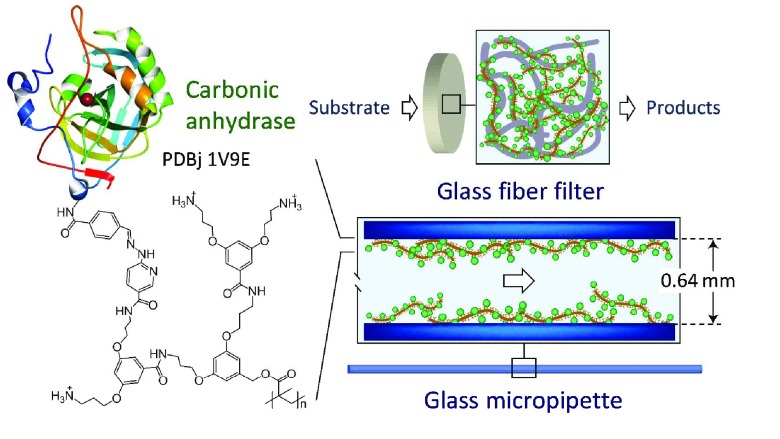
Abstract
There are various ways of immobilizing carbonic anhydrase (CA) on solid materials. One of the final aims is to apply immobilized CA for the catalytic hydration of carbon dioxide (CO2) as a first step in the conversion of gaseous CO2 into solid products. The immobilization method investigated allows a straightforward, stable, and quantifiable immobilization of bovine erythrocyte carbonic anhydrase (BCA) on silicate surfaces. The method is based on the use of a water-soluble, polycationic second-generation dendronized polymer with on average 1000 repeating units, abbreviated as de-PG21000. Several copies of BCA were first covalently linked to de-PG21000 through stable bisaryl hydrazone (BAH) bonds. Then, the de-PG21000-BAH-BCA conjugates obtained were adsorbed noncovalently either on microscopy glass coverslips, inside glass micropipettes, or in porous glass fiber filters. The apparent density of the immobilized BCA on the glass surfaces was about 8–10 pmol/cm2. In all three cases, the immobilized enzyme was highly active and stable when tested with p-nitrophenyl acetate as a model enzyme substrate at room temperature. The micropipettes and the glass fiber filters were applied as flow-through systems for continuous operation at room temperature. In the case of the glass fiber filters, the filters were placed inside a homemade flow-through filter holder which allows flow-through runs with more than one filter connected in series. This offers the opportunity of increasing the substrate conversion by increasing the number of BCA-containing filters.
1. Introduction
Carbonic anhydrase (CA) is a metalloenzyme which is ubiquitously present in the tissues of animals and plants as well as in bacteria.1,2 Although there are multiple forms of CA, such as α-, β-, and γ-CA, which differ in their primary structures, the functional importance of CA is the same for the diverse living systems.2 This is because CA reversibly catalyzes a biologically critical reaction, namely, between carbon dioxide and water, to yield bicarbonate and hydronium ions, CO2(g) + 2H2O(l) → HCO3–(aq) + H3O+(aq).3 This CA-catalyzed reaction is the first step of the biological fixation of atmospheric carbon into organic compounds, occurring in vivo within carboxysomes.4 Furthermore, it is essential for the removal of carbon dioxide from animal tissues.3,5 Among the various forms of CA, α-CA is the best characterized form. It is a monomeric protein with a zinc ion coordinated by three histidine residues at the active site.2 α-CA is structurally robust,2 easily quantifiable on the basis of its esterase activity,6 and commercially available, which are advantageous features if one considers practical applications of CA-catalyzed reactions. Since controlling the atmospheric concentration of carbon dioxide at a global scale is an important issue,7 various approaches were reported concerning the efficient capturing and sequestration of carbon dioxide under mild conditions with the help of α-CA.8−11
Apart from the carrier-free immobilization of CA,12,13 there are several reports on carrier-dependent CA immobilization.13−24 Stable immobilization of CA on a solid carrier material (“support”) is essential for the development of economical and scalable systems for capturing carbon dioxide with a continuous reactor or a batch process in which CA can be recycled. In a number of previous studies it was shown that α-CA molecules can be immobilized on solid materials through covalent or noncovalent bonding. For this carrier-dependent enzyme immobilization, porous silica particles are often used,14−16 partly because the particles possess pores which are large enough for entrapping the CA molecules. Moreover, the silica surface can be modified with functional groups for a covalent bonding of the enzyme. In other approaches, CA was immobilized covalently to other solid or soft colloidal materials, such as polyurethane foams,17 graphite rods,14 iron filings,18 and phospholipid vesicles.19 For the noncovalent immobilization, biospecific20 or electrostatic21,22 interactions between the support material and the CA molecules were utilized. CA immobilization via electrostatic interactions was demonstrated by using the layer-by-layer deposition of polyelectrolytes21 and with inner surface-modified mesoporous silica.22 However, the direct noncovalent immobilization of CA under retention of the activity of the enzyme is not straightforward due to a homogeneous distribution of positive and negative charges on the enzyme surface.22 Therefore, the orientation and conformation of CA on charged solid surfaces were investigated23,24 since both, conformation and orientation, are of general significance for the catalytic performance of immobilized enzymes.25
In the work presented, α-CA was immobilized on silicate surfaces via simple adsorption of conjugates consisting of a polycationic dendronized polymer (denpol)26 to which CA molecules were first covalently bound through bisaryl hydrazone (BAH) bonds.27,28 With this methodology, horseradish peroxidase (HRP),29 glucose oxidase (GOD),29 and proteinase K (proK)30,31 could be immobilized successfully on the inner surface of glass micropipettes for using them as continuous-flow reactors.29,30 For each enzyme, the optimal conditions for the formation of the denpol–enzyme conjugate and for the adsorption step had to be elaborated. We used CA from bovine erythrocytes (Mr ≈ 29 000, pI = 5.9),2,32 abbreviated as BCA. It was conjugated to the deprotected (de) second-generation dendronized polymer de-PG2 through the mentioned BAH bonds yielding different denpol-BAH-BCA conjugates, abbreviated as de-PG2-BAH-BCA (Figure 1). The denpol used had a number-average degree of polymerization of n = 1000, and the experimentally determined average number of BCA molecules per denpol chain was between 100 and about 300. Then, the conjugates were immobilized (i) onto glass coverslips, (ii) inside glass micropipettes of different sizes, and (iii) in glass fiber filters (Figure 1) through noncovalent interactions between the conjugates and the supports. The immobilization was carried out at different pH values below and above the pI value of BCA. In some experiments, the silicate surface was first precoated with de-PG2 before adsorption of de-PG2-BAH-BCA. The flow-through catalytic hydrolysis of p-nitrophenyl acetate as a model substrate of BCA was performed with glass micropipettes and glass fiber filters containing immobilized conjugates in order to clarify the influence of the reactor type on the efficiency of the catalyzed reaction. With the glass micropipettes and the glass fiber filters, the operational stability of the immobilized enzyme was also determined in simple flow reactor systems.
Figure 1.
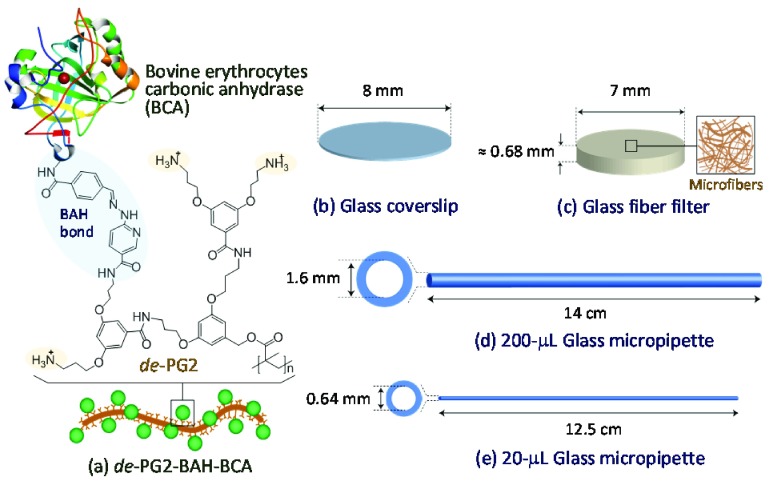
Schematic illustration of (a) the deprotected second-generation dendronized polymer (denpol) de-PG2 to which bovine erythrocyte carbonic anhydrase (BCA) molecules are bound along the polymer chain via bisaryl hydrazone (BAH) linker units (de-PG2-BAH-BCA). The chemical structure of one repeating unit (r.u.) carrying one BCA molecule is shown: n = 1000 for the denpol used in this work. Schematic drawings of the various silicate glass supports which were used for the immobilization of de-PG2-BAH-BCA are shown in (b)–(e): (b) glass coverslip, (c) porous glass fiber filter, and (d, e) two different glass micropipettes. At the pH used in the work (pH = 7.2 and 5.0), the free amino groups of de-PG2-BAH-BCA are expected to be positively charged.31 The image of the three-dimensional structure of BCA with an incorporated zinc ion (red ball) was taken from the Protein Data Bank Japan, PDBj (PDB code 1V9E).32 In the illustration (a), Lys35 of the BCA molecule is assumed to be modified and linked to the de-PG2 molecule.
Although it was shown in this work that the preparation of the conjugates and their immobilization on different silicate surfaces are highly reproducible and the results obtained are positive, the details of preparation and analysis are very important. This is why we try to provide all necessary information so that the same reactions and characterizations can also be performed in other laboratories.
2. Materials and Methods
2.1. Carbonic Anhydrase, Dendronized Polymer, and Other Chemicals
Carbonic anhydrase from bovine erythrocytes (BCA, EC 4.2.1.1, catalog number C2624, lot #SLBL1750 V and lot #SLBR4228 V) was obtained from Sigma-Aldrich (ε280 = 56 000 M–1·cm–1).33 The deprotected dendronized polymer (de-PG2, number-average degree of polymerization, Pn = 1000, polydispersity index, PDI = 2.4, molar mass of the repeating unit (r.u.) including trifluoroacetic acids = 1279 g/mol), N-succinimidyl 4-formylbenzoate (S-4FB), and N-succinimidyl 6-hydrazinonicotinate acetone hydrazone (S-HyNic) were synthesized and characterized at ETH Zürich by Daniel Messmer (denpol) or Dr. Chengmin Hou (S-4FB and S-HyNic), as reported previously.31 4-Formyl-N-methyl-benzamide (methyl-4FB) was from Fluorochem (Hadfield, UK). For other chemicals used, see Supporting Information.
2.2. Glass Coverslips, Glass Micropipettes, and Glass Fiber Filters
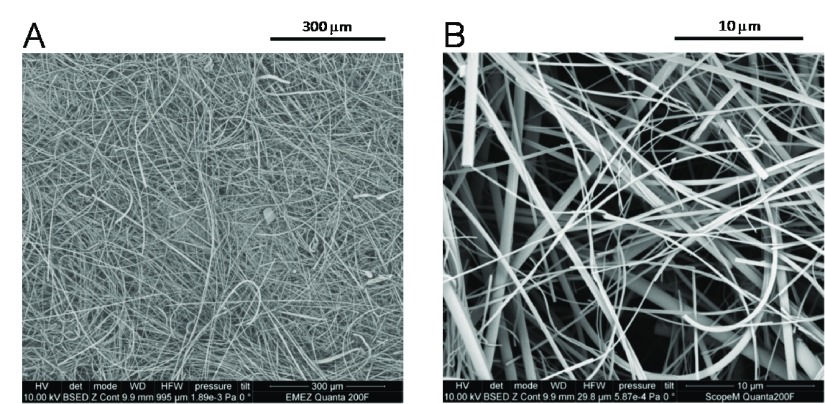
(a) Glass coverslips. Round glass coverslips (diameter 8 mm, thickness 0.16–0.19 mm, catalog number 72296-08) were obtained from Science Services. (b) Glass micropipettes. Two types of glass micropipettes (BRAND disposable BLAUBRAND, intraMark, obtained from Sigma-Aldrich) were used. Usually, they are applied for pipetting a precise volume of 200 μL (catalog number 708757) or 20 μL (catalog number 708718), and they are abbreviated in this paper as “200 μL micropipettes” and “20 μL micropipettes”, respectively. The length L, the inner diameter din, and the maximum volume Vm of the 200 μL micropipette were 14 cm, 1.6 mm, and 280 μL, respectively. For the 20 μL micropipette, L = 12.5 cm, din = 0.64 mm, and Vm = 40 μL (Figure 1). (c) Glass fiber filters. Binder-free glass fiber filters (grade GF/D, diameter 7 mm, catalog number 1823-007, lot 12887121, Whatman) were obtained from GE Healthcare. The filters consist of borosilicate glass microfibers. The fibers had a wide variety of lengths and thicknesses, as observed with a field emission gun scanning electron microscope (FEG-SEM) analysis at 10 kV by using an FEI Quanta 200F instrument (Figure 2).
Figure 2.
SEM images of the glass microfiber filters used. The horizontal field width (HFW) is for (A) 995 μm and for (B) 29.8 μm. For the SEM analysis, the filter was washed with an ethanol solution (10 min, 3 times) followed by drying at atmospheric pressure in air.
2.3. Buffer Solutions Used
MOPSB: 0.1 M MOPS buffer solution (pH = 7.6) containing 0.15 M NaCl. MESB1: 0.1 M MES buffer solution (pH = 4.7) containing 0.15 M NaCl. MESB2: 0.1 M MES buffer solution (pH = 5.0). PB1: 0.1 M sodium phosphate buffer solution (pH = 7.2) containing 0.15 M NaCl. PB2: 0.1 M sodium phosphate buffer solution (pH = 7.2) containing 1.15 M NaCl. PB3: 0.01 M sodium phosphate buffer solution (pH = 7.2). PB4: 0.01 M sodium phosphate buffer solution (pH = 5.0). PB5: 0.01 M sodium phosphate buffer solution (pH = 5.0) containing 0.15 M NaCl.
2.4. Preparation of de-PG2-BAH-BCA
Details about the modification of de-PG2 with S-HyNic,31 of BCA with S-4FB, and about the preparation of the conjugate de-PG2-BAH-BCA are given in the Supporting Information (Figure S-1–Figure S-7).
2.5. Determination of the Activity and Thermal Stability of Dissolved, Free BCA, and Dissolved de-PG2-BAH-BCA
The enzymatic activity was determined with p-nitrophenylacetate (p-NA) as a substrate34−36 (see Supporting Information (Figure S-8–Figure S-11)). BCA catalyzes the hydrolysis of p-NA into acetate and p-nitrophenol/p-nitrophenolate at pH = 7.2 (see Figure S-8C).
2.6. Immobilization of de-PG21000-BAH-BCA on Microscopy Glass Coverslips
The obtained solution of purified conjugate de-PG21000-BAH175-BCA115 (see Supporting Information) was desalted by ultrafiltration with the centrifugal filter unit Amicon Ultra-0.5 (MWCO 50 kDa or 10 kDa) as follows. The conjugate solution (130 μL) was first diluted with PB3 to yield a total volume of 0.5 mL. Next, the conjugate solution was centrifuged in the filter unit to a volume of less than 100 μL. Then, this concentrated conjugate solution was diluted about 5 times with PB3 for the next centrifugation. This process was performed 4 times. Finally, the desalted conjugate solution was diluted with PB3 to yield a BAH concentration of 3.3 μM. Before immobilizing the conjugates, the round glass coverslips were cleaned by sonication for 10 min in ethanol. This process was performed 3 times with fresh ethanol, followed by drying under a flow of nitrogen gas. Each dry glass coverslip was immersed in 250 μL of the desalted conjugate solution ([BAH] = 3.3 μM) in a 2.0 mL polypropylene tube, followed by incubation for 1 h at room temperature. Then, the glass coverslip was recovered from the conjugate solution and immersed in 1.5 mL of PB3 for 5 min for washing. This process was performed 3 times with fresh PB3. In a control measurement, a glass coverslip was prepared with the same procedure as described above, including the washing treatments, except that the dry coverslip was immersed in a conjugate-free PB3 solution. The glass coverslips with immobilized conjugates and the control glass coverslip were stored in 1.5 mL of PB3 inside 2.0 mL polypropylene tubes at 4 °C until use.
2.7. Immobilization of de-PG21000-BAH-BCA Inside Glass Micropipettes
The two types of micropipettes were first cleaned by sonication in ethanol followed by drying as described above. The immobilization of the conjugates in the “200 μL micropipettes” was carried out with PB3 or PB4. For each pH value, the conjugates were immobilized in two different ways: (i) directly on the inner surface of the micropipettes or (ii) on the inner surface of micropipettes which were first coated with de-PG2. The conjugate solution was desalted prior to the immobilization, as described above at the pH value to be employed for the following immobilization step. For the direct immobilization, case (i), the “200 μL micropipettes” were wetted with PB3 or PB4 and then filled with the conjugate solution ([BAH] = 3.3 μM) using a 1.0 mL polypropylene syringe (HSW Norm-Ject, Henke Sass Wolf, 4010-200V0) with a needle. The silicon tubes connected to both ends of the micropipette were sealed for keeping the solution inside the micropipettes for 1 h at room temperature. Then, the conjugate solution was removed from the micropipettes, followed by washing 3 times with the buffer solution. Immobilization tests with free BCA were also carried out at pH = 7.2 or 5.0 with the same procedure as described above using the free BCA solution instead of the conjugate solution. The concentration of BCA in the free enzyme solution was the same as the concentration of BCA in the conjugate solution prepared at [BAH] = 3.3 μM. For the immobilization of the conjugates on the de-PG2-modified surface, case (ii), de-PG2 was first dissolved in PB5 at [de-PG2] = 10 μg/mL.26 The micropipettes were filled with this de-PG2 solution followed by incubation for 1 h at room temperature and then 3 times washing with PB5. The de-PG2-modified micropipettes thus prepared were then used for the immobilization of desalted conjugates or for immobilization tests with free BCA at pH = 7.2 or 5.0 as in the case of the direct immobilization. For the micropipettes with and without de-PG2 modification, control micropipettes were also prepared with PB3 or PB4 in the absence of enzyme. The prepared micropipettes containing immobilized conjugates or free BCA and the control micropipettes were filled with the respective buffer solution and then stored at 4 °C until further use.
With the “20-μL micropipettes”, only the direct immobilization of the conjugates or free BCA was performed at pH = 7.2. The conjugate solution ([BAH] = 3.3 μM) or the solution of free BCA was aspirated into the micropipette using a 1.0 mL polypropylene syringe which was connected to the micropipette. The washing step was carried out by passing the buffer solution continuously through the micropipette at a flow rate of 5.7 μL/min using a syringe pump AL-1000 from World Precision Instruments.
2.8. Immobilization of de-PG21000-BAH-BCA in Glass Fiber Filters
The glass fiber filters were cleaned by incubation in ethanol for 10 min (3 times). Evaporation of the ethanol molecules from the porous filters under atmospheric pressure at room temperature was analyzed by measuring the weight of the filters until it became practically unchanged. The immobilization of de-PG21000-BAH175-BCA115 conjugates (desalted solution, see Supporting Information) or the adsorption of free BCA was performed by immersing the dry filter in PB3 containing either the conjugate or free BCA. The conditions used for the immobilization, washing, and storage were the same as the ones employed for the glass coverslips (see section 2.6).
2.9. Determination of the Activity of de-PG21000-BAH-BCA Immobilized on Microscopy Glass Coverslips
The BCA activity of the glass coverslips onto which the de-PG21000-BAH175-BCA115 conjugates were immobilized was measured with p-NA as substrate. The reaction was initiated at room temperature in a 2.0 mL polypropylene tube by immersing the coverslip in 280 μL of PB3 containing 1.0 mM p-NA. The absorbance at 405 nm of a freshly prepared 1.0 mM p-NA solution (A405,0) was measured with a NanoDrop ND-1000 instrument (l = 0.1 cm). After a reaction time of 15 min, the coverslip was recovered from the reaction mixture, and A405,15 min of the solution was immediately measured. The apparent rate of formation of product, rapp [mol·L–1·min–1], was calculated as rapp = (A405,15 – A405,0)/(t·l·ε405), with t = 15 min, l = 0.1 cm, and ε405 = 10 510 M–1 cm–1.34 The same measurements were also performed with the control glass coverslip to obtain the rate of hydrolysis without enzyme, rcontrol. The difference rapp – rcontrol was used for the determination of the apparent concentration of active BCA molecules in the reaction solution on the basis of a comparison with a standard curve (Figure S-8A), which allowed estimating the apparent density of active BCA molecules on the surface of the coverslip with a total surface area of 1.0 cm2, Γapp [pmol/cm2]. In this estimation, the reaction mechanism including the catalytic efficiency is assumed to be the same for the free and conjugated BCA molecules. Since the estimated apparent amount of immobilized BCA may be lower than the real total amount of immobilized BCA, another way of expressing immobilization efficiencies is to use enzyme activity units (U) instead of enzyme amounts (see Supporting Information). With this, the calculated amount of 1 pmol of BCA per cm2 corresponds to 4.66 × 10–5 U per cm2.
2.10. Determination of the Activity of Different de-PG21000-BAH-BCA Conjugates Immobilized Inside Glass Micropipettes
For the “200 μL and 20 μL micropipettes” containing immobilized conjugates, the BCA activity was determined in two different ways, either (i) in a batchwise mode or (ii) in a continuous mode. For the micropipettes which were treated with free BCA instead of the conjugates, exactly the same types of analyses were carried out. All reactions were performed at room temperature in PB3 at an initial p-NA concentration of 1.0 mM. To initiate the batch-wise reaction, case (i), for the estimation of Γapp, 280 and 40 μL of the substrate solution was introduced into the “200 μL and 20 μL micropipettes”, respectively, and incubated for 3 min. The solution was recovered, and the absorption spectrum was then immediately measured with a NanoDrop ND-1000 instrument (l = 0.1 cm). The initial rate of p-NA hydrolysis was determined from A405 by taking into account the rate obtained with the control micropipettes (empty, no BCA used at all), and Γapp was estimated. To examine the stability of immobilized conjugates or adsorbed free BCA inside the “200 μL micropipettes”, 30 min reactions were performed repeatedly. The amount of product formed for 30 min was determined from A405 by taking into account the amount obtained with the control micropipettes. After each reaction, the micropipettes were washed 3 times with PB3. For the continuous flow reaction, case (ii), the outlet of a syringe pump (from World Precision Instruments) was connected directly to the inlet of the “200 μL or 20 μL micropipettes” with a silicon tube fitting. Contact between the p-NA solution and the silicon tube was avoided because of the non-negligible interaction between p-NA and the tube. The p-NA-containing PB3 was passed through the micropipette at a constant flow rate of about 18.4 μL/min for a “200 μL micropipette” and 2.7 or 5.7 μL/min for a “20 μL micropipette”. The reaction solution eluting from the outlet of a micropipette was pooled every 3 min in a polypropylene tube for the “200 μL micropipette” or collected directly with a Gilson pipetman P-20 every 2 min at the flow rate of 2.7 μL/min or every 1 min at 5.7 μL/min for the “20 μL micropipettes” for determining A405 with the NanoDrop instrument.
2.11. Determination of the Activity of de-PG21000-BAH-BCA Immobilized in Glass Fiber Filters
The apparent rate of product formation, rapp, in the presence of glass fiber filters containing immobilized conjugate de-PG21000-BAH175-BCA115 or adsorbed free BCA was determined at room temperature in PB3 as follows. The weight of a wet filter was first measured by placing the filter on a plastic weighing dish to determine the liquid phase volume VL [L] contained in the filter based on its dry weight (≈4.6 mg) and the density of water at 23 °C (0.998 g/cm3). PB3 (1.0 mL) containing 1.0 mM p-NA was prepared, and A405,0 was measured at l = 0.1 cm. Then, the filter was immersed in 280 μL of the above substrate solution to initiate the reaction. After a reaction time t = 15 min, the filter was separated from the reaction solution, and A405,15 was immediately measured. For the glass fiber filters which were treated with free BCA instead of the conjugates, exactly the same type of analysis was carried out. Since the concentration of p-NA, Cp-NA, is much lower than the Michaelis constant Km (Cp-NA ≪ Km) (Figure S-10), the rate of reaction assuming Michaelis–Menten kinetics can be approximated as first-order with respect to both Cp-NA and enzyme concentration CBCA; i.e., rapp ≈ (kcat/Km)Cp-NACCA = kappCp-NACBCA, where kcat is the rate constant. The measured value of A405,15 was corrected by a factor of {1 + VL/(2.8 × 10–4)}2 to yield a value which takes into account the dilution of the reaction solution. With this and the initial p-NA concentration of 1.0 mM, the conditions used for the standard curve obtained with known amounts of BCA (Figure S-8A), rapp [mol·L–1·min–1] for the reaction time t (= 15 min) was determined with the following equation.
In the above calculation, the enzyme molecules are assumed to be dispersed homogeneously in the reaction system. The rcontrol value was determined with the control filter, assuming that the rate is first order with respect to Cp-NA. The apparent concentration of active BCA per surface area, Γapp, was then calculated as rapp – rcontrol and taking into account the total outer surface area of the filter (0.92 cm2).
2.12. Flow-Through Reactor Unit Consisting of One or More Glass Fiber Filters
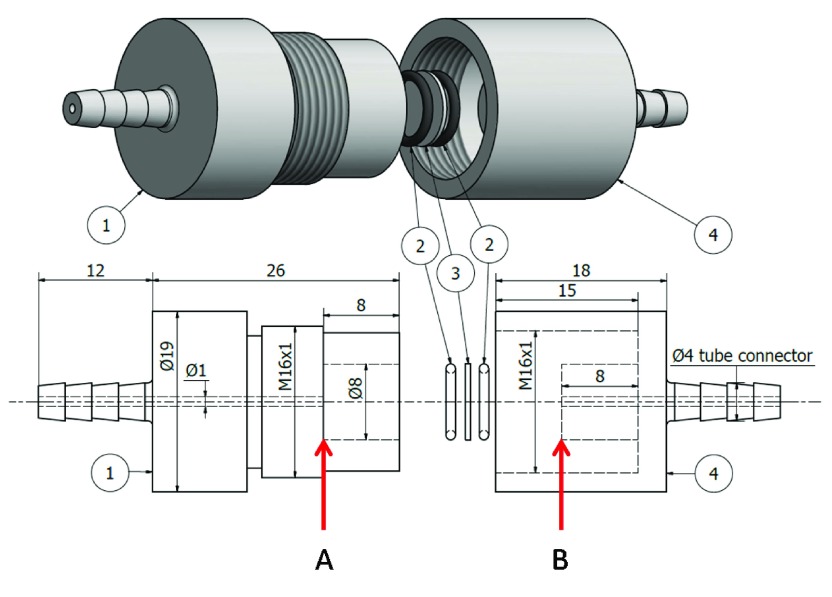
Continuous flow reactions using glass fiber filters were performed with a homemade reactor unit in which single or stacked filters can be fixed with very soft O-rings (Silicone O-ring, 30 Sh A, K+D AG, Hombrechtikon, Switzerland), and the substrate solution can be passed continuously through the filters from the inlet to the outlet (Figure 3). The reactor unit was manufactured by turning at ETH Zürich from Delrin (= poly(oxymethylene) from DuPont), a material which is inert toward the substrate. The design of the reactor unit is based on a small volume apparatus for the preparation of large unilamellar vesicles (liposomes).37 The inlet nozzle of the reactor unit was directly connected to the outlet of the syringe pump. PB3 initially containing 1.0 mM p-NA was continuously passed through the reactor unit at a constant flow rate. The concentration of product was measured on the basis of A405 (l = 0.1 cm) of the droplets directly collected every 2 min at the reactor outlet with a Gilson Pipetman P-20.
Figure 3.
Home-made reactor unit for the continuous hydrolysis of p-NA with glass microfiber filters containing immobilized conjugates or adsorbed free BCA. The reactor unit consists of two filter holder units (1 and 4), a glass fiber filter (3), and two O-rings (2). One filter (3) with one O-ring on each side was first mounted on the plane A within unit 1, and then, unit 4 was screwed finger tight into unit 1 to fix the filter with the O-rings between the planes A and B. The distance between the plane A and B can be adjusted manually so as not to induce breakage of the filters along the O-rings. Two stacked filters can also be fixed in the holder unit by adjusting the above distance adequately. The distances in the drawing are given in mm.
3. Results and Discussion
3.1. Modification of de-PG21000 with S-HyNic
The deprotected denpol de-PG21000 was partially modified with S-HyNic in aqueous solution at pH = 7.6 (MOPSB) and then purified by repetitive ultrafiltration (see Supporting Information). The concentration of r.u. in the obtained purified de-PG21000-HyNic solution was determined as 1.93 mM on the basis of the Trypan Blue assay.38,39 The concentration of HyNic in this solution was 0.85 mM, as determined through reaction with 4-nitrobenzaldehyde (Figure S-1). Since each r.u. carries four amino groups, the molar substitution ratio MSR (HyNic) of the prepared de-PG21000-HyNic on the basis of the amino group content was 0.11 (= 0.85 mM/(4·1.93 mM)). This means that on average 440 HyNic moieties were introduced per 1000 r.u. and that such 1000 r.u. long HyNic-modified chain still had on average 3560 free amino groups. This solution of purified de-PG21000-HyNic was stored at 4 °C and used throughout this work.
3.2. Modification of BCA with S-4FB
BCA (80 μM) was modified with S-4FB (160 μM) at pH = 7.2 (PB1) (see Supporting Information). These buffer conditions were found to be optimal for the modification of BCA. After purification by repetitive ultrafiltration, the concentration of 4FB in the obtained purified BCA-4FB solution was determined as 74 ± 18 μM through reaction with 2-HP (2-hydrazinopyridine) (mean value ± standard deviation from five independent preparations) (Figure S-2). The concentration of BCA in this BCA-4FB solution was estimated as 76 ± 3 μM through enzyme activity measurements by assuming that the activity of the modified enzyme was not different from the activity of the unmodified enzyme. This assumption is supported experimentally (Figure S-11). Accordingly, the molar substitution ratio MSR (4FB) was calculated as 0.97 ± 0.26, meaning that one BCA molecule was modified with on average about one 4FB moiety.
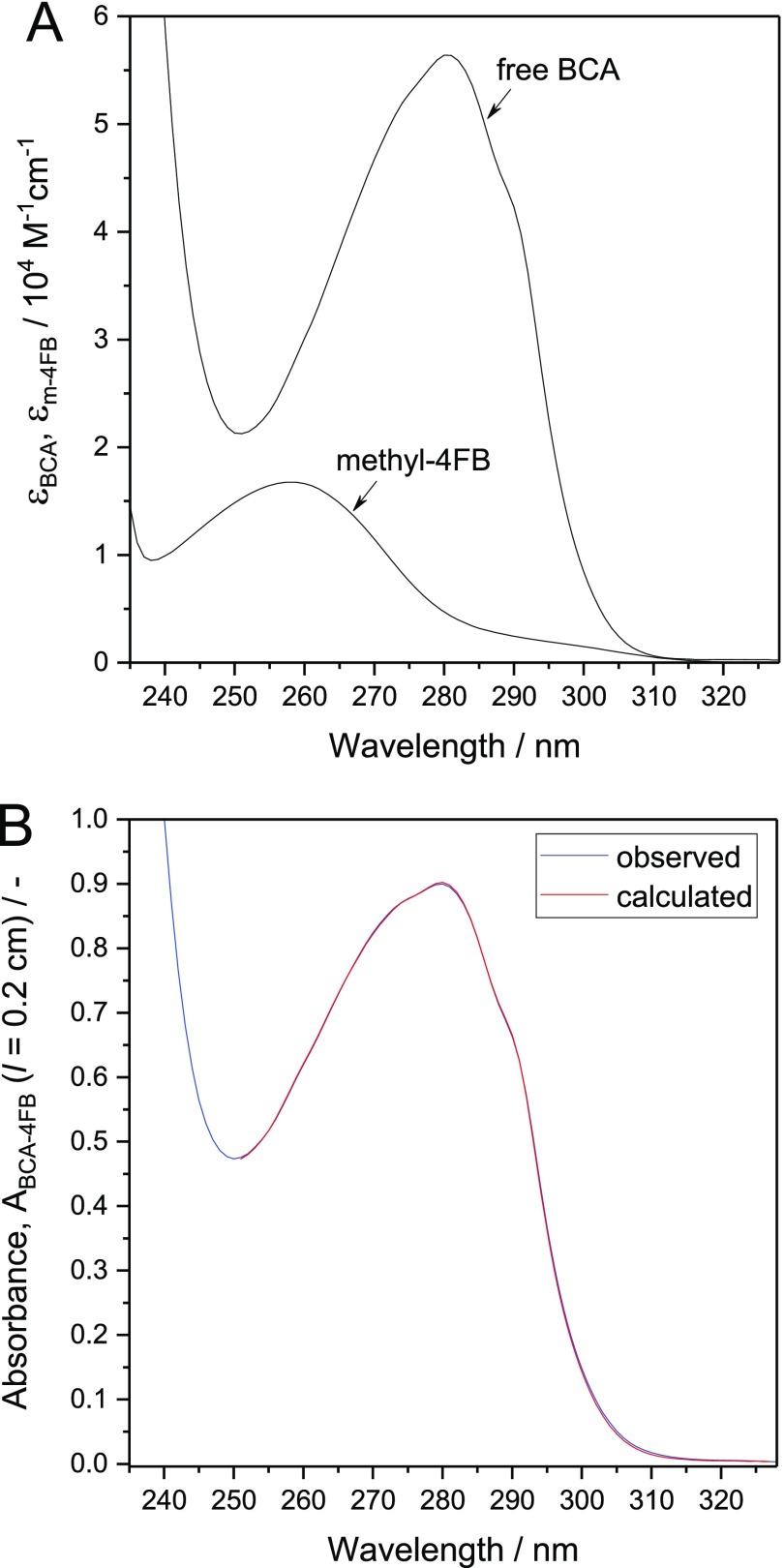
For one of the preparations, the MSR was also determined with an alternative spectrophotometric method (see Supporting Information). This method is based on fitting the measured absorption spectrum of the obtained solution of purified BCA-4FB with the absorption spectra of solutions of the model compound methyl-4FB and free (unmodified) BCA, both spectra recorded at known concentrations in PB1. Figure 4A shows the UV-absorption spectra of methyl-4FB and free BCA, plotted as molar absorption coefficient, ε, vs wavelength. In Figure 4B, the absorption spectrum of a solution of purified BCA-4FB at an unknown concentration is compared with the absorption spectrum which was obtained by fitting (linear combination of the spectra of free BCA and methyl-4FB). The measured and fitted spectra agree well with each other for 49.5 μM methyl-4FB and 75.9 μM BCA, corresponding to MSR (4FB) = 0.65 (= 49.5 μM/75.9 μM). The concentration of 4FB in this purified BCA-4FB solution was separately determined as 54 ± 14 μM (n = 9) through reaction with 2-HP. This concentration is consistent with the concentration determined by the spectral fitting (≈50 μM). Furthermore, the concentration of BCA resulting from the spectral fitting (≈76 μM) agrees with the enzymatic determination of the BCA concentration in solutions of purified BCA-4FB (76 ± 3 μM, see above). Therefore, the spectra analysis method can be considered as a reliable and simple alternative method for the determination of MSR (4FB) of purified 4-FB-modified enzymes.
Figure 4.
(A) Molar absorption coefficient of free (unmodified) BCA, εBCA, and of methyl-4FB, εm-4FB, as a function of wavelength: PB1 (with 1 vol % DMF). (B) Measured absorption spectrum of a solution of purified BCA-4FB (blue curve) at unknown concentrations of 4FB and BCA and the fitted spectrum of BCA-4FB (red curve), obtained as a linear combination of the individual spectra of methyl-4FB and free BCA shown in (A). Wavelength range: 251–327 nm. For this wavelength range, the measured absorbance of the purified BCA-4FB solution, Ameas, was assumed to linearly depend on the concentrations and molar absorption coefficients of free BCA (CBCA and εBCA) and BCA-4FB (CBCA-4FB and εBCA-4FB), whereby for BCA-4FB the molar absorption coefficient, εBCA-4FB, is taken as the sum of εBCA and εm-4FB: Ameas = CBCA·l·εBCA + Cm-4FB·l·εm-4FB + Cm-4FB·l·εBCA = CBCA·l·εBCA + Cm-4FB·l·(εm-4FB + εBCA); l is the optical path length of the cuvette used (l = 0.2 cm). The fitting was made with the software “R”, resulting in the best fit with CBCA = 75.9 and Cm-4FB = 49.5 μM, corresponding to MSR (4FB) = 0.65 (see text for details).
Another preparation of purified BCA-4FB was analyzed by ESI-MS. In Figure S-5A, the unmodified BCA molecules show a molecular mass of 29 024 Da which corresponds to the value calculated on the basis of the primary sequence (29 024.5 Da) without zinc ion.2 A similar value (29 024.34 Da) was reported in the literature.40 For the BCA-4FB molecules (Figure S-5B), peaks with masses of 29 025, 29 157, and 29 289 are seen. This shows that the purified BCA-4FB solution analyzed contained a mixture of unmodified BCA molecules (Mr(BCA) = 29 025), BCA molecules which were modified with one 4FB moiety (Mr(BCA-4FB) = Mr(BCA) + 132 = 29 157), and BCA molecules which were modified with two 4FB moieties (Mr(BCA-4FB2) = Mr(BCA) + 132 × 2 = 29 289).
3.3. Formation of the Conjugate de-PG2-BAH-BCA
The type of denpol-BAH-enzyme conjugate which we used in the work was prepared by simple mixing of an aqueous solution of de-PG21000-HyNic and an aqueous solution of BCA-4FB at pH = 7.2 (PB2). Details for the synthesis and characterization of the obtained conjugates de-PG21000-BAHy-BCAz are summarized in Table 1A and Figure S-12 (for de-PG21000-BAH175-BCA115). During the course of our investigations, five de-PG2-BAH-BCA conjugates were synthesized by using the same de-PG21000-HyNic preparation; the different conjugates vary in the average number of BAH units (y) and BCA molecules (z) per denpol chain. The conjugate solutions were further characterized in terms of BCA activity and storage stability. Both are essential if one aims at applying the conjugates on silicate surfaces.
Table 1A. Characteristics of the Reaction Mixtures for the Preparation of de-PG2-BAH-BCA from de-PG21000-HyNica.
| no. | MSR (4FB) = [4FB]/[BCA]/– | [4FB]/μM | [BCA]/μM | 4FB:HyNic (mol/mol) | [BAH]/μM |
|---|---|---|---|---|---|
| 1 | 1.02 | 66.5 | 65.1 | 1:1.8 | 48.4 |
| 2 | 1.17 | 65.0 | 55.6 | 1:1.8 | 48.4 |
| 3 | 0.54 | 36.7 | 68.5 | 1:1.8 | 26.7 |
| 4 | 0.78b | 50.0 | 64.3 | 1.6:1 | 31.2 |
| 5 | 0.78b | 49.5 | 63.6 | 1:2.3 | 45.5 |
See Supporting Information for details.
In the following, the preparation and characterization of one of the five conjugates are described in great detail for explaining the meaning and determination of the entries of Table 1A. The conjugate (entry 1 of Table 1A) was prepared by mixing a portion of the de-PG21000-HyNic solution (258 μL, 1.93 mM r.u., 0.85 mM HyNic, MSR (HyNic) = 0.11) with a portion of a BCA-4FB solution (1542 μL, 76 μM BCA, 78 μM 4FB, MSR (4FB) = 1.02) to induce conjugate formation under the following reaction (t) conditions: total volume = 1800 μL, [HyNic]r = 122 μM, [r.u.]r = 277 μM, [BCA]overall,r (= [BCA-4FB] + [free BCA]) = 65.1 μM, and [4FB]r = 66.5 μM (4FB:HyNic = 1:1.8) (Table 1A and Figure S-12). A portion of the reaction mixture (350 μL) was immediately placed into a quartz cell (l = 0.2 cm) for spectrophotometrically following the reaction. The rest of the solution (1450 μL) was incubated in a polypropylene tube for 20 h, and then, a portion (1300 μL) was used for the purification. The total amount of BCA-4FB and free BCA in the reaction mixture employed for the purification (1.3 mL) was 84.6 nmol, while that removed from the mixture by repetitive ultrafiltration was 43.0 nmol (Figure S-7B). Therefore, based on simple mass balance consideration, the amount of BCA-4FB conjugated to de-PG21000 in the conjugate was 41.6 nmol (= 84.6 nmol – 43.0 nmol). The concentration of BAH in the purified conjugate solution [BAH]p was determined as [BAH]p = {(A354,p – A354,pb)/(A354,r – A354,rb)} [BAH]r, where A354,p and A354,r represent the absorbance at 354 nm of the purified solution (p) and the reaction mixture (r), respectively; the terms with additional subscript b correspond to the background absorbance. The above equation can be simplified to [BAH]p = (A354,p/A354,r) [BAH]r because (A354,r/A354,rb) is equal to (A354,p/A354,pb). Assuming that the ratio of [BAH]/[r.u.] remained unchanged during the purification, the concentration of r.u. in the solution of the purified conjugate, [r.u.]p, was [r.u.]p = (A354,p/A354,r) [r.u.]r, where [r.u.]r is the concentration of r.u. in the reaction mixture. The ratio A354,p/A354,r for the discussed conjugate preparation was 0.92, yielding [r.u.]p = 255 μM and [BAH]p = 44.5 μM (Table 1B). The [BAH]p/[r.u.]p ratio was 0.175, meaning that 175 BAH bonds were present per 1000 r.u. The concentration of BCA in the purified conjugate solution was [BCA]p = (41.6 × 10–9 mol × 0.92)/(1.3 × 10–3 L) = 29.4 × 10–6 M = 29.4 μM. With this, [BCA]p/[BAH]p = 0.66 (<1), which indicates that a part of the BCA molecules was conjugated to de-PG21000 through multiple BAH bonds/per enzyme molecule. This is reasonable because BCA molecules with multiple 4FB units were present in the purified BCA-4FB solution, as indicated by the ESI-MS analysis (Figure S-5B). The [BCA]p/[r.u.]p ratio in the purified conjugate solution was 0.115 (= 29.4 μM/255 μM), which means that on average 115 BCA molecules were conjugated per 1000 r.u. Based on this analysis, the purified conjugate was abbreviated as de-PG21000-BAH175-BCA115.
Table 1B. Characteristics of the Purification of de-PG2-BAH-BCAa.
| no. | BCA charged/nmol | BCA separated/nmol | BCA conjugated/nmol | recovery yield/% | volume/mL | [BCA]/μM | [BAH]/μM | [r.u.]/μM |
|---|---|---|---|---|---|---|---|---|
| 1 | 84.6 | 43.0 | 41.6 | 92 | 1.30 | 29.4 | 44.5 | 255 |
| 2 | 72.3 | 37.1 | 35.2 | 84 | 1.30 | 22.7 | 40.7 | 228 |
| 3 | 68.5 | 44.7 | 23.8 | 82 | 1.05 | 18.6 | 20.9 | 121 |
| 4 | 73.4 | 50.3 | 23.1 | 72 | 1.14 | 14.6 | 22.5 | 49.9 |
| 5 | 44.0 | 24.2 | 19.8 | 86 | 0.69 | 24.7 | 39.1 | 222 |
Quantification of the conjugates obtained resulted in the following specification. Entry 1: de-PG21000-BAH175-BCA115, Entry 2: de-PG21000-BAH179-BCA100, Entry 3: de-PG21000-BAH173-BCA154, Entry 4: de-PG21000-BAH451-BCA293, Entry 5: de-PG21000-BAH176-BCA111.
In the next step, the activity of the conjugated BCA was examined and compared with the spectrophotometrically determined BAH concentration. For this, the conjugate solution was desalted by repetitive ultrafiltration. From the measured A354 value of this purified and desalted conjugate solution and by taking into account [BCA]p/[BAH]p = 0.66 (see above), the BCA concentration in the desalted solution of the conjugate was determined as 26.0 μM, a bit lower than the concentration determined before desalting (29.4 μM, see above). A determination of the BCA concentration in this solution through measurements of the esterase activity with p-NA and considering a calibration curve made with known amounts of BCA yielded an apparent concentration of BCA of 18.7 μM. This indicates that either the BCA molecules underwent a partial inactivation during the conjugation reaction or denpol binding of BCA altered the reaction kinetics, possibly due to a decreased substrate access to the enzyme’s active site. However, a large fraction (72%, i.e., 18.7 μM of 26.0 μM) of the original BCA activity remained in the desalted conjugate solution.
With the same 4FB:HyNic ratio of 1:1.8 applied for the conjugation reaction which yielded de-PG21000-BAH175-BCA115, the two conjugates de-PG21000-BAH179-BCA100 and de-PG21000-BAH173-BCA154 were obtained by using a solution of either BCA-4FB with MSR (4FB) = 1.17 or BCA-4FB with MSR (4FB) = 0.54 (Table 1A, Figure S-13). Although the few preparations did not allow a true statistical analysis, it seems that the differences in the [BAH]/[BCA] ratio in the conjugates resulted from an increase in MSR (4FB) of the BCA-4FB used, i.e., from an increase of the fractional amount of BCA modified with multiple 4FB units per enzyme molecule. If the conjugates were prepared at different 4FB:HyNic ratios of 1.6:1 and 1:2.3, de-PG21000-BAH451-BCA293 and de-PG21000-BAH176-BCA111 were obtained, respectively (Table 1A, Figure S-13).
In summary, with the different denpol-BAH-BCA conjugates prepared in this work, we have proven that the general protocols which we developed for the synthesis and purification of these types of conjugates are reliable. From the same de-PG21000-HyNic different de-PG21000-BAHy-BCAz conjugates can be prepared which differ in y and z by choosing the experimental conditions in terms of (i) degree of BCA modification with 4FB, (ii) the concentrations of 4FB and HyNic applied during the conjugate preparation, and (iii) the used ratio of 4FB to HyNic.
3.4. Stability of de-PG2-BAH-BCA in Aqueous Solution
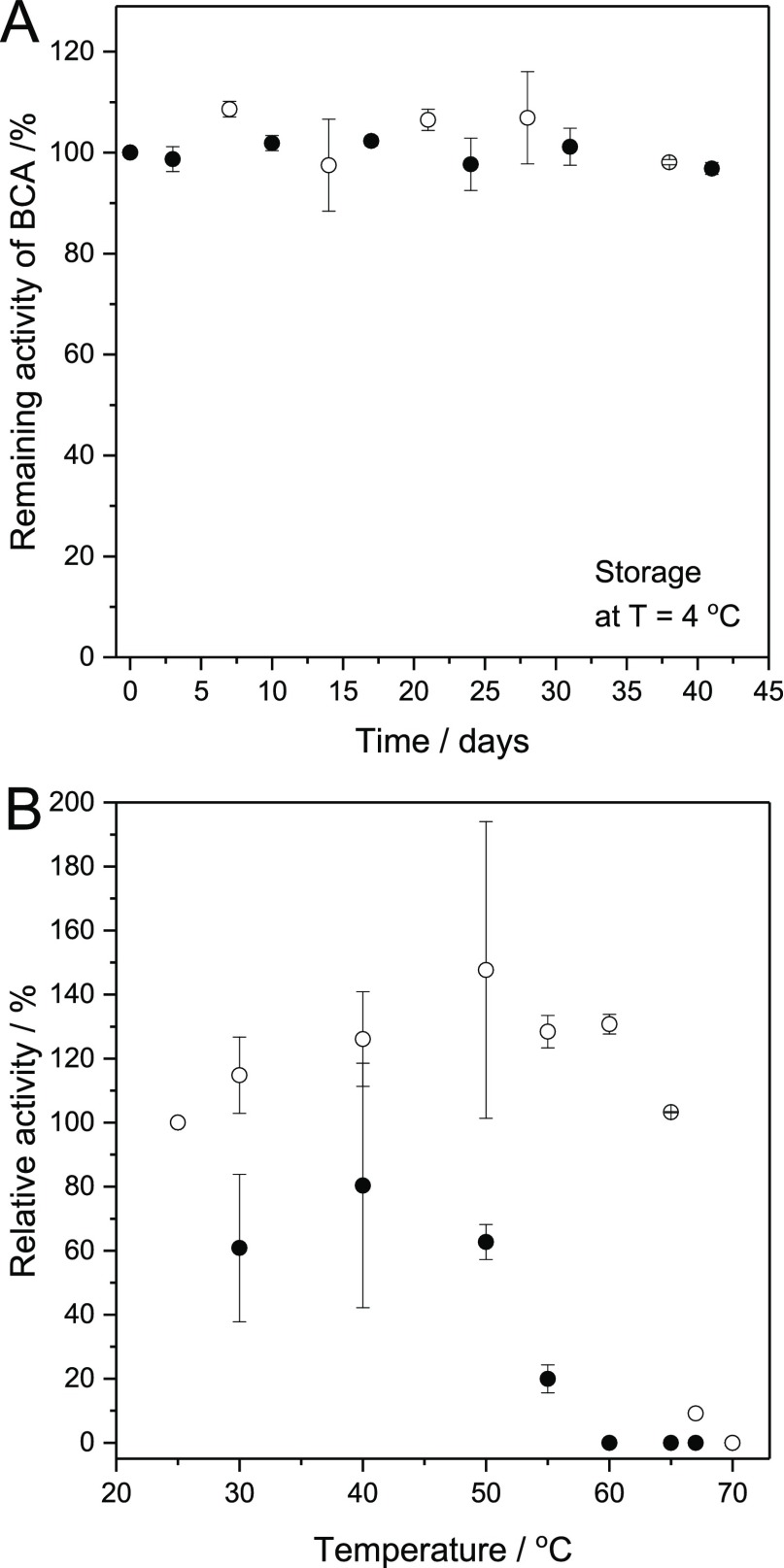
The activity of the conjugate de-PG21000-BAH175-BCA115 during storage in solution (PB2) at 4 °C was measured by withdrawing small volumes which were analyzed with p-NA as substrate. Under these conditions (ca. 30 μM BCA, pH = 7.2, presence of 1.15 M NaCl), the conjugate was very stable: after a period of 41 days, 97% of the initial activity remained (Figure 5A, filled circles). From a practical point of view, this finding is very important since once prepared the same conjugate solution can be used over an extended period of time if stored at 4 °C. In the case of experiments with free BCA, dissolved in the same buffer solution at about the same BCA concentration, it was shown that the free enzyme is also stable (Figure 5A, empty circles).
Figure 5.
Storage stability of de-PG2-BAH-BCA (●) and free BCA (○) in aqueous solution at pH = 7.2 for T = 4 °C (A) and 25 °C ≤ T ≤ 70 °C (B). (A) Remaining relative activity of a de-PG21000-BAH175-BCA115 solution (●) and of free BCA (○), which was stored at 4 °C in PB2 (pH = 7.2) in a polypropylene tube at an initial volume of 1.3 mL. The overall concentration of BCA was 29.4 μM. For the activity measurements, aliquots (10 μL) were withdrawn and diluted with 980 μL of PB1 (pH = 7.2), followed by addition of 10 μL of substrate solution (100 mM p-NA in acetonitrile). The total concentration of NaCl during the activity measurement at 25 °C was 0.16 M. The background hydrolysis of 1.0 mM p-NA was also measured in a 0.1 M phosphate buffer solution containing 0.16 M NaCl in the absence of enzyme and then subtracted from the value obtained with the enzyme. The data thus obtained and shown represent mean values ± standard deviations (n = 3). The activity measured before storage was taken as 100%. (B) Effect of temperature on the relative activity of de-PG21000-BAH451-BCA293 (●) and free BCA (○) dissolved in PB3 (pH = 7.2). In both cases, the BCA concentration was about 3.4 μM. The samples were incubated at the indicated temperature for 30 min followed by incubation at 25 °C for 30 min. Then, the BCA activity was measured with 1.0 mM p-NA at 25 °C (see Supporting Information). The measured activity at 25 °C in PB3 without heat treatment was taken as 100%. The rates of hydrolysis in the presence of the conjugate at T ≥ 60 °C and in the presence of free BCA at T = 70 °C were smaller than the rate of the background hydrolysis. In these cases, the relative activity of BCA was taken as zero. The number of measurements n was n = 3 at 25–55 °C for both free BCA and conjugates, and n = 2 at 60–65 °C and n = 1 at 67 °C for free BCA. For the cases of n ≥ 2, the data represent mean values ± standard deviations.
The heat stabilities of dissolved de-PG21000-BAH451-BCA293 and free BCA were examined (Figure 5B). Although for some data points the standard deviations are high, it is evident that the free enzyme is more stable at high temperature (T > 55 °C) than the conjugated enzyme. A significant deactivation of free BCA is seen at about 65 °C, in agreement with previous literature data,41,42 whereas the deactivation of BCA in the conjugate occurs already at about 50 °C. The kinetics of BCA inactivation for the free enzyme and the denpol-bound enzyme were compared at 60 °C, and this comparison confirmed again a lower stability for the conjugate enzyme as compared to free BCA (Figure S-14). The reason for the lower thermostability of the conjugated enzyme, as compared to free BCA, is not clear. It may be that interactions of the many free amino groups of the denpol chain with the conjugated BCA molecules have a negative effect. However, this is only a hypothesis and deserves further investigations if one would aim at applying such conjugates in aqueous solution at high temperatures. One approach toward increasing the thermostability of CA is to modify the enzyme’s shape through directed evolution. Alvizo et al. have shown that the stability of CA from Desulfovibrio vulgaris could be significantly improved with this approach for applying the enzyme to capturing carbon dioxide in flue gas.43
3.5. Activity of de-PG21000-BAH175-BCA115 Immobilized on Microscopy Glass Coverslips
The enzyme activity of glass coverslips which contained immobilized de-PG21000-BAH175-BCA115 was measured at pH = 7.2 (PB3) with 1.0 mM p-NA as substrate. The initial rate of reaction was determined by quantifying the conversion of p-NA, xp-NA, after 15 min, corresponding to about 2% of the initially present p-NA. From this determination and by taking into account the contribution from the nonenzymatic hydrolysis and a calibration curve made with known amounts of free BCA (Figure S-8), the apparent surface density of active BCA molecules, Γapp, was then calculated by considering the total surface area (≈1.0 cm2) of one coverslip. The obtained value was Γapp = (3.5 ± 0.1) × 10–4 U/cm2 (= 7.6 ± 0.3 pmol/cm2) (mean and standard deviation from the analysis of n = 3 different freshly prepared coverslips). This analysis shows that the immobilization of the conjugate is very reproducible and that about 4.6 × 1012 active BCA molecules were immobilized in the form of conjugates on the surface of one coverslip. It is likely that the positively charged free amino groups of the denpol chain contribute significantly to the immobilization of the conjugate on the negatively charged silicate glass coverslips. If one assumes that the glass surface is molecularly flat and if it would be completely covered by a monolayer of BCA molecules with a hydrodynamic diameter of 4.1 nm,44 then one obtains an occupancy of Γ = 6.1 × 10–4 U/cm2 (13 pmol/cm2). This value is higher than Γapp = 3.5 ± 0.1 U/cm2 (7.6 ± 0.3 pmol/cm2) for the conjugate but still of the same order of magnitude. It is expected that a part of the BCA molecules of the conjugate is in close contact to the surface, possibly undergoing (partial) inactivation, and another part of the BCA molecules is exposed to the aqueous solution.
3.6. Activity of de-PG21000-BAHy-BCAz Immobilized Inside Glass Micropipettes
The conjugate de-PG21000-BAH175-BCA115 was immobilized inside the “200 μL micropipettes” at pH = 7.2 (PB3) and [BAH] = 3.3 μM. In control measurements it was shown that the glass surface of the micropipettes without immobilized enzyme had little effect on the initial hydrolysis of p-NA under the conditions employed (xp-NA < 0.1% after a reaction time of 3 min). The calculated Γapp value obtained for BCA of the immobilized conjugate was (4.5 ± 0.2) × 10–4 U/cm2 (9.7 ± 0.5 pmol/cm2) on the basis of initial activity measurements (xp-NA ≈ 4% after a reaction time of 3 min). This value is very similar to the value obtained for the glass coverslips (3.5 ± 0.1 U/cm2 (7.6 ± 0.3 pmol/cm2); see section 3.5). If free BCA molecules were used during the immobilization procedure instead of the conjugate, a much smaller Γapp value of 4.7 × 10–7 U/cm2 (0.01 pmol/cm2) was obtained. Similarly, in the case of “20 μL micropipettes”, the Γapp values were (4.1 ± 0.4) × 10–4 U/cm2 (8.7 ± 0.8 pmol/cm2) and 9.3 × 10–7 U/cm2 (0.02 pmol/cm2), for the immobilized conjugates and free BCA, respectively. These results clearly demonstrate that the conjugates can be efficiently immobilized inside the micropipettes under conditions where the noncovalent adsorption of free BCA is very low. The Γapp value decreased when the conjugates were immobilized at lower concentrations of BAH but at an identical immobilization time of 1 h, as examined with the “200 μL micropipettes” (see Figure S-15).
To examine more details about the interaction between the conjugates and the glass surface, the conjugate de-PG21000-BAH179-BCA100 was immobilized inside “200 μL micropipettes” at pH = 5.0 (PB4) in addition to pH = 7.2 (PB3). As shown in Table 2, for de-PG21000-BAH179-BCA100, Γapp for the pH = 5.0 conditions was slightly larger than for the pH = 7.2 conditions. The reason for this difference may be the higher positive charge of the BCA molecules at pH = 5.0, as compared to pH = 7.2 (pI(BCA) = 5.9),2 which would favor electrostatic interactions with the negatively charged glass surface. However, the many positively charged amino groups from the denpol (pKa ≈ 8–9)31 of the conjugate system seem to be the determinants for the strong noncovalent binding of the conjugate to the glass surface. Therefore, the effect of precoating the inner surface of the micropipettes with de-PG21000 on the immobilization of the conjugate at pH = 7.2 or 5.0 was also examined. This precoating is expected to modify the negatively charged silicate surface significantly,26 as in the case of the coating with polylysine.45−47 As shown in Table 2, the conjugate did also adsorb on the de-PG21000-modified surface, both at pH = 7.2 and 5.0. Based on the relatively large Γapp value of 6.5 × 10–4 U/cm2 (13.9 pmol/cm2) at pH = 7.2 > pI(BCA), one is tempted to conclude that the (overall) negatively charged BCA molecules which are bound along the denpol chain contribute to the immobilization of the conjugate to those areas of the glass surface which were coated with positively charged de-PG21000. However, the conjugate also bound to the de-PG21000-modified surface at pH = 5.0 < pI(BCA), although in this case Γapp (= 3.9 × 10–4 U/cm2 (8.4 pmol/cm2)) was lower than at pH = 7.2 (Γapp = 6.5 × 10–4 U/cm2 (13.9 pmol/cm2)). If one assumes that de-PG21000 was relatively densely adsorbed on the glass surface, as concluded from a previous study with de-PG21400,29 then it seems that the immobilization of the conjugate not only is based on electrostatic interactions but must also involve nonelectrostatic contributions. On the other hand, both positively and negatively charged sites distinctly exist on the surface of BCA at pH values below and above pI.23 Therefore, negatively charged local sites of BCA23 may contribute to the interaction of the conjugate with the positively charged denpol coat, even at pH = 5.0.
Table 2. Apparent Density of Enzymatically Active BCA on the Inner Surface of “200 μL Glass Micropipettes” (Γapp) onto Which Either the Conjugate de-PG21000-BAH179-BCA100 or Free BCA Was Immobilized.
| immobilized catalyst | modification of micropipette | pH at immobilization of catalyst | Γapp/× 10–4 U·cm–2 (pmol·cm–2) |
|---|---|---|---|
| conjugate | none | 7.2 | 5.2 (11.2) |
| conjugate | none | 5.0 | 6.0 (12.9) |
| conjugate | de-PG21000 | 7.2 | 6.5 (13.9) |
| conjugate | de-PG21000 | 5.0 | 3.9 (8.4) |
| free BCA | none | 7.2 | 0.005 (0.01) |
| free BCA | none | 5.0 | 0.10 (0.21) |
| free BCA | de-PG21000 | 7.2 | 0.09 (0.19) |
| free BCA | de-PG21000 | 5.0 | 0.07 (0.16) |
The immobilization (adsorption) of free BCA was also examined at pH = 7.2 or 5.0 using micropipettes with and without preadsorbed de-PG1000 (Table 2). Under all conditions examined, only trace amounts of free BCA molecules remained inside the micropipettes if the same immobilization procedure was applied as in the case of the conjugates (see section 2.7). This is in very clear contrast to the results obtained with the conjugates. Notably, even under conditions where the free BCA molecules have a net charge opposite to that of the solid surface (pH = 7.2 > pI(BCA)), only small amounts of active enzyme were adsorbed. Therefore, the interaction of single BCA molecules with the glass surface may not be strong enough for a stable immobilization. In contrast, one de-PG21000-BAH179-BCA100 molecule has on average 100 conjugated BCA molecules and as much as 3560 free amino groups from the denpol (see section 3.1). Therefore, the conjugate provides many opportunities to interact with a negatively or positively charged surface through a large number of weak interactions between the surface and the denpol or the surface and the enzyme. To which extent the BCA molecules contribute to the binding of the conjugate to the surface—with a possible concomitant partial BCA inactivation—is not clear at the moment. However, the three-dimensional “worm-like” structure of the conjugate certainly contributes to their high immobilization efficiency because a large fraction of the conjugate’s BCA molecules is expected to be without direct contact with the surface.
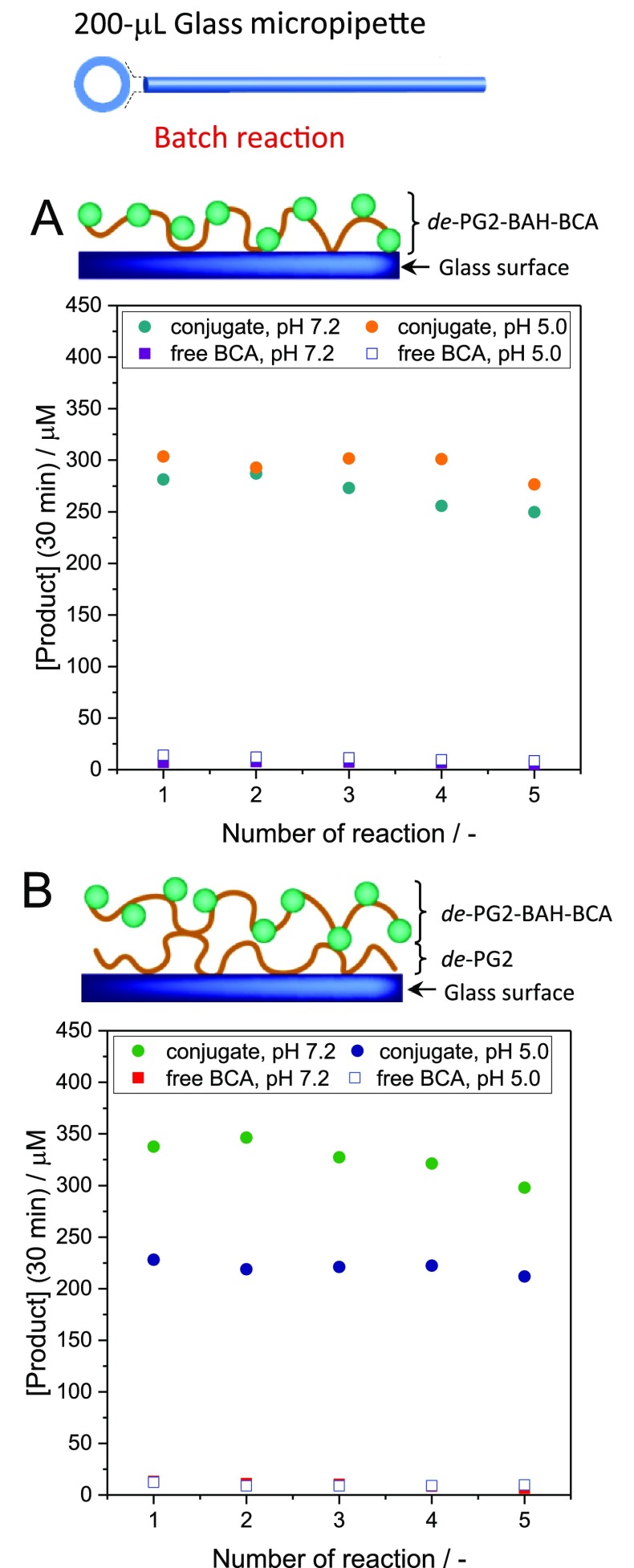
The “200 μL micropipettes” containing either immobilized de-PG21000-BAH179-BCA100 or adsorbed free BCA were used for a batch reaction analysis at pH = 7.2 (PB3). The extent of p-NA hydrolysis was determined upon filling the micropipettes with a 1 mM p-NA solution and keeping it inside the pipettes for 30 min. The micropipettes were used repeatedly for 5 times to check the stability of the immobilized enzymes (Figure 6). The immobilized conjugates remained active even after the fifth reaction, although the amount of product formed during the 30 min incubation slightly decreased with increasing number of repeated reactions. Similar results were obtained in a separate preparation with “200 μL micropipettes” inside which a slightly different conjugate, de-PG21000-BAH173-BCA154, was immobilized at pH = 7.2 and used with 6 repeated batch reactions (Figure S-16).
Figure 6.
Schematic illustration of (A) a glass surface with an immobilized conjugate and (B) a de-PG2-modified glass surface with an immobilized conjugate. Results from batchwise repeated reactions with (A) “200 μL micropipettes” containing directly immobilized de-PG21000-BAH179-BCA100 or adsorbed free BCA at pH 7.2 (PB3) or 5.0 (PB4) and with (B) de-PG21000-modified “200 μL micropipettes” containing immobilized de-PG21000-BAH179-BCA100 or adsorbed free BCA, both at pH = 7.2 (PB3) or 5.0 (PB4). Each reaction was performed for 30 min at an initial concentration of p-NA of 1.0 mM in PB3 (pH = 7.2). [Product] = [p-nitrophenolate] + [p-nitrophenol].
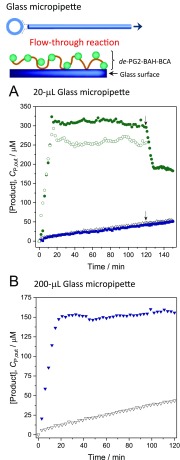
The flow-through reaction with a 1 mM p-NA solution was performed using “20 μL micropipettes” containing either immobilized de-PG21000-BAH175-BCA115 or adsorbed free BCA. The reaction was also performed with a control micropipette without BCA for determining the nonenzymatic background reaction. The flow rate was set to 2.7 μL/min corresponding to a mean residence time τ of 15 min, followed by an increase to 5.7 μL/min (τ = 7.0 min) after 120 min. Figure 7A shows the time course of the concentration of product, CP,out, at the outlet of the micropipette. For the control micropipette, CP,out gradually increased with time, independent of the flow rate, confirming that the glass surface is catalytically inert. The micropipette containing adsorbed free BCA gave practically the same reaction time course as the control micropipette, meaning that the activity of possibly adsorbed free BCA was negligible. On the other hand, the micropipette containing immobilized conjugates clearly catalyzed the hydrolysis of p-NA, yielding an almost constant CP,out of about 300 μM for the chosen observation time of 120 min. Considering the background hydrolysis of p-NA (empty triangles in Figure 7A), the amount of enzymatically produced product continuously decreased with increasing operation time (Figure S-17). From the data shown in Figure 7A it is clear that CP,out obtained with a micropipette containing immobilized conjugates depends on the flow rate, which is expected from the kinetics in a plug flow (Figure S-17). The reaction was also performed with a larger “200 μL micropipette”, again containing immobilized de-PG21000-BAH175-BCA115, by setting the flow rate to 18.4 μL/min (τ = 15 min). The results obtained are shown in Figure 7B. After 15 min, CP,out was about 125 μM. For the smaller “20 μL micropipette”, CP,out reached 313 μM after 14 min at the same mean residence time τ of 15 min. In another set of measurements, the conjugate de-PG21000-BAH451-BCA293 was also immobilized in a “200 μL micropipette” and tested as a flow reactor; the results obtained are in agreement with the ones obtained with de-PG21000-BAH175-BCA115 (see Figure S-18).
Figure 7.
(A) Time courses of flow-through reactions with “20 μL micropipettes” containing immobilized conjugate de-PG21000-BAH175-BCA115 (filled circles) or adsorbed free BCA (filled squares); results from a “20 μL control micropipette” are also shown (empty triangles, no BCA). PB3 initially containing 1.0 mM p-NA (pH = 7.2) was continuously passed through the micropipettes. The absorption spectrum of the solution eluting from the micropipettes was measured, and A405 was converted into Cp,out = [p-nitrophenolate] + [p-nitrophenol] (see section 2.10). The flow rate was 2.7 μL/min (mean residence time τ = 15 min) for the first 120 min, and then at the time indicated by the arrows, the flow rate was increased to 5.7 μL/min (τ = 7.0 min). The empty circles are the data for a “20 μL micropipette” containing immobilized de-PG21000-BAH175-BCA115, which was washed first with PB3 before the substrate solution was pumped trough. This washing was at a flow rate of 14.3 μL/min for 218 min. (B) Reaction time courses for a “200 μL micropipette” containing immobilized de-PG21000-BAH175-BCA115 (filled reverse triangles) and for a “200-μL control micropipette” (no, BCA, empty reverse triangles). The reaction was performed by passing the substrate solution at a flow rate of 18.4 μL/min (τ = 15 min) through the micropipette.
Since the catalytic reaction occurs at the solid–liquid interface inside the micropipettes, the ratio of surface area to volume is expected to affect CP,out. The present results clearly demonstrate that the “20 μL micropipette” system with a surface area to volume ratio of 6.3 × 103 m–1 is much more efficient than the “200 μL micropipette” system with its smaller surface area to volume ratio of 2.5 × 103 m–1. For the “200 μL micropipette” system, the concentration of enzymatically produced product gradually decreased as the operation time elapsed (Figure S-17 and Figure S-18B), in agreement with what was found for the “20 μL micropipette” (see above). Concerning this point, the following prewashing tests are important to mention. The “20 μL micropipette” containing immobilized conjugate de-PG21000-BAH175-BCA115 was washed with PB3 at a high flow rate of 14.3 μL/min for 218 min prior to running the flow-through reaction with p-NA. Although after this washing step the micropipette still contained immobilized active BCA which catalyzed the hydrolysis of p-NA, as shown in Figure 7A, the outlet conversion decreased to about 70% of that obtained with the micropipette prior to washing. Γapp decreased from 4.2 × 10–4 U/cm2 (9.0 pmol/cm2) (before washing) to 3.0 × 10–4 U/cm2 (6.5 pmol/cm2) (after washing). This indicates that a fraction of the originally immobilized BCA became inactive or desorbed from the glass surface under the shear stress generated in the micropipettes.
3.7. Activity of de-PG21000-BAH175-BCA115 Immobilized in Glass Fiber Filters
The immobilization of the conjugate de-PG21000-BAH175-BCA115 in binder-free glass fiber filters was carried out by simply immersing the purified and dry filter in an aqueous solution of the conjugate (PB3), followed by washing with the same buffer solution (see section 2.11). For a direct comparison with flat, nonporous glass coverslips, the apparent density of active BCA molecules on the filter surface, Γapp, was determined through BCA activity measurements and by taking into account the calculated total “outer” surface area of the filter (0.92 cm2) with the assumption of a flat surface. For comparison, the filters were also immersed in a solution of free BCA instead of the conjugate, followed by the same washing procedure as in the case of the conjugate. As a result, Γapp for the conjugate was (14.7 ± 1.5) × 10–4 U/cm2 (31.6 ± 3.2 pmol/cm2) (n = 4), which was larger than the values determined for the conjugates immobilized on glass coverslips ((3.5 ± 0.1) × 10–4 U/cm2 (7.6 ± 0.3 pmol/cm2)) or on the inner surface of micropipettes (5.2 × 10–4 U/cm2 (11.2 pmol/cm2)) (see above). This is reasonable since the glass fiber filters have a porous structure (Figure 2), and thus the conjugates can adsorb to the microfibers not only at the area which is exposed to the bulk liquid but also at the inner parts of the filter. Notably, the filters containing adsorbed free BCA molecules yielded Γapp = (8.4 ± 1.4) × 10–4 U/cm2 (18.1 ± 2.9 pmol/cm2) (n = 3), whereas practically no free BCA adsorbed in the case of the coverslips or micropipettes (see above). This indicates that free BCA molecules probably can diffuse into the pores of the fiber matrix of the filter, where they have several opportunities to adsorb through multiple interactions. Furthermore, the release of free BCA molecules from the filter during the washing step may be sterically hindered.
In a next step, we investigated whether BCA-containing glass fiber filters can be applied as flow-through reactor systems. For this, one filter containing immobilized conjugate was first mounted in a homemade reactor unit (Figure 3). This device was then used as a flow-through reactor with monitoring of the hydrolysis of p-NA (Figure 8A, curve 1). The reaction was also studied with a single filter with adsorbed free BCA (Figure 8A, curve 2) and with a control filter (no BCA, Figure 8A, curve 3). The use of the control filter yielded a comparable time course of CP,out as in the case of the control glass micropipette (Figure 7A), demonstrating that the glass fiber filter is catalytically inert toward the hydrolysis of p-NA, as in the case of the glass micropipettes (see above). With the filter containing immobilized conjugates, the hydrolysis of p-NA was clearly accelerated; i.e., significantly larger CP,out values were obtained as compared to the BCA-free control system. However, from the data shown in Figure 8A it is clear that with the filter containing adsorbed free BCA the p-NA hydrolysis reaction was also catalyzed, although CP,out at 180 min reaction was about one-half of the value obtained with the filter containing immobilized conjugates (compare curves 1 and 2). To examine the stability of the immobilized conjugates and of adsorbed free BCA, freshly prepared filters were prewashed with PB3 at high flow rate (30–32 μL/min for 4.5 h). As shown in Figure 8A, the time course of CP,out in the case of the prewashed filters containing immobilized conjugates was not significantly different from the filter which was not prewashed (compare curves 1 and 4). This demonstrates that the inactivation or desorption of the conjugates from the filter during the prewashing was negligible, while for the filter containing adsorbed free BCA (compare curves 2 and 5), the catalytic performance clearly decreased after the high-flow washing step (Figure 8A). This suggests that a part of the adsorbed free BCA molecules desorbed from the filter during the prewashing process. However, the remaining adsorbed free BCA molecules still exhibited a stable catalytic activity at an operational flow rate of 2.7 μL/min. The time courses of the “true” enzymatically produced product by using filters with immobilized conjugates or adsorbed free BCA were then calculated by subtracting the nonenzymatic contribution. The data obtained show that the filter with the immobilized conjugate was clearly more efficient than the filter with adsorbed free BCA (Figure S-19), but the difference was by no means as large as in the case of the micropipettes (Figure 7A).
Figure 8.
(A) Time courses of the hydrolysis of p-NA in a continuous flow reactor system which consisted of a single glass fiber filter containing immobilized conjugate de-PG21000-BAH175-BCA115 (1 and 4) or adsorbed free BCA (2 and 5). A control filter without BCA was also used under the same conditions (3). The reaction was performed without (1 and 2) or with (4 and 5) prewashing with PB3 (pH = 7.2). The prewashing was performed at a flow rate of 30.4 μL/min for 4.5 h for the immobilized conjugates and 31.8 μL/mL for 4.5 h for the adsorbed free BCA. For the reaction, PB3 which initially contained 1.0 mM p-NA was continuously passed through the filter. After initial small adjustments of the flow rate, it was set to a fixed value of 2.7 μL/min after about 60 min for a further operation during 120 min. (B) The same flow-through reaction as in (A) with a flow rate of 2.7 μL/min but by using either two stacked glass fiber filters containing immobilized conjugate de-PG21000-BAH175-BCA115 (filled reverse triangles) or two stacked control filters (no BCA, empty reverse triangles). For a drawing of the filter holder, see Figure 3.
From a practical point of view, it is important to mention that for all reactions without and with high-flow prewashing, no breakage of the glass fiber filters was observed when the filters were removed from the flow reactor unit (Figure 3).
Finally, we tested whether two stacked glass fiber filters can be used sequentially. Figure 8B shows the reaction operation with two stacked filters, both containing either immobilized conjugate de-PG21000-BAH175-BCA115 or no enzyme at all (used as control). The analysis with the latter control filters gave a similar time course of CP,out as in the case of the single filter (Figure 8A). This confirmed that the filters as such were catalytically inert. On the other hand, the use of the two stacked filters containing immobilized conjugates resulted in significantly higher CP,out values than in the case of the corresponding single filter use. This is reasonable since the amount of immobilized enzyme molecules is higher for two filters as compared to one filter. Moreover, the successful use of stacked filters containing immobilized enzymes opens a door for applications in enzymatic cascade reactions with filters containing different types of enzymes.
4. Conclusions
Different de-PG21000-BAH-BCA conjugates were prepared by linking BCA-4FB molecules to de-PG2-HyNic chains through stable BAH bonds. The conjugates were prepared in aqueous solution at room temperature and pH = 7.2 in the presence of 1.15 M NaCl. The use of high amounts of NaCl was found to be critical for preventing precipitation during conjugate formation. The advantage of the “BAH chemistry”27,28 is that the individual steps of the conjugate formation can be quantified spectrophotometrically. This is usually not the case for other linker chemistries.
Although the general methodology of conjugate formation by using a water-soluble polycationic dendronized polymer was developed a few years ago already by using horseradish peroxidase (HRP) and bovine erythrocytes superoxide dismutase (SOD),39 for each new enzyme of interest, the experimental conditions have to be adopted. Furthermore, once succeeded with the conjugate formation, the conjugate stability in aqueous solution is important to know if one considers applications. In the work presented, we have shown that de-PG21000-BAH-BCA conjugates can be prepared under the elaborated conditions with high reproducibility and that these conjugates are stable in aqueous solution for more than one month if stored at pH = 7.2 and 4 °C (Figure 5A). Since there is a continuous desire for optimizing and possibly simplifying the quantification of the different steps of the conjugate formation, we tried to apply for the first time a simple spectrophotometric fitting procedure for the quantification of 4FB-modified BCA by using BCA and methyl-4FB as reference compounds (Figure 4). Since the spectrophotometric fitting procedure gave reliable results, it may become a generally applicable, alternative 4-FB quantification method that does not require the use of 2-HP (2-hydrazinopyridine) as quantification reagent.
Although the stability of the conjugates in aqueous solution above T ≈ 50 °C was found to be lower than the stability of free BCA (Figure 5B), the immobilization of de-PG21000-BAH-BCA inside glass micropipettes and porous glass fiber filters at room temperature was successful. Similar to the required optimization for the conjugate preparation, the conditions for the immobilization of the conjugate had to be elaborated (pH = 7.2, low salt content). For simple, small-scale flow-through reactor applications, the use of the “20 μL micropipettes” containing immobilized BCA (Figure 7) resulted in higher substrate conversions than in the case of the “200 μL micropipettes”, which clearly confirmed the expected increase in conversion with increase of surface-to-volume ratio.
So far, BCA is the fifth enzyme which could be immobilized successfully on silicate surfaces through simple noncovalent de-PG2-BAH-enzyme conjugate adsorption. The enzymes previously used are HRP,29Aspergillus sp. glucose oxidase (GOD),29Engyodontium album proteinase K,30 and a microbial transglutaminase (from Streptomyces mobaraensis).48 In the last part of the work, we have shown that this immobilization method can also be applied for immobilizing BCA in porous glass fiber filters (Figure 2 and Figure 8). Similar filters were already used previously for the covalent immobilization of trypsin for developing affinity membranes for chromatographic separation of biomolecules.49 In our work, we used a homemade reactor unit with porous glass filters containing immobilized BCA as flow-through system (Figure 3). By placing more than one filter in series, it is possible to either increase the amount of immobilized enzyme or to use filters containing different types of enzymes for enzymatic cascade reactions. This latter possibility will be elaborated in one of our future studies.
Acknowledgments
We thank Daniel Messmer for providing us with the denpol PG2; Dr. Chengmin Hou for her experimental support concerning the general procedures for the preparation, characterization, and immobilization of the conjugates; Dr. Erich Meister for help with the spectrophotometric fitting; and Dr. Karsten Kunze for the SEM analysis of the porous glass fiber filter used. This work was supported in part by Japan Society of Promotion of Science (JSPS) KAKENHI Grant Number 15KK0241.
Glossary
Abbreviations
- BAH
bisaryl hydrazone
- BCA
bovine erythrocyte carbonic anhydrase
- CBCA
concentration of bovine erythrocyte carbonic anhydrase
- CA
carbonic anhydrase
- Cp-NA
concentration of p-nitrophenyl acetate
- CP,out
concentration of product at reactor outlet
- de-PG2
second-generation dendronized polymer
- de-PG2-BAH-BCA
dendronized polymer–BCA conjugates through a BAH bond
- din
inner diameter of a glass micropipette
- DMF
N,N-dimethylformamide
- ESI-MS
electrospray ionization mass spectrometry
- 2-HP
2-hydrazinopyridine dihydrochloride
- kcat
rate constant of Michaelis–Menten-type enzyme reactions
- Km
Michaelis constant
- L
length of a glass micropipette
- l
optical path length
- methyl-4FB
4-formyl-N-methyl-benzamide
- MSR
molar substitution coefficient
- p-NA
p-nitrophenyl acetate
- r
rate of hydrolysis of p-NA
- r.u.
repeating units of a dendronized polymer
- SEM
scanning electron microscope
- S-4FB
N-succinimidyl 4-formylbenzoate
- S-HyNic
N-succinimidyl 6-hydrazinonicotinate acetone hydrazone
- U
BCA activity unit
- VL
liquid-phase volume of glass fiber filter
- Vm
maximum volume of a glass micropipette
- xp-NA
conversion of p-NA at reactor outlet
- ε
molar absorption coefficient
- Γapp
apparent density of biologically active enzymes on the surface of supports.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01517.
Modification of de-PG2 with S-HyNic; modification of BCA with S-4FB, quantification of HyNic in de-PG2-HyNic and 4FB in BCA-4FB; ESI-MS analysis of BCA-4FB; spectrophotometric properties of methyl-4FB and free BCA; formation of de-PG21000-BAH-BCA conjugates; separation of free BCA-4FB from conjugates; details on the esterase activity of free BCA; schematic illustration for the preparation and purification of conjugates; thermal stability of conjugates; effect of the concentration of BAH on the immobilization of conjugates; activity of conjugates in repeated hydrolysis of p-NA; flow-through reactions with the micropipette and glass fiber filters immobilized with conjugates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Maren T. H. Carbonic Anhydrase: Chemistry, Physiology, and Inhibition. Physiol. Rev. 1967, 47, 595–781. 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy V. M.; Kaufman G. K.; Urbach A. R.; Gitlin I.; Gudiksen K. L.; Weibel D. B.; Whitesides G. M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein-Ligand Binding. Chem. Rev. 2008, 108, 946–1051. 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J. Carbonic Anhydrase: Isoenzymes, Properties, Distribution, and Functional Significance. Biol. Rev. 1972, 47, 465–513. 10.1111/j.1469-185X.1972.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Yeates T. O.; Kerfeld C. A.; Heinhorst S.; Cannon G. C.; Shively J. M. Protein-Based Organelles in Bacteria: Carboxysomes and Related Microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691. 10.1038/nrmicro1913. [DOI] [PubMed] [Google Scholar]
- Geers C.; Gros G. Carbon Dioxide Transport and Carbonic Anhydrase in Blood and Muscle. Physiol. Rev. 2000, 80, 681–715. 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- Verpoorte J. A.; Mehta S.; Edsall J. T. Esterase Activities of Human Carbonic Anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [PubMed] [Google Scholar]
- D’Alessandro D. M.; Smit B.; Long J. R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem., Int. Ed. 2010, 49, 6058–6082. 10.1002/anie.201000431. [DOI] [PubMed] [Google Scholar]
- Gao S.; Mohammad M.; Yang H.-C.; Xu J.; Liang K.; Hou J.; Chen V. Janus Reactors with Highly Efficient Enzymatic CO2 Nanocascade at Air-Liquid Interface. ACS Appl. Mater. Interfaces 2017, 9, 42806–42815. 10.1021/acsami.7b14465. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Lu Y.; Ye X. Catalytic Behavior of Carbonic Anhydrase Enzyme Immobilized onto Nonporous Silica Nanoparticles for Enhancing CO2 Adsorption into a Carbonate Solution. Int. J. Greenhouse Gas Control 2013, 13, 17–25. 10.1016/j.ijggc.2012.12.010. [DOI] [Google Scholar]
- Forsyth C.; Yip T. W. S.; Patwardhan S. V. CO2 Sequestration by Enzyme Immobilized onto Bioinspired Silica. Chem. Commun. 2013, 49, 3191–3193. 10.1039/C2CC38225C. [DOI] [PubMed] [Google Scholar]
- Woo K. M.; Lee I.; Hong S.-G.; An S.; Lee J.; Oh E.; Kim J. Crosslinked Chitosan Coating on Magnetic Mesoporous Silica with Pre-Adsorbed Carbonic Anhydrase for Carbon Dioxide Conversion. Chem. Eng. J. 2015, 276, 232–239. 10.1016/j.cej.2015.04.057. [DOI] [Google Scholar]
- Shanbhag B. K.; Liu B.; Fu J.; Haritos V. S.; He L. Self-Assembled Enzyme Nanoparticles for Carbon Dioxide Capture. Nano Lett. 2016, 16, 3379–3394. 10.1021/acs.nanolett.6b01121. [DOI] [PubMed] [Google Scholar]
- Rozbeský D.; Rosůlek M.; Kukačka Z.; Chmelík J.; Man P.; Novák P. Impact of Chemical Cross-Linking on Protein Structure and Function. Anal. Chem. 2018, 90, 1104–1113. 10.1021/acs.analchem.7b02863. [DOI] [PubMed] [Google Scholar]
- Crumbliss A. L.; McLachlan K. L.; O’Daly J. P.; Henkens R. W. Preparation and Activity of Carbonic Anhydrase Immobilized on Porous Silica Beads and Graphite Rods. Biotechnol. Bioeng. 1988, 31, 796–801. 10.1002/bit.260310806. [DOI] [PubMed] [Google Scholar]
- Méndez J.; Monteagudo A.; Griebenow K. Stimulus-Responsive Controlled Release System by Covalent Immobilization of an Enzyme into Mesoporous Silica Nanoparticles. Bioconjugate Chem. 2012, 23, 698–704. 10.1021/bc200301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinoba M.; Bhagiyalakshmi M.; Jeong S. K.; Yoon Y. II; Nam S. C. Capture and Sequestration of CO2 by Human Carbonic Anhydrase Covalently Immobilized onto Amine-Functionalized SBA-15. J. Phys. Chem. C 2011, 115, 20209–20216. 10.1021/jp204661v. [DOI] [Google Scholar]
- Ozdemir E. Biomimetic CO2 Sequestration: 1. Immobilization of Carbonic Anhydrase within Polyurethane Foam. Energy Fuels 2009, 23, 5725–5730. 10.1021/ef9005725. [DOI] [Google Scholar]
- Bhattacharya S.; Schiavone M.; Chakrabarti S.; Bhattacharya S. K. CO2 Hydration by Immobilized Carbonic Anhydrase. Biotechnol. Appl. Biochem. 2003, 38, 111–117. 10.1042/BA20030060. [DOI] [PubMed] [Google Scholar]
- Maeshima K.; Yoshimoto M. Preparation and Characterization of Carbonic Anhydrase-Conjugated Liposomes for Catalytic Synthesis of Calcium Carbonate Particles. Enzyme Microb. Technol. 2017, 105, 9–17. 10.1016/j.enzmictec.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Datz S.; Argyo C.; Gattner M.; Weiss V.; Brunner K.; Bretzler J.; von Schirnding C.; Torrano A. A.; Spada F.; Vrabel M.; Engelke H.; Bräuchle C.; Carell T.; Bein T. Genetically Designed Biomolecular Capping System for Mesoporous Silica Nanoparticles Enables Receptor-Mediated Cell Uptake and Controlled Drug Release. Nanoscale 2016, 8, 8101–8110. 10.1039/C5NR08163G. [DOI] [PubMed] [Google Scholar]
- Yong J. K. J.; Cui J.; Cho K. L.; Stevens G. W.; Caruso F.; Kentish S. E. Surface Engineering of Polypropylene Membranes with Carbonic Anhydrase-Loaded Mesoporous Silica Nanoparticles for Improved Carbon Dioxide Hydration. Langmuir 2015, 31, 6211–6219. 10.1021/acs.langmuir.5b01020. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Chen B.; Qi W.; Li X.; Shin Y.; Lei C.; Liu J. Enzymatic Conversion of CO2 to Bicarbonate in Functionalized Mesoporous Silica. Microporous Mesoporous Mater. 2012, 153, 166–170. 10.1016/j.micromeso.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.; Wang X.; Birch L.; Rayment T.; Abell C. AFM Study on Protein Immobilization on Charged Surfaces at the Nanoscale: Toward the Fabrication of Three-Dimensional Protein Nanostructures. Langmuir 2003, 19, 10557–10562. 10.1021/la035491q. [DOI] [Google Scholar]
- Karlsson M.; Ekeroth J.; Elwing H.; Carlsson U. Reduction of Irreversible Protein Adsorption on Solid Surfaces by Protein Engineering for Increased Stability. J. Biol. Chem. 2005, 280, 25558–25564. 10.1074/jbc.M503665200. [DOI] [PubMed] [Google Scholar]
- Secundo F. Conformational Changes of Enzymes upon Immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. 10.1039/c3cs35495d. [DOI] [PubMed] [Google Scholar]
- Fornera S.; Balmer T. E.; Zhang B.; Schlüter A. D.; Walde P. Immobilization of Peroxidase on SiO2 Surfaces with the Help of a Dendronized Polymer and the Avidin-Biotin System. Macromol. Biosci. 2011, 11, 1052–1067. 10.1002/mabi.201100035. [DOI] [PubMed] [Google Scholar]
- For general information about the BAH bond formation and stability, see http://www.solulink.com.
- Hermanson G. T.Bioconjugate Techniques, 3rd ed.; Elseviers, 2013; Chapter 17. [Google Scholar]
- Küchler A.; Adamcik J.; Mezzenga R.; Schlüter A. D.; Walde P. Enzyme Immobilization on Silicate Glass through Simple Adsorption of Dendronized Polymer-Enzyme Conjugates for Localized Enzymatic Cascade Reactions. RSC Adv. 2015, 5, 44530–44544. 10.1039/C5RA06268C. [DOI] [Google Scholar]
- Küchler A.; Bleich J. N.; Sebastian B.; Dittrich P. S.; Walde P. Stable and Simple Immobilization of Proteinase K Inside Glass Tubes and Microfluidic Channels. ACS Appl. Mater. Interfaces 2015, 7, 25970–25980. 10.1021/acsami.5b09301. [DOI] [PubMed] [Google Scholar]
- Küchler A.; Messmer D.; Schlüter A. D.; Walde P. Preparation and Applications of Dendronized Polymer-Enzyme Conjugates. Methods Enzymol. 2017, 590, 445–474. 10.1016/bs.mie.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Saito R.; Sato T.; Ikai A.; Tanaka N. Structure of Bovine Carbonic Anhydrase II at 1.95 Å Resolution. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004, 60, 792–795. 10.1107/S0907444904003166. [DOI] [PubMed] [Google Scholar]
- Lindskog S. Purification and Properties of Bovine Erythrocyte Carbonic Anhydrase. Biochim. Biophys. Acta 1960, 39, 218–226. 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- Innocenti A.; Scozzafava A.; Parkkila S.; Puccetti L.; De Simone G.; Supuran C. T. Investigations of the Esterase, Phosphatase, and Sulfatase Activities of the Cytosolic Mammalian Carbonic Anhydrase Isoforms I, II, and XIII with 4-Nitrophenyl Esters as Substrates. Bioorg. Med. Chem. Lett. 2008, 18, 2267–2271. 10.1016/j.bmcl.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Garg L. C. Catalytic Activity and Inhibition of Carbonic Anhydrase of Rat Tissues. Biochem. Pharmacol. 1974, 23, 3153–3161. 10.1016/0006-2952(74)90601-7. [DOI] [PubMed] [Google Scholar]
- Pocker Y.; Stone J. T. The Catalytic Versatility of Erythrocyte Carbonic Anhydrase. III. Kinetic Studies of the Enzyme-Catalyzed Hydrolysis of p-Nitrophenyl Acetate. Biochemistry 1967, 6, 668–678. 10.1021/bi00855a005. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C.; MacDonald R. I.; Menco B. Ph. M.; Takeshita K.; Subbarao N. K.; Hu L.-R. Small-volume extrusion apparatus for preparation of large unilamellar vesicles. Biochim. Biophys. Acta, Biomembr. 1991, 1061, 297–303. 10.1016/0005-2736(91)90295-J. [DOI] [PubMed] [Google Scholar]
- Grotzky A.; Manaka Y.; Fornera S.; Willeke M.; Walde P. Quantification of α-Polylysine: a Comparison of Four UV/Vis Spectrophotometric Methods. Anal. Methods 2010, 2, 1448–1455. 10.1039/c0ay00116c. [DOI] [Google Scholar]
- Grotzky A.; Nauser T.; Erdogan H.; Schlüter A. D.; Walde P. A Fluorescently Labeled Dendronized Polymer–Enzyme Conjugate Carrying Multiple Copies of Two Different Types of Active Enzymes. J. Am. Chem. Soc. 2012, 134, 11392–11395. 10.1021/ja304837f. [DOI] [PubMed] [Google Scholar]
- O’Connor P. B.; Speir J. P.; Senko M. W.; Little D. P.; McLafferty F. W. Tandem Mass Spectrometry of Carbonic Anhydrase (29 kDa). J. Mass Spectrom. 1995, 30, 88–93. 10.1002/jms.1190300114. [DOI] [Google Scholar]
- Schön A.; Clarkson B. R.; Siles R.; Ross P.; Brown R. K.; Freire E. Denatured state aggregation parameters derived from concentration dependence of protein stability. Anal. Biochem. 2015, 488, 45–50. 10.1016/j.ab.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Gitlin I.; Gudiksen K. L.; Whitesides G. M. Effects of Surface Charge on Denaturation of Bovine Carbonic Anhydrase. ChemBioChem 2006, 7, 1241–1250. 10.1002/cbic.200600191. [DOI] [PubMed] [Google Scholar]
- Alvizo O.; Nguyen L. J.; Savile C. K.; Bresson J. A.; Lakhapatri S. L.; Solis E. O. P.; Fox R. J.; Broering J. M.; Benoit M. R.; Zimmerman S. A.; Novick S. J.; Liang J.; Lalonde J. J. Directed Evolution of an Ultrastable Carbonic Anhydrase for Highly Efficient Carbon Capture from Flue Gas. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 16436–16441. 10.1073/pnas.1411461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.; Whitesides G. M. Using Protein Charge Ladders to Estimate the Effective Charges and Molecular Weights of Proteins in Solution. Anal. Chem. 1997, 69, 575–580. 10.1021/ac9608073. [DOI] [PubMed] [Google Scholar]
- Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. 10.1126/science.277.5330.1232. [DOI] [Google Scholar]
- West J. K.; Latour R. Jr.; Hench L. L. Molecular Modeling Study of Adsorption of Poly-L-lysine onto Silica Glass. J. Biomed. Mater. Res. 1997, 37, 585–591. . [DOI] [PubMed] [Google Scholar]
- Huang N.-P.; Vörös J.; De Paul S. M.; Textor M.; Spencer N. D. Biotin-Derivatized Poly(L-lysine)-g-poly(ethylene glycol): A Novel Polymeric Interface for Bioaffinity Sensing. Langmuir 2002, 18, 220–230. 10.1021/la010913m. [DOI] [Google Scholar]
- Spycher P. R.; Amann C. A.; Wehrmüller J. E.; Hurwitz D. R.; Kreis O.; Messmer D.; Ritler A.; Küchler A.; Blanc A.; Béhé M.; Walde P.; Schibli R. Dual, Site-Specific Modification of Antibodies by Using Solid-Phase Immobilized Microbial Transglutaminase. ChemBioChem 2017, 18, 1923–1927. 10.1002/cbic.201700188. [DOI] [PubMed] [Google Scholar]
- Ruckenstein E.; Guo W. Cellulose and Glass Fiber Affinity Membranes for the Chromatographic Separation of Biomolecules. Biotechnol. Prog. 2004, 20, 13–25. 10.1021/bp030055f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.