ganglionic eminences has previously been demonstrated in premature infants with IVH of 24–28 weeks gestation, although the specific identity of the dividing progenitors was not ascertained (Del Bigio 2011). Also, a study on hippocampal neurogenesis in neonatal rodents (P10) found that cerebral ischemia leads to acute reduction in proliferating nestin+ progenitors, total NeuroD1+ cells, and doublecortin+ neurons in the dentate gyrus (Kwak et al. 2015), which is consistent with our findings in the IVH model. In contrast, a study of hypoxia-ischemia in neonatal mice (P10) revealed that proliferation and neurogenesis were increased in the SVZ and peri-infarct striatum (Plane et al. 2004; Ong et al. 2005). Subsequent studies on cerebral ischemia have also shown increased proliferation of neural cells in the cortical SVZ, and in the dentate gyrus subgranular zone (SGZ) in P7 rats, however, these proliferating cells were astrocytic precursors (Spiegler et al. 2007). Nevertheless, an increase in the density of doublecortin (marker of immature neurons) was reported at 7–14 days after ischemia. Differences in the effects of hypoxia-ischemia between reports could be because of the dissimilarities in the animal model, method of induction of hypoxia-ischemia and brain region under evaluation. In the present study, results were consistent in showing that IVH suppresses neocortical neurogenesis in premature humans and rabbits. This result contrasts with reports that hypoxia-ischemia increases hippocampal neurogenesis 1–2 weeks after injury later in development (Plane et al. 2004; Ong et al. 2005; Spiegler et al. 2007).
Developmental follow-up studies have reported that premature infants with even low grade IVH are at greater risk of impaired neurodevelopmental outcome relative to preterm infants without IVH (Pinto-Martin et al. 1995; Whitaker et al. 1997; Vasileiadis et al. 2004). Volumetric MRI techniques have shown that premature infants with uncomplicated IVH display a major reduction in the cortical gray matter volume at near-term age, and these infants continue to exhibit impaired cortical gray matter growth even in childhood and adolescence (de Kieviet et al. 2012). Consistent with these data and imaging studies, we found that the population of pyramidal cell in the upper cortical layers, including Cux1+ and Satb2+ neurons, were reduced in rabbits with IVH compared with controls without IVH at D14.
Another major finding in the present study was that GSK3β inhibition enhanced the population of total and proliferating Sox2+ radial glia at D3, and Tbr2+ IP cells at D7 in kits with IVH, but not in control kits without IVH. An increase in the number of radial glia and IP cells was attributed to ARA-induced restoration of Wnt signaling in kits with IVH, which increased proliferation and maturation of the progenitors. Consistent with our findings, GSK3β inhibition promotes neurogenesis, reduces inflammation, and ameliorates cognitive deficits in a number of animal models of CNS diseases, including Alzheimer’s disease, Down syndrome, Parkinson’s disease, and traumatic brain injury (King et al. 2014). Activation of Wnt signaling has been shown to expand neuronal progenitor pool in transgenic mouse models expressing a stabilized β catenin (Chenn and Walsh 2002; Zechner et al. 2003; Mutch et al. 2009). Indeed, inhibition of GSK3β, an activator of Wnt signaling, enhances neurogenesis in both cell culture studies and in vivo experiments (Hirsch et al. 2007; Munji et al. 2011; Seib et al. 2013). GSK3β inhibition also reduces apoptotic neuronal death following glutamate exposure or oxygen-glucose deprivation (OGD) in cell culture experiments and a rodent model of cerebral hypoxia-ischemia (Kelly et al. 2004). This reinforces our finding of reduced apoptosis in ARA-treated rabbits with IVH, which would contribute to restoration of progenitor populations in kits with hemorrhage. Together, the data suggest that GSK3β inhibition restored radial glia and IPC population by enhancing neuronal survival, proliferation, and differentiation by recruiting Wnt and other signaling pathways.
The cerebral cortex is the multilayered sheet of neurons that orchestrates our highest cognitive abilities. Canonical Wnt/β-catenin signaling and GSK3β enzyme, mutually interlinked, plays key roles in the development and organization of layers of cerebral cortex (Chenn and Walsh 2002; Mutch et al. 2009). GSK3β heterozygote mouse or knocking down GSK3β using siRNA reduces differentiation of radial glia cells into Tbr2+ cells (Ma et al. 2017). Wnt/β-catenin signaling directs switching of neuronal progenitors in the SVZ from a proliferative to migratory mode (Ishizuka et al. 2011). It is also essential for neuronal positioning during cortical development (Boitard et al. 2015). A defective canonical Wnt signaling delays neuronal migration and results in abnormal interhemispheric connectivity (Bocchi et al. 2017). Indeed, the occurrence of IVH in the present study reduced Wnt signaling and the population of Cux1+ and Satb2+ neurons in the upper cortical layers; conversely, activation of Wnt signaling by ARA treatment restored the population of neurons in upper layers. Consistent with this study, transient pharmacological activation of Wnt signaling during the period of early corticogenesis, rescues the β-catenin/Brn2/Tbr2 cascade and reverses abnormal brain structure in Dvl mutant mice (Belinson et al. 2016). Together, a reduction in the density of Cux1+ and Satb2+ cell in the upper cortical layers is attributed to downregulation of Wnt signaling in IVH, which is reversed by activation of Wnt signaling by GSK3β inhibitor.
The Pax6 gene is pivotal for neurogenesis and cerebral cortical development. Pax6 mutation is associated with a range of neuropsychiatric disorders including autism, intellectual disability, nystagmus, abnormal auditory processing, and working memory (Manuel et al. 2015). Pax6 regulates proliferation and differentiation of neuronal progenitors in a highly context- and concentration-dependent manner. Pax6 overexpression is associated with reduced proliferation and an increased cell cycle length, while Pax6 reduction leads to increased proliferation, and shortening of the cell cycle (Manuel et al. 2015). Accordingly, we observed increased expression of Pax6 in rabbits with IVH, where total and cycling Sox2+ and Tbr2+ cells were reduced in the dorsal SVZ and population of Cux1+ cells was diminished in the upper cortical layer. More importantly, GSK3β inhibition by ARA treatment upregulated Wnt signaling, reduced Pax6 levels and restored neurogenesis in our rabbits with IVH. Consistent with these findings, increased Wnt signaling delays expression of Pax6 in mouse model of β-catenin overexpression (Machon et al. 2007).
AR-A014418, an ATP-competitive and specific GSK3 inhibitor (Cohen and Goedert 2004), restored neurogenesis and corticogenesis in rabbits with IVH. GSK3β inhibitors are currently employed for the treatment of various diseases, including traumatic brain injury, Alzheimer’s disease, diabetes, cancer, and other neurodegenerative diseases (Avila and Hernandez 2007). Some of these inhibitors are under clinical trial (Avila and Hernandez 2007). To our knowledge, ARA has only been used in animals and has not undergone a clinical trial. However, a suitable GSK3β inhibitor, if translated into human investigation, might positively impact the neurological outcome premature infants with IVH.
In conclusion, the present study demonstrated suppression of neurogenesis and corticogenesis in preterm rabbits with IVH, which we ascribe to downregulation of Wnt signaling and Tbr2 transcription as well as elevation in Pax6 transcription factor. In addition, GSK3β inhibition restored neurogenesis, and population of neurons in the upper cortical layer, which we attribute to activation of Wnt signaling and diminution in Pax6 levels. We speculate that strategies directed to GSK3β inhibition might restore neurological outcome of premature infants with IVH.
Supplementary Material
Funding
NIH/NINDS (Grant # R01NS083947-01 to P.B., R01 NS092339 to R.H., and R01 NS085081 to R.H.).
Notes
Conflict of Interest: None declared.
References
- Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, Network NR. 2008. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 121:e1167–e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Jang J, Choi J, Lee J, Oh SH, Lee J, Yoon K, Kim S. 2014. GSK3beta, but not GSK3alpha, inhibits the neuronal differentiation of neural progenitor cells as a downstream target of mammalian target of rapamycin complex1. Stem Cells Dev. 23:1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni E, Shapira M, Eldar-Finkelman H, Barnea A. 2015. GSK-3beta inhibition affects singing behavior and neurogenesis in adult songbirds. Brain Behav Evol. 85:233–244. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Mirnics K. 2007. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci. 25:1843–1854. [DOI] [PubMed] [Google Scholar]
- Avila J, Hernandez F. 2007. GSK-3 inhibitors for Alzheimer’s disease. Expert Rev Neurother. 7:1527–1533. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, et al. 2007. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 13:477–485. [DOI] [PubMed] [Google Scholar]
- Belinson H, Nakatani J, Babineau BA, Birnbaum RY, Ellegood J, Bershteyn M, McEvilly RJ, Long JM, Willert K, Klein OD, et al. 2016. Prenatal beta-catenin/Brn2/Tbr2 transcriptional cascade regulates adult social and stereotypic behaviors. Mol Psychiatry. 21:1417–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. 2008. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 26:2361–2371. [DOI] [PubMed] [Google Scholar]
- Bocchi R, Egervari K, Carol-Perdiguer L, Viale B, Quairiaux C, De Roo M, Boitard M, Oskouie S, Salmon P, Kiss JZ. 2017. Perturbed Wnt signaling leads to neuronal migration delay, altered interhemispheric connections and impaired social behavior. Nature Commun. 8:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard M, Bocchi R, Egervari K, Petrenko V, Viale B, Gremaud S, Zgraggen E, Salmon P, Kiss JZ. 2015. Wnt signaling regulates multipolar-to-bipolar transition of migrating neurons in the cerebral cortex. Cell Rep. 10:1349–1361. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. 2008. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 57:378–392. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. 2002. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 297:365–369. [DOI] [PubMed] [Google Scholar]
- Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. 2009. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 40:3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Goedert M. 2004. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 3:479–487. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. 2012. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 54:313–323. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. 2011. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 134:1344–1361. [DOI] [PubMed] [Google Scholar]
- Dummula K, Vinukonda G, Chu P, Xing Y, Hu F, Mailk S, Csiszar A, Chua C, Mouton P, Kayton RJ, et al. 2011. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J Neurosci. 31:12068–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. 2005. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 25:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. 2008. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 39:3378–3388. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. 2001. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 65:391–426. [DOI] [PubMed] [Google Scholar]
- Guo W, Murthy AC, Zhang L, Johnson EB, Schaller EG, Allan AM, Zhao X. 2012. Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum Mol Genet. 21:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Rubenstein JL, Kriegstein AR. 2011. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 70:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C, Campano LM, Wohrle S, Hecht A. 2007. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 313:572–587. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. 2010. GSK3 signalling in neural development. Nat Rev Neurosci. 11:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM. 2010. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 31:286–294. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, Murdoch H, Dunlop AJ, Kubo K, Furukori K, et al. 2011. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 473:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Dawson LA. 2011. Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. Int J Neuropsychopharmacol. 14:1051–1067. [DOI] [PubMed] [Google Scholar]
- Kelly S, Zhao H, Hua Sun G, Cheng D, Qiao Y, Luo J, Martin K, Steinberg GK, Harrison SD, Yenari MA. 2004. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Exp Neurol. 188:378–386. [DOI] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. 2009. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 12:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. 2014. Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol Ther. 141:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. 2009. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 19:2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M, Lim S, Kang E, Furmanski O, Song H, Ryu YK, Mintz CD. 2015. Effects of neonatal hypoxic-ischemic injury and hypothermic neuroprotection on neural progenitor cells in the mouse hippocampus. Dev Neurosci. 37:428–439. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim K, Yu SW, Kim EK. 2016. Wnt3a upregulates brain-derived insulin by increasing NeuroD1 via Wnt/beta-catenin signaling in the hypothalamus. Mol Brain. 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. 2011. Development and evolution of the human neocortex. Cell. 146:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YX, Wang XL, Chen JQ, Li B, Hur EM, Saijilafu . 2017. Differential roles of glycogen synthase kinase 3 subtypes alpha and beta in cortical development. Front Mol Neurosci. 10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. 2007. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 311:223–237. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. 2013. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 33:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel MN, Mi D, Mason JO, Price DJ. 2015. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC. 2012. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS One. 7:e30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MH, Bromme JO, Feldmann RE Jr., Jarve A, Sabouri F, Burgers HF, Schelshorn DW, Kruger C, Schneider A, Kuschinsky W. 2007. Glycogen synthase kinase 3beta (GSK3beta) regulates differentiation and proliferation in neural stem cells from the rat subventricular zone. J Proteome Res. 6:1198–1208. [DOI] [PubMed] [Google Scholar]
- Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, Santos A, Martinez A, Perez-Castillo A. 2012. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci. 3:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. 2011. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci. 31:1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch CA, Funatsu N, Monuki ES, Chenn A. 2009. Beta-catenin signaling levels in progenitors influence the laminar cell fates of projection neurons. J Neurosci. 29:13710–13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR. 2016. Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron. 91:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J, Plane JM, Parent JM, Silverstein FS. 2005. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr Res. 58:600–606. [DOI] [PubMed] [Google Scholar]
- Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. 1995. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics. 95:249–254. [PubMed] [Google Scholar]
- Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. 2004. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 16:585–595. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. 2008. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 30:24–32. [DOI] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. 2013. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 12:204–214. [DOI] [PubMed] [Google Scholar]
- Seira O, Del Rio JA. 2014. Glycogen synthase kinase 3 beta (GSK3beta) at the tip of neuronal development and regeneration. Mol Neurobiol. 49:931–944. [DOI] [PubMed] [Google Scholar]
- Shim SS, Stutzmann GE. 2016. Inhibition of glycogen synthase kinase-3: an emerging target in the treatment of traumatic brain injury. J Neurotrauma. 33:2065–2076. [DOI] [PubMed] [Google Scholar]
- Spiegler M, Villapol S, Biran V, Goyenvalle C, Mariani J, Renolleau S, Charriaut-Marlangue C. 2007. Bilateral changes after neonatal ischemia in the P7 rat brain. J Neuropathol Exp Neurol. 66:481–490. [DOI] [PubMed] [Google Scholar]
- Stephens BE, Vohr BR. 2009. Neurodevelopmental outcome of the premature infant. Pediatr Clin North Am. 56:631–646, Table of Contents. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. , Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N . 2010. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiadis GT, Gelman N, Han VK, Williams LA, Mann R, Bureau Y, Thompson RT. 2004. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 114:e367–e372. [DOI] [PubMed] [Google Scholar]
- Vinukonda G, Csiszar A, Hu F, Dummula K, Pandey NK, Zia MT, Ferreri NR, Ungvari Z, LaGamma EF, Ballabh P. 2010. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain. 133:2264–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, Kayton R, Hascall VC, Lauer ME, Ballabh P. 2016. Hyaluronidase and hyaluronan oligosaccharides promote neurological recovery after intraventricular hemorrhage. J Neurosci. 36:872–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose LR, Vinukonda G, Jo S, Miry O, Diamond D, Korumilli R, Arshad A, Zia MT, Hu F, Kayton RJ, et al. 2013. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci. 33:17232–17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS. 2002. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci USA. 99:7951–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, Pinto-Martin J, Shaffer D, Paneth N. 2011. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 68:742–752. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, Shaffer D, Paneth N. 1997. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Arch Gen Psychiatry. 54:847–856. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB 3rd, Birchmeier W, Birchmeier C. 2003. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 258:406–418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.