Abstract

Discrimination of illegal cooking oil is a conundrum in the fields of analytical chemistry and food safety due to complicated sample systems, lack of common targets, and stringent demand of ultrahigh detection sensitivity for corresponding analytical methods. Capsaicin, one of the exogenous molecules that is subsistent in recycled kitchen waste oils, can be regarded as a target for illegal cooking oil identification. Nowadays, tracing capsaicin in oils is implemented mainly by high-performance liquid chromatography–mass spectrometry, which displays shortcomings in high costs and incapableness for field test. Here, we established a surface-enhanced resonance Raman scattering approach to detect capsaicin and identify illegal cooking oils by means of the molecular derivatization treatment of capsaicin. This method features high detection sensitivity with the detection limit of 1.0 × 10–8 M, rapid response (<7 min detection duration), and simplicity in sample pretreatment, which is available for fast field test of illegal cooking oils.
Introduction
In recent years, cooking oil safety accidents have occurred frequently in developing countries increasing the attention toward food safety inspection. Illegal cooking oils, generally referring to all kinds of abandoned inferior inedible oils, mainly consists of kitchen waste oils, also called “underground drainage oils” in China. Reuse of these grease wastes for cooking is legally prohibited even if they have been purified by filtration, distillation, decoloration, deodorizing, and refining processes. Inedible oils are usually contaminated by hazardous additives, such as condiments, heavy metal ions, biotoxins, phthalates, and oil oxides,1−5 which are extremely harmful to human health if consumed in large amounts or small doses chronically. The complete removal of contaminants or additives in waste oils is almost impossible in most illicit refining industries. It has been reported that up to 3 million tons of waste oils are distributed back to Chinese dining tables annually.6 The main reason for the long-term existence of blended waste oils with edible ones or mixing the spurious with the genuine is due to the lack of an effective, fast, and cheap testing method. Methods and indicators for identifying illegal cooking oil have attracted widespread attention and are also in urgent demand. Current efforts have been devoted to discriminating oils based on the evaluation of compositions (cholesterol and fatty acid) and physical parameters (e.g., conductivity), as well as the detection of foreign contaminants (polycyclic aromatic hydrocarbons, benzopyrene, plasticizers, aflatoxin, etc.) according to National Food Safety Standards.7 However, these parameters can only tell the oil quality and cannot give a definite conclusion that the oil is from the recycled “drainage oils” or not. Also, exogenous pollutions that come from raw materials or the process of storage and refining can also lead to a false judgment. Therefore, a gold standard for the discrimination of drainage oils is required.
Capsaicine, including capsaicin and dihydrocapsaicin, is the main active chemical extract of the fruits and seeds of capsaicum plants, which gives the hot/burning sensation of spicy peppers. The concentration of capsaicin can reach 4.249 mg g–1 in some kind of peppers.8 As the basic five tastes, pungent condiments are indispensable and generally used in different types of dishes. Capsicine will most likely remain in the cooking oil after being used in dishes due to its oleophilic feature, no matter what kinds of retreatments had been implemented in the oil recovery. Therefore, capsaicin can be a qualitative basis for the identification of drainage oil.9 Different from other hazardous contaminants in cooking oils, capsaicin is very safe for human body even in a high concentration as much as many hot peppers. To trace capsaicin in edible oils, Chen’s group established a detection method by high-performance liquid chromatography–mass spectrometry (HPLC–MS).10,11 A total of 67 blind samples from the National Food Safety Risk Assessment Center were analyzed. One of the important things is that they have proven that capsaicin is an excellent indicator for the identification of drainage oils. The correct recognition rates for positive and negative samples were 75 and 100%, respectively.12 Recently, the method of solid-phase extraction-HPLC–MS has become one of the four instrument examination methods for identifying drainage oils according to the latest information publicized by the National Ministry of Health, China.13 However, owing to the large and expensive MS equipment and high testing cost per sample, these MS-based methods face difficulties for rapid field analysis in food safety and market monitoring.
Herein, surface-enhanced resonance Raman scattering (SERRS) technique was adopted to establish an ultrasensitive detection method for an exogenous substance (capsaicin) in illegal cooking oils. With a molecule derivative technology based on a diazotization procedure for the aromatic amines of capsaicin, the product displays colors and SERRS activity. We adapted magnetic surface-enhanced Raman scattering (SERS)-active particles to enhance the signal of the capsaicin derivatives, and a portable Raman instrument is enough for the measurement (Scheme S2). This SERRS method for the identification of drainage oil is simple, rapid, and ultrasensitive, which are required for accurate field analysis.
Surface-enhanced Raman scattering (SERS) spectroscopy is based on the smart combination of nanomaterials and Raman spectrum technology and becomes a very important spectroscopic technique in modern analysis and detection fields with its single molecule level detection capability.14−17 SERRS, a combination of resonance Raman and SERS, can further amplify Raman signal as a fold of 104 relative to SERS, which results in a higher detection sensitivity and provides broader applications in analysis of ultralow concentrations. As is well known, the prerequisite condition for SERRS is to resonate the electronic energy levels of probed molecules with the wavelengths of the incident lasers.18−20 Thus, it is convenient to implement molecular derivatization strategies to convert colorless analytes into species with absorption band that match the wavelength of a commonly used laser.
Results and Discussion
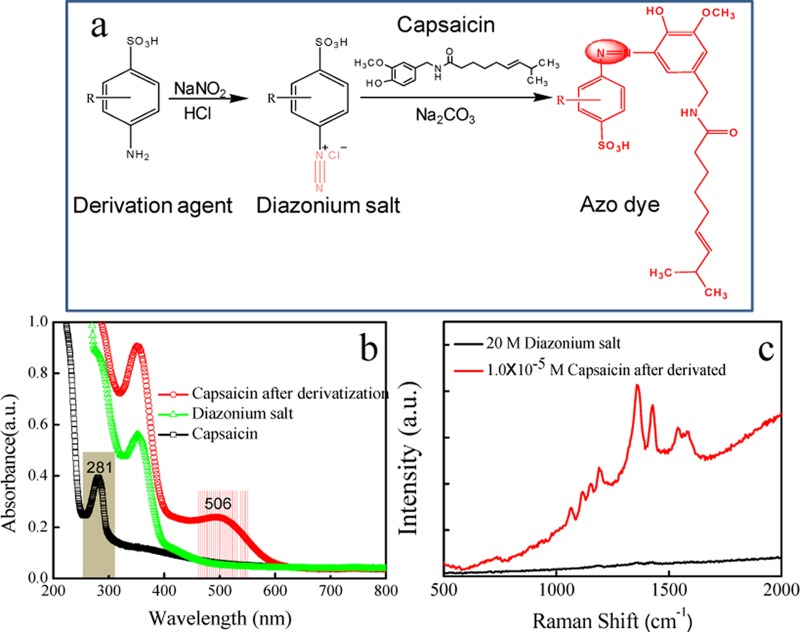
Capsaicin has a low SERS cross section and faces difficulty in chemical and physical adsorption on metal surfaces. Therefore, the direct detection of capsaicin in oils by SERS is infeasible. Owing to the molecular character of capsaicin, that is, a phenolic compound, a derivatization strategy was designed to convert it into an azo dye, which fortunately supports high SERS activity. The procedures follow the Pauly reaction21,22 that involves two steps (Figure 1): (1) pretreating the derivation agents (many aromatic amines have been tried and 2,2′-benzidinedisulfonic acid is preferred; see Figure S2 in SI) to produce a diazonium salt with the addition of NaNO2 in aromatic amine in solution, and (2) reacting capsaicin with the diazonium salt under basic conditions (NaCO3 added). Then, the capsaicin derivative is obtained. The total derivation time can be completed within 1 min. To investigate the resonance absorption of targets at excitation light, capsaicin derivatives were prepared by adding different aromatic amines, including 2,5-diaminobenzenesulfonic acid, sodium diphenylamine-4-sulfonate, 1-naphthylamine-4-sulfonic acid, 6-amino-1-naphthalenesulfonic acid, sulfanilic acid, and 2,2′-benzidinedisulfonic acid. Their structural formulas are shown as (a–f) in Figure S2. SERRS signal amplification is closely related to the surface plasmon resonance of metal nanoparticles, laser excitation wavelength, and molecular resonance.23 If the probed molecule contains a molecular resonance that overlaps with the laser excitation, additional orders of magnitude in signal intensity can be gained.24,25 SERS spectra of the capsaicin derivative under different incident laser wavelengths at 514, 532, 633, and 785 nm were recorded and are shown in Figure S3a. It should be noted that the peak intensities are normalized by the laser power. Figure S3b displays the histogram of the SERS peak intensity at 1361 cm–1 of capsaicin derivative. By comparing SERS intensities, the strong enhancement was obtained when the excitation wavelengths of 532 nm were used. The structure of the target derivative was confirmed by matrix-assisted laser desorption/ionization–time-of-flight–mass spectrometry (MALDI–TOF–MS) (Figure S4).
Figure 1.
Derivatization reaction route of capsaicin (a). UV–vis spectra (b) and SERRS spectra (c) of capsaicin and its derivation agent.
UV–vis absorption spectra of capsaicin before and after derivatization with 2,2′-benzidinedisulfonic acid and of the intermediate product (diazonium salt) are illustrated in Figure 1b, which indicates that the derivatization reagent and the intermediate product (diazonium salt) have no absorption band in the visible range, whereas the capsaicin derivative shows a new band at about 506 nm. The SERS spectra of diazonium salt and capsaicin derivative were both measured (Figure 1c) using a B&W Tek Raman spectrometer with a portable optical fiber probe and 532 nm excitation wavelength. It can be found that the diazonium salt does not show SERS signal, indicating neither 2,2′-benzidinedisulfonic acid nor the diazonium salt intermediate gives background interference in the SERRS spectrum. Compared to that of diazonium salt, the SERRS spectrum of capsaicin derivative is remarkably strong and identifiable. The main peaks are located at 1191, 1361, 1429, 1574, and 1606 cm–1, which are assigned to υ(C–C) from phenol groups, δ(C–H), ν(N=N), ν(C=C) from phenyl rings, and υ(C–N), respectively, according to the previous reports.26 It should be noted that this derivative supports a broad background in its SERRS spectrum due to the fluorescence emission.
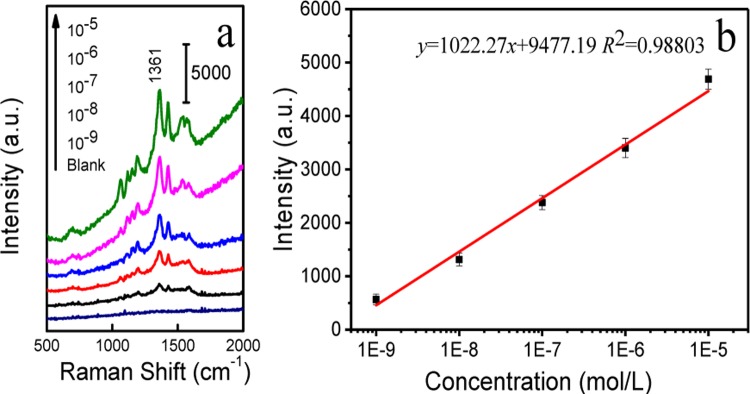
The derivation reaction makes capsaicin traceable in the SERRS detection. However, as derivatization reaction must be performed in water phase, capsaicin should be extracted from the vegetable oil to water phase using different extraction solvents via liquid–liquid extraction. More discussions on capsaicin extraction and HPLC characterization are provided in Figure S5. The capsaicin extraction liquid was next used for the derivatization treatment as stated above. Then, the capsaicin derivative sample was mixed with magnetic SERS-active beads for the enrichment, separation, and SERRS detection of analytes. Figure 2 shows SERRS spectra of the capsaicin derivative at different concentrations and the linear calibration curve obtained by plotting the SERS intensity of capsaicin derivative at 1361 cm–1 (each data point represents an average of three trials). The linear regression equation is expressed as y = 1022.27x + 9477.19 (R2 = 0.9880), and the detection range is 1.0 × 10–5–1.0 × 10–9 M. For a fast field detection purpose, a higher detection sensitivity and reliability of testing are much more important for a qualitative detection. To evaluate the lowest detection limit of the method, we chose three kinds of vegetable oil samples, soybean oil, maize oil, and blend oil (six samples for one oil, total trials n = 18). The blank samples were spiked to 1.0 × 10–8 M with a standard capsaicin solution as illegal cooking oils for subsequent extraction and detection. The operation procedures of the proposed method are illustrated in Scheme S1, and the detection results are listed in Table S1. The accuracy of this SERRS method for capsaicin is 100% (n = 18). According to Wang’ study, cooking oil samples could be identified as illegal ones when the contents of capsaicin are over 1 μg L–1.9 In the present study, SERRS method can achieve a detection limit of 1.0 × 10–8 M (0.31 μg L–1), indicating this detection limit will be applicable for the identification of most illegal cooking oils.
Figure 2.
Concentration-dependent SERRS spectra of the capsaicin derivative (a). Intensity of the band at 1361 cm–1 versus the concentration of the capsaicin derivative (b).
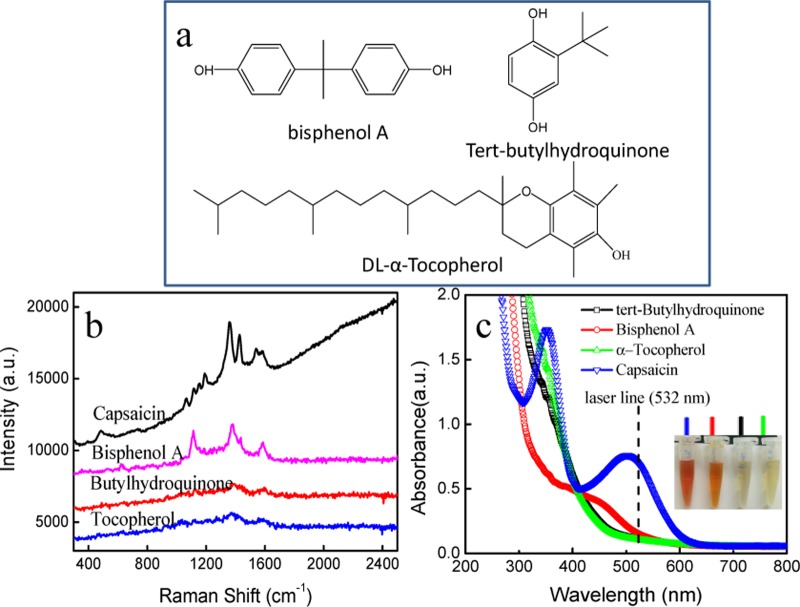
To evaluate the selectivity and feasibility of derivative SERRS method for capsaicin, the interference responses of various foreign species in vegetable oils were tested, involving tert-butylhydroquinone,27,28dl-α-tocopherol,29,30 and bisphenol A,31,32 and their chemical structures are shown in Figure 3a. Among them, tert-butylhydroquinone and dl-α-tocopherol are highly effective food-grade phenolic antioxidants that may be added during oil processing and storage. Bisphenol A is one of the common additives used in the plastics industry, and it causes food (oil) contamination by contacting with packages. These interfering substances in vegetable oils were tested by SERRS spectroscopy with the assistance of the same molecular derivatization reaction. As shown in Figure 3b, there are no SERS signals when tert-butylhydroquinone and dl-α-tocopherol were added in sample at the same concentration (1.0 × 10–5 M), indicating they cannot bring interference to capsaicin SERRS determination. Differently, bisphenol A can be converted into an azo dye. However, the bisphenol A derivative provides totally different SERS fingerprints that are distinguishable from that of the capsaicin derivative. Figure 3c shows the UV–vis spectra of three derivatives, in which neither color changes nor the absorption bands in the visible range were observed for tert-butylhydroquinone and dl-α-tocopherol, implying both of them cannot be converted into azo dyes using this derivative method, probably due to the large space resistance and electrophilic substitution effect of substitutes on benzene. Figure 3c also shows the obvious color change of bisphenol A derivative and a new band appearing at about 450 nm, which is different from the capsaicin derivative that exhibits the absorption maximum at 506 nm. Therefore, we can conclude that there are no interferences from these three substances and that the molecular derivatization-assisted SERRS technique has excellent selectivity toward capsaicin.
Figure 3.
Molecular structures of three phenolic compounds (a). SERS spectra (b) and UV–vis spectra (c) of azo dyes derived from capsaicin, tert-butylhydroquinone, dl-α-tocopherol, and bisphenol A. The inset in (c) shows a photo of four derivatives.
Conclusions
In summary, aiming at the identification of illegal cooking oil, we established an ultrasensitive detection method for the exogenous target, capsaicin, based on a molecular derivatization strategy via SERRS spectroscopy. Combined with a magnetic separation SERS substrate, this method can achieve a detection limit of 1.0 × 10–8 M. Batches of interference experiments show that our proposed SERRS method has a good selectivity for capsaicin in vegetable oils, and it can be a gold standard for the identification of drainage oils due to its trivialness, rapidity, and ultrasensitivity. The whole sensing duration can be completed within 7 min. Compared to the HPLC–MS method, this method not only shows superiority in time and cost savings, but also displays applicability in fast field detections when equipped with portable Raman instruments.
Experimental Section
Reagents and Materials
Capsaicin, tert-butylhydroquinone, α-tocopherol, bisphenol A, iron(III) chloride hexahydrate (FeCl3·6H2O), ethylene glycol (EG), sodium acetate anhydrous (NaAc), silver nitrate (AgNO3), sodium citrate (C6H5Na3O7·2H2O), anhydrous ethanol, 2,5-diaminobenzenesulfonic acid, sodium diphenylamine-4-sulfonate, sulfonic acid, 1-naphthylamine-4-sulfonic acid, 6-amino-1-naphthalenesulfonic acid, 2,2′-benzidinedisulfonic acid, sodium nitrite, sodium carbonate hydrochloride, methanol (chromatographic grade), and ethylene imine polymer (PEI 10 000, 99%) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). Capsaicin stock solution (1.0 × 10–3 M) was prepared by accurately weighing a certain quantity of reference materials and dissolving them in methanol. Different concentrations of standard solutions were prepared by diluting the stock solutions with vegetable oil and stored in the dark at 4 °C before use.
Instruments
X-ray diffraction data were taken from a Bruker D8 Focus with Cu Kα radiation (k = 1.5406 Å). UV–vis spectra were taken at 200–800 nm from a Shimadzu UV-2550 spectrometer. High-performance liquid chromatography (HPLC) analysis was carried out on a Shimadzu LC-20AB system (Kyoto, Japan) equipped with a CTO-10AS column oven and an RF-10Axl fluorescence detector. The chromatographic separation of analytes was performed on a VP-ODS C18 column (5 μm, 4.6 mm × 150 mm i.d.). The mobile phase used was methanol/water (62:38). Its flow rate was 1.0 mL min–1, the excitation wavelength was 275 nm, and the emission wavelength was 352 nm for capsaicin. The injection volume was 20 μL. SERS spectra of analytes were recorded using a B&W Tek Raman spectrometer with a portable optical fiber probe and 532 nm excitation wavelength. The integration time was 5 s. The laser power reaching the samples was 5.01 mW. In addition, Raman spectra under other different excitation lasers (514, 633, and 785 nm) were separately obtained by a Horiba Jobin Yvon T64000 Raman spectrometer equipped with a water-cooled Ar ion laser as excitation source (514 nm), a Horiba Jobin Yvon LabRAM Aramis Raman microscopy system, which is equipped with a water-cooled He–Ne-ion laser (λex = 633 nm) and another B&W Tek Raman spectrometer with a portable optical fiber probe and 785 nm excitation wavelength. Mass spectra of capsaicin derivatives were obtained on matrix-assisted laser desorption/ionization–time-of-flight–mass spectrometry (MALDI–TOF–MS, Bruker Daltonics, Bremen, Germany). Capsaicin derivative samples (1 μL) were overlaid with 1 μL of MALDI matrix (a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile–2.5% trifluoroacetic acid) and detected by MALDI–TOF–MS. Scanning electron microscopy (SEM) analysis was acquired on a Hitachi SU8020 scanning electron microscopy (Tokyo, Japan). The shape and size of the produced AgNPs were measured by a JEM-2100F field emission transmission electron microscopy (JEOL, Tokyo, Japan).
Preparation of Fe3O4@Ag Particles
Fe3O4@Ag particles were fabricated on the basis of a reported method.33 AgNPs with the size of about 40 nm (Figure S1a) were first synthesized according to the Lee and Meisel method.34 Briefly, 27 mg of AgNO3 was dissolved in ultrapure water (150 mL) and brought to boiling. Then, 3.0 mL of 1% trisodium citrate was added and boiling was continued for 40 min. Next, Fe3O4 particles were prepared according to the solvothermal reduction method.35 After that, 50 mg of the as-prepared Fe3O4 particles was functionalized with PEI by suspending them in 100 mL of 5.0 mg mL–1 positively charged PEI solution. PEI supplies amino groups around Fe3O4 particles. Then, AgNPs were aggregated on the amino-functionalized Fe3O4 microspheres to produce Fe3O4@Ag particles (Figure S1b). The produced Fe3O4@Ag particles were characterized by energy-dispersive X-ray spectroscopy via a casting film, and the result shows the existence of Fe and Ag elements (Figure S1c).
Detection Procedures of Capsaicin
Capsaicin detection from vegetable oil mainly includes the following processes: capsaicin extraction, derivatization, and SERS detection. Capsaicin extraction: 2 mL of vegetable oil and 4 mL of extraction reagent were added to a centrifuge tube (10 mL) and static stratification was conducted after vortexing for 5 min to obtain solvent layer containing capsaicin. Derivatization: the capsaicin derivative was synthesized by adding NaNO2 (1 mL), aromatic amines (1 mL), capsaicin extract (1 mL), and NaCO3 (1 mL) solution at a certain concentration to the centrifuge tube (10 mL) and then manually vortexing for 1 min at room temperature. SERS detection: magnetic SERS substrate particles (50 mg mL–1, 20 μL) were dispersed in the above sample solution (1 mL). To make capsaicin derivative molecules adsorbed onto magnetic beads, the sample solution and beads were incubated for 1 min at room temperature in a vortex mixer set at the lowest speed. The capsaicin derivative molecules adsorbed on Fe3O4@Ag particles were then separated and enriched by a 16-hole magnetic rack. SERS measurement of capsaicin derivative molecules was conducted using a B&W Tek Raman spectrometer with a portable optical fiber probe and 532 nm excitation wavelength.
Evaluation of Accuracy Experiment
The accuracy of derivative SERRS method for capsaicin detection in vegetable oil was verified by the spike-and-recovery experiment. Three kinds of vegetable oils, soybean oil, maize oil, and blend oil, were collected from supermarkets. Real samples were obtained by adding capsaicin standard solutions in vegetable oil with the final concentration of 1.0 × 10–8 M, which were used as analogues of illegal cooking oils for next extraction and detection.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, Grant Nos. 21373096, 91441105, 21573087, and 21573092), National Instrumentation Program (NIP) of the Ministry of Science and Technology of China (No. 2011YQ03012408), and Science and Technology Development Program Funded Projects of Jilin province.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01457.
Characterization of Fe3O4@Ag particles, scheme of operation procedures, selection of excitation wavelength, mass spectrometric characterization of capsaicin derivative, capsaicin extraction from vegetable oils, SERS determination of capsaicin in real samples (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhou R.-Z.; Jiang J.; Mao T.; Zhao Y. S.; Lu Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography-tandem mass spectrometry. Food Chem. 2016, 207, 43–50. 10.1016/j.foodchem.2016.03.071. [DOI] [PubMed] [Google Scholar]
- Barp L.; Purcaro G.; Franchina F. A.; Zoccali M.; Sciarrone D.; Tranchida P. Q.; Mondello L. Determination of phthalate esters in vegetable oils using direct immersion solid-phase microextraction and fast gas chromatography coupled with triple quadrupole mass spectrometry. Anal. Chim. Acta 2015, 887, 237–244. 10.1016/j.aca.2015.06.039. [DOI] [PubMed] [Google Scholar]
- Pschenitza M.; Hackenberg R.; Niessner R.; Knopp D. Analysis of Benzo[a]pyrene in Vegetable Oils Using Molecularly Imprinted Solid Phase Extraction (MISPE) Coupled with Enzyme-Linked Immunosorbent Assay (ELISA). Sensors 2014, 14, 9720–9737. 10.3390/s140609720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcaro G.; Morrison P.; Moret S.; Conte L. S.; Marriott P. J. Determination of polycyclic aromatic hydrocarbons in vegetable oils using solid-phase microextraction-comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1161, 284–291. 10.1016/j.chroma.2007.05.105. [DOI] [PubMed] [Google Scholar]
- Zhao W.-J.; Chen X. B.; Fang L.; Li C. L.; Zhao D. Y. Determination of light-medium-heavy polycyclic aromatic hydrocarbons in vegetable oils by solid-phase extraction and high-performance liquid chromatography with diode array and fluorescence detection. J. Agric. Food Chem. 2013, 61, 1804–1809. 10.1021/jf3052779. [DOI] [PubMed] [Google Scholar]
- http://news.sohu.com/20100318/n270904146.shtml.
- Local Standard of People’s Republic of China , DB33/T 430-2003, 2003.
- Al Othman Z. A.; Ahmed Y. B.; Habila M. A.; Ghafar A. A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 16, 8919–8929. 10.3390/molecules16108919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Jin J.; Wang S.; Wang X.; Tian Y.; Chen J. A novel method for the identification of illegal cooking oil(1): detection of three capsaicinoids with liquid chromatography-mass spectrometry. Chin. J. Chromatogr. 2012, 30, 1094–1099. 10.3724/SP.J.1123.2012.08051. [DOI] [PubMed] [Google Scholar]
- Jin J.; Wang L.; Chen J.; Tian Y.; Zou L.; Zhang B.; Wang S.; Wang X. A novel method for the identification of illegal cooking oil(2): determination of special odd-chain fatty acids by multidimensional gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2012, 30, 1100–1107. 10.3724/SP.J.1123.2012.08052. [DOI] [PubMed] [Google Scholar]
- You Y.; Uboh C. E.; Soma L. R.; Guan F.; Taylor D.; Li X.; Liu Y.; Chen J. Validated UHPLC-MS-MS method for rapid analysis of capsaicin and dihydrocapsaicin in equine plasma for doping control. J. Anal. Toxicol. 2013, 37, 122–132. 10.1093/jat/bks098. [DOI] [PubMed] [Google Scholar]
- Ma F.; Yang Q.; Matthaus B.; Li P.; Zhang Q.; Zhang L. Simultaneous determination of capsaicin and dihydrocapsaicin for vegetable oil adulteration by immunoaffinity chromatography cleanup coupled with LC-MS/MS. J. Chromatogr. B 2016, 137–144. 10.1016/j.jchromb.2015.12.017. [DOI] [PubMed] [Google Scholar]
- http://www.sda.gov.cn/WS01/CL1605/172005.html.
- Cecchini M. P.; Turek V. A.; Paget J.; Kornyshev A. A.; Edel J. B. Self-assembled nanoparticle arrays for multiphase trace analyte detection. Nat. Mater. 2013, 12, 165–171. 10.1038/nmat3488. [DOI] [PubMed] [Google Scholar]
- Qian X. M.; Nie S. M. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. 10.1039/b708839f. [DOI] [PubMed] [Google Scholar]
- Li J. F.; Huang Y. F.; Ding Y.; Yang Z. L.; Li S. B.; Zhou X. S.; Fan F. R.; Zhang W.; Zhou Z. Y.; Wu D. Y.; Ren B.; Wang Z. L.; Tian Z. Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. 10.1038/nature08907. [DOI] [PubMed] [Google Scholar]
- Yang S.; Dai X. M.; Stogin B. B.; Wong T. S. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 268–273. 10.1073/pnas.1518980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman E. J.; Carron K. T.; Schatz G. C.; Van Duyne R. P. A surface enhanced resonance Raman study of cobalt phthalocyanine on rough Ag films: Theory and experiment. J. Chem. Phys. 1987, 87, 4189–4200. 10.1063/1.452923. [DOI] [Google Scholar]
- Deb S. K.; Davis B.; Knudsen G. M.; Gudihal R.; Ben-Amotz D.; Davisson V. J. Detection and relative quantification of proteins by surface enhanced Raman using isotopic labels. J. Am. Chem. Soc. 2008, 130, 9624–9525. 10.1021/ja800772p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour S. O.; Rocks L.; Faulds K.; Graham D.; Parchansky V.; Bour P.; Blanch E. W. Through-space transfer of chiral information mediated by a plasmonic nanomaterial. Nat. Chem. 2015, 7, 591–596. 10.1038/nchem.2280. [DOI] [PubMed] [Google Scholar]
- Mcanally G.; Mclaughlin C.; Brown R.; Robson D. C.; Faulds K.; Tackley D. R.; Smith W. E.; Graham D. SERRS dyes. Part I. Synthesis of benzotriazole monoazo dyes as model analytes for surface enhanced resonance Raman scattering. Analyst 2002, 127, 838–841. 10.1039/b201598f. [DOI] [PubMed] [Google Scholar]
- Szele I.; Zollinger H.. Azo Coupling Reactions Structures and Mechanisms; Springer: Berlin, 1983; pp 1–66. [Google Scholar]
- Zhao J.; Jensen L.; Sung J.; Zou S.; Schatz G. C.; Van Duyne R. P. Interaction of plasmon and molecular resonances for rhodamine 6G adsorbed on silver nanoparticles. J. Am. Chem. Soc. 2007, 129, 7647. 10.1021/ja0707106. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Dieringer J. A.; Zhang X. Y.; Schatz G. C.; Van Duyne R. P. Wavelength-scanned surface-enhanced resonance Raman excitation spectroscopy. J. Phys. Chem. C 2008, 112, 19302–19310. 10.1021/jp807837t. [DOI] [Google Scholar]
- Haes A. J.; Zou S. L.; Zhao J.; Schatz G. C.; Van Duyne R. P. Localized surface plasmon resonance spectroscopy near molecular resonances. J. Am. Chem. Soc. 2006, 128, 10905–10914. 10.1021/ja063575q. [DOI] [PubMed] [Google Scholar]
- Han X. X.; Pienpinijtham P.; Zhao B.; Ozaki Y. Coupling Reaction-Based Ultrasensitive Detection of Phenolic Estrogens Using Surface-Enhanced Resonance Raman Scattering. Anal. Chem. 2011, 83, 8582–8588. 10.1021/ac2019766. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Lai K. Q.; Fan Y. X.; Li C. Y.; Pei L.; Rasco B. A.; Huang Y. Q. Determination of Tert-Butylhydroquinone in Vegetable Oils Using Surface-Enhanced Raman Spectroscopy. J. Food Sci. 2014, 79, T1225–T1230. 10.1111/1750-3841.12482. [DOI] [PubMed] [Google Scholar]
- Hao P. P.; Ni J. R.; Sun W. L.; Huang W. Determination of tertiary butylhydroquinone in edible vegetable oil by liquid chromatography/ion trap mass spectrometry. Food Chem. 2007, 105, 1732–1737. 10.1016/j.foodchem.2007.04.058. [DOI] [Google Scholar]
- Feng S.; Gao F.; Chen Z. W.; Grant E.; Kitts D. D.; Wang S.; Lu X. N. Determination of alpha-Tocopherol in Vegetable Oils Using a Molecularly Imprinted Polymers-Surface-Enhanced Raman Spectroscopic Biosensor. J. Agric. Food Chem. 2013, 61, 10467–10475. 10.1021/jf4038858. [DOI] [PubMed] [Google Scholar]
- Sagratini G.; Allegrini M.; Caprioli G.; Cristalli G.; Giardina D.; Maggi F.; Ricciutelli M.; Sirocchi V.; Vittori S. Simultaneous Determination of Squalene, alpha-Tocopherol and beta-Carotene in Table Olives by Solid Phase Extraction and High-Performance Liquid Chromatography with Diode Array Detection. Food Anal. Methods 2013, 6, 54–60. 10.1007/s12161-012-9422-6. [DOI] [Google Scholar]
- Yang Y.; Yu J.; Yin J.; Shao B.; Zhang J. Molecularly Imprinted Solid-Phase Extraction for Selective Extraction of Bisphenol Analogues in Beverages and Canned Food. J. Agric. Food Chem. 2014, 62, 11130–11137. 10.1021/jf5037933. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Zhang J.; Wu Y.; Shao B. Simultaneous determination of bisphenol A and alkylphenol in plant oil by gel permeation chromatography and isotopic dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 5248–5253. 10.1016/j.chroma.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Tang X. H.; Dong R. L.; Yang L. B.; Liu J. H. Fabrication of Au nanorod-coated Fe3O4 microspheres as SERS substrate for pesticide analysis by near-infrared excitation. J. Raman Spectrosc. 2015, 46, 470–475. 10.1002/jrs.4658. [DOI] [Google Scholar]
- Lee P. C.; Meisel D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. 10.1021/j100214a025. [DOI] [Google Scholar]
- Deng H.; Li X. L.; Peng Q.; Wang X.; Chen J. P.; Li Y. D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem., Int. Ed. 2005, 44, 2782–2785. 10.1002/anie.200462551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.