Abstract

Biofortification of crops to enhance provitamin A carotenoids is a strategy to increase the intake where vitamin A deficiency presents a widespread problem. Heat, light, and oxygen cause isomerization and oxidation of carotenoids, reducing provitamin A activity. Understanding provitamin A retention is important for assessing efficacy of biofortified foods. Retention of carotenoids in high-xanthophyll and high-β-carotene maize was assessed after a long-term storage at three temperatures. Carotenoid retention in high-β-cryptoxanthin maize was determined in muffins, non-nixtamalized tortillas, porridge, and fried puffs made from whole-grain and sifted flour. Retention in eggs from hens fed high-β-cryptoxanthin maize was assessed after frying, scrambling, boiling, and microwaving. Loss during storage in maize was accelerated with increasing temperature and affected by genotype. Boiling whole-grain maize into porridge resulted in the highest retention of all cooking and sifting methods (112%). Deep-fried maize and scrambled eggs had the lowest carotenoid retention rates of 67–78 and 84–86%, respectively.
Introduction
Vitamin A (VA) is a fat-soluble essential micronutrient playing critical roles in sight, cellular differentiation, development, and immunity. VA deficiency is a global burden affecting an estimated 190 million preschool-aged children and associated with blindness and increased mortality.1 Biofortification with provitamin A carotenoids is a food-based strategy to combat VA deficiency. Biofortified crops have been selectively bred to have a greater carotenoid content, with special emphasis on β-carotene, and are being disseminated in many African countries.2 Since 2013, biofortified hybrids are being grown in Zambia, Nigeria, and Ghana,3−5 with recent releases in Malawi, Zimbabwe, and Tanzania.6,7 A high-β-carotene maize variety was as efficacious as a VA supplement at increasing VA stores in Zambian children.8
In 2013, ∼1 billion metric tons of maize was produced.9 Maize supplies an estimated 800–1000 kcal/capita/day in Mexico, Central America, Zambia, and other parts of Africa,9 providing about one-third of the total caloric intake. Therefore, improving the nutritional quality of maize will positively impact the micronutrient intake and potentially VA status. In addition, egg consumption has increased globally. Nearly 65 million metric tons of eggs were produced in 2013, providing 53–75 kcal/capita/day in South America, Mexico, and China.9 Biofortification of eggs with carotenoids through feeding β-cryptoxanthin-enhanced maize to laying hens increases the provitamin A content in yolk, suggesting that eggs may be a novel, sustainable strategy to increase consumption.10 High-β-cryptoxanthin eggs are not yet available commercially.
The HarvestPlus Challenge Program’s target for provitamin A content of biofortified maize is 15 μg β-carotene equiv/g fresh weight [17 μg/g dry weight (DW)].11 Maize typically undergoes several processing steps postharvest for consumption, such as cleaning, milling, storage, and cooking (Figure 1).12,13 Processing of grain disrupts the food matrix and increases bioaccessibility of nutrients.14 However, exposure to heat, light, and air causes isomerization and oxidation of carotenoids in foods, reducing the amount in the final food.15
Figure 1.
Major processes and end products involved in raw kernel processing (reproduced from Nuss and Tanumihardjo12).
Provitamin A degradation in many genotypes of biofortified maize grain may reach 60–65% when stored for 6 months under traditional conditions.16−18 A 40% reduction in β-carotene equivalents occurred during the first 2 weeks of maize kernel storage in ambient conditions for four maize varieties.19 Retention also depends on flour type, where coarser hammer meal retention (∼73%) was higher than in finer refined meal (64–69%) after 4 months.13 β-Cryptoxanthin may be more stable than β-carotene in some genotypes. The retention of carotenoids with prolonged storage should be compared across several temperatures that simulate household methods used in sub-Saharan Africa, Mexico, and South and Central America.
Baking maize muffins resulted in 65–75% retention of carotenoids compared with 52–60% retention after cooking porridge and 56–65% after extrusion into corn puffs.20 In stiff porridge, 78–129% of carotenoids were retained compared with 64–78% in thin porridge.21 Nixtamalized maize retained 85–100% provitamin A as tortillas22 and 64% after deep-frying.23 However, only 20% retention occurred after frying flour into tortilla chips.24 Because of the variation in the methods and genotypes, additional retention and characterization studies are needed on biofortified maize varieties that are being introduced to alleviate VA deficiency, using typical household storage conditions and processing methods. Furthermore, although several studies have reported differing results of maize carotenoid retention, no studies have investigated postcooking retention in β-cryptoxanthin-biofortified eggs. Eggs contain a different food matrix that differs substantially from that of maize, which may influence retention. This series of studies evaluated carotenoid losses during storage in maize flour and several household cooking methods using β-cryptoxanthin-biofortified maize flour and β-cryptoxanthin-enhanced eggs.
Materials and Methods
Preparation of Biofortified Maize Flour
For maize flour storage, dried whole maize cobs from two maize genotypes, the KUI synthetic high in xanthophylls (i.e., lutein and zeaxanthin) and F2 seed of the C17/DEexp F1 that is high in β-carotene (Table 1), were received from the University of Illinois at Urbana-Champaign and stored at −80 °C upon arrival. These two genotypes were selected due to high carotenoid content and potential for implementation in breeding programs to improve VA status in Zambia and other locations. These genotypes were developed through conventional breeding and grown in Champaign, Illinois. Kernels were removed from the cobs, ground using a C&N hammer mill #8 (Christy-Norris, Ltd., Ipswich, U.K.), and sifted with a 1 mm sieve.
Table 1. Initial Carotenoid Concentrations (nmol/g DW) of High Xanthophyll (KUI Synthetic) and High β-Carotene (C17/DEexp) Maize Genotypes Used in a Maize Flour Storage Retention Studya.
| lutein + zeaxanthin | β-cryptoxanthin | total β-caroteneb | |
|---|---|---|---|
| high xanthophyll | 66.8 ± 0.8a | 5.9 ± 0.0 | 6.8 ± 0.1 |
| high β-carotene | 27.3 ± 1.1 | 1.1 ± 0.1 | 26.1 ± 1.4 |
All of the values shown are means of three determinations ± standard deviation (SD).
Sum of all (E)- and (Z)-β-carotenes.
A high-β-cryptoxanthin synthetic maize was used to assess carotenoid retention after household cooking. High β-cryptoxanthin maize was conventionally bred at the International Maize and Wheat Improvement Center through the HarvestPlus program and grown in Agua Fria, Puebla, Mexico. Ears were harvested, dried, and stored at −20 °C prior to shipping as grain to University of Wisconsin—Madison. Whole kernels were milled into course meal using a Meadows 8-in. stone-burr mill in Lone Rock, WI. Mill stones were set 1.6 mm apart. All of the ground material was manually sifted; the fraction that passed through a 1.7 mm sieve was considered “whole-grain” or coarse maize meal, and the fraction that passed through a 0.60 mm sieve was considered “sifted” or refined maize meal. All of the maize kernels and flour were stored at −30 °C for the duration of this study.
Production of Biofortified Eggs
Biofortified eggs were produced in Single Comb White Leghorn hens as previously described.25 All of the procedures involving animals were approved by the University of Wisconsin—Madison College of Agricultural and Life Sciences’ Animal Care and Use Committee. Hen feed consisted of either biofortified high-β-cryptoxanthin maize or white maize with freeze-dried tangerine peel (as a source of β-cryptoxanthin) to produce feed equivalent in β-cryptoxanthin content after saponification of the feed. Fresh eggs were collected daily and stored in a conventional refrigerator at 2 °C in standard cardboard or Styrofoam egg cartons until cooking.
Maize Flour Storage
Maize carotenoid stability in flour during storage was assessed at three temperatures and five sampling times. The storage conditions included −20 °C freezer, ∼22 °C room temperature, and 37 °C simulated tropical temperature in an oven. Maize flour was prepackaged into air- and light-impermeable vacuum-sealed plastic bags using a Selovac vacuum chamber (model 200B) and stored inside opaque secondary containers. Maize flour was analyzed for carotenoids at time 0 to establish baseline (Table 1) before transferring the sealed bags to their respective long-term storage temperatures. The individual bags were opened and analyzed in triplicate at 0.5, 1, 2, 4, 6, 8, and 12 months.
Cooking Methods
Seven replicates of each recipe for eggs and maize flour type were prepared. Retention of carotenoids from biofortified maize was assessed in muffins, tortillas, thick porridge, and deep-fried puffs made from whole-grain or sifted flour. Household cooking methods for maize flour were selected because variants of these foods are consumed across cultures worldwide. Aliquots of uncooked and cooked maize dishes were weighed and then freeze-dried using a VirTis benchtop lyophilizer (6K BT EL-85). Samples were again weighed when dry and held at −80 °C until high-performance liquid chromatography (HPLC) analysis.
For porridge, a typical Zambian “nshima” recipe was used.26,27 Briefly, water (175 g) was brought to boil. An additional 25 g water was added to 25 g cornmeal and stirred to form a paste, which was added to the pot of boiling water and stirred. After 2 min, another 25 g maize meal was added. Porridge was stirred constantly for an additional 8 min. The final temperature at the end of cooking was 95–98 °C.
A standardized muffin recipe was used that included sugar, egg white (yolks were discarded to not add other carotenoids to the muffins), milk, peanut oil, salt, and baking powder to produce baked muffins that weighed 48.4 ± 1.6 g. Muffins were baked for 14 min at 204 °C.
For each tortilla recipe, 63 g non-nixtamalized maize meal was mixed with 37.5 g water; from this mixture, 50 g spheres of dough were formed and pressed in a tortilla press to 13 cm diameter, approximately 3 mm thick. Non-nixtamalized maize flour is used in unleavened flatbreads, such as maize roti in Indian cuisine and arepa in South America (Figure 1). Tortillas were cooked in a frying pan preheated to 150 °C for 1.5 min on each side. Final temperature was 104–127 °C.
For maize meal puffs, a standardized recipe was used including sugar, egg white, milk, oil, and baking powder. Scoops of batter (15 g) were deep-fried individually in peanut oil for 3 min, turning periodically. Oil temperature was maintained between 177 and 185 °C. Excess oil was strained back into the pot. Frying oil was weighed before and after cooking and used to adjust carotenoid content of puffs to account for absorption of oil into the cooked food. Peanut oil was chosen because it contains minimal carotenoids.28
Carotenoid retention in eggs from chickens fed either biofortified high-β-cryptoxanthin orange maize or white maize with tangerine peel fortificant was assessed after frying, scrambling, boiling, and microwaving. Eggs were chosen at random for each cooking treatment. All of the eggs, except those that were hard-boiled, were cooked individually. For scrambled eggs, whites and yolks were separated and weighed individually. The yolks and whites were then whisked together and poured into a nonstick skillet that had been preheated for 3 min. The eggs were cooked for 3.5 min while the pan was tilted and scraped. Fried eggs were cracked into a nonstick skillet that had been preheated for 3 min and were cooked for 3 min on each side. To microwave, whole eggs without shells were placed in a microwave-safe glass dish and cooked for 75 s on 50% power in a GE Profile 60 Hz household microwave oven (Louisville, KY). To hard-boil the eggs, whole-shelled eggs were placed in a single layer in a large pot and covered with 3–5 cm cold water. Eggs were boiled for 10 min, cooled with running tap water for 5 min, and peeled. Except for scrambled eggs, cooked yolks were separated from whites. Cooked eggs and yolks were then placed in amber vials, flushed with nitrogen, and stored at −80 °C.
Carotenoid Extraction and Analysis
All of the extraction protocols were performed under yellow light to reduce photodegradation of carotenoids. Maize flour and food samples were ground with a mortar and pestle to a fine powder, and extracted using a modified method.29 Briefly, 6 mL ethanol with 0.1% butylated hydroxytoluene as antioxidant was added to 0.5 g sample and incubated for 5 min at 85 °C. Samples were saponified with potassium hydroxide/water (500 μL, 80:20, w/v) for 10 min, mixed every 5 min. The reaction was quenched with 3 mL deionized water and mixed with a vortex. Two hundred microliters of β-apo-8′-carotenal was added after saponification as an internal standard to account for mechanical losses. Carotenoids were extracted four times with hexanes. Extracts were pooled, dried under nitrogen, reconstituted in 500 μL 50:50 methanol/dichloroethane (v/v), and 50 μL was injected into the HPLC system. Extraction efficiency ranged from 85 to 100%.
For egg extractions, 0.1–0.2 g microwaved, hard-boiled, and fried yolks or 0.3–0.4 g scrambled eggs were used. Two milliliters of Milli-Q water was added, mixed by vortex, followed by 3 mL ethanol (0.1% butylated hydroxytoluene), and mixed. Tubes were placed in a 60 °C water bath for 5 min, mixed by vortex, and potassium hydroxide/water (700 μL, 30:70, w/v) was added. Tubes were mixed and returned to the 60 °C water bath for 30 min, mixing every 15 min. Samples were immediately placed on ice and 3 mL cold deionized water was added and mixed. Two hundred microliters of β-apo-carotenal was added to the samples as an internal standard, mixed with a vortex, and 100 μL 2-propanol was added. Carotenoids were extracted three times with 4 mL hexanes/ethyl acetate (9:1). Organic layers were pooled, dried under nitrogen, reconstituted with 500 μL 50:50 methanol/dichloroethane, and 50 μL was injected into the HPLC system.
HPLC analysis for all of the samples was conducted according to a method used by Davis et al.30 The Waters HPLC system was composed of a binary pump, autosampler, and photodiode array detector (Milford, MA). A 40 min gradient system using two mobile phases was employed, with solvent A consisting of methanol/water 92:8 (v/v) with 10 mmol/L ammonium acetate and solvent B consisting of 100% methyl-tert-butyl ether. The system was run at 1 mL/min with solvent A at 70% transitioning to 40% over 30 min, with a 10 min equilibration before the next injection. Chromatograms were produced at 450 and 515 nm for quantification of carotenoids and the β-apo-carotenal internal standard. Efficiency of extraction was used to adjust the final carotenoid concentration values. Standard curves were generated regularly for identification and quantification of lutein, zeaxanthin, β-cryptoxanthin, α-carotene, and β-carotene using HPLC-purified standards.
Percent nutrient retention was calculated for each of the carotenoids of interest on a dry weight (DW) basis according to the following equation
| 1 |
Statistical Analysis
Statistical analysis was conducted using SAS statistical software (version 9.4; SAS Institute, Cary, NC) using Proc Mixed. Fisher’s least significant difference (LSD) pairwise comparisons were used to compare the carotenoid content and retention across storage treatments at final time point, across maize milling and cooking methods, and across egg type and cooking method. Confidence intervals for retention means were calculated for each storage time point to determine significant differences from 100% at baseline.
Results
Maize Storage
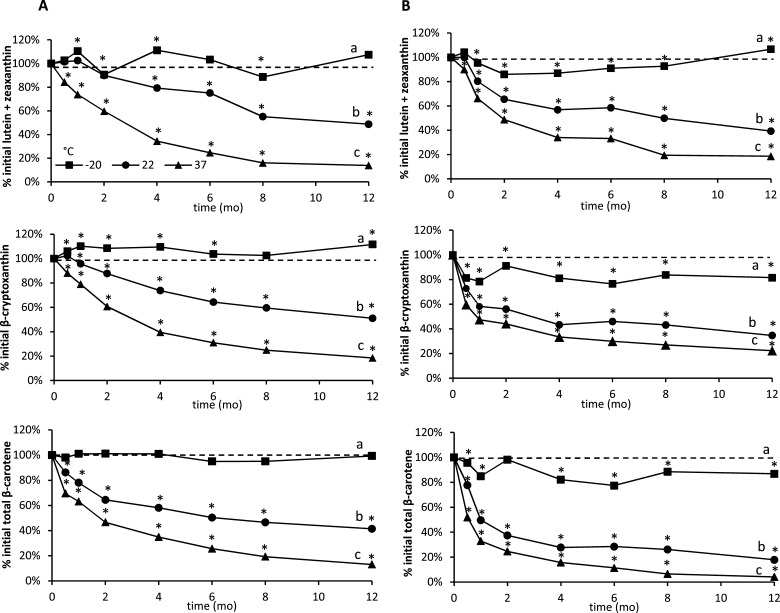
Carotenoid retentions over time in the high-xanthophyll and high-β-carotene maize flours are shown in Figure 2 compared with baseline values represented as 100%. Significant differences in percent carotenoid retention across storage temperatures for each line of maize were observed as early as 0.5 month, especially at 37 °C. Generally, carotenoid concentrations for both maize genotypes decreased over time and were significantly different across all of the storage temperatures at 12 months. Total carotenoid retention varied by storage temperature as well as genotype, with the high-xanthophyll maize retaining higher amounts of carotenoids as a percentage of the original at all of the temperature conditions than the high β-carotene maize. As expected, freezer storage at −20 °C resulted in the highest retention of all of the carotenoids, followed by 22 and 37 °C, in both maize lines with little change in the high-xanthophyll line and only a modest loss in provitamin A activity (<20% loss at 12 months).
Figure 2.
Effect of storage temperature and time on carotenoid degradation in (A) high-xanthophyll maize (KUI synthetic) and (B) high-β-carotene maize (C17/DEexp) flours. All of the values are shown as mean of three determinations. Carotenoid retention was calculated by dividing carotenoid concentration at each time point by baseline values. Significant changes in carotenoid concentration from baseline were determined using 95% confidence intervals, and are indicated with an asterisk. Different letters indicate Fisher’s LSD-adjusted significant differences (P < 0.05) in carotenoid retention between storage temperatures for each graph.
Trendlines were developed to model carotenoid degradation. Decay of carotenoids in the high-xanthophyll maize followed a linear decay model at 22 °C and a quadratic model at 37 °C. Decay of carotenoids in the high-β-carotene maize occurred in two phases for the 22 and 37 °C storage treatments. The first phase was exponential lasting for 2 months followed by a linear decay for the last 10 months. Decay of β-carotene in the high-xanthophyll variety followed a quadratic decay model at 22 and 37 °C. Decay of β-carotene in the high-β-carotene variety also occurred in two phases as noted for total carotenoids. Decay of all of the xanthophylls (i.e., lutein, zeaxanthin, and β-cryptoxanthin) in the high-xanthophyll maize followed a linear model at 22 °C and a quadratic model at 37 °C. Decay of all of the xanthophylls (i.e., lutein, zeaxanthin, and β-cryptoxanthin) in the high-β-carotene maize occurred in two phases as observed for total carotenoids. For the first 2 months, decay was exponential, followed by linear decay.
Maize Flour Cooking
Carotenoid retention after cooking the four maize foods prepared from high-β-cryptoxanthin maize is shown in Table 2. The whole-grain maize flour had significantly higher lutein, zeaxanthin, β-carotene, and total carotenoids compared with the sifted maize flour; however, the flours did not differ in the β-cryptoxanthin content. Total carotenoid retention was close to or exceeded 100% across most food types, ranging from 67% (sifted flour puffs) to 112% (whole-grain flour porridge). Carotenoid retention rates of >100% are common19,21 and often attributed to better extractability after processing. For porridge, puffs, and muffins, the whole-grain maize retained a significantly higher proportion of the original β-cryptoxanthin content compared with the sifted flour versions of these foods. For β-carotene and β-cryptoxanthin, but not lutein and zeaxanthin, there was a significant interaction between cooking and milling methods, such that lower retention of these provitamin A carotenoids was observed for sifted flour puffs (86 and 88%, respectively) compared with whole-grain puffs (118 and 110%, respectively). Conversely, significantly lower retention of lutein and zeaxanthin was observed after deep-frying for both flour types, compared with the other cooking methods. Final carotenoid content in the cooked foods differed significantly across all of the food types of a given milling method, with porridge containing 33–44 nmol/g DW, followed by tortillas (27–35 nmol/g DW), muffins (13–19 nmol/g DW), and puffs (9–10 nmol/g DW). (Note: 1 nmol β-carotene equivalents is 537 ng.)
Table 2. Final Carotenoid Concentrations (nmol/g DW) of Whole-Grain and Sifted High-β-Cryptoxanthin-Biofortified Maize after Four Different Cooking MethodsA.
| carotenoid
concentration |
||||||
|---|---|---|---|---|---|---|
| cooking method | lutein | zeaxanthin | β-cryptoxanthin | all-(E)-β-carotene | total carotenoids | |
| whole-grain | ||||||
| flour | 4.8 ± 0.2a | 20.9 ± 0.5b | 9.6 ± 0.2b | 4.0 ± 0.1b | 39.2 ± 0.5b | |
| porridge | 5.2 ± 0.2a | 23.3 ± 0.8a | 10.6 ± 0.3a | 5.0 ± 0.2a | 44.0 ± 1.4a | |
| tortillas | 3.8 ± 1.2b | 16.7 ± 4.9c | 10.3 ± 0.9a | 4.1 ± 0.4b | 34.8 ± 7.0c | |
| puffs | 0.6 ± 0.2f | 3.2 ± 0.5g | 3.8 ± 0.2f | 1.7 ± 0.1e | 9.3 ± 0.9g | |
| muffins | 2.0 ± 0.2d | 9.2 ± 0.8e | 5.8 ± 0.5d | 2.6 ± 0.3d | 19.6 ± 1.7e | |
| sifted flour | ||||||
| flour | 3.8 ± 0.3b | 17.4 ± 1.5c | 9.5 ± 0.4b | 3.6 ± 0.2c | 34.3 ± 2.3c | |
| porridge | 3.7 ± 0.4b | 17.3 ± 1.8c | 8.7 ± 0.3c | 3.5 ± 0.1c | 33.2 ± 2.5c | |
| tortillas | 2.9 ± 0.1c | 13.0 ± 0.4d | 8.3 ± 0.3c | 2.8 ± 0.2d | 27.0 ± 0.9d | |
| puffs | 0.8 ± 0.1f | 3.9 ± 0.4g | 3.9 ± 0.2f | 1.5 ± 0.1e | 10.0 ± 0.7g | |
| muffins | 1.4 ± 0.1e | 6.0 ± 0.6f | 4.7 ± 0.4e | 1.6 ± 0.3e | 13.7 ± 1.0f | |
| carotenoid
retention (%)B |
||||||
|---|---|---|---|---|---|---|
| cooking method | lutein | zeaxanthin | β-cryptoxanthin | all-(E)-β-carotene | total carotenoidsC | |
| whole-grain | ||||||
| flour | 100 | 100 | 100 | 100 | 100 | |
| porridge | 108 ± 7.2a | 112 ± 4.6a | 110. ± 2.1ab | 125 ± 4.9a | 112 ± 3.7a | |
| tortillas | 82.7 ± 29.6b | 80.7 ± 26.3c | 93.1 ± 11.6c | 92.8 ± 13.2de | 85.6 ± 20.4cd | |
| puffs | 43.9 ± 12.9c | 55.8 ± 11.5d | 119 ± 11.8a | 111 ± 8.7b | 77.9 ± 10.7de | |
| muffins | 98.5 ± 20.9ab | 103 ± 25.5ab | 106 ± 18.2b | 108 ± 19.0bc | 103 ± 19.1ab | |
| sifted flour | ||||||
| flour | 100 | 100 | 100 | 100 | 100 | |
| porridge | 98.2 ± 11.0ab | 99.8 ± 11.7ab | 91.6 ± 4.2c | 98.2 ± 5.4cd | 97.1 ± 8.4bc | |
| tortillas | 99.0 ± 4.6ab | 92.6 ± 3.17bc | 91.7 ± 2.2c | 86.7 ± 2.3de | 93.8 ± 3.3bc | |
| puffs | 51.7 ± 7.3c | 53.4 ± 7.0d | 88.0 ± 6.5c | 86.1 ± 8.3de | 67.2 ± 6.7e | |
| muffins | 98.1 ± 16.1ab | 91.2 ± 13.1bc | 89.9 ± 6.6c | 82.8 ± 14.9e | 90.1 ± 9.7b,c,d | |
All of the values are shown as the mean of seven determinations ± SD. Different letters within a column indicate Fisher’s LSD-adjusted significant differences (P < 0.05) between means across all maize milling and cooking preparations.
Percent retention is calculated by dividing the concentration (nmol/g DW) of each carotenoid species in the raw maize food samples by carotenoid concentration (nmol/g DW) of the cooked samples.
Total carotenoids represents the sum of the carotenoids quantified; lutein, zeaxanthin, β-cryptoxanthin, and all-(E)-β-carotene.
Egg Cooking
Table 3 shows the carotenoid content of both biofortified egg types, raw and cooked. Hen eggs accumulate xanthophyll carotenoids better than it does hydrocarbons.25 Very low amounts of α- and β-carotene were present in the eggs from either biofortified maize-fed or tangerine peel-fed chickens, i.e., ≤0.8 and ≤1.1 nmol/g DW for α- and β-carotene, respectively. Thus, percent retention was not determined for these carotenoids. For retention of total carotenoids, a significant main effect of diet on retention was observed, with biofortified maize eggs retaining higher concentrations of carotenoids after cooking than tangerine peel-derived eggs, which contained <50% of total carotenoids of the maize-derived eggs, for all of the cooking methods excluding scrambling (Table 3). Values close to 100% retention were observed for all of the egg cooking methods, with the exception of scrambling.
Table 3. Final Carotenoid Concentrations (nmol/g DW) of Biofortified Chicken Eggs Produced from Hens Fed High-β-Cryptoxanthin Maize or Tangerine Peel-fortified Feed after Four Different Cooking MethodsA.
| carotenoid
concentration |
|||||
|---|---|---|---|---|---|
| hen diet | cooking method | lutein | zeaxanthin | β-cryptoxanthin | total carotenoidsC |
| tangerine peel | raw | 12.8 ± 1.1 | 11.9 ± 1.1 | 4.7 ± 0.8c | 30.2 ± 2.9 |
| microwaved | 10.6 ± 1.5 | 9.9 ± 1.4 | 3.8 ± 0.5d | 25.2 ± 3.2 | |
| hard boiled | 11.0 ± 1.2 | 10.2 ± 1.2 | 4.0 ± 0.6cd | 26.2 ± 2.9 | |
| fried | 11.1 ± 1.4 | 10.1 ± 1.3 | 4.2 ± 0.8cd | 26.5 ± 3.2 | |
| scrambled | 10.5 ± 1.7 | 9.9 ± 1.4 | 3.9 ± 0.8cd | 25.4 ± 4.0 | |
| biofortified maize | raw | 17.7 ± 3.6 | 40.3 ± 8.1 | 8.3 ± 0.8a | 67.8 ± 12.6 |
| microwaved | 18.5 ± 2.0 | 39.5 ± 6.6 | 8.1 ± 0.8a | 67.7 ± 9.2 | |
| hard boiled | 18.7 ± 2.5 | 41.9 ± 5.2 | 8.0 ± 0.5a | 70.3 ± 8.0 | |
| fried | 18.3 ± 3.2 | 40.4 ± 7.0 | 8.4 ± 0.6a | 68.9 ± 10.8 | |
| scrambled | 15.4 ± 1.5 | 34.7 ± 3.7 | 6.9 ± 0.6b | 58.5 ± 5.7 | |
| xanthophyll
retention (%)B |
|||||
|---|---|---|---|---|---|
| hen diet | cooking method | lutein | zeaxanthin | β-cryptoxanthin | total carotenoids |
| tangerine peel | raw | 100 | 100 | 100 | 100 |
| microwaved | 84.5 ± 11.0b | 82.8 ± 10.7c | 80.2 ± 9.3c | 83.5 ± 9.9c | |
| hard boiled | 86.6 ± 90.2b | 85.7 ± 9.0bc | 85.7 ± 12.3bc | 86.7 ± 9.0c | |
| fried | 87.1 ± 10.1b | 84.6 ± 10.1bc | 90.0 ± 15.6abc | 87.6 ± 9.8bc | |
| scrambled | 82.4 ± 12.3b | 82.9 ± 11.1c | 84.1 ± 15.7c | 83.9 ± 12.1c | |
| biofortified maize | raw | 100 | 100 | 100 | 100 |
| microwaved | 104 ± 10.2a | 98.0 ± 15.1ab | 97.5 ± 8.5ab | 100. ± 12.5ab | |
| hard boiled | 105 ± 13.3a | 104 ± 11.9a | 97.4 ± 5.2ab | 104 ± 10.9a | |
| fried | 103 ± 16.8a | 100. ± 16.0a | 102 ± 6.2a | 102 ± 14.7a | |
| scrambled | 86.8 ± 7.9b | 86.2 ± 8.5bc | 84.0 ± 7.1c | 86.4 ± 7.8c | |
All of the values are shown as the mean of seven determinations ± SD, except for raw eggs (mean of five determinations). Different letters within a column indicate Fisher’s LSD-adjusted significant differences (P < 0.05) between means across egg types and cooking treatments.
Percent retention is calculated by dividing carotenoid content of cooked eggs by carotenoid content of raw eggs.
Total carotenoids represents the sum of the carotenoids quantified; lutein, zeaxanthin, and β-cryptoxanthin.
Discussion
This study demonstrated significant effects of genotype and storage temperature on carotenoid retention in maize, and differential effects of several common cooking methods on carotenoid retention in biofortified maize and β-cryptoxanthin-enhanced eggs. Depending on the cooking method, carotenoids were well-preserved, as in the case of maize porridge, or degraded as a result of cooking, such as after deep-frying. Retention values greater than 100% after cooking could reflect changes in the food matrix, with cooking leading to improved extractability of carotenoids, or deactivation of oxidative enzymes that would degrade carotenoids, rather than true increases in carotenoid content.
Regarding carotenoid retention with storage temperature, genotypic variation was observed as previously published.13,16,19 Significant differences in retention occurred between the two genotypes and across storage conditions, with high β-carotene maize exhibiting greater losses of carotenoids at all of the storage conditions compared with high-xanthophyll maize, and an inverse trend of carotenoid content with increasing temperature. Even though the high β-carotene maize initially had higher amounts of the provitamin A carotenoid, by 12 months at 37 °C storage, both maize genotypes had less than 1 nmol/g of β-carotene, emphasizing the importance of avoiding prolonged heat exposure when storing biofortified maize flour. In both genotypes, decay occurred at a faster rate in the first month and gradually plateaued; this is a similar pattern to that observed by Mugode et al. and Ortiz et al. but different from that observed by Burt et al., who noted relative stability of carotenoids in high-carotentoid maize lines from 0 to 3 months and significant degradation starting at 3–6 months.16,18,19 Genotype effects have also been observed for provitamin A bioefficacy in Mongolian gerbils.31 Thus, during breeding and germplasm development, germplasm exhibiting lower rates of degradation should be identified, and ideally, maize genotypes close to release should be evaluated for both storage and efficacy effects.
Xanthophyll content increased during −20 °C storage in the high-xanthophyll maize line; Scott and Eldridge also observed increases in maize carotenoid content after cold storage,32 which may preserve the enzymatic pathways for biosynthetic carotenoid flux. Apparent retention values greater than 100% for one or more individual carotenoids were also observed for porridge, puffs, and muffins. Processing and cooking maize flour may separate carotenoids from the food matrix and increase their extractability from the food, resulting in an apparent increase in their content.15 Higher carotenoid concentration in cooked foods compared with the raw foods has been observed in sweet potato33 and maize food products previously.13,19,22
For porridge, tortillas, and muffins, final carotenoid content was higher in the coarser whole-grain versions of these foods compared with the sifted maize meal versions. This finding suggests that milling and sifting methods could be an important consideration to maximize biofortification efforts targeting areas that consume large amounts of maize. Coarser flours could potentially lead to greater amounts of carotenoids in the final food products due to greater integrity of the cellular components and decreased oxygen exposure.22 In this study, greater than 100% retention was observed for carotenoids in maize porridge, higher than 86% retention of β-carotene equivalents noted by Li et al.34 but similar to Mugode et al.19 who observed over 100% retention of β-carotene equivalents after cooking provitamin A carotenoid biofortified maize into thin and thick porridges. For tortillas and muffins, over 80% retention of total carotenoids was documented, which is in contrast to Muzhingi et al., who observed 30–50% retention of carotenoids after baking yellow maize flour into muffins.35 Similar to the current results, Rosales et al. observed >80% retention of carotenoids in tortillas; however, the latter study used traditionally nixtamalized flour,22 which was not investigated in the current study.
The biofortified maize-derived eggs retained more carotenoids than the tangerine-peel eggs, which may have been due to much higher concentrations in the maize-derived eggs before cooking, even though the hen feeds were equalized for β-cryptoxanthin concentration.25 Microwaving, hard-boiling, and pan-frying eggs preserved carotenoids, whereas scrambling resulted in greater carotenoid loss. Continuous agitation and loss of matrix integrity in combination with high heat increases the surface area exposed to heat and atmospheric oxygen, accelerating carotenoid loss in foods.13,22 Because scrambling retention differed significantly from pan-frying, which used the same pan and stovetop conditions and twice the cooking time, the process of scrambling decreased retention of carotenoids between the two methods. Scrambling of biofortified eggs in areas with VA deficiency should be discouraged if maximal carotenoid retention is desired.
Deep-frying maize dough in hot oil into puffs resulted in lower total carotenoid retention and 86–110% β-carotene retention. Other common names for this food are hush-puppies and fritters. It was previously suggested that frying decreases carotenoid content due to the high heat used, or leaching into the frying oil.36 Large carotenoid losses were also observed when nixtamalized tortillas were fried to produce chips.23 Notably, the provitamin A carotenoids β-carotene and β-cryptoxanthin were well-preserved relative to lutein and zeaxanthin. However, the retention found with the maize puffs is slightly higher than the 77–86% β-carotene retention reported by Vimala et al. after frying orange-fleshed sweet potatoes.33
Different particle-sized maize products are produced by milling methods, improving digestibility and usability. In many sub-Saharan countries, milling is accomplished using either hammer or roller mills.37 “Super meal” for porridge has been produced previously for retention research by roller-milling whole kernels and sifting out the <0.5 mm particle size fraction.21 Stone milling and grinding maize by hand (pounding) is practiced as well; the latter being particularly important in rural areas and situations where commercial milling services are not economically feasible.37 Given the diversity of milling and processing methods utilized worldwide, this study included flours of two different particle-sized fractions; however, both whole-grain meal and sifted meal were shown to have good retention of provitamin A carotenoids, suggesting that sifting the maize flour prior to cooking did not diminish subsequent provitamin A content of porridge. Furthermore, hammer versus refined milling of biofortified high-β-carotene maize did not affect the provitamin A bioefficacy in Mongolian gerbils.38 Because maize is milled in batches and stored in flour form for varying time periods, additional studies need to be done in milled, biofortified maize to understand the effect of milling method and particle size on storage retention; although in red pepper powder, coarse grinding resulted in greater retention of carotenoids during cold storage.39 As genotype affects the extent to which maize retains carotenoids with storage; future studies should also probe the effect of maize genotype on carotenoid retention from cooking biofortified maize and eggs.
In this study, carotenoids were observed to degrade rapidly when maize flour was stored at room temperature or above, emphasizing the importance of the postmilling storage step in the processing of high-carotenoid biofortified foods into the final consumed products. It is noteworthy that biofortified maize and eggs retained >80% provitamin A carotenoids with cooking, given that both of these foods are not widely consumed in the raw state. Overall, lutein, zeaxanthin, and the provitamin A carotenoids β-carotene and β-cryptoxanthin were retained well after household cooking of biofortified maize and eggs into widely consumed dishes with the exception of fried maize puffs. These findings further support the likely effectiveness of the biofortification strategy to increase consumption of provitamin A from maize and egg dishes.
Acknowledgments
The authors thank Emily Heying for overseeing egg production, Kevin Pixley for assisting with the development of the high β-cryptoxanthin maize, and Peter Crump for statistical consultation.
Glossary
Abbreviation
- VA
vitamin A
Author Present Address
# Department of Agronomy, Purdue University, 915 West State Street, 47907 West Lafayette, Indiana, United States (T.R.).
Author Present Address
⊥ Soil and Crop Sciences Department, Texas A&M University, 2474 TAMU, 77843 College Station, Texas, United States (J.A.H.).
Author Present Address
∥ Global R&D Nutrition Sciences, PepsiCo, 555 West Monroe Street, 60661 Chicago, Illinois, United States (S.R.G.).
Author Contributions
M.S. conducted research on maize flour cooking, analyzed samples and data, and wrote the first draft of the manuscript. J.Y. assisted in maize flour cooking and sample analysis. N.P-R. oversaw grain production and assisted in procuring funding and research design. S.R.G. conducted the egg retention work. J.A.H. developed maize analysis procedures, conducted the maize flour degradation work, and assisted in procuring funding. C.R.D. analyzed samples. T.R. produced the maize used in the maize flour retention study. S.A.T. designed the research, procured funding, and revised the manuscript. All of the authors read and approved the final manuscript.
This research was supported by Modernizacion Sustentable de la Agricultura (MASAGRO), a program of SAGARPA-Mexico in collaboration with the International Center of Wheat and Maize Improvement, USDA Hatch WIS01528 and WIS01804, and HarvestPlus contract number 8029. HarvestPlus (www.harvestplus.org) is a global alliance of agriculture and nutrition research institutions working to increase the micronutrient density of staple food crops through biofortification. The views expressed do not necessarily reflect those of HarvestPlus.
The authors declare no competing financial interest.
References
- WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005. WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, 2009. [Google Scholar]
- Tanumihardjo S. A.; Ball A. M.; Kaliwile C.; Pixley K. V. The research and implementation continuum of biofortified sweet potato and maize in Africa. Ann. N. Y. Acad. Sci. 2017, 1390, 88–103. 10.1111/nyas.13315. [DOI] [PubMed] [Google Scholar]
- Pixley K.; Palacios-Rojas N.; Babu R.; Mutale R.; Surles R.; Simpungwe E.. Biofortification of Maize with Provitamin A Carotenoids. In Carotenoids and Human Health; Tanumihardjo S. A., Ed.; Springer Science: New York, 2013; pp 271–292. [Google Scholar]
- Alamu E. O.; Maziya-Dixon B.; Menkir A.; Olaofe O. Effects of husk and harvesting on provitamin A activity and sensory properties of boiled fresh orange maize hybrids. J. Food Qual. 2015, 38, 387–395. 10.1111/jfq.12158. [DOI] [Google Scholar]
- Smale M.; Simpungwe E.; Biro E.; Kassie G. T.; de Groote H.; Mutale R. The changing structure of the maize seed industry in Zambia: Prospects for orange maize. Agribusiness 2015, 31, 132–146. 10.1002/agr.21384. [DOI] [Google Scholar]
- Simpungwe E.; Dhliwayo T.; Palenberg M.; Taleon V.; Birol E.; Oparinde A.; Saltzman A.; Diressie M. T. Orange maize in Zambia: Crop development and delivery experience. Afr. J. Food, Agric., Nutr. Dev. 2017, 17, 11973–11999. 10.18697/ajfand.78.HarvestPlus08. [DOI] [Google Scholar]
- Hwang T.; Ndolo V. U.; Katundu M.; Nyirenda B.; Bezner-Kerr R.; Arntfield S.; Beta T. Provitamin A potential of landrace orange maize variety (Zea mays L.) grown in different geographical locations of central Malawi. Food Chem. 2016, 196, 1315–1324. 10.1016/j.foodchem.2015.10.067. [DOI] [PubMed] [Google Scholar]
- Gannon B.; Kaliwile C.; Arscott S. A.; Schmaelzle S.; Chileshe J.; Kalungwana N.; Mofu M.; Pixley K.; Masi C.; Tanumihardjo S. A. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. 10.3945/ajcn.114.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT. Food Balance Sheets, 2013. http://faostat3.fao.org/download/FB/FBS/E (accessed April 12, 2017).
- Liu Y. Q.; Davis C. R.; Schmaelzle S. T.; Rocheford T.; Cook M. E.; Tanumihardjo S. A. β-Cryptoxanthin biofortified maize (Zea mays) increases β-cryptoxanthin concentration and enhances the color of chicken egg yolk. Poult. Sci. 2012, 91, 432–438. 10.3382/ps.2011-01719. [DOI] [PubMed] [Google Scholar]
- Mulualem T. Application of biofortification through plant breeding to improve the value of staple crops. Biomed. Biotechnol. 2015, 3, 11–19. 10.12691/bb-3-1-3. [DOI] [Google Scholar]
- Nuss E. T.; Tanumihardjo S. A. Maize: A paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. 10.1111/j.1541-4337.2010.00117.x. [DOI] [PubMed] [Google Scholar]
- Taleon V.; Mugode L.; Cabrera-Soto L.; Palacios-Rojas N. Carotenoid retention of biofortified maize in different post-harvest storage and packaging methods. Food Chem. 2017, 232, 60–66. 10.1016/j.foodchem.2017.03.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martinez L. X.; Garcia H. S.; Autónoma U.; De Química F.; Colón P.. Processing of Corn (Maize) and Compositional Features. Processing and Impact on Active Components in Food; Elsevier: London, 2015; Chapter 39, pp 329–336. [Google Scholar]
- Rodriguez-Amaya D. B.Carotenoids and Food Preparation: The Retention of Provitamin A Carotenoids in Prepared, Processed, and Stored Foods; OMNI/USAID: Washington, DC, 1997; pp 1–93. [Google Scholar]
- Burt A. J.; Grainger C. M.; Young J. C.; Shelp B. J.; Lee E. A. Impact of postharvest handling on carotenoid concentration and composition in high-carotenoid maize (Zea mays L.) kernels. J. Agric. Food Chem. 2010, 58, 8286–8292. 10.1021/jf100161r. [DOI] [PubMed] [Google Scholar]
- De Moura F. F.; Miloff A.; Boy E. Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: Cassava, maize and sweet potato. Crit. Rev. Food Sci. Nutr. 2015, 55, 1246–69. 10.1080/10408398.2012.724477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D.; Rocheford T.; Ferruzi M. G. Influence of temperature and humidity on the stability of carotenoids in biofortified maize (Zea mays L.) genotypes during controlled post-harvest storage. J. Agric. Food Chem. 2015, 64, 2727–2736. 10.1021/acs.jafc.5b05698. [DOI] [PubMed] [Google Scholar]
- Mugode L.; Ha B.; Kaunda A.; Sikombe T.; Phiri S.; Mutale R.; Davis C.; Tanumihardjo S. A.; De Moura F. F. Carotenoid retention of biofortified provitamin A maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage. J. Agric. Food Chem. 2014, 62, 6317–6325. 10.1021/jf501233f. [DOI] [PubMed] [Google Scholar]
- Kean E. G.; Hamaker B. R.; Ferruzzi M. G. Carotenoid bioaccessibility from whole grain and degermed maize meal products. J. Agric. Food Chem. 2008, 56, 9918–9926. 10.1021/jf8018613. [DOI] [PubMed] [Google Scholar]
- Pillay K.; Siwela M.; Derera J.; Veldman F. J. Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods. J. Food Sci. Technol. 2014, 51, 634–644. 10.1007/s13197-011-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales A.; Agama-Acevedo E.; Bello-Perez L.; Gutiérrez-Dorado R.; Palacios-Rojas N. Effect of traditional and extrusion nixtamalization on carotenoid retention in tortillas made from provitamin A-enriched maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 8289–8295. 10.1021/acs.jafc.6b02951. [DOI] [PubMed] [Google Scholar]
- Lozano-Alejo N.; Carrillo G. V.; Pixley K.; Palacios-Rojas N. Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innovative Food Sci. Emerging Technol. 2007, 8, 385–389. 10.1016/j.ifset.2007.03.015. [DOI] [Google Scholar]
- De La Parra C.; Serna Saldivar S. O.; Liu R. H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 2007, 55, 4177–4183. 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- Heying E. K.; Tanumihardjo J. P.; Vasic V.; Cook M.; Palacios-Rojas N.; Tanumihardjo S. A. Biofortified orange maize enhances ß-cryptoxanthin concentrations in egg yolks of laying hens better than tangerine peel fortificant. J. Agric. Food Chem. 2014, 62, 11892–11900. 10.1021/jf5037195. [DOI] [PubMed] [Google Scholar]
- Schmaelzle S.; Kaliwile C.; Arscott S. A.; Gannon B.; Masi C.; Tanumihardjo S. A. Nutrient and non-traditional food intakes by Zambian children in a controlled feeding trial. Food Nutr. Bull. 2014, 35, 60–67. 10.1177/156482651403500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss E. T.; Arscott S. A.; Bresnahan K.; Pixley K. V.; Rocheford T.; Hotz C.; Siamusantu W.; Chileshe J.; Tanumihardjo S. A. Comparative intake of white- versus orange-colored maize by Zambian children in the context of promotion of biofortified maize. Food Nutr. Bull. 2012, 33, 63–71. 10.1177/156482651203300106. [DOI] [PubMed] [Google Scholar]
- Pattee H. E.; Purcell A. E. Carotenoid pigments of peanut oil. J. Am. Oil Chem. Soc. 1967, 44, 328–330. 10.1007/BF02635627. [DOI] [Google Scholar]
- Howe J. A.; Tanumihardjo S. A. Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J. Agric. Food Chem. 2006, 54, 7992–7997. 10.1021/jf062256f. [DOI] [PubMed] [Google Scholar]
- Davis C.; Jing H.; Howe J. A.; Rocheford T.; Tanumihardjo S. A. ß-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to ß-carotene supplements. Br. J. Nutr. 2008, 100, 786–793. 10.1017/S0007114508944123. [DOI] [PubMed] [Google Scholar]
- Schmaelzle S.; Gannon B.; Crawford S.; Arscott S. A.; Goltz S.; Palacios-Rojas N.; Pixley K. V.; Simon P. W.; Tanumihardjo S. A. Maize genotype and food matrix affect the provitamin A carotenoid bioefficacy from staple and carrot-fortified feeds in Mongolian gerbils (Meriones unguiculatus). J. Agric. Food Chem. 2014, 62, 136–143. 10.1021/jf403548w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. E.; Eldridge A. L. Comparison of carotenoid content in fresh, frozen and canned corn. J. Food Compos. Anal. 2005, 18, 551–559. 10.1016/j.jfca.2004.04.001. [DOI] [Google Scholar]
- Vimala B.; Nambisan B.; Hariprakash B. Retention of carotenoids in orange-fleshed sweet potato during processing. J. Food Sci. Technol. 2011, 48, 520–524. 10.1007/s13197-011-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Tayie F. A. K.; Young M. F.; Rocheford T.; White W. S. Retention of provitamin A carotenoids in high ß-carotene maize (Zea mays) during traditional African household processing. J. Agric. Food Chem. 2007, 55, 10744–10750. 10.1021/jf071815v. [DOI] [PubMed] [Google Scholar]
- Muzhingi T.; Yeum K-J.; Russell R. M.; Johnson E. J.; Qin J.; Tang G. Determination of carotenoids in yellow maize, the effects of saponification and food preparations. Int. J. Vitam. Nutr. Res. 2008, 78, 112–120. 10.1024/0300-9831.78.3.112. [DOI] [PubMed] [Google Scholar]
- Miglio C.; Chiavaro E.; Visconti A.; Fogliano V.; Pellegrini N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. 10.1021/jf072304b. [DOI] [PubMed] [Google Scholar]
- Clarke B.; Rottger A.. Small Mills in Africa; FAO: Rome, Italy, 2006; pp 1–23. [Google Scholar]
- Gannon B. M.; Pixley K. V.; Tanumihardjo S. A. Maize milling method affects growth and zinc status, but not provitamin A carotenoid bioefficacy, in male Mongolian gerbils. J. Nutr. 2017, 147, 337–345. 10.3945/jn.116.241935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. S.; Chung S. K.; Yam K. L. Carotenoid loss in dried red pepper products. Int. J. Food Sci. Technol. 1992, 27, 179–185. 10.1111/j.1365-2621.1992.tb01194.x. [DOI] [Google Scholar]