Abstract
Background
This is an update of the review last published in 2011. It focuses on early postoperative enteral nutrition after lower gastrointestinal surgery. Traditional management consisted of 'nil by mouth', where patients receive fluids followed by solids after bowel function has returned. Although several trials have reported lower incidence of infectious complications and faster wound healing upon early feeding, other trials have shown no effect. The immediate advantage of energy intake (carbohydrates, protein or fat) could enhance recovery with fewer complications, and this warrants a systematic evaluation.
Objectives
To evaluate whether early commencement of postoperative enteral nutrition (within 24 hours), oral intake and any kind of tube feeding (gastric, duodenal or jejunal), compared with traditional management (delayed nutritional supply) is associated with a shorter length of hospital stay (LoS), fewer complications, mortality and adverse events in patients undergoing lower gastrointestinal surgery (distal to the ligament of Treitz).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library 2017, issue 10), Ovid MEDLINE (1950 to 15 November 2017), Ovid Embase (1974 to 15 November 2017). We also searched for ongoing trials in ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (15 November 2017). We handsearched reference lists of identified studies and previous systematic reviews.
Selection criteria
We included randomised controlled trials (RCT) comparing early commencement of enteral nutrition (within 24 hours) with no feeding in adult participants undergoing lower gastrointestinal surgery.
Data collection and analysis
Two review authors independently assessed study quality using the Cochrane 'Risk of bias' tool tailored to this review and extracted data. Data analyses were conducted according to the Cochrane recommendations. We rated the quality of evidence according to GRADE. Primary outcomes were LoS and postoperative complications (wound infections, intraabdominal abscesses, anastomotic dehiscence, pneumonia). Secondary outcomes were: mortality, adverse events (nausea, vomiting), and quality of life (QoL). LoS was estimated using mean difference (MD (presented as mean +/‐ SD). For other outcomes we estimated the common risk ratio (RR) and calculated the associated 95% confidence intervals. For analysis, we used an inverse‐variance random‐effects model for the primary outcome (LoS) and Mantel‐Haenszel random‐effects models for the secondary outcomes. We also performed Trial Sequential Analyses (TSA).
Main results
We identified 17 RCTs with 1437 participants undergoing lower gastrointestinal surgery. Most studies were at high or unclear risk of bias in two or more domains. Six studies were judged as having low risk of selection bias for random sequence generation and insufficient details were provided for judgement on allocation concealment in all 17 studies. With regards to performance and deception bias; 14 studies reported no attempt to blind participants and blinding of personnel was not discussed either. Only one study was judged as low risk of bias for blinding of outcome assessor. With regards to incomplete outcome data, three studies were judged to be at high risk because they had more than 10% difference in missing data between groups. For selective reporting, nine studies were judged as unclear as protocols were not provided and eight studies had issues with either missing data or incomplete reporting of results.
LOS was reported in 16 studies (1346 participants). The mean LoS ranged from four days to 16 days in the early feeding groups and from 6.6 days to 23.5 days in the control groups. Mean difference (MD) in LoS was 1.95 (95% CI, ‐2.99 to ‐0.91, P < 0.001) days shorter in the early feeding group. However, there was substantial heterogeneity between included studies (I2 = 81, %, Chi2 = 78.98, P < 0.00001), thus the overall quality of evidence for LoS is low. These results were confirmed by the TSA showing that the cumulative Z‐curve crossed the trial sequential monitoring boundary for benefit. We found no differences in the incidence of postoperative complications: wound infection (12 studies, 1181 participants, RR 0.99, 95%CI 0.64 to 1.52, very low‐quality evidence), intraabdominal abscesses (6 studies, 554 participants, RR 1.00, 95%CI 0.26 to 3.80, low‐quality evidence), anastomotic leakage/dehiscence (13 studies, 1232 participants, RR 0.78, 95%CI 0.38 to 1.61, low‐quality evidence; number needed to treat for an additional beneficial outcome (NNTB) = 100), and pneumonia (10 studies, 954 participants, RR 0.88, 95%CI 0.32 to 2.42, low‐quality evidence; NNTB = 333).
Mortality was reported in 12 studies (1179 participants), and showed no between‐group differences (RR = 0.56, 95%CI, 0.21 to 1.52, P = 0.26, I2 = 0%, Chi2 = 3.08, P = 0.96, low‐quality evidence). The most commonly reported cause of death was anastomotic leakage, sepsis and acute myocardial infarction.
Seven studies (613 participants) reported vomiting (RR 1.23, 95%CI, 0.96 to 1.58, P = 0.10, I2 = 0%, Chi2 = 4.98, P = 0.55, low‐quality evidence; number needed to treat for an additional harmful outcome (NNTH) = 19), and two studies (118 participants) reported nausea (RR 0.95, 0.71 to 1.26, low‐quality evidence). Four studies reported combined nausea and vomiting (RR 0.94, 95%CI 0.51 to 1.74, very low‐quality evidence). One study reported QoL assessment; the scores did not differ between groups at 30 days after discharge on either QoL scale EORTC QLQ‐C30 or EORTC QlQ‐OV28 (very low‐quality evidence).
Authors' conclusions
This review suggests that early enteral feeding may lead to a reduced postoperative LoS, however cautious interpretation must be taken due to substantial heterogeneity and low‐quality evidence. For all other outcomes (postoperative complications, mortality, adverse events, and QoL) the findings are inconclusive, and further trials are justified to enhance the understanding of early feeding for these. In this updated review, only a few additional studies have been included, and these were small and of poor quality. To improve the evidence, future trials should address quality issues and focus on clearly defining and measuring postoperative complications to allow for better comparison between studies. However due to the introduction of fast track protocols which already include an early feeding component, future trials may be challenging. A more feasible trial may be to investigate the effect of differing postoperative energy intake regimens on relevant outcomes.
Plain language summary
The effect of having nutrition within the first 24 hours after bowel surgery on length of hospital stay and postoperative complications
Review question
To look at whether feeding patients early after surgery (orally or through a tube) can help them to leave hospital sooner with fewer complications.
Background
Traditionally, after gastrointestinal surgery, it was usual for patients not to be given any food until their bowel regained some function (e.g. bowel sounds, passing wind, bowel motion). Studies have looked at whether feeding patients sooner after surgery can help reduce complications (e.g. pneumonia), but there are mixed results. It is important to do this update of the review because the evidence in previous reviews is not extensive. The relevance of early feeding following its incorporation within a programme of patient care (also known as the enhanced recovery after surgery (ERAS)), remains an important question to answer.
Study characteristics
This review found 17 relevant studies that recruited 1437 participants in total who had undergone lower gastrointestinal surgery (distal to the ligament of Treitz).
Key results
We found evidence that patients who received nutrition within the first 24 hours after their surgery were able to leave hospital almost two days sooner than those patients who were not given any nutrition until their bowel activity returned. However, the quality of the evidence is low and therefore early feeding after surgery may not lead to patients leaving hospital sooner. They may also have a reduced risk of dying. However, we found weak evidence that patients who were given nutrition within the first day after their operation were more at risk of vomiting. There were no differences in complication rates (such as wound infection or pneumonia) between patients who were fed early and those that were not.
Quality of the evidence
All studies were of low quality, which may mean their results are less reliable. To explore further early feeding after surgery, more studies are needed which are larger and of better quality.
Summary of findings
Background
Description of the condition
Traditionally, it was standard in many elective surgical practices to keep patients ‘nil by mouth’ (NBM) from the previous evening (six to 12 hours preoperatively) and postoperatively for several days until resolution of ileus: a period of loss of peristalsis that is often experienced after lower gastrointestinal surgery (Weissenfluh 2006). Evidence of bowel motility (such as the reappearance of bowel sounds and passage of flatus or bowel movement) may (although are poor markers of resolution of ileus), signify resolution of ileus, following which individuals would begin with a clear liquid diet and slowly advance to a regular diet (Warren 2011). This period of bowel rest (Grizas 2008) was considered important to prevent complications such as anastomotic dehiscence, aspiration pneumonia, bowel distension, bowel obstruction and nausea and vomiting (Maessen 2009). Surgical patients in the past were however often malnourished (Hill 1977; Lennard‐Jones 1992; McWhirter 1994), which may have caused an increase in morbidity and mortality (Vet's Affairs 1991). In recent years this practice has been challenged as studies have shown that both pre‐ and postoperative fasting may be periods of unnecessary starvation that result in adverse consequences on patient outcomes (Andersen 2011; Ljungqvist 2009; Viganò 2012). Avoidance of long periods of preoperative fasting, the use of preoperative carbohydrate loading and re‐establishment of oral feeding as soon as possible after surgery have been incorporated within the Enhanced Recovery After Surgery (ERAS) programme (Department of Health 2010).
Description of the intervention
Prolonged fasting after surgery may deplete the patient of vital nutrients (McWhirter 1994). A common practice in the postoperative management of patients who undergo gastrointestinal surgery has been to withhold nutrition until bowel function is restored. The fear being that early enteral nutrition may encourage ileus, anastomotic leakage/dehiscence or aspiration pneumonia. It has been suggested that feeding in the postoperative management of patients may actually help reduce postoperative complications and their stay in hospital (da Fonseca 2011).
How the intervention might work
Within 24 hours of starvation, changes in the body's metabolism are evident including increased insulin resistance and reduced muscle function. Experimental data from both humans and animals (Fukuzawa 2007) suggest that providing nutrition in the postoperative period improves wound healing (relevant to the integrity of the intestinal anastomosis), muscle function and reduces sepsis. Furthermore, it has been suggested that early enteral nutrition is useful for recovering gastrointestinal motility and maintaining the nutritional status for patients undergoing gastrointestinal surgery (Kawasaki 2009).
Why it is important to do this review
Studies have shown improved wound healing (Karl 2014; Schroeder 1991), and reduced infectious complications (Moore 1989) with early nutrition. The first randomised study to show jejunal feeding was tolerated within 24 hours of surgery was published in 1979 and showed a reduced length of hospital stay (LoS) in patients fed early (Sagar 1979). Subsequently, there have been further studies which have explored the safety and benefits of early postoperative feeding (Chatterjee 2012; Dag 2011) with mixed results in terms of benefits in relation to LoS and postoperative complications such as pneumonia, anastomotic dehiscence, abdominal abscess and wound infection. Early postoperative feeding has now been incorporated into the UK ERAS programme. However, the extent to which early postoperative feeding is implemented clinically is unclear, and it is a care practice that is not always carried out. Ahmed and colleagues (Ahmed 2012) conducted a systematic review of 11 studies in a variety of countries reporting compliance to individual ERAS elements in a clinical setting in colorectal surgical patients. In the nine studies which reported relevant data, compliance with early postoperative feeding ranged from 13% to 100%. Another study showed that of the 861 colorectal surgical patients enrolled in an ERAS programme, 65% had ‘normal food’ but this was on postoperative day two (Maessen 2009). These studies show that early feeding is not consistently implemented. The previous reviews of early postoperative feeding were limited by the available evidence and so an update is warranted. This systematic review summarises the available evidence on early enteral nutrition within the first 24 hours post lower gastrointestinal surgery (distal to the ligament of Treitz) on LoS and complications.
Objectives
To evaluate whether early commencement of postoperative enteral nutrition (within 24 hours), oral intake and any kind of tube feeding (gastric, duodenal or jejunal), compared with traditional management (delayed nutritional supply) is associated with a shorter length of hospital stay (LoS), fewer complications, mortality and adverse events in patients undergoing lower gastrointestinal surgery (distal to the ligament of Treitz).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs), including cluster‐RCTS, of early enteral nutrition (defined as oral intake or using any kind of tube feeding: gastric, duodenal or jejunal) containing energy (carbohydrate, protein or fat) that commenced within 24 hours postoperatively in patients undergoing either acute or elective lower gastrointestinal surgery (distal to the ligament of Treitz). Trials were considered irrespective of whether blinding or placebo treatment was performed. Studies reporting comparison of treatment between parenteral, enteral nutrition and controls were included only if data could be provided separately for patients in the feeding group and control group. Studies with multiple arms were included if data allowed a clear distinction between each single group. Unpublished studies and abstracts were considered and included if we were able to obtain complete manuscripts from the author(s).
Types of participants
We included studies of adult participants (above 18 years of age and both gender) undergoing lower gastrointestinal surgery, with malignant or benign disease including inflammatory bowel diseases. We defined lower gastrointestinal surgery as where an anastomosis was formed distal to the ligament of Treitz. Studies including participants with surgery distal as well as proximal to the ligament of Treitz were included.
Types of interventions
This review focused on early enteral feeding, regardless of method (oral or tube), defined as nutrition given within the first 24 hours postoperatively to patients undergoing surgery in the lower gastrointestinal tract. The control arm was traditional management, defined as no oral intake or any kind of tube feeding containing energy before bowel function returns. The following were excluded: studies in children (i.e. age less than 18 years); trials comparing different types of enteral nutrition with each other; trials in which patients served as their own controls and cross‐over trials; trials reporting only upper gastrointestinal surgery; and trials on parenteral nutrition.
Types of outcome measures
Primary outcomes
Length of hospital stay (days)
-
Postoperative complications using the Clavien‐Dindo classification for surgical complications (Dindo 2004):
wound infections (e.g. a standardised criteria to assess wound infection (Buzby II‐IV:Buzby 1988)
intraabdominal abscesses
anastomotic leakage/dehiscence
pneumonia (within hospital stay)
Secondary outcomes
Mortality
Adverse events: nausea, vomiting (within hospital stay)
Quality of Life (standardised scale)
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished randomised controlled trials with no language or date of publication restrictions. The following electronic databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, issue 10) in the Cochrane Library (15 November 2017) (Appendix 1);
Ovid MEDLINE (from 1946 to 15 November 2017) (Appendix 2);
Ovid Embase (from 1974 to 15 November 2017) (Appendix 3);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 15 November 2017);
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/ searched 15 November 2017
LILACS (Latin American and Caribbean Health Science Information database) (from 1979 to 20 December 2013) (Appendix 4)
A sensitivity‐ and precision‐maximising search filter (RCT) was applied to the MEDLINE and Embase search strategies as recommended by the Cochrane Handbook or Systematic Reviews of Interventions (Chapter 6.4.11, Higgins 2011).
We searched for ongoing trials in Clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) database (WHO ICTRP database), by using combinations of search terms including "early AND feeding" and "enteral AND nutrition". We also searched conference abstracts from the most recent gastrointestinal conferences (DDW, ASCO, UEGW).
Searching other resources
We searched Google and Google Scholar, and handsearched reference lists of identified studies, previous reviews and systematic reviews for additional relevant articles.
Data collection and analysis
Selection of studies
Two review authors (CA and SL) independently screened titles and abstracts of all studies identified from searches. Each study was coded as either eligible for inclusion (potentially eligible/unclear) or excluded. The review authors were blinded to each others' codes. We retrieved full‐text copies of all eligible and potentially eligible studies, and the same two review authors (CA and SL) independently screened full texts, identified studies for inclusion, and recorded reasons for exclusion of the ineligible studies. Disagreements were resolved through discussion or consultation with a third and fourth author (HA and ST). We collated multiple reports that related to the same study, so that each study rather than each report was the unit of interest in the review. For any studies reported in multiple publications, we used the reference that provided the most comprehensive information. We contacted authors of studies eligible for inclusion to clarify inclusion/exclusion criteria of their research, where further information was needed. Articles in foreign languages were translated by a person (not in the byline) who could read and write that language, if they matched the inclusion criteria, data were extracted onto the data extraction form (CA and SL). Justification for excluding studies has been reported in the Characteristics of excluded studies. We have presented the overall selection process in detail in the PRISMA flow diagram (Figure 1).
1.

Study flow diagram.
Data extraction and management
We developed a data extraction form adapted for this review (Appendix 5) from an original form provided by Cochrane Colorectal Cancer Group and a previous Cochrane review (Short 2015). Two review authors (GH and RP) piloted it on two studies selected for inclusion, prior to its use for all studies. The same review authors independently extracted the study characteristics and outcome from the included studies. Titles and abstracts that were not available in English were translated by a person (not in the byline) who could read and write that language. If a trial in a non‐English language matched the inclusion criteria, it was directly extracted onto the data extraction form, confirmed by two review authors (GH and RP). Hereafter, two review authors (GH and RP) transferred all data into the Review Manager 5.3 software (RevMan 2014). A third review author (ST) checked all the extracted data for accuracy against the trial reports. For included studies with multiple reports, we extracted data from the report with the most recent data for a specific outcome.
Assessment of risk of bias in included studies
Two review authors (GH and RP) independently assessed risk of bias for each study. The 'Risk of bias' tool that we used was based on the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8: Higgins 2011; Appendix 6) and was tailored to this review (see Appendix 5). We developed this as data extraction continued. We judged each potential source of bias as high, low or unclear risk, using the criteria provided in Appendix 6 for the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Two review authors (GH and RP) discussed the risk of bias for all studies to ensure uniformity and agreement. For each outcome we summarised the 'Risk of bias' judgements across different studies for the domains listed.Single studies were categorised as overall low risk of bias if they has low risk across all domains; overall unclear risk of bias with unclear risk in one or more key domains; and overall high risk of bias with high risk in one or more key domains. For the sensitivity analysis we listed four of the seven standard domains (random sequence generation, allocation concealment, incomplete outcome data and selective outcome reporting), considered the most important for determining the validity of our findings. For the outcome 'mortality' we judged and categorised lack of blinding of participants as low risk of bias.
Measures of treatment effect
We considered continuous variables (LoS) as weighted mean differences (WMD), and included 95% confidence intervals (95% CIs). We considered frequency of postoperative complications and adverse events related to the intervention, and presented dichotomous data using a risk ratio (RR) with 95% confidence intervals (CIs).
Unit of analysis issues
We found no unit of analysis issues. The individual participant is the unit of analysis in all the included trials. No studies used cluster‐randomisation, and as cluster‐RCT's do not work well with surgery; this study design is unlikely for future updates of this review.
Dealing with missing data
Regarding trials with incompletely reported outcomes, two review authors (SL and GH) contacted the lead authors of the primary study to request further information (Chapter 16: Higgins 2011). Details of those studies which include results from additional correspondence are included in Table 3. With regards to participants missing from included studies, we have based analyses on intention‐to‐treat for relevant outcome measures. Where primary outcome data were not provided in the form of a mean and standard deviation, we derived these from the reported test statistics or estimated them from the reported data if suitable test statistics were not reported. We used the following methods to estimate or impute missing data (see Table 4 for further details).
1. Characteristics of the seventeen included trials of early enteral feeding.
| Study | Site of surgery | Elective or acute surgery | Feed type | Route of feeding | Feed timing and amount in intervention group | Pathology | Outcomes | Additional data |
| Beier Holgersen (1996) | Lower GI (87%), Upper GI (13%) | Elective surgery | Nutritional supplement (Nutridrink, Nutricia) | Nasoduodenal tube (2nd and 3rd part of duodenum) and oral | Intervention: Nutritional supplement (Nutridrink, Nutricia) within four hours postoperatively. POD 1 = 1000ml nutrition (median); POD 2 = 1200 mL (median) nutrition; POD 3 = 1000mL (median) nutrition; POD 4 = 1000mL nutrition. Normal diet from 5th POD. | 65% Malignant; 35% Benign | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, postoperative complications (pneumonia, dehiscence, pulmonary failure, myocardial infarction), LoS, adverse events (nausea, vomiting). Patients were seen every day and all complications were recorded using Buzby's classification. | Yes (data set provided) |
| Binderow (1994) | 27% small bowel, 73% large bowel | Elective surgery | Regular diet | Oral | Intervention: Patients told to eat as much of the diet as they wanted. | Not reported | LoS, postoperative complications (ileus), adverse events (vomiting). Patients completed a daily symptom log, in addition to a daily interview, examination and review of nursing records. | No |
| Carr (1996) | Intestinal only | Elective surgery | Enteral feed (Fresubin, Fresenius). Oral fluids started on passage of flatus and progressed to regular diet over 48 hours. | Double lumen Nasojejunal tube and oral | Feeding was started two to three hours after surgery and continued until normal diet was possible. Energy and water requirements were calculated from the weight of the patient, and a mixture of Fresubin and water provided the full basic fluid requirements (35 mL/kg body weight/day). Initially feeding was at 25 mlLan hour and was increased by 25 mL four hourly until the target volume was reached, at which point intravenous fluids were stopped. | Not reported | Mortality, LoS, postoperative complications (bleeding duodenal ulcer, infection), adverse events (nausea and vomiting, distension, diarrhoea) | No |
| Chatterjee (2012) | Large bowel, small bowel, gastric, and biliary | Emergency or elective surgery | Enteral feed progressed to regular diet | Nasogastric tube ‐ progressed to oral | Oral liquids (25 mL/hour) were started within 24 hours of operation with clamping the NGT and the feed was increased by 25 mL/hour at 12 hours interval. When the patients started tolerating the liquid diet, NGT was removed and the semisolid diet and then normal oral diet were started to reach the nutritional goal (25 kcal/ kg/ day) as soon as possible. | 38% Malignant, 62% Benign | Wound infection, wound dehiscence, anastomotic leakage (detected by clinical exam‐ feature of septicaemia, distention of abdomen, change in character and measure of drain output/0 and radiological investigations (USG, CT scan), mortality, LoS, postoperative complications (respiratory tract infection, urinary tract infection, incidence of re‐exploration) adverse events (nausea and vomiting) | Yes (half data set provided) |
| Da Fonseca (2011) | Lower GI (100%) ‐ | Elective surgery | Liquid diet progressed to regular diet | Oral | POD 1 patients received an oral liquid diet (approximately 500 cm3) and were advanced to a regular diet within the next 24 hours) | 88% Malignant, 12% Benign | Wound infection, anastomotic leak, mortality, LoS, postoperative complications (aspiration pneumonia, angina pectoris, prolonged ileus, catheter sepsis, deep vein thrombosis, pancreatitis), adverse events (nausea and vomiting, hyporexia, readmission) | Yes (confirmed study met inclusion criteria) |
| Dag (2011) | Lower GI (100%) ‐ elective open colorectal surgery | Elective surgery | Fluid diet gradually increased to a solid diet | Oral | Fluid diet (12 hours after surgery) | Not reported | Wound infection, anastomotic leakage, postoperative complications (pneumonia, toxic hepatitis, sepsis, evisceration, cerebral infarct), LoS | Yes (provided mortality data) |
| Hartsell (1997) | Lower GI (100%) | Elective surgery | Liquid diet progressed to regular diet | Oral | Full liquid diet on POD 1. If patient consumed 1000 mL or more in a 24‐hour period, they were advanced to a regular diet next day. | 64% Malignant, 36% Benign | Wound infection, anastomotic leakage, mortality, postoperative complications (pneumonia), LoS, adverse events (nausea, vomiting) | No |
| Lucha (2005) | Lower GI (100%) | Elective surgery | Regular diet | Oral | Not reported | Not reported | Anastomotic leakage, postoperative complications (pneumonia), LoS | No |
| Minig (2009) | Gynaecological patients with malignancy. 37/40 had either rectosigmoid or rectosigmoid and small bowel resection. 3/40 had small bowel resection |
Elective surgery | Liquids, mineral water (no gas), tea, chamomile infusion or apple juice. Progressed to regular diet of boiled or grilled beef, chicken or fish on POD1. | Oral | Liquids, mineral water (no gas), tea, chamomile infusion or apple juice during first 24 hours. Progressed to regular diet of boiled or grilled beef, chicken or fish on POD1. | Malignant (100%) | Wound infection, intraabdominal abscesses, anastomotic leakage, mortality, postoperative complications (infectious complications, intestinal complications, ileus, bleeding, pleural effusion, thromboembolic complications, pneumothorax), LoS, adverse events (nausea and vomiting, diarrhoea, readmission), QoL (EORTC QLQ‐C30 and EORTC QLQ‐QV28). | No |
| Mulrooney 2004 | Lower GI (100%) | NR | Enteral feed (Nutrison Standard by Nutricia Clinical Care) | Nasojejunal tube and oral | Jejunal feeding was started within 24 hours from the end of the operation at 25 mL/hour. This rose to 50 mL/hour on day 2 and 75 mL/hour on day 3 etc. The feed ran continuously with no break. The nutritional goal (final feeding rate) was calculated using the dietetic department guidelines at DRI. The feed was stopped once oral feeding recovery was adequate (nutritional intake meets at least half of the calorie and protein requirement). | 73% malignant and 27% benign | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, postoperative complication (pneumonia, urinary tract infection), LoS | Yes (provided data on outcomes of interest) |
| Nakeeb (2009) | Lower GI (100%) | Elective surgery | Fluids POD1 progressed to regular diet within next 24 to 48 hours | Oral | Not reported | 100% cancer | Wound infection, anastomotic leakage (diagnosis was from symptoms such as fever and leakage of intestinal contents), mortality, LoS, postoperative complications (burst abdomen, abnormal serum electrolyte, pulmonary infection), adverse events (vomiting, readmission). Follow up occurred 10‐14 days postoperatively in the form of clinical, lab and radiological evaluations. |
Yes (confirmed study met inclusion criteria) |
| Ortiz (1996) | Lower GI (100%) | Elective surgery | Regular diet | Oral | On postoperative evening patients allowed ab libitum intake of clear liquids; this continued until POD 1 at which time they progressed to a regular diet as desired. | 87% Malignant, 13% Benign | Wound infection, anastomotic leakage, intraabdominal abscess, mortality, postoperative complication (haemorrhage, pneumonia, venous thrombosis, urinary infection, intestinal obstruction, ileostomy necrosis), LoS | Yes (LoS provided) |
| Reissman (1995) | Lower GI (100%) | Elective surgery | Clear liquid diet | Oral | Clear liquid diet on POD 1 and advanced to a regular diet within the next 24 to 48 hours, as tolerated. | Not reported | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, postoperative complications (recorded daily) (pneumonia, intestinal obstruction, urinary tract infection, pelvic abscess), LoS, adverse events (vomiting) (recorded daily) | Yes (confirmed study met inclusion criteria) |
| Sagar (1979) | Lower GI (73%), Upper GI (27%) | NR | Enteral feed (elemental, Flexical Mead Johnson Laboratories) | Nasojejunal tube and oral | For the first 24 hours a half strength solution was infused at 25 mL/hour. Thereafter, undiluted. Flexical was infused at 25 mL/hour on POD 2, 50 mL/hour on POD 3, and 100 mL/hour on POD 4 and 5. If there were no complications the double lumen tube was removed on the sixth day and the patient given as much Flexical as they could take by mouth on POD 6 and 7. Patients given 2 L dextrose (50, w/v) and 1 L saline (0.9% w/v) intravenously from POD 1 ‐3. | Not reported | Wound infection (inspected on the 10th day or earlier if infection was suspected), anastomotic leakage, intraabdominal abscess, LoS | No |
| Schroeder (1991) | 30/32 had colonic resection and 2/32 had small bowel resection | NR | Enteral feed (Osmolite, Ross Laboratories) | Nasojejunal tube | Immediate infusion with full‐strength Osmolite at a rate of 50 mL/hour via a continuous infusion pump. Patient encouraged to drink water. If absorption was occurring with no problems, the infusion rate was then increased to 80 mL/hour and the iv rate decreased accordingly. On the morning of POD 3 (i.e., either 72 or 67 hours after commencement of operation) both tubes were removed and the patient allowed to take whatever they liked by mouth. | Not reported | Postoperative complications (pneumonia, intestinal obstruction, urinary tract infection, pelvic abscess), LoS, adverse events (diarrhoea, small bowel obstruction) | No |
| Stewart (1998) | Lower GI (100%) | Elective surgery | Free fluids progressed to a solid diet. | Oral | Free fluids from 4 hours after the operation and progressed to a solid diet from POD 1 at their own discretion. | Not reported | Wound infection, anastomotic leakage, mortality, postoperative complications (respiratory complications, cardiovascular complications, urinary tract infection, pneumonia), LoS, adverse events (vomiting) | Yes (data set provided, LoS and pneumonia extracted) |
| Yang (2013) | Lower GI (100%) | Elective surgery | Nutritional supplement (Ensure, Abbott) progressed to regular diet | Oral | Nutritional supplement (Ensure, Abbott) progressed to regular diet within 12 hours. 30 mL to 50 mL Ensure 6 to 12 hours post‐surgery at 1‐ to 2‐hour intervals. POD 2, 100 mL to 200 mL Ensure at ‐ to 3‐hour intervals. | 100% cancer | LoS, adverse events (vomiting, stomach distension) | No |
2. Estimated results and assumptions for length of hospital stay outcome.
| Study | Estimated result |
| Binderow (1994) | SD not reported, SD imputed using mean SD of other studies (by treatment arm) |
| Lucha (2005) | SD not reported, SD imputed using mean SD of other studies (by treatment arm) |
| Mulrooney (2004) | Results extracted from additional correspondence ‐ thesis |
| Nakeeb (2009) | SD appear to be from paired t‐tests, SD imputed using mean SD of other studies (by treatment arm) |
| Ortiz (1996) | Results provided from additional correspondence |
| Reissman (1995) | SEMs converted to SD |
| Sagar (1979) | Estimated mean LoS and SDs using Wan et al (2014) |
| Stewart (1998) | Results calculated from additional correspondence |
Where LoS results were presented as median and range, we estimated mean and standard deviation using the formulae described by Wan 2014.
We imputed missing standard deviations for LoS using the mean of the standard deviations reported by other studies within that treatment arm.
Where LoS were presented as Kaplan‐Meier graphs, we extracted the following LoS data for each trial arm where available: median (50%), inter‐quartile range (25% to 75%) and range (minimum and maximum). Mean LoS and its associated standard deviation were subsequently derived as described above. We did not use results for LoS presented as hazard ratios without further descriptive LoS measures to estimate median LoS, due to potentially high uncertainty in estimation (Cortes 2014).
Where complications were reported as percent incidence, we converted this into the number of participants who experienced complications.
Assessment of heterogeneity
We determined clinical heterogeneity on the basis of both the participant demographic data and methodology of the studies. We assessed statistical heterogeneity across studies by visual inspection of the forest plot and using the Chi2 measurement. Heterogeneity is more difficult to detect when sample sizes and number of events are small, so we used a cut off of P < 0.01 for the Chi2 measurement to decide if there was statistical evidence of heterogeneity as described in the Cochrane Handbook for Systematic Reviews of Intervention (Chapter 9.5, Deeks 2011). As a measure of the variation in intervention effect due to statistical heterogeneity, we also assessed the I2 statistic. We applied the following thresholds for the interpretation of the I2 statistic (Deeks 2011).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Clinical heterogeneity was explored through post‐hoc subgroup analyses, as described in the Subgroup analysis and investigation of heterogeneity section.
Assessment of reporting biases
We assessed reporting bias using funnel plot asymmetry of included studies. We did not assess funnel plot asymmetry for outcomes reported in fewer than 10 studies, since it is considered unreliable as described in the Cochrane Handbook for Systematic Reviews of Intervention (Chapter 10, Sterne 2011). We visually inspected funnel plots for asymmetry, and for continuous outcomes we used Egger's regression test. There is currently no guidance for testing funnel plot asymmetry for outcomes measured as risk ratios (Sterne 2011). The application of funnel plot asymmetry tests to detect publication bias is considered appropriate and meaningful for outcomes with more than 10 included trial as recommended in the Cochrane Handbook for Systematic Reviews of Intervention (Sterne 2011).
Data synthesis
We performed analyses in RevMan 5.3 (RevMan 2014). Analyses comprised only within‐study comparisons rather than individual‐level data. For analysis, we used an inverse‐variance random‐effects model for the primary outcome (LoS) and Mantel‐Haenszel random‐effects models without zero‐cell correction (Higgins 2011) for dichotomous outcomes (Bradburn 2007), since the event risks >1%. The effect of a continuity correction (0.5) for trial arms with zero events was assessed in our sensitivity analyses.
We undertook Trial Sequential Analysis (TSA) using TSA software (Thorlund 2011) to calculate the required information size, and to determine whether the cumulative Z‐curve of the trial sequential analysis boundaries for benefit, harm, or futility were crossed. The required information size for the primary outcome (LoS) was based on a reduction in mean LoS of one day with an associated variance of nine days, using results from a comparable population of people receiving colorectal surgery (Short 2015). We also used an alpha of 3.3% (accounting for multiple outcomes Jakobsen 2016), a beta of 20%, and the observed diversity in the trials in the meta‐analysis (Jakobsen 2014). For postoperative complications and secondary outcomes, which were all dichotomous outcomes, we estimated the required information size based on the proportion of participants in the control group with the outcome, a relative risk reduction of 30%, an alpha of 2.5% (accounting for multiple outcomes Jakobsen 2016), a beta of 20%, and the observed diversity in the trials in the meta‐analysis. To maintain approximate consistency with our main data synthesis, we included a continuity correction of 0.001 for the TSA (the TSA software does not allow no zero‐cell correction).
We performed the analyses using Review Manager 5 (RevMan 2014), R with the 'meta' package (Schwarzer 2007), and Trial Sequential Analysis (TSA ‐ Trial Sequential Analysis).
Subgroup analysis and investigation of heterogeneity
We conducted two post‐hoc subgroup analyses to explore the effect of clinical diversity on LoS. Given that some trials had included patients undergoing surgery either distal or proximal to the ligament of Treitz (but which could not be separated out for analysis), we conducted a post‐hoc subgroup analysis for LoS to determine the sensitivity of overall conclusions to the surgical site. We created two subgroups: one which encompassed trials that had only included patients having surgery distal to the ligament of Treitz (this group was termed 'distal to ligament of Treitz'), and the other group, which encompassed trials that had included patients undergoing surgery either distal or proximal to the ligament of Treitz (this group was termed 'distal and proximal to ligament of Treitz'). We also created two subgroups relating to whether the route of postoperative feeding used in the intervention was 'oral feeding only' or by 'tube feeding with or without oral feeding'. We also planned to conduct two further subgroup analyses; one comparing patients who have elective surgery versus acute surgery and the other comparing patients with cancer versus non‐cancerous conditions.
Sensitivity analysis
We conducted sensitivity analyses based on methodological and reporting quality of the studies analysed. We considered the impact of methodological quality by excluding studies of high risk of bias and we assessed how robust our overall results were to the use of estimates for missing data. We therefore conducted the following sensitivity analyses.
We repeated meta‐analysis of the primary outcome LoS excluding studies found to be at high risk of bias for at least two of the following components: random sequence generation, allocation concealment, incomplete outcome data and selective outcome reporting.
-
In order to determine whether imputation or estimation of missing data biased results, the meta‐analysis of LoS was repeated with the following changes:
we excluded studies with imputed results;
instead of using the mean of the standard deviations reported by other studies, we used the maximum (worst‐case scenario) and the minimum (best‐case scenario) reported standard deviations.
To determine the effect of including trials with zero events in one of the arms, we repeated our analysis of dichotomous outcomes with a continuity correction of 0.5 was added to an arm of a study with zero events. Studies with zero events in both arms were not included in the data synthesis.
'Summary of findings' tables
We assessed the overall quality of evidence of all outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in 'Summary of findings' tables (Schünemann 2009). The quality of evidence can be downgraded one level (serious concern) or two levels (very serious concern) for one of the following reasons: study limitations (risk of bias), inconsistency of evidence, indirectness (indirect outcomes, interventions, controls), imprecision (wide confidence interval, small sample size) and publication bias. Judgements about the quality of the evidence (high, moderate, low or very low) were justified, documented and incorporated into the reporting of results for each outcome.
The GRADE system classifies the quality of evidence in one of four grades.
| Grade | Definition |
| High | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence on the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
Results
Description of studies
Results of the search
The original version of this review was published in 2001 (Lewis 2001). It was published as a Cochrane Review in 2006 (Andersen 2006) and updated in 2011 (Andersen 2011), including the following 14 studies: (Beier‐Holgersen 1996; Binderow 1994; Carr 1996; Hartsell 1997; Heslin 1997; Lucha 2005; Mulrooney 2004; Ortiz 1996; Reissman 1995; Sagar 1979; Schroeder 1991; Smedley 2004; Stewart 1998; Watters 1997). Three of these studies have been removed from the present update.Two because they were dealing with upper gastrointestinal surgery only (Heslin 1997; Watters 1997), and one study did not have a control group that was nil by mouth (NBM)/received no energy (Smedley 2004). See tables of Characteristics of included studies, Characteristics of excluded studies. Regarding seven trials which did not fully report on outcomes, we contacted the trial authors and received four usable responses (Dag 2011; Mulrooney 2004; Ortiz 1996; Stewart 1998): three with further information about length of hospital stay (LoS) (Mulrooney 2004; Ortiz 1996; Stewart 1998), and three about both primary and secondary outcomes (Dag 2011; Mulrooney 2004; Stewart 1998). Regarding three trials where it was unclear if they met the inclusion criteria, all authors responded and confirmed they met inclusion criteria (da Fonseca 2011; Nakeeb 2009; Reissman 1995). We contacted first authors for two included studies for additional information, but the correspondence did not provide usable extra information (Beier‐Holgersen 1996; Chatterjee 2012).
For this update, the electronic search identified 3956 records of potential relevance to our review. We identified 62 additional records through handsearching (Google and Google Scholar, and scanning reference lists of included studies and relevant systematic reviews). After removal of 1681 duplicates, 2337 records remained. After initial screening of the titles and abstracts, 2193 records were excluded. We sought full texts for the remaining 144, and upon screening we excluded 117 records with reasons (see Characteristics of excluded studies). In total for this review update, we identified 17 RCT's (27 records) for inclusion, comprising 1437 participants. We provide full details of the search results in the PRISMA flow diagram (Figure 1).
Included studies
We provide full characteristics of the 17 independent studies in the Characteristics of included studies table. For five studies reported in multiple publications, we used the reference that provided the most comprehensive information (Beier‐Holgersen 1996; Binderow 1994; Carr 1996; da Fonseca 2011; Ortiz 1996). Published data were available for all 17 studies for one primary outcome measure (LoS). Additional unpublished data were obtained for three studies for further information about LoS (Mulrooney 2004; Ortiz 1996; Stewart 1998).
All trials included in the review were randomised controlled trials (RCTs). Studies varied in sample size, although the majority of studies were small. The number of participants in the studies ranged from 28 (Carr 1996) to 199 (Dag 2011). Studies were conducted in 12 countries; four in the USA (Binderow 1994; Hartsell 1997; Lucha 2005; Reissman 1995), three in the UK (Carr 1996; Mulrooney 2004; Sagar 1979), one in Denmark (Beier‐Holgersen 1996), one in India (Chatterjee 2012), one in Brazil (da Fonseca 2011), one in Turkey (Dag 2011), one in Italy (Minig 2009), one in Egypt (Nakeeb 2009), one in Spain (Ortiz 1996), one in New Zealand (Schroeder 1991), one in Australia (Stewart 1998), and one in China (Yang 2013). One of the included studies (Yang 2013) was published in Chinese.
The studies adopted different feeding regimens in the intervention groups: in three studies (Binderow 1994; Lucha 2005; Ortiz 1996), participants began on a regular diet; in seven studies (da Fonseca 2011; Dag 2011; Hartsell 1997; Minig 2009; Nakeeb 2009; Reissman 1995; Stewart 1998), participants began with a liquid diet and progressed to a regular/solid diet; in two studies (Beier‐Holgersen 1996, Yang 2013) participants began on a nutritional supplement and progressed to a regular diet; in five studies (Carr 1996; Chatterjee 2012; Mulrooney 2004; Sagar 1979; Schroeder 1991), participants were on enteral feed. The routes of feeding within the intervention group were either through a nasoduodenal (Beier‐Holgersen 1996), nasojejunal (Carr 1996; Mulrooney 2004; Sagar 1979; Schroeder 1991) or nasogastric (Chatterjee 2012) tube, or oral (Binderow 1994; da Fonseca 2011; Dag 2011; Hartsell 1997; Lucha 2005; Minig 2009; Nakeeb 2009; Ortiz 1996; Reissman 1995; Stewart 1998; Yang 2013).
The 17 included studies all describe early enteral feeding after elective lower gastrointestinal surgery, but with a wide variety of gastrointestinal conditions (Table 3). Nine trials reported patients undergoing colorectal surgery, (da Fonseca 2011; Dag 2011; Hartsell 1997; Lucha 2005; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Stewart 1998; Yang 2013). Four reported all surgery distal to the ligament of Trietz (Binderow 1994; Carr 1996; Reissman 1995; Schroeder 1991) and four reported some surgery proximal to the ligament of Trietz as well as distal (Beier‐Holgersen 1996; Chatterjee 2012; Minig 2009; Sagar 1979). Eight studies did not state the underlying pathology of disease of the study participants, whereas nine stated that they included both benign and malignant conditions. See Table 3 for an overview of study characteristics regarding site of surgery, feed type, route of feeding, feed timing and amount, pathology and outcomes, definitions and time frames, depending on reporting in the individual studies.
Controls received traditional care, which is where no energy is given until bowel activity/resolution of ileus. Of our outcomes, LoS was the most commonly reported, followed by anastomotic leakage, wound infection, mortality, pneumonia, vomiting, intraabdominal abscess, nausea and vomiting (as a combined outcome), and nausea. Quality of life (QoL) was only reported in one study (Minig 2009). Additional reported outcomes included postoperative complications such as ileus, thrombosis and urinary tract infection (see Table 5) and adverse events such as readmission and diarrhoea (see Table 6).
3. Other postoperative complications.
| Study | Complication | Intervention group | Control group |
| Beier‐Holgersen (1996) | Dehiscence | 3 | 0 |
| Pulmonary failure (ARDS) | 0 | 1 | |
| Myocardial infarction | 1 | 0 | |
| Binderow (1994) | Ileus (duration in days) | 3.6 | 4 |
| Carr (1996) | Bleeding duodenal ulcer | 0 | 1 |
| Infection (wound, urinary) | 0 | 3 | |
| Chatterjee (2012) | Respiratory tract infection | 10 | 5 |
| Urinary tract infection | 5 | 8 | |
| Incidence of re‐exploration | 4 | 1 | |
| Dag (2011) | Toxic hepatitis | 1 | 0 |
| Sepsis | 1 | 2 | |
| Evisceration | 0 | 1 | |
| Cerebral infarct | 0 | 1 | |
| Da Fonseca (2011) | Angina pectoris | 0 | 1 |
| Prolonged ileus | 0 | 2 | |
| Catheter sepsis | 0 | 1 | |
| Deep vein thrombosis | 0 | 1 | |
| Pancreatitis | 1 | 0 | |
| Hartsell (1997) | Sepsis (anastomotic leak, death) | 0 | 1 |
| Minig (2009) | Ileus | 0 | 0 |
| Intestinal complications | 0 | 3 | |
| Infectious complications | 0 | 3 | |
| Bleeding | 6 | 7 | |
| Bleeding requiring surgical reexploration | 1 | 1 | |
| Pleural effusion | 1 | 3 | |
| Thromboembolic complications | 1 | 2 | |
| Pneumothorax | 0 | 3 | |
| Mulrooney (2004) | Urinary tract infection | 0 | 1 |
| Nakeeb (2009) | Burst abdomen | 1 | 2 |
| Abnormal serum electrolyte | 5 | 6 | |
| Pulmonary infection | 2 | 7 | |
| Ortiz (1996) | Haemorrhage | 2 | 2 |
| Venous thrombosis | 2 | 2 | |
| Urinary infection | 0 | 1 | |
| Intestinal obstruction | 2 | 0 | |
| Ileostomy necrosis | 1 | 0 | |
| Reissman (1995) | Intestinal obstruction | 1 | 0 |
| Urinary tract infection | 2 | 1 | |
| Pelvic abscess | 1 | 1 | |
| Schroeder (1991) | Myocardial infarction | 1 | 1 |
| Atelectasis | 2 | 2 | |
| Stewart (1998) | Respiratory complications | 4 | 3 |
| Cardiovascular complications | 4 | 3 | |
| Urinary tract infection | 1 | 2 |
4. Other adverse effects.
| Study | Adverse event | Intervention | Control |
| Carr (1996) | Distension (n) | 2 | 4 |
| Diarrhoea (n) | 0 | 1 | |
| Da Fonseca (2011) | Hyporexia (n) | 1 | 1 |
| Readmission (n) | 0 | 4 | |
| Minig (2009) | Diarrhoea (mean, EORTC C‐30) | 13.9 | 6.7 |
| Readmission (n) | 0 | 1 | |
| Nakeeb (2009) | Readmission (n) | 3 | 4 |
| Schroeder (1991) | Diarrhoea (n) | 1 | 0 |
| Small bowel obstruction (n) | 0 | 4 | |
| Yang (2013) | Stomach distension (n) | 6 | 11 |
Excluded studies
Of the 146 full texts assessed for eligibility, we excluded 122 records. The most common justifications for exclusion were not being lower gastrointestinal surgery (N = 22) and not having an appropriate control group (N = 18), followed by not a relevant topic (N = 16), feeding started greater than 24 hours after surgery (N = 15), the study was actually a review (N = 17), the study was not an RCT (N = 12), the study used total parenteral nutrition (N = 9), examined multiple variables not just feeding (N = 3), could not be sourced/not readable (N = 3), or was only available as an abstract (N = 5), feeding commenced post‐discharge (n = 1) and one study is still awaiting classification (N = 1).
Risk of bias in included studies
Risk of bias for each included study is described in detail in the Characteristics of included studies section. Details of 'Risk of bias' judgements for each study are presented in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged six studies to be at low risk of bias for random sequence generation (da Fonseca 2011; Dag 2011; Minig 2009; Sagar 1979; Stewart 1998; Yang 2013). Randomisation was undertaken using a computer‐generated random number generator (da Fonseca 2011; Dag 2011; Minig 2009; Stewart 1998), a statistical table (Sagar 1979) or a random number table (Yang 2013). For all other studies (Beier‐Holgersen 1996; Binderow 1994; Carr 1996; Chatterjee 2012; Hartsell 1997; Lucha 2005; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Schroeder 1991) insufficient details were provided, resulting in a judgement of unclear risk of bias.
Allocation concealment
Insufficient details were provided in all 17 studies with regards to their allocation concealment methods; we therefore judged all to have an unclear risk of bias. Two studies (Carr 1996; Nakeeb 2009) mentioned using sealed envelopes, however they did not report whether they were sequentially numbered or opaque; they were therefore judged as unclear.
Blinding
Participants
Fourteen of the studies (Binderow 1994; Carr 1996; Chatterjee 2012; Dag 2011; Hartsell 1997; Lucha 2005; Minig 2009; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Schroeder 1991; Stewart 1998; Yang 2013) reported no attempt to blind participants. They were judged to be at high risk of bias as they were unlikely to be adequately blinded with an intervention of this nature. One study reported that participants were informed of the group to which they were assigned on the first postoperative day (da Fonseca 2011) and was also judged to be at high risk of bias. One study (Mulrooney 2004) reported, through author correspondence, that participants were not aware of group allocation until postoperatively, but insufficient detail was provided on the blinding procedure and the timing postoperatively that blinding was broken; this study was judged to be of unclear risk of bias. One study, (Beier‐Holgersen 1996) where the intervention group received a nutrition supplement, was judged to be at low risk of bias for blinding of participants as they used an identical placebo (flavoured orange drink) to blind their control participants.
Personnel
Personnel blinding was not discussed in 14 studies (Binderow 1994; Carr 1996; Chatterjee 2012; Dag 2011; Hartsell 1997; Lucha 2005; Minig 2009; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Schroeder 1991; Stewart 1998; Yang 2013) and were therefore judged to be at high risk of bias as they are unlikely to be adequately blinded with an intervention of this nature. Two studies (da Fonseca 2011; Mulrooney 2004 author correspondence) insufficiently described methods used to minimise possible bias, and were consequently judged to be at high risk of bias. Only one study, (Beier‐Holgersen 1996) was judged to be at low risk of bias for blinding of personnel.
Outcome assessment
One study (Beier‐Holgersen 1996) was judged at low risk of bias as it was reported as double‐blind and the randomisation code was not broken until patients had been followed up for 30 days after surgery. In the remaining 16 studies (Binderow 1994; Carr 1996; Chatterjee 2012; da Fonseca 2011; Dag 2011; Hartsell 1997; Lucha 2005; Minig 2009; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Schroeder 1991; Stewart 1998; Yang 2013), the blinding of the outcome assessor for wound infection, intraabdominal abscesses, postoperative complications (all complications considered, not just the primary and secondary outcomes of interest), anastomotic leakage/dehiscence, adverse events (all considered) and LoS was not discussed and were judged to be at high risk of bias as they are unlikely to be adequately blinded with an intervention of this nature. Twelve studies (Beier‐Holgersen 1996; Carr 1996; Chatterjee 2012; da Fonseca 2011;Dag 2011; Hartsell 1997; Minig 2009; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Stewart 1998) reported mortality data, and were judged to be at low risk of bias for this outcome because the outcome assessor is unlikely to affect this outcome.
Incomplete outcome data
Three studies were judged to be at high risk of bias (Minig 2009; Mulrooney 2004; Yang 2013) because they had greater than 10% difference in missing data between groups. Four trials were judged unclear risk of bias as either information was not provided (Lucha 2005;Sagar 1979; Schroeder 1991) or dropouts after randomisation were not reported (Dag 2011). The remaining 10 studies were judged to be at low risk of bias.
Selective reporting
We judged 10 studies to be at unclear risk of bias as protocols were not available. However seven studies (Beier‐Holgersen 1996; Binderow 1994; Carr 1996; Lucha 2005; Nakeeb 2009; Sagar 1979; Schroeder 1991) did have some issues.that resulted in bias. Five had missing data for primary or secondary outcomes (Beier‐Holgersen 1996; Binderow 1994; Lucha 2005; Nakeeb 2009; Sagar 1979) and five (Beier‐Holgersen 1996; Carr 1996; Lucha 2005; Nakeeb 2009; Schroeder 1991) either reported results that were not stated in the methods or did not report results that had been stated in the methods.
Effects of interventions
Summary of findings for the main comparison. Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement.
| Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement | ||||||
| Patient or population: lower gastrointestinal surgery Setting: hospital Intervention: early enteral nutrition Comparison: later commencement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with no calories | Corresponding risk with early enteral nutrition | |||||
| Length of hospital stay (days) | The mean LoS of control groups ranged from six to 24 days | MD 1.95 lower (2.99 lower to 0.91 lower) | ‐ | 1346 (16 RCTs) | ⊕⊕⊝⊝ LOW1 | Trial Sequential Analysis showed that the boundary for benefit was crossed. This indicates that early feeding seems to decrease the mean length of hospital stay by at least one day if risk of bias and other threats to the validity can be disregarded |
| Wound infection | 91 per 1,000 | 91 per 1,000
(58 to 138) (33 fewer to 47 more) |
RR 0.99 (0.64 to 1.52) | 1181 (12 RCTs) | ⊕⊝⊝⊝ VERY LOW2 | Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of wound infection by 30% or more |
| Intraabdominal abscess | 29 per 1,000 | 29 per 1,000
(8 to 110) (21 fewer to 81 more) |
RR 1.00 (0.26 to 3.80) | 554 (6 RCTs) | ⊕⊕⊝⊝ LOW3 | It was not possible to perform Trial Sequential Analysis due to limited data and too few events |
| Anastomotic leakage/dehiscence | 47 per 1,000 | 37 per 1,000
(18 to 76) (19 fewer to 29 more) |
RR 0.78 (0.38 to 1.61) | 1232 (13 RCTs) | ⊕⊕⊝⊝ LOW3 | Absolute risk reduction: 0.01
Thus, for every 1000 participants receiving early feeding, 10 less anastomotic leakage compared to later commencement. Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of anastomotic leakage/dehiscence by 30% or more |
|
Pneumonia (within hospital stay) |
21 per 1,000 | 18 per 1,000
(7 to 51) (14 fewer to 30 more) |
RR 0.88 (0.32 to 2.42) | 954 (10 RCTs) | ⊕⊕⊝⊝ LOW3 | Absolute risk reduction: 0.003 Thus, for every 1000 participants receiving early feeding, 3 less pneumonia compared to later commencement.Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of pneumonia by 30% or more |
| Mortality | 30 per 1,000 | 17 per 1,000
(6 to 46) (24 fewer to 16 more) |
RR 0.56 (0.21 to 1.52) | 1179 (12 RCTs) | ⊕⊕⊝⊝ LOW4 | Absolute risk reduction: 0.013 Thus, for every 1000 participants receiving early feeding, 13 less mortality compared to later commencement.Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of mortality by 30% or more |
| Vomiting | 231 per 1,000 | 284 per 1,000
(222 to 365) (9 fewer to 134 more) |
RR 1.23 (0.96 to 1.58) | 613 (7 RCTs) | ⊕⊕⊝⊝ LOW5 | Absolute risk reduction: ‐ 0.053 So for every 1000 participants receiving early feeding, 53 more vomiting compared to later commencement.Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of vomiting by 30% or more |
| Nausea | 627 per 1,000 | 596 per 1,000 (445 to 790) (172 fewer to 163 more) |
RR 0.95 (0.71 to 1.26) | 118 (2 RCTs) |
⊕⊕⊝⊝ LOW5 | Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of nausea by 30% or more |
| Nausea and vomiting | 262 per 1,000 | 241 per 1,000 (157 to 369) (105 fewer to 107 more) |
RR 0.92 (0.60 to 1.41) | 238 (4 RCTs) |
⊕⊝⊝⊝ VERY LOW6 | Trial Sequential Analysis showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of nausea and vomiting by 30% or more |
| Quality of Life | 1 study: EORTC OV‐28 (global):Traditional feeding 28.6(13.7) versus early feeding 26.5(14.9), P= 0.68. EORTC C‐30 (global):Traditional feeding 56.1(22.2) versus early feeding 64.6(17.1), P=0.172. |
⊕⊕⊝⊝ LOW7 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbrevations: CI: Confidence interval; WMD: weighted mean differences; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by two levels because of the following: risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment were poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2)
2Downgraded by three levels because of the following: risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment were poorly reported, and selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement), imprecision (confidence intervals are wide) and indirectness (included various definitions e.g. some incorporated pelvic abscesses whilst others did not; unclear definition of outcome)
3Downgraded by two levels because of the following: risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment were poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement) and imprecision (confidence intervals are wide and very few events occurred)
4Downgraded by two levels because of the following: indirectness (unclear definition whether mortality occurred prior or after discharge) and imprecision (confidence intervals are wide and very few events occurred)
5Downgraded by two levels because of the following: risk of bias (blinding did not occur in any study (expect one), random sequence generation and allocation concealment were poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement) and imprecision (confidence intervals are wide)
6 Downgraded by three levels because of the following: risk of bias (blinding did not occur in any study, allocation concealment was poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement) and imprecision (confidence intervals are wide) and indirectness (included various definitions of nausea and vomiting)
7 Downgraded by two levels because of the following: risk of bias (blinding did not occur in this study, allocation concealment was poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement) and imprecision (just one study available)
Summary of findings 2. Subgroup and Sensitivity analyses.
| Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement | ||||||
| Patient or population: lower gastrointestinal surgery Setting: hospital Intervention: early enteral nutrition Comparison: later commencement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with no calories | Corresponding risk with early enteral nutrition | |||||
|
Subgroup 1: Length of hospital stay (days) distal to ligament of Treitz |
The mean LoS of control groups ranged from 6 to 17 days | MD 1.77 shorter (2.95 shorter to 0.59 shorter) |
‐ | 1156 (13 RCTs) |
⊕⊕⊝⊝ LOW1 | |
|
Subgroup 2: Length of hospital stay (days) distal and proximal to ligament of Treitz |
The mean LoS of control groups ranged from 9 to 24 days | MD 2.58 days shorter (4.40 days shorter to 0.76 days shorter) |
‐ | 190 (3 RCTs) |
⊕⊕⊝⊝ LOW2 | |
|
Subgroup 3: Length of hospital stay (days) oral feeding only |
The mean LoS of control groups ranged from 6 to 17 days. | MD 2.06 days shorter (3.26 days shorter to 0.87 days shorter) |
‐ | 1075 (11 RCTs) |
⊕⊕⊝⊝ LOW1 | |
|
Subgroup 4: Length of hospital stay (days) tube feeding with/without oral feeding |
The mean LoS of control groups ranged from 9 to 24 days. | MD 1.75 days shorter (4.32 days shorter to 0.82 days shorter) |
‐ | 271 (5 RCTs) |
⊕⊝⊝⊝ VERY LOW3 | |
|
Sensitivity analysis 2: Removing studies with imputed results |
The mean LoS of control groups ranged from seven to 17 days | MD 2.27 days shorter (3.62 days shorter to 0.92 days shorter) |
‐ | 396 (5 RCTs) |
⊕⊝⊝⊝ VERY LOW3 | |
|
Senstivity analysis 3: Worst case scenario imputation |
The mean LoS of control groups ranged from 6 to 24 days | MD 2.06 days shorter (3.18 days shorter to 0.94 days shorter) | ‐ | 235 (3 RCTs) |
⊕⊕⊝⊝ LOW1 | |
|
Senstivity analysis 4: Best case scenario imputation |
The mean LoS of control groups ranged from 6 to 24 days | MD 1.80 days shorter (2.61 days shorter to 1.00 day shorter) | ‐ | 235 (3 RCTs) |
⊕⊕⊝⊝ LOW1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbrevations: CI: Confidence interval; WMD: weighted mean differences; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by two levels because of the following: risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment were poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2)
2 Downgraded by two levels because of the following: risk of bias (blinding did not occur in any study and allocation concealment was poorly reported) and imprecision (confidence intervals are wide and only based on 3 studies)
3 Downgraded by three levels because of the following: risk of bias (blinding did not occur in any study and allocation concealment was poorly reported) and imprecision (confidence intervals are wide) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2)
The 17 randomised controlled trials represent a total of 1437 patients, all undergoing lower gastrointestinal surgery but with a wide variety of clinical gastrointestinal conditions.
1. Primary outcomes
1.1 Length of hospital stay
Length of hospital stay (LoS) was reported in all 17 studies, but one was excluded from meta‐analysis (Beier‐Holgersen 1996) due to not reporting LoS in a compatible format (median only, without range or interquartile range (IQR)). We included estimated results for LoS which differed from the published results in eight of the included studies (Binderow 1994; Lucha 2005; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Stewart 1998), details of which are presented in Table 4. The mean LoS ranged from four days to 16 days in early feeding groups and from 6.6 days to 23.5 days in control groups. Mean difference (MD) from the random‐effects model in LoS was 1.95 (95% CI, ‐2.99 to ‐0.91, P = 0.0002) days shorter in the intervention group (Analysis 1.1). However, there was a high level of heterogeneity between studies (I2 = 81%, Chi2 = 78.98, P < 0.00001) (Table 7; Table 8, and Figure 4). The results from Beier‐Holgersen (Beier‐Holgersen 1996) (median LoS of eight days in the early feeding and 11.5 days in the control group), support a reduction in LoS with early feeding. These results were confirmed by the Trial Sequential Analysis showing that the cumulative Z‐curve crossed the trial sequential monitoring boundary for benefit (Figure 5). Although the required information size to support or reject an a priori intervention effect of a reduction in LoS of one day (var = 9 days, alpha of 3.3% and beta of 20%) has not yet been reached, which was 2444 people. The Trial Sequential Analysis‐adjusted CI was ‐3.57 to ‐0.33 days. Overall, using GRADE criteria, we judged the quality of the evidence to be low. This outcome was downgraded by two levels because of risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment was poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, substantial statistical heterogeneity).
1.1. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 1 Length of hospital stay.
5. Early enteral nutrition versus later commencement after gastrointestinal surgery, surgical site, outcome: 1.1 Length of hospital stay.
| Study or subgroup | Experimental | Control | Weight | Mean difference | ||||
| Mean | SD | N | Mean | SD | N | IV, Random, 95%CI | ||
| 1.1.1 Surgical site distal to ligament of Treitz | ||||||||
| Binderow 1994 | 6.7 | 3.7 | 32 | 8.0 | 5.5 | 32 | 6.3% | ‐1.30 [‐3.60, 1.00] |
| Carr 1996 | 9.8 | 6.6 | 14 | 9.3 | 2.8 | 14 | 4.2% | 0.50 [‐3.26, 4.26] |
| Da Fonseca 2011 | 4.0 | 3.7 | 24 | 7.6 | 8.1 | 26 | 4.6% | ‐3.60 [‐7.05, ‐0.15] |
| Dag 2011 | 5.55 | 2.35 | 99 | 9.0 | 6.5 | 100 | 7.9% | ‐3.45 [‐4.81, ‐2.09] |
| Hartsell 1997 | 7.2 | 3.3 | 29 | 8.1 | 2.3 | 29 | 7.7% | ‐0.90 [‐2.36, 0.56] |
| Lucha 2005 | 6.3 | 3.7 | 26 | 6.6 | 5.5 | 25 | 5.8% | ‐0.30 [‐2.88, 2.28] |
| Mulrooney 2004 | 11.79 | 4.46 | 32 | 10.57 | 4.64 | 29 | 6.3% | 1.22 [‐1.07, 3.51] |
| Nakeeb 2009 | 6.2 | 3.7 | 60 | 6.9 | 5.5 | 60 | 7.4% | ‐0.70 [‐2.38, 0.98] |
| Ortiz 1996 | 13.13 | 4.23 | 95 | 16.57 | 9.23 | 95 | 6.8% | ‐3.44 [‐5.48, ‐1.40] |
| Reissman 1995 | 6.2 | 1.8 | 80 | 6.8 | 1.8 | 81 | 8.9% | ‐0.60 [‐1.16, ‐0.04] |
| Schroeder 1991 | 10.0 | 4.0 | 16 | 15.0 | 10.0 | 16 | 2.7% | ‐5.00 [‐10.28, 0.28] |
| Stewart 1998 | 9.36 | 4.11 | 36 | 10.08 | 2.55 | 36 | 7.5% | ‐0.72 [‐2.30, 0.86] |
| Yang 2013 | 6.0 | 1.0 | 35 | 11.7 | 3.8 | 35 | 8.0% | ‐5.70 [‐7.00, ‐4.40] |
| Subtotal (95% CI) | 578 | 578 | 84.2% | ‐1.77 [‐2.95, ‐0.59] | ||||
| Heterogeneity: Tau² = 3.41; Chi² = 74.33, df = 12 (P < 0.00001); I² = 84% | ||||||||
| Test for overall effect: Z = 2.95 (P = 0.003) | ||||||||
| 1.1.2 Surgical site distal and proximal to ligament of Treitz | ||||||||
| Chatterjee 2012 | 8.45 | 5.14 | 60 | 10.53 | 4.95 | 60 | 7.2% | ‐2.08 [‐3.89, ‐0.27] |
| Minig 2009 | 6.9 | 2.6 | 18 | 9.1 | 4.5 | 22 | 6.4% | ‐2.20 [‐4.43, 0.03] |
| Sagar 1979 | 16.0 | 4.8 | 15 | 23.5 | 10.9 | 15 | 2.2% | ‐7.50 [‐13.53, ‐1.47] |
| Subtotal (95% CI) | 93 | 97 | 15.8% | ‐2.58 [‐4.40, ‐0.76] | ||||
| Heterogeneity: Tau² = 0.83; Chi² = 2.90, df = 2 (P = 0.23); I² = 31% | ||||||||
| Test for overall effect: Z = 2.78 (P = 0.005) | ||||||||
| Total (95% CI) | 671 | 675 | 100.0% | ‐1.95 [‐2.99, ‐0.91] | ||||
| Heterogeneity: Tau² = 3.08; Chi² = 78.98, df = 15 (P < 0.00001); I² = 81% | ||||||||
| Test for overall effect: Z = 3.68 (P = 0.0002) | ||||||||
| Test for subgroup differences: Chi² = 0.54, df = 1 (P = 0.46), I² = 0% | ||||||||
6. Early enteral nutrition versus later commencement after gastrointestinal surgery, feeding route, outcome: 1.2 Length of hospital stay.
| Study or subgroup | Experimental | Control | Weight | Mean difference | ||||
| Mean | SD | N | Mean | SD | N | IV, Random, 95%CI | ||
| 1.2.1 Oral feeding only | ||||||||
| Binderow 1994 | 6.7 | 3.7 | 32 | 8.0 | 5.5 | 32 | 6.3% | ‐1.30 [‐3.60, 1.00] |
| Da Fonseca 2011 | 4.0 | 3.7 | 24 | 7.6 | 8.1 | 26 | 4.6% | ‐3.60 [‐7.05, ‐0.15] |
| Dag 2011 | 5.55 | 2.35 | 99 | 9.0 | 6.5 | 100 | 7.9% | ‐3.45 [‐4.81, ‐2.09] |
| Hartsell 1997 | 7.2 | 3.3 | 29 | 8.1 | 2.3 | 29 | 7.7% | ‐0.90 [‐2.36, 0.56] |
| Lucha 2005 | 6.3 | 3.7 | 26 | 6.6 | 5.5 | 25 | 5.8% | ‐0.30 [‐2.88, 2.28] |
| Minig 2009 | 6.9 | 2.6 | 18 | 9.1 | 4.5 | 22 | 6.4% | ‐2.20 [‐4.43, 0.03] |
| Nakeeb 2009 | 6.2 | 3.7 | 60 | 6.9 | 5.5 | 60 | 7.4% | ‐0.70 [‐2.38, 0.98] |
| Ortiz 1996 | 13.13 | 4.23 | 95 | 16.57 | 9.23 | 95 | 6.8% | ‐3.44 [‐5.48, ‐1.40] |
| Reissman 1995 | 6.2 | 1.8 | 80 | 6.8 | 1.8 | 81 | 8.9% | ‐0.60 [‐1.16, ‐0.04] |
| Stewart 1998 | 9.36 | 4.11 | 36 | 10.08 | 2.55 | 36 | 7.5% | ‐0.72 [‐2.30, 0.86] |
| Yang 2013 | 6.0 | 1.0 | 35 | 11.7 | 3.8 | 35 | 8.0% | ‐5.70 [‐7.00, ‐4.40] |
| Subtotal (95% CI) | 534 | 541 | 77.4% | ‐2.06 [‐3.26, ‐0.87] | ||||
| Heterogeneity: Tau² = 5.14; Chi² = 12.18, df = 10 (P<0.00001); I² = 85% | ||||||||
| Test for overall effect: Z = 3.38 (P = 0.00073) | ||||||||
| 1.2.2 Tube +/‐ oral feeding | ||||||||
| Carr 1996 | 9.8 | 6.6 | 14 | 9.3 | 2.8 | 14 | 4.2% | 0.50 [‐3.26, 4.26] |
| Chatterjee 2012 | 8.45 | 5.14 | 60 | 10.53 | 4.95 | 60 | 7.2% | ‐2.08 [‐3.89, ‐0.27] |
| Mulrooney 2004 | 11.79 | 4.46 | 32 | 10.57 | 4.64 | 29 | 6.3% | 1.22 [‐1.07, 3.51] |
| Sagar 1979 | 16.0 | 4.8 | 15 | 23.5 | 10.9 | 15 | 2.2% | ‐7.50 [‐13.53, ‐1.47] |
| Schroeder 1991 | 10.0 | 4.0 | 16 | 15.0 | 10.0 | 16 | 2.7% | ‐5.00 [‐10.28, 0.28] |
| Subtotal (95% CI) | 137 | 134 | 22.6% | ‐1.75 [‐4.32, 0.82] | ||||
| Heterogeneity: Tau² = 5.14; Chi² = 12.18, df = 4 (P = 0.02); I² = 67% | ||||||||
| Test for overall effect: Z = 1.34 (P = 0.18) | ||||||||
| Total (95% CI) | 671 | 675 | 100.0% | ‐1.95 [‐2.99, ‐0.91] | ||||
| Heterogeneity: Tau² = 3.08; Chi² = 78.98, df = 15 (P<0.00001); I² = 81% | ||||||||
| Test for overall effect: Z = 3.68 (P = 0.0002) | ||||||||
| Test for subgroup differences: Chi² = 0.54, df = 1 (P = 0.46), I² = 0% | ||||||||
4.

Funnel plot of comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.1 Length of hospital stay.
5.
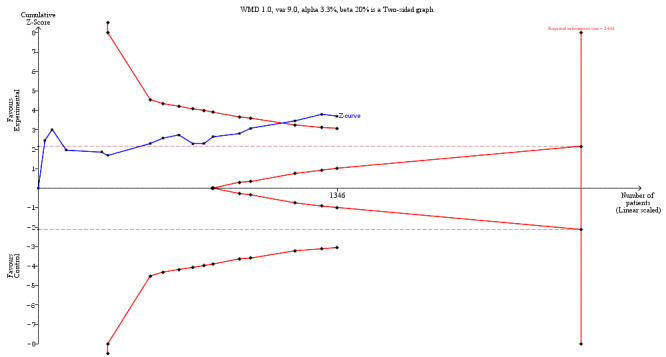
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.1 Length of hospital stay
1.2 Postoperative complications
Wound infection
Wound infection was reported in 12 of the included studies (Beier‐Holgersen 1996; Chatterjee 2012; da Fonseca 2011; Dag 2011; Hartsell 1997; Minig 2009; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Stewart 1998). Absolute risks ranged from 0% (0/40) to 25% (15/60) in the intervention groups and from 0% (0/29) to 33.3% (10/30) in the control groups. Using the Mantel‐Haenszel methods the combined RR was 0.99 (0.64 to 1.52, P = 0.95), with some heterogeneity between trials (I2 = 5%, Chi2 = 10.54, P = 0.39) (Table 9 and Figure 6). The study by Beier‐Holgersen (Beier‐Holgersen 1996) differs from the 11 other studies by having a considerably higher proportion of patients with wound infection in the control group (33%). Excluding this study (results not presented) resulted in less heterogeneity between trials (I2 = 0%, Chi2 = 4.43, P = 0.55) and a RR 1.12 (95% CI, 0.73 to 1.71, P = 0.62). TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of wound infection events by 30% or more (Figure 7). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction (a priori estimate) of wound infection (with a control group proportion of 9.1%, an alpha of 3.3%, and a beta of 20%) is 3,388 patients. The TSA‐adjusted CI was 0.48 to 2.11. Using GRADE criteria, wound infection was judged as having very low‐quality evidence. This outcome was downgraded by three levels because of risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment was poorly reported and selective reporting, (where insufficient information was provided due to lack of protocol or reporting outcomes not mentioned in methods or having to input missing data)), imprecision (confidence intervals are wide) and indirectness (included various definitions e.g. some incorporated pelvic abscesses whilst others did not; unclear definition of outcome). We tested for publication bias by visual inspection of the funnel plot for wound infection (Figure 8), although this had only nine included trials after excluding those with zero events in one or both arms.
7. Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: Wound infection.
| Study | Experimental | Control | Weight | Risk Ratio | ||
| Events | Total | Events | Total | MH, Random, 95% CI | ||
| Beier‐Holgersen 1996 | 1 | 30 | 10 | 30 | 4.7% | 0.10 [0.01; 0.73] |
| Chatterjee 2012 | 15 | 60 | 8 | 60 | 26.5% | 1.88 [0.86; 4.09] |
| Da Fonseca 2011 | 2 | 24 | 2 | 26 | 5.2% | 1.08 [0.17; 7.10] |
| Dag 2011 | 5 | 99 | 7 | 100 | 14.1% | 0.72 [0.24; 2.20] |
| Hartsell 1997 | 0 | 29 | 0 | 29 | 0.0% | |
| Minig 2009 | 0 | 18 | 3 | 22 | 0.05 | 0.00 |
| Mulrooney 2004 | 4 | 36 | 3 | 37 | 8.9% | 1.37 [0.33; 5.70] |
| Nakeeb 2009 | 5 | 60 | 5 | 60 | 12.5% | 1.00 [0.31; 3.28] |
| Ortiz 1996 | 5 | 95 | 6 | 95 | 13.2% | 0.83 [0.26; 2.64] |
| Reissman 1995 | 2 | 80 | 1 | 81 | 3.3% | 2.03 [0.19, 21.89] |
| Sagar 1979 | 3 | 15 | 5 | 15 | 11.6% | 0.60 [0.17; 2.07] |
| Stewart 1998 | 0 | 40 | 4 | 40 | 0.0% | 0.00 |
| Total (95% CI) | 42 | 586 | 54 | 595 | 100.0% | 0.99 [0.64; 1.52] |
| Heterogeneity: Tau² = 0.0290; Chi² = 10.54, df = 10 (P = 0.39); I² = 5% | ||||||
| Test for overall effect: Z = ‐0.07 (P = 0.95) | ||||||
6.
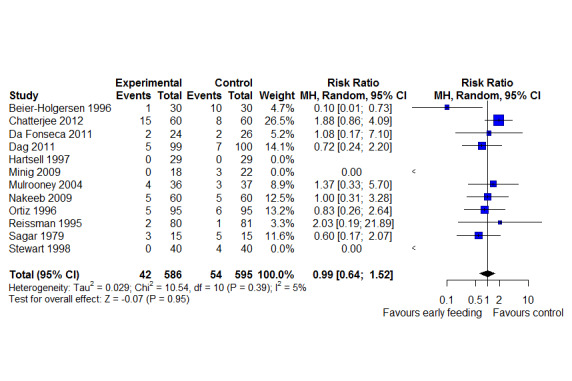
Comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome: 3 Wound infection
7.
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.3 Wound infection.
8.
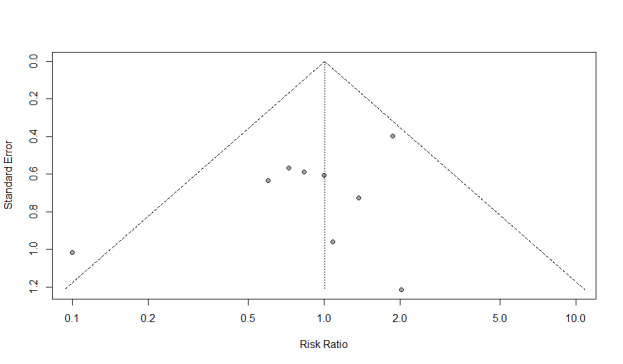
Funnel plot of comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.3 Wound infection.
Intraabdominal abscess
Intraabdominal abscess was reported in six studies (Beier‐Holgersen 1996; Minig 2009; Mulrooney 2004; Ortiz 1996; Reissman 1995; Sagar 1979). Absolute risks ranged from 0% (0/36) to 13% (2/15) in both the early feeding groups and the control groups. Using a random‐effects method, the combined RR for this outcome was 1.00 (95% CI, 0.26 to 3.80, P = 1.00), with very little heterogeneity between trials (I2 = 0%, Chi2 = 0.97, P = 0.91) (Table 10 and Figure 9). It was not possible to do TSA for intraabdominal abscess due to limited data and too few events. We judged the quality of the evidence for intraabdominal abscess to be low. This outcome was downgraded by two levels because of risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment was poorly reported, and selective reporting, (where insufficient information was provided due to lack of protocol or reporting outcomes not mentioned in methods or having to input missing data)) and imprecision (confidence intervals are wide and very few events occurred).
8. Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: Intraabdominal abscess.
| Study | Experimental | Control | Weight | Risk Ratio | ||
| Events | Total | Events | Total | MH, Random, 95% CI | ||
| Beier‐Holgersen 1996 | 0 | 30 | 2 | 30 | 0.0% | 0.00 |
| Minig 2009 | 0 | 18 | 2 | 22 | 0.0% | 0.00 |
| Mulrooney 2004 | 0 | 36 | 0 | 37 | 0.0% | |
| Ortiz 1996 | 1 | 95 | 1 | 95 | 23.3% | 1.00 [0.06; 15.76] |
| Reissman 1995 | 1 | 80 | 1 | 81 | 23.4% | 1.01 [0.06; 15.91] |
| Sagar 1979 | 2 | 15 | 2 | 15 | 53.3% | 1.00 [0.16; 6.20] |
| Total (95% CI) | 4 | 274 | 8 | 280 | 100.0% | 1.00 [0.26; 3.80] |
| Heterogeneity: Tau² = 0; Chi² = 0.97, df = 4 (P = 0.91); I² = 0% | ||||||
| Test for overall effect: Z = 0.00 (P = 1.00) | ||||||
9.
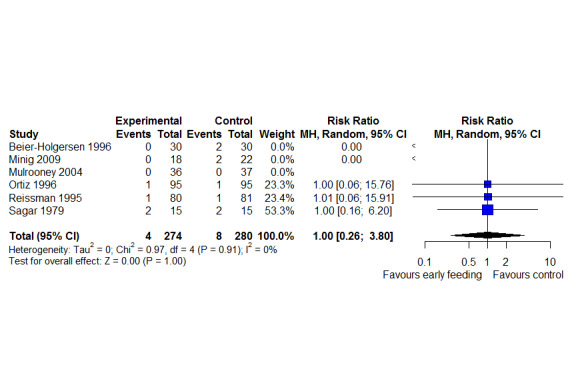
Comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome: 4 Intraabdominal abscess
Anastomotic leakage/dehiscence
Anastomotic leakage/dehiscence was reported in 13 studies (Beier‐Holgersen 1996; Chatterjee 2012; da Fonseca 2011; Dag 2011; Hartsell 1997; Lucha 2005; Minig 2009; Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Sagar 1979; Stewart 1998).The risk of dehiscence ranged from 0% (0/80) to 8.3% (3/36) in early feeding groups and from 0% (0/40) to 13.3% (4/30) in control groups. The combined RR from the random‐effects model was 0.78 (95% CI 0.38 to 1.61, P = 0.51), with some evidence of heterogeneity between studies (I2 = 0%, Chi2 = 5.43, P = 0.94) (Table 11 and Figure 10). The absolute risk reduction (ARR) was 0.01. Thus, for every 100 participants receiving early feeding, one less anastomotic leakage compared to 100 participants receiving later commencement of feeding (NNTB). TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of anastomotic leakage/dehiscence events by 30% or more (Figure 11). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction (a priori estimate) of anastomotic leakage/dehiscence (with a control group proportion of 27.6%, an alpha of 3.3%, and a beta of 20%) is 6,824 patients (vertical red dashed line). The TSA‐adjusted CI was 0.04 to 14.75. Anastomotic leak/dehiscence was also judged as having low‐quality evidence. It was also downgraded by two levels because of risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment was poorly reported and selective reporting, (where insufficient information was provided due to lack of protocol or reporting outcomes not mentioned in methods or having to input missing data)) and imprecision (confidence intervals are wide and very few events occurred).
9. Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: Anastomotic leakage.
| Study | Experimental | Control | Weight | Risk Ratio | ||
| Events | Total | Events | Total | MH, Random, 95% CI | ||
| Beier‐Holgersen 1996 | 2 | 30 | 4 | 30 | 19.7% | 0.50 [0.10; 2.53] |
| Chatterjee 2012 | 8 | 60 | 3 | 60 | 31.7% | 2.67 [0.74; 9.57] |
| Da Fonseca 2011 | 0 | 24 | 4 | 26 | 0.0% | 0.00 |
| Dag 2011 | 2 | 99 | 6 | 100 | 20.9% | 0.34 [0.07; 1.63] |
| Hartsell 1997 | 0 | 29 | 1 | 29 | 0.0% | 0.00 |
| Lucha 2005 | 1 | 26 | 0 | 25 | 0.0% | Inf |
| Minig 2009 | 0 | 18 | 3 | 22 | 0.0% | 0.00 |
| Mulrooney 2004 | 3 | 36 | 0 | 37 | 0.0% | Inf |
| Nakeeb 2009 | 1 | 60 | 2 | 60 | 9.2% | 0.50 [0.05; 5.37] |
| Ortiz 1996 | 2 | 95 | 4 | 95 | 18.5% | 0.50 [0.09; 2.67] |
| Reissman 1995 | 0 | 80 | 1 | 81 | 0.0% | 0.00 |
| Sagar 1979 | 0 | 15 | 1 | 15 | 0.0% | 0.00 |
| Stewart 1998 | 1 | 40 | 0 | 40 | 0.0% | Inf |
| Total (95% CI) | 20 | 612 | 29 | 620 | 100.0% | 0.78 [0.38; 1.61] |
| Heterogeneity: Tau² = 0; Chi² = 5.43, df = 12 (P = 0.94); I² = 0% | ||||||
| Test for overall effect: Z = ‐0.67 (P = 0.51) | ||||||
10.
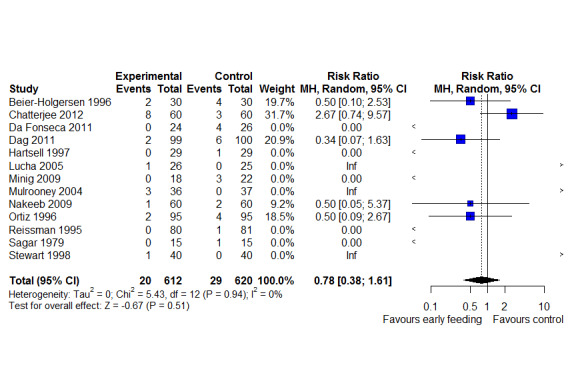
Comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome: 5 Anastomotic leakage
11.
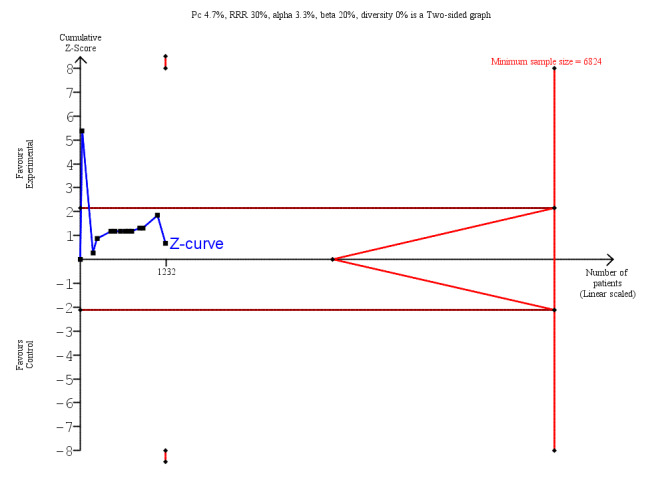
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.5 Anastomotic leakage.
Pneumonia
Post‐surgical pneumonia was reported in 10 studies (Beier‐Holgersen 1996; da Fonseca 2011; Dag 2011; Hartsell 1997; Lucha 2005; Mulrooney 2004; Ortiz 1996; Reissman 1995; Schroeder 1991; Stewart 1998). Pneumonia events ranged from none to 6.3% (1/16) in the intervention group and from none to 6.7% (2/30) in the control group. The combined RR using the random‐effects model was 0.88 (95% CI, 0.32 ‐ 2.42, P = 0.81) with little evidence of heterogeneity between studies (I2 = 0%, Chi2 = 0.65, P = 1.00) (Table 12 and Figure 12). The absolute risk reduction (ARR) was 0.003. Thus, for every 1000 participants receiving early feeding, 3 less pneumonia compared to later commencement of feeding (NNTB).
10. Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: Pneumonia.
| Study | Experimental | Control | Weight | Risk Ratio | ||
| Events | Total | Events | Total | MH, Random, 95% CI | ||
| Beier‐Holgersen 1996 | 1 | 30 | 2 | 30 | 18.5% | 0.50 [0.05; 5.22] |
| Da Fonseca 2011 | 1 | 24 | 0 | 26 | 0.0% | Inf |
| Dag 2011 | 3 | 99 | 3 | 100 | 40.9% | 1.01 [0.21; 4.88] |
| Hartsell 1997 | 1 | 29 | 0 | 29 | 0.0% | Inf |
| Lucha 2005 | 0 | 26 | 1 | 25 | 0.0% | 0.00 |
| Mulrooney 2004 | 2 | 36 | 0 | 37 | 0.0% | Inf |
| Ortiz 1996 | 2 | 95 | 2 | 95 | 27.0% | 1.00 [0.14; 6.95] |
| Reissman 1995 | 0 | 80 | 1 | 81 | 0.0% | 0.00 |
| Schroeder 1991 | 1 | 16 | 0 | 16 | 0.0% | Inf |
| Stewart 1998 | 1 | 40 | 1 | 40 | 13.6% | 1.00 [0.06; 15.44] |
| Total (95% CI) | 12 | 475 | 10 | 479 | 100.0% | 0.88 [0.32; 2.42] |
| Heterogeneity: Tau² = 0; Chi² = 0.65, df = 9 (P = 1.00); I² = 0% | ||||||
| Test for overall effect: Z = ‐0.24 (P = 0.81) | ||||||
12.

Comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome: 6 Pneumonia
TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of pneumonia by 30% or more (Figure 13). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction (a priori estimate) of anastomotic leakage/dehiscence (with a control group proportion of 2.1%, an alpha of 3.3%, and a beta of 20%) is 15,624 patients (vertical red dashed line). The TSA‐adjusted CI was 0.01 to 54.17. Using GRADE criteria, we judged the quality of the evidence for pneumonia to be low. This outcome was downgraded by two levels because of risk of bias (blinding did not occur in any study (except one), random sequence generation and allocation concealment was poorly reported and selective reporting, (where insufficient information was provided due to lack of protocol reporting outcomes not mentioned in methods or having to input missing data)) and imprecision (confidence intervals are wide and very few events occurred).
13.
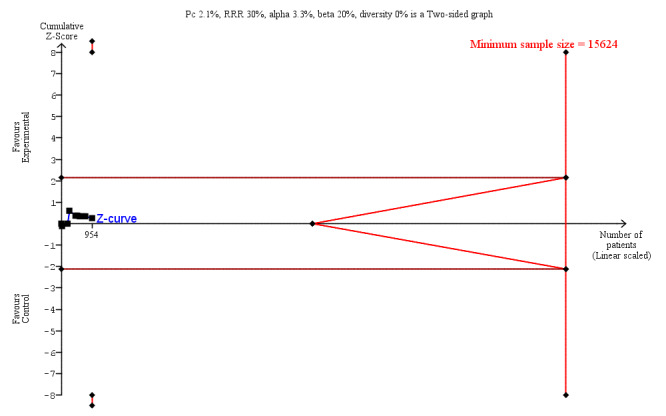
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.6 Pneumonia.
Other complications
A range of other complications were reported in the included studies, which included pulmonary failure, myocardial infarction, urinary tract infection, toxic hepatitis, deep vein thrombosis, bleeding, pelvic abscess. See Table 5 for full list of other complications.
2. Secondary outcomes
2.1 Mortality
Mortality was reported in 12 studies (Beier‐Holgersen 1996; Carr 1996; Chatterjee 2012; da Fonseca 2011; Dag 2011; Hartsell 1997; Minig 2009;.Mulrooney 2004; Nakeeb 2009; Ortiz 1996; Reissman 1995; Stewart 1998). Mortality ranged from none to 6.7% (2/30) in the early feeding groups, and from none to 19% (7/37) in the control groups. Mortality was reported predominantly in the hospital unit during the entire experimental period, except for three studies in which mortality was reported after 30 days (Beier‐Holgersen 1996, da Fonseca 2011) or 60 days (Mulrooney 2004). The most commonly reported cause of death was anastomotic leakage/dehiscence, sepsis, and acute myocardial infarction. There was weak evidence of a decrease in the relative risk (RR) of mortality among patients fed early from the random‐effects model (RR = 0.56, 95% CI, 0.21 to 1.52, P = 0.26), with little statistical heterogeneity between trials (I2 = 0%, Chi2 = 3.08, P = 0.96) (Table 13 and Figure 14). TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of mortality by 30% or more (Figure 15). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction (a priori estimate) of mortality (with a control group proportion of 3.0%, an alpha of 3.3%, and a beta of 20%) is 11,665 patients (vertical red dashed line). The TSA‐adjusted CI was 0.01 to 31.66. Overall, using GRADE criteria, this outcome was downgraded by two levels to low quality of evidence because of indirectness (unclear definition whether mortality occurred prior or after discharge) and imprecision (confidence intervals are wide and very few events occurred). We did not consider downgrading for risk of bias issues (i.e. lack of blinding) for this outcome.
11. Early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: Mortality.
| Study | Experimental | Control | Weight | Risk Ratio | ||
| Events | Total | Events | Total | MH, Random, 95% CI | ||
| Beier‐Holgersen 1996 | 2 | 30 | 4 | 30 | 37.2% | 0.50 [0.10; 2.53] |
| Carr 1996 | 0 | 14 | 1 | 14 | 0.0% | 0.00 |
| Chatterjee 2012 | 3 | 60 | 1 | 60 | 19.6% | 3.00 [0.32; 28.03] |
| Da Fonseca 2011 | 1 | 24 | 0 | 26 | 0.0% | Inf |
| Dag 2011 | 0 | 99 | 1 | 100 | 0.0% | 0.00 |
| Hartsell 1997 | 0 | 29 | 1 | 29 | 0.0% | 0.00 |
| Minig 2009 | 0 | 18 | 1 | 22 | 0.0% | 0.00 |
| Mulrooney 2004 | 2 | 36 | 7 | 37 | 43.2% | 0.29 [0.07; 1.32] |
| Nakeeb 2009 | 0 | 60 | 1 | 60 | 0.0% | 0.00 |
| Ortiz 1996 | 0 | 95 | 0 | 95 | 0.0% | |
| Reissman 1995 | 0 | 80 | 0 | 81 | 0.0% | |
| Stewart 1998 | 0 | 40 | 1 | 40 | 0.0% | 0.00 |
| Total (95% CI) | 8 | 585 | 18 | 594 | 100.0% | 0.56 [0.21; 1.52] |
| Heterogeneity: Tau² = 0; Chi² = 3.08, df = 9 (P = 0.96); I² = 0% | ||||||
| Test for overall effect: Z = ‐1.14 (P = 0.26) | ||||||
14.
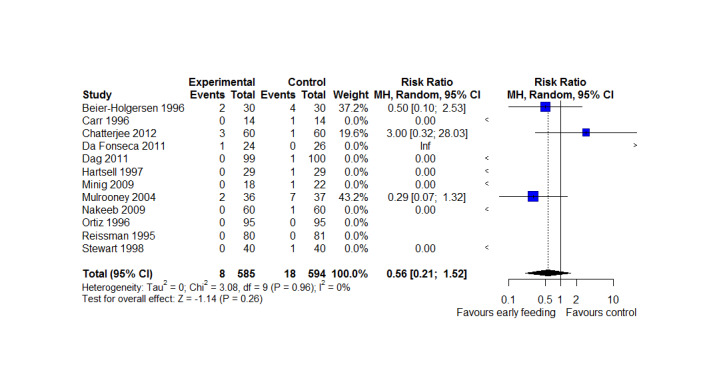
Comparison: 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome: 7 Mortality
15.
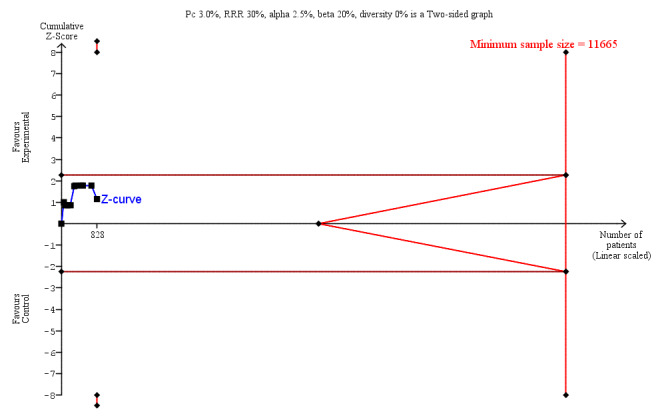
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.7 Mortality.
2.2 Adverse events: Vomiting, nausea, and combined nausea and vomiting
A total of seven studies described incidence of vomiting (Beier‐Holgersen 1996; Binderow 1994; Hartsell 1997; Nakeeb 2009; Reissman 1995; Stewart 1998; Yang 2013), and the absolute risks ranged from 8.6% (3/35) to 50% (15/30) in the early feeding groups and from 2.9% (1/35) to 57% (17/30) in the control groups. There was weak evidence of an increase in the RR of vomiting among patients fed early using a random‐effects model (RR = 1.23, 95% CI, 0.96 to 1.58, P = 0.10) with little heterogeneity between trials (I2 = 0%, Chi2 = 4.98, P = 0.55) (Analysis 1.8). TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of vomiting events by 30% or more (Figure 16). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction of vomiting (with a control group proportion of 23.1%, an alpha of 3.3%, and a beta of 20%) is 1,250 patients. The TSA‐adjusted CI was 0.80 to 1.91. Vomiting was judged as having low‐quality evidence. This outcome was downgraded by two levels because of risk of bias (blinding did not occur in any study (expect one), random sequence generation and allocation concealment was poorly reported, selective reporting (where insufficient information was provided due to lack of protocol or reporting outcomes not mentioned in methods or having to input missing data)), and imprecision (wide confidence intervals).
1.8. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 8 Vomiting.
16.
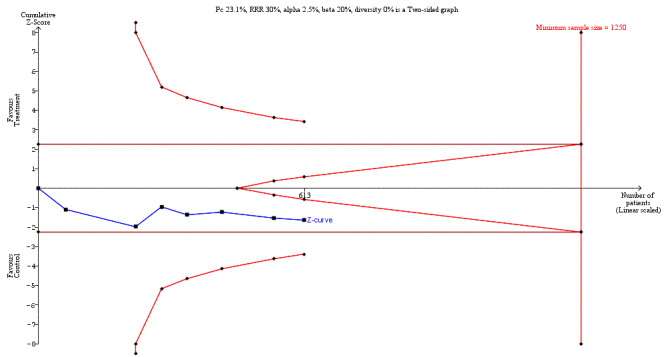
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.8 Vomiting.
Two studies with 118 participants reported incidence of nausea (Beier‐Holgersen 1996; Hartsell 1997).The risk ratio (RR) was 0.93, 95%CI 0.70 to 1.23, with no statistical heterogeneity (I2 = 0%). TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the RR of nausea events by 30% or more (Figure 17). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction of nausea (with a control group proportion of 62.7%, an alpha of 3.3%, and a beta of 20%) is 268 patients. Although the threshold for utility has nearly been crossed, which would indicate that the inclusion of further trials is unlikely to lead to a conclusion of increased or decreased risk of nausea events with early feeding. The TSA‐adjusted CI was 0.55 to 1.55. This outcome was downgraded by two levels because of risk of bias (blinding only occurred in one study, random sequence generation and allocation concealment was poorly reported, selective reporting (where insufficient information was provided due to lack of protocol or reporting outcomes not mentioned in methods or having to input missing data)), and imprecision (wide confidence intervals).
17.
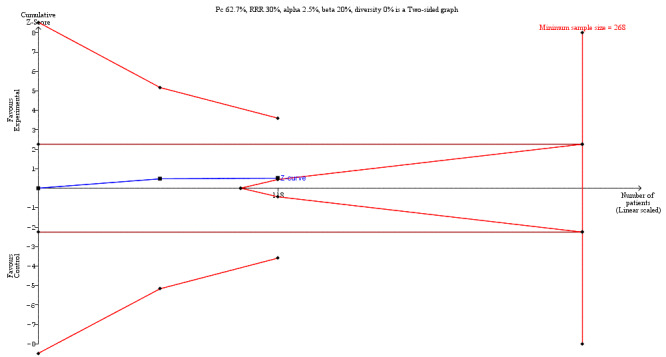
Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.9 Nausea.
Four studies (Carr 1996; da Fonseca 2011; Chatterjee 2012; Minig 2009) reported incidence of nausea and vomiting as a combined variable, which was included post‐hoc as an additional adverse event. There was no evidence of treatment effects for either outcome (nausea RR = 0.95, 95% CI, 0.71 ‐ 1.26, P = 0.70; Analysis 1.9; nausea and vomiting RR = 0.94, 95% CI, 0.51 ‐ 1.74, P = 0.84; Analysis 1.10), with little heterogeneity between trials (nausea I2 = 40%, Chi2=4.97, P = 0.17; nausea and vomiting I2 = 40%, Chi2=4.97, P = 0.17) although estimates of heterogeneity should be interpreted with caution given the small number of studies. TSA showed that the information size was not large enough to rule out that early feeding versus control reduces the risk ratio of nausea and vomiting events by 30% or more (Figure 18). The heterogeneity‐adjusted required information size to demonstrate or reject a 30% relative risk reduction of nausea and vomiting (with a control group proportion of 26.2%, an alpha of 3.3%, and a beta of 20%) is 2,180 patients. The TSA‐adjusted CI was 0.08 to 11.08.This outcome was downgraded by three levels because of risk of bias (blinding did not occur in any study, allocation concealment was poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement)) and imprecision (wide confidence intervals) and indirectness (included various definitions of nausea and vomiting).
1.9. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 9 Nausea.
1.10. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 10 Nausea and vomiting.
18.

Trial sequential analysis of early enteral nutrition versus later commencement after gastrointestinal surgery, outcome: 1.10 Nausea and vomiting.
A number of trials reported other adverse events (Table 6), rare events and not subject for analyses in this review. These included distention, diarrhoea, hyporexia and small bowel obstruction.
2.3 Quality of Life
Just one study (Minig 2009) assessed QoL using two separate tools. The European Organisation for research and treatment of Cancer (EORTC) is often used to measure overall health status. The authors opted for EORTC QLQ‐C30 (Aaronson 1993) which was supplemented by the EORTC QlQ‐OV28 (Griemel 1998) questionnaire (specifically for ovarian cancer). They were both administered before surgery and 30 days after discharge. The scores did not differ between groups at 30 days after discharge: EORTC OV‐28 (global) traditional feeding (28.6 (13.7) versus early feeding 26.5 (14.9), P = 0.68) and EORTC C‐30 (Global) traditional feeding (56.1 (22.2) versus early feeding 64.6 (17.1), P = 0.17). This outcome was downgraded by two levels because of risk of bias (blinding did not occur in this study, allocation concealment was poorly reported, selective reporting (as some outcomes were reported in methods but not in results/vice versa or no protocol was available to make a judgement) and also downgraded for imprecision.
3. Subgroup analysis
The post‐hoc subgroup analysis, using a random‐effects model, of studies in patients having surgery distal to the ligament of Treitz (13 studies) compared with studies that included patients having surgery distal or proximal to the ligament of Treitz (three studies) confirmed the main finding. Compared with usual care, in the intervention arm LoS was reduced by 1.77 (95% CI, ‐2.95 to ‐0.59, P = 0.003) days in patients having surgery distal to the ligament of Treitz and by 2.58 (95% CI, ‐4.40 to ‐0.76, P = 0.005) days in patients having surgery distal or proximal to the ligament of Treitz (Analysis 1.1, Table 7). Heterogeneity remained high in the 'distal only' subgroup (I2 = 84%, Chi2 = 74.33, P < 0.001) but was less in the 'distal or proximal' subgroup (I2 = 31%, Chi2 = 2.90, P = 0.23).
For the subgroup of patients having surgery distal to the ligament of Treitz, using GRADE criteria, we judged the quality of the evidence to be low. This outcome was downgraded by two levels because of risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment was poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, substantial statistical heterogeneity). For the subgroup of patients having surgery distal or proximal to the ligament of Treitz, using GRADE criteria, we also judged the quality of the evidence to be low. This outcome was downgraded by two levels because of the following: risk of bias (blinding did not occur in any study and allocation concealment was poorly reported) and imprecision (confidence intervals are wide).
The post‐hoc subgroup analysis of studies in which patients in the intervention arm were fed orally only (11 studies) compared with studies in which patients were fed through a tube with or without oral feeding (five studies) found conflicting results for the effectiveness of early feeding on LoS. In studies using only oral feeding in their early feeding arm, compared with usual care, LoS was reduced by 2.06 (95% CI, ‐3.26 to ‐0.87, P = 0.0007) days (Analysis 1.2, Table 8). However, in studies which used tube feeding (with or without oral feeding) in their early feeding arm, the evidence supporting a reduction in LoS in the early feeding arm compared with usual care by 1.75 (95% CI, ‐4.32 to 0.82, P = 0.18) days was weak. Heterogeneity remained high in both the 'oral feeding only' (I2 = 85%, Chi2 = 66.53, P < 0.00001) and 'tube feeding +/‐ oral feeding' (I2 = 67%, Chi2 = 12.18, P = 0.02) subgroups. The oral feeding only subgroup was judged to be low in terms of quality of evidence. It was downgraded by two levels because of the following: risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment were poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2). Whereas the tube feeding with/without oral feeding subgroup was judged to be very low quality. It was downgraded by three levels because of the following:risk of bias (blinding did not occur in any study and allocation concealment was poorly reported) and imprecision (confidence intervals are wide) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2).
1.2. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 2 Length of hospital stay.
Given that the clinical populations of only two studies (Nakeeb 2009; Yang 2013) were 100% cancer and the rest were either a mixture of cancer and non‐cancer or did not specify, it was deemed impossible to conduct this subgroup analysis.
Only one study (Chatterjee 2012), reported that their population would include patients having either acute or elective surgery, two studies did not specify and the remaining 14 studies were all elective surgery, so it was again deemed impossible to conduct a subgroup analysis.
Subgroup analyses are presented in Table 2.
4. Sensitivity analysis
We conducted the following sensitivity analyses for the continuous outcomes included in this review and presented these in Table 2.
4.1 Removing studies with at least two domains with high risk of bias
None of the included studies were classified as having a high risk of bias in two or more of the following components: random sequence generation, allocation concealment, incomplete outcome data or selective outcome reporting. Therefore we were unable to carry out this sensitivity analysis.
4.2a Removing studies with imputed results
Results were estimated or imputed for LoS in five studies (Binderow 1994; Lucha 2005; Nakeeb 2009; Reissman 1995; Sagar 1979). After excluding these results from the meta‐analysis of LoS we found an overall reduction in LoS in favour of early feeding of 2.27 days (95% CI, ‐3.62 to ‐0.92, P = 0.001), with substantial heterogeneity between trials (I2 = 80%, Chi2 = 49.43, P < 0.00001). Using GRADE criteria this analysis was downgraded by three levels because of the following: risk of bias (blinding did not occur in any study and allocation concealment was poorly reported) and imprecision (confidence intervals are wide) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2).
4.2b Worst/best case scenario imputation
Results were imputed for the standard deviations of LoS in three studies (Binderow 1994; Lucha 2005; Nakeeb 2009) using the mean of the standard deviations reported by other studies. Under the worst case scenario (maximum of reported standard deviations) we found an overall reduction in LoS in favour of early feeding of 2.06 days (95% CI, ‐3.18 to ‐0.94, P = 0.0003), with substantial heterogeneity between trials (I2 = 81%, Chi2 = 77.59, P < 0.00001). Under the best case scenario (minimum of reported standard deviations) we found an overall reduction in LoS in favour of early feeding of 1.80 days (95% CI, ‐2.61 to ‐1.00, P < 0.0001), with substantial heterogeneity between trials (I2 = 83%, Chi2 = 89.28, P < 0.00001). This analysis was downgraded by two levels because of the following: risk of bias (blinding did not occur in any study; random sequence generation and allocation concealment were poorly reported) and inconsistency (large variation in size of effect, some confidence intervals do not overlap, very high I2) (Table 2).
4.3 Inclusion of continuity correction for zero cell counts
The inclusion of a continuity correction for zero cell counts resulted in narrower confidence intervals around the relative risk estimate for the effect of the intervention on mortality. The combined RR was 0.53 (95% CI 0.25 to 1.13, P = 0.10), with no evidence of heterogeneity between trials (I2 = 0%, Chi2 = 4.8, P = 0.85). The increased precision provides weak evidence that early feeding may reduce the risk of postoperative mortality. Relative risk estimates for the remaining outcomes were changed only minimally after continuity correction. For wound infection the combined RR was 0.84 (0.51 to 1.39, P = 0.50), with some heterogeneity between trials (I2 = 25%, Chi2 = 13.36, P = 0.20). For intraabdominal abscess the combined RR was 0.65 (0.21 to 2.01, P = 0.46), with minimal heterogeneity between trials (I2 = 0%, Chi2 = 1.60, P = 0.81). For anastomotic leak the combined RR was 0.74 (0.40 to 1.36, P = 0.33), with minimal heterogeneity between trials (I2 = 3%, Chi2 = 12.42, P = 0.41). For pneumonia the combined RR was 1.12 (0.51 to 2.47, P = 0.78), with minimal heterogeneity between trials (I2 = 0%, Chi2 = 3.85, P = 0.92).
5. Publication bias
We tested for publication bias by visual inspection of the funnel plot for LoS (Figure 4), which had more than 10 included trials, and wound infection (Figure 8), although this had only nine included trials after excluding those with zero events in one or both arms. Neither of the presented funnel plots as well as Egger's regression test for LoS (z = ‐1.00, P = 0.32) indicate plot asymmetry, thus suggesting that the results are unlikely to be affected by reporting biases (including publication bias). However, these results should be interpreted with caution due to the generally high level of heterogeneity between studies. We did not perform funnel plots for the following outcomes: Intraabdominal abscess, anastomotic leakage, pneumonia, mortality, vomiting, nausea, and nausea and vomiting (reported in combination), due to limited number of included studies (less than 10) for any of the outcomes.
Discussion
Summary of main results
Our review shows that there is evidence for a reduction in length of hospital stay (LoS) with early feeding (reduction of 1.95 days, Analysis 1.1). However, evidence to support this is of low quality, thus the results should be interpreted with caution. We also found weak evidence of a 23% increase in risk of vomiting, but with confidence intervals which include no effect (Analysis 1.8). There was no clear difference between early feeding versus control regarding the postoperative complications wound infection (Analysis 1.3), intraabdominal abscess (Analysis 1.4), anastomotic leakage (Analysis 1.5), or pneumonia (Analysis 1.6). Likewise for mortality and the adverse events nausea (Analysis 1.9), or nausea and vomiting as a combined variable (Analysis 1.10). Findings are summarised in the Table 1. Sensitivity analyses for use of estimated data showed no clinically important changes to the findings. Subgroup analysis indicated that LoS did not differ when excluding studies that had participants undergoing surgery proximal to the ligament of Treitz as well as participants having surgery distal to the ligament of Treitz (Analysis 1.1). One study assessed quality of life (QoL) and no between‐group difference was reported at 30 days post‐discharge.
1.3. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 3 Wound infection.
1.4. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 4 Intraabdominal abscess.
1.5. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 5 Anastomotic leakage.
1.6. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 6 Pneumonia.
Overall completeness and applicability of evidence
We attempted to identify and synthesise all existing research to provide a comprehensive estimate of the effect of early feeding on LoS and postoperative complications. The previous update (Andersen 2011) search for trials comparing early commencement of postoperative enteral nutrition with traditional management (no nutritional supply before bowel function) included 14 studies representing a total of 1224 patients. The current review updated this with the addition of six trials (Chatterjee 2012; da Fonseca 2011; Dag 2011; Minig 2009; Nakeeb 2009; Yang 2013) and removal of three (Heslin 1997; Smedley 2004; Watters 1997), to give 17 studies, involving 1437 participants. Our search strategies however may not have identified all of the existing literature. Additionally, two identified publications could not be located through our library resources, two could only be found in abstract format and so were excluded after attempting to contact authors, and one study was not in a readable format in Chinese (Characteristics of excluded studies). One study (Beier‐Holgersen 1996) reported LoS in a format that could not be used in the review and so was excluded from the meta‐analysis for LoS. However, where possible we estimated and made assumptions about the data, which allowed us to use the majority of identified information (Table 4).
Surgical and anaesthetic practice has changed dramatically since publication of the first randomised controlled trial in 1979 (Sagar 1979). Surgery is less 'stressful' especially if done laparoscopically. Patients recover from anaesthetics faster and combined with improved postoperative analgesia enable patients to mobilise and return to usual activities much faster than previously. Thus patients today may be more able to tolerate early enteral feeding than in previous studies. It is also possible that in an era where multi‐model programmes such as the Enhanced Recovery After Surgery (ERAS) programme are encouraged, the benefits of early enteral feeding may not be so obvious but may still add to the overall improvements in recovery.
Quality of the evidence
Assessments of quality of evidence for each outcome are presented in the Table 1. Overall, using GRADE criteria, there is a low level of evidence that early feeding leads to a shorter period of LoS. There is also a low level of evidence that early feeding reduces the risk of mortality. There is a low level of evidence of a weak increased risk of vomiting in those who are fed early. There are low levels of evidence that early feeding does not reduce the following postoperative outcomes (intraabdominal abscesses, anastomotic dehiscence, pneumonia) or adverse events (nausea) compared to traditional feeding. There is also a very low level of evidence that early feeding does not reduce wound infection compared to traditional feeding.
1. Methodology
Methodological quality and risk of bias were difficult to assess in many studies due to poor reporting. Those with available information were of poor methodological rigour. Randomisation sequence generation was the most reported of all evaluated risk of bias elements, however other elements such as allocation concealment were more poorly reported and there were few reports of attempts to blind patients, outcome assessors and other personnel. Incomplete outcome reporting was mainly low risk but selective reporting was judged to be either high or unclear risk of bias. Overall, the majority of 'Risk of bias' assessments were therefore judged as ‘unclear’ or ‘high’ risk.
2. Outcome assessment
LoS was the clinical outcome of interest in this research area. However, it was likely to be influenced by variation in discharge criteria, which may result in differences between studies. Timing of the mortality assessment was also not clearly defined or consistent between studies. For example, nine studies reported mortality within the hospital unit, whilst three used 30‐day mortality or mortality within two months. This lack of uniformity across centres will have caused rates of reporting to differ between studies. Furthermore, the definition of some of the other outcomes of interest were not clear and often differed between studies, which may introduce variability among some outcomes. For example, for the wound infection outcome, one study (Sagar 1979) reported incorporating pelvic abscesses (as well as subphrenic abscesses and anastomotic leak) within its definition, whilst others (e.g. Chatterjee 2012; Ortiz 1996; Reissman 1995; Stewart 1998) separated pelvic abscess from wound infection. Carr 1996 had an overall outcome of infection which incorporated wound and urinary together so was excluded from the meta‐analysis for wound infection because effects on wound infection alone could not be separated out. One study (Beier‐Holgersen 1996), reported using a standardised criteria to assess wound infection (Buzby II‐IV), whilst the surgical team decided the definition in another (Mulrooney 2004 ‐ author correspondence). The definition reported in Beier‐Holgersen (Beier‐Holgersen 1996) may have been more sensitive than in the other studies as they reported a third of controls had experienced a wound infection, whereas in other studies, minimal infection rates were noted. The outcomes nausea and vomiting relied on participants self‐report. Postoperative participants may feel unwell or disorientated; hence these self‐reported outcomes may be open to misreporting or bias. However, this risk of bias is applicable to all studies reporting nausea and vomiting and cannot be more accurately recorded by any other means. Additionally, blinding of participants and outcome assessors with this intervention is difficult, and an awareness of treatment allocation may result in participants and outcome assessors misreporting the outcomes.
3. Heterogeneity
The 17 randomised trials identified were clinically heterogeneous, which was reflected in the considerable evidence of heterogeneity in one of the primary outcomes, LoS. Combining trials that differ in terms of underlying condition, operation, and intervention may be inappropriate. However, we were interested in the pragmatic comparison of early versus deferred feeding strategies after lower gastrointestinal surgery and not in differences between feed types or specific routes of feeding. Our ability to detect statistical heterogeneity was limited by the small number of trials and by the often inadequate reporting. The post‐hoc subgroup analyses, which compared surgical sites ('distal to the ligament of Treitz only' compared with 'distal and proximal to the ligament of Treitz') and oral feeding route ('oral feeding only' compared with 'nasojejunal tube +/‐ oral feeding') did not explain the observed heterogeneity in LoS.
Potential biases in the review process
The review process may have been biased. Although we believe that our electronic and handsearching strategies identified the majority of relevant trials, it is possible that we may have missed some available literature or unpublished material. We stopped handsearching at the end of December 2017, and in the time period until publication other trials may have been published or made available. These will be incorporated into future updates to this review. We made judgements of risk of bias according to predefined rules, but the information required to make these judgements was infrequently published. Judgements of risk of bias, however, were completed following all data collection, so did not impact on the review process. We may have introduced bias in the review process as we had to make estimations and assumptions using the available data to be able to conduct meta‐analyses. We conducted a sensitivity analysis removing all results that had been estimated, and the direction and extent of effect for LoS remained the same, indicating that the quality of our statistical manipulations did not substantially change our findings. We found no evidence of funnel plot asymmetry, but several outcomes (intraabdominal abscess, vomiting, nausea, and nausea and vomiting) were reported in only a small number of trials, which precluded assessment of funnel plot asymmetry for these outcomes. Many of the outcomes were rare events (mortality, wound infection, intraabdominal abscess, anastomotic leak, pneumonia) and the methods for handling these events may have biased the pooled estimates of the effect of early feeding. We conducted sensitivity analyses in which we used a zero‐cell continuity correction method and this made a small difference to the findings relating to mortality, but no other clinically meaningful differences. None of the reviewed studies accounted for competing risks, the scenario in which an alternative outcome occurs which prevents or delays the primary outcome from occurring, and may have resulted in biased results. In the reviewed studies wound infections may extend a hospital stay and mortality will mean a patient is not discharged, both of which may lead to biased estimates of intervention effects on LoS.
We made a number of assumptions about the comparability of study methodology, including the control and intervention protocols. The exact timing of feeding and type of feed received in the intervention and control groups was not always clear, and likely differed between studies (Table 3 and Characteristics of included studies). In addition, the experience of the surgeon, the length of the operation, postoperative pain control, the use of antibiotics, and the success of the operation, may have affected outcomes. Control group participants likely began receiving energy at different times as for most studies it was introduced after evidence of return to normal bowel function e.g. bowel sounds, flatus; for some, calories will have been introduced on postoperative day one (the same day as the intervention group) as explicitly reported in one study (da Fonseca 2011).
Agreements and disagreements with other studies or reviews
The key message of this systematic review is that there is no obvious benefit for keeping patients 'nil by mouth' (NBM) after lower gastrointestinal surgery, and confirms the conclusion from our previous reviews (Andersen 2011; Lewis 2001; Lewis 2009). The first review in this area (Lewis 2001) was published in 2001 and included 11 studies. We published subsequent updates in 2009 (Lewis 2009) and 2011 (Andersen 2011) which included 13 and 14 studies, respectively. Other systematic reviews that have looked at early postoperative feeding in gastrointestinal tract resection or colorectal surgery include Osland 2011, Zhuang 2013 and Shu 2016. Osland 2011 included 15 trials involving 1240 patients undergoing gastrointestinal tract resection. Zhuang 2013 included seven trials involving 587 patients undergoing elective colorectal surgery, and Shu 2016 included 11 trials involving 1095 patients undergoing digestive tract surgery.
The present review included 17 studies representing a total of 1437 patients all undergoing lower gastrointestinal surgery. All the outcomes we assessed were clinically relevant in the context of lower gastrointestinal surgery. Compared to previous reviews including ours (Andersen 2011; Lewis 2001; Lewis 2009), and those done by others (Osland 2011; Zhuang 2013), this review showed a greater reduction in LoS in the early feeding group (1.96 days compared to 0.89, 0.89, 0.84, 1.28, and 1.58 days, respectively). The reduction in mortality with early postoperative feeding in this review (RR = 0.56, 95% confidence interval (CI) 0.21 to 1.52, P = 0.26) was similar to that reported in our earliest review (Lewis 2001) and also by Osland 2011 (RR = 0.48, 95% CI 0.18 to 1.29, P = 0.15, and OR = 0.71, 95% CI, 0.32 to 1.56, P = 0.39, respectively). Our previous reviews (Andersen 2011; Lewis 2009), however, found stronger statistical evidence that mortality was reduced with early postoperative feeding (RR = 0.42, 95% CI 0.18 to 0.96, P = 0.03, and RR = 0.41, 95% CI 0.18 to 0.93, P = 0.03, respectively).
Similar to our previous reviews (Andersen 2011;Lewis 2001; Lewis 2009), the current review showed no evidence of treatment effects for mortality or for the following clinical complications: intraabdominal abscess, pneumonia, wound infections or anastomotic leakage. Compared to the most recent version of our review (Andersen 2011), we found a similar effect size for an increased risk of vomiting among those fed early, but a wider confidence interval, suggesting weaker evidence of a treatment effect (RR = 1.27, 95% CI 1.01 to 1.61, P = 0.05 versus RR = 1.23, 95% CI 0.96 to 1.58, P=0.10, respectively). Although not entirely comparable with our findings (given that we did not look at infectious and non‐infectious complications as combined groups) Shu 2016 found that early enteral nutrition in patients with digestive tract surgery was more effective than no early feeding in decreasing the incidence of infectious (RR = 0.50, 95% CI 0.38 to 0.67, P < 0.01) and non‐infectious (RR = 0.72, 95% CI 0.43 to 1.22, P < 0.05) complications. In addition, Zhuang 2013 found a reduction in total postoperative complications (RR = 0.70, 95% CI 0.50 to 0.98, P = 0.04).
Authors' conclusions
Implications for practice.
Traditionally, it has been standard in many elective surgical practices to keep patients ‘nil by mouth’ postoperatively for several days. In recent years this practice has been challenged as studies have shown that this may be a period of unnecessary starvation (Andersen 2011). On this evidence, re‐establishment of oral feeding as soon as possible after surgery is now encouraged and has been incorporated within the enhanced recovery after surgery (ERAS) programme. This update supports the notion of early commencement of feeding, and the inclusion of early feeding within ERAS programmes for patients undergoing lower gastrointestinal surgery, as there was no evidence that keeping patients nil by mouth is beneficial, expect perhaps in terms of reducing vomiting. This review strengthens the evidence that early feeding reduces LoS. Overall, the reduction corresponded to almost two days, which is both clinically and economically important. Postoperative feeding after non‐gastrointestinal surgery has also been shown to reduce postoperative inpatient stay (Delmi 1990; Schilder 1997). A reduction in complication rates may help to explain this observation, as might a faster return of gastrointestinal function. Although previous reviews have reported that early feeding leads to a reduction in mortality (Andersen 2011; Lewis 2009), the current review did not support this finding and the earliest version of this review (Lewis 2001) found weaker evidence. Future studies should focus their attention on the reporting of postoperative outcomes. Unfortunately, it was impossible to comment on many factors, which may have influenced the clinical complication endpoints such as the fitness of the patient, experience of the surgeon, whether resections were on the small or large bowel, the operation time, pain control, use of antibiotics and the success of the operation in removing the underlying pathology. Surgical and anaesthetic practice has changed since publication of the first randomised controlled trial (Sagar 1979). Surgery has become less ‘stressful’ especially if undertaken laparoscopically. Patients recover from anaesthetics faster and when combined with improved postoperative analgesia and attention to fluid balance, patients mobilise and recover more rapidly. Thus, patients today may be more able to tolerate early enteral feeding than was seen in previous studies. It is also possible that the potential benefits of early enteral feeding may not be so obvious as it has been incorporated within the ERAS programme which aims to reduce postoperative complications and LoS through a multi‐modal perioperative care pathway. Challenges to the implementation of ERAS also need to be considered. For example, a recent qualitative investigation in healthcare providers reported a number of potential barriers, such as keeping ERAS visible, gaining the buy‐in of relevant stakeholders and spreading the programme (Herbert 2017). Nonetheless, if early postoperative feeding does provide benefit, then the identification and implementation of strategies to improve uptake of this ERAS component should be a priority.
Implications for research.
The earliest review conducted in 2001 (Lewis 2001) suggested that an adequately powered clinical trial was required to assess early enteral feeding on appropriate outcomes. This review suggests that we now have sufficient evidence to indicate that early enteral feeding leads to a reduced postoperative length of hospital stay. However, the findings were inconclusive for all other outcomes, and further clinical trials are therefore justified to further understand the effect of early enteral feeding on postoperative complications and other secondary outcomes. This review further suggests that trials should clearly define their outcomes in terms of timeframe and definition to allow for better comparison between studies. However, the examination of the effect of early nutrition alone in the hospital context today would be difficult, due to the nature of surgery changing with fast track surgery protocols, and the incorporation of the recommendation to feed early post‐surgery within this. The trials reviewed used different feeding routes and amounts of energy, which make it unclear which is the best approach to consider in clinical practice. Of benefit, would be to investigate if different feeding regimens affect outcomes post‐surgery. However, any trial would need to be large and would therefore be expensive.
What's new
| Date | Event | Description |
|---|---|---|
| 4 July 2019 | Amended | Minor changes to plain language summary |
| 10 October 2018 | New citation required and conclusions have changed |
|
| 9 October 2018 | New search has been performed |
|
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 5 January 2011 | New search has been performed | Updated review with one more included study |
| 21 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Vanessa Er for her support in extracting the studies in Chinese, and the Information Specialist in CCCG for running the searches.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Gastrointestinal Tract] explode all trees
#2 MeSH descriptor: [Lower Gastrointestinal Tract] explode all trees
#3 MeSH descriptor: [Intestines] explode all trees
#4 MeSH descriptor: [Gastrointestinal Diseases] explode all trees
#5 MeSH descriptor: [Intestinal Diseases] explode all trees
#6 #1 or #2 or #3 or #4 or #5
#7 (surgery or surgical* or operat* or postoperativ* or perioperativ* or resect*):ti,ab
#8 #6 and #7
#9 MeSH descriptor: [Digestive System Surgical Procedures] explode all trees
#10 MeSH descriptor: [Colectomy] explode all trees
#11 MeSH descriptor: [Colorectal Surgery] explode all trees
#12 MeSH descriptor: [Ileostomy] explode all trees
#13 MeSH descriptor: [Colostomy] explode all trees
#14 ((colorectal* or colon* or rectal* or rectum or abdomin* or gastrointestin* or intestin* or bowel*) near/5 (surgery or surgical* or operat* or postoperativ* or perioperativ* or resect*)):ti,ab
#15 colectom*:ti,ab
#16 colostom* or ileostom*:ti,ab
#17 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Enteral Nutrition] explode all trees
#19 MeSH descriptor: [Feeding Methods] explode all trees
#20 ((tube or tubes or sip or sips or oral or orally or enteral*) near/5 (nutrition* or feed* or nutrient*)):ti,ab
#21 MeSH descriptor: [Nutritional Support] explode all trees
#22 MeSH descriptor: [Diet Therapy] explode all trees
#23 MeSH descriptor: [Nutrition Therapy] explode all trees
#24 MeSH descriptor: [Dietary Supplements] explode all trees
#25 nutrition* next support*
#26 ((postoperativ* or post‐operativ* or perioperativ* or peri‐operativ*) near/1 (nutrition* or feed* or nutrient*)):ti,ab
#27 (early near/2 (nutrition* or feed* or nutrient* or intake))
#28 "enhanced recovery" :ti,ab
#29 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #17 and #29
Appendix 2. MEDLINE
1 Gastrointestinal Tract/
2 exp Lower Gastrointestinal Tract/
3 exp Intestines/
4 Gastrointestinal Diseases/
5 exp intestinal diseases/
6 or/1‐5
7 su.fs.
8 (surgery or surgical$ or operat$ or post‐operativ$ or postoperativ$ or resect$).tw.
9 7 or 8
10 6 and 9
11 digestive system surgical procedures/
12 exp Colectomy/
13 Colorectal Surgery/
14 ileostomy/
15 colostomy/
16 ((colorectal$ or colon$ or rectal$ or rectum or abdomin$) adj5 (surgery or surgical$ or operat$ or postoperativ$ or perioperativ$ or resect$)).tw.
17 ((gastrointestin$ or intestin$ or bowel$) adj5 (surgery or surgical$ or operat$ or perioperativ$ or postoperativ$ or resect$)).tw.
18 colectom$.tw.
19 (colostom$ or ileostom$).tw.
20 or/10‐19
21 Enteral Nutrition/
22 Feeding Methods/
23 ((tube or tubes or sip or sips) adj5 (nutrition$ or feed$ or nutrient$)).tw.
24 ((oral or orally) adj5 (nutrition$ or feed$ or nutrient$)).tw.
25 (enteral$ adj5 (nutrition$ or feed$ or nutrient$)).tw.
26 Nutritional Support/
27 Diet Therapy/
28 Nutrition Therapy/
29 exp Dietary Supplements/
30 nutrition$ support$.tw.
31 ((postoperativ$ or post‐operativ$ or perioperativ$ or peri‐operativ$) adj1 (nutrition$ or feed$ or nutrient$)).tw.
32 (early adj2 (nutrition$ or feed$ or nutrient$ or intake)).tw.
33 enhanced recovery.tw.
34 or/21‐33
35 20 and 34
36 randomized controlled trial.pt.
37 controlled clinical trial.pt.
38 randomized.ab.
39 placebo.ab.
40 clinical trials as topic.sh.
41 randomly.ab.
42 trial.ti.
43 or/36‐42
44 exp animals/ not humans.sh.
45 43 not 44
47 35 and 45
Appendix 3. Embase
1 Gastrointestinal Tract/
2 gastrointestinal tumor/
3 exp Intestines/
4 Gastrointestinal Diseases/
5 exp enteropathy/
6 or/1‐5
7 su.fs.
8 (surgery or surgical$ or operat$ or post‐operativ$ or postoperativ$ or resect$).tw.
9 7 or 8
10 6 and 9
11 abdominal surgery/
12 gastrointestinal surgery/
13 exp intestine surgery/
14 ((colorectal$ or colon$ or rectal$ or rectum or abdomin$ or gastrointestin$ or intestin$ or bowel$) adj5 (surgery or surgical$ or operat$ or postoperativ$ or perioperativ$ or resect$)).tw.
15 colectom$.tw.
16 (colostom$ or ileostom$).tw.
17 or/10‐16
18 enteric feeding/
19 food intake/
20 ((tube or tubes or sip or sips or oral or orally or enteral$) adj5 (nutrition$ or feed$ or nutrient$)).tw.
21 nutritional support/
22 Diet Therapy/
23 diet supplementation/
24 nutrition$ support$.tw.
25 ((postoperativ$ or post‐operativ$ or perioperativ$ or peri‐operativ$) adj1 (nutrition$ or feed$ or nutrient$)).tw.
26 (early adj2 (nutrition$ or feed$ or nutrient$ or intake)).tw.
27 enhanced recovery.tw.
28 or/18‐27
29 17 and 28
30 CROSSOVER PROCEDURE.sh.
31 DOUBLE‐BLIND PROCEDURE.sh.
32 SINGLE‐BLIND PROCEDURE.sh.
33 (crossover* or cross over*).ti,ab.
34 placebo*.ti,ab.
35 (doubl* adj blind*).ti,ab.
36 allocat*.ti,ab.
37 trial.ti.
38 RANDOMIZED CONTROLLED TRIAL.sh.
39 random*.ti,ab.
40 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
41 (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.
42 40 not 41
43 29 and 42
Appendix 4. LILACS (Bireme)
"enhanced recovery" or "enteral nutrition" or (early and (nutrition$ or feed$ or nutrient$ or intake)) [Words]
and
random$ or trial or placebo or double‐blind or single‐blind [Words]
and
COLORECT$ or COLON or RECTAL or ABDOMINAL$ or post‐operativ$ or post operativ$ or surgery [Words]
Appendix 5. Data extraction form
Data collection form
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
1. General Information
| Date form completed | |
| Name/ID of person extracting data | |
| Report title | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study Eligibility
| Study Characteristics | Eligibility criteria | Yes | No | Unclear | Location in text | |
| Type of study | Randomised controlled trial | |||||
| Cluster‐randomised trial | ||||||
| Completed study | ||||||
| Participants | Adults (Studies solely on paediatric patients (i.e. age less than 18 years) will be excluded). Patients undergoing colorectal surgery, both males and female, with malignant and benign disease including inflammatory bowel diseases. |
|||||
| Types of intervention | ‐ Trials comparing feeding patients retrospectively pair‐matched with a contemporary group of patients not receiving feeding are excluded. ‐ Trials comparing different types of enteral nutrition with each other are excluded. ‐ Trials in which patients served as their own controls are excluded, and cross‐over trials are excluded. |
|||||
|
Included: Patients undergoing surgery in the gastrointestinal tract; Early enteral nutrition is defined as all oral intakes (i.e. registered oral intake, supplemented oral feeding) and any kind of tube feeding (gastric, duodenal or jejunal) containing caloric content commenced within 24 hours postoperatively.) No feeding is traditional management, defined as none caloric oral intake or any kind of tube feeding before bowel function. The definition ’no nutrition’ includes non caloric placebo and water. |
Postoperative enteral nutrition: | |||||
| Parenteral nutrition given: | ||||||
| Colorectal surgery: | ||||||
| Colon surgery alone: | ||||||
| Rectal surgery alone: | ||||||
| Upper gastrointestinal surgery: | ||||||
| Nutrition initiated < 24 hours: | ||||||
| Nutrition initiated > 24 hours | ||||||
| Route of feeding: ‐ Oral ‐ Nasojejunal tube ‐ Nasoduodenal tube ‐ Nasogastric tube ‐ Jejunostomy |
||||||
| Feed type: ‐ Normal diet ‐ Polymeric ‐ Polymeric supplements |
||||||
| Nutrition given pr. days ( mL, calories, other) | ||||||
| Additional information on nutrition: | ||||||
| Information on BMI, weight loss or other information | ||||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
3. Participants and setting
| Description | Location in text | |
|
Participants (total number randomised, and for each group) |
||
| Site of surgery e.g. small bowel, large bowel, both | ||
| Pathology e.g. 10% malignant 90% benign | ||
| Setting/Location | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Baseline imbalances | ||
| Were the groups treated identical except for the named interventions? | ||
| Withdrawals and exclusions | ||
| Age (median, interquartile range) | ||
|
Sex Male:Female |
||
| Race/Ethnicity | ||
| Specific surgery types | ||
|
Severity of illness Dukes(A‐D) TNM |
F | |
|
Co‐morbidities (e.g. diabetes mellitus) |
||
|
Subgroups reported (e.g. laparoscopic vs. open surgery) |
||
| Study groups not included in review comparison (e.g. placebo groups, alternate treatment groups) | ||
| Sample size/Power calculations | ||
| Study start date to end date | ||
| Primary outcome e.g. length of hospital stay | ||
| ERAS protocol used? | ||
| Number of NG tube reinserted | ||
| Ethics | ||
| Notes: | ||
4. Intervention groups
Intervention Group
| Description as stated in report/paper | Location in text | |
| Group name | ||
|
Intervention Description (e.g. type of gum, frequency of chewing gum, how long gum chewed for each time) |
||
|
Duration of treatment period (e.g. when started chewing gum, how long for) |
||
|
Providers (e.g. profession) |
||
| Study groups included in the overall intervention group (e.g. different types of gum) | ||
| Notes: | ||
Control Group
| Description as stated in report/paper | Location in text | |
| Group name | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Notes: | ||
5. Results
Number of Wound infections Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | ||||
| Outcome | |||||
|
Method of reporting (e.g. medical records, patients asked to tell clinicians) |
|||||
| Unit of measurement(days/hrs) | |||||
| Results | Intervention | Comparison | |||
| Mean | SD or SE (delete one) | Mean | SD or SE (delete one) | ||
| Median | Range or IQR (delete one) | Median | Range or IQR (delete one) | ||
| P value = | |||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||
Number of Intraabdominal abscesses Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | ||||
| Outcome | |||||
|
Method of reporting (e.g. medical records, patients asked to tell clinicians) |
|||||
| Unit of measurement(days/hrs) | |||||
| Results | Intervention | Comparison | |||
| Mean | SD or SE (delete one) | Mean | SD or SE (delete one) | ||
| Median | Range or IQR (delete one) | Median | Range or IQR (delete one) | ||
| P value = | |||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||
Anastomotic leakage (dehiscence) Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | ||||
| Outcome | |||||
|
Method of reporting (e.g. medical records, patients asked to tell clinicians) |
|||||
|
Unit of measurement (days/hrs) |
|||||
| Results | Intervention | Comparison | |||
| Mean | SD or SE (delete one) | Mean | SD or SE (delete one) | ||
| Median | Range or IQR (delete one) | Median | Range or IQR (delete one) | ||
| P value = | |||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||
Mortality (within 30 days PO) Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | ||||
| Outcome | (or other description) | ||||
|
Method of reporting (e.g. medical records, patients asked to tell clinicians) |
|||||
| Unit of measurement(days/hrs) | |||||
| Results | Intervention | Comparison | |||
| Mean | SD or SE (delete one) | Mean | SD or SE (delete one) | ||
| Median | Range or IQR delete one) | Median | Range or IQR delete one) | ||
| P value | |||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||
Length of hospital stay Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | ||||
| Outcome | |||||
|
Method of reporting (e.g. medical records, patients asked to tell clinicians) |
|||||
| Unit of measurement(days/hrs) | |||||
| Results | Intervention | Comparison | |||
| Mean | SD or SE (delete one) | Mean | SD or SE (delete one) | ||
| Median | Range or IQR delete one) | Median | Range or IQR delete one) | ||
| P value = | |||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||
POST OP COMPLICATIONS (e.g. acute myocardial infarction, postoperative thrombosis or pneumonia) Investigated in study (Y/N):
| Intervention result (unit) | Control result (unit) | Statistical result | |
| Notes: | |||
ADVERSE EVENTS (e.g. nausea, vomiting, abdominal distension, aspiration, tube blockage) Investigated in study (Y/N):
| Intervention result (unit) | Control result (unit) | Statistical result | |
| Notes: | |||
Economic effect Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | |
| Outcome | Economic effect (or other description) |
|
| Method of reporting | ||
| Description | ||
| Notes: | ||
Quality of Life Investigated in study (Y/N):
| Description as stated in report/paper | Location in text | |
| Outcome | (or other description) | |
| Method of reporting | ||
| Description | ||
| Notes: | ||
6. 'Risk of bias' assessment
| Domain | Risk of bias | Support for judgement | Location in text | ||
| Low risk | High risk | Unclear | |||
| Random sequence generation(selection bias) | |||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of patients (performance bias) All outcomes |
|||||
| Blinding of personnel(performance bias) All outcomes | |||||
| Blinding of outcome assessor(detection bias) – 1. wound infections | |||||
| Blinding of outcome assessor(detection bias) – 2.intraabdominal abscesses | |||||
| Blinding of outcome assessor(detection bias) – 3. postoperative complications e.g. acute myocardial infarction, postop thrombosis or pneumonia | |||||
| Blinding of outcome assessor(detection bias) – 4. anastomotic leakage/dehiscence | |||||
| Blinding of outcome assessor(detection bias) – 5. mortality within 30 days postop | |||||
| Blinding of outcome assessor(detection bias) – 6. adverse events such as nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | |||||
|
Blinding of outcome assessor 7. (Length of Hospital Stay) |
|||||
|
Incomplete outcome data (attrition bias, intention to treat and as‐treated analysis) |
|||||
| Selective outcome reporting?(reporting bias) | |||||
| Other bias | |||||
| Notes: | |||||
7. Applicability
| Have important populations been excluded from the study? (disadvantaged populations etc.) | Yes No Unclear | Justification: |
|
Does the study directly address the review question? (issues of partial/indirect applicability) |
Yes No Unclear | Justification |
| Notes: | ||
8. Other information
| Key conclusions of study authors | |
| References to other RCTS for inclusion | |
| Key general information references | |
| Notes: | |
Appendix 6. 'Risk of bias' tool
RANDOM SEQUENCE GENERATION
|
LOW RISK The investigators describe a random component in the sequence generation process such as: • Referring to a random number table • Using a computer random number generator • Coin tossing • Shuffling cards or envelopes • Throwing dice • Drawing of lots/slips |
|
HIGH RISK The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: • Sequence generated by odd or even date of birth • Sequence generated by some rule based on date (or day) of admission • Sequence generated by some rule based on hospital or clinic record number. • Allocation by judgement of the clinician • Allocation by preference of the participant • Allocation based on the results of a laboratory test or a series of tests • Allocation by availability of the intervention |
|
UNCLEAR RISK Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’. |
ALLOCATION CONCEALMENT
| LOW RISK Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: • Central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation) • Sequentially numbered drug containers of identical appearance • Sequentially numbered, opaque, sealed envelopes ‐ all 3 features of the envelopes must be described |
| HIGH RISK Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: • Using an open random allocation schedule (e.g. a list of random numbers) • Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered) • Alternation or rotation • Date of birth • Case record number • Any other explicitly unconcealed procedure |
| UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement ‐ for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
BLINDING OF PARTICIPANTS AND PERSONNEL
|
LOW RISK • Methods of blinding of participants and personnel described sufficiently and deemed adequate e.g. “The patients in group I received a nutrition supplement of Nutridrink (orange flavour), Nutricia, the Netherlands within four hours postoperatively. Patients in group II received placebo (water with orange flavour, no energy, vitamins or trace elements) during the same period. Nutridrink or placebo in identical containers was administered by the nursing staff.” |
|
HIGH RISK Any one of the following: • Methods of blinding of outcome assessors described sufficiently but deemed inadequate • No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding e.g. “This study was limited by small number of cases and the absence of blinding, placebo arm” |
|
UNCLEAR RISK • Insufficient information to permit judgement of ‘Yes’ or ‘No’ |
BLINDING OF OUTCOME ASSESSORS
|
LOW RISK • Methods of blinding of outcome assessors described sufficiently and deemed adequate e.g. “The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified.” |
|
HIGH RISK Any one of the following: • Methods of blinding of outcome assessors described sufficiently but deemed inadequate • No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding e.g. “This study was limited by small number of cases and the absence of blinding, placebo arm” |
|
UNCLEAR RISK • Insufficient information to permit judgement of ‘Yes’ or ‘No’ |
INCOMPLETE OUTCOME DATA
|
LOW RISK Any one of the following: • No missing outcome data • Missing outcome data less than 10% of the total randomised population • Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) • Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups • For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate • For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size |
|
HIGH RISK Any one of the following: • Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups • Difference in missing data between the groups greater than 10% • Overall missing data greater than 10% of the total randomised population • Stated as ‘intention‐to‐treat analysis’ but does not use this • For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate • For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size • ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation • Potentially inappropriate application of simple imputation |
|
UNCLEAR RISK Any one of the following: • Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomised not stated, no reasons for missing data provided) • Dropouts not mentioned |
SELECTIVE OUTCOME REPORTING
|
LOW RISK The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
|
HIGH RISK Any one of the following: • Not all of the study’s pre‐specified primary outcomes have been reported • One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis, or any data excluded from the analysis despite the data being available • The study report fails to include results for a key outcome that would be expected to have been reported for such a study |
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’. • No protocol is available |
Data and analyses
Comparison 1. Early enteral nutrition versus later commencement after gastrointestinal surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 16 | 1346 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐2.99, ‐0.91] |
| 1.1 Surgical site distal to ligament of Treitz | 13 | 1156 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.95, ‐0.59] |
| 1.2 Surgical site distal and proximal to ligament of Treitz | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐4.40, ‐0.76] |
| 2 Length of hospital stay | 16 | 1346 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐2.99, ‐0.91] |
| 2.1 Oral feeding only | 11 | 1075 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.26, ‐0.87] |
| 2.2 Tube +/‐ oral feeding | 5 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐4.32, 0.82] |
| 3 Wound infection | 12 | 1181 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.39] |
| 4 Intraabdominal abscess | 6 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.21, 2.01] |
| 5 Anastomotic leakage | 13 | 1232 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.40, 1.36] |
| 6 Pneumonia | 10 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.51, 2.47] |
| 7 Mortality | 12 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.13] |
| 8 Vomiting | 7 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.96, 1.58] |
| 9 Nausea | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.23] |
| 10 Nausea and vomiting | 4 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.74] |
1.7. Analysis.
Comparison 1 Early enteral nutrition versus later commencement after gastrointestinal surgery, Outcome 7 Mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beier‐Holgersen 1996.
| Methods | Randomised double‐blind controlled trial No information provided about duration of study |
|
| Participants | 60 participants (30 in each group); mainly colorectal surgery (87%) Bowel resection Route of feeding: nasoduodenal Median age: 66.5 years (range 27‐93) (intervention group), 61.5 years (range 27‐80) (control group) Male:Female 18:12 (intervention group), 20:10 (control group) |
|
| Interventions | Intervention group: received a nutrition supplement of Nutridrink (orange flavour) within four hours postoperatively. Nutridrink contained 150 kcal/100 mL and 5 g protein/100 mL. Nutritional supplement given until fourth day. Control group: received placebo (water with orange flavour, no energy, vitamins or trace elements) during same period. |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, postoperative complications (pneumonia, dehiscence, pulmonary failure), LoS, adverse events (nausea, vomiting). | |
| Notes | Country of study: Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | Low risk | Reported as double‐blinded. Use of an identical placebo flavoured orange. Groups received different volume of drink (4000 mL vs. 4700 mL). Unlikely to unblind. |
| Blinding of personnel (performance bias) All outcomes | Low risk | Reported as double‐blinded. Nutridrink or placebo in identical containers was administered by the nursing staff. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications (pneumonia, dehiscence, pulmonary failure, myocardial infarction) were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | Low risk | (Nausea, vomiting) The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | Low risk | The principal investigator saw the patients every day during their stay in the ward, and all complications were recorded by the same investigator. The randomisation code was not broken until all results were available and all patients had been followed up for 30 days after surgery and all complications classified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. |
| Selective reporting (reporting bias) | High risk | No protocol available. The outcome LoS was reported in results but not explicitly stated as an outcome of interest in methods (just PO course). |
Binderow 1994.
| Methods | Randomised controlled trial Between July 1st 1992 and October 31st 1992 |
|
| Participants | 64 participants (32 in each group). Elective laparotomy with either a colonic or ileal resection. Route of feeding: oral. Mean age: 52 years (range 15‐81) (intervention group), 52 years (range 15‐87) (control group) Male:Female 14:18 (intervention group), 18:14 (control group) |
|
| Interventions | Intervention group: received "regular solid food diet" on first postoperative morning. Control group: received a maximum of 8 oz of ice chips per day until return of bowel activity. Patients then begun on diet of clear liquids and if tolerated for 24 hours advanced to a regular diet. |
|
| Outcomes | LoS, vomiting. For LoS outcome standard deviations not reported | |
| Notes | Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Ileus) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ mortality | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | No protocol available. All outcomes listed in methods were reported in the results. |
Carr 1996.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 30 participants. 2 participants did not proceed to resection, and their data were not included. 28 participants (14 in each group). Elective gastrointestinal resection Route of feeding: nasojejunal tube Mean (SD) age: 60.1 years (7.5) (intervention group), 51.1 years (8.2) (control group) Male:Female 9:5 (intervention group), 8:6 (control group) |
|
| Interventions | Intervention group: feeding started on return from operating theatre by using standard isocaloric feed (Fresubin, Fresenius, Cheshire). Energy and water requirements were calculated from the weight of the patient, and a mixture of Fresubin and water provided the full basic fluid requirements. Initially feeding was at 25 mL an hour and was increased by 25 mL four‐hourly until the target volume was reached, at which point intravenous fluids were stopped. Control group: received intravenous fluids with NBM until passage of flatus. Oral fluids started on passage of flatus and increased to normal diet over 48 hours. Intravenous fluids and enteral feeding were stopped with introduction of diet. |
|
| Outcomes | Mortality, LoS, nausea and vomiting | |
| Notes | Country of study: UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly allocated by closed envelope. Not reported if sequentially numbered or opaque. |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Bleeding duodenal ulcer, infection) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Nausea and vomiting, distension, diarrhoea) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | No protocol available. Some outcomes were reported in methods but not in results (sepsis) and there was a very general statement of clinical outcomes in the methods section (but more detail of complications, adverse events, LoS given in results) |
Chatterjee 2012.
| Methods | Randomised controlled trial Study conducted April 2008 to March 2010 |
|
| Participants | 120 participants (60 in each group). Gastric, small bowel, large bowel and uncomplicated simple biliary‐enteric anastomosis. Route of feeding: oral Mean age (SD): 38.18 (11.9) (intervention group), 36.23 (12.88) (control group) Sex ratio (M:F): 42:18 (intervention group), 46:14 (control group) |
|
| Interventions | Intervention group: oral liquids (25 mL/hour) started within 24 hours of operation with clamping the NGT and the feed was increased by 25 mL/hour at 12‐hour intervals. When patient tolerated liquid diet, NGT removed and semi‐solid diet and then normal oral diet were started to reach nutritional goal (25 kcal/kg/day). Control group: kept NBM and were then fed 48‐72 hours after or more following enteric anastomosis depending upon return of full peristaltic sounds. NGT removed when output was less than 20 mL to 30 mL/day and there was no paralytic ileus. Gradually shifted to semi‐solid and then to solid normal diet. |
|
| Outcomes | Wound infection, anastomotic leakage, mortality, LoS, nausea and vomiting | |
| Notes | Country of study: India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Respiratory tract infection, urinary tract infection, incidence of re‐exploration) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting and nausea) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
da Fonseca 2011.
| Methods | Randomised controlled trial Study conducted May 2006 to February 2009 |
|
| Participants | 50 participants: intervention (n = 24), control (n = 26) Elective colonic resections. Route of feeding: oral Mean age (SD): 57.4 years (16.3) (intervention group), 51.7 years (13.3) (control group) Sex: (M:F) 8:16 (intervention group), 10:16 (control group) |
|
| Interventions | Intervention group: On postoperative day (POD) 1 patients received an oral liquid diet (approximately 500 cm³) and were advanced to a regular diet within the next 24 hours. Control group: patients NBM until elimination of the first flatus and then received an oral liquid diet, followed by a regular diet within the next 24 hours. |
|
| Outcomes | Wound infection, anastomotic leakage, mortality, LoS, pneumonia, nausea and vomiting | |
| Notes | Country of study: Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to groups by a computer program. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants (performance bias) All outcomes | High risk | Not blinded. All participants were informed of the group to which they were assigned on first POD, when the oral diet was offered to certain participants. |
| Blinding of personnel (performance bias) All outcomes | High risk | Reported the difficulty of having a physician blinded to the groups. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author (LMF). |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA ‐ not assessed. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Aspiration pneumonia, angina pectoris, prolonged ileus, catheter sepsis, deep vein thrombosis, pancreatitis). Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author (LMF). |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author (LMF). |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author (LMF). Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Nausea and vomiting, hyporexia, readmission). Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Reported the difficulty of having a physician blinded to the groups. To minimise possible bias, outcome data for both groups were recorded prospectively by just one author (LMF). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
Dag 2011.
| Methods | Randomised controlled trial Study conducted August 2007 to September 2009 |
|
| Participants | 199 participants: intervention (n = 99), control (n = 100) Elective open colorectal surgery Route of feeding: oral Mean age (range): 62 years (35‐85) (intervention group), 61 years (17‐89) (control group) Sex (M:F) 52:47 (intervention group), 61:39 (control group) |
|
| Interventions | Intervention group: oral feeding commenced approximately 12 hours after operation with a fluid diet; this was gradually increased to a solid diet as tolerated by the patient. Control group: fasted until passage of flatus or stool. |
|
| Outcomes | Wound infection, anastomotic leakage, pneumonia, mortality, LoS. | |
| Notes | Country of study: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed according to a computer generated list immediately after surgery by an independent computer consultant. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Pneumonia, toxic hepatitis, sepsis, evisceration, cerebral infarct) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | Unclear risk | NA‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts after randomisation not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Hartsell 1997.
| Methods | Randomised controlled trial Study conducted between May 1st 1995 and February 1st 1996 |
|
| Participants | 58 participants (29 in each group). Elective colorectal surgery Route of feeding: oral Mean age: 66 years (range 40‐83) (intervention group), 68 years (40‐83) (control group) |
|
| Interventions | Intervention group: began a full liquid diet on postoperative day 1. If the patient consumed 1000 mL or more in a 24‐hour period, they were advanced to regular diet the next day. Control group: began a liquid diet after evidence of return to normal bowel function. These patients were advanced to a regular diet when they consumed 1000mL or more in a 24‐hour period. |
|
| Outcomes | Wound infection, anastomotic leakage, mortality, pneumonia, LoS, nausea, vomiting. | |
| Notes | Country of study: USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Pneumonia) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Nausea, vomiting) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data less than 10%. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Lucha 2005.
| Methods | Randomised controlled trial Study conducted July 1999 to December 2001 |
|
| Participants | 51 participants Elective open gastrointestinal surgery, Route of feeding: oral Age: both groups were similar with an average age of 51 (range, 22 to 74 years old). Sex: 65% males |
|
| Interventions | Intervention group: bowel rest for 8 hours after completion of surgery followed by a regular diet. Control group: bowel rest until passage of flatus, followed by 24 hours of clear liquids and advancement to a regular diet. |
|
| Outcomes | Anastomotic leakage, pneumonia, LoS. For LoS outcome standard deviations not reported. | |
| Notes | Country of study: USA The RCT also focused on tolerance of diet |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Pneumonia) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | Unclear risk | NA – not assessed |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported |
| Selective reporting (reporting bias) | High risk | No protocol available. One outcome was reported in the results but not stated in methods (complications) |
Minig 2009.
| Methods | Randomised controlled trial Study conducted January 1st 2007 to March 15th 2008 |
|
| Participants | 51 participants: intervention (n = 27), control (n = 24) Gynaecologic oncology patients undergoing laparotomy with associated intestinal resection Route of feeding: oral Median age (range): 54 (42, 62) (intervention group), 58 (47, 66) (control group) Female |
|
| Interventions | Intervention group: offered liquids: mineral water (no gas), tea, chamomile infusion or apple juice, during the first 24 PO hours (day 0). If well tolerated, as evidenced by absence of nausea and vomiting, they were then fed a regular diet of boiled or grilled beef, chicken or fish starting on day 1 and for the entire duration of hospital stay. Control group: NBM awaiting resolution of PO ileus. After resumption of normal bowel function, as expressed by presence of bowel sound and passage of flatus, and if patients did not have nausea and emesis, they were switched to oral liquid diet for 24 hours. If tolerated, they were placed on semi‐solid diet of rice or pasta, vegetable soup, boiled potatoes or bread for 1 day. Regular diet was finally prescribed provided the semisolid diet had been well tolerated. |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, LoS, nausea and vomiting. | |
| Notes | Country of study: Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using the web‐based TENALEA randomisation system (https://it.tenalea.net/ieo). |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Infectious complications, intestinal complications, ileus, bleeding, pleural effusion, thromboembolic complications, pneumothorax) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Nausea and vomiting, diarrhoea, readmission) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Mulrooney 2004.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 73 participants, intervention (n = 37), control (n = 36) Colonic resection Route of feeding: nasojejunal |
|
| Interventions | Intervention group: uniform feeding regimen, until oral intake met at least half of the nutritional requirements. Control group: NBM and intravenous fluids |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, pneumonia, LoS. | |
| Notes | Country of study: UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | Unclear risk | Participants were not aware of group allocation until postoperatively (author correspondence), but insufficient detail was provided on the blinding procedure and the timing postoperatively that blinding was broken |
| Blinding of personnel (performance bias) All outcomes | High risk | Surgeon not aware of group allocation until surgery (author correspondence); surgeon therefore not blinded at time of surgery. Other personnel unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. (Correspondance regarding this outcome from author.) |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. (Correspondance regarding this outcome from author.) |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Pnuemonia, urinary tract infection) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. (Correspondance regarding this outcome from author.) |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. (Correspondence regarding these outcomes from author.) |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | Vomitting information collected via a log, however group numbers are not reported (author correspondence). Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 61% of the 64 patients assessed for nausea or vomiting reported no symptoms. However 73 participants were randomised. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Nakeeb 2009.
| Methods | Randomised controlled trial Study conducted June 2005 to April 2008 |
|
| Participants | 120 participants (60 in each group) Elective open colorectal surgery Route of feeding: oral Mean age (SD, range): 52.3 (12.5, 21‐70) (intervention group), 56.3 (11.6, 25‐69) (control group) Sex ratio (M:F): 39:21 (intervention group), 42:18 (control group) |
|
| Interventions | Intervention group: Began fluids on the first POD and advanced to a regular diet within the next 24 to 48 hours, as tolerated (indicated by an absence of vomiting or abdominal distension). Control group: Managed in traditional way: NMB until resolution of ileus, then a fluid diet, followed by a regular diet. |
|
| Outcomes | Wound infection, anastomotic leakage, mortality, LoS, vomiting. | |
| Notes | Country of study: Egypt | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Did not report being sequentially numbered or opaque |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Burst abdomen, abnormal serum electrolyte, pulmonary infection) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting, readmission) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | High risk | No protocol available. Some outcomes were reported in the results but not stated in methods (patient satisfaction, readmission rates) Standard deviations appear to be from paired t tests – should be unpaired (identified by statistician, CP) |
Ortiz 1996.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 190 participants: intervention (95 in each group). Elective colorectal surgery. Diagnosed with either neoplasm (165), inflammatory bowel disease (21) or diverticular disease (4) Route of feeding: oral Mean age: 65.54 years (range 22‐90) (intervention group), 65.07 years (range 39‐88) (control group) Male: Female 57:38 (intervention group), 55:40 (control group) |
|
| Interventions | Intervention group: on postoperative evening were allowed ad libitum clear liquids, which continued to first postoperative day and progressed to a regular diet as desired. Control group: after resolution of ileus patients were started on a clear liquid diet if this was tolerated for 24 hours they were advanced to a regular diet (traditional way). |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, pneumonia, LoS. | |
| Notes | Country of Study: Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Haemorrhage, pneumonia, venous thrombosis, urinary infection, intestinal obstruction, ileostomy necrosis) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | Unclear risk | NA ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. (Correspondance regarding this outcome from author.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. LoS was not listed as an outcome of interest nor was it reported. We accessed this information through author correspondence. |
Reissman 1995.
| Methods | Randomised controlled trial Study conducted November 1992 to April 1994 |
|
| Participants | 161 participants: intervention (n = 80), control (n = 81). Elective laparotomy with bowel resection of colon or small bowel. Route of feeding: oral Mean age: 51 years (range 16‐82) (intervention group), 56 years (range 20‐90) (control group) Male: Female 34:46 (intervention group), 43:38 (control group) |
|
| Interventions | Intervention group: began a clear liquid diet on first postoperative day and advanced to a regular diet within next 24‐48 hours. Control group: were nil by mouth until resolution of ileus. Then a clear liquid diet followed by a regular diet (traditional way). |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, mortality, pneumonia, LoS, vomiting. | |
| Notes | Country of Study : USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Pneumonia, intestinal obstruction, urinary tract infection, pelvic abscess) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants are included in the results (author correspondence). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Sagar 1979.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 30 participants (15 in each group) Gastrointestinal surgery (oesophago‐gastrectomy, gastrectomy, colectomy, anterior resection, abdominoperineal resection) Route of feeding: nasojejunal tube Mean age (years) ± SE: 54.8 ± 2.9 (intervention group), 54.5 ± 2.8 (control group) Male:Female 8:7 (intervention group), 9:6 (control group) |
|
| Interventions | Intervention group: Flexical was infused through the feeding channel, beginning on the first POD. For the first 24 hours a half strength solution was infused at 25 mL/hour. Thereafter, undiluted Flexical was infused at 25 mL/hour on the second POD, 50 mL/hour on the third POD, and 100 mL/hour on the fourth and fifth days. If there were no complications the double lumen tube was removed on the sixth day and the patient given as much Flexical as they could take by mouth on the sixth and seventh days. In addition all patients given 2 L dextrose (5% w/v) and 1 L saline (0.9% w/v) intravenously from the first to third POD. Control group: patient given 1 L saline (0.9%) and 2 L dextrose (5%) intravenously and were NBM for two days. On third POD they were given a 30 mL drink of water if there were no contraindications. Thereafter, oral intake was gradually increased until by the fifth day the patients could take as much fluid as desired. IV fluids stopped on the fifth POD, and light diet introduced on sixth and seventh days. |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leakage, LoS. | |
| Notes | Country of study: UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospective randomised study from statistical tables |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | High risk | No protocol available. All outcomes listed in methods were reported in the results. Missing standard deviations for LoS (imputed by statistician, CP). |
Schroeder 1991.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 32 participants; 16 in each group. Small or large bowel resection with reanastomosis. Route of feeding: Nasojejunal tube Mean age: 51±18 years (intervention group), 53 ± 22 years (control group) Male:Female 8:8 (intervention group), 9:7 (control group) |
|
| Interventions | Intervention: full strength Osmolite solution for 56 hours. On morning of 3rd PO day tubes removed and reintroduction of diet. Control: hypo‐caloric fluids and gradual reintroduction of diet. |
|
| Outcomes | Pneumonia, LoS. | |
| Notes | Country of study: New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Myocardial infarction, pneumonia, atelectasis) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ mortality | Unclear risk | N/A ‐not assessed |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Diarrhea, small bowel obstruction) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | High risk | No protocol available. Some outcomes were reported in the results, without being stated in methods (LoS and time to flatus) |
Stewart 1998.
| Methods | Randomised controlled trial No information provided about duration of study |
|
| Participants | 80 participants (40 in each group) Elective intraperitoneal colorectal resections without stoma formation (Ileocolic resection (10); hemicolectomy (right or left) (35); subtotal colectomy (11); anterior resection (24)) Route of feeding: oral Mean age (range): 58 (25‐89) (intervention group), 59 (17‐88) (control group) Sex ratio (M:F): 19/21 (intervention group), 18/22 (control group) |
|
| Interventions | Intervention group: allowed free fluids from 4 hours after operation and progressed to a solid diet from the first POD at their own discretion. Control group: remained fasting until passage of flatus or bowel motion, and were then commenced on clear fluids and progressed to a solid diet over 24 to 48 hours at the surgeon’s discretion. |
|
| Outcomes | Wound infection, anastomotic leakage, mortality, pneumonia, LoS, vomiting. | |
| Notes | Country of study: Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned according to a computer number generator to one of two groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | High risk | Assessors were not blinded. |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | NA ‐ not assessed. |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | High risk | (Respiratory complications, cardiovascular complications, urinary tract infection, pneumonia) Assessors were not blinded. |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | High risk | Assessors were not blinded. |
| Blinding of outcome assessor (detection bias) ‐ mortality | Low risk | Asessors were not blinded. Outcome assessor unlikely to affect this outcome. |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting). Assessors were not blinded. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Asessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% missing data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
Yang 2013.
| Methods | Randomised controlled trial Study conducted May to December 2008 |
|
| Participants | 70 participants (35 in each group) Colorectal cancer Route of feeding: oral (nutritional drinks) Mean age (SD): 57.2 (11.7) (intervention group), 59.5 (12.1) (control group) Sex ratio (M:F): 20:12 (intervention group), 23:7 (control group) |
|
| Interventions | Intervention group: oral intake of 30 mL to 50 mL Ensure (US Abbott; 4.184 kJ energy) 6 to 12 hours post‐surgery at 1‐ to 2‐hour intervals. Amount adjusted based on participant’s tolerance. Participants also provided with IV fluid. Most participants stopped having IV fluid 3‐4 days PO. Control group: food/drink intake commenced after first flatus. Started with liquid diet and gradually changed to normal diet. |
|
| Outcomes | LoS, vomiting. | |
| Notes | Country of study: China Article directly extracted from Chinese |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Calculator generated random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants (performance bias) All outcomes | High risk | No reports of attempts to blind participants. Participants are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of personnel (performance bias) All outcomes | High risk | No reports of attempts to blind personnel. Personnel are unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ wound infections | Unclear risk | N/A ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ intraabdominal abscesses | Unclear risk | N/A ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ postoperative complications e.g. acute myocardial infarction, thrombosis or pneumonia | Unclear risk | N/A ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ anastomotic leakage/dehiscence | Unclear risk | N/A ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ mortality | Unclear risk | N/A ‐ not assessed |
| Blinding of outcome assessor (detection bias) ‐ adverse events (e.g. nausea, vomiting, abdominal distention, aspiration, tube blockage and any other adverse events | High risk | (Vomiting, stomach distension) Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Blinding of outcome assessor (detection bias) ‐ length of hospital stay | High risk | Blinding of outcome assessor not discussed. Outcome assessor unlikely to be adequately blinded with an intervention of this nature. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes listed in methods were reported in the results. |
IV: intravenous LoS: length of hospital stay NBM: nil by mouth NGT: nasogastric tube POD: postoperative day RCT: randomised controlled trial SD: standard deviation SE: standard error w/v: weight/volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abela 2017 | Narrative review |
| Barletta 2011 | Not an RCT or feeding |
| Barlow 2011 | Not lower GI surgery; upper GI surgery |
| Beati 1998 | Abstract only |
| Beattie 2000 | Feeding did not start until a mean of 6.5 days after GI surgery, and the trial compared normal diet versus normal diet plus supplement |
| Behrns 2000 | Both groups received calories |
| Bernardes 2008 | Upper GI surgery |
| Beyer 1991 | Total parenteral nutrition |
| Bickel 1992 | Nutrition began greater than 24 hours postoperatively |
| Bisgaard 2002 | Review |
| Boelens 2014 | Compared enteral with parenteral nutrition |
| Buchmann 1996 | An abstract. Results presented in the 1998 reference |
| Buchmann 1998 | Not randomised on early enteral feeding. The comparisons focus on post enteral nutrition for two surgical techniques, whether there is any difference between open and minimal invasive procedures with respect to the start of oral intake. |
| Bufo 1994 | Not a randomised trial |
| Choudhry 1996 | Variety of indications for placement of a PEG tube |
| Dardai 1985 | Total parenteral nutrition |
| de Aguilar‐Nascimento 2002 | Both comparison groups start feeding more than 24 hours after surgery. |
| Delaney 2003 | This is a well performed RCT, but reported multiple variables not just diet. |
| Feo 2004 | Both treatment groups were allowed liquid diet, therefore no control group to early enteral feeding |
| Gianotti 2011 | Not RCT (early feeding group compared with historical controls) |
| Gustafsson 2011 | Review |
| Gómez Sánchez 2010 | Compares two types of fluids (immunonutrition and ‘conventional’) |
| Gómez Sánchez 2011 | Immunonutrition and does not specifically state that it is looking at early postoperative feeding versus NBM |
| Han‐Geurts 2001 | Patients undergoing colonic and vascular surgery were randomly allocated to choose when they wanted to start an oral diet (patient‐controlled group) or a fixed‐feeding regimen where diet was introduced on postoperative day four |
| Han‐Geurts 2007 | It is not clear if some or all patients are common to the 2001 publication, but reason for exclusion the same: atients undergoing colonic and vascular surgery were randomly allocated to choose when they wanted to start an oral diet (patient‐controlled group) or a fixed‐feeding regimen where diet was introduced on postoperative day four |
| Hedberg 1999 | Not randomised |
| Henriksen 2002 | All patients received the same nutrition (the comparison was in regards to other factors) |
| Heslin 1997 | Includes only upper GI |
| Holst 2015 | Review |
| Hoover 1980 | Patients receiving enteral nutrition compared with a control group, receiving intravenous nutrition. Main outcome is cumulative 10‐day nitrogen balance. |
| Hyltander 2005 | Upper GI and total parental nutrition |
| Kaur 2005 | Non‐traumatic GI perforation. 44% had DU’s, 39% ileal (TB or typhoid). Closure was direct, no resections and GI anastamosis formed. Feeding was started 24 hours after surgery. |
| Keele 1997 | Not dealing with early enteral feeding, rather whether oral diet supplements have an effect or not. |
| Kemen 1995 | Both comparison groups receives early postoperative enteral nutrition. One with arginine‐omega‐3 fatty acids and ribonucleic acid‐supplemented diet versus placebo. |
| Klappenbach 2013 | Not lower GI surgery: Appendicectomy where there is no anastomosis. |
| Klek 2011 | Gastrectomy and pancreas. All groups received some form of nutrition – no control group. |
| Kompan 1999 | Study on trauma patients, admitted in shock and stabilised in 6 hours. |
| Kong 2017 | Feeding started after hospital discharge |
| Lau 2014 | Two types of calorie‐containing diets compared |
| Le Guen 2014 | Review |
| Lee 2010 | Early feeding and early mobilisation versus traditional care |
| Lee 2014 | Not lower GI surgery |
| Li 2015 (a) | Not lower GI surgery |
| Li 2014 | Article cannot be sourced |
| Li 2015 | Not lower GI surgery |
| Lidder 2013 | All groups were allowed calories postoperatively, so there can not be a comparison of calories versus no calories |
| Liu 2013 | Not lower GI surgery |
| Liu 2014 | Review |
| Mahla 2016 | Surgery does not involve a GI resection with re‐anastamosis |
| Mahmoodzadeh 2015 | Not lower GI surgery |
| Malhotra 2004 | GI perforation also feeding > 48 hours |
| Mari 2011 | Multi‐modal intervention: unable to separate effects of feeding alone |
| Maňásek 2016 | Not an RCT |
| McCarter 1998 | No control group was used, as the comparisons groups begin tube feedings at 4 hours (group A) or at 24 hours (group B) after intervention |
| Mirea 2015 | Compared enteral with parenteral nutrition |
| Moore 1991 | Review on multisystem trauma, a decade perspective |
| Moss 1981 | Not able to determine if the study was randomised |
| Murchan 1995 | Can only obtain abstract but does not state that comparison is early versus traditional nutrition |
| Nakajima 2009 | Not relevant |
| Neri 2001 | Total parental nutrition |
| Nessim 1999 | This is a study examining the benefits of bowel confinement where the interventional group received clear liquids with high dose loperamide and codeine with the control group of patients started a regular dietary intake on the day of surgery |
| Nguyen 2009 | Not relevant |
| Niu 2015 | Compared enteral with parenteral nutrition |
| Osland 2011 | Review |
| Palmer 1998 | Both groups receive calorie‐containing diets. Compares supplement with no supplement. |
| Pappa 2011 | Does not look at early feeding |
| Peng 2014 | Not lower GI surgery |
| Pragatheeswarane 2014 | The provision of fluids was not within 24 hours postsurgery: it was given "at the 24th hour". The 'clear liquid diet' was water, coconut water, clear vegetable soup etc (author correspondence). For those who were initially given water, their fluids would not have contained any calories. |
| Rosales 2009 | Gastrectomy |
| Ryan 1981 | All three groups undergoing elective partial colectomy received early feeding, randomised to receive either jejunal feeding of elemental diet (ED) or isotonic intravenous infusions of dextrose (IV). |
| Schwenk 1998 | A comparison of laparoscopic or conventional resection of colorectal tumours. Major endpoints were the postoperative time to the first bowel movement and the time until oral feeding without parenteral alimentation was tolerated. |
| Seenu 1995 | No method stated. Individual protocol for commencement of postoperative feeding. |
| Seri 1984 | Both groups received early postoperative feeding |
| Sharma 2013 | Two types of calorie‐containing diets compared |
| Shen 2013 | Review |
| Shoar 2013 | Not lower GI surgery |
| Shrikhande 2009 | Review |
| Shu 2013 | Review |
| Shu 2016 | Systematic review |
| Singh 1998 | Patients in the early feeding group did not start receiving 'feed' until after 24 hours (before 24 hours they received saline/dextrose). Our study criteria is that patients receive enteral feed within 24 hours of surgery. |
| Smedley 2004 | No control group that was NBM/received no calories |
| Smeets 2017 | Systematic review |
| Smith 2002 | Pseudo randomised on an intention to treat. Decision on treatment group was determined individually |
| Soliani 2001 | Comparison of three different methods of feeding administration |
| Stein 2002 | Not GI surgery; PEG feeding |
| Takala 1985 | Not a randomised study |
| Tong 2006 | Article could not be located |
| Ugarte 2000 | Not RCT |
| Vaithiswaran 2008 | Feeding started via NJ tube 12 to 24 hours saline & 5% dextrose then 24 to 48 hours 1L and half strength feed at 50 mL/hour. Thus patients were not fed within 24 hours of surgery |
| Velez 1997 | Not a randomised study |
| Vlug 2009 | Not analysed as an RCT; ERAS study |
| Wallström 2014 | Review |
| Wang 2013 | Nutrition began greater than 24 hours postoperatively |
| Wang 2014 | Nutrition began greater than 24 hours postoperatively |
| Watters 1997 | Includes only upper GI |
| Weber 1999 | Not relevant |
| Weinstein 1993 | Caesarean section |
| Wiedeck 1984 | Comparison between enteral and parenteral nutrition. |
| Wiren 2002 | This study evaluates the feasibility of alpha‐ketoglutarate enrichment of enteral feeding and the effect on protein metabolism (nitrogen balance). |
| Wong 1995 | Trial of growth hormone |
| Wu 1996 | Chinese ‐ article not in readable format |
| Wu 2000 | Chinese ‐ not clear if feeding started within 24 hours post surgery |
| Wu 2004 | Gastric cancer – not relevant |
| Wu 2007 | Chinese ‐ not clear if feeding started within 24 hours post surgery. |
| Yu 2013 | Not lower GI surgery |
| Yuanlong 1999 | Compared enteral with parenteral nutrition |
| Zaidi 2016 | Mixed upper and lower GI, no definition of early oral feeding |
| Zhang 2014 | Nutrition began greater than 24 hours postoperatively |
| Zhang 2017 | Systematic review |
| Zhao 2014 | Not lower GI surgery |
| Zhou 2006 | Compares early versus late removal of naso‐gastric tubes after colorectal surgery |
| Zhuang 2013 | Systematic review |
| Zitta 2012 | Unclear what "conventional postoperative treatment" is, and involved mechanical bowel preparation |
DU: duodenal Ulcer ED: elemental diet ERAS:enhanced recovery after surgery GI: gastrointestinal GI: gastrointestinal IV: intravenous NBM: 'nil by mouth' NJ: nasojejunal PEG:percutaneous endoscopic gastrostomy RCT: randomised controlled trial TB: tuberculosis
Characteristics of studies awaiting assessment [ordered by study ID]
Fanaie 2005.
| Methods | Randomised controlled trial Duration of study: August 2003 and November 2004 |
| Participants | 110 participants (55 in each group) Route of feeding: oral Sex ratio (M:F): 31/24 (intervention group), 38/17 (control group) |
| Interventions | Intervention group: early feeding beginning with liquid diet, 8 hours postoperatively. Control group: given a regular diet with normal bowel sounds. |
| Outcomes | Postoperative complications: wound infection, leakage of anastomosis, obstruction, mesenteric emboli, upper gastrointestinal bleeding, wound dehiscence, prolonged ileus and mortality. |
| Notes | Country of study: Iran |
GI: gastrointestinal
Differences between protocol and review
We have made the following changes from the last update of this review (Andersen 2011):
Authorship has changed from: Andersen HK, Lewis SJ, Thomas S in the last update to: Herbert G, Perry R, Andersen HK, Atkinson C, Penfold C, Lewis SJ, Ness, A, Thomas S
Length of hospital stay and postoperative complications were designated as primary outcomes.
We included secondary outcomes that had been meta‐analysed in the previous review. We also included data for other postoperative complications and adverse events.
Studies reporting only upper gastrointestinal surgery were excluded as the review update focus on lower gastrointestinal surgery.
Clearly defined population of interest: instead of 'colorectal' or 'gastrointestinal', we define the group of interest as lower gastrointestinal surgery (distal to the ligament of Treitz)
To reflect the above changes, the title changed from: Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications to: Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications.
Previous review mentioned subgroup analysis comparing malnourished and well‐nourished patients but no analysis was conducted. We decided not to follow this up in this review.
Previous review mentioned subgroup analysis comparing studies with adequate allocation concealment and inadequate allocation concealment. We extended this to include high risk of bias in two of the following components: random sequence generation, allocation concealment, incomplete outcome data or selective outcome reporting. We conducted this as a sensitivity analysis.
We conducted two post‐hoc subgroup analyses to explore the effect of clinical diversity on length of hospital stay (LoS). We created two subgroups: one which encompassed trials that had only included patients having surgery distal to the ligament of Treitz (this group was termed 'distal to ligament of Treitz'), and the other which encompassed trials that had included patients undergoing surgery either distal or proximal to the ligament of Treitz (this group was termed 'distal and proximal to ligament of Treitz'). We also created two subgroups relating to whether the route of postoperative feeding used in the intervention was 'oral feeding only' or by 'tube feeding with or without oral feeding'. We also planned to conduct two further subgroup analyses; one comparing patients that have elective surgery versus acute surgery and the other comparing patients with cancer versus non‐cancerous conditions.
Revised methods including Trial Sequential Analyses (TSA ) and no zero‐cell continuity correction.
Contributions of authors
Search strategies were developed by Henning Keinke Andersen (HKA) and Stephen John Lewis (SJL). Charlotte Atkinson (CA) and SJL screened the titles and abstracts of all studies identified by the updated search. Georgia Herbert (GH) and Rachel Perry (RP) designed the data extraction form and extracted full texts of the included studies. Steven Thomas (ST) checked the extracted data for accuracy against the trial reports. Christopher Penfold (CP) carried out the statistical analyses. GH drafted the update of the review and all authors (HKA, CA, SJL, AN, CP, RP, ST) added valuable comments and participated in the revision of the draft review.
Sources of support
Internal sources
RegionH, Copenhagen, Denmark, Denmark.
-
NIHR Biomedical Research Unit in Nutrition, Diet and Lifestyle, Bristol, UK.
This research was funded by the National Institute for Health Research (NIHR) Bristol Nutrition Biomedical Research Unit. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
External sources
Danish Institute for Health Technology Assessment, Denmark.
Declarations of interest
All authors: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Beier‐Holgersen 1996 {published and unpublished data}
- Beier‐Holgersen R, Boesby S. Effect of early postoperative enteral nutrition on postoperative infections. [Danish]. Ugeskrift for Laeger 1998;160(22):3223‐6. [PubMed] [Google Scholar]
- Beier‐Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut 1996;39(6):833‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier‐Holgersen R, Brandstrup B. Influence of early postoperative enteral nutrition versus placebo on cell‐mediated immunity, as measured with the Multitest CMI. Scandinavian Journal of Gastroenterology 1999;34(1):98‐102. [DOI] [PubMed] [Google Scholar]
- Beier‐Holgersen R, Brandstrup B. Influence of postoperative enteral nutrition on cellular immunity. A random double‐blinded placebo controlled clinical trial. International Journal of Colorectal Disease 2012;27(4):513‐20. [DOI] [PubMed] [Google Scholar]
Binderow 1994 {published data only}
- Binderow SR, Cohen SM, Wexner SD, Nogueras JJ. Must early postoperative oral intake be limited to laparoscopy?. Diseases of the Colon & Rectum 1994;37(6):584‐9. [DOI] [PubMed] [Google Scholar]
- Binderow SR, Cohen SM, Wexner SD, Schmitt SL, Nogueras JJ, Jagelman DG. Must early postoperative oral intake be limited to laparoscopy? [Abstract]. International Journal of Colorectal Disease 1993;8:230. [Google Scholar]
Carr 1996 {published data only}
- Carr CS, Boulos PB. Immediate postoperative enteral feeding following bowel resection. [Abstract]. International Journal of Colorectal Disease 1996;11:142. [Google Scholar]
- Carr CS, Ling KD, Boulos P, Singer M. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ 1996;312(7035):869‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Carr C. Immediate enteral feeding after gastrointestinal resection [Letter; author's reply]. BMJ 1996;313:230. [Google Scholar]
Chatterjee 2012 {published data only}
- Chatterjee S, Bala SK, Chakrabory P, Dey R, Sinha S, Ray R, et al. A comparative study between early enteral feeding (within 24 hours) versus conventional enteral feeding after enteric anastomosis. Bangladesh Journal of Medical Science 2012;11(4):273‐83. [Google Scholar]
da Fonseca 2011 {published and unpublished data}
- Consoli MLD, Fonseca LM, Silva RG, Davisson Correia MIT. Early postoperative oral feeding impacts positively in patients undergoing colonic resection: results of a pilot study. Nutrición Hospitalaria 2010;25(5):806‐9. [PubMed] [Google Scholar]
- Fonseca LM, Profeta da Luz MM, Lacerda‐Filho A, Davisson Correia MIT, Silva RG. A simplified rehabilitation program for patients undergoing elective colonic surgery ‐ randomized controlled clinical trial. International Journal of Colorectal Disease 2011;26(5):609‐16. [DOI] [PubMed] [Google Scholar]
Dag 2011 {published and unpublished data}
- Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo) 2011;66(12):2001‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartsell 1997 {published data only}
- Hartsell PA, Frazee RC, Harrison JB, Smith RW. Early postoperative feeding after elective colorectal surgery. Archives of Surgery 1997;132(5):518‐21. [DOI] [PubMed] [Google Scholar]
Lucha 2005 {published data only}
- Lucha PA Jr, Butler R, Plichta J, Francis M. The economic impact of early enteral feeding in gastrointestinal surgery: a prospective survey of 51 consecutive patients. American Surgeon 2005;71(3):187‐90. [PubMed] [Google Scholar]
Minig 2009 {published data only}
- Minig L, Biffi R, Zanagnolo V, Attanasio A, Beltrami C, Bocciolone L, et al. Early oral versus "traditional" postoperative feeding in gynecologic oncology patients undergoing intestinal resection: a randomized controlled trial. Annals of Surgical Oncology 2009;16(6):1660‐8. [DOI] [PubMed] [Google Scholar]
Mulrooney 2004 {unpublished data only}
- Mulrooney LJ, Bagley JS, Wilkinson JA, Kelty C, Coker AO, Jacob G. Nasojejunal feeding does not improve clinical outcomes and is poorly tolerated following colorectal surgery [Abstract]. Proceedings of the Nutrition Society 2004; Vol. 64:9A.
Nakeeb 2009 {published and unpublished data}
- Nakeeb A, Fikry A, Metwally T, Fouda E, Youssef M, Ghazy H, et al. Early oral feeding in patients undergoing elective colonic anastomosis. International Journal of Surgery 2009;7(3):206‐9. [DOI] [PubMed] [Google Scholar]
Ortiz 1996 {published and unpublished data}
- Ortiz H, Armendariz P, Yarnoz C. Early postoperative feeding after elective colorectal surgery is not a benefit unique to laparoscopy‐assisted procedures. International Journal of Colorectal Disease 1996;11(5):246‐9. [DOI] [PubMed] [Google Scholar]
- Ortiz H, Armendariz P, Yarnoz C. Is early postoperative feeding feasible in elective colon and rectal surgery?. International Journal of Colorectal Disease 1996;11(3):119‐21. [DOI] [PubMed] [Google Scholar]
- Wexner SD. Is early postoperative feeding feasible in elective colon and rectal surgery?. International Journal of Colorectal Disease 1996;11(6):303. [DOI] [PubMed] [Google Scholar]
Reissman 1995 {published and unpublished data}
- Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Annals of Surgery 1995;222(1):73‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexner SD. Is early postoperative feeding feasible in elective colon and rectal surgery?. International Journal of Colorectal Disease 1996;11(6):303. [DOI] [PubMed] [Google Scholar]
Sagar 1979 {published data only}
- Sagar S, Harland P, Shields R. Early postoperative feeding with elemental diet. British Medical Journal 1979;1(6159):293‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroeder 1991 {published data only}
- Schroeder D, Gillanders L, Mahr K, Hill GL. Effects of immediate postoperative enteral nutrition on body composition, muscle function, and wound healing. Journal of Parenteral & Enteral Nutrition 1991;15(4):376‐83. [DOI] [PubMed] [Google Scholar]
Stewart 1998 {published and unpublished data}
- Stewart BT, Woods RJ, Collopy BT, Fink RJ, Mackay JR, Keck JO. Early feeding after elective open colorectal resections: a prospective randomized trial. Australian & New Zealand Journal of Surgery 1998;68(2):125‐8. [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang DJ, He WL, Wang L, Xu JB, Peng JJ, Wu H, et al. Effect of postoperative early enteral nutrition on the recovery of humoral immune function in patients with colorectal carcinoma undergoing elective resection. Chinese Journal of Gastrointestinal Surgery 2013;16(11):1051‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Abela 2017 {published data only}
- Abela G. The potential benefits and harms of early feeding post surgery: a literature review. International Wound Journal 2017;14:870‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barletta 2011 {published data only}
- Barletta JF, Asgeirsson T, Senagore AJ. Influence of intravenous opioid dose on postoperative ileus. Annals of Pharmacotherapy 2011;45(7‐8):916‐23. [DOI] [PubMed] [Google Scholar]
Barlow 2011 {published data only}
- Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clinical Nutrition 2011;30(5):560‐6. [DOI] [PubMed] [Google Scholar]
Beati 1998 {published data only}
- Beati C, Samori G, Gentile MG, Corradi E, Gastaldo L, Confalonieri MA. A prospective, randomised trial of early enteral feeding after resection for colorectal cancer. British Journal of Surgery 1998;85:69. [Google Scholar]
Beattie 2000 {published data only}
- Beattie AH, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46(6):813‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Behrns 2000 {published data only}
- Behrns KE, Kircher AP, Galanko JA, Brownstein MR, Koruda MJ. Prospective randomized trial of early initiation and hospital discharge on a liquid diet following elective intestinal surgery. Journal Gastrointestinal Surgery 2000;4(2):217‐21. [DOI] [PubMed] [Google Scholar]
Bernardes 2008 {published data only}
- Bernardes LFM, Filho AD. Early enteral feeding after upper digestive tract surgeries and clinical assessment of post‐operative ileus. Bioscience Journal 2008;24(4):100‐7. [Google Scholar]
Beyer 1991 {published data only}
- Beyer PA, Thomas J, Muller Cl, Encke A. The immediate postoperative diet after colon resection ‐ enteral or parenteral?. Lagenbecks Archive for Surgery united with Bruns' Contributions for Clinical Surgery 1991;91:481‐4. [Google Scholar]
Bickel 1992 {published data only}
- Bickel A, Shtamler B, Mizrahi S. Early oral feeding following removal of nasogastric tube in gastrointestinal operations. A randomized prospective study. Archives of Surgery 1992;127(3):287‐9. [DOI] [PubMed] [Google Scholar]
Bisgaard 2002 {published data only}
- Bisgaard T, Kehlet H. Early oral feeding after elective abdominal surgery ‐ what are the issues?. Nutrition 2002;18(11‐12):944‐8. [DOI] [PubMed] [Google Scholar]
Boelens 2014 {published data only}
- Boelens PG, Heesakkers FF, Luyer MD, Barneveld KW, Hingh IH, Nieuwenhuijzen GA, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Annals of Surgery 2014;259(4):649‐55. [DOI] [PubMed] [Google Scholar]
Buchmann 1996 {published data only}
- Buchmann P, Bischofberger U, Lorenzi D, Sartoretti C. Early enteral nutrition after colorectal carcinoma resections ‐ a prospective, controlled study. Schweizerische Medizinische Wochenschrift 1996;126:33. [Google Scholar]
Buchmann 1998 {published data only}
- Buchmann P, Bischofberger U, Lorenzi D, Christen D. Early postoperative nutrition after laparoscopic and open colorectal resection [German]. Swiss Surgery 1998;4(3):146‐55. [PubMed] [Google Scholar]
Bufo 1994 {published data only}
- Bufo AJ, Feldman S, Daniels GA, Lieberman RC. Early postoperative feeding. Diseases of the Colon and Rectum 1994;37(12):1260‐5. [DOI] [PubMed] [Google Scholar]
Choudhry 1996 {published data only}
- Choudhry U, Barde CJ, Markert R, Gopalswamy N. Percutaneous endoscopic gastrostomy: a randomized prospective comparison of early and delayed feeding. Gastrointestinal Endoscopy 1996;44(2):164‐7. [DOI] [PubMed] [Google Scholar]
Dardai 1985 {published data only}
- Dardai E, Bite A, Bedo M. Enteral artifical nutrition in the early post‐operative phase by gastro‐intestinal surgery. Anaesthesist 1985;34:155. [Google Scholar]
de Aguilar‐Nascimento 2002 {published data only}
- Aguilar‐Nascimento JE, Goelzer J. Early feeding after intestinal anastomoses: risks or benefits? [Portuguese]. Revista Da Associacao Medica Brasileira 2002;48(4):348‐52. [PubMed] [Google Scholar]
Delaney 2003 {published data only}
- Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Diseases of the Colon and Rectum 2003;46(7):851‐9. [DOI] [PubMed] [Google Scholar]
Feo 2004 {published data only}
- Feo CV, Romanini B, Sortini D, Ragazzi R, Zamboni P, Pansini GC, et al. Early oral feeding after colorectal resection: A randomized controlled study. ANZ Journal of Surgery 2004;74(5):298‐301. [DOI] [PubMed] [Google Scholar]
Gianotti 2011 {published data only}
- Gianotti L, Nespoli L, Torselli L, Panelli M, Nespoli A. Safety, feasibility, and tolerance of early oral feeding after colorectal resection outside an enhanced recovery after surgery (ERAS) program. International Journal of Colorectal Disease 2011;26(6):747‐53. [DOI] [PubMed] [Google Scholar]
Gómez Sánchez 2010 {published data only}
- Gómez Sánchez MB, García‐Talavera Espín NV, Sánchez Álvarez C, Zomeño Ros AI, Hernández MN, Gómez Ramos MJ, et al. Perioperative nutritional support in patients with colorectal neoplasms [Spanish]. Nutricion Hospitalaria 2010;25(5):797‐805. [PubMed] [Google Scholar]
Gómez Sánchez 2011 {published data only}
- Gómez Sánchez MB, García Talavera Espín NV, Monedero Saiz T, Sánchez Álvarez C, Zomeño Ros AI, Nicolás Hernández M, et al. Evaluation of perioperative nutritional therapy in patients with gastrointestinal tract neoplasms. Nutricion Hospitalaria 2011;26(5):1073‐80. [DOI] [PubMed] [Google Scholar]
Gustafsson 2011 {published data only}
- Gustafsson UO, Ljungqvist O. Perioperative nutritional management in digestive tract surgery. Current Opinion in Clinical Nutrition & Metabolic Care 2011;14(5):504‐9. [DOI] [PubMed] [Google Scholar]
Han‐Geurts 2001 {published data only}
- Han‐Geurts I, Jeekel J, Tilanus H, Brouwer K. Randomized clinical trial of patient‐controlled versus fixed regimen feeding after elective abdominal surgery. British Journal of Surgery 2001;88(12):1578‐82. [DOI] [PubMed] [Google Scholar]
Han‐Geurts 2007 {published data only}
- Han‐Geurts IJ, Hop WC, Kok NF, Lim A, Brouwer KJ, Jeekel J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. British Journal of Surgery 2007;94(5):555‐61. [DOI] [PubMed] [Google Scholar]
Hedberg 1999 {published data only}
- Hedberg AM, Lairson DR, Aday LA, Chow J, Suki R, Houston S, et al. Economic implications of an early postoperative enteral feeding protocol. Journal of the American Dietetic Association 1999;99(7):802‐7. [DOI] [PubMed] [Google Scholar]
Henriksen 2002 {published data only}
- Henriksen MG, Hansen HV, Hessov I. Early oral nutrition after elective colorectal surgery: Influence of balanced analgesia and enforced mobilization. Nutrition 2002;18(3):263‐7. [DOI] [PubMed] [Google Scholar]
Heslin 1997 {published and unpublished data}
- Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, et al. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Annals of Surgery 1997;226(4):567‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holst 2015 {published data only}
- Holst M, Rasmussen HH. Irtun O. Advances in clinical nutrition in GI surgery. Expert Review of Gastroenterology and Hepatology 2015;9(4):467‐73. [DOI] [PubMed] [Google Scholar]
Hoover 1980 {published data only}
- Hoover HC Jr, Ryan JA, Anderson EJ, Fischer JE. Nutritional benefits of immediate postoperative jejunal feeding of an elemental diet. American Journal of Surgery 1980;139(1):153‐9. [DOI] [PubMed] [Google Scholar]
Hyltander 2005 {published data only}
- Hyltander A, Bosaeus I, Svedlund J, Liedman B, Hugosson I, Wallengren O, et al. Supportive nutrition on recovery of metabolism, nutritional state, health‐related quality of life, and exercise capacity after major surgery: a randomized study. Clinical Gastroenterology and Hepatology 2005;3(5):466‐74. [DOI] [PubMed] [Google Scholar]
Kaur 2005 {published data only}
- Kaur N, Gupta MK, Minocha VR. Early enteral feeding by nasoenteric tubes in patients with perforation peritonitis. World Journal of Surgery 2005;29(8):1023‐7. [DOI] [PubMed] [Google Scholar]
Keele 1997 {published data only}
- Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DB. Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut 1997;40(3):393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemen 1995 {published data only}
- Kemen M, Senkal M, Homann HH, Mumme A, Dauphin AK, Baier J, et al. Early postoperative enteral nutrition with arginine‐omega‐3 fatty acids and ribonucleic acid‐supplemented diet versus placebo in cancer. Critical Care Medicine 1995;23(4):652‐9. [DOI] [PubMed] [Google Scholar]
Klappenbach 2013 {published data only}
- Klappenbach RF, Yazyi FJ, Alonso Quintas F, Horna ME, Alvarez Rodríguez J, Oría A. Early oral feeding versus traditional postoperative care after abdominal emergency surgery: a randomized controlled trial. World Journal of Surgery 2013;37(10):2293‐9. [DOI] [PubMed] [Google Scholar]
Klek 2011 {published data only}
- Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. Perioperative nutrition in malnourished surgical cancer patients ‐ A prospective, randomized, controlled clinical trial. Clinical Nutrition 2011;30(6):708‐13. [DOI] [PubMed] [Google Scholar]
Kompan 1999 {published data only}
- Kompan L, Kremzar B, Gadzijev E, Prosek M. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Medicine 1999;25(2):157‐61. [DOI] [PubMed] [Google Scholar]
Kong 2017 {published and unpublished data}
- Kong S‐H, Park JS, Lee IK, Ryu S‐W, Park Y‐K, Yang H‐K, et al. Postoperative oral nutritional supplementation after major gastrointestinal surgery: a randomized controlled clinical trial.. Asia Pacific Journal of Clinical Nutrition 2017;26(5):811‐9. [DOI] [PubMed] [Google Scholar]
- Lee H‐J, Kong S‐H. Randomized controlled trial evaluating postoperative oral nutritional supplementation after major gastrointestinal surgery. Poster tour: Nutrition and Chronic Disease. 2016; Vol. PTO8 6. [DOI] [PubMed]
Lau 2014 {published data only}
- Lau C, Phillips E, Bresee C, Fleshner P. Early use of low residue diet is superior to clear liquid diet after elective colorectal surgery: a randomized controlled trial. Annals of Surgery 2014;260(4):641‐7. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee T, Kang S, Hong S, Kim D. A prospective randomized controlled trial of enhanced recovery program in patients undergoing laparoscopic resection for colon cancer. Diseases of the Colon and Rectum. 2010:556. [Google Scholar]
Lee 2014 {published data only}
- Lee HJ, Na JR, Suh YS, Kong SH, Yang HK. Effect of perioperative oral nutritional supplementation in malnourished patients who will receive gastrectomy: A prospective randomized trial. Clinical Nutrition 2014;33(1):S249. [DOI] [PubMed] [Google Scholar]
Le Guen 2014 {published data only}
- Guen M, Fessler J, Fischler M. Early oral feeding after emergency abdominal operations: another paradigm to be broken?. Current Opinion in Clinical Nutrition and Metabolic Care 2014;17(5):477‐82. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li Y, Sun HB, Liu XB, Wang ZF, Zhang RX, Qin JJ. The feasibility of "non‐tube no‐fasting" after oesophagectomy for oesophageal cancer in the thoracolaparoscopic era. Diseases of the Esophagus 2014;27:78A. [Google Scholar]
Li 2015 {published data only}
- Li Y, L, Ma L, Fan XQ. Clinical effects of early standardized enteral nutrition through a nasogastric tube combined with effective monitoring in patients with gastric cancer. [Chinese]. World Chinese Journal of Digestology 2015;23(21):3446‐50. [Google Scholar]
Li 2015 (a) {published data only}
- Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. The postoperative clinical outcomes and safety of early enteral nutrition in operated gastric cancer patients. Journal of the Balkan Union of Oncology 2015;20(2):468‐72. [PubMed] [Google Scholar]
Lidder 2013 {published data only}
- Lidder P, Thomas S, Fleming S, Hosie K, Shaw S, Lewis S. A randomized placebo controlled trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Disease 2013;15(6):737‐45. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu ZH, Su GQ, Zhang SY, Zhang JB, Huang XR. Study on early postoperative nutritional support in elderly patients with gastric cancer. Chinese Journal of Gastrointestinal Surgery 2013;16(11):1063‐6. [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu X, Wang D, Zheng L, Mou T, Liu H, Li G. Is early oral feeding after gastric cancer surgery feasible? A systematic review and meta‐analysis of randomized controlled trials. PLOS One 2014;9 (11):e112062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahla 2016 {published data only}
- Mahla V, Khan S, Ahmad R, Jenaw RK. Early feeding after loop ileostomy reversal: A prospective study. Formosan Journal of Surgery 2016;49(5):178‐82. [Google Scholar]
Mahmoodzadeh 2015 {published data only}
- Mahmoodzadeh H, Shoar S, Sirati F, Khorgami Z. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surgery Today 2015;45(2):203‐8. [DOI] [PubMed] [Google Scholar]
Malhotra 2004 {published data only}
- Malhotra A, Mathur AK, Gupta S. Early enteral nutrition after surgical treatment of gut perforations: a prospective randomised study. Journal of Postgraduate Medicine 2004;50(2):102‐6. [PubMed] [Google Scholar]
Maňásek 2016 {published data only}
- Maňásek V, Bezděk K, Foltys A, Klos K, smitka J, Smehlik D. The impact of high protein nutritional support on clinical outcomes and treatment costs of patients with colorectal cancer. Klinicka Onkologie 2016;29(5):351‐7. [PubMed] [Google Scholar]
Mari 2011 {published data only}
- Mari G, Costanzi A, Maggioni A, Ferrari G, Citterio D, Origi M, et al. Fast track vs conventional care in laparoscopic left hemicolectomy. A pilot prospective randomized study. Surgical Endoscopy and Other Interventional Techniques 2011;25(S2). [Google Scholar]
McCarter 1998 {published data only}
- McCarter TL, Condon SC, Aguilar RC, Gibson DJ, Chen YK. Randomized prospective trial of early versus delayed feeding after percutaneous endoscopic gastrostomy placement. American Journal of Gastroenterology 1998;93(3):419‐21. [DOI] [PubMed] [Google Scholar]
Mirea 2015 {published data only}
- Mirea L, Pavelescu D, Grintescu I. Could preoperative and postoperative optimal nutrition support modulate the inflammatory response and clinical outcome of severe malnourished surgical patients with gastrointestinal neoplasia?. Critical Care 2015;19(S1):396. [Google Scholar]
Moore 1991 {published data only}
- Moore EE, Moore FA. Immediate enteral nutrition following multisystem trauma: a decade perspective. Journal of the American College of Nutrition 1991;10(6):633‐48. [DOI] [PubMed] [Google Scholar]
Moss 1981 {published data only}
- Moss G. Maintenance of gastrointestinal function after bowel surgery and immediate enteral full nutrition. II. Clinical experience, with objective demonstration of intestinal absorption and motility. Journal of Parenteral and Enteral Nutrition 1981;5(3):215‐20. [DOI] [PubMed] [Google Scholar]
Murchan 1995 {published data only}
- Murchan PM, Bradford I, Palmer D, Townsend S, Harrison JD, Mitchell CJ, et al. Value of preoperative and postoperative supplemental enteral nutrition in patients undergoing major gastrointestinal surgery. Clinical Nutrition 1995;14(S2):8. [Google Scholar]
Nakajima 2009 {published data only}
- Nakajima S, Iijima H, Inoue T, Shinzaki S, Egawa, S, Hayashi Y, et al. Correlation between vitamin K deficiency and clinical activity index in patients with inflammatory bowel disease. Gastroenterology. 2009:A361.
Neri 2001 {published data only}
- Neri A, Mariani F, Piccolomini A, Testa M, Vuolo G, Cosmo L. Glutamine‐supplemented total parenteral nutrition in major abdominal surgery. Nutrition 2001;17(11‐12):968‐9. [DOI] [PubMed] [Google Scholar]
Nessim 1999 {published data only}
- Nessim A, Wexner SD, Agachan F, Albaz O, Weiss EG, Nogueras JJ, et al. Is bowel confinement necessary after anorectal reconstructive surgery? A prospective, randomized, surgeon‐blinded trial. Diseases of the Colon and Rectum 1999;42(1):16‐23. [DOI] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Nguyen NQ, Fraser RJ, Bryant L, Burgstad C, Burnett J, Holloway RH. Absorption of lipid is impaired longer than that of carbohydrate after abdominal aortic aneurysm (AAA) repair: An implication for nutritional management in surgical patients. Gastroenterology 2009;136(5):A539. [Google Scholar]
Niu 2015 {published data only}
- Niu WB, Li ZY, Zhou CX, Wang GY, Yu YM. Clinical effects of early enteral nutrition in patients after laparoscopic surgery for colorectal cancer. World Chinese Journal of Digestology 2015;23(5):857‐61. [Google Scholar]
Osland 2011 {published data only}
- Osland E, Yunus RM, Khan S, Memon MA. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta‐analysis. Journal of Parenteral and Enteral Nutrition 2011;35(4):473‐87. [DOI] [PubMed] [Google Scholar]
Palmer 1998 {published data only}
- MacFie J, Woodcock NP, Palmer MD, Walker A, Townsend S, Mitchell CJ. Oral dietary supplements in pre‐ and postoperative surgical patients: a prospective and randomized clinical trial. Nutrition 2000;16(9):723‐8. [DOI] [PubMed] [Google Scholar]
- Palmer MD, Townsend S, Harrison JD, Mitchell CJ, MacFie J. Value of preoperative and postoperative supplemental enteral nutrition in patients undergoing major gastrointestinal surgery. British Journal of Surgery 1998;85:74. [Google Scholar]
Pappa 2011 {published data only}
- Pappa HM, Saslowsky TM, Filip‐Dhima R, DiFabio D, Lahsinoui HH, Akkad A, et al. Efficacy and harms of nasal calcitonin in improving bone density in young patients with inflammatory bowel disease: a randomized, placebo‐controlled, double‐blind trial. American Journal of Gastroenterology 2011;106(8):1527‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2014 {published data only}
- Peng SS, Duan J, Huang HF, Lin J, Xu WG, Huang Z, et al. Clinical effects of early postoperative oral feeding versus traditional oral feeding after bilioenteric anastomosis. World Chinese Journal of Digestology 2014;22(9):1312‐6. [Google Scholar]
Pragatheeswarane 2014 {published and unpublished data}
- Pragatheeswarane M, Muthukumarassamy R, Kadambari D, Kate V. Early oral feeding vs. traditional feeding in patients undergoing elective open bowel surgery‐ a randomized controlled trial. Journal of Gastrointestinal Surgery 2014;18(5):1017‐23. [DOI] [PubMed] [Google Scholar]
Rosales 2009 {published data only}
- Rosales VB, Morales BV, Campano MB, Aranda WCh, Kehr JS. Comparison between early enteral nutrition and late enteral nutrition in the nutritional status of patients with gastrectomy [Comparación entre nutrición enteral precoz y nutrición enteral tardía en el estado nutricional de pacientes gastrectomizados]. Revista Chilena De Nutricion 2009;36(1):15‐22. [Google Scholar]
Ryan 1981 {published data only}
- Ryan JA Jr, Page CP, Babcock L. Early postoperative jejunal feeding of elemental diet in gastrointestinal surgery. American Surgeon 1981;47(9):393‐403. [PubMed] [Google Scholar]
Schwenk 1998 {published data only}
- Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Archives of Surgery 1998;383(1):49‐55. [DOI] [PubMed] [Google Scholar]
Seenu 1995 {published data only}
- Seenu V, Goel AK. Early oral feeding after elective colorectal surgery: is it safe. Tropical Gastroenterology 1995;16(4):72‐3. [PubMed] [Google Scholar]
Seri 1984 {published data only}
- Seri S, Aquilio E. Effects of early nutritional support in patients with abdominal trauma. Italian Journal of Surgical Sciences 1984;14(3):223‐7. [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma M, Wahed S, O'Dair G, Gemmell L, Hainsworth P, Horgan AF. A randomized controlled trial comparing a standard postoperative diet with low‐volume high‐calorie oral supplements following colorectal surgery. Colorectal Disease 2013;15(7):885‐91. [DOI] [PubMed] [Google Scholar]
Shen 2013 {published data only}
- Shen Y, Jin W. Early enteral nutrition after pancreatoduodenectomy: a meta‐analysis of randomized controlled trials. Langenbecks Archives of Surgery 2013;398(6):817‐23. [DOI] [PubMed] [Google Scholar]
Shoar 2013 {published data only}
- Shoar S, Mahmoodzadeh H, Hosseini Araghi N, Mahboobi N, Sirati F, Khorgami Z. Early postoperative oral feeding in gastresophageal tumors surgery: A randomized controlled trial. Journal of Surgical Research 2013;179(2):201. [Google Scholar]
Shrikhande 2009 {published data only}
- Shrikhande SV, Shetty GS, Singh K, Ingle S. Is early feeding after major gastrointestinal surgery a fashion or an advance? Evidence‐based review of literature. Journal of Cancer Research & Therapeutics 2009;5(4):232‐9. [DOI] [PubMed] [Google Scholar]
Shu 2013 {published data only}
- Shu XL, Kang K, Zhong JX, Ji SR, Wang MH, Zhang YS, et al. Prognosis of patients with early enteral nutrition after gastrointestinal operation: a meta‐analysis. Chinese Journal of Gastrointestinal Surgery 2013;16(11):1035‐40. [PubMed] [Google Scholar]
Shu 2016 {published data only}
- Shu XL, Kang K, Gu LJ, Zhang YS. Effect of early enteral nutrition on patients with digestive tract surgery: A meta‐analysis of randomized controlled trials. Experimental and Therapeutic Medicine 2016;12(4):2136‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 1998 {published data only}
- Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. Journal of the American College of Surgeons 1998;187(2):142‐6. [DOI] [PubMed] [Google Scholar]
Smedley 2004 {published and unpublished data}
- Smedley F, Bowling T, James M, Stokes E, Goodger C, O'Connor O, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. British Journal of Surgery 2004;91(8):983‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smedley F, Goodger C, Oldale C, James M, Jones P, Bowling T, et al. Benefits of extended pre‐ and post‐operative oral nutritional supplementation (ONS): A prospective randomised controlled trial. Clinical Nutrition 2002;21:43. [Google Scholar]
Smeets 2017 {published data only}
- Smeets BJJ, Peters EG, Horsten ECJ, Weijs TJ, Rutten HJT, Buurman WA, et al. Effects of early vs late start of oral intake on anastomotic leakage following elective lower intestinal surgery: a systematic review. Nutrition in Clinical Practice 2017;XX:1‐9. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Smith JJ, Mathur P, Burke CC, Ramesh S, Dawson PM. Early feeding post major colonic surgery: why we should be all doing it [Abstract]. British Journal of Surgery 2002;89:69. [Google Scholar]
Soliani 2001 {published data only}
- Soliani P, Dell'Abate P, Rio P, Arcuri MF, Salsi P, Cortellini P, et al. Early enteral nutrition in patients treated with major surgery of the abdomen and the pelvis [Italian]. Chirurgia Italiana 2001;53(5):619‐32. [PubMed] [Google Scholar]
Stein 2002 {published data only}
- Stein J, Schulte‐Bockholt A, Sabin M, Keymling M. A randomized prospective trial of immediate vs. next‐day feeding after percutaneous endoscopic gastrostomy in intensive care patients. Intensive Care Medicine 2002;28(11):1656‐60. [DOI] [PubMed] [Google Scholar]
Takala 1985 {published data only}
- Takala J, Havia T, Heinonen R, Renvall S. Immediate enteral feeding after abdominal surgery. Acta Chirurgica Scandinavica 1985;151(2):143‐5. [PubMed] [Google Scholar]
Tong 2006 {published data only}
- Tong Q, Wang GB, Lu XM, Tao KX, Chen DD. Safety and clinical efficacy of enteral nutrition in elderly postoperative patients with malignant gastrointestinal tumor. Chinese Journal of Clinical Nutrition 2006;14(3):154‐8. [Google Scholar]
Ugarte 2000 {published data only}
- Ugarte F. Prospective randomized trial of early initiation and hospital discharge on a liquid diet following elective intestinal surgery. Journal of Gastrointestinal Surgery : official journal of the Society for Surgery of the Alimentary Tract 2000;4(6):649. [DOI] [PubMed] [Google Scholar]
Vaithiswaran 2008 {published data only}
- Vaithiswaran V, Srinivasan K, Kadambari D. Effect of early enteral feeding after upper gastrointestinal surgery. Tropical Gastroenterology 2008;29(2):91‐4. [PubMed] [Google Scholar]
Velez 1997 {published data only}
- Velez JP, Lince LF, Restrepo JI. Early enteral nutrition in gastrointestinal surgery: A pilot study. Nutrition 1997;13(5):442‐5. [DOI] [PubMed] [Google Scholar]
Vlug 2009 {published data only}
- Vlug MS, Wind J, Zaag E, Ubbink DT, Cense HA, Bemelman WA. Systematic review of laparoscopic vs open colonic surgery within an enhanced recovery programme. Colorectal Disease 2009;11(4):335‐43. [DOI] [PubMed] [Google Scholar]
Wallström 2014 {published data only}
- Wallström A, Frisman GH. Facilitating early recovery of bowel motility after colorectal surgery: a systematic review. Journal of Clinical Nursing 2014;23(1‐2):22‐44. [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang ZH, Zhong B, Xiang JY, Zhou YB, Wang DS. Effect of early oral enteral nutrition on clinical outcomes after colorectal cancer surgery. Chinese Journal of Gastrointestinal Surgery 2013;16(8):735‐8. [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang D, Zhong B, Zhao P, Liu X, Zhou Y. A randomized control study of early oral enteral nutrition after colorectal cancer operation. Chinese Journal of Gastrointestinal Surgery 2014;17(10):977‐80. [PubMed] [Google Scholar]
Watters 1997 {published data only}
- Watters JM, Kirkpatrick SM, Norris SB, Shamji FM, Wells GA. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Annals of Surgery 1997;226(3):369‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 1999 {published data only}
- Weber TR. Toupét fundoplication for gastroesophageal reflux in childhood. Archives of Surgery 1999;134(7):720‐1. [DOI] [PubMed] [Google Scholar]
Weinstein 1993 {published data only}
- Weinstein L, Dyne PL, Duerbeck NB. The PROEF diet‐ a new postoperative regimen for oral early feeding. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 1):128‐31. [DOI] [PubMed] [Google Scholar]
Wiedeck 1984 {published data only}
- Wiedeck H, Merkle N, Herfarth C, Grunert A. Postoperative enteral nutrition following resections of the colon. Der Anaesthesist 1984;33(1):63‐7. [PubMed] [Google Scholar]
Wiren 2002 {published data only}
- Wiren M, Ernerudh J, Permert J, Larsson J. Early postoperative enteral feeding supplemented with alpha‐ketoglutarate after major abdominal surgery [Abstract]. Clinical Nutrition 1998;17(S1):68. [Google Scholar]
- Wiren M, Permert J, Larsson J. Alpha‐ketoglutarate‐supplemented enteral nutrition: Effects on postoperative nitrogen balance and muscle catabolism. Nutrition 2002;18(9):725‐8. [CN‐00443995] [DOI] [PubMed] [Google Scholar]
Wong 1995 {published data only}
- Wong WK, Soo KC, Nambiar R, Tan YS, Yo SL, Tan IK. The effect of recombinant growth hormone on nitrogen balance in malnourished patients after major abdominal surgery. Australian and New Zealand Journal of Surgery 1995;65(2):109‐13. [DOI] [PubMed] [Google Scholar]
Wu 1996 {published data only}
- Wu GH, Jin DY, Wu ZH, Huang DX, Wu ZG. A study on the effect and safety of early enteral nutrition after gastrointestinal operation. Chinese Journal of Enteral and Parenteral Nutrition 1996;4(3):123‐7. [Google Scholar]
Wu 2000 {published data only}
- Wu GH, Zhang YW, Wu ZH. The effect of early enteral nutrition on postoperative patients with gastrointestinal cancer. Chinese Journal of Clinical Nutrition 2000;8(1):56‐7. [Google Scholar]
Wu 2004 {published data only}
- Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang‐Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. British Journal of Surgery 2004;91(3):283‐7. [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu GH, Zhang YW, Pan HT, Zhang B, Liu ZH, Wu ZH. A randomized controlled trial of postoperative artificial nutrition in malnourished patients with gastrointestinal cancer. Chinese Journal of Gastrointestinal Surgery 2007;10(6):546‐9. [PubMed] [Google Scholar]
Yu 2013 {published data only}
- Yu G, Chen G, Huang B, Shao W, Zeng G. Effect of early enteral nutrition on postoperative nutritional status and immune function in elderly patients with esophageal cancer or cardiac cancer. Chinese Journal of Cancer Research 2013;25(3):299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuanlong 1999 {published data only}
- Yuanlong H, Xuemin X, Chuanyong Y. Early enteral nutrition after gastrointestinal surgery clinical research. Journal of Clinical Surgery 1999;1:14‐6. [Google Scholar]
Zaidi 2016 {published data only}
- Zaidi SAA, Rehman SU. The outcome of hospital stay in early and delayed enteral feeding after bowel anastomosis. Pakistan Journal of Medicine and Dentistry 2016;5:4. [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Liu H, Li Y, Wang W. Effect of early enteral nutrition on postoperative recovery in patients with colon cancer. Cancer Research and Clinic 2014;26(7):470‐2. [Google Scholar]
Zhang 2017 {published data only}
- Zhang K, Cheng S, Zhu Q, Han Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta‐analysis of safety and efficacy. Chinese Journal of Gastrointestinal Surgery 2017;20(9):1060‐6. [PubMed] [Google Scholar]
Zhao 2014 {published data only}
- Zhao G, Cao S, Zhang K, Xin Y, Han J, Dong Q, et al. Effect of early enteral nutrition on immune response and clinical outcomes after esophageal cancer surgery. Chinese Journal of Gatrointestinal Surgery 2014;17(4):356‐60. [PubMed] [Google Scholar]
Zhou 2006 {published data only}
- Zhou T, Wu XT, Zhou YJ, Huang X, Fan W, Li YC. Early removing gastrointestinal decompression and early oral feeding improve patients' rehabilitation after colorectostomy. World Journal of Gastroenterology 2006;12(15):2459‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhuang 2013 {published data only}
- Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC, Yu Z. Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: A meta‐analysis of randomized clinical trials. Digestive Surgery 2013;30(3):225‐32. [DOI] [PubMed] [Google Scholar]
Zitta 2012 {published data only}
- Zitta D, Subbotin V, Busirev Y. Early oral feeding and avoidance of mechanical bowel preparation in elective major colorectal surgery. Colorectal Disease 2012;14(2):19. [Google Scholar]
References to studies awaiting assessment
Fanaie 2005 {published data only}
- Fanaie SA, Ziaee SA. Safety of early oral feeding after gastrointestinal anastomosis: a randomized controlled trial. Indian Journal of Surgery 2005;67(4):185‐8. [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of National Cancer Institute 1993;85:365–7. [DOI] [PubMed] [Google Scholar]
Ahmed 2012
- Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols – compliance and variations in practice during routine colorectal surgery. Colorectal Disease 2012;14(9):1045‐51. [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Localio RA. Much ado about nothing: A comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. [DOI] [PubMed] [Google Scholar]
Buzby 1988
- Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, McNeal GE, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients.. Amercian Juornal of Clinical Nutrition 1988;47:366‐81. [DOI] [PubMed] [Google Scholar]
Cortes 2014
- Cortes J, Gonzalez JA, Campbell MJ, Cobo E. A hazard ratio was estimated by a ratio of median survival times, but with considerable uncertainty. Journal of Clinical Epidemiology 2014;67(10):1172‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delmi 1990
- Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990;335(8696):1013‐6. [DOI] [PubMed] [Google Scholar]
Department of Health 2010
- Department of Health. Delivering Enhanced Recovery: Helping patients to get better sooner after surgery. London, Department of Health 2010.
Dindo 2004
- Dindo D, Demartines N, Clavien P‐A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukuzawa 2007
- Fukuzawa J, Terashima H, Ohkohchi N. Early postoperative oral feeding accelerates upper gastrointestinal anastomotic healing in the rat model. World Journal of Surgery 2007;31(6):1234‐9. [DOI] [PubMed] [Google Scholar]
Griemel 1998
- Greimel E, Bottomley A, Cull A, Waldenstrom AC, Arraras J, Chauvenet L, et al. An international field study of the reliability and validity of a disease‐specific questionnaire module (the QLQ‐OV28) in assessing the quality of life of patients with ovarian cancer. European Journal of Cancer 2003;39:1402‐8. [DOI] [PubMed] [Google Scholar]
Grizas 2008
- Grizas S, Gulbinas A, Barauskas G, Pundzius J. A comparison of the effectiveness of the early enteral and natural nutrition after pancreatoduodenectomy. Medicina 2008;44(9):678‐86. [PubMed] [Google Scholar]
Herbert 2017
- Herbert G, Sutton E, Burden S, Lewis S, Thomas S, Ness A, et al. Healthcare professionals’ views of the enhanced recovery after surgery programme: a qualitative investigation. BMC Health Service Research 2017;17:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org 2011.
Hill 1977
- Hill GL, Blackett RL, Pickford I, Burkinshaw L, Young GA, Warren JV. Malnutrition in surgical patients; an unrecognised problem. Lancet 1977;1(8013):689‐92. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2016
- Jakobsen JC, Wetterslev J, Lange T, Gluud C. Viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews. Cochrane Database of Systematic Reviews. ED000111. doi: 10.1002/14651858.ED000111. No abstract available.PMID: 27030037 2016, issue 3. [PUBMED: PMID: 27030037] [DOI] [PMC free article] [PubMed]
Karl 2014
- Karl A, Buchnera A, Beckera A, Staehlera M, Seitza M, Khodera W, et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a prospective randomized study. Journal of Urology. 2014;191(2):335‐40. [DOI] [PubMed] [Google Scholar]
Kawasaki 2009
- Kawasaki N, Suzuki Y, Nakayoshi T, Hanyu N, Nakao M, Takeda A, et al. Early postoperative enteral nutrition is useful for recovering gastrointestinal motility and maintaining the nutritional status. Surgery Today 2009;39(3):225‐30. [DOI] [PubMed] [Google Scholar]
Lennard‐Jones 1992
- Lennard‐Jones JE. A positive approach to nutrition as a treatment. London: King's Fund Centre Report 1992.
Ljungqvist 2009
- Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Practice & Research 2009;23(4):401‐9. [DOI] [PubMed] [Google Scholar]
Maessen 2009
- Maessen JM, Hoff C, Jottard K, Kessels AG, Bremers AJ, Havenga K, et al. To eat or not to eat: facilitating early oral intake after elective colonic surgery in the Netherlands. Clinical Nutrition 2009;28(1):29‐33. [DOI] [PubMed] [Google Scholar]
McWhirter 1994
- McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308(6934):945‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1989
- Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma‐reduced septic morbidity. Journal of Trauma 1989;29(7):916‐22. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3.. Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schilder 1997
- Schilder JM, Hurteau JA, Look KY, Moore DH, Raff G, Stehman FB, et al. A prospective controlled trial of early postoperative oral intake following major abdominal gynaecologic surgery. Gynecologic Oncology 1997;67(3):235‐40. [DOI] [PubMed] [Google Scholar]
Schwarzer 2007
- Schwarzer G. Meta: An R package for meta‐analysis. R news 2007;7(3):40‐5. [Google Scholar]
Schünemann 2009
- Schünemann H, Brozek J, Oxman A, editors of The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendation. www.cc‐ims.net/gradepr (accessed 08/02/16);Version 3.2([updated March 2009]). Available from http://www.cc‐ims.net/gradepro.
Short 2015
- Short V, Herbert G, Perry R, Atkinson C, Ness AR, Penfold C, et al. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD006506.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Thorlund 2011 [Computer program]
- Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, 2011.
TSA ‐ Trial Sequential Analysis [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Copenhagen: Copenhagen Trial Unit, 2016.
Vet's Affairs 1991
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. New England Journal of Medicine 1991;325(8):525‐32. [DOI] [PubMed] [Google Scholar]
Viganò 2012
- Viganò J, Cereda E, Caccialanza R, Carini R, Cameletti B, Spampinato M, et al. Effects of preoperative oral carbohydrate supplementation on postoperative metabolic stress response of patients undergoing elective abdominal surgery. World Journal of Surgery 2012;36(8):1738‐43. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2011
- Warren J, Bhalla V, Cresci G. Postoperative diet advancement: surgical dogma vs evidence‐based medicine. Nutrition in Clinical Practice 2011;26(2):115‐25. [DOI] [PubMed] [Google Scholar]
Weissenfluh 2006
- Weissenfluh GM, Brundage SI, Spain DA. Early enteral nutrition after abdominal trauma: effects on septic morbidity and practicality. Nutrition in Clinical Practice 2006;21(5):479‐84. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Andersen 2003
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004080] [DOI] [PubMed] [Google Scholar]
Andersen 2006
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004080.pub2] [DOI] [PubMed] [Google Scholar]
Andersen 2011
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 2011;18(4):CD004080. [DOI] [PubMed] [Google Scholar]
Lewis 2001
- Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus 'nil by mouth' after gastrointestinal surgery: systematic review and meta‐analysis of controlled trials. BMJ 2001;323(7316):773‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2009
- Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta‐analysis. Journal of Gastrointestinal Surgery 2009;13(3):569‐75. [DOI] [PubMed] [Google Scholar]


