Abstract

The possible mechanisms of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)-catalyzed chemoselective insertion of N-methyl isatin into aryl difluoronitromethyl ketone to synthesize 3,3-disubstituted and 2,2-disubstituted oxindoles have been studied in this work. As revealed by calculated results, the reaction occurs via two competing paths, including α and β carbonyl paths, and each path contains five steps, that is, nucleophilic addition of DBU to ketone, C–C bond cleavage affording difluoromethylnitrate anion and phenylcarbonyl–DBU cation, nucleophilic addition of difluoromethylnitrate anion to carbonyl carbon of N-methyl isatin, acyl transfer process, and dissociation of DBU and product. The computational results suggest that nucleophilic additions on different carbonyl carbons of N-methyl isatin via α and β carbonyl paths would lead to different products in the third step, and β carbonyl path associated with the main product 3,3-disubstituted oxindole is more energetically favorable, which is consistent with the experimental observations. Noteworthy, electrophilic Parr function can be successfully applied for exactly predicting the activity of reaction site and reasonably explaining the chemoselectivity. In addition, the distortion/interaction and noncovalent interaction analyses show that much more hydrogen bond interactions should be responsible for the lower energy of the transition state associated with β carbonyl path. The obtained insights would be valuable for the rational design of efficient organocatalysts for this kind of reactions with high selectivities.
1. Introduction
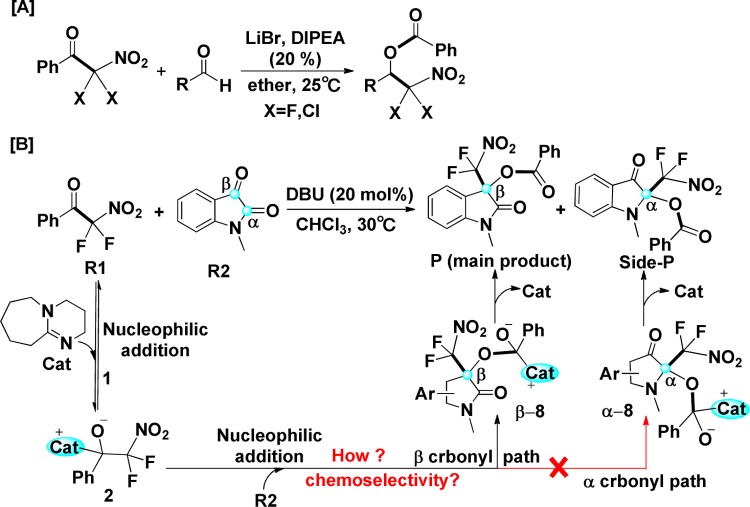
In the past decade, the development of synthetic methodologies for multiple fluorinated nitroalkanes, which have the advantages of general availability, high synthetic versatility, and a wide range of applications in pharmaceutical and agrochemical fields, has received widespread attention.1−11 Bissell first prepared difluoronitromethane, which was carefully isolated at very low temperature by thermal decomposition of difluoronitroacetamidine.12 After that, there are a few reports on the C–C bond formation with difluoronitromethane.13−15 Until 2016, Wolf and coauthors, who had their longstanding interest in aldol reactions with nitroalkanes16−19 and fluorinated enolates,8,20,21 reported a new synthetic methodology to prepare dihalonitronates from aryl dihalonitromethyl ketones catalyzed by LiBr/N(i-Pr)2Et (depicted in Scheme 1A).22 Although they contributed many efforts to explore the reaction mechanism by using Fourier transform infrared spectroscopy and NMR analyses, the detailed mechanism of the Lewis acid LiBr/N(i-Pr)2Et-catalyzed insertion reaction and the role of catalyst have still been not very clear by now.
Scheme 1. (A) LiBr/Amine-Catalyzed and (B) DBU-Catalyzed Insertions.
LiBr/amine-catalyzed insertion of aldehyde into aryl dihalonitromethyl ketone. DBU-catalyzed insertion of N-methyl isatin into aryl difluoronitromethyl ketone, and its possible mechanism.
More recently, Wolf et al. reported a Lewis base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)-catalyzed insertion of isatins into aryl difluoronitromethyl ketones to give difluoronitromethylated oxindoles with high yields and 100% atom economy using chloroform as solvent under mild conditions (depicted in Scheme 1B).23 Similarly, this reaction might also follow on a C–C bond cleavage–aldol reaction–acyl transfer sequence. As depicted in Scheme 1B, some efforts had been made by Wolf’s group to speculate the possible catalytic mechanism, and the experimental explorations of this kind of novel insertion reactions are quite useful and constructive, but there are still some key issues that need to be solved: (1) Why does the catalyst DBU nucleophilically attack the carbonyl carbon of aryl difluoronitromethyl ketone (R1) rather than those of isatin (R2)? (2) How does the catalyst promote the breaking of C–C bond in ketone (R1)? (3) What is the role of DBU catalyst? (4) Why is the main product P rather than Side-P depicted in Scheme 1B? (5) What are the key factors for controlling the chemoselectivity of this reaction? To the best of our knowledge, no related theoretical investigation on this kind of organocatalytic insertion of isatins into ketones has been reported to date. The above questions and our interest in organocatalysis24−33 have promoted us to pursue a density functional theory (DFT) study on the title reaction. In this theoretical work, we not only investigated the detailed mechanisms of the catalytic reaction, but also explored the factors that govern the chemoselectivity of this kind of reaction. Moreover, we would predict and validate which carbonyl carbon of reactants would be the best electrophilic sites by using electrophilic and nucleophilic Parr function analysis. We believe that this study will be useful for understanding the organocatalytic insertion reactions, and it provides valuable insights for recognizing the key role of catalyst and origin of chemoselectivity.
In the present study, as illustrated in Scheme 1B, the insertion of N-methyl isatin into aryl difluoronitromethyl ketone generating difluoronitromethylated oxindole was selected as the reaction model to investigate the detailed mechanism and the origin of chemoselectivity for this kind of reaction. The reactants aryl difluoronitromethyl ketone and N-methyl isatin, the catalyst DBU, and the possible products 3-difluoronitromethyl-3-phenylcarboxyl-2-oxindole and 2-difluoronitromethyl-2-phenylcarboxyl-2-oxindole were denoted as R1, R2, Cat, P, and Side-P, respectively.
2. Results and Discussion
2.1. Reaction Mechanisms
As shown in Scheme 2, we suggested and studied two possible competing reaction paths, including α and β carbonyl paths, for the entire catalytic insertion of N-methyl isatin into aryl difluoronitromethyl ketone. It can be seen that both these paths contain five steps. The first step is the nucleophilic addition of Cat to R1, affording intermediate 2. The second step is C2–C4 bond cleavage of 2, forming intermediate 4, in which difluoromethylnitrate anion is dissociated with phenylcarbonyl–DBU cation. Then, α carbonyl path diverges with β carbonyl path in the following three steps, which is because of the existence of two different reactive sites (carbonyl carbons) in R2. In the third step, nucleophilic additions on the different carbonyl carbons of R2 by the difluoromethylnitrate anion afford intermediates α-6 and β-6 via α and β carbonyl paths, respectively. The fourth step is the acyl transfer to the different carbonyl oxygen forming intermediates α-8 and β-8. The fifth step is the regeneration of catalyst Cat, which is accompanied with the dissociation of possible products P and Side-P. The associated energy profiles are shown in Figure 1, in which the Gibbs free energies (GFEs) of R1 + R2 + Cat as 0.00 kcal/mol were set as references and the geometries of Cat, R1, and R2 were optimized separately. Figure 2 depicts the optimized structures of all of the stationary points in the reaction. The combined energy profiles of the insertion reactions and the optimized geometries can be found in Figure S1 of Supporting Information.
Scheme 2. Possible Mechanisms for the Catalytic Reaction.
Figure 1.
Energy profiles of the DBU-catalyzed insertion reaction. All of the energies were calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level of theory.
Figure 2.
Optimized geometries of all of the stationary points involved in the catalytic reaction (distance in angstrom, all of the hydrogens were omitted for the sake of clarity). All of the geometries were optimized at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level.
Actually, as shown in Scheme 2, both reactants R1 and R2 have carbonyl groups, so which carbonyl carbon is in the most favorable position for nucleophilic attack by catalyst Cat remains unclear. To solve this question, electrophilic and nucleophilic Parr functions (Pk+ and Pk–)34 have been calculated and are summarized in Table 1. The electrophilic and nucleophilic Parr functions (Pk+ and Pk–, respectively) were obtained through the analysis of the Mulliken atomic spin density of the radical anion and the radical cation by single-point energy calculations over the optimized neutral geometries for radical species at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level. The computed results revealed that the nucleophilic Parr functions (Pk–) of C2, C6, and C8 atoms are similar; however, the value of the electrophilic Parr function (Pk+) of C2 atom in R1 (0.31) is obviously higher than the corresponding values of C6 (0.10) and C8 (0.22) atoms in R2, indicating that C2 atom of R1 has the strongest electrophilicity among the three carbonyl carbons. So, we thought the reactant R1 would like to act as the initial electrophile and was absorbed easily by the nucleophilic catalyst Cat. That is to say, C8 atom should be nucleophilically attacked more easily than C6 atom in R2. For validating the prediction of local activities of reactants by using electrophilic Parr functions, we have considered and investigated the possible and competing pathways in a step-by-step fashion as follows.
Table 1. Electrophilic and Nucleophilic Parr Functions (Pk+ and Pk–, Respectively) at the Atoms (C2, C6, and C8) in Reactants R1 and R2.
| R1 |
R2 |
||
|---|---|---|---|
| Parr function | C2 | C6 | C8 |
| Pk+ | 0.31a | 0.10 | 0.22 |
| Pk– | –0.01 | –0.07 | –0.02 |
All of the electrophilic and nucleophilic Parr functions (Pk+ and Pk–) were calculated at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level of theory.
2.1.1. First Step: Nucleophilic Addition of Cat to R1
As depicted in Scheme 2, because both Cat and R1 do not have a chiral center, the nucleophilic attack of Cat on re or si face of R1 can generate R or S configurational intermediates, which should be a pair of enantiomers that have the same energy in theory; thus, we only considered the attack of Cat on one of the prochiral faces of R1. Similarly, we have only considered a nucleophilic attack on one of the prochiral faces of R2 by Cat. Initially, the reaction precursor 0 is formed by weak interactions between Cat and R1 and then the N1 atom in Cat can nucleophilically attack C2 atom of R1 via transition state 1, generating intermediate 2. The energy barrier via 1 is 12.7 kcal/mol, and the relative Gibbs free energy of 2 is 12.4 kcal/mol, suggesting that this step is an endothermic process and 2 is not very stable. Noteworthy, the dihedral angle O2–C3–C4–N5 may change in the reaction process, so four possible conformations have been investigated and compared in the Supporting Information to confirm that the lowest energy conformation was selected in the energy profile.
As mentioned above, the N1 atom in Cat may also nucleophilically attack C6 and C8 atoms in R2, so we have tried but failed to locate both the corresponding intermediates and transition states for the formations of N1–C6 and N1–C8 bonds.35 To explain the experimental equilibrium between difluoronitroacetophenone and difluoronitroacetophenone–DBU adduct (1.2:1), the ΔΔG between them was calculated to be 0.1 kcal/mol, according to the Boltzmann distribution equation. As shown in Figure 1, the energies of possible difluoronitroacetophenone–DBU complexes (i.e., precursor 0 and intermediate 2) locate 3.5 and 12.4 kcal/mol above that of reactant, respectively. The energy difference between the reactant and precursor 0 (3.5 kcal/mol), which is calculated to be 1.5 kcal/mol at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level and 3.5 kcal/mol at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level, is close to the ΔΔG based on the experiment, so we think the calculated results should be reasonable.
2.1.2. Second Step: C2–C4 Bond Cleavage
The second step is the C2–C4 bond cleavage, in which 2 is dissociated into two parts, including difluoronitromethyl anion and phenylcarbonyl–DBU cation. As presented in Scheme 2, the single bond in 2 can be directly broken between C2 and C4 atoms via transition state 3, affording intermediate 4. Furthermore, as shown in Scheme 2, we have considered another possible pathway, in which 2 can be structurally transformed to intermediate β-6 via an SN2 type of transition state β-3N2. As shown in Figure 1, the energy barrier via 3 (24.4 kcal/mol) is remarkably lower than that via β-3N2 (33.2 kcal/mol), indicating that the pathway associated with β-3N2 can be safely excluded.
2.1.3. Third Step: Nucleophilic Addition of Difluoromethylnitrate Anion to R2
The third step is the nucleophilic addition of difluoromethylnitrate anion on carbonyl carbon of R2. As discussed above, there are two carbonyl carbons in R2 that can be nucleophilically attacked by nitro carbon of difluoromethylnitrate anion, so the following reaction divides into two possible α and β carbonyl paths. As shown in Scheme 2, because both participated parts (i.e., difluoromethylnitrate anion and reactant R2) have no chiral center, obviously, the nucleophilic attack on the re and si face of C6=O7 and C8=O9 carbonyl groups of R2 by difluoromethylnitrate anion would generate a pair of R and S configurational enantiomers with the same energy in theory. Thus, we only need to consider the nucleophilic attack on one of the prochiral faces of R2. During this process, the intermediates α-6 and β-6 were obtained by the nucleophilic attack of C4 atom in difluoromethylnitrate anion on C6 and C8 atoms in R2 via their corresponding transition states α-7 and β-7, which results in chemoselectivity of the reaction.
In this step, a single bond between C4 atom and C6/C8 atom is formed in α-6/β-6. As shown in Scheme 2, the C6=O7 double bond lengthens to C6–O7 single bond in α-6, whereas the C8=O9 double bond changes to C8–O9 single bond in β-6. Notably, the different chemoselectivities were initiated in this step, meaning this process would be the key for chemoselectivity of the reaction. As depicted in Figure 1, the energy barriers via α-5 and β-5 are 3.7 and 0.4 kcal/mol, respectively, with respect to 4, and the relative Gibbs free energies of α-6 and β-6 are 18.7 and 4.8 kcal/mol, respectively, indicating that β-6 is more stable than α-6 in thermodynamics. The energy of β-5 is significantly lower (∼3.3 kcal/mol) than that of α-5, and the energy of β-6 is extremely lower (∼13.9 kcal/mol) than that of α-6. Therefore, the β carbonyl path is more energetically favorable than the α carbonyl path, which is consistent with the prediction of electrophilic Parr function (Pk+) analysis results, that is, C8 atom can be nucleophilically attacked more easily than C6 atom in R2.
2.1.4. Fourth Step: Acyl Transfer Process
The next reaction process is the acyl transfer from the phenylcarbonyl–DBU cation to the two different carbonyl oxygens (O7 and O9 atoms) for the formation of zwitterion intermediates α-8 and β-8 via α and β carbonyl paths, respectively. As shown in Scheme 2, a negative charge center is formed at O7 and O9 atoms in α-6 and β-6, respectively, which facilitates the nucleophilic attack on C2 atom via transition states α-7 and β-7, affording intermediates α-8 and β-8, respectively. The natural bond orbital (NBO) analysis has been conducted for the key atoms during this process. As summarized in Table 2, both the charge values of O7 and O9 atoms are significantly reduced in α-6/β-6, indicating that the negative charge centers would be formed in this step. As depicted in Figure 1, the energy barriers associated with α-7 and β-7 are 7.9 and 3.8 kcal/mol, corresponding to α-6 and β-6, respectively, and the energies of α-8 and β-8 are located 20.7 and 4.5 kcal/mol above those of reactants, respectively, indicating that the β carbonyl path is more energetically favorable than α carbonyl path during this step.
Table 2. Values of NBO Charges (Units of e) on the Key Atoms of O7 and O9 in Reactant R2 and Intermediates α-6 and β-6.
| O7 | O9 | |
|---|---|---|
| R2 | –0.606a | –0.524 |
| α-6 | –0.924 | –0.597 |
| β-6 | –0.692 | –0.912 |
All of the NBO charges were calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level.
2.1.5. Fifth Step: Regeneration of Cat
As presented in Scheme 2, the last step of this reaction is the dissociation of catalyst Cat and the possible products P/Side-P via transition states α-9/β-9, leading to the regeneration of the catalyst. As calculated by single-point energies, the energy barriers without any corrections associated with α-9 and β-9 are only 0.6 and 0.7 kcal/mol, respectively, indicating that the two processes can occur smoothly under the experimental condition. As can be seen in Figure 1, the energy differences between α-9/β-9 and α-8/β-8 are separately −1.2/–0.1 kcal/mol, indicating that the two processes are barrierless. As concluded as above, the β carbonyl path is more energetically preferred than the α carbonyl path and the second step (via 3) in the β carbonyl path has the highest energy barrier, indicating that the C2–C4 bond cleavage is the rate-determining step.
2.2. Role of the Catalyst
The previous theoretical work has provided evidence that Lewis base can not only act as base but also work as nucleophile.28,31,32,36−38 To confirm the role of Lewis base DBU, we have performed global reactivity index (GRI) analysis of the related reactants and intermediates. Table 3 provides the electrophilicity index ω,39,40 electronic chemical potential μ, chemical hardness η, energies of highest occupied molecular orbital (HOMO) (EH) and lowest unoccupied molecular orbital (LUMO) (EL), and nucleophilicity index N.34,41−44 The nucleophilicity index N, which is based on tetracyanoethylene (TCNE) taken as a reference, is defined as N = EHOMO(2) – EHOMO(TCNE). As summarized in Table 3, the electrophilicity of ketone R1 is dramatically decreased after the absorption of catalyst, but its nucleophilicity is remarkably increased from 1.145 to 2.279 eV in 2 and finally to 3.328 eV in 4. Therefore, the GRI analysis demonstrates that the DBU catalyst mainly works as Lewis base to strengthen the nucleophilicity of R1, which would further facilitate the nucleophilic attack on the carbonyl carbons of R2 in the following processes.
Table 3. Energies of HOMO (EH, au) and LUMO (EL, au), Electronic Potential (μ, au), Chemical Hardness (η, eV), Global Electrophilicity (ω, eV), and Global Nucleophilicity (N, eV) of Reactant R1 and Intermediates 2 and 4.
| EH (au) | EL (au) | μ (au) | η (au) | ω (eV) | N (eV) | |
|---|---|---|---|---|---|---|
| R1 | –0.33619a | –0.06971 | –0.20295 | 0.26648 | 2.103 | 1.145 |
| 2 | –0.29451 | –0.03270 | –0.16361 | 0.26181 | 1.391 | 2.279 |
| 4 | –0.25594 | –0.03500 | –0.14547 | 0.22094 | 1.303 | 3.328 |
All of the data were calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level of theory.
To verify the fact that DBU shows the highest catalytic efficiency than the other Lewis bases for this insertion reaction in experiments, we have computed the reactions catalyzed by other two Lewis bases (e.g., 2,6-lutidine and 4-dimethylaminopyridine (DMAP)) to investigate and compare the relative energy barriers of the rate-determining step (Table 4). As summarized in Table 4, the energy barrier via transition state 3DBU (24.4 kcal/mol) is obviously lower than that via transition states 32,6-lutidine (46.3 kcal/mol) and 3DMAP (26.9 kcal/mol). The extremely high energy barrier (46.3 kcal/mol) indicates that the insertion reaction catalyzed by 2,6-lutidine is impossible, and the insertion reaction catalyzed by DMAP can occur probably under mild condition but with the lower efficiency relative to that catalyzed by DBU, which is well consistent with the experimental observations.23 Furthermore, we have predicted the efficiency of some new catalysts in theory, and the results are referenced in Supporting Information.
Table 4. Relative Gibbs Free Energies (kcal/mol) of Transition States 3DBU, 32,6-lutidine, and 3DMAP.
The energy barrier was calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level of theory.
The energy barriers were calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMdichloromethane//M06-2X/6-31G(d, p)/IEF-PCMdichloromethane level of theory.
In addition, Wiberg bond order analyses on N1–C2, C2–O3, and C2–C4 bonds in R1 and 2 have been performed on the basis of NBO analysis at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level using the software of Mutiwfn,45 and the computed data are provided in Table 5. As summarized in Table 5, the bond order of C2–C4 slightly reduces from 0.95 in R1 to 0.92 in 2, whereas the bond length increases from 1.55 to 1.57 Å. The bond order of C2–O3 reduces significantly from 2.63 in R1 to 2.22 in 2, whereas the bond length increases from 1.21 to 1.28 Å, suggesting that both C2–O3 and C2–C4 bonds weakened after the absorption of the catalyst. As concluded as above, the DBU catalyst can not only act as the nucleophile, but also weaken the bond order to promote the C–C bond cleavage in the next process.
Table 5. Wiberg Bond Order Analyses on the N1–C2, C2–O3, and C2–C4 Bonds of Reactant R1 and Intermediate 2.
| N1–C2 | C2–O3 | C2–C4 | |
|---|---|---|---|
| R1 | 2.63 (1.21 Å)a | 0.95 (1.55 Å) | |
| 2 | 0.84 (1.64 Å) | 2.22 (1.28 Å) | 0.92 (1.57 Å) |
The distance within parentheses refers to the bond length. All of the Wiberg bond orders were calculated at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level of theory.
2.3. Origin of Chemoselectivity
To disclose the origin of chemoselectivity from a theoretical perspective is of great value for experimental scientists to design the reactions with high selectivities. In the competing α and β carbonyl paths, nucleophilic center (C4 atom) of difluoromethylnitrate anion attacks on one of the electrophilic sites (C6 or C8 atoms) of R2 in the third step; therefore, the stabilities of the relevant transition states (α-5/β-5) would be important for explaining chemoselectivity of the reaction. The distortion/interaction analysis of α-5 and β-5 has been carried out to value the preference of β-5. As summarized in Table 6, the distortion energy (ΔE‡dist) of β-5 (141.8 kcal/mol) is slightly larger (1.7 kcal/mol) than that of α-5 (140.1 kcal/mol), whereas the interaction energy (ΔE‡int) of β-5 (−121.1 kcal/mol) is significantly lower (5.0 kcal/mol) than that of α-5 (−116.1 kcal/mol), indicating that the stronger interaction would be responsible for the energy favorability of β-5.
Table 6. Distortion/Interaction Analysis for the Chemoselectivity-Determining Stepa.
| ΔE‡dist |
||||||
|---|---|---|---|---|---|---|
| ΔΔG‡ | ΔE‡dist (Cat) | ΔE‡dist (R1) | ΔE‡dist (R2) | ΔE‡act | ΔE‡int | |
| α-5 | 3.3b | 8.4 | 127.2 | 4.5 | 24.0 | –116.2 |
| β-5 | 0 | 9.5 | 127.9 | 4.4 | 20.7 | –121.1 |
All of the values are in kcal/mol.
All of the data were calculated at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level.
For further understanding the preference of β-5, noncovalent interaction (NCI) analyses of α-5/β-5 were then performed to explore the effects of their existing interactions, including strong interactions, van der Waals interactions, and repulsive steric interactions. As Figure 3 shows, β-5 has more hydrogen bond interactions and less repulsive steric hindrances than α-5, indicating that the hydrogen bond interactions play critical roles in the determination of the chemoselectivity of this reaction. As concerned as above, the NCI analysis would be a powerful tool for exploring the origin of chemoselectivity and thus provide some valuable insights for experimental chemists on rational design of this kind of reactions with high selectivities.
Figure 3.
NCI analyses of the key transition states β-5 and α-5 computed at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level (blue, green, and red represent strong interaction, weak interaction, and steric hindrance, respectively).
3. Conclusions
In this work, DFT calculations have been performed to investigate the possible mechanisms and chemoselectivities of the synthesis of functionalized difluoronitromethylated oxindole, utilizing the N-methyl isatin and aryl difluoronitromethyl ketone as substrates catalyzed by DBU. Two possible paths, including α and β carbonyl paths, were studied in detail. The computed results suggested that the β carbonyl path is more energetically favorable than the α carbonyl path and the C–C bond cleavage is the rate-determining step, thereby leading to P as the main product, which is in accord with the experimental observations. The favorable path contains five steps, that is, nucleophilic addition of Cat to the carbonyl carbon of R1, C–C bond cleavage for the formation of difluoromethylnitrate anion and phenylcarbonyl–DBU cation, nucleophilic addition of difluoromethylnitrate anion to β carbonyl carbon of R2, acyl transfer process, and the regeneration of Cat coupled with the formation of product P.
The computational results indicate that the third step is the key for the chemoselectivity, and the distortion/interaction and NCI analyses demonstrate that the chemoselectivity has been significantly affected by the hydrogen bond interactions. Interestingly, the convenient calculations of Parr function would be successfully used for predicting the chemoselectivity of this kind of reactions, which gives a new clue for rational design and pathway searches for the electrophilic and nucleophilic reactions. This work would provide some valuable insights for theoretical and experimental investigations into such kind of organocatalytic chemoselective insertion reaction in future.
4. Computational Details
All of the theoretical calculations were carried out in the Gaussian 09 suite of programs.46 All of the stationary points were optimized by DFT method, which has been proved to be a powerful tool for clarifying the detailed reaction mechanisms in enzymes,47−51 transition metals,30,52−58 organocatalysts27−33,36,53,59−68 catalyzed reactions, and other theoretical studies.69−76 All of the species were optimized with M06-2X77−79 density functional and 6-31G(d, p) basis set in chloroform solvent using the integral equation formalism polarizable continuum model (IEF-PCM).80,81 Harmonic vibrational frequency calculations were performed at the same level of theory as that used for geometry optimizations to provide thermal corrections of Gibbs free energies and to make sure that the local minima had no imaginary frequencies, whereas the saddle points had only one imaginary frequency. Intrinsic reaction coordinates82,83 were calculated to confirm that the transition-state structure connected the correct reactant and product. The single-point energies accompanied with natural bond orbital (NBO) analyses of all of the species have been computed at the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform level. Noncovalent interaction (NCI) analysis was plotted using Multiwfn (version 3.3.8).84 The optimized structures were rendered using the CYLview software.85 The calculated results were discussed above on the basis of the relative Gibbs free energies (GFE) attributed by the addition of Gibbs free energy corrections at the M06-2X/6-31G(d, p)/IEF-PCMchloroform level to the single-point energies at the M06-2X-D386/6-311++G(2df, 2pd)/IEF-PCMchloroform level, which is denoted as the M06-2X-D3/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform level.
To test the accuracy of the selected method, we have performed additional calculations at the M06-2X/6-311++G(2df, 2pd)/IEF-PCMchloroform//M06-2X/6-31G(d, p)/IEF-PCMchloroform and ωB97X-D87/6-311++G(2df, 2pd)/IEF-PCMchloroform//ωB97X-D/6-31G(d, p)/IEF-PCMchloroform levels. The computed results indicate that there are small differences in energy and structures obtained by using the different methods, and more details can be found in the Supporting Information.
In the distortion/interaction analysis,88−91 the activation energy (ΔE‡act) of transition state is attributed by two main components: distortion (ΔE‡dist) and interaction (ΔE‡int) energy. The distortion energy composed of geometric and electronic changes is generally used to value the deformation of reactants (DBU + R1 + R2) within transition-state geometry, and the interaction energy contains repulsive and stabilizing electrostatic, polarization, and orbital effects in the transition-state structure, which is recovered by the relationship: ΔE‡int = ΔE‡act – ΔE‡dist.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21303167 and 21773214), the China Postdoctoral Science Foundation (Nos. 2013M530340 and 2015T80776), and the Outstanding Young Talent Research Fund of Zhengzhou University (No. 1521316001).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00907.
Combined energy profiles of the insertion reactions and the optimized geometries (1), energy scans along with the changes of N1–C6 and N1–C8 distances (2), different conformations of 1 and 2 (3), design of DBU catalysts (4), test of DFT methods (5), the two reaction models (6), the protocol used to generate NCI plot (7), absolute single-point energies and GFE of all of the optimized structures/transition states in models 1/2 (8/9), list of all of the vibrational frequencies of the optimized structures in model 1 (10), and list of the Cartesian coordinates of the optimized structures (11) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rieck H.; Helmchen G. Palladium complex catalyzed asymmetric allylic substitutions with nitromethane: Enantioselectivities exceeding 99.9% ee. Angew. Chem., Int. Ed. 1996, 34, 2687–2689. 10.1002/anie.199526871. [DOI] [Google Scholar]
- Trost B. M.; Surivet J. P. Asymmetric alkylation of nitroalkanes. Angew. Chem., Int. Ed. 2000, 39, 3122–3124. . [DOI] [PubMed] [Google Scholar]
- Seebach D.; Lehr F. α,α-Doubly Deprotonated Nitroalkanes. Enhancement of the C-Nucleophilicity of Nitronates. Angew. Chem., Int. Ed. 1976, 15, 505–506. 10.1002/anie.197605051. [DOI] [Google Scholar]
- Vogl E. M.; Buchwald S. L. Palladium-catalyzed monoarylation of nitroalkanes. J. Org. Chem. 2002, 67, 106–111. 10.1021/jo010953v. [DOI] [PubMed] [Google Scholar]
- Katritzky A. R.; Kashmiri M. A.; De Ville G. Z.; Patel R. C. Kinetics and mechanism of the C-alkylation of nitroalkane anions by 1-alkyl-2, 4, 6-triphenylpyridiniums: a nonchain reaction with radicaloid characteristics. J. Am. Chem. Soc. 1983, 105, 90–96. 10.1021/ja00339a016. [DOI] [Google Scholar]
- Gildner P. G.; Gietter A. A. S.; Cui D.; Watson D. A. Benzylation of nitroalkanes using copper-catalyzed thermal redox catalysis: toward the facile C-alkylation of nitroalkanes. J. Am. Chem. Soc. 2012, 134, 9942–9945. 10.1021/ja304561c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T.; Neumann C. N.; Ritter T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Wolf C. Catalytic Enantioselective Difluoroalkylation of Aldehydes. Angew. Chem., Int. Ed. 2013, 52, 7869–7873. 10.1002/anie.201303551. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wu T.; Phipps R. J.; Toste F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015, 115, 826–870. 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H. B.; Xie C.; Aceña J. L.; Soloshonok V. A.; Röschenthaler G. V.; Han J. L. Recent Progress in the in situ Detrifluoroacetylative Generation of Fluoro Enolates and Their Reactions with Electrophiles. Eur. J. Org. Chem. 2015, 2015, 6401–6412. 10.1002/ejoc.201500787. [DOI] [Google Scholar]
- Fugard A. J.; Thompson B. K.; Slawin A. M. Z.; Taylor J. E.; Smith A. D. Organocatalytic Synthesis of Fused Bicyclic 2,3-Dihydro-1,3,4-oxadiazoles through an Intramolecular Cascade Cyclization. Org. Lett. 2015, 17, 5824–5827. 10.1021/acs.orglett.5b02997. [DOI] [PubMed] [Google Scholar]
- Bissell E. R. Fluorine-Containing Nitrogen Compounds. V. Difluoronitroacetamidines and Difluoronitromethyl-1, 2, 4-triazoles1, 2. J. Org. Chem. 1963, 28, 1717–1720. 10.1021/jo01041a516. [DOI] [Google Scholar]
- Butler P.; Golding B. T.; Laval G.; Loghmani-Khouzani H.; Ranjbar-Karimi R.; Sadeghi M. M. Fluorination and chlorination of nitroalkyl groups. Tetrahedron 2007, 63, 11160–11166. 10.1016/j.tet.2007.08.020. [DOI] [Google Scholar]
- West T. H.; Spoehrle S. S. M.; Kasten K.; Taylor J. E.; Smith A. D. Catalytic Stereoselective [2,3]-Rearrangement Reactions. ACS Catal. 2015, 5, 7446–7479. 10.1021/acscatal.5b02070. [DOI] [Google Scholar]
- Attaba N.; Taylor J. E.; Slawin A. M. Z.; Smith A. D. Enantioselective NHC-Catalyzed Redox [4 + 2]-Hetero-Diels–Alder Reactions Using α,β-Unsaturated Trichloromethyl Ketones as Amide Equivalents. J. Org. Chem. 2015, 80, 9728–9739. 10.1021/acs.joc.5b01820. [DOI] [PubMed] [Google Scholar]
- Xu H. H.; Wolf C. Asymmetric Synthesis of Chiral 1, 3-Diaminopropanols: Bisoxazolidine-Catalyzed C-C Bond Formation with α-Keto Amides. Angew. Chem., Int. Ed. 2011, 50, 12249–12252. 10.1002/anie.201105778. [DOI] [PubMed] [Google Scholar]
- Xu H.; Wolf C. Synthesis of chiral tertiary trifluoromethyl alcohols by asymmetric nitroaldol reaction with a Cu (II)-bisoxazolidine catalyst. Chem. Commun. 2010, 46, 8026–8028. 10.1039/c0cc02378g. [DOI] [PubMed] [Google Scholar]
- Spangler K. Y.; Wolf C. Asymmetric copper (I)-catalyzed Henry reaction with an aminoindanol-derived bisoxazolidine ligand. Org. Lett. 2009, 11, 4724–4727. 10.1021/ol9018612. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wolf C. Asymmetric nitroaldol reaction catalyzed by a C2-symmetric bisoxazolidine ligand. Org. Lett. 2008, 10, 1831–1834. 10.1021/ol800442s. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Wolf C. Synthesis of Pentafluorinated β-Hydroxy Ketones. J. Org. Chem. 2012, 77, 8840–8844. 10.1021/jo3017583. [DOI] [PubMed] [Google Scholar]
- Balaraman K.; Moskowitz M.; Liu Y.; Wolf C. Detrifluoroacetylative Generation of Halogenated Enolates: Practical Access to Perhalogenated Ketones and Alkenes. Synthesis 2016, 48, 2376–2384. 10.1055/s-0035-1561433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R.; Wolf C. Catalytic insertion of aldehydes into dihalonitroacetophenones via sequential bond scission-aldol reaction-acyl transfer. Chem. Commun. 2016, 52, 3576–3579. 10.1039/C5CC09753C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R.; Bakhshi P. R.; Wolf C. Organocatalytic Insertion of Isatins into Aryl Difluoronitromethyl Ketones. J. Org. Chem. 2017, 82, 1273–1278. 10.1021/acs.joc.6b02704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Zhu Y.; Zhang W.; Wei D.; Ran Y.; Zhao Q.; Tang M. A DFT study on the reaction mechanism of dimerization of methyl methacrylate catalyzed by N-heterocyclic carbene. Phys. Chem. Chem. Phys. 2014, 16, 20001–20008. 10.1039/C4CP02186J. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wei D.; Wang Y.; Zhu Y.; Tang M. DFT study on the mechanisms and stereoselectivities of the [4 + 2] cycloadditions of enals and chalcones catalyzed by N-heterocyclic carbene. J. Org. Chem. 2014, 79, 3069–3078. 10.1021/jo500194d. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wei D. H.; Li Z. Y.; Zhu Y. Y.; Tang M. S. DFT Study on the Mechanisms and Diastereoselectivities of Lewis Acid-Promoted Ketene–Alkene [2 + 2] Cycloadditions: What is the Role of Lewis Acid in the Ketene and C= X (X= O, CH2, and NH) [2 + 2] Cycloaddition Reactions?. J. Phys. Chem. A 2014, 118, 4288–4300. 10.1021/jp500358m. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Guo X. K.; Tang M. S.; Wei D. H. Theoretical Investigations toward the Asymmetric Insertion Reaction of Diazoester with Aldehyde Catalyzed by N-Protonated Chiral Oxazaborolidine: Mechanisms and Stereoselectivity. J. Phys. Chem. A 2015, 119, 8422–8431. 10.1021/acs.jpca.5b04793. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zheng L.; Wei D.; Tang M. A quantum mechanical study of the mechanism and stereoselectivity of the N-heterocyclic carbene catalyzed [4 + 2] annulation reaction of enals with azodicarboxylates. Org. Chem. Front. 2015, 2, 874–884. 10.1039/C5QO00121H. [DOI] [Google Scholar]
- Wang Y.; Wu B. H.; Zhang H. Y.; Wei D. H.; Tang M. S. A computational study on the N-heterocyclic carbene-catalyzed Csp2–Csp3 bond activation/[4 + 2] cycloaddition cascade reaction of cyclobutenones with imines: a new application of the conservation principle of molecular orbital symmetry. Phys. Chem. Chem. Phys. 2016, 18, 19933–19943. 10.1039/C6CP03180C. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wu B. H.; Zheng L. J.; Wei D. H.; Tang M. S. DFT perspective toward [3 + 2] annulation reaction of enals with α-ketoamides through NHC and Brønsted acid cooperative catalysis: mechanism, stereoselectivity, and role of NHC. Org. Chem. Front. 2016, 3, 190–203. 10.1039/C5QO00338E. [DOI] [Google Scholar]
- Zhang X.; Tang M.; Wang Y.; Ran Y. Y.; Wei D. H.; Zhu Y. Y.; Zhang W. J. DFT Study on the Mechanism and Stereoselectivity of NHC-Catalyzed Synthesis of Substituted Trifluoromethyl Dihydropyranones with Contiguous Stereocenters. J. Org. Chem. 2016, 81, 868–877. 10.1021/acs.joc.5b02439. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Wang Y.; Wei D. H.; Qiao Y. Insights into N-Heterocyclic Carbene-Catalyzed [4 + 2] Annulation Reaction of Enals with Nitroalkenes: Mechanisms, Origin of Chemo- and Stereoselectivity, and Role of Catalyst. Chem. – Asian J. 2016, 11, 3046–3054. 10.1002/asia.201601022. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Zhao X. Y.; Qiao Y.; Guo X. K.; Wang Y. Y.; Wei D. H.; Tang M. S.; Niu J. L. A DFT study on the reaction mechanisms of phosphonation of heteroaryl N-oxides with H-phosphonates. Comput. Theor. Chem. 2015, 1071, 33–38. 10.1016/j.comptc.2015.08.012. [DOI] [Google Scholar]
- Domingo L. R.; Pérez P.; Sáez J. A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. 10.1039/C2RA22886F. [DOI] [Google Scholar]
- Biju A. T.; Padmanaban M.; Wurz N. E.; Glorius F. N-Heterocyclic Carbene Catalyzed Umpolung of Michael Acceptors for Intermolecular Reactions. Angew. Chem., Int. Ed. 2011, 50, 8412–8415. 10.1002/anie.201103555. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Tang M. S.; Wang Y. Y.; Wei D. H. Insights into Stereoselective Aminomethylation Reaction of α, β-Unsaturated Aldehyde with N, O-Acetal via N-Heterocyclic Carbene and Brønsted Acid/Base Cooperative Organocatalysis. J. Org. Chem. 2016, 81, 5370–5380. 10.1021/acs.joc.6b00656. [DOI] [PubMed] [Google Scholar]
- Ran Y. Y.; Tang M. S.; Wang Y.; Wang Y. Y.; Zhang X. L.; Zhu Y. Y.; Wei D. H.; Zhang W. J. Theoretical investigations towards the [4 + 2] cycloaddition of ketenes with 1-azadienes catalyzed by N-heterocyclic carbenes: mechanism and stereoselectivity. Tetrahedron 2016, 72, 5295–5300. 10.1016/j.tet.2016.06.057. [DOI] [Google Scholar]
- Zhang W.; Qiao Y.; Wang Y.; Tang M. S.; Wei D. H. Theoretical investigation toward organophosphine-catalyzed [3 + 3] annulation of Morita-Baylis-Hillman carbonates with azomethine imines: Mechanism, origin of stereoselectivity, and role of catalyst. Int. J. Quantum Chem. 2017, 117, e25367 10.1002/qua.25367. [DOI] [Google Scholar]
- Parr R. G.; Pearson R. G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. 10.1021/ja00364a005. [DOI] [Google Scholar]
- Domingo L. R.; Picher M. T.; Sáez J. A. Toward an Understanding of the Unexpected Regioselective Hetero-Diels–Alder Reactions of Asymmetric Tetrazines with Electron-Rich Ethylenes: A DFT Study. J. Org. Chem. 2009, 74, 2726–2735. 10.1021/jo802822u. [DOI] [PubMed] [Google Scholar]
- Kohn W.; Sham L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- Sham L. J.; Kohn W. One-particle properties of an inhomogeneous interacting electron gas. Phys. Rev. 1966, 145, 561. 10.1103/PhysRev.145.561. [DOI] [Google Scholar]
- Domingo L. R.; Chamorro E.; Pérez P. An Analysis of the Regioselectivity of 1, 3-Dipolar Cycloaddition Reactions of Benzonitrile N-Oxides Based on Global and Local Electrophilicity and Nucleophilicity Indices. Eur. J. Org. Chem. 2009, 2009, 3036–3044. 10.1002/ejoc.200900213. [DOI] [Google Scholar]
- Domingo L. R.; Chamorro E.; Perez P. An Understanding of the Electrophilic/Nucleophilic Behavior of Electro-Deficient 2, 3-Disubstituted 1, 3-Butadienes in Polar Diels–Alder Reactions. A Density Functional Theory Study. J. Phys. Chem. A 2008, 112, 4046–4053. 10.1021/jp711704m. [DOI] [PubMed] [Google Scholar]
- Lu T.; Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, revision C.01; Gaussian, Inc.: Wallingford, CT, 2010.
- Wei D.; Lei B. L.; Tang M. S.; Zhan C. G. Fundamental reaction pathway and free energy profile for inhibition of proteasome by epoxomicin. J. Am. Chem. Soc. 2012, 134, 10436–10450. 10.1021/ja3006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.; Tang M. S.; Zhan C. G. Fundamental reaction pathway and free energy profile of proteasome inhibition by syringolin A (SylA). Org. Biomol. Chem. 2015, 13, 6857–6865. 10.1039/C5OB00737B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.; Huang X. Q.; Liu J. J.; Tang M. S.; Zhan C. G. Reaction pathway and free energy profile for papain-catalyzed hydrolysis of N-acetyl-Phe-Gly 4-nitroanilide. Biochemistry 2013, 52, 5145–5154. 10.1021/bi400629r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.; Fang L.; Tang M. S.; Zhan C. G. Fundamental reaction pathway for peptide metabolism by proteasome: insights from first-principles quantum mechanical/molecular mechanical free energy calculations. J. Phys. Chem. B 2013, 117, 13418–13434. 10.1021/jp405337v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.; Huang X. Q.; Qiao Y.; Rao J. J.; Wang L.; Liao F.; Zhan C. G. Catalytic Mechanisms for Cofactor-Free Oxidase-Catalyzed Reactions: Reaction Pathways of Uricase-Catalyzed Oxidation and Hydration of Uric Acid. ACS Catal. 2017, 7, 4623–4636. 10.1021/acscatal.7b00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. K.; Zhang L. B.; Wei D. H.; Niu J. L. Mechanistic insights into cobalt (II/III)-catalyzed C–H oxidation: a combined theoretical and experimental study. Chem. Sci. 2015, 6, 7059–7071. 10.1039/C5SC01807B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y.; Wei D. H.; Wang Y.; Zhang W. J.; Tang M. S. N-Heterocyclic Carbene (NHC)-Catalyzed sp3 β-C–H Activation of Saturated Carbonyl Compounds: Mechanism, Role of NHC, and Origin of Stereoselectivity. ACS Catal. 2016, 6, 279–289. 10.1021/acscatal.5b01710. [DOI] [Google Scholar]
- Wang Y.; Guo X. K.; Wu B. H.; Wei D. H.; Tang M. S. Mechanistic and stereoselectivity study for the reaction of trifluoropyruvates with arylpropenes catalyzed by a cationic Lewis acid rhodium complex. RSC Adv. 2015, 5, 100147–100158. 10.1039/C5RA21074G. [DOI] [Google Scholar]
- Harrison J. G.; Gutierrez O.; Jana N.; Driver T. G.; Tantillo D. J. Mechanism of Rh2(II)-Catalyzed Indole Formation: The Catalyst Does Not Control Product Selectivity. J. Am. Chem. Soc. 2016, 138, 487–490. 10.1021/jacs.5b11427. [DOI] [PubMed] [Google Scholar]
- Mustard T. J. L.; Wender P. A.; Cheong P. H.-Y. Catalytic Efficiency Is a Function of How Rhodium(I) (5 + 2) Catalysts Accommodate a Conserved Substrate Transition State Geometry: Induced Fit Model for Explaining Transition Metal Catalysis. ACS Catal. 2015, 5, 1758–1763. 10.1021/cs501828e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard T. J. L.; Mack D. J.; Njardarson J. T.; Cheong P. H.-Y. Mechanism and the Origins of Stereospecificity in Copper-Catalyzed Ring Expansion of Vinyl Oxiranes: A Traceless Dual Transition-Metal-Mediated Process. J. Am. Chem. Soc. 2013, 135, 1471–1475. 10.1021/ja310065z. [DOI] [PubMed] [Google Scholar]
- Spoehrle S. S. M.; West T. H.; Taylor J. E.; Slawin A. M. Z.; Smith A. D. Tandem Pd and Isothiourea Relay Catalysis: Enantioselective Synthesis of α-Amino Acid Derivatives via Allylic Amination and [2,3]-Sigmatropic Rearrangement. J. Am. Chem. Soc. 2017, 139, 11895–11902. 10.1021/jacs.7b05619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y.; Wei D. H.; Chang J. B. Insights into the Unexpected Chemoselectivity for the N-Heterocyclic Carbene-Catalyzed Annulation Reaction of Allenals with Chalcones. J. Org. Chem. 2015, 80, 8619–8630. 10.1021/acs.joc.5b01222. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang Y.; Wei Dh; Qiao Y.; Tang M. S. A DFT study on the reaction mechanisms of N-heterocyclic carbene catalyzed homodimerization of styrenes. Commun. Comput. Chem. 2016, 4, 49–59. 10.4208/cicc.2016.v4.n2.2. [DOI] [Google Scholar]
- Zhang W.; Wang Y.; Zheng L. J.; Wei D. H.; Tang M. S. Insights into the NHC-catalyzed formal [2 + 2 + 2] cycloaddition of ketenes with C=S double bond of isothiocyanate. Commun. Comput. Chem. 2016, 4, 59–72. 10.4208/cicc.2016.v4.n2.3. [DOI] [Google Scholar]
- Cheong P. H.-Y.; Legault C. Y.; Um J. M.; Çelebi-Ölçüm N.; Houk K. N. Quantum Mechanical Investigations of Organocatalysis: Mechanisms, Reactivities, and Selectivities. Chem. Rev. 2011, 111, 5042–5137. 10.1021/cr100212h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O.; Tantillo D. J. Analogies between Synthetic and Biosynthetic Reactions in Which [1,2]-Alkyl Shifts Are Combined with Other Events: Dyotropic, Schmidt, and Carbocation Rearrangements. J. Org. Chem. 2012, 77, 8845–8850. 10.1021/jo301864h. [DOI] [PubMed] [Google Scholar]
- Cheong P. H.-Y.; Houk K. N. Origins of Selectivities in Proline-Catalyzed α-Aminoxylations. J. Am. Chem. Soc. 2004, 126, 13912–13913. 10.1021/ja0464746. [DOI] [PubMed] [Google Scholar]
- Cheong P. H.-Y.; Morganelli P.; Luzung M. R.; Houk K. N.; Toste F. D. Gold-Catalyzed Cycloisomerization of 1,5-Allenynes via Dual Activation of an Ene Reaction. J. Am. Chem. Soc. 2008, 130, 4517–4526. 10.1021/ja711058f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T. H.; Walden D. M.; Taylor J. E.; Brueckner A. C.; Johnston R. C.; Cheong P. H.-Y.; Lloyd-Jones G. C.; Smith A. D. Catalytic Enantioselective [2,3]-Rearrangements of Allylic Ammonium Ylides: A Mechanistic and Computational Study. J. Am. Chem. Soc. 2017, 139, 4366–4375. 10.1021/jacs.6b11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y.; Chen X. H.; Wei D. H.; Chang J. B. Insights into the Competing Mechanisms and Origin of Enantioselectivity for N-Heterocyclic Carbene-Catalyzed Reaction of Aldehyde with Enamide. Sci. Rep. 2016, 6, 38200 10.1038/srep38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Qiao Y.; Wei D. H.; Tang M. S. Computational study on NHC-catalyzed enantioselective and chemoselective fluorination of aliphatic aldehydes. Org. Chem. Front. 2017, 4, 1987–1998. 10.1039/C7QO00436B. [DOI] [Google Scholar]
- Ríos-Gutiérrez M.; Darù A.; Tejero T.; Domingo L. R.; Merino P. A molecular electron density theory study of the [3 + 2] cycloaddition reaction of nitrones with ketenes. Org. Biomol. Chem. 2017, 15, 1618–1627. 10.1039/C6OB02768G. [DOI] [PubMed] [Google Scholar]
- Domingo L. R.; Ríos-Gutiérrez M.; Emamian S. Understanding the domino reaction between 1-diazopropan-2-one and 1, 1-dinitroethylene. A molecular electron density theory study of the [3 + 2] cycloaddition reactions of diazoalkanes with electron-deficient ethylenes. RSC Adv. 2017, 7, 15586–15595. 10.1039/C7RA00544J. [DOI] [Google Scholar]
- Chamorro E.; Duque-Noreña M.; Ríos-Gutiérrez M.; Domingo L. R.; Pérez P. Intrinsic relative nucleophilicity of indoles. Theor. Chem. Acc. 2016, 135, 202. 10.1007/s00214-016-1974-x. [DOI] [Google Scholar]
- Lodewyk M. W.; Siebert M. R.; Tantillo D. J. Computational Prediction of 1 H and 13 C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. 10.1021/cr200106v. [DOI] [PubMed] [Google Scholar]
- Harrison J. G.; Zheng Y. B.; Beal P. A.; Tantillo D. J. Computational Approaches to Predicting the Impact of Novel Bases on RNA Structure and Stability. ACS Chem. Biol. 2013, 8, 2354–2359. 10.1021/cb4006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S. R.; Pemberton R. P.; Tantillo D. J. Navigating Past a Fork in the Road: Carbocation−π Interactions Can Manipulate Dynamic Behavior of Reactions Facing Post-Transition-State Bifurcations. J. Am. Chem. Soc. 2017, 139, 7485–7493. 10.1021/jacs.7b01042. [DOI] [PubMed] [Google Scholar]
- Siebert M. R.; Osbourn J. M.; Brummond K. M.; Tantillo D. J. Differentiating Mechanistic Possibilities for the Thermal, Intramolecular [2 + 2] Cycloaddition of Allene–Ynes. J. Am. Chem. Soc. 2010, 132, 11952–11966. 10.1021/ja102848z. [DOI] [PubMed] [Google Scholar]
- Walden D. M.; Ogba O. M.; Johnston R. C.; Cheong P. H.-Y. Computational Insights into the Central Role of Nonbonding Interactions in Modern Covalent Organocatalysis. Acc. Chem. Res. 2016, 49, 1279–1291. 10.1021/acs.accounts.6b00204. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Truhlar D. G. Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. 10.1021/ct800246v. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Truhlar D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- Zhang W. J.; Truhlar D. G.; Tang M. S. Tests of exchange-correlation functional approximations against reliable experimental data for average bond energies of 3d transition metal compounds. J. Chem. Theory Comput. 2013, 9, 3965–3977. 10.1021/ct400418u. [DOI] [PubMed] [Google Scholar]
- Mennucci B.; Tomasi J. Continuum solvation models: a new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. 10.1063/1.473558. [DOI] [Google Scholar]
- Barone V.; Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. 10.1021/jp9716997. [DOI] [Google Scholar]
- Gonzalez C.; Schlegel H. B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. 10.1063/1.456010. [DOI] [Google Scholar]
- Gonzalez C.; Schlegel H. B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. 10.1021/j100377a021. [DOI] [Google Scholar]
- Lu T.; Chen F. W. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Legault C. Y.CYLview, version 1.0 b; Université de Sherbrooke, 2009. http://www.cylview.org/.
- Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Chai J.-D.; Head-Gordon M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]
- Gordillo R.; Houk K. N. Origins of Stereoselectivity in Diels–Alder Cycloadditions Catalyzed by Chiral Imidazolidinones. J. Am. Chem. Soc. 2006, 128, 3543–3553. 10.1021/ja0525859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. D.; Dudding T.; Gordillo R.; Houk K. N. Origin of Enantioselection in Hetero-Diels– Alder Reactions Catalyzed by Naphthyl-TADDOL. Org. Lett. 2008, 10, 2749–2752. 10.1021/ol800875k. [DOI] [PubMed] [Google Scholar]
- Gutierrez O.; Iafe R. G.; Houk K. N. Origin of Stereoselectivity in the Imidazolidinone-Catalyzed Reductions of Cyclic α, β-Unsaturated Ketones. Org. Lett. 2009, 11, 4298–4301. 10.1021/ol901586t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi M. A. M.; Centrone C.; Fracchiolla G.; Naso F.; Cardellicchio C. A study of factors affecting enantioselectivity in the oxidation of aryl benzyl sulfides in the presence of chiral titanium catalysts. Eur. J. Org. Chem. 2011, 2011, 4327–4334. 10.1002/ejoc.201100310. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.