Abstract

An efficient, cost-effective, and earth-abundant catalyst that could drive the production of hydrogen from water without or with little external energy is the ultimate goal toward hydrogen economy. Herein, nanoplates of tungsten oxide and its hydrates (WO3·H2O) as promising electrocatalysts for the hydrogen evolution reaction (HER) are reported. The square-shaped and stacked WO3·H2O nanoplates are synthesized at room temperature under air in ethanol only, making it as a promising green synthesis strategy. The repeated electrochemical cyclic voltammetry cycles modified the surface of WO3·H2O nanoplates to WO3 as confirmed by X-ray photoelectron and Auger spectroscopy, which leads to an improved HER activity. Hydrogen evolution is further achieved from distilled water (pH 5.67) producing 1 mA cm–2 at an overpotential of 15 mV versus the reversible hydrogen electrode. Moreover, WO3·H2O and WO3 nanoplates demonstrate excellent durability in acidic and neutral media, which is highly desirable for practical application. Improved hydrogen evolution by WO3(200) when compared to that by Pt(111) is further substantiated by the density functional theory calculations.
Introduction
Rapid depletion of fossil fuels and ever-increasing energy demand prompt the researchers to explore clean, renewable, sustainable, and environmentally friendly energy sources.1 On that prospect, hydrogen is considered as an efficient, earth-abundant, and renewable clean energy carrier that has potential to play a major role. Hydrogen can be produced through electrochemical water splitting with zero emission of CO2, which makes the process green and sustainable.2 Two important half reactions of water splitting are the hydrogen evolution reaction (HER), that is, 2H+(aq) + 2e– → H2(g) and the oxygen evolution reaction, that is, 2H2O(l) → O2(g) + 4H+(aq) + 4e–, occurring at the cathode and the anode, respectively.3 To lower the overpotential of these reactions while increasing their reaction rate, electrocatalysts at the cathode and anode surface play the central role, thus improving the overall efficiency of the water-splitting reaction. Noble metals such as platinum (Pt), palladium (Pd), and ruthenium (Ru) have so far been recognized as effective and efficient HER catalysts with negligible overpotential and excellent kinetics in acidic electrolyte solutions.4,5 However, high cost and poor earth-abundance of these noble metals are the major hindrance for their practical utilization.6,7 Therefore, it is indispensable to develop noble metal-free HER catalysts which are not only efficient but also earth-abundant, low cost, and stable in the electrolyte which is being used. Several noble metal-free electrocatalysts, particularly Mo- and W-based materials, have been reported recently and have been reckoned to be promising. In particular, chalcogenides such as MoS2,8−12 WS2,13−16 WSe2,17 and MoSe217 have been successfully investigated as cost-effective potential substitution to the noble metal-based catalysts in acidic solutions. Although several chalcogenides have been studied for the HER in acidic media, little emphasis has been directed toward metal hydroxides and/or oxides and/or in neutral media.
Hydrogen evolution in neutral electrolyte solutions is desirable for practical applications. Thus, 0.1 M acetate buffer solution (pH 4.5) and phosphate buffer solution (PBS, pH 7) have been used as electrolytes for hydrogen evolution by Andreiadis et al.18 with a cobalt-based complex and Karunadasa et al.19 with a molybdenum-oxo-complex, respectively. Helm et al. also demonstrated hydrogen production using nickel complexes in both aqueous and acidic electrolytes.20 The synthesis of these metal complexes is not only complex but also involves multiple steps and several chemicals. However, the stability of these complexes for long-term uses is an issue. Therefore, it is of immense importance to explore suitable inorganic electrocatalysts for hydrogen generation in neutral media.
Here, competent HER activity of tungsten trioxide (WO3) nanoplates is demonstrated in acidic and neutral electrolytes, and compared with standard platinum on carbon (Pt/C) catalysts. Coincidently, WO3 was obtained by electrochemical surface oxidization of WO3·H2O nanoplates through repeated electrochemical cyclic voltammetry (CV) cycles for the first time. The electrochemically surface-modified WO3·H2O exhibits a performance similar to WO3 obtained separately by annealing WO3·H2O nanoplates under air at 400 °C. It is noteworthy that WO3·H2O nanoplates were synthesized at room temperature using only ethanol as a solvent thus excluding the use of corrosive HCl or HNO3 along with other organic chemicals previously reported for the synthesis of hydrated WO3.21−24 To demonstrate the potential of the present electrocatalysts, we further demonstrate hydrogen evolution in a neutral medium (distilled water; pH, 5.67) and their remarkable stabilities in acidic and neutral electrolytes. The superior HER activity of WO3 when compared to that of Pt is additionally supported by reaction energetics obtained by the density functional theory (DFT) calculations.
Results and Discussion
Structural Analysis
The structural analysis of the as-synthesized samples was carried out by powder X-ray diffraction (XRD) as shown in Figure 1. The diffraction features of the precipitate formed at room temperature (Figure 1a) are readily indexed to the orthorhombic WO3·H2O with lattice parameters a = 5.25 Å, b = 10.7 Å, and c = 5.11 Å, which are in good agreement with those of JCPDS file no. 00-043-0679 (a = 5.24 Å, b = 10.7 Å, and c = 5.12 Å). The XRD pattern (Figure 1b) of WO3·H2O annealed at 400 °C for 2 h under air matches that of monoclinic WO3 with lattice parameters a = 7.3 Å, b = 7.51 Å, and c = 7.71 Å (JCPDS file no. 01-083-0951, a = 7.3 Å, b = 7.54 Å, and c = 7.69 Å). No other phases and/or impurities such as WO3·2H2O and WO3·0.33H2O were found in any of these samples indicating the phase-pure product. The XRD analysis suggests that the WO3·H2O powder formed at room temperature is completely phase-transformed to WO3 upon annealing at 400 °C for 2 h under air.
Figure 1.
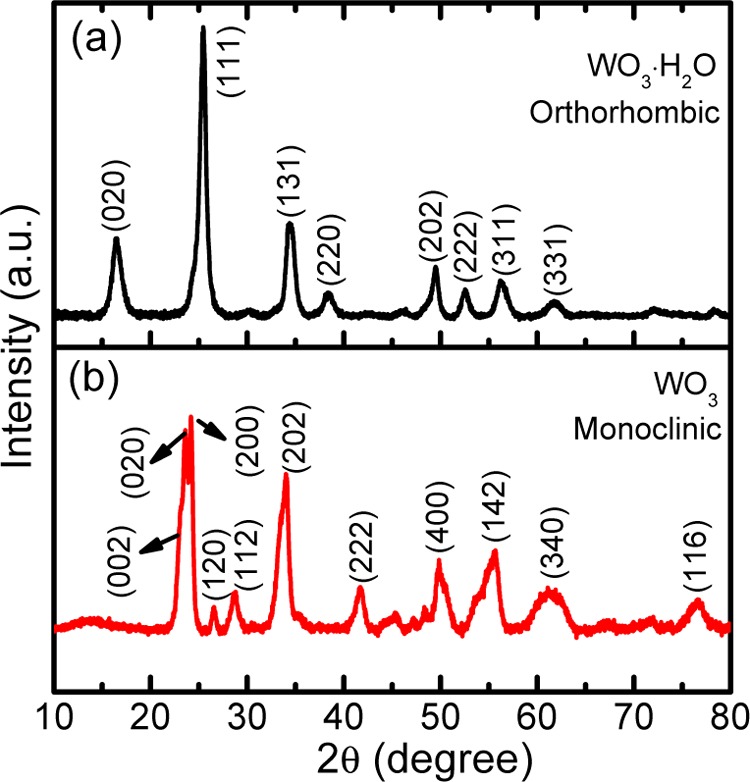
XRD patterns of (a) orthorhombic WO3·H2O nanoplates and (b) monoclinic WO3 nanoplates obtained by annealing WO3·H2O at 400 °C for 2 h under air.
Thermal Stability
Complete conversion of WO3·H2O to WO3 upon annealing was further confirmed by thermogravimetry (TG) analysis. Figure 2 shows the TG and its derivative plot of WO3·H2O nanoplates in a temperature range of 26–800 °C at a heating rate of 10 °C min–1 under air. A major weight loss of 7.2% occurred up to 230 °C matches the theoretical value of 7.2% confirming the phase transformation of WO3·H2O to WO3.25 The subsequent weight loss of 1.1% between 230 and 400 °C is believed to be due to the final water decomposition in the crystallization of minor amorphous contents.23 The TG analysis corroborates the XRD results on the complete phase transformation of WO3·H2O to WO3 under air at 400 °C for 2 h (Figure 1).
Figure 2.

TG curve and its derivative plot of WO3·H2O nanoplates performed under air with a heating rate of 10 °C min–1.
Morphology and Microstructure
The morphology and microstructure of the as-synthesized orthorhombic WO3·H2O and monoclinic WO3 were examined in detail using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM), respectively. Figure 3a,b shows the FESEM images of WO3·H2O nanoplates at different magnifications synthesized by mixing WCl6 in ethanol at room temperature. These nanoplates were found to be square-shaped, highly uniform, and stacked. The length/width of these nanoplates was measured to be 100 nm with a thickness <30 nm, measured from the magnified SEM image (shown as an inset in Figure 3b). Figure 3c presents a TEM image of stacked WO3·H2O nanoplates in accordance with the FESEM images. The high-resolution TEM (HRTEM) image of a WO3·H2O nanoplate shows a lattice spacing of 3.5 Å corresponding to the (111) plane as shown in Figure 3d. Additional TEM images (Figure S1, Supporting Information) clearly depict the stacked arrangement and thickness of individual WO3·H2O nanoplates. The powder XRD pattern (Figure 1a) of WO3·H2O nanoplates also shows maximum intensity for the same (111) plane indicating their growth direction. The regular spot selected area diffraction (SAED) pattern (inset of Figure 3d) obtained from the WO3·H2O nanoplate confirms its single crystalline nature. Although synthesis of WO3·H2O nanoplates has been reported earlier,21,23,24 they were of much larger sizes and synthesized at a higher temperature than the room temperature used in the present work. In particular, Huang et al.23 synthesized rectangular slab-like WO3·H2O of size 2–3 μm by taking Na2WO4 and HCl at 70 °C for 10 h and Kalantar-zadeh et al.24 reported hydrated WO3 platelets of 0.2–2 μm size obtained at 80 °C for 6 h using 0.5 M HNO3. Recently, Guo et al. reported orthorhombic WO3·H2O nanoplates of size >200 nm by the hydrothermal method at 100 °C for 10 h.21 The formation of nanosized WO3·H2O plates (∼100 nm) is thus attributed to the lower synthesis temperature (room temperature) employed in the present work. Figure 3e,f shows the FESEM images of stacked WO3 nanoplates at different magnifications obtained by annealing WO3·H2O nanoplates at 400 °C for 2 h under air. The annealing not only produced phase-pure WO3 but also resulted in nanoplates to be separated in the stack as shown in Figure 3f. A TEM image of WO3 nanoplates is shown in Figure 3g, which reveals the formation of gap between consecutive nanoplates (marked by arrows) and voids (marked by a circle) in the nanoplates. The formation of gaps and voids in WO3 nanoplates is due to the annealing of WO3·H2O nanoplates at a higher temperature (400 °C). In WO3·H2O, water molecules are present between layers of WO6 octahedral units and the W–OH2 bond is weak.26,27 Thus, water molecules in WO3·H2O nanoplates are evaporated upon annealing as confirmed from the mass loss (Figure 2) forming gaps between nanoplates and voids in the resulting WO3 nanoplates. The HRTEM image of a WO3 nanoplate shows a lattice spacing of 3.8 Å, corresponding to the (002) plane of monoclinic WO3. The regular spot SAED pattern (inset of Figure 3h) confirmed the single crystalline nature of the WO3 nanoplates. In addition, the elemental energy dispersive X-ray (EDX) mapping of WO3·H2O and WO3 nanoplates was performed (Figure S2, Supporting Information) in a FESEM which shows a uniform distribution of W and O throughout the sample.
Figure 3.
FESEM images of (a,b) square-shaped WO3·H2O nanoplates of length/width 100 nm, synthesized by just mixing WCl6 in ethanol under ambient conditions, inset of (b) shows the corresponding magnified image revealing stacked arrangement of nanoplates, and (c) TEM and (d) HRTEM images of WO3·H2O nanoplates. Inset of (d) shows a SAED pattern of WO3·H2O nanoplates. FESEM images of (e,f) WO3 nanoplates obtained by calcining WO3·H2O at 400 °C for 2 h. (g) TEM and (h) HRTEM images of WO3 nanoplates. Inset of (h) shows a SAED pattern of WO3 nanoplates.
Electrocatalytic HER Study
The electrochemical HER performance of WO3·H2O and WO3 nanoplates was examined by linear sweep voltammetry (LSV), CV, and chronoamperometry in a highly acidic (0.5 M H2SO4) electrolyte. Figure 4a presents the LSV plots for the HER in 0.5 M H2SO4 at 1 mV s–1 with WO3·H2O and WO3 nanoplates along with Pt/C and glassy carbon electrodes (GCE) for comparison. An overpotential of 147, 73, and 24 mV was measured to obtain 10 mA cm–2 with WO3·H2O, WO3, and Pt/C, respectively. An 73 mV overpotential with WO3 nanoplates was found to be smaller than that reported for several nonnoble metal electrocatalysts including Ni2P (117 mV),28 MoS2/Au (226 mV),29 MoS2 (190 mV),30 WS2 (233 mV),13 and other electrocatalysts (Table S1, Supporting Information). Moreover, a similar superior HER activity was reported by Phuruangrat et al. with hexagonal WO3 nanowires synthesized by the microwave-assisted hydrothermal method.31 This suggests WO3 as a potential HER electrocatalyst. The cyclic stability of WO3·H2O and WO3 nanoplates was tested by performing CV for 4000 cycles in 0.5 M H2SO4 at 100 mV s–1 (Figure S3, Supporting Information). A slight positive current on Pt/C, WO3·H2O, and WO3 prior to onset potential of the HER (Figure 4a) was found to disappear after CV cycles, which is believed to be due to oxidative surface cleaning and surface oxidation of hydroxide. Furthermore, the HER current density of WO3·H2O was found to be significantly increased (Figure S3a) with repeated CV cycles toward the value of WO3 nanoplates (Figure S3b), which is attributed to surface oxidation of WO3·H2O to WO3 (discussed later). A smaller increase in the HER current density for WO3 nanoplates (Figure S3b) can be termed to surface cleaning during CV cycles and conversion of surface hydroxide to oxide.17Figure 4b shows the Tafel plots of the electrocatalysts from which a slope was measured. The Tafel slopes (and exchange current densities) were measured to be 43.9 mV dec–1 (6.1 mA cm–2), 39.5 mV dec–1 (12.58 mA cm–2), and 29 mV dec–1 (17.78 mA cm–2) for WO3·H2O, WO3, and Pt/C, respectively, in the first cycle. The Tafel slope and exchange current density were, respectively, smaller and larger with WO3 nanoplates than those of several other chalcogenides reported recently (Table S1, Supporting Information) for the HER. After 4000 CV cycles, the Tafel slope was found to be further decreased while the exchange current density increased for WO3·H2O (Table 1). The estimated Tafel slopes suggested the Volmer–Tafel HER mechanism of these electrocatalysts.32 The superior HER activity of W-based hydroxide and oxide to that of other recently studied materials (Table S1, Supporting Information) makes it a potential candidate for hydrogen generation.
Figure 4.
(a) LSV and (b) Tafel plots of WO3·H2O and WO3 nanoplates for the HER in 0.5 M H2SO4.
Table 1. HER Performance of WO3·H2O and WO3 Nanoplates in Different Electrolytes.
| catalysts | electrolyte | overpotential (mV at 10 mA cm–2 vs RHE) | Tafel slope (mV dec–1) | exchange current density (A cm–2) |
|---|---|---|---|---|
| WO3·H2O (1st LSV) | 0.5 M H2SO4 | 147 | 43.9 | 6.11 × 10–3 |
| WO3 (1st LSV) | 0.5 M H2SO4 | 73 | 39.5 | 12.58 × 10–3 |
| WO3·H2O (4001st LSV) | 0.5 M H2SO4 | 66 | 34.8 | 14.2 × 10–3 |
| WO3 (4001st LSV) | 0.5 M H2SO4 | 70 | 38.53 | 12.82 × 10–3 |
| Pt/C | 0.5 M H2SO4 | 24 | 29 | 17.78 × 10–3 |
| WO3·H2O (1st LSV) | distilled water | 55.7 | 0.02 × 10–3 | |
| WO3 (1st LSV) | distilled water | 331 at 1 mA cm–2 | 51.59 | 0.021 × 10–3 |
| WO3·H2O (10001st LSV) | distilled water | 177 at 1 mA cm–2 | 32 | 0.032 × 10–3 |
| WO3 (10001st LSV) | distilled water | 193 at 1 mA cm–2 | 47.73 | 0.052 × 10–3 |
To understand the better HER performance of WO3 than that of WO3·H2O, the electrochemical active surface area (ECSA) was calculated from the electrochemical double layer capacitance measurement using eq 1.33,34
| 1 |
where Cdl is the electrochemical double layer capacitance and Cs refers the specific electrochemical double layer capacitance of an atomically smooth surface (typically 15–50 μF cm–2).34 In the present case, the value of Cs is 30 μF cm–2 for all the electrodes in 0.5 M H2SO4 electrolyte. Cdl was calculated by measuring the CVs at different scan rates in the nonfaradaic region. Figure 5a,b shows the CVs of WO3·H2O and WO3 nanoplates at different scan rates (10–100 mV s–1) in 0.5 M H2SO4 electrolyte, respectively. The capacitive currents for WO3·H2O and WO3 nanoplates at 0.7 V versus the reversible hydrogen electrode (RHE) were plotted as a function of scan rates (Figure 5c) and their slope is known as Cdl. The ECSA (and Cdl) of WO3·H2O and WO3 nanoplates was measured to be 1.0 cm2 (0.03 mF) and 2.83 cm2 (0.085 mF), respectively. The larger ECSA of WO3 nanoplates clearly indicates more active sites in them, which resulted in higher HER performance compared to that of WO3·H2O nanoplates. Furthermore, the specific activity (SA) of WO3·H2O and WO3 nanoplates was calculated to compare their intrinsic catalytic performance by normalizing the HER current density to the specific surface area (Figure S4, Supporting Information) as per eq 2.35,36
| 2 |
where SA stands for specific activity (mA cm–2), j refers to the current density (mA cm–2) at 0.35 V (vs RHE), m stands for the catalyst loading mass (28.57 mg cm–2), and SBET is the Brunauer-Emmett-Teller surface area (m2 g–1). The SA values normalized to the specific surface area were calculated to be 2.58 and 5.38 in magnitude for WO3·H2O and WO3 nanoplates, respectively. This further confirms the higher HER performance of WO3 nanoplates. The electrochemical impedance spectroscopy (EIS) study was carried out to understand the charge transfer behavior at the electrode/electrolyte interface. Figure 5d shows the Nyquist plots of WO3·H2O and WO3 nanoplates before and after 4000 CV cycles at an applied potential of 0.06 V versus the RHE in 0.5 M H2SO4. All the EIS spectra show semicircles and straight lines in the high-frequency and low-frequency regions, respectively. The diameter of the semicircles infers to the charge-transfer resistance (Rct) at the electrode–electrolyte interface. A smaller semicircle confirms superiority of the electrode because of a smaller Rct. The Rct values for WO3·H2O and WO3 nanoplates were measured to be 9.3 and 5.5 Ω, respectively, by fitting the experimental data with an equivalent circuit model shown as an inset in Figure 5d. As expected, the Rct value was decreased to 4.6 and 4.8 Ω for WO3·H2O and WO3 nanoplates, respectively, after 4000 cycle CV test. A smaller Rct further validates an increased current density with the electrocatalysts after the stability test.
Figure 5.
CVs at different scan rates in the potential range of 0.54–0.84 V vs the RHE (nonfaradaic region) in 0.5 M H2SO4 solution with (a) WO3·H2O and (b) WO3 nanoplates. (c) Capacitive currents measured at 0.7 V vs the RHE as a function of scan rates with WO3·H2O and WO3 nanoplates. (d) Nyquist plots of WO3·H2O and WO3 nanoplates at 0.06 V (vs RHE) before and after the stability test and the inset shows the equivalent circuit diagram used to fit the experimental data.
The quantitative estimation of hydrogen evolution was finally performed with WO3 nanoplates using a gas chromatograph. The hydrogen generation rate was found to increase and reach 20.4 mmol·cm–2 in 10 h (Figure 6) with WO3 nanoplates at −0.76 V versus the RHE in 0.5 M H2SO4. The repeated 4000 CV cycles (Figure S3, Supporting Information) and hydrogen generation for 10 h suggests the good stability of WO3·H2O and WO3 for the HER.
Figure 6.
H2 evolution measured by a gas chromatograph at −0.76 V vs the RHE using WO3 nanoplates as the electrocatalyst and 0.5 M H2SO4 as the electrolyte.
The HER investigation was further extended to a neutral medium using normal distilled water (pH 5.67). Figure 7a–c shows the LSV, Tafel, and chronoamperometry plots of WO3·H2O and WO3 nanoplates in water. The HER was found to begin at ∼0.5 V versus the RHE [−0.076 V vs saturated calomel electrode (SCE)] during the cathodic scan and HER current sharply increased below 0 V versus the RHE (−0.576 V vs SCE) in the presence of the catalyst, as shown in Figure 7a. As expected, WO3 exhibited a higher HER activity in the neutral medium as well. The obtained HER current of 1.0 mA cm–2 at the overpotential of 15 mV in the first cycle is significantly lower than recently reported electrocatalysts studied in the PBS electrolyte.18,37 The Tafel slopes (and exchange current densities) of 49.75 mV dec–1 (1.0 mA cm–2) and 43.43 mV dec–1 (1.15 mA cm–2) were measured for WO3·H2O and WO3 nanoplates in the first cycle, respectively. The repeated CV cycles showed an increase in the HER current density of WO3·H2O to the value obtained for WO3 nanoplates in water as well (Figure S5a,b, Supporting Information). After 10 000 CV cycles, LSV collected with WO3·H2O and WO3 nanoplates revealed improved performance, that is, a higher current density (3.6 mA cm–2 at 15 mV overpotential for both WO3·H2O and WO3 nanoplates), smaller Tafel slope (30.0 mV dec–1 for WO3·H2O and 41.98 mV dec–1 for WO3), and higher exchange current density (1.5 mA cm–2 for WO3·H2O and 1.1 mA cm–2 for WO3) as shown in Figure S6 (Supporting Information) and Table 1. Similar to the HER study in acidic electrolyte, a higher positive current was found with WO3·H2O than with WO3 nanoplates prior to the onset of HER (Figure 7a), which is due to the oxidation of surface hydroxide. However, after repeated CV cycles, the positive current prior to the onset of the HER remained (Figure S6, Supporting Information), which suggests that further study is needed to understand this behavior. The chronoamperometry measurement further confirmed excellent stability of the electrocatalysts as studied for 20 h without a decrease in current (Figure 7c). The quantitative hydrogen generation was also measured using gas chromatography and was found to be 550 μmol·cm–2 in 10 h with WO3 nanoplates at −0.42 V versus the RHE (or −1.0 V vs SCE) in distilled water (Figure 7d).
Figure 7.
(a) LSV, (b) Tafel plots, and (c) chronoamperometry plots of WO3·H2O and WO3 nanoplates for hydrogen evolution in distilled water at pH 5.67. (d) H2 evolution measured by a gas chromatograph at −0.76 V vs the RHE using WO3 nanoplates as the electrocatalyst and distilled water as the electrolyte.
Surface Composition
Improvements in the HER performance of WO3·H2O after repeated CV cycles is attributed to its surface modification to WO3. The change in the surface composition was confirmed by X-ray photoelectron spectroscopic (XPS) and Auger electron spectroscopic (AES) measurements. Figure 8 shows the W 4f and O 1s region XPS spectra of WO3·H2O, WO3·H2O after repeated CV cycles (in 0.5 M H2SO4), and WO3. The W 4f binding energy positions are well-matched to the literature values.38,39 The two O 1s XPS peaks at ∼530.5 and ∼531.7 eV are assigned to oxide and surface hydroxide, respectively.39 The W and O atomic compositions estimated using CasaXPS software are presented in Table 2, which clearly indicate the change in the surface composition of WO3·H2O to WO3 after CV cycles. Figure S7 (Supporting Information) shows the AES spectra of WO3·H2O, WO3·H2O after CV cycles (in 0.5 M H2SO4), and WO3. The surface composition (Table 2) measured by AES correlates the XPS results and confirmed the surface oxidation of WO3·H2O to WO3 during electrochemical CV cycles. This signifies the important role of room temperature synthesized WO3·H2O nanoplates for the HER. However, the XRD pattern (not shown) of WO3·H2O after CV cycles showed no change in its phase, thus indicating the surface modification only.
Figure 8.
(a–c) W 4f and (a1–c1) O 1s region XPS spectra of (a) WO3·H2O nanoplates, (b) WO3·H2O nanoplates after 4000 CV cycles in 0.5 M H2SO4, and (c) WO3 nanoplates.
Table 2. Surface Composition (Atomic Percentage) of WO3·H2O Nanoplates before and after CV Cycles in 0.5 M H2SO4, and WO3 Nanoplates Measured by XPS and AES.
| atomic % by XPS |
atomic % by AES |
|||
|---|---|---|---|---|
| sample | W % | O % | W % | O % |
| WO3·H2O | 19.83 | 80.17 | 20.9 | 79.1 |
| WO3·H2O after CV cycles | 24.47 | 75.53 | 22.4 | 77.6 |
| WO3 | 23.88 | 76.72 | 25.4 | 74.6 |
DFT Study
The mechanism of hydrogen evolution was further investigated using periodic plane wave DFT calculations on the (200) plane of the P21/n monocline phase of WO3 nanoplates as per experimental observation and their thermodynamic stability40 and compared with those of the well-established Pt(111) catalyst.41 The protons adsorbed on the hcp site of Pt(111) with the Pt–H bond distance of 1.912, 1.886, and 1.872 Å whereas 0.98 Å atop WO3(200). The reaction coordinates for proton adsorption followed by its recombination (H + e– → H* → 1/2H2) have been reported to be the indicator of HER catalyst activity.42 Therefore, energy landscapes indicating the above reactions were developed as shown in Figure 9. The adsorption energy of proton on Pt(111) was calculated to be −2.52 eV matching the values calculated by Nobuhara et al.43 Moreover, the proton adsorption energy on WO3(200) was found to be −1.8 eV, which was lower than that of Pt, establishing the reason behind the superior HER performance of WO3 as experimentally observed.
Figure 9.

Calculated energy landscapes of the HER on WO3(200) and Pt(111).
Conclusions
The present work spotlights the importance of hydroxide and oxide for the water-splitting reaction and generation of hydrogen. The inherent stability of oxides makes them highly suitable and efficient materials for the water-splitting reaction. The superior HER performance of WO3·H2O and WO3 was affirmed through high hydrogen evolution current densities and smaller Tafel slopes as demonstrated here. These catalysts also exhibited excellent durability in acidic and neutral electrolytes. The synthesis of WO3·H2O at room temperature and its surface evolution to WO3 during repeated electrochemical CV cycles were evidenced from surface characterization and electrochemical performances. In addition, the high hydrogen generation rate in acidic (20.4 mmol·cm–2) and neutral (550 μmol·cm–2) electrolytes as measured quantitatively demonstrated potential of these electrocatalysts for hydrogen generation through water splitting.
Experimental Details
Chemicals
Tungsten (VI) chloride (WCl6), platinum on carbon (Pt/C) (Sigma-Aldrich, USA), and ethanol (C2H5OH) and H2SO4 (Merck, India) were used for synthesis and activity tests. All chemicals were of analytical grade and used without further purification.
Synthesis of WO3·H2O and WO3 Nanoplates
In a typical synthesis, 40 mL (25 mM) of WCl6 was prepared in ethanol and kept under ambient temperature (at ∼30 °C) for 1 h to obtain pine green color precipitate of WO3·H2O nanoplates, which was washed with ethanol and dried at 60 °C for 4 h. WO3 nanoplates were obtained by annealing WO3·H2O nanoplates at 400 °C in a muffle furnace for 2 h under air and cooling the furnace naturally to room temperature.
Characterization
The crystal structures of the samples were examined with a PANalytical high-resolution XRD (PW 3040/60) operated at 40 kV and 30 mA with Cu Kα X-rays (1.54 Å). TG analysis was performed with a TA Instrument (TGA Q50) under synthetic air (N2/O2 = 80:20) at a heating rate of 10 °C per min. The surface morphology of the as-synthesized WO3·H2O and WO3 powder was examined using a Carl Zeiss SUPRA 40 FESEM. The detailed microstructures of the samples were analyzed using a Tecnai G2 TEM (FEI) at an accelerating voltage of 200 kV. The surface analysis of the samples was carried out by XPS using a PHI 5000 VersaProbe II scanning XPS microprobe with a monochromatic Al Kα source (1486.6 eV). The AES measurements were performed with a scanning Auger nanoprobe (PHI 710, ULAC-PHI Inc.) equipped with a coaxial cylindrical mirror analyzer.
Electrochemical Study
The HER activity of the as-synthesized catalysts was studied using a BioLogic SP-150 potentiostat with Pt-foil as the counter electrode, SCE as the reference electrode, and a GCE coated with the catalyst as the working electrode. Prior to loading the catalyst, the GCE was cleaned by polishing with alumina powder and sonicating in distilled water and ethanol. A slurry was prepared by sonicating the catalyst powder (WO3·H2O or WO3 nanoplates) in a mixture of distilled water (10 mg mL–1) and one drop of diluted polytetrafluoroethylene (PTFE) (10 μL of 1% PTFE dispersion) for 30 min. Then, 50 μL of slurry was coated on the GCE by drop-cast and dried under vacuum overnight. The electrochemical measurements such as LSV and CV were carried out with a three-electrode system in 0.5 M H2SO4 electrolyte at a scan rate of 1–100 mV s–1. The HER study was also performed in a neutral electrolyte using distilled water (pH 5.67). The electrode potential was calibrated with respect to the RHE by using the equation, E(RHE) = E(SCE) + 0.241 + 0.0591 pH. Stabilities of the as-synthesized electrocatalysts were measured by chronoamperometry for 20 h at a selected applied potential. The hydrogen generation rate was measured using a gas chromatograph (7890B, Agilent Technologies) both in acidic (0.5 M H2SO4) and neutral electrolytes (distilled water, pH 5.67) at −0.36 V and −0.42 V versus the RHE, respectively.
DFT Calculations
The HER mechanism was analyzed using DFT calculations on the (200) plane of P21/n monoclinic WO3 following the experimental observation. DFT calculations were implemented with a Quantum ESPRESSO package.44 For optimization, a 2 × 2 surface unit cell was used with three layers while freezing bottom two layers. To avoid the interaction of the adsorbate and periodic image of the slab, a vacuum of 10 Å was incorporated. Plane wave self-consistent field calculations were carried out with the Perdew–Burke–Ernzerhof exchange–correlation functional,42 and ultrasoft pseudopotentials were used. For the wave functions (and charge densities), a kinetic energy cutoff of 37 Ry (370 Ry) was used. The convergence threshold of 1 × 10–6 arb units and 2 × 2 × 1 k-mesh were used for energy and Brillouin zone sampling, respectively. DFT analysis was also done with a stable (111) plane of Pt within a Pt 2 × 2 super cell. A comparative energy landscape for the reaction H+ + e– → H* was developed for the synthesized catalyst and the established Pt catalyst to corroborate the difference in their activities.
Acknowledgments
This work was supported by the Science and Engineering Research Board of India through grant number SB/S1/IC-15/2013.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01151.
TEM images, EDX mapping, CVs, N2 adsorption–desorption isotherms, and AES spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chow J.; Kopp R. J.; Portney P. R. Energy Resources and Global Development. Science 2003, 302, 1528–1531. 10.1126/science.1091939. [DOI] [PubMed] [Google Scholar]
- Turner J. A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. 10.1126/science.1103197. [DOI] [PubMed] [Google Scholar]
- Jin H.; Wang J.; Su D.; Wei Z.; Pang Z.; Wang Y. In situ Cobalt–Cobalt Oxide/N-Doped Carbon Hybrids As Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. 10.1021/ja5127165. [DOI] [PubMed] [Google Scholar]
- Greeley J.; Jaramillo T. F.; Bonde J.; Chorkendorff I.; Nørskov J. K. Computational High-throughput Screening of Electrocatalytic Materials for Hydrogen Evolution. Nat. Mater. 2006, 5, 909–913. 10.1038/nmat1752. [DOI] [PubMed] [Google Scholar]
- Wu M.; Shen P. K.; Wei Z.; Song S.; Nie M. High Activity PtPd-WC/C Electrocatalyst for Hydrogen Evolution Reaction. J. Power Sources 2007, 166, 310–316. 10.1016/j.jpowsour.2006.12.108. [DOI] [Google Scholar]
- Chen W.-F.; Sasaki K.; Ma C.; Frenkel A. I.; Marinkovic N.; Muckerman J. T.; Zhu Y.; Adzic R. R. Hydrogen-Evolution Catalysts Based on Non-Noble Metal Nickel-Molybdenum Nitride Nanosheets. Angew. Chem., Int. Ed. 2012, 51, 6131–6135. 10.1002/anie.201200699. [DOI] [PubMed] [Google Scholar]
- Gordon R. B.; Bertram M.; Graedel T. E. Metal Stocks and Sustainability. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 1209–1214. 10.1073/pnas.0509498103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Wang H.; Xie L.; Liang Y.; Hong G.; Dai H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. 10.1021/ja201269b. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Cummins D.; Reinecke B. N.; Clark E.; Sunkara M. K.; Jaramillo T. F. Core–shell MoO3–MoS2 Nanowires for Hydrogen Evolution: A Functional Design for Electrocatalytic Materials. Nano Lett. 2011, 11, 4168–4175. 10.1021/nl2020476. [DOI] [PubMed] [Google Scholar]
- Kibsgaard J.; Chen Z.; Reinecke B. N.; Jaramillo T. F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. 10.1038/nmat3439. [DOI] [PubMed] [Google Scholar]
- Lukowski M. A.; Daniel A. S.; Meng F.; Forticaux A.; Li L.; Jin S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. 10.1021/ja404523s. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Salehi M.; Silva R.; Fujita T.; Chen M.; Asefa T.; Shenoy V. B.; Eda G.; Chhowalla M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. 10.1021/nl403661s. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Yamaguchi H.; Li J.; Silva R.; Alves D. C. B.; Fujita T.; Chen M.; Asefa T.; Shenoy V. B.; Eda G.; Chhowalla M. Enhanced Catalytic Activity in Strained Chemically Exfoliated WS2 Nanosheets for Hydrogen Evolution. Nat. Mater. 2013, 12, 850–855. 10.1038/nmat3700. [DOI] [PubMed] [Google Scholar]
- Yang J.; Voiry D.; Ahn S. J.; Kang D.; Kim A. Y.; Chhowalla M.; Shin H. S. Two-Dimensional Hybrid Nanosheets of Tungsten Disulfide and Reduced Graphene Oxide as Catalysts for Enhanced Hydrogen Evolution. Angew. Chem., Int. Ed. 2013, 52, 13751–13754. 10.1002/anie.201307475. [DOI] [PubMed] [Google Scholar]
- Cheng L.; Huang W.; Gong Q.; Liu C.; Liu Z.; Li Y.; Dai H. Ultrathin WS2 Nanoflakes as a High-Performance Electrocatalyst for the Hydrogen Evolution Reaction. Angew. Chem., Int. Ed. 2014, 53, 7860–7863. 10.1002/anie.201402315. [DOI] [PubMed] [Google Scholar]
- Choi C. L.; Feng J.; Li Y.; Wu J.; Zak A.; Tenne R.; Dai H. WS2 Nanoflakes from Nanotubes for Electrocatalysis. Nano Res. 2013, 6, 921–928. 10.1007/s12274-013-0369-8. [DOI] [Google Scholar]
- Wang H.; Kong D.; Johanes P.; Cha J. J.; Zheng G.; Yan K.; Liu N.; Cui Y. MoSe2 and WSe2 Nanofilms with Vertically Aligned Molecular Layers on Curved and Rough Surfaces. Nano Lett. 2013, 13, 3426–3433. 10.1021/nl401944f. [DOI] [PubMed] [Google Scholar]
- Andreiadis E. S.; Jacques P.-A.; Tran P. D.; Leyris A.; Chavarot-Kerlidou M.; Jousselme B.; Matheron M.; Pécaut J.; Palacin S.; Fontecave M.; Artero V. Molecular Engineering of a Cobalt-based Electrocatalytic Nanomaterial for H2 Evolution under Fully Aqueous Conditions. Nat. Chem. 2013, 5, 48–53. 10.1038/nchem.1481. [DOI] [PubMed] [Google Scholar]
- Karunadasa H. I.; Chang C. J.; Long J. R. A Molecular Molybdenum-oxo Catalyst for Generating Hydrogen from Water. Nature 2010, 464, 1329–1333. 10.1038/nature08969. [DOI] [PubMed] [Google Scholar]
- Helm M. L.; Stewart M. P.; Bullock R. M.; DuBois M. R.; DuBois D. L. A Synthetic Nickel Electrocatalyst with a Turnover Frequency above 100,000 s-1 for H2 Production. Science 2011, 333, 863–866. 10.1126/science.1205864. [DOI] [PubMed] [Google Scholar]
- Guo S.-Q.; Zhen M.-M.; Sun M.-Q.; Zhang X.; Zhao Y.-P.; Liu L. Controlled Fabrication of Hierarchical WO3·H2O Hollow Microspheres for Enhanced Visible Light Photocatalysis. RSC Adv. 2015, 5, 16376–16385. 10.1039/c4ra14312d. [DOI] [Google Scholar]
- Liu B.; Wang J.; Wu J.; Li H.; Li Z.; Zhou M.; Zuo T. Controlled Fabrication of Hierarchical WO3 Hydrates with Excellent Adsorption Performance. J. Mater. Chem. A 2014, 2, 1947–1954. 10.1039/c3ta13897f. [DOI] [Google Scholar]
- Huang J.; Xu X.; Gu C.; Yang M.; Yang M.; Liu J. Large-scale Synthesis of Hydrated Tungsten Oxide 3D Architectures by a Simple Chemical Solution Route and Their Gas-sensing Properties. J. Mater. Chem. 2011, 21, 13283–13289. 10.1039/c1jm11292a. [DOI] [Google Scholar]
- Kalantar-zadeh K.; Vijayaraghavan A.; Ham M.-H.; Zheng H.; Breedon M.; Strano M. S. Synthesis of Atomically Thin WO3 Sheets from Hydrated Tungsten Trioxide. Chem. Mater. 2010, 22, 5660–5666. 10.1021/cm1019603. [DOI] [Google Scholar]
- Liu Y.; Li Q.; Gao S.; Shang J. K. Template-free Solvothermal Synthesis of WO3/WO3 H2O Hollow Spheres and Their Enhanced Photocatalytic Activity From the Mixture Phase Effect. CrystEngComm 2014, 16, 7493–7501. 10.1039/c4ce00857j. [DOI] [Google Scholar]
- Daniel M. F.; Desbat B.; Lassegues J. C.; Gerand B.; Figlarz M. Infrared and Raman Study of WO3 Tungsten Trioxides and WO3, xH2O Tungsten Trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. 10.1016/0022-4596(87)90359-8. [DOI] [Google Scholar]
- Nayak A. K.; Lee S.; Choi Y. I.; Yoon H. J.; Sohn Y.; Pradhan D. Crystal Phase and Size-Controlled Synthesis of Tungsten Trioxide Hydrate Nanoplates at Room Temperature: Enhanced Cr(VI) Photoreduction and Methylene Blue Adsorption Properties. ACS Sustainable Chem. Eng. 2017, 5, 2741–2750. 10.1021/acssuschemeng.6b03084. [DOI] [Google Scholar]
- Popczun E. J.; McKone J. R.; Read C. G.; Biacchi A. J.; Wiltrout A. M.; Lewis N. S.; Schaak R. E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. 10.1021/ja403440e. [DOI] [PubMed] [Google Scholar]
- Wang T.; Liu L.; Zhu Z.; Papakonstantinou P.; Hu J.; Li M. Enhanced Electrocatalytic Activity for Hydrogen Evolution Reaction from Self-Assembled Monodispersed Molybdenum Sulfide Nanoparticles on an Au Electrode. Energy Environ. Sci. 2013, 6, 625–633. 10.1039/c2ee23513g. [DOI] [Google Scholar]
- Xie J.; Zhang H.; Li S.; Wang R.; Sun X.; Zhou M.; Zhou J.; Lou X. W.; Xie Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. 10.1002/adma.201302685. [DOI] [PubMed] [Google Scholar]
- Phuruangrat A.; Ham D. J.; Hong S. J.; Thongtem S.; Lee J. S. Synthesis of Hexagonal WO3 Nanowires by Microwave-Assisted Hydrothermal Method and Their Electrocatalytic Activities for Hydrogen Evolution Reaction. J. Mater. Chem. 2010, 20, 1683–1690. 10.1039/b918783a. [DOI] [Google Scholar]
- Conway B. E.; Tilak B. V. Interfacial Processes Involving Electrocatalytic Evolution and Oxidation of H2, and the Role of Chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. 10.1016/s0013-4686(02)00329-8. [DOI] [Google Scholar]
- Benck J. D.; Chen Z.; Kuritzky L. Y.; Forman A. J.; Jaramillo T. F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into The Origin of Their Catalytic Activity. ACS Catal. 2012, 2, 1916–1923. 10.1021/cs300451q. [DOI] [Google Scholar]
- Bikkarolla S. K.; Papakonstantinou P. CuCo2O4 Nanoparticles on Nitrogenated Graphene as Highly Efficient Oxygen Evolution Catalyst. J. Power Sources 2015, 281, 243–251. 10.1016/j.jpowsour.2015.01.192. [DOI] [Google Scholar]
- Huang J.; Chen J.; Yao T.; He J.; Jiang S.; Sun Z.; Liu Q.; Cheng W.; Hu F.; Jiang Y.; Pan Z.; Wei S. CoOOH Nanosheets with High Mass Activity for Water Oxidation. Angew. Chem., Int. Ed. 2015, 54, 8722–8727. 10.1002/anie.201502836. [DOI] [PubMed] [Google Scholar]
- Song F.; Hu X. Exfoliation of Layered Double Hydroxides for Enhanced Oxygen Evolution Catalysis. Nat. Commun. 2014, 5, 4477. 10.1038/ncomms5477. [DOI] [PubMed] [Google Scholar]
- Cobo S.; Heidkamp J.; Jacques P.-A.; Fize J.; Fourmond V.; Guetaz L.; Jousselme B.; Ivanova V.; Dau H.; Palacin S.; Fontecave M.; Artero V. A Janus Cobalt-based Catalytic Material for Electro-splitting of Water. Nat. Mater. 2012, 11, 802–807. 10.1038/nmat3385. [DOI] [PubMed] [Google Scholar]
- Nayak A. K.; Ghosh R.; Santra S.; Guha P. K.; Pradhan D. Hierarchical Nanostructured WO3–SnO2 for Selective Sensing of Volatile Organic Compounds. Nanoscale 2015, 7, 12460–12473. 10.1039/c5nr02571k. [DOI] [PubMed] [Google Scholar]
- Baek Y.; Yong K. Controlled Growth and Characterization of Tungsten Oxide Nanowires Using Thermal Evaporation of WO3 Powder. J. Phys. Chem. C 2007, 111, 1213–1218. 10.1021/jp0659857. [DOI] [Google Scholar]
- Li Y. H.; Liu P. F.; Pan L. F.; Wang H. F.; Yang Z. Z.; Zheng L. R.; Hu P.; Zhao H. J.; Gu L.; Yang H. G. Local Atomic Structure Modulations Activate Metal Oxide as Electrocatalyst for Hydrogen Evolution in Acidic Water. Nat. Commun. 2015, 6, 8064. 10.1038/ncomms9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skúlason E.; Karlberg G. S.; Rossmeisl J.; Bligaard T.; Greeley J.; Jónsson H.; Nørskov J. K. Density Functional Theory Calculations for the Hydrogen Evolution Reaction in an Electrochemical Double Layer on the Pt(111) Electrode. Phys. Chem. Chem. Phys. 2007, 9, 3241–3250. 10.1039/b700099e. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/physrevlett.77.3865. [DOI] [PubMed] [Google Scholar]
- Nobuhara K.; Nakanishi H.; Kasai H.; Okiji A. Interactions of Atomic Hydrogen with Cu(111), Pt(111), and Pd(111). J. Appl. Phys. 2000, 88, 6897–6901. 10.1063/1.1322067. [DOI] [Google Scholar]
- Giannozzi P.; et al. QUANTUM ESPRESSO: A Modular and Open-source Software Project for Quantum Simulations of Materials. J. Phys.: Condens. Matter 2009, 21, 395502. 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








