Abstract
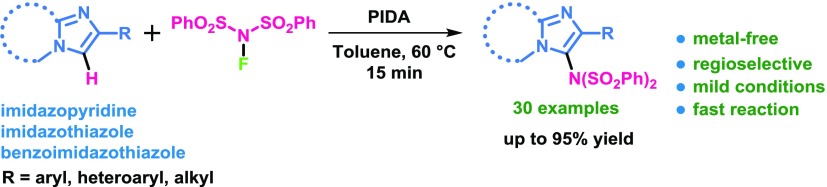
Metal-free (diacetoxy)iodobenzene-mediated regioselective imidation of imidazoheterocycles using commercially available N-fluorobenzenesulfonimide as an imidating reagent has been developed. This protocol exhibits broad substrate scope with good to excellent yields of the imidated imidazopyridines under mild conditions in short reaction times. The present protocol also represents an efficient way to access the imidated derivatives of imidazo[2,1-b]thiazole, benzo[d]imidazo-[2,1-b]thiazole, indoles, and indolizines. A radical mechanistic pathway has been proposed for the present protocol.
Introduction
Imidazopyridine, an important class of nitrogen-containing fused heterocyclic motif, is widely present in plant alkaloids and natural products.1 It is used in pharmaceutical chemistry as well as in materials science.2 Thus, the development of versatile methods for the synthesis and functionalization of imidazopyridines is an important field of research in medicinal and synthetic organic chemistry.3,4 Over the past decades, considerable efforts have been devoted for the incorporation of nitrogen functionality in heterocyclic motifs because of their wide applications in industry and pharmacology.5 Conventionally, aromatic C–N bond formation relies on the transition-metal-catalyzed C–N cross-coupling reactions, such as Ullmann–Goldberg, Buchwald–Hartwig, and Chan–Lam amination.6 These reactions require high temperature and prefunctionalization of starting materials. Transition-metal-catalyzed or visible light-induced direct C–H aminations are another efficient atom-economical coupling processes.7,8 Direct amination is the direct incorporation of amine or amine derivatives to a carbon atom through the formation of C–N bond. Beside these, one more promising strategy of direct C–N couplings of heteroarenes with preactivated amino precursors has also been reported.9
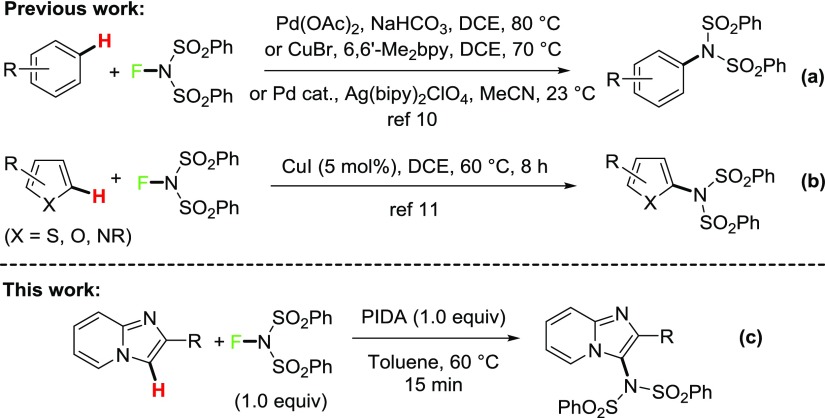
Over the past decade, the use of N-fluorobenzenesulfonimide (NFSI) for the amination of aromatic C(sp2)–H bond,10,11 alkenes or unsaturated ketones,12 and benzylic or allylic C–H bond13 has been intensely studied by using Pd and Cu catalysts. NFSI can act as a source of both fluoronium cation for fluorination and nucleophilic nitrogen or nitrogen radical for amination.14 So far, few groups have explored the transition-metal-catalyzed amination of arenes with NFSI (Scheme 1a).10 Recently, Pan and co-workers described a copper-catalyzed imidation of heterocycles such as thiophene, furan, and pyrrole with NFSI (Scheme 1b).11 Despite these successes, the imidation of other biologically relevant heterocycles using NFSI under metal-free mild conditions still remains an interesting and challenging subject for researchers.
Scheme 1. Direct Amination of Aromatic C–H Bond Using NFSI.
Hypervalent iodines(III) are useful oxidants in various coupling reactions.15 Moreover, iodine(III)-mediated intramolecular and intermolecular oxidative aminations have also been explored to construct diverse heterocyclic compounds.16,17 In this regard, solely di(pivaloyloxy)iodobenzene [PhI(OPiv)2]-mediated oxidative C–H imidation by Wang et al. is a mentionable work.18 Here, the imidation of 8-acylaminoquinolines and anilides by NFSI has been carried out in tetrahydrofuran (THF) at 80 °C for 8 h under metal-free condition. Very recently, our group reported an efficient method for a regioselective (diacetoxy)iodobenzene (PIDA)-mediated oxidative amination of imidazopyridines through C(sp2)–H functionalization leading to 3-amino-substituted imidazopyridines.4c As a part of our ongoing investigations on functionalization of imidazoheterocycles,4 herein, we described a metal-free PIDA-mediated regioselective imidation of imidazoheterocycles with NFSI (Scheme 1c).
Results and Discussion
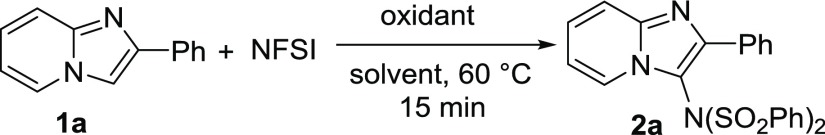
2-Phenylimidazo[1,2-a]pyridine (1a) was initially selected as a model substrate with NFSI as an imidating reagent (Table 1). At first, the reaction was carried out by taking 1a (0.2 mmol) with NFSI (1.0 equiv) at 60 °C in 1,2-DCE for 15 min. However, no reaction took place, and both 1a and NFSI were recovered (Table 1, entry 1). To our delight, in the presence of 1 equiv of PIDA, the reaction afforded N-(2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2a) in 68% yield after 15 min (Table 1, entry 2). Further, the yield was not affected when the reaction was carried out for 1 h. Other solvents, such as THF, CH3CN, toluene, 1,4-dioxane, ethanol, chlorobenzene, and dimethylformamide (DMF), were also screened under the same conditions (Table 1, entries 3–9). Better result was obtained in toluene affording 89% of the desired product (Table 1, entry 5), whereas very low yield of the desired product was formed with bis(trifluoroacetoxy)iodobenzene (PIFA) (Table 1, entry 10). Next, we checked the effect of different oxidants such as K2S2O8, TBHP, and H2O2 instead of PIDA (Table 1, entries 11–13). In all cases, only trace amount of imidated products were detected. The yield of the reaction was decreased with diminished loading of PIDA to 0.5 equiv but remained unchanged by increasing the amount to 1.5 equiv (Table 1, entries 14 and 15). No significant improvement of the yield was found with increasing the reaction temperature, but the yield was dropped when the reaction was carried out at 40 °C (Table 1, entries 16 and 17). A trace amount of the desired product 2a were obtained at room temperature (Table 1, entry 18). Direct imidation product of toulene was not detected. Summing up, our study led to the following optimized conditions: 1 equiv of PIDA in toluene at 60 °C for 15 min (Table 1, entry 5).
Table 1. Optimization for the Amination Reactiona.
| entry | oxidant (equiv) | solvent | yieldb (%) |
|---|---|---|---|
| 1 | 1,2-DCE | 0 | |
| 2 | PIDA (1) | 1,2-DCE | 68 |
| 3 | PIDA (1) | THF | 61 |
| 4 | PIDA (1) | CH3CN | 66 |
| 5 | PIDA (1) | toluene | 89 |
| 6 | PIDA (1) | 1,4-dioxane | 78 |
| 7 | PIDA (1) | EtOH | 33 |
| 8 | PIDA (1) | chlorobenzene | 72 |
| 9 | PIDA (1) | DMF | 67 |
| 10 | PIFA (1) | toluene | 18 |
| 11 | K2S2O8 (1) | toluene | trace |
| 12 | TBHP (1) | toluene | trace |
| 13 | H2O2 (1) | toluene | trace |
| 14 | PIDA (0.5) | toluene | 41 |
| 15 | PIDA (1.5) | toluene | 90 |
| 16c | PIDA (1) | toluene | 88 |
| 17d | PIDA (1) | toluene | 59 |
| 18e | PIDA (1) | toluene | trace |
Reaction conditions: 1a (0.2 mmol), NFSI (1.0 equiv), and oxidant (1.0 equiv) in the presence of solvent (1 mL) at 60 °C for 15 min.
Isolated yields.
Reaction was carried out at 80 °C.
Reaction was carried out at 40 °C.
Reaction was carried out at rt.
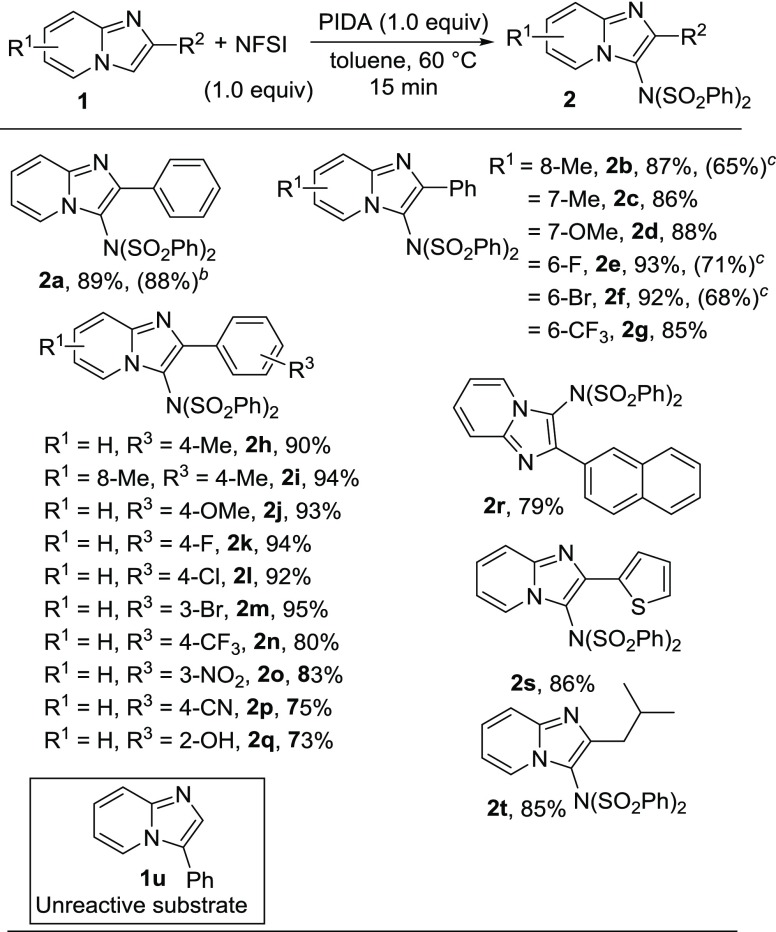
With the optimized reaction conditions, we explored the substrate scope of the present protocol, as shown in Scheme 2. A series of C-3 imidated imidazo[1,2-a]-pyridine products were obtained in good to excellent yields (2a–2t). Imidazo[1,2-a]-pyridines containing electron-donating substituents such as −CH3 and −OCH3 at different positions of the pyridine ring produced the desired products in excellent yields (2b–2d). Substrates possessing halogen substituents attached to the six-membered ring successfully underwent the reaction (2e–2f). 2-Phenyl-6-(trifluoromethyl)imidazo[1,2-a]pyridine successfully reacted with NFSI to furnish the desired product (2g). Next, we investigated the effect of substituents at the phenyl ring at the C-2 position of imidazo[1,2-a]pyridine. The phenyl moiety containing both electron-donating substituents and electron-withdrawing groups gave the corresponding products in high yields (2h–2p). Hydroxy-containing imidazo[1,2-a]pyridine was also tolerable for such transformation (2q). Both naphthyl- and heteroaryl-substituted imidazopyridines produced the desired products without any difficulties (2r and 2s). The single-crystal X-ray diffraction study of N-(phenylsulfonyl)-N-(2-(thiophen-2-yl)imidazo[1,2-a]pyridin-3-yl)benzenesulfonamide (2s) was performed to confirm the structure of imidated imidazopyridine.19 Isopropyl-substituted (C-2) imidazo[1,2-a]pyridine could also be successfully converted to the corresponding product in excellent yield (2t). Moreover, no desired imidated product was obtained when 3-phenylimidazo[1,2-a]pyridine (1u) was reacted with NFSI under the optimized reaction conditions. This result suggests that the reaction selectively took place at the C-3 position of imidazo[1,2-a]pyridine. We also performed the reactions of 1b, 1e, and 1f with dibenzenesulfonimide [HN(SO2Ph)2] instead of NFSI under the same optimized conditions (Scheme 2). Here, the corresponding products (2b, 2e, and 2f) were obtained in moderate yields. The gram-scale reaction was also carried out under the normal laboratory setup by taking 2-phenylimidazo[1,2-a]pyridine (1a) and NFSI under the standard reaction conditions on a 10 mmol scale. Gratifyingly, N-(2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2a) was obtained without significant decrease in yield which clearly signifies the practicability of our present protocol.
Scheme 2. Substrate Scope of Imidazopyridinesa,b,c.
Reaction conditions: 1 (0.2 mmol), NFSI (1.0 equiv), and PIDA (1.0 equiv) in toluene (1 mL) at 60 °C for 15 min.
10 mmol scale.
Reaction with HN(SO2Ph)2 instead of NFSI.
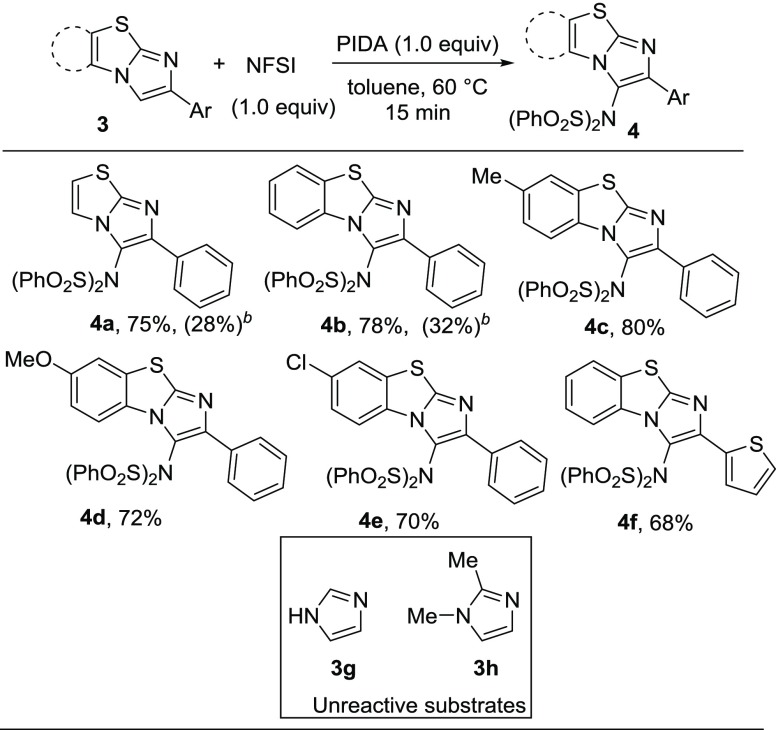
To extend the generality of this methodology, we investigated the present imidation reaction with other heterocycles such as imidazo[2,1-b]thiazole and benzo[d]imidazo[2,1-b]thiazoles (Scheme 3). Both 6-phenylimidazo[2,1-b]thiazole (3a) and 2-phenylbenzo[d]imidazo[2,1-b]thiazole (3b) reacted well to afford the desired products in satisfactory yields (4a and 4b). 2-Phenylbenzo[d]imidazo[2,1-b]thiazole containing both electron-donating and electron-withdrawing substituents underwent the reaction with NFSI without any difficulties (4c–4e). Heteroaryl-substituted benzoimidazothiazole was also effective for such transformation to afford the desired imidated product in good yields (4f). However, 4,5-unsubstituted imidazoles (3g and 3h) did not provide imidated products under the optimized reaction conditions. The reactions of imidazothiazole (3a) and benzoimidazothiazole (3b) with HN(SO2Ph)2 produced the desired imidated products (4a and 4b) in lower yields (Scheme 3).
Scheme 3. Coupling of Imidazothiazole and Benzoimidazothiazole with NFSIa,b.
Reaction conditions: 3 (0.2 mmol), NFSI (1.0 equiv), and PIDA (1.0 equiv) in toluene (1 mL), at 60 °C for 15 min.
Reaction with HN(SO2Ph)2 instead of NFSI.
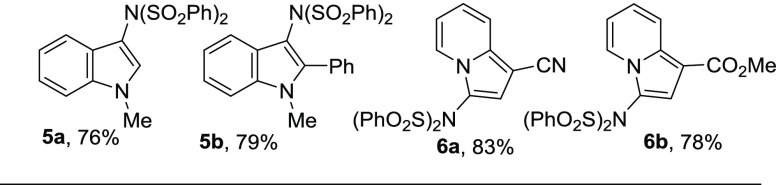
The current methodology is also applicable for N-substituted indole and indolizine derivatives (Figure 1). Both N-methylindole and 1-methyl-2-phenyl indole produced the desired products in high yields (5a and 5b). Moreover, indolizine derivatives smoothly participated in this reaction to give the desired imidated products in good yields (6a and 6b).
Figure 1.
Substrate scope with other heterocycles. Reaction conditions: N-substituted indoles or indolizines (0.2 mmol), NFSI (1.0 equiv), and PIDA (1.0 equiv) in toluene (1 mL) at 60 °C for 15 min.
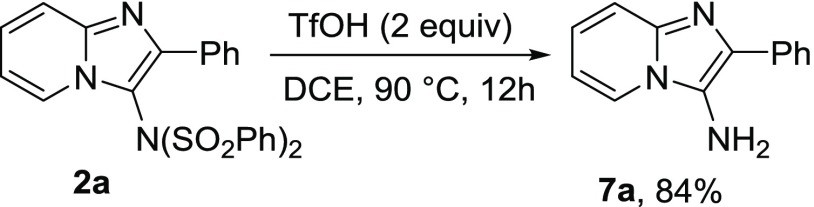
Amino derivative of imidazopyridine (7a) could be easily synthesized from N-(2 phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2a) using TfOH (Scheme 4).14b
Scheme 4. Synthetic Utility.
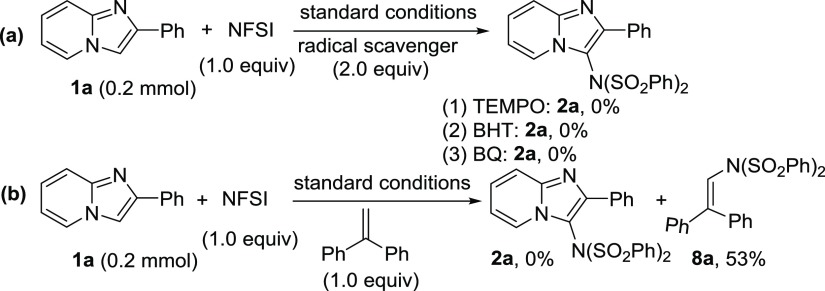
To acquire the mechanistic insights into the reaction pathway, few control experiments were carried out (Scheme 5). It was found that imidazo[1,2-a]pyridine failed to give the corresponding product in the presence radical scavengers such as 2,2,6,6-tetramethylpiperidine-1-oxyl, 2,6-di-tert-butyl-4-methylphenol, and p-benzoquinone (Scheme 5a). The use of a stoichiometric amount of 1,1-diphenylethylene was also completely suppressed the imidation reaction. Moreover, N-(2,2-diphenylvinyl)-N-(phenylsulfonyl)benzenesulfonamide (8a) was obtained in 53% yield along with almost full recovery of 1a (Scheme 5b). These observations imply that the reaction proceeds through a radical pathway.
Scheme 5. Control Experiments.
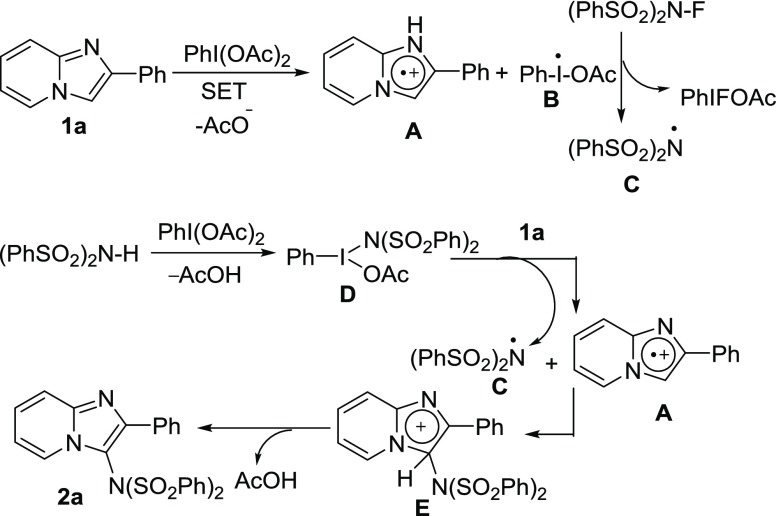
On the basis of the control experiments (Scheme 5) as well as previous reports,4c,18,20 a plausible mechanism of PIDA-mediated C–H imidation of imidazopyridines with NFSI is presented in Scheme 6. Probably, the first step is the PIDA-mediated oxidation of imidazopyridine (1a) to imidazopyridine radical cation (A) along with the formation of radical (B) through a single electron-transfer process. After that the radical (B) is oxidized by NFSI via one-electron F-atom transfer pathway, affording the bis-sulfonylamidyl radical (C). On the other hand, dibenzenesulfonimide [HN(SO2Ph)2] could also produce N-iodoamido species (D) in the presence of PIDA which subsequently gives bis-sulfonylamidyl radical (C) and generates imidazopyridine radical cation (A) from 1a. Finally, the resulting imidazopyridine radical cation (A) regioselectively coupled with the bis-sulfonylamidyl radical (C) to produce the imidazolenium ion (E) which consequently affords the product (2a) through the elimination of AcOH.
Scheme 6. Plausible Mechanistic Pathway.
Conclusions
In summary, we have developed an efficient and simple methodology for PIDA-mediated regioselective imidation of imidazo[1,2-a]pyridines using NFSI as the nitrogen source under metal-free conditions in short reaction times. The present methodology is also applicable to other heterocycles such as imidazo[2,1-b]thiazole, benzo[d]imidazo-[2,1-b]thiazole, indole, and indolizine derivatives. Metal-free conditions, broad substrates scope, fast reactions, and gram-scale synthesis are the notable advantages of our methodology. To the best of our knowledge, this is the first report of metal-free imidation of imidazoheterocycles using NFSI featuring the incorporation of −N(SO2Ph)2 group. We believe that our present protocol will open a new possibility for the forthcoming more practical and selective C–N bond formation route of heterocycles under metal-free conditions.
Experimental Section
General Information
All reagents were purchased from commercial sources and used without further purification. 1H NMR spectra were determined on a 400 MHz spectrometer as solutions in CDCl3. Chemical shifts are expressed in parts per million (δ), and the signals were reported as s (singlet), d (doublet), t (triplet), m (multiplet), dd (double of doublet), and coupling constants (J) were given in hertz. 13C{1H} NMR spectra were recorded at 100 MHz in CDCl3 solution. Chemical shifts as an internal standard are referenced to CDCl3 (δ = 7.26 for 1H and δ = 77.16 for 13C{1H} NMR) as an internal standard. Thin-layer chromatography (TLC) was done on a silica gel-coated glass slide. All solvents were dried and distilled before use. Commercially available solvents were freshly distilled before the reaction. All reactions involving moisture sensitive reactants were executed using an oven-dried glassware. X-ray single crystal data were collected using Mo Kα (λ = 0.71073 Å) radiation with a CCD area detector. Melting points were determined on a glass disk with an electrical bath and are uncorrected. All of the imidazoheterocycles were prepared by our reported method.4b,4d
General Experimental Procedures for the Synthesis of 2a–2t and 4a–4f
Imidazoheterocycles (0.2 mmol), NFSI (1.0 equiv, 63 mg), and PIDA (1.0 equiv, 65 mg) were taken in an oven-dried reaction vessel equipped with a magnetic stir bar. Then, toluene (1 mL) was added and stirred at 60 °C for 15 min. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was diluted with 8 mL of water/ethyl acetate (1:1). Then, the reaction mixture was extracted with ethyl acetate, and the organic phase was dried over anhydrous Na2SO4. After evaporating the solvent under reduced pressure, the crude residue was obtained. Finally, it was purified by column chromatography on silica gel (60–120 mesh) using petroleum ether/ethylacetate as an eluent to afford the pure imidated products (2a–2t and 4a–4f).
N-(2-Phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2a)
White solid (87 mg, 89%), Rf 0.5 (PET/EtOAc = 3:2), mp 128–130 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.90 (m, 4H), 7.72–7.70 (m, 2H), 7.64 (d, J = 8.8 Hz, 1H), 7.59–7.55 (m, 2H), 7.52–7.50 (m, 1H), 7.41–7.37 (m, 4H), 7.29–7.24 (m, 1H), 7.18–7.14 (m, 1H), 7.12–7.08 (m, 2H), 6.66–6.63 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.6, 144.6, 139.2, 134.7, 131.9, 129.2, 129.1, 128.5, 128.2, 127.6, 126.9, 124.0, 118.0, 112.8, 111.1; Anal. Calcd for C25H19N3O4S2: C, 61.34; H, 3.91; N, 8.58%. Found: C, 61.13; H, 4.02; N, 8.67%.
N-(8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2b)
White solid (88 mg, 87%), Rf 0.6 (PET/EtOAc = 4:1), mp 116–118 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.90 (m, 4H), 7.75–7.73 (m, 2H), 7.56 (t, J = 7.6 Hz, 2H), 7.38 (t, J = 8.0 Hz, 5H), 7.17–7.08 (m, 3H), 7.05–7.03 (m, 1H), 6.55 (t, J = 6.8 Hz, 1H), 2.65 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.3, 144.8, 139.3, 134.6, 132.3, 129.2, 129.1, 128.3, 128.1, 128.0, 127.8, 125.5, 121.7, 112.7, 111.3, 16.6; Anal. Calcd for C26H21N3O4S2: C, 62.01; H, 4.20; N, 8.34%. Found: C, 62.21; H, 4.09; N, 8.25%.
N-(7-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2c)
White solid (87 mg, 86%), Rf 0.7 (PET/EtOAc = 4:1), mp 78–80 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.90 (m, 4H), 7.69–7.67 (m, 2H), 7.59–7.55 (m, 2H), 7.41–7.37 (m, 6H), 7.15–7.13 (m, 1H), 7.10–7.06 (m, 2H), 6.50–6.48 (m, 1H), 2.40 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 149.0, 145.0, 139.2, 134.7, 132.9, 132.0, 131.8, 129.2, 129.1, 128.4, 128.1, 127.6, 123.2, 116.5, 115.4, 21.4; Anal. Calcd for C26H21N3O4S2: C, 62.01; H, 4.20; N, 8.34%. Found: C, 62.27; H, 4.32; N, 8.13%.
N-(7-Methoxy-2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2d)
White solid (91 mg, 88%), Rf 0.5 (PET/EtOAc = 4:1), mp 96–98 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.90 (m, 4H), 7.68–7.66 (m, 2H), 7.58–7.55 (m, 2H), 7.41–7.37 (m, 4H), 7.28 (d, J = 7.6 Hz, 1H), 7.16–7.12 (m, 1H), 7.10–7.06 (m, 2H), 6.92 (d, J = 2.4 Hz, 1H), 6.35 (dd, J = 2.4, 7.2 Hz, 1H), 3.87 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 159.5, 146.3, 145.3, 139.2, 134.7, 131.9, 129.2, 129.1, 128.4, 128.1, 127.4, 124.4, 110.1, 107.7, 95.3, 55.8; Anal. Calcd for C26H21N3O5S2: C, 60.10; H, 4.07; N, 8.09%. Found: C, 59.95; H, 4.15; N, 7.99%.
N-(6-Fluoro-2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2e)
White solid (94 mg, 93%), Rf 0.5 (PET/EtOAc = 3:2), mp 130–132 °C; 1H NMR (CDCl3, 400 MHz): δ 7.93–7.91 (m, 4H), 7.71–7.69 (m, 2H), 7.62–7.58 (m, 3H), 7.43–7.39 (m, 4H), 7.36–7.35 (m, 1H), 7.19–7.16 (m, 2H), 7.12–7.09 (m, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 153.4 (d, JC–F = 238.0 Hz), 142.1, 139.1, 134.9, 134.1, 131.7, 129.2 (d, JC–F = 19.0 Hz) 129.0, 128.7, 128.3, 127.5, 119.0, 118.8, 118.6 (d, JC–F = 9.0 Hz), 111.4, 110.9; Anal. Calcd for C25H18FN3O4S2: C, 59.16; H, 3.57; N, 8.28%. Found: C, 59.30; H, 3.68; N, 8.12%.
N-(6-Bromo-2-phenylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2f)
White solid (104 mg, 92%), Rf 0.4 (PET/EtOAc = 3:2), mp 136–138 °C; 1H NMR (CDCl3, 400 MHz): δ 7.94–7.92 (m, 4H), 7.74–7.72 (m, 2H), 7.63–7.58 (m, 2H), 7.51 (d, J = 9.6 Hz, 1H), 7.44–7.40 (m, 5H), 7.32–7.29 (m, 1H), 7.18–7.16 (m, 1H), 7.13–7.09 (m, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 142.6, 139.1, 135.1, 130.9, 129.3, 129.0, 128.8, 128.5, 128.4, 127.7, 127.0, 126.1, 124.2, 118.4, 108.1; Anal. Calcd for C25H18BrN3O4S2: C, 52.82; H, 3.19; N, 7.39%. Found: C, 52.61; H, 3.22; N, 7.48%.
N-(2-Phenyl-6-(trifluoromethyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2g)
White solid (95 mg, 85%), Rf 0.6 (PET/EtOAc = 3:2), mp 124–126 °C; 1H NMR (CDCl3, 400 MHz): δ 7.93 (d, J = 7.6 Hz, 4H), 7.77–7.75 (m, 2H), 7.72 (d, J = 9.6 Hz, 1H), 7.65 (s, 1H), 7.62–7.59 (m, 2H), 7.43–7.37 (m, 5H), 7.22–7.19 (m, 1H), 7.13 (t, J = 7.6 Hz, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.8 (d, JC–F = 296.0 Hz), 139.0, 135.1, 131.2, 129.6, 129.4, 129.1, 129.0, 128.8, 128.7, 128.4, 127.7, 126.7, 122.9 (q, JC–F = 6.0, 11.0 Hz), 122.7 (d, JC–F = 2.0 Hz), 118.8; Anal. Calcd for C26H18F3N3O4S2: C, 56.01; H, 3.25; N, 7.54%. Found: C, 56.18; H, 3.21; N, 7.40%.
N-(Phenylsulfonyl)-N-(2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)benzenesulfonamide (2h)
White solid (91 mg, 90%), Rf 0.5 (PET/EtOAc = 7:3), mp 120–122 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.90 (m, 4H), 7.64–7.56 (m, 5H), 7.49–7.47 (m, 1H), 7.41–7.37 (m, 4H), 7.27–7.23 (m, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.64–6.61 (m, 1H), 2.28 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.8, 144.5, 139.3, 138.8, 138.3, 134.5, 129.1, 129.0, 128.9, 127.5, 126.8, 123.9, 117.9, 112.6, 110.8, 21.2; Anal. Calcd for C26H21N3O4S2: C, 62.01; H, 4.20; N, 8.34%. Found: C, 61.78; H, 4.15; N, 8.46%.
N-(8-Methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2i)
White solid (97 mg, 94%), Rf 0.5 (PET/EtOAc = 4:1), mp 132–134 °C; 1H NMR (CDCl3, 400 MHz): δ 7.91 (dd, J = 0.6, 8.4 Hz, 4H), 7.26–7.55 (m, 4H), 7.40–7.33 (m, 5H), 7.03 (d, J = 6.8 Hz, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.53 (t, J = 6.8 Hz, 1H), 2.64 (s, 3H), 2.28 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.4, 144.8, 139.3, 138.0, 134.4, 129.3, 129.15, 129.11, 128.9, 127.9, 127.6, 125.5, 121.6, 112.6, 111.1, 21.3, 16.6; Anal. Calcd for C27H23N3O4S2: C, 62.65; H, 4.48; N, 8.12%. Found: C, 62.49; H, 4.41; N, 8.30%.
N-(2-(4-Methoxyphenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2j)
White solid (96 mg, 93%), Rf 0.3 (PET/EtOAc = 4:1), mp 119–121 °C; 1H NMR (CDCl3, 400 MHz): δ 7.94–7.92 (m, 4H), 7.67 (d, J = 8.8 Hz, 2H), 7.63–7.58 (m, 3H), 7.47–7.39 (m, 5H), 7.27–7.23 (m, 1H), 6.64–6.60 (m, 3H), 3.78 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 159.9, 145.5, 144.5, 139.3, 134.6, 129.29, 129.22, 129.1, 129.0, 126.8, 124.5, 123.8, 117.8, 113.7, 112.6, 55.3; Anal. Calcd for C26H21N3O5S2: C, 60.10; H, 4.07; N, 8.09. Found: C, 59.84; H, 4.15; N, 8.23%.
N-(2-(4-Fluorophenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2k)
White solid (95 mg, 94%), Rf 0.6 (PET/EtOAc = 7:3), mp 143–145 °C; 1H NMR (CDCl3, 400 MHz): δ 7.94–7.92 (m, 4H), 7.74–7.70 (m, 2H), 7.61 (t, J = 7.6 Hz, 3H), 7.48 (d, J = 6.8 Hz, 1H), 7.42 (t, J = 8.0 Hz, 4H), 7.30–7.26 (m, 1H), 6.79 (t, J = 8.8 Hz, 2H), 6.67–6.64 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 162.9 (d, JC–F = 247.0 Hz), 144.7, 144.6, 139.2, 134.8, 129.5 (d, JC–F = 8.0 Hz), 129.2, 129.1, 128.2 (d, JC–F = 2.0 Hz), 127.0, 123.9, 118.0, 115.1 (d, JC–F = 22.0 Hz), 112.8, 111.0; HRMS (ESI–TOF) m/z: [M + H+] calcd for C25H18FN3O4S2, 508.0795; found, 508.0798.
N-(2-(4-Chlorophenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2l)
White solid (96 mg, 92%), Rf 0.6 (PET/EtOAc = 7:3), mp 153–155 °C; 1H NMR (CDCl3, 400 MHz): δ 7.93–7.90 (m, 4H), 7.66–7.60 (m, 5H), 7.48–7.46 (m, 1H), 7.44–7.40 (m, 4H), 7.30–7.27 (m, 1H), 7.07–7.04 (m, 2H), 6.67–6.64 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 144.6, 144.5, 139.1, 134.8, 134.6, 130.4, 129.3, 129.1, 128.9, 128.4, 127.2, 126.5, 124.0, 118.1, 113.0; Anal. Calcd for C25H18ClN3O4S2: C, 57.30; H, 3.46; N, 8.02. Found: C, 57.43; H, 3.38; N, 8.11%.
N-(2-(3-Bromophenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2m)
White solid (108 mg, 95%), Rf 0.5 (PET/EtOAc = 7:3), mp 145–147 °C; 1H NMR (CDCl3, 400 MHz): δ 7.84–7.82 (m, 4H), 7.75–7.74 (m, 1H), 7.61–7.49 (m, 5H), 7.34 (t, J = 8.0 Hz, 4H), 7.24–7.18 (m, 2H), 6.89 (t, J = 8.0 Hz, 1H), 6.64–6.61 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 144.6, 144.0, 138.8, 134.8, 134.0, 132.2, 131.5, 130.4, 129.6, 129.2, 129.0, 127.2, 126.1, 124.1, 122.4, 118.1, 113.1; Anal. Calcd for C25H18BrN3O4S2: C, 52.82; H, 3.19; N, 7.39%. Found: C, 52.59; H, 3.07; N, 7.28%.
N-(Phenylsulfonyl)-N-(2-(4-(trifluoromethyl)phenyl)imidazo[1,2-a]pyridin-3-yl)benzenesulfonamide (2n)
White solid (89 mg, 80%), Rf 0.6 (PET/EtOAc = 3:2), mp 131–133 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92 (dd, J = 0.8, 8.0 Hz, 4H), 7.84 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 9.2 Hz, 1H), 7.61–7.57 (m, 2H), 7.50 (d, J = 6.8 Hz, 1H), 7.42–7.34 (m, 7H), 6.71–6.68 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.8 (d, JC–F = 218.0 Hz), 139.1, 135.0, 130.3, 130.0, 129.2 (d, JC–F = 18.0 Hz), 128.7, 128.5, 127.8, 127.4, 126.6, 125.4, 125.1 (q, JC–F = 5.0, 9.0 Hz), 124.0, 118.3, 113.2; Anal. Calcd for C26H18F3N3O4S2: C, 56.01; H, 3.25; N, 7.54%. Found: C, 56.20; H, 3.33; N, 7.45%.
N-(2-(3-Nitrophenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2o)
White solid (89 mg, 83%), Rf 0.6 (PET/EtOAc = 3:2), mp 155–157 °C; 1H NMR (CDCl3, 400 MHz): δ 8.47–7.46 (m, 1H), 8.06–8.04 (m, 1H), 7.94–7.91 (m, 1H), 7.86 (d, J = 7.6 Hz, 4H), 7.62–7.59 (m, 1H), 7.52–7.48 (m, 3H), 7.33 (t, J = 8.0 Hz, 4H), 7.29–7.19 (m, 2H), 6.68–6.65 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 148.2, 144.7, 142.8, 138.9, 134.9, 133.2, 132.2, 132.1, 129.3, 129.1, 128.6, 127.6, 124.1, 123.1, 122.4, 118.2, 113.4; Anal. Calcd for C25H18N4O6S2: C, 56.17; H, 3.39; N, 10.48%. Found: C, 56.03; H, 3.36; N, 10.53%.
N-(2-(4-Cyanophenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2p)
White solid (77 mg, 75%), Rf 0.7 (PET/EtOAc = 3:2); mp 156–158 °C; 1H NMR (CDCl3, 400 MHz): δ 7.96–7.94 (m, 4H), 7.88–7.86 (m, 2H), 7.68–7.64 (m, 3H), 7.51–7.44 (m, 5H), 7.41–7.39 (m, 2H), 7.36–7.31 (m, 1H), 6.73–6.69 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 144.8, 143.2, 139.0, 136.5, 135.0, 132.7, 131.9, 129.4, 129.1, 127.9, 127.5, 124.0, 118.8, 118.3, 113.3, 111.7; Anal. Calcd for C26H18N4O4S2: C, 60.69; H, 3.53; N, 10.89%. Found: C, 60.54; H, 3.44; N, 10.76%.
N-(2-(2-Hydroxyphenyl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2q)
White solid (74 mg, 73%), Rf 0.4 (PET/EtOAc = 7:3), mp 139–140 °C; 1H NMR (CDCl3, 400 MHz): δ 12.9 (br s, 1H), 7.96 (dd, J = 0.8, 8.4 Hz, 4H), 7.64–7.56 (m, 4H), 7.45 (t, J = 8.0 Hz, 4H), 7.35–7.31 (m, 1H), 7.23–7.21 (m, 1H), 7.07–7.02 (m, 1H), 6.90–6.88 (m, 1H), 6.73–6.69 (m, 1H), 6.28–6.24 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 158.1, 144.2, 142.2, 139.1, 135.2, 135.0, 130.6, 129.3, 129.2, 127.7, 126.7, 123.8, 118.4, 117.5, 117.0, 114.2, 113.4; Anal. Calcd for C25H19N3O5S2: C, 59.39; H, 3.79; N, 8.31%. Found: C, 59.16; H, 3.84; N, 8.17%.
N-(2-(Naphthalen-2-yl)imidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2r)
White solid (85 mg, 79%), Rf 0.5 (PET/EtOAc = 7:3), mp 150–152 °C; 1H NMR (CDCl3, 400 MHz): δ 8.14 (s, 1H), 7.84–7.78 (m, 5H), 7.65–7.53 (m, 3H), 7.50 (d, J = 8.0 Hz, 2H), 7.38–7.28 (m, 4H), 7.22–7.15 (m, 5H), 6.62–6.58 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 145.5, 144.7, 139.1, 134.5, 133.3, 133.0, 132.2, 129.1, 129.0, 128.8, 128.6, 127.7, 127.3, 127.09, 127.06, 126.5, 125.8, 125.2, 124.0, 118.0, 112.9; Anal. Calcd for C29H21N3O4S2: C, 64.55; H, 3.92; N, 7.79%. Found: C, 64.41; H, 3.85; N, 7.92%.
N-(Phenylsulfonyl)-N-(2-(thiophen-2-yl)imidazo[1,2-a]pyridin-3-yl)benzenesulfonamide (2s)
White solid (85 mg, 86%), Rf 0.4 (PET/EtOAc = 7:3); mp 137–139 °C; 1H NMR (CDCl3, 400 MHz): δ 7.90–7.88 (m, 4H), 7.53 (t, J = 8.0 Hz, 3H), 7.38–7.34 (m, 5H), 7.20–7.16 (m, 1H), 7.09 (d, J = 5.2 Hz, 1H), 6.99–6.98 (m, 1H), 6.62–6.55 (m, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 144.6, 141.2, 139.5, 134.8, 134.6, 129.2, 129.0, 128.9, 127.8, 127.1, 126.7, 126.1, 123.7, 117.8, 112.9; HRMS (ESI–TOF) m/z: [M + H+] calcd for C23H17N3O4S3, 496.0454; found, 496.0455.
N-(2-Isobutylimidazo[1,2-a]pyridin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (2t)
White solid (80 mg, 85%), Rf 0.5 (PET/EtOAc = 8:2), mp 83–85 °C; 1H NMR (CDCl3, 400 MHz): δ 7.97 (d, J = 7.2 Hz, 4H), 7.73–7.69 (m, 2H), 7.55 (t, J = 7.6 Hz, 6H), 7.24–7.20 (m, 1H), 6.67–6.63 (m, 1H), 2.23–2.20 (m, 1H), 1.89 (d, J = 7.2 Hz, 2H), 0.84 (d, J = 6.4 Hz, 6H); 13C{1H} NMR (CDCl3, 100 MHz): δ 149.3, 144.7, 139.1, 134.7, 129.4, 129.1, 128.9, 126.3, 123.4, 117.5, 112.5, 35.7, 27.2, 22.8; Anal. Calcd for C23H23N3O4S2: C, 58.83; H, 4.94; N, 8.95%. Found: C, 58.97; H, 5.02; N, 8.83%.
N-(6-Phenylimidazo[2,1-b]thiazol-5-yl)-N-(phenylsulfonyl)benzenesulfonamide (4a)
White solid (74 mg, 75%), Rf 0.4 (PET/EtOAc = 4:1), mp 118–120 °C; 1H NMR (CDCl3, 400 MHz): δ 7.92–7.89 (m, 4H), 7.59–7.54 (m, 4H), 7.42–7.38 (m, 4H), 7.13–7.06 (m, 3H), 6.70–6.66 (m, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 150.1, 147.2, 138.9, 134.7, 131.9, 129.9, 129.2, 128.9, 128.2, 128.1, 126.8, 118.1, 113.1; Anal. Calcd for C23H17N3O4S3: C, 55.74; H, 3.46; N, 8.48%. Found: C, 55.62; H, 3.50; N, 8.31%.
N-(2-Phenylbenzo[d]imidazo[2,1-b]thiazol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (4b)
White solid (85 mg, 78%), Rf 0.5 (PET/EtOAc = 4:1), mp 144–146 °C; 1H NMR (CDCl3, 400 MHz): δ 7.97–7.95 (m, 4H), 7.67–7.65 (m, 2H), 7.62 (d, J = 8.0 Hz, 1H), 7.56–7.52 (m, 2H), 7.35 (t, J = 8.4 Hz, 4H), 7.22–7.19 (m, 1H), 7.11–7.09 (m, 1H), 7.07–7.03 (m, 2H), 6.91–6.89 (m, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 147.4, 138.9, 134.7, 131.8, 130.2, 129.9, 129.6, 129.0, 128.9, 128.5, 128.2, 128.1, 127.0, 125.7, 124.9, 123.8, 114.5; Anal. Calcd for C27H19N3O4S3: C, 59.43; H, 3.51; N, 7.70%. Found: C, 59.69; H, 3.64; N, 7.61%.
N-(7-Methyl-2-phenylbenzo[d]imidazo[2,1-b]thiazol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (4c)
White solid (90 mg, 80%), Rf 0.6 (PET/EtOAc = 4:1); mp 135–137 °C; 1H NMR (CDCl3, 400 MHz): δ 7.95 (dd, J = 0.8, 8.4 Hz, 4H), 7.65–7.62 (m, 2H), 7.54 (t, J = 7.6 Hz, 2H), 7.41 (s, 1H), 7.35 (t, J = 8.0 Hz, 4H), 7.10–7.02 (m, 3H), 6.78 (d, J = 8.4 Hz, 1H), 6.70 (dd, J = 0.8, 8.8 Hz, 1H), 2.37 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 147.1, 138.9, 135.1, 134.7, 131.9, 130.0, 129.9, 129.6, 129.0, 128.5, 128.16, 128.11, 126.9, 126.7, 123.8, 114.1, 113.9, 21.3; Anal. Calcd for C28H21N3O4S3: C, 60.09; H, 3.78; N, 7.51%. Found: C, 59.80; H, 3.71; N, 7.65%.
N-(7-Methoxy-2-phenylbenzo[d]imidazo[2,1-b]thiazol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (4d)
White solid (83 mg, 72%), Rf 0.4 (PET/EtOAc = 4:1), mp 170–172 °C; 1H NMR (CDCl3, 400 MHz): δ 7.95 (d, J = 7.2 Hz, 4H), 7.64–7.62 (m, 2H), 7.54 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 8.0 Hz, 4H), 7.12–7.01 (m, 4H), 6.80 (d, J = 8.8 Hz, 1H), 6.46 (dd, J = 2.4, 9.2 Hz, 1H), 3.80 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 157.0, 138.9, 134.7, 132.0, 131.3, 129.5, 129.1, 128.8, 128.3, 128.1, 128.0, 126.8, 126.5, 126.1, 115.1, 113.1, 107.9, 55.9; Anal. Calcd for C28H21N3O5S3: C, 58.42; H, 3.68; N, 7.30%. Found: C, 58.64; H, 3.74; N, 7.22%.
N-(7-Chloro-2-phenylbenzo[d]imidazo[2,1-b]thiazol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (4e)
White solid (81 mg, 70%), Rf 0.3 (PET/EtOAc = 4:1), mp 125–127 °C; 1H NMR (CDCl3, 400 MHz): δ 7.96–7.94 (m, 4H), 7.65–7.60 (m, 2H), 7.58–7.54 (m, 2H), 7.37 (t, J = 8.0 Hz, 4H), 7.31–7.28 (m, 1H), 7.12–7.10 (m, 1H), 7.08–7.03 (m, 2H), 6.89–6.86 (m, 1H), 6.82 (d, J = 8.8 Hz, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 138.8, 134.8, 131.6, 130.5, 129.6, 129.5, 129.49, 129.44, 129.1, 128.3, 128.2, 128.0, 127.5, 126.9, 126.0, 123.5, 115.1; Anal. Calcd for C27H18ClN3O4S3: C, 55.90; H, 3.13; N, 7.24%. Found: C, 56.14; H, 3.05; N, 7.08%.
N-(Phenylsulfonyl)-N-(2-(thiophen-2-yl)benzo[d]imidazo[2,1-b]thiazol-3-yl)benzenesulfonamide (4f)
White solid (75 mg, 68%), Rf 0.7 (PET/EtOAc = 7:3); mp 132–134 °C; 1H NMR (CDCl3, 400 MHz): δ 8.01 (dd, J = 1.2, 8.8 Hz, 4H), 7.62–7.56 (m, 3H), 7.42–7.38 (m, 4H), 7.21 (t, J = 7.2 Hz, 1H), 7.11 (dd, J = 0.8, 4.8 Hz, 1H), 6.97–6.96 (m, 1H), 6.93–6.89 (m, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.64–6.62 (m, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 139.2, 134.7, 131.8, 129.9, 129.5, 129.1, 127.8, 127.5, 126.0, 125.8, 125.1, 125.0, 124.4, 123.8, 123.7, 114.3, 110.9; Anal. Calcd for C25H17N3O4S4: C, 54.43; H, 3.11; N, 7.62%. Found: C, 54.68; H, 3.02; N, 7.47%.
N-(1-Methyl-1H-indol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (5a)
White solid (65 mg, 76%), Rf 0.4 (PET/EtOAc = 4:1); mp 78–80 °C; 1H NMR (CDCl3, 400 MHz): δ 7.94–7.91 (m, 4H), 7.72–7.68 (m, 2H), 7.59–7.53 (m, 5H), 7.31–7.27 (m, 2H), 7.17–7.13 (m, 1H), 6.23 (s, 1H), 3.33 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 138.8, 136.4, 134.4, 129.2, 128.9, 127.2, 125.7, 123.7, 121.7, 120.4, 110.2, 104.7, 29.2; Anal. Calcd for C21H18N2O4S2: C, 59.14; H, 4.25; N, 6.57. Found: C, 59.05; H, 4.19; N, 6.64%.
N-(1-Methyl-2-phenyl-1H-indol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (5b)
White solid (79 mg, 79%), Rf 0.5 (PET/EtOAc = 4:1), mp 155–157 °C; 1H NMR (CDCl3, 400 MHz): δ 7.76–7.74 (m, 3H), 7.51 (t, J = 7.6 Hz, 3H), 7.37–7.25 (m, 10H), 7.22–7.16 (m, 1H), 6.93 (t, J = 7.6 Hz, 1H), 6.76 (d, J = 8.0 Hz, 1H), 3.54 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 142.2, 139.9, 135.9, 133.6, 130.5, 129.3, 129.1, 129.0, 128.9, 128.6, 128.4, 125.7, 122.7, 120.9, 119.3, 109.9, 31.4; HRMS (ESI–TOF) m/z: [M + Na+] calcd for C27H22N2O4S2, 525.0913; found, 525.0917.
N-(1-Cyanoindolizin-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (6a)
White solid (73 mg, 83%), Rf 0.6 (PET/EtOAc = 4:1), mp 120–122 °C; 1H NMR (CDCl3, 400 MHz): δ 7.87–7.85 (m, 4H), 7.76–7.70 (m, 3H), 7.68–7.65 (m, 1H), 7.58–7.54 (m, 4H), 7.21–7.17 (m,1H), 6.75–6.72 (m, 1H), 6.63 (s, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 138.3, 137.7, 134.9, 129.5, 128.7, 124.8, 123.5, 119.5, 118.0, 115.6, 114.0, 113.9, 82.2; Anal. Calcd for C21H15N3O4S2: C, 57.65; H, 3.46; N, 9.61. Found: C, 57.89; H, 3.41; N, 9.51%.
Methyl-3-(N-(phenylsulfonyl)phenylsulfonamido)indolizine-1-carboxylate (6b)
White solid (73 mg, 78%), Rf 0.5 (PET/EtOAc = 4:1), mp 106–108 °C; 1H NMR (CDCl3, 400 MHz): δ 8.21 (dd, J = 0.8, 8.8 Hz, 1H), 7.86 (dd, J = 0.8, 8.0 Hz, 4H), 7.72–7.67 (m, 3H), 7.53 (t, J = 8.0 Hz, 4H), 7.16–7.12 (m, 1H), 6.91 (s, 1H), 6.68–6.64 (m, 1H), 3.87 (s, 3H); 13C{1H} NMR (CDCl3, 100 MHz): δ 164.7, 138.6, 135.8, 134.6, 129.3, 128.8, 124.6, 123.0, 119.8, 118.9, 113.4, 103.7, 51.2; Anal. Calcd for C22H18N2O6S2: C, 56.16; H, 3.86; N, 5.95%. Found: C, 56.39; H, 3.81; N, 6.04%.
2-Phenylimidazo[1,2-a]pyridin-3-amine (7a)4f
Gummy mass (35 mg, 84%), Rf 0.4 (PET/EtOAc = 3:2); 1H NMR (CDCl3, 400 MHz): δ 7.98–7.95 (m, 3H), 7.53 (d, J = 9.2 Hz, 1H), 7.47–7.43 (m, 2H), 7.33–7.29 (m, 1H), 7.11–7.07 (m, 1H), 6.79 (t, J = 6.8 Hz, 1H), 3.40 (br s, 2H); 13C{1H} NMR (CDCl3, 100 MHz): δ 143.9, 141.1, 134.6, 130.4, 129.1, 128.8, 127.2, 123.3, 121.9, 117.5, 111.8.
N-(2,2-Diphenylvinyl)-N-(phenylsulfonyl)benzenesulfonamide (8a)12a
White solid (50 mg, 53%), Rf 0.3 (PET/EtOAc = 9:1); mp 122–124 °C; 1H NMR (CDCl3, 400 MHz): δ 8.00–7.98 (m, 3H), 7.69–7.67 (m, 3H), 7.59–7.55 (m, 5H), 7.38 (d, J = 8.0 Hz, 3H), 7.30 (s, 1H), 7.24–7.20 (m, 5H), 6.11 (s, 1H); 13C{1H} NMR (CDCl3, 100 MHz): δ 139.9, 138.7, 135.9, 133.9, 130.0, 129.5, 128.9, 128.7, 128.5, 116.4.
Acknowledgments
A.H. acknowledges the financial support from SERB-DST (grant no. EMR/2016/001643). M.S. thanks to UGC-New Delhi (UGC-NFHE-SRF), S.M. thanks CSIR-New Delhi (CSIR-SRF), and R.S. thanks SERB (N-PDF) for their fellowships.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02088.
The authors declare no competing financial interest.
Supplementary Material
References
- Enguehard-Gueiffier C.; Gueiffier A. Recent Progress in the Pharmacology of Imidazo[1,2-a]pyridines. Mini-Rev. Med. Chem. 2007, 7, 888–899. 10.2174/138955707781662645. [DOI] [PubMed] [Google Scholar]
- Stasyuk A. J.; Banasiewicz M.; Cyrański M. K.; Gryko D. T. Imidazo[1,2-a]pyridines Susceptible to Excited State Intramolecular Proton Transfer: One-Pot Synthesis via an Ortoleva-King Reaction. J. Org. Chem. 2012, 77, 5552–5558. 10.1021/jo300643w. [DOI] [PubMed] [Google Scholar]
- a Bagdi A. K.; Santra S.; Monir K.; Hajra A. Synthesis of Imidazo[1,2-a]pyridines: a Decade Update. Chem. Commun. 2015, 51, 1555–1575. 10.1039/c4cc08495k. [DOI] [PubMed] [Google Scholar]; b Pericherla K.; Kaswan P.; Pandey K.; Kumar A. Recent Developments in the Synthesis of Imidazo[1,2-a]pyridines. Synthesis 2015, 47, 887–912. 10.1055/s-0034-1380182. [DOI] [Google Scholar]; c Koubachi J.; El Kazzouli S.; Bousmina M.; Guillaumet G. Functionalization of Imidazo[1,2-a]pyridines by Means of Metal-Catalyzed Cross-Coupling Reactions. Eur. J. Org. Chem. 2014, 5119–5138. 10.1002/ejoc.201400065. [DOI] [Google Scholar]; d Lei S.; Mai Y.; Yan C.; Mao J.; Cao H. A Carbonylation Approach Toward Activation of Csp2-H and Csp3-H Bonds: Cu-Catalyzed Regioselective Cross Coupling of Imidazo[1,2-a]pyridines with Methyl Hetarenes. Org. Lett. 2016, 18, 3582–3585. 10.1021/acs.orglett.6b01588. [DOI] [PubMed] [Google Scholar]; e Yang D.; Yan K.; Wei W.; Li G.; Lu S.; Zhao C.; Tian L.; Wang H. Catalyst-Free Regioselective C-3 Thiocyanation of Imidazopyridines. J. Org. Chem. 2015, 80, 11073–11079. 10.1021/acs.joc.5b01637. [DOI] [PubMed] [Google Scholar]; f Chang Q.; Liu Z.; Liu P.; Yu L.; Sun P. Visible-Light-Induced Regioselective Cyanomethylation of Imidazopyridines and Its Application in Drug Synthesis. J. Org. Chem. 2017, 82, 5391–5397. 10.1021/acs.joc.7b00750. [DOI] [PubMed] [Google Scholar]; g Cao H.; Liu X.; Zhao L.; Cen J.; Lin J.; Zhu Q.; Fu M. One-Pot Regiospecific Synthesis of Imidazo[1,2-a]pyridines: A Novel, Metal-Free, Three-Component Reaction for the Formation of C-N, C-O, and C-S Bonds. Org. Lett. 2014, 16, 146–149. 10.1021/ol4031414. [DOI] [PubMed] [Google Scholar]; h Donthiri R. R.; Pappula V.; Reddy N. N. K.; Bairagi D.; Adimurthy S. Copper-Catalyzed C-H Functionalization of Pyridines and Isoquinolines with Vinyl Azides: Synthesis of Imidazo Heterocycles. J. Org. Chem. 2014, 79, 11277–11284. 10.1021/jo5021618. [DOI] [PubMed] [Google Scholar]; i Chernyak N.; Gevorgyan V. General and Efficient Copper-Catalyzed Three-Component Coupling Reaction towards Imidazoheterocycles: One-Pot Synthesis of Alpidem and Zolpidem. Angew. Chem., Int. Ed. 2010, 49, 2743–2746. 10.1002/anie.200907291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bagdi A. K.; Hajra A. Design, Synthesis, and Functionalization of Imidazoheterocycles. Chem. Rec. 2016, 16, 1868–1885. 10.1002/tcr.201600057. [DOI] [PubMed] [Google Scholar]; b Bagdi A. K.; Rahman M.; Santra S.; Majee A.; Hajra A. Copper-Catalyzed Synthesis of Imidazo[1,2-a]pyridines through Tandem Imine Formation-Oxidative Cyclization under Ambient Air: One-Step Synthesis of Zolimidine on a Gram-Scale. Adv. Synth. Catal. 2013, 355, 1741–1747. 10.1002/adsc.201300298. [DOI] [Google Scholar]; c Mondal S.; Samanta S.; Jana S.; Hajra A. (Diacetoxy)iodobenzene-Mediated Oxidative C-H Amination of Imidazopyridines at Ambient Temperature. J. Org. Chem. 2017, 82, 4504–4510. 10.1021/acs.joc.7b00564. [DOI] [PubMed] [Google Scholar]; d Mishra S.; Monir K.; Mitra S.; Hajra A. FeCl3/ZnI2-Catalyzed Synthesis of Benzo[d]imidazo[2,1-b]thiazole through Aerobic Oxidative Cyclization between 2-Aminobenzothiazole and Ketone. Org. Lett. 2014, 16, 6084–6087. 10.1021/ol5028893. [DOI] [PubMed] [Google Scholar]; e Monir K.; Bagdi A. K.; Ghosh M.; Hajra A. Regioselective Oxidative Trifluoromethylation of Imidazoheterocycles via C(sp2)-H Bond Functionalization. J. Org. Chem. 2015, 80, 1332–1337. 10.1021/jo502928e. [DOI] [PubMed] [Google Scholar]; f Monir K.; Ghosh M.; Jana S.; Mondal P.; Majee A.; Hajra A. Regioselective Synthesis of Nitrosoimidazoheterocycles using tert-Butyl Nitrite. Org. Biomol. Chem. 2015, 13, 8717–8722. 10.1039/c5ob01345c. [DOI] [PubMed] [Google Scholar]
- a Amino Group Chemistry, from Synthesis to the Life Sciences; Ricci A., Ed.; Wiley-VCH: Weinheim, 2008. [Google Scholar]; b Hili R.; Yudin A. K. Making Carbon-Nitrogen Bonds in Biological and Chemical Synthesis. Nat. Chem. Biol. 2006, 2, 284–287. 10.1038/nchembio0606-284. [DOI] [PubMed] [Google Scholar]; c Hartwig J. F. Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res. 2008, 41, 1534–1544. 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Brasche G.; Buchwald S. L. CH Functionalization/CN Bond Formation: Copper-Catalyzed Synthesis of Benzimidazoles from Amidines. Angew. Chem., Int. Ed. 2008, 47, 1932–1934. 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; b Monnier F.; Taillefer M. Catalytic CC, CN, and CO Ullmann-Type Coupling Reactions. Angew. Chem., Int. Ed. 2009, 48, 6954–6971. 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]; c Park Y.; Kim Y.; Chang S. Transition Metal-Catalyzed C-H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]
- For selected reviews on direct C–H amination of arenes, see:; a Louillat M.-L.; Patureau F. W. Oxidative C-H Amination Reactions. Chem. Soc. Rev. 2014, 43, 901–910. 10.1039/c3cs60318k. [DOI] [PubMed] [Google Scholar]; b Sharma U.; Kancherla R.; Naveen T.; Agasti S.; Maiti D. Palladium-Catalyzed Annulation of Diarylamines with Olefins through C-H Activation: Direct Access to N-Arylindoles. Angew. Chem., Int. Ed. 2014, 53, 11895–11899. 10.1002/anie.201406284. [DOI] [PubMed] [Google Scholar]; c Sadhu P.; Punniyamurthy T. Copper(II)-Mediated Regioselective N-Arylation of Pyrroles, Indoles, Pyrazoles and Carbazole via Dehydrogenative Coupling. Chem. Commun. 2016, 52, 2803–2806. 10.1039/c5cc08206d. [DOI] [PubMed] [Google Scholar]; d Song C.; Yi H.; Dou B.; Li Y.; Singh A. K.; Lei A. Visible-Light-Mediated C2-Amination of Thiophenes by Using DDQ as Organophotocatalyst. Chem. Commun. 2017, 53, 3689–3692. 10.1039/c7cc01339f. [DOI] [PubMed] [Google Scholar]
- a Tran B. L.; Li B.; Driess M.; Hartwig J. F. Copper-Catalyzed Intermolecular Amidation and Imidation of Unactivated Alkanes. J. Am. Chem. Soc. 2014, 136, 2555–2563. 10.1021/ja411912p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shang M.; Sun S.-Z.; Dai H.-X.; Yu J.-Q. Cu(II)-Mediated C-H Amidation and Amination of Arenes: Exceptional Compatibility with Heterocycles. J. Am. Chem. Soc. 2014, 136, 3354–3357. 10.1021/ja412880r. [DOI] [PubMed] [Google Scholar]; c Gao Y.; Chen S.; Lu W.; Gu W.; Liu P.; Sun P. Visible Light-Induced C3-Sulfonamidation of Imidazopyridines with Sulfamides. Org. Biomol. Chem. 2017, 15, 8102–8109. 10.1039/c7ob02029e. [DOI] [PubMed] [Google Scholar]; d Sun K.; Li Y.; Zhang Q. Copper-Catalyzed Arenes Amination with Saccharins. Sci. China: Chem. 2015, 58, 1354–1358. 10.1007/s11426-015-5385-y. [DOI] [Google Scholar]
- a Allen L. J.; Cabrera P. J.; Lee M.; Sanford M. S. N-Acyloxyphthalimides as Nitrogen Radical Precursors in the Visible Light Photocatalyzed Room Temperature C-H Amination of Arenes and Heteroarenes. J. Am. Chem. Soc. 2014, 136, 5607–5610. 10.1021/ja501906x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rit R. K.; Shankar M.; Sahoo A. K. C-H imidation: a distinct perspective of C-N bond formation. Org. Biomol. Chem. 2017, 15, 1282–1293. 10.1039/c6ob02162j. [DOI] [PubMed] [Google Scholar]
- a Sun K.; Li Y.; Xiong T.; Zhang J.; Zhang Q. Palladium-Catalyzed C–H Aminations of Anilides withN-Fluorobenzenesulfonimide. J. Am. Chem. Soc. 2011, 133, 1694–1697. 10.1021/ja1101695. [DOI] [PubMed] [Google Scholar]; b Kawakami T.; Murakami K.; Itami K. Catalytic C-H Imidation of Aromatic Cores of Functional Molecules: Ligand-Accelerated Cu Catalysis and Application to Materials-and Biology-Oriented Aromatics. J. Am. Chem. Soc. 2015, 137, 2460–2463. 10.1021/ja5130012. [DOI] [PubMed] [Google Scholar]; c Boursalian G. B.; Ngai M.-Y.; Hojczyk K. N.; Ritter T. Pd-Catalyzed Aryl C-H Imidation with Arene as the Limiting Reagent. J. Am. Chem. Soc. 2013, 135, 13278–13281. 10.1021/ja4064926. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ito E.; Fukushima T.; Kawakami T.; Murakami K.; Itami K. Catalytic Dehydrogenative C–H Imidation of Arenes Enabled by Photo-generated Hole Donation to Sulfonimide. Chem 2017, 2, 383–392. 10.1016/j.chempr.2017.02.006. [DOI] [Google Scholar]
- Wang S.; Ni Z.; Huang X.; Wang J.; Pan Y. Copper-Catalyzed Direct Amidation of Heterocycles with N-Fluorobenzenesulfonimide. Org. Lett. 2014, 16, 5648–5651. 10.1021/ol502724u. [DOI] [PubMed] [Google Scholar]
- a Xie J.; Wang Y.-W.; Qi L.-W.; Zhang B. Access to Aminated Saturated Oxygen Heterocycles via Copper-Catalyzed Aminooxygenation of Alkenes. Org. Lett. 2017, 19, 1148–1151. 10.1021/acs.orglett.7b00182. [DOI] [PubMed] [Google Scholar]; b Li D.; Mao T.; Huang J.; Zhu Q. Copper-Catalyzed Regioselective 1,2-Thioamidation of Alkenes. Chem. Commun. 2017, 53, 3450–3453. 10.1039/c7cc00083a. [DOI] [PubMed] [Google Scholar]
- a Ni Z.; Zhang Q.; Xiong T.; Zheng Y.; Li Y.; Zhang H.; Zhang J.; Liu Q. Highly Regioselective Copper-Catalyzed Benzylic C-H Amination by N-Fluorobenzenesulfonimide. Angew. Chem. 2012, 124, 1270–1273. 10.1002/ange.201107427. [DOI] [PubMed] [Google Scholar]; b Xiong T.; Li Y.; Lv Y.; Zhang Q. Remote Amide-Directed Palladium-Catalyzed Benzylic C-H Amination with N-Fluorobenzenesulfonimide. Chem. Commun. 2010, 46, 6831–6833. 10.1039/c0cc02175j. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhang Q. N-Fluorobenzenesulfonimide: An Efficient Nitrogen Source for C-N Bond Formation. Synthesis 2015, 47, 159–174. 10.1055/s-0034-1379396. [DOI] [Google Scholar]; b Reddy C. R.; Prajapti S. K.; Ranjan R. Cu(I)-Catalyzed Aminative Aza-Annulation of Enynyl Azide using N-Fluorobenzenesulfonimide: Synthesis of 5-Aminonicotinates. Org. Lett. 2018, 20, 3128–3131. 10.1021/acs.orglett.8b01228. [DOI] [PubMed] [Google Scholar]
- a Yoshimura A.; Zhdankin V. V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. 10.1021/acs.chemrev.5b00547. [DOI] [PubMed] [Google Scholar]; b Merritt E. A.; Olofsson B. Diaryliodonium Salts: A Journey from Obscurity to Fame. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. 10.1002/anie.200904689. [DOI] [PubMed] [Google Scholar]; c Liu D.; Lei A. Iodine-Catalyzed Oxidative Coupling Reactions Utilizing C-H and X-H as Nucleophiles. Chem.—Asian J. 2015, 10, 806–823. 10.1002/asia.201403248. [DOI] [PubMed] [Google Scholar]
- a Kalbandhe A. H.; Kavale A. C.; Karade N. N. Ring-Opening Reaction of Imidazo[1,2-a]pyridines Using (Diacetoxyiodo)benzene and NaN3: The Synthesis of α-Iminonitriles. Eur. J. Org. Chem. 2017, 1318–1322. 10.1002/ejoc.201601480. [DOI] [Google Scholar]; b Manna S.; Matcha K.; Antonchick A. P. Metal-Free Annulation of Arenes with 2-Aminopyridine Derivatives: The Methyl Group as a Traceless Non-Chelating Directing Group. Angew. Chem., Int. Ed. 2014, 53, 8163–8166. 10.1002/anie.201403712. [DOI] [PubMed] [Google Scholar]; c Maiti S.; Bose A.; Mal P. Oxidative N-Arylation for Carbazole Synthesis by C-C Bond Activation. J. Org. Chem. 2018, 83, 8127–8138. 10.1021/acs.joc.8b00921. [DOI] [PubMed] [Google Scholar]
- a Manna S.; Serebrennikova P. O.; Utepova I. A.; Antonchick A. P.; Chupakhin O. N. Hypervalent Iodine(III) in Direct Oxidative Amination of Arenes with Heteroaromatic Amines. Org. Lett. 2015, 17, 4588–4591. 10.1021/acs.orglett.5b02320. [DOI] [PubMed] [Google Scholar]; b Kantak A. A.; Potavathri S.; Barham R. A.; Romano K. M.; DeBoef B. Metal-Free Intermolecular Oxidative C-N Bond Formation via Tandem C-H and N-H Bond Functionalization. J. Am. Chem. Soc. 2011, 133, 19960–19965. 10.1021/ja2087085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Wang Y.; Guo Z.; Zhang Q.; Li D. Metal-Free Oxidative C-H Amination of 8-Acylaminoquinolines and Anilides with N-Fluorobenzenesulfonimide. Asian J. Org. Chem. 2016, 5, 1438–1441. 10.1002/ajoc.201600389. [DOI] [Google Scholar]
- Further information can be found in the CIF file. This crystal was deposited in the Cambridge Crystallographic Data Centre and assigned as CCDC 1846489.
- a Xiong T.; Zhang Q. New Amination Strategies Based on Nitrogen-Centered Radical Chemistry. Chem. Soc. Rev. 2016, 45, 3069–3087. 10.1039/c5cs00852b. [DOI] [PubMed] [Google Scholar]; b Xiang D.; Xia L.; Zhang Y.; Zhang Q.; Li D. Remote Oxidative C–H Amidation of Anilides with Dibenzenesulfonimides under Metal-Free Conditions. Synlett 2018, 29, 1400–1404. 10.1055/s-0036-1591970. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.