Abstract
Background: The relationship of endogenous sex hormones (SH) with vascular endothelial function and with cardiovascular disease (CVD) is incompletely understood. We examined the associations between SH and endothelial function measured by brachial artery flow-mediated dilation (FMD).
Materials and Methods: We included 1368 postmenopausal women and 1707 men, free of clinical CVD, participating in MESA Visit 1 (2000–2002). Serum SH [total testosterone, SH binding globulin (SHBG), dehydroepiandrosterone (DHEA), estradiol] were measured; free testosterone was calculated. The percent FMD difference (%FMD) was measured by high-resolution ultrasound. Using multivariable-adjusted linear regression, we tested the cross-sectional associations of SH (log transformed, compared per one SD increment) with %FMD.
Results: The mean age of women and men were 64.2 and 61.4 years, respectively. Among women, after adjusting for demographics, CVD risk factors, and hormone therapy, higher SHBG was associated with greater %FMD [β = 0.215% (95% CI 0.026–0.405)], whereas higher free testosterone was associated with a smaller %FMD [−0.209% (−0.402, −0.017)]. Estradiol and DHEA were not associated with %FMD in women after multivariable adjustment. There was an age interaction, with higher free testosterone and lower SHBG associated with worse FMD in women <65 years of age, but not in those ≥65 years (p = 0.04). We did not see similar associations in men.
Conclusions: A more androgenic SH profile of higher free testosterone and lower SHBG was associated with worse %FMD in postmenopausal women. Changes in SH with aging and menopause may result in vascular changes in women. Further studies are needed to assess longitudinal changes in SH levels and their association with vascular function.
Keywords: endothelial function, sex hormones, menopause, cardiovascular disease
Introduction
Atherosclerotic cardiovascular disease (CVD) is the leading cause of morbidity and mortality in women and men in the United States and worldwide.1 Premenopausal women have lower rates of CVD events compared to age-matched men and older postmenopausal women.2 This phenomenon is thought to be due to the protective vascular effects of endogenous estrogens on the development of atherosclerosis.3,4 In fact, women who experience premature menopause are at a higher risk for coronary heart disease (CHD) and CVD-related mortality compared to those who undergo natural menopause.3 However, postmenopausal hormone therapy (HT) has not been shown to be beneficial in CVD primary or secondary prevention randomized clinical trials.5,6
The vascular endothelium is a single-cell layer that lines blood vessels and is important in regulating coagulation, platelet reactivity, inflammation, oxidative stress, vascular tone, and smooth muscle cell proliferation by releasing various factors to chemical and physical stimuli, including nitric oxide (NO), prostaglandins, and endothelins.7–9 Endothelial dysfunction is a marker of subclinical atherosclerosis10 and is strongly associated with future CVD events, particularly in women.11
Sex hormones (SHs) exert a wide variety of effects on the vascular endothelium. In animal and human models, endogenous estrogen has beneficial effects on endothelial cells, including upregulation of NO synthase,12,13 protection from lipid oxidation,14 enhanced endothelial cell vasoreactivity through smooth muscle vasodilation,15 reduced oxidative stress,16–18 and protection from vascular injury.19 In addition, in humans, short-term estrogen supplementation improves brachial endothelial function.20 Estrogen also has beneficial effects on cardiometabolic risk factors, such as lipids21 and on the metabolic syndrome,22 which contribute to endothelial injury.23,24
In women, after menopause, while estrogen levels decline, the ovary continues to produce testosterone and this may be associated with the increase in atherosclerotic CVD after menopause.25,26 We previously found that postmenopausal women with higher androgen levels relative to estradiol had an increased risk of CVD events.26 In contrast, in men, the opposite pattern is seen with low testosterone being associated with endothelial dysfunction and CHD.27,28 However, whether the levels of the different endogenous SHs differ in their association with endothelial function, and whether there is a difference between men and post-menopausal women, is not completely understood.
Our objective was to assess the cross-sectional associations between endogenous SH levels and brachial artery endothelial function as measured by percent flow-mediated dilation (%FMD) in adults free of clinical CVD. We hypothesized that a more androgenic SH profile (higher testosterone and lower SH binding globulin [SHBG]) would be associated with abnormal endothelial function among postmenopausal women, but not in similarly aged men.
Materials and Methods
Study population
We used cross-sectional data from the baseline exam of the Multi-Ethnic Study of Atherosclerosis (MESA). MESA is a prospective cohort study of asymptomatic individuals in the community from six field centers in the United States. MESA is assessing the natural history of CVD and specifically the prevalence of, and risk factors associated with, subclinical progression of CVD. The study design and methods have been previously described.29 The baseline visit was conducted from 2000 to 2002 and included 6814 individuals 45–84 years of ages without known clinical CVD at baseline.
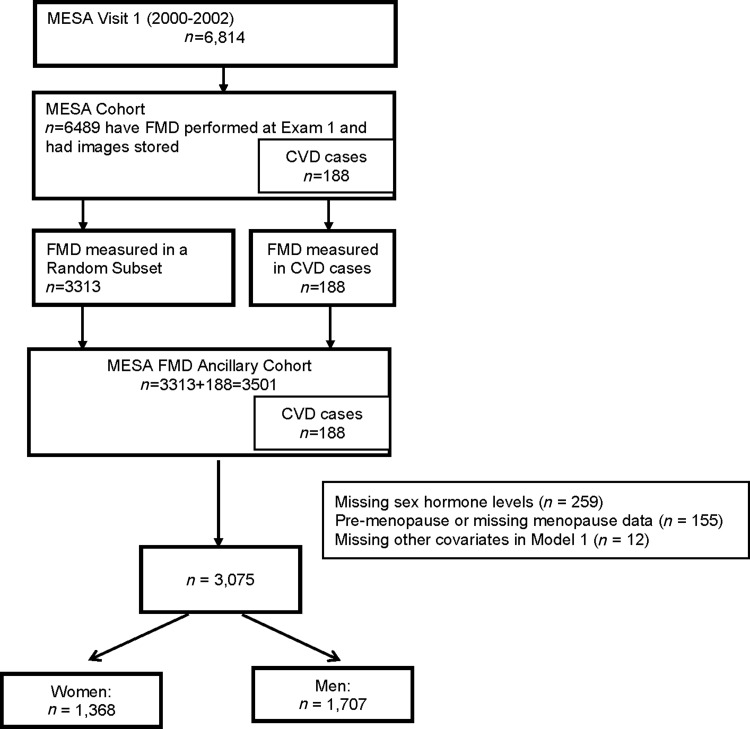
Although the majority of MESA participants (n = 6489) had measurements of FMD performed at the baseline examination, due to cost reasons, only a subset had their tapes of FMD read and included in the MESA FMD ancillary study using a case-cohort sampling design.30 A nested case cohort allows for an efficient study design when the cost of measuring an exposure is prohibitive. The findings, while efficient and economical, are still representative of the whole cohort. The case-cohort subset described in this work included all participants who had an adjudicated CVD event in MESA by October 2005 (n = 188) and a random subset of MESA participants (n = 3313) (Fig. 1).
FIG. 1.
Flowchart of study participants, Multi Ethnic Study of Atherosclerosis (MESA) Visit 1 (2000–2002).
For our female sample, we included only post-menopausal women because SH levels are very different between premenopausal and postmenopausal women, and there were few premenopausal women in MESA, limiting our ability to adequately study this subgroup. An algorithm was used in MESA to determine menopausal status, which has been previously reported.31 Among our 3501 participants in our nested case-cohort FMD sample, we additionally excluded individuals missing SH data (n = 259), missing menopausal status or premenopausal (n = 155), and missing baseline covariates from our model 1 (n = 12). Our final analysis included 3075 participants (Fig. 1).
The Institutional Review Board approved the study at each MESA field center and all participants provided written informed consent.
Sex hormones
Fasting blood samples for SH measurements were collected and stored at the time of the baseline visit. Serum hormone levels were measured at the Steroid Hormone Laboratory of the University of Massachusetts Medical Center (Worcester, MA) as previously described.32,33 Total estradiol was measured using an ultrasensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX). Total testosterone and total dehydroepiandrosterone (DHEA) were measured using radioimmunoassay kits, and SHBG was measured using a chemiluminescence enzyme immunometric assay using Immulite kits (Diagnostic Products Corporation, Los Angeles, CA). Free testosterone was calculated from total testosterone and SHBG as previously described.34 The total Testosterone/Estradiol (T/E) ratio26 was also calculated. The reliability of the SH assays was determined using 5% of randomly selected duplicate samples and quality control samples. The intra-assay coefficients of variation for total T, SHBG, DHEA, and estradiol were 12.3%, 9.0%, 11.2%, and 10.5%, respectively.35
Flow-mediated dilation of the brachial artery
The methods for FMD measurement by high-resolution ultrasound in MESA have been previously described30,36 and a detailed protocol is available on the MESA website (www.mesa-nhlbi.org). Briefly, brachial FMD was measured at Visit 1 in participants without uncontrolled blood pressure, arm blood pressures that were discordant by 15 mmHg, Raynaud's phenomenon, congenital abnormalities of the arms or hand, or history of radical mastectomy. The test was performed in the supine position after 15 minutes of rest and 6 hours of fasting. A blood pressure cuff was positioned on the right arm two inches below the antecubital fossa with imaging of the brachial artery 5–9 cm above the antecubital fossa. The blood pressure cuff was inflated to greater than 50 mmHg above the participants' systolic blood pressure for 5 minutes. An ultrasound transducer was used to acquire the images of the right brachial artery continuously for 30 seconds before cuff inflation and for 2 minutes before cuff deflation to assess the vasodilator response. Videotapes of the images were analyzed at the Wake Forest Cardiology Image Processing Laboratory.
Our primary outcome measure was percent FMD difference (%FMD) = [(maximum diameter−baseline diameter)/baseline diameter] × 100%. This method accounts for baseline diameter and has been used in other MESA studies.30,36,37
Reliability of the FMD measurements were evaluated by a comparison of original and blinded quality control re-reads of the ultrasounds from 40 participants. The interobserver correlation coefficients were 0.99, 0.99, and 0.93 for the baseline diameter, maximal diameter, and % FMD, respectively. In addition, intraobserver variability was measured by repeated examinations in 19 participants on 2 days, 2 weeks apart, and the correlation coefficient was 0.90, 0.90, and 0.54 respectively.36
Other covariates
Baseline covariates were assessed at Visit 1 by trained examiners using standardized questionnaires, physical exams, and laboratory testing. We considered demographic variables (age, race/ethnicity, sex, and MESA field center), menopausal variables (years since menopause, history of oophorectomy, and current use of HT), parity variables (number of live births and age at first birth), socioeconomic variables (education and income), physical activity (total of moderate plus vigorous activities in Metabolic Equivalents-min per week), smoking status (never, former, and current), diabetes status (measured by the 2003 American Diabetes Association criteria as normal, impaired fasting glucose, and treated diabetes), systolic blood pressure, use of antihypertensive medications, body mass index (BMI), estimated glomerular filtration rate (eGFR) using the CKD-EPI equation,38 total cholesterol, high-density lipoprotein (HDL) cholesterol, and the use of lipid-lowering therapy.
Statistical analysis
All analyses were stratified by sex. The inverse of the probability of selection was used in a weighted analysis to account for the sampling structure, for the nested case-cohort study design. Continuous approximately symmetric variables were expressed as mean and standard deviation, right-skewed variables were expressed as median and interquartile ranges, and categorical variables were expressed as percentages. The differences in the distributions of participant characteristics were assessed by Student's t-tests and Wilcoxon rank sum tests for approximately symmetric and right-skewed continuous variables, respectively, and χ2-tests for categorical variables.
The %FMD was modeled as a continuous outcome in linear regression models and presented as beta-coefficients (95% confidence intervals). SH levels are right-skewed and thus were log transformed. The association of each of the SH levels and the T/E2 ratio with %FMD was assessed separately. We did not find any evidence of nonlinearity of the associations of SHs with FMD; therefore, in primary analyses, SHs were assessed continuously and compared per one standard deviation (SD) of their log-transformed distribution (which are shown in Supplementary Table S1). Nonlinear associations of SH levels with %FMD were evaluated using restricted cubic splines for log-transformed SH levels with knots at the 5th, 35th, 65th, and 95th percentiles of their distributions.
We used two multivariable-adjusted linear regression models to evaluate the association of SHs with %FMD. Model 1 (limited) was adjusted for age, race/ethnicity, and MESA study center. Model 2 (our primary fully adjusted model) was additionally adjusted for socioeconomic factors (education level and income), lifestyle (smoking status and physical activity), and CVD risk factors (BMI, diabetes, systolic blood pressure, use of antihypertensive medications, eGFR, total and HDL cholesterol, and use of lipid lowering therapy). In postmenopausal women, model 2 was additionally adjusted for years since menopause and use of HT.
We performed sensitivity analysis additionally adjusting for parity in women, and performed exploratory subgroup analyses among postmenopausal women, assessing for additive interactions between SHs and %FMD with age (≥65 vs. <65 years), race/ethnicity, HT use (yes/no), and years since menopause (≥10 vs. <10 years).
Results
Among the 3075 participants, 1368 (44.5%) were women (Table 1). The mean (SD) age of study participants was 64.2 (8.9) and 61.4 (10.0) years in women and men, respectively. The race/ethnicity distribution was 33.6% white, 18.6% Chinese, 21.7% black, and 26.1% Hispanic. Of the women, 33.3% were on HT. Compared to men, women were less likely to have completed high school, have diabetes, and be current smokers, and were more likely to have lower physical activity, higher BMI, more hypertension, higher total cholesterol, and higher HDL cholesterol. As expected, SH levels differed by sex with women having lower total and free testosterone, lower T/E2 ratio, lower DHEA, and higher SHBG. Estradiol levels were low in both postmenopausal women and men. Compared to men, women also had lower baseline brachial artery diameter and higher %FMD (Table 1).
Table 1.
Baseline Characteristics of Study Participants: The Multi-Ethnic Study of Atherosclerosis Visit 1 (2000–2002)
| Baseline characteristics | Total | Women | Men | p-value |
|---|---|---|---|---|
| No. of participants | 3075 | 1368 (44.5) | 1707 (55.5) | |
| Age, years | 62.6 (9.6) | 64.2 (8.9) | 61.4 (10.0) | <0.001 |
| Race/ethnicity | ||||
| White | 1033 (33.6) | 453 (33.1) | 580 (34.0) | 0.68 |
| Chinese | 572 (18.6) | 254 (18.6) | 318 (18.6) | |
| Black | 666 (21.7) | 289 (21.1) | 377 (22.1) | |
| Hispanic | 804 (26.1) | 372 (27.2) | 432 (25.3) | |
| High school education and higher | 1914 (62.2) | 755 (55.2) | 1159 (67.9) | <0.001 |
| Smoking | <0.001 | |||
| Never | 1590 (51.7) | 856 (62.6) | 734 (43.0) | |
| Former | 1104 (35.9) | 360 (26.3) | 744 (43.6) | |
| Current | 381 (12.4) | 152 (11.1) | 229 (13.4) | |
| Moderate/vigorous physical activity total, MET-min/week | 3915 (1985, 7365) | 3435 (1740, 6307.5) | 4395 (2205, 8460) | <0.001 |
| Hormone therapy use | 455 (33.3) | 455 (33.3) | n/a | |
| Body mass index, kg/m2 | 27.7 (5.0) | 28.0 (5.7) | 27.5 (4.3) | 0.019 |
| Impaired fasting glucose/diabetes status | 0.033 | |||
| Normal | 2261 (73.6) | 1041 (76.1) | 1220 (71.6) | |
| Impaired fasting glucose | 444 (14.5) | 174 (12.7) | 270 (15.9) | |
| Treated diabetes mellitus | 366 (11.9) | 153 (11.2) | 213 (12.5) | |
| Systolic blood pressure, mmHg | 125.9 (19.8) | 126.9 (21.7) | 125.1 (18.1) | 0.012 |
| Antihypertensive medication use | 1016 (33.0) | 496 (36.3) | 520 (30.5) | <0.001 |
| Estimated GFR, 60 mL/min per 1.73 min2 | 77.3 (15.4) | 75.9 (15.4) | 78.4 (15.4) | <0.001 |
| Total cholesterol, mg/dL | 194.4 (35.4) | 200.9 (34.7) | 189.2 (35.1) | <0.001 |
| HDL cholesterol, mg/dL | 50.1 (14.5) | 56.5 (15.4) | 45.0 (11.4) | <0.001 |
| Cholesterol-lowering medication use | 510 (16.6) | 247 (18.1) | 263 (15.4) | 0.050 |
| Baseline arterial diameter, mm | 4.4 (0.8) | 3.9 (0.6) | 4.8 (0.7) | <0.001 |
| Percent FMD, % | 4.2 (2.6) | 4.5 (2.8) | 3.9 (2.4) | <0.001 |
| Total testosterone, nmol/L | 9.0 (1.0, 14.9) | 0.9 (0.6, 1.3) | 14.2 (11.5, 17.7) | <0.001 |
| Bioavailable testosterone, nmol/L | 3.4 (0.2, 5.6) | 0.2 (0.1, 0.3) | 5.3 (4.3, 6.6) | <0.001 |
| Estradiol, nmol/L | 0.07 (0.05, 0.15) | 0.1 (0.0, 0.2) | 0.11 (0.09, 0.14) | <0.001 |
| Dehydroepiandrosterone, nmol/L | 11.8 (8.3, 16.3) | 10.5 (7.0, 14.9) | 12.9 (9.4, 17.3) | <0.001 |
| Free testosterone, % | 1.7 (1.3, 2.2) | 1.3 (0.9, 1.7) | 2.0 (1.7, 2.4) | <0.001 |
| Sex hormone binding globulin, nmol/L | 45.6 (33.5, 65.2) | 59.5 (39.9, 92.3) | 40.4 (30.9, 50.9) | <0.001 |
| Testosterone estrogen ratio | 74.1 (13.8, 136.4) | 12.2 (5.2, 22.1) | 128.1 (94.1, 171.3) | <0.001 |
Values in the Table are number (percent), mean (standard deviation), or median (interquartile range).
FMD, flow-mediated dilation; GFR, glomerular filtration rate; HDL, high-density lipoprotein.
The measures of brachial reactivity in men and women by tertiles of free testosterone and SHBG are shown in Supplementary Table S2. The distribution of measures of obstetric/gynecologic history in women, such as number of live births, age at first live birth, history of oophorectomy, and age at menopause, is shown in Supplementary Table S3 by tertiles of free testosterone and SHBG.
Primary analysis
Among women, in models adjusted only for age, race/ethnicity, and study center (model 1), higher estradiol and SHBG levels were significantly associated with a greater %FMD, while higher free testosterone and T/E2 ratio were significantly associated with a smaller %FMD (Table 2). After adjusting for lifestyle and CVD risk factors (model 2), only lower free testosterone and higher SHBG were significantly associated with favorable %FMD. The fully adjusted average difference in %FMD was −0.209% (95% CI −0.402 to −0.017) for a one SD increment in log-free testosterone, and 0.215% (0.026 to 0.405) for a one SD increment in log SHBG. Results were also essentially unchanged in a sensitivity model where we additionally adjusted for parity (Supplementary Table S4). In contrast, we did not find similar associations of SHs with %FMD in men (Table 2).
Table 2.
Percent Change in Flow-Mediated Dilation Associated with a One Standard Deviation Increment in Log-Transformed Sex Hormone Levels
| Total testosterone | Estradiol | DHEA | Free testosterone | SHBG | T/E ratio | |
|---|---|---|---|---|---|---|
| Women | ||||||
| Model 1 (N = 1367) | 0.049 (−0.107, 0.206) | 0.275c(0.119, 0.431) | −0.110 (−0.268, 0.049) | −0.207b(−0.357, −0.057) | 0.208b(0.059, 0.358) | −0.189a(−0.351, −0.027) |
| Model 2 (N = 1321) | 0.113 (−0.049, 0.275) | 0.145 (−0.055, 0.345) | −0.044 (−0.208, 0.121) | −0.209a(−0.402, −0.017) | 0.215a(0.026, 0.405) | −0.009 (−0.210, 0.192) |
| Men | ||||||
| Model 1 (N = 1707) | −0.066 (−0.185, 0.053) | 0.011 (−0.099, 0.122) | −0.027 (−0.153, 0.100) | 0.032 (−0.080, 0.145) | −0.036 (−0.149, 0.077) | −0.068 (−0.188, 0.051) |
| Model 2 (N = 1647) | −0.104 (−0.235, 0.026) | 0.017 (−0.098, 0.132) | −0.028 (−0.158, 0.103) | 0.097 (−0.030, 0.223) | −0.104 (−0.231, 0.023) | −0.109 (−0.241, 0.023) |
Results presented in beta-coefficients (95% confidence interval).
Model 1 adjusted for age, race/ethnicity, and site.
Model 2 additionally adjusted for education, income, cigarette status, physical activity, systolic blood pressure, antihypertensive use, cholesterol medication use, body mass index, diabetes, total and HDL cholesterol, and estimated GFR (plus hormone therapy and years since menopause in women).
Values in bold are statistically significant (p < 0.05).
p < 0.05.
p < 0.01.
p < 0.001.
DHEA, dehydroepiandrosterone; SHBG, sex hormone binding globulin; T/E ratio, testosterone/estradiol ratio.
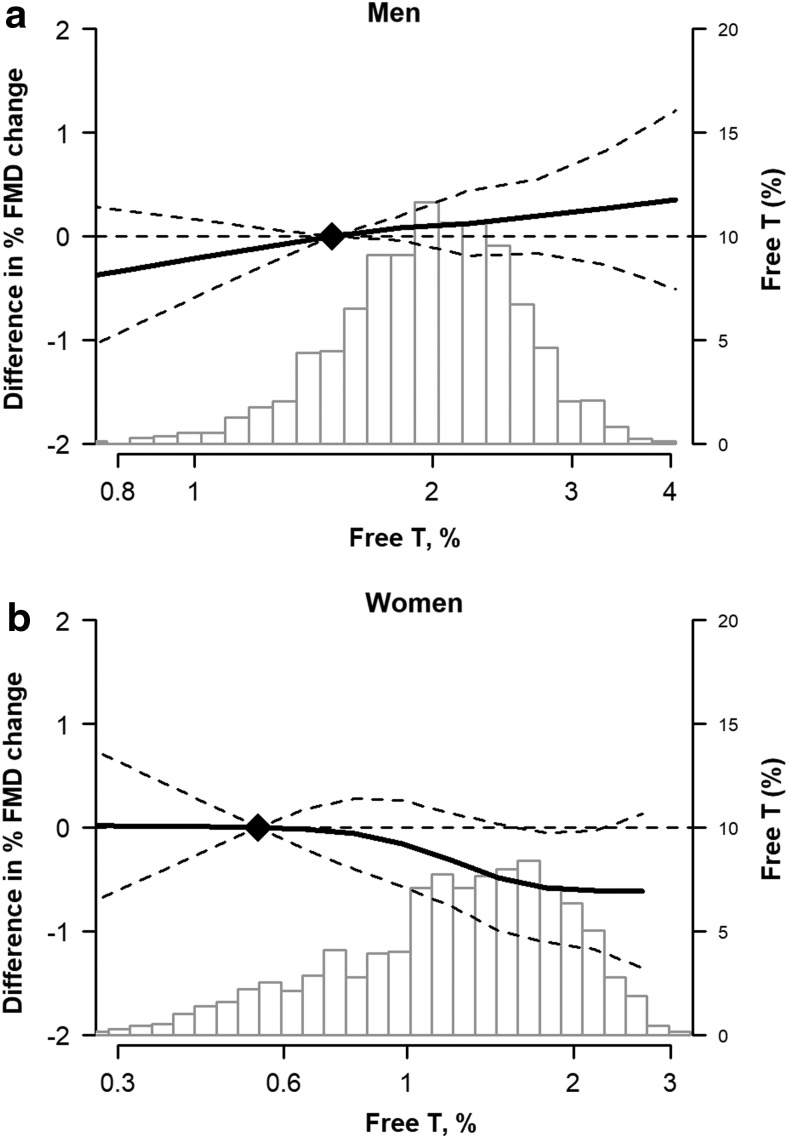
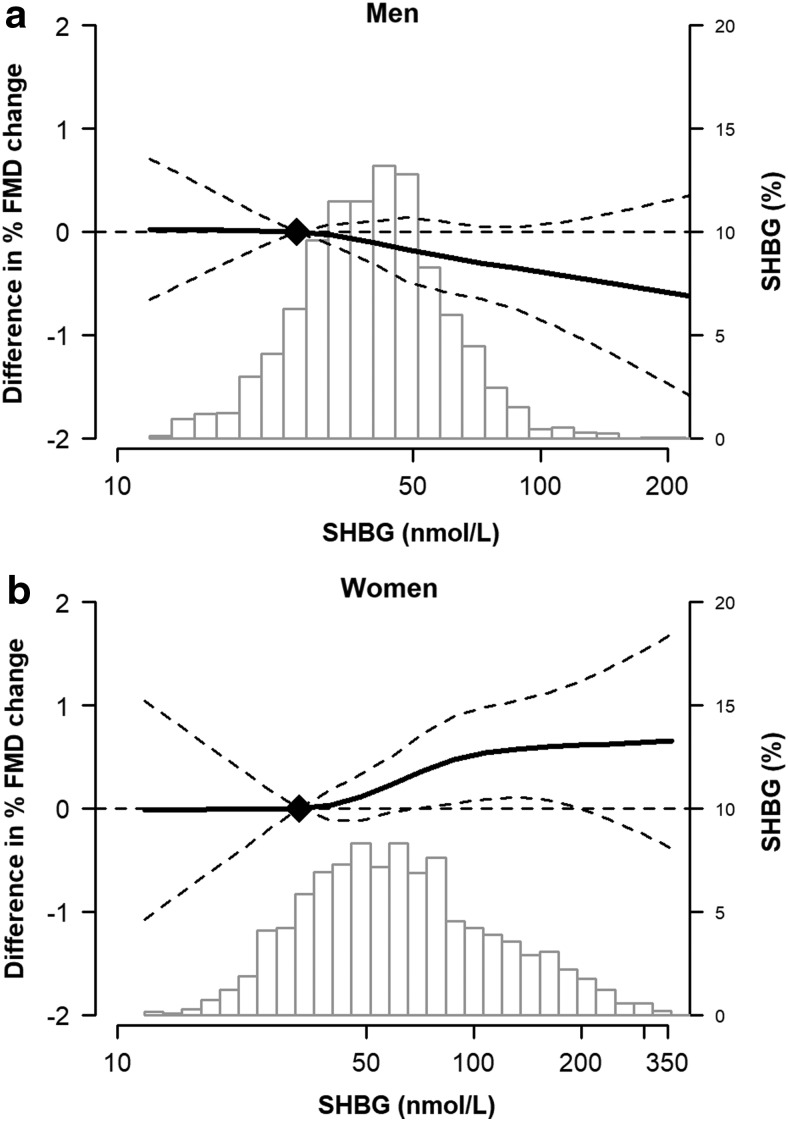
Restricted cubic spline models confirmed a generally linear inverse relationship of %FMD with higher free testosterone levels (Fig. 2) and a direct relationship with SHBG (Fig. 3) in women. An opposite pattern was seen for men, although the associations in men were not statistically significant.
FIG. 2.
Adjusted change in %FMD (and 95% confidence intervals) associated with free testosterone levels in men (a) and women (b). The histograms represent the distribution of the sex hormone in the sample. The curves represent the estimates from multivariable linear regression models of the association of %FMD by sex hormone level, adjusted for age, race/ethnicity, site, cigarette status, education, income, physical activity, systolic blood pressure, antihypertensive use, cholesterol medication use, body mass index, diabetes, total and HDL cholesterol, and estimated GFR (plus hormone therapy and years since menopause in women). The dotted lines represent the 95% CI. The knots were placed at 5th, 35th, 65th and 95th percentile of the exposures. FMD, flow mediated dilation; Free T, free testosterone; SHBG, sex hormone binding globulin.
FIG. 3.
Adjusted change in %FMD (and 95% confidence intervals) associated with SHBG levels in men (a) and women (b). The histograms represent the distribution of the sex hormone in the sample. The curves represent the estimates from multivariable linear regression models of the association of %FMD by sex hormone level, adjusted for age, race/ethnicity, site, cigarette status, education, income, physical activity, systolic blood pressure, antihypertensive use, cholesterol medication use, body mass index, diabetes, total and HDL cholesterol, and estimated GFR (plus hormone therapy and years since menopause in women). The dotted lines represent the 95% CI. The knots were placed at 5th, 35th, 65th and 95th percentile of the exposures.
Subgroup analyses among postmenopausal women
In exploratory subgroup analyses among women, we stratified by age, race/ethnicity, years since menopause, and HT use, and assessed for interactions. There were no interactions by race/ethnicity, years since menopause, or HT use (Table 3), but there were significant interactions of free testosterone and SHBG with age. In the fully adjusted model, in younger postmenopausal women (<65 years), the average difference in %FMD was −0.341% (95% CI −0.611, −0.070) for a one SD increment in log-free testosterone and 0.331% (0.067, 0.595) for a one SD increment in log SHBG, but no clear associations were seen in women ≥65 years (p-interaction for age <0.05 for both free testosterone and SHBG).
Table 3.
Percent Change in Flow-Mediated Dilation Associated with a One Standard Deviation Increment in Log-Transformed Sex Hormone Levels by Subgroup of Age, Race/Ethnicity, and Use of Hormone Therapy
| Total testosterone | Estradiol | DHEA | Free testosterone | SHBG | T/E ratio | |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≥65 years (N = 677) | 0.126 (−0.102, 0.360) | 0.232 (−0.053, 0.519) | 0.156 (−0.077, 0.390) | 0.012 (−0.277, 0.301) | −0.003 (−0.293, 0.286) | −0.049 (−0.346, 0.248) |
| <65 years (N = 691) | 0.121 (−0.098, 0.340) | 0.200 (−0.094, 0.493) | −0.104 (−0.339, 0.130) | −0.341 (−0.611, −0.070) | 0.331 (0.067, 0.595) | −0.053 (−0.334, 0.228) |
| P for interaction | 0.526 | 0.120 | 0.239 | 0.041 | 0.044 | 0.487 |
| Years since menopause | ||||||
| ≥10 years (N = 892) | 0.156 (−0.048, 0.360) | 0.242 (−0.003, 0.487) | 0.060 (−0.139, 0.258) | −0.120 (−0.370, 0.129) | 0.136 (−0.111, 0.383) | −0.034 (−0.282, 0.213) |
| <10 years (N = 476) | 0.046 (−0.236, 0.326) | −0.022 (−0.393, 0.348) | −0.191 (−0.486, 0.104) | −0.362 (−0.670, −0.054) | 0.343 (0.040, 0.646) | −0.053 (−0.309, 0.416) |
| P for interaction | 0.280 | 0.405 | 0.095 | 0.099 | 0.117 | 0.970 |
| Race/ethnicity | ||||||
| Black (N = 263) | 0.315 (−0.112, 0.742) | −0.066 (−0.469, 0.337) | −0.245 (−0.683, 0.187) | −0.034 (−0.383, 0.451) | −0.008 (−0.411, 0.395) | 0.372 (−0.112, 0.856) |
| White (N = 445) | 0.079 (−0.179, 0.336) | 0.066 (−0.302, 0.434) | 0.085 (−0.178, 0.349) | −0.164 (−0.466, 0.138) | 0.191 (−0.118, 0.500) | 0.018 (−0.316, 0.352) |
| Chinese American (N = 249) | −0.046 (−0.451, 0.360) | 0.577 (0.001, 1.153) | −0.208 (−0.556, 0.140) | −0.367 (−0.938, 0.203) | 0.330 (−0.237, 0.897) | −0.431 (−1.004, 0.141) |
| Hispanic (N = 364) | 0.261 (−0.009, 0.532) | 0.194 (−0.145, 0.533) | −0.008 (−0.301, 0.284) | −0.413 (−0.805, −0.020) | 0.381 (0.012, 0.750) | 0.077 (−0.282, 0.436) |
| P for interaction | 0.630 | 0.930 | 0.716 | 0.651 | 0.719 | 0.677 |
| Hormone therapy | ||||||
| HT use (N = 441) | 0.017 (−0.260, 0.293) | 0.113 (−0.222, 0.449) | 0.113 (−0.176, 0.402) | −0.191 (−0.474, 0.093) | 0.213 (−0.081, 0.507) | −0.067 (−0.391, 0.257) |
| No HT use (N = 913) | 0.168 (−0.022, 0.357) | 0.162 (−0.108, 0.431) | −0.135 (−0.340, 0.070) | −0.211 (−0.486, 0.064) | 0.192 (−0.066, 0.450) | 0.054 (−0.213, 0.321) |
| P for interaction | 0.623 | 0.588 | 0.047 | 0.559 | 0.705 | 0.380 |
Results presented in beta-coefficients (95% confidence interval).
Adjusted for age, race/ethnicity, site, education, income, cigarette status, physical activity, systolic blood pressure, antihypertensive use, cholesterol medication use, body mass index, diabetes, total and HDL cholesterol, estimated GFR, hormone therapy, and years since menopause in women.
Values in bold are statistically significant (p < 0.05).
p < 0.05.
p < 0.01.
p < 0.001.
Discussion
In a group of individuals without clinical CVD, a more androgenic SH profile characterized by higher free testosterone and lower SHBG was associated with worse endothelial function (as assessed by %FMD) in postmenopausal women. We did not find similar associations in men. Among women, the association between a more androgenic profile and worse endothelial function was largely restricted to younger postmenopausal women (<65 years), and it was not evident in older women.
Endothelial dysfunction assessed by %FMD predicts incident CVD risk.37 There are known age and sex differences in endothelial function.39 NO is the most potent vasodilator produced by endothelial cells, resulting in smooth muscle relaxation and arterial dilation. It is released in response to various stimuli, including acetylcholine, ischemia, and mechanical sheer stress.8 Endothelial dysfunction is marked by the loss of NO production and evidenced by reduced vasodilation in response to these stimuli such as sheer stress.9 NO is also a critical molecule that inhibits the development of atherosclerosis, plaque rupture, and thrombosis.40
The pathophysiology, presentation, and prognosis of CVD differ between men and women. Women present with CVD at older ages, have a higher prevalence of ischemia with nonobstructive coronary artery disease,41 microvascular coronary dysfunction, and plaque erosion rather than plaque rupture,42 have unique risk factors, including those related to pregnancy complications43 and premature menopause, and are affected differently by conventional risk factors when compared to men.44 These sex differences in CVD have been attributed, at least in part, to the effects of SHs.
SHs exert a wide variety of effects on myocardial cells, endothelial cells, and vascular tone directly through binding to sex steroid hormone receptors and/or interacting with other regulatory proteins.45 Estrogen reduces coronary vasoreactivity,15,46 oxidative stress,16–18 lipid oxidation,14 exercise-induced ischemia,47,48 and endothelial injury,19 and thus may be protective against atherosclerosis.49,50 In women, estrogen therapy after menopause was shown to have beneficial effects on subclinical CVD markers such as endothelial function20,51 and carotid intimal media thickness,51,52 but it did not affect the progression of atherosclerosis or reduce cardiovascular events in randomized clinical trials.6,53–56
In men, estrogen treatment may also have some benefit on endothelial function.57,58 However, while short-term administration of exogenous androgens results in vascular dilation,59 studies of long-term androgen administration have conflicting results with some studies showing benefit and others showing deleterious changes in endothelial function.60 In men with coronary disease, estrogen supplementation has not shown improvement in coronary vasoreactivity.48
Prior work in MESA has found that a more androgenic pattern of SHs in postmenopausal women was associated with a more adverse cardiovascular phenotype, including concentric left ventricular remodeling,32 aortic stiffness,33 progression of coronary artery calcification,61 and greater incidence of clinical CVD, CHD, and heart failure events.26 In contrast, in men, low testosterone levels were associated with subclinical coronary atherosclerosis.62 Thus, our results are consistent with prior findings in MESA. However, we also found a significant interaction with age among women, with an androgenic SH profile being associated with less favorable endothelial function in women <65 years of age compared to women ≥65 years of age. The reasons for this age interaction are unclear. The arteries of older individuals may be stiffer and have less capacity for further dynamic dilation following a flow stimulus. This could be related to abnormal endothelial independent vasodilation from arterial stiffness or increased sympathetic tone that occurs in late menopause, resulting in a higher vasomotor tone.63 In sum, our findings may further help understand the mechanisms behind the lack of benefit of HT in older postmenopausal women seen in the randomized clinical trials.64
Our study has many strengths including using data from a high-quality large community-based multi-ethnic cohort of men and women that has been well characterized. The large number of variables collected allowed us to adjust for multiple potentially confounding variables, including HT use. However, our findings must be considered in context of several limitations. The observational study design limits the ability to attribute causation. In MESA, both SHs and FMD were measured once at the baseline examination, and thus we were unable to assess for difference in either factor over time. Use of single measurements of SHs and FMD is also affected by higher intraindividual variability and measurement error, which may attenuate the observed associations toward the null. The %FMD is a measure of the difference in arterial diameter after provocation compared to the baseline diameter. In fact, the difference in arterial diameter was found to negatively correlate with baseline diameter, and consequently a smaller difference is noted in larger arteries.65,66 However we feel this is not a source of systematic bias by sex because in general, arteries get larger with age for both men and women.67,68 In addition, the MESA cohort enrolled very few premenopausal women, whom we excluded from our analysis due to small numbers; therefore, we were unable to assess whether the relationship of SHs with brachial reactivity differed between premenopausal vs. postmenopausal women.
Conclusion
In summary, we found that a more androgenic SH profile was inversely associated with endothelial function, as determined by %FMD, in women. We did not find similar associations in men. Among women, this adverse association was restricted to younger postmenopausal women (<65 years). Further studies are needed to assess longitudinal changes in SH levels and their association with vascular aging in both premenopausal and postmenopausal women. At this time, it does not appear that HT in postmenopausal women can mitigate CVD risk.64 Our findings, however, may provide further mechanistic insight regarding increased CVD risk after menopause. Our findings also suggest that a more androgenic profile might help identify women at higher CVD risk due to endothelial dysfunction, who might benefit from other risk-reducing interventions.
Supplementary Material
Acknowledgments
The authors thank the MESA participants, staff, and other investigators for their valuable contributions to the MESA study. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Dr. Mathews is supported by Grant Number T32 HL007024 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Drs. Michos and Zhao are supported by the Blumenthal Scholars Program in Preventive Cardiology and by the American Heart Association Go Red for Women Strategic Focused Research Network contract AHA 16SFRN27870000. This research was funded by R01 HL074406 and R01 HL074338 from the National Heart, Lung, and Blood Institute (NHLBI). The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
Author Disclosure Statement
Dr. Budoff reports receiving grant support from General Electric. The other authors report no conflicts of interest related to this work.
Supplementary Material
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J 1986;111:383–390 [DOI] [PubMed] [Google Scholar]
- 3. Muka T, Oliver-Williams C, Kunutsor S, et al. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol 2016;1:767–776 [DOI] [PubMed] [Google Scholar]
- 4. Muka T, Vargas KG, Jaspers L, et al. Estrogen receptor beta actions in the female cardiovascular system: A systematic review of animal and human studies. Maturitas 2016;86:28–43 [DOI] [PubMed] [Google Scholar]
- 5. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333 [DOI] [PubMed] [Google Scholar]
- 6. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605–613 [DOI] [PubMed] [Google Scholar]
- 7. Traish AM, Galoosian A. Androgens modulate endothelial function and endothelial progenitor cells in erectile physiology. Korean J Urol 2013;54:721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iantorno M, Weiss RG. Using advanced noninvasive imaging techniques to probe the links between regional coronary artery endothelial dysfunction and atherosclerosis. Trends Cardiovasc Med 2014;24:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vita JA. Endothelial Function. Circulation 2011;124:e906-e912 [DOI] [PubMed] [Google Scholar]
- 10. Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993;362:801–809 [DOI] [PubMed] [Google Scholar]
- 11. von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–725 [DOI] [PubMed] [Google Scholar]
- 12. Hayashi T, Yamada K, Esaki T, et al. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun 1995;214:847–855 [DOI] [PubMed] [Google Scholar]
- 13. MacRitchie AN, Jun SS, Chen Z, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 1997;81:355–362 [DOI] [PubMed] [Google Scholar]
- 14. Keaney JF, Jr., Shwaery GT, Xu A, et al. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 1994;89:2251–2259 [DOI] [PubMed] [Google Scholar]
- 15. Bell DR, Rensberger HJ, Koritnik DR, Koshy A. Estrogen pretreatment directly potentiates endothelium-dependent vasorelaxation of porcine coronary arteries. Am J Physiol 1995;268:H377–383 [DOI] [PubMed] [Google Scholar]
- 16. Florian M, Freiman A, Magder S. Treatment with 17-beta-estradiol reduces superoxide production in aorta of ovariectomized rats. Steroids 2004;69:779–787 [DOI] [PubMed] [Google Scholar]
- 17. Muehlfelder M, Arias-Loza PA, Fritzemeier KH, Pelzer T. Both estrogen receptor subtypes, ERalpha and ERbeta, prevent aldosterone-induced oxidative stress in VSMC via increased NADPH bioavailability. Biochem Biophys Res Commun 2012;423:850–856 [DOI] [PubMed] [Google Scholar]
- 18. Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch 2013;465:739–746 [DOI] [PubMed] [Google Scholar]
- 19. Rocha de Sousa PT, Breithaupt-Faloppa AC, de Jesus Correia C, et al. 17beta-Estradiol prevents mesenteric injury induced by occlusion of the proximal descending aorta in male rats. J Vasc Surg 2018;67:597–606 [DOI] [PubMed] [Google Scholar]
- 20. Hurtado R, Celani M, Geber S. Effect of short-term estrogen therapy on endothelial function: A double-blinded, randomized, controlled trial. Climacteric 2016;19:448–451 [DOI] [PubMed] [Google Scholar]
- 21. El Khoudary SR, Brooks MM, Thurston RC, Matthews KA. Lipoprotein subclasses and endogenous sex hormones in women at midlife. J Lipid Res 2014;55:1498–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaspers L, Dhana K, Muka T, et al. Sex Steroids, Sex hormone-binding globulin and cardiovascular health in men and postmenopausal women: The Rotterdam Study. J Clin Endocrinol Metab 2016;101:2844–2852 [DOI] [PubMed] [Google Scholar]
- 23. Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis 2010;20:140–146 [DOI] [PubMed] [Google Scholar]
- 24. Kim JA, Montagnani M, Chandrasekran S, Quon MJ. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin 2012;8:589–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rech CM, Clapauch R, de Souza M, Bouskela E. Low testosterone levels are associated with endothelial dysfunction in oophorectomized early postmenopausal women. Eur J Endocrinol 2016;174:297–306 [DOI] [PubMed] [Google Scholar]
- 26. Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 2018;71:2555–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosano GM, Spoletini I, Vitale C. Cardiovascular disease in women, is it different to men? The role of sex hormones. Climacteric 2017;20:125–128 [DOI] [PubMed] [Google Scholar]
- 28. Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 2007;30:1029–1034 [DOI] [PubMed] [Google Scholar]
- 29. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 30. Yeboah J, Crouse JR, Bluemke DA, et al. Endothelial dysfunction is associated with left ventricular mass (assessed using MRI) in an adult population (MESA). J Hum Hypertens 2011;25:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ying W, Zhao D, Ouyang P, et al. Sex Hormones and Change in N-Terminal Pro-B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab 2018;103:4304–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018;108:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Subramanya V, Ambale-Venkatesh B, Ohyama Y, et al. Relation of Sex Hormone Levels with Prevalent and 10-year Change in Aortic Distensibility Assessed by MRI: The Multi-Ethnic Study of Atherosclerosis. Am J Hypertens 2018; 31:774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810 [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2012;224:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009;120:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007;115:2390–2397 [DOI] [PubMed] [Google Scholar]
- 38. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skaug EA, Aspenes ST, Oldervoll L, et al. Age and gender differences of endothelial function in 4739 healthy adults: The HUNT3 Fitness Study. Eur J Prev Cardiol 2013;20:531–540 [DOI] [PubMed] [Google Scholar]
- 40. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 2015;14:623–641 [DOI] [PubMed] [Google Scholar]
- 41. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017;135:1075–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–1275 [DOI] [PubMed] [Google Scholar]
- 43. Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol 2018;41:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKibben RA, Al Rifai M, Mathews LM, Michos ED. Primary prevention of atherosclerotic cardiovascular disease in women. Curr Cardiovasc Risk Rep 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller VM, Duckles SP. Vascular actions of estrogens: Functional implications. Pharmacol Rev 2008;60:210–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosano GM, Collins P, Gerbara O, et al. Effect of estradiol 17beta upon coronary artery vasoconstrictor response to methylergometrine maleate in female menopausal patients. Int J Cardiol 2006;107:254–259 [DOI] [PubMed] [Google Scholar]
- 47. Webb CM, Rosano GM, Collins P. Oestrogen improves exercise-induced myocardial ischaemia in women. Lancet 1998;351:1556–1557 [DOI] [PubMed] [Google Scholar]
- 48. Collins P, Rosano GM, Sarrel PM, et al. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation 1995;92:24–30 [DOI] [PubMed] [Google Scholar]
- 49. Collins P, Rosano GM, Jiang C, Lindsay D, Sarrel PM, Poole-Wilson PA. Cardiovascular protection by oestrogen—a calcium antagonist effect? Lancet 1993;341:1264–1265 [DOI] [PubMed] [Google Scholar]
- 50. Nofer JR. Estrogens and atherosclerosis: Insights from animal models and cell systems. J Mol Endocrinol 2012;48:R13–29 [DOI] [PubMed] [Google Scholar]
- 51. Reis SE, Gloth ST, Blumenthal RS, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation 1994;89:52–60 [DOI] [PubMed] [Google Scholar]
- 52. Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 2000;343:522–529 [DOI] [PubMed] [Google Scholar]
- 54. Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 2002;288:2432–2440 [DOI] [PubMed] [Google Scholar]
- 55. Clarke SC, Kelleher J, Lloyd-Jones H, Slack M, Schofiel PM. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: The Papworth HRT atherosclerosis study. BJOG 2002;109:1056–1062 [DOI] [PubMed] [Google Scholar]
- 56. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297:1465–1477 [DOI] [PubMed] [Google Scholar]
- 57. New G, Timmins KL, Duffy SJ, et al. Long-term estrogen therapy improves vascular function in male to female transsexuals. J Am Coll Cardiol 1997;29:1437–1444 [DOI] [PubMed] [Google Scholar]
- 58. McCrohon JA, Walters WA, Robinson JT, et al. Arterial reactivity is enhanced in genetic males taking high dose estrogens. J Am Coll Cardiol 1997;29:1432–1436 [DOI] [PubMed] [Google Scholar]
- 59. Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1999;100:1690–1696 [DOI] [PubMed] [Google Scholar]
- 60. Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol 1997;17:2004–2009 [DOI] [PubMed] [Google Scholar]
- 61. Subramanya V, Zhao D, Ouyang P, et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr 2019;13:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khazai B, Golden SH, Colangelo LA, et al. Association of endogenous testosterone with subclinical atherosclerosis in men: The multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf) 2016;84:700–707 [DOI] [PubMed] [Google Scholar]
- 63. Huber DA, Bazilio D, Lorenzon F, et al. Cardiovascular autonomic responses in the VCD rat model of menopause: Effects of short- and long-term ovarian failure. Reprod Sci 2018;25:1093–1105 [DOI] [PubMed] [Google Scholar]
- 64. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol 2006;47:1741–1753 [DOI] [PubMed] [Google Scholar]
- 65. Atkinson G, Batterham AM. The percentage flow-mediated dilation index: A large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med 2013;18:354–365 [DOI] [PubMed] [Google Scholar]
- 66. Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA, Liu K. Chronic Stress and Endothelial Dysfunction: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens 2017;30:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am 2009;93:583–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dibble CT, Shimbo D, Barr RG, et al. Brachial artery diameter and the right ventricle: The Multi-Ethnic Study of Atherosclerosis-right ventricle study. Chest 2012;142:1399–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.