Abstract
One-compartment H2O2-photofuel cells using monoclinic scheelite BiVO4 film deposited on fluorine-doped tin oxide (ms-BiVO4/FTO) as the photoanode, Prussian blue film-coated FTO cathode, and deaerated aqueous electrolyte solution of 0.1 M NaClO4 and 0.1 M H2O2 were constructed. Mesoporous TiO2 photoanode cells with the same cathode and electrolyte solution were also prepared for comparison. The ms-BiVO4/FTO photoanode was prepared by a two-step route consisting of spin coating of a precursor solution on FTO and subsequent heating at 500 °C in the air. The thickness of the ms-BiVO4 film was controlled in the range from 50 to 500 nm by the number of the spin-coating times. There is an optimum thickness of the ms-BiVO4 film in the cell performances under illumination of simulated sunlight (AM 1.5, 100 mW cm–2, 1 sun). Under the optimum conditions, the ms-BiVO4/FTO photoanode cell provides a short-circuit current (Jsc) = 0.81 mA cm–2 and an open-circuit voltage (Voc) = 0.61 V, far surpassing the values of Jsc = 0.01 mA cm–2 and Voc = 0.31 V for the conventional mesoporous TiO2 photoanode cell. The striking cell performance is ascribable to the high visible-light activity of ms-BiVO4 for H2O2 oxidation and its low thermocatalytic activity for the decomposition.
Introduction
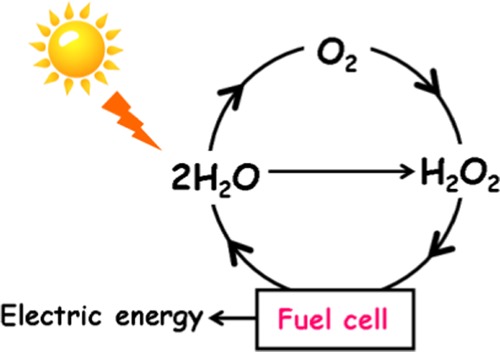
A sustainable “solar oxygen cycle” can be completed by combining the technology for synthesizing H2O2 from H2O and/or O2 and H2O2-fuel cell (FC) (Scheme 1).1 At present, most H2O2 is industrially produced by the anthraquinone method with the consumption of a large amount of energy.2 As an alternative method, photocatalytic synthesis of H2O2 via O2 reduction3−7 and H2O oxidation8 are currently in rapid progress. On the other hand, photofuel cells (PFCs) using TiO2 photoanode have recently been developed as a chemical-to-electric energy conversion device.9,10 The PFCs have attracted considerable interest because of the availability of biomass derivatives as the fuel, but the operation emits CO2.11,12 Meanwhile, clean one-compartment FCs can be constructed without the expensive separator by only using H2O2 as the fuel and oxidant.13 We have recently reported a prototype of one-compartment H2O2-PFC consisting of mesoporous TiO2 film coated on fluorine-doped tin oxide (mp-TiO2/FTO, photoanode), glassy carbon (cathode), and an aqueous electrolyte solution of H2O2 (pH 3).14 In this cell, H2O2 is oxidized to O2 on the mp-TiO2/FTO photoanode (eq 1), whereas H2O2 is reduced to H2O at the cathode (eq 2).
| 1 |
| 2 |
where the electrode potentials are values with respect to the standard hydrogen electrode (SHE).
Scheme 1. Solar Oxygen Cycle with H2O2 as the Key Component.
The H2O2-FC and H2O2-PFC afford a thermodynamic electromotive force (=Ec – Ea) of 1.07 V comparable with the value for the H2/O2-FC (1.23 V). In addition, the H2O2-PFC has various advantages over the H2/O2-FC, i.e., it can be easily handled at ambient temperature and pressure and does not need separator and electrocatalysts such as Pt. The most serious drawback common with these PFCs is that TiO2 only responds to UV-light occupying a few percent of the solar spectrum.15 The photoanode material for the H2O2-PFC should possess the following properties: (1) high visible-light activity for H2O2 oxidation, (2) low thermocatalytic activity for H2O2 decomposition, (3) high stability, and (4) nontoxicity. In the biomass-PFC, the cell performances have been improved by using a visible-light-responsive TiO2 photoanode.16,17 The simultaneous fulfillment of conditions (1) and (2) is a difficult subject because H2O2 undergoes catalytic decomposition by various materials. Among the visible-light-responsive semiconductor photocatalysts, bismuth vanadate (BiVO4) is a promising material because it is known to have a high level of activity for H2O oxidation18 as well as high stability (condition 3) and nontoxicity (condition 4),19 whereas Prussian blue (PB) can be used as the cathode material effectively improving the cell performances of H2O2-FC20 and H2O2-PFC.21 Here, we report a visible-light-driven one-compartment H2O2-PFC using BiVO4/FTO photoanode and PB/FTO cathode and the performances under simulated sunlight.
Results and Discussion
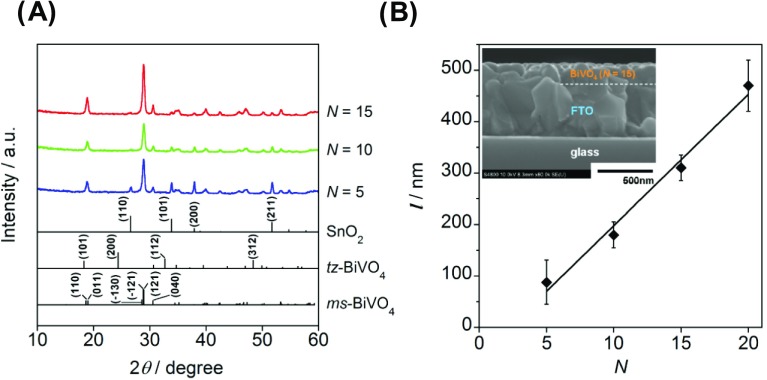
BiVO4 is an n-type semiconductor with three crystal polymorphs, i.e., monoclinic scheelite (ms), tetragonal scheelite (ts), and tetragonal zircon (tz) structures. Among them, ms-BiVO4 was reported to show the highest photocatalytic activity due to its narrower band gap energy (Eg) of 2.4 eV against tz-BiVO4 with Eg = 2.9 eV22 and the more significant distortion of the metal polyhedra than ts-BiVO4.23 The crystal form of the present BiVO4 film was determined by X-ray diffraction (XRD) measurements. Figure 1A shows the XRD patterns for the samples prepared at various spin-coating cycles (N). In the XRD patterns, sharp diffraction peaks are present at 2θ = 18.9, 28.9, and 30.6°. The first peak is derived from the overlapping of the diffraction peaks assignable to the (110) and (011) crystal planes of ms-BiVO4, the second peak to the (1̅30), (1̅21), and (121) planes, and the third peak to the (040) plane (ICDD 00-014-0688). Also, the diffraction peaks observed at 26.6, 33.9, 37.9, and 51.8° are assignable to the diffraction from the (110), (101), (200), and (211) crystal planes of SnO2 (ICDD 01-070-6995), respectively. Clearly, films consisting of single-phase ms-BiVO4 are formed on FTO by the spin-coating technique. As shown in the inset in Figure 1B, the scanning electron microscopy (SEM) image confirms the formation of uniform ms-BiVO4 layer on the FTO surface. Figure 1B shows the relation between the thickness of ms-BiVO4 (l) and N. The l increases linearly with respect to N according to the following equation of l (nm) = 25.5N – 57.0 at N ≥ 5. In Figure 1A, the intensity of the diffraction peaks of SnO2 monotonically decreases with an increase in N or l.
Figure 1.
(A) XRD patterns of BiVO4/FTO electrodes prepared at various spin-coating cycles. (B) Plots of the thickness of BiVO4 film (l) vs the number of spin-coating cycle (N). The inset is the cross-sectional SEM image of the BiVO4/FTO electrode.
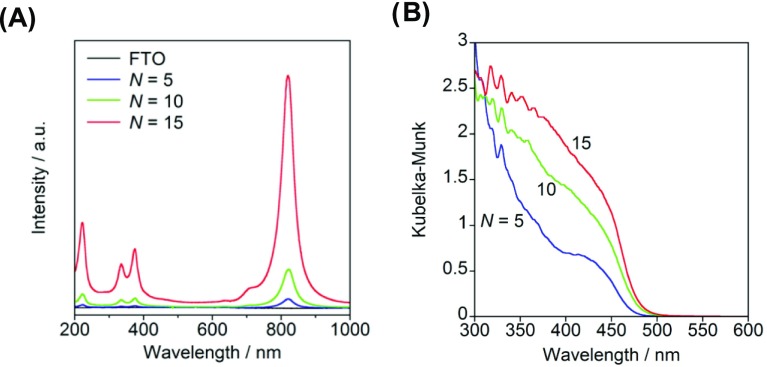
Raman spectra were recorded to gain information about the bulk and local structures of the ms-BiVO4 film. Figure 2A shows the Raman spectra for ms-BiVO4/FTO with varying N. Every spectrum has several signals that increase in intensity with an increase in N. In the spectrum of the sample (N = 15), the signals at 375 and 335 cm–1 are due to the symmetric and antisymmetric bending modes of VO43– anion (δs(VO43–) and δas(VO43–)), respectively. Also, the intense signal at 822 cm–1 and the weak signal around 710 cm–1 are assignable to the symmetric and antisymmetric stretching vibrations of the VO43– anion (νs(V–O) and νas(V–O)), respectively.24 The emergence of the forbidden νas(V–O) band can be induced by the distortion of the local symmetry of VO43– tetrahedron in ms-BiVO4. Yu and Kudo reported that the local structure as well as crystallinity affects the photocatalytic activity of the O2 evolution from an AgNO3 aqueous solution.25 The absence of the νas(V–O) signal in the spectrum of the sample (N = 5) is probably due to the small film thickness of ∼70 nm.26Figure 2B shows the UV–vis absorption spectra of ms-BiVO4/FTO with varying N. Every sample has a strong absorption due to the interband transition at λ < 500 nm, whose intensity increases with increase in N. Density functional theory calculations for the ms-BiVO4 crystal indicated that the valence band (VB) maximum and conduction band (CB) minimum mainly consist of the nonbonding O 2p and nonbonding V 3d states, and the interband transition is allowed along the polarization direction of E//a and E//c.27 The direct band gap (Eg) was determined from the [F(R∞)hν]2 vs (hν – Eg) plot, where hν is the photon energy.28 The Eg lies in the range from 2.6 to 2.7 eV, and there is a trend that it increases with decreasing N or l. The Eg values are somewhat larger than the value of 2.45 ± 0.05 eV reported for ms-BiVO4 particles at room temperature (Figure S1).29
Figure 2.
Raman spectra (A) and UV–vis absorption spectra (B) of BiVO4/FTO electrode prepared at various spin-coating cycle. The inset shows the Tauc plots for the same samples.
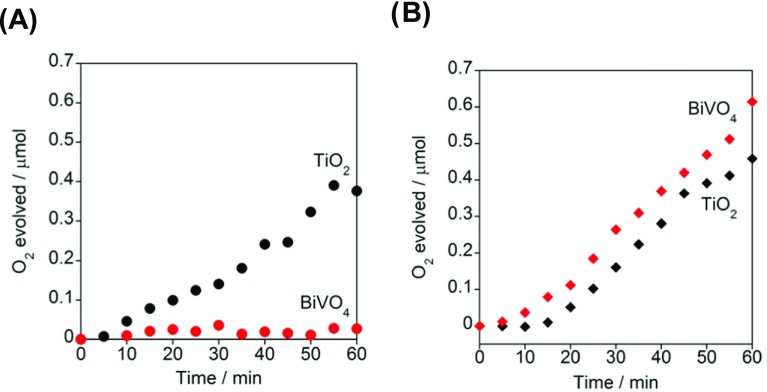
The thermocatalytic and photocatalytic activities of TiO2 and ms-BiVO4 particles for H2O2 decomposition were examined in a deaerated 0.1 M H2O2 aqueous solution (pH 3). The rates of H2O2 decomposition in the dark and under irradiation are denoted as vdark and vph, respectively, below. Figure 3A compares the time courses of O2 evolution from 0.1 M H2O2 aqueous solution in the TiO2 and ms-BiVO4 systems in the dark. In the TiO2 system, the amount of O2 increases with a vdark of 0.36 μmol h–1, whereas the decomposition is very sluggish in the ms-BiVO4 system (vdark < 0.03 μmol h–1). Clearly, ms-BiVO4 is almost inert for H2O2 decomposition in the dark, whereas TiO2 has a significant thermocatalytic activity.30Figure 3B shows time courses for O2 evolution from 0.1 M H2O2 aqueous solution in the TiO2 and ms-BiVO4 systems under simulated sunlight (AM 1.5, 19 mW cm–2). Irradiation of ms-BiVO4 causes H2O2 decomposition with a vph of 0.45 μmol h–1. In the TiO2 system, O2 is produced with a vph of 0.42 μmol h–1. The vph/vdark ratio for ms-BiVO4 reaches 16.7, which is larger than the value for TiO2 by a factor of 14. The high level of visible-light activity (condition 1) and low thermocatalytic activity (condition 2) of ms-BiVO4 for the H2O2 decomposition lead us to expect its application as the photoanode material for the H2O2-PFC. In the absence of H2O2, O2 is hardly generated in the ms-BiVO4 system (Figure S2). Previously, the flatband potential of ms-BiVO4 or the approximate conduction band (CB) minimum potential (ECB) was determined to be −0.064 V at pH 3 (SHE) by the slurry method.31 By using this value and the Eg of 2.60 eV, the valence band (VB) maximum potential (EVB) can be estimated to be +2.54 V at pH 3 (vs SHE). Thus, the potential of the excited electrons in the CB of ms-BiVO4 is insufficient for H2O reduction (electrode potential, E(H2O/H2) = −0.177 V vs SHE at pH 3),32 whereas the VB-holes can oxidize H2O oxidation (E(O2/H2O) = +1.05 V vs SHE at pH 3).32
Figure 3.
Time courses for O2 evolution with the decomposition of H2O2 in the presence of TiO2 particles (10 mg) or BiVO4 particles (10 mg) in the dark (A) and under illumination of simulated sunlight (B, AM 1.5, 19 mW cm–2).
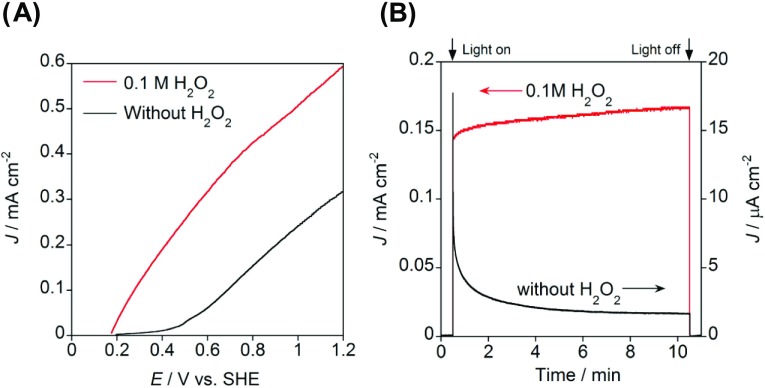
To study the H2O2 additive effect on the photoelectrochemical (PEC) properties of ms-BiVO4/FTO, three-electrode PEC cells with a structure of ms-BiVO4/FTO (N = 5, working electrode)|deaerated aqueous electrolyte solution containing 0.1 M NaClO4 (pH 3)|Ag/AgCl (reference electrode)|glassy carbon (counter electrode) were fabricated, and the photocurrent (J)–electrode potential (E) curves were measured for the cells under the illumination of simulated solar light (AM 1.5, 100 mW cm–2, 1 sun). Figure 4A compares the J–E curves of the ms-BiVO4/FTO electrode in the electrolyte solutions with (red) and without 0.1 M H2O2 (black). In the H2O2-free cell, the photocurrent starts to flow around +0.4 V vs SHE. In the H2O2-cell, the photocurrent sharply increases at the onset potential of +0.2 V vs SHE. Figure 4B shows the photochronoamperometry curves for the PEC cells. In the H2O2-free cell, a sharp decay in the photocurrent is observed immediately after photoirradiation. This feature indicates that significant surface recombination occurs via surface peroxo species generated during the H2O oxidation by the VB-holes. On the other hand, the initial anodic photocurrent spike and its subsequent decay disappear in the H2O2-cell, providing a very stable photocurrent. Thus, H2O2 works as an excellent scavenger for the VB-holes in ms-BiVO4, effectively suppressing the surface recombination.33
Figure 4.
(A) Current (J)–electrode potential (E) curves for the three-electrode cell with the structure of BiVO4/FTO photoanode (N = 5)|deaerated electrolyte solution of 0.1 M NaClO4 without (black) and with (red) 0.1 M H2O2 (pH 3)|Ag/AgCl reference electrode|glassy carbon counter electrode under the illumination of simulated solar light (AM 1.5, 100 mW cm–2, 1 sun). (B) Photochronoamperometry curves at the rest potential for the three-electrode cell with the same cell configuration as in (A).
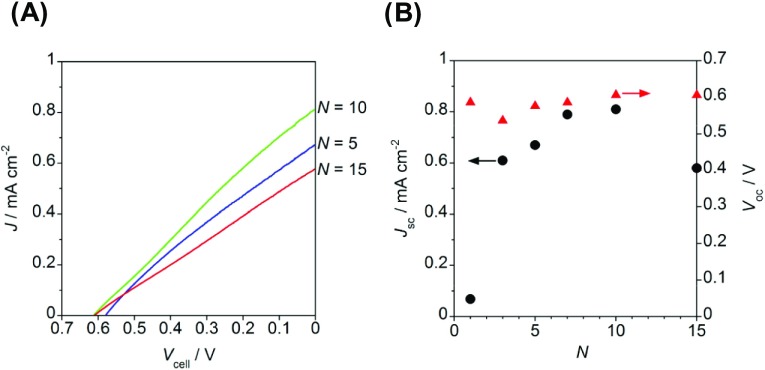
The performance of one-compartment H2O2-PFCs were examined for the cell with a structure of ms-BiVO4/FTO photoanode|deaerated aqueous electrolyte solution containing 0.1 M NaClO4 and 0.1 M H2O2 (pH 3)|PB/FTO cathode under irradiation of simulated sunlight (AM 1.5, 100 mW cm–2, 1 sun). Figure 5A exhibits the photocurrent (J)–cell voltage (Vcell) curves for the H2O2-PFCs using ms-BiVO4/FTO photoanodes prepared at varying N. The J–Vcell curves are highly dependent on N. Figure 5B shows the N-dependences of the short-circuit current (Jsc) and the open-circuit voltages (Voc). The numerical data are also summarized in Table 1. The Jsc–N curve exhibits a volcano shape, whereas Voc is almost independent of N at ≥ 1. The ms-BiVO4/FTO (N = 10) photoanode cell provides Jsc = 0.81 mA cm–2 and Voc = 0.61 V much greater than the values for the mp-TiO2/FTO photoanode cell (Jsc = 0.01 mA cm–2 and Voc = 0.31 V) under the same irradiation conditions. Clearly, there exists an optimal thickness of ms-BiVO4. These experiments were carried out in deaerated electrolyte solution to prove that H2O2 works both as a fuel and an oxidant in this cell. However, the presence of O2 was confirmed to hardly affect the cell performances (Figure S3).
Figure 5.
(A) J–Vcell curves for the two-electrode cell with the structure of BiVO4/FTO photoanode|deaerated electrolyte solution of 0.1 M NaClO4 with 0.1 M H2O2 (pH 3)|PB/FTO cathode under the illumination of simulated solar light (AM 1.5, 100 mW cm–2, 1 sun). (B) Jsc and Voc of the H2O2-PFCs as functions of N.
Table 1. Cell Parameters of the H2O2-PFCs under Illumination of Simulated Sunlight (AM 1.5, 100 mW cm–2, 1 sun).
| photoanode | N | Voc (V) | Jsc (mA cm–2) | Jmax (mA cm–2) | Pmax (mW cm–2) |
|---|---|---|---|---|---|
| ms-BiVO4 | 1 | 0.59 | 0.068 | 0.034 | 0.0092 |
| ms-BiVO4 | 3 | 0.54 | 0.61 | 0.26 | 0.075 |
| ms-BiVO4 | 5 | 0.58 | 0.67 | 0.37 | 0.11 |
| ms-BiVO4 | 7 | 0.59 | 0.79 | 0.44 | 0.13 |
| ms-BiVO4 | 10 | 0.61 | 0.81 | 0.46 | 0.13 |
| ms-BiVO4 | 15 | 0.61 | 0.58 | 0.29 | 0.09 |
| mp-TiO2 | a | 0.31 | 0.01 | 0.007 | 0.0015 |
The thickness of mp-TiO2 film was ∼4 μm.
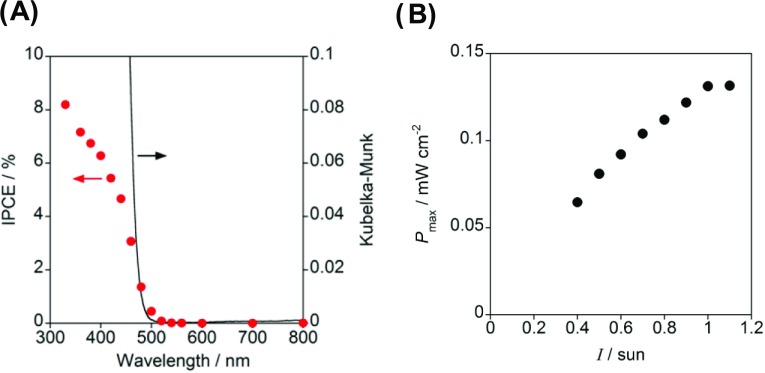
The photocurrent was measured under irradiation of monochromatic light whose wavelength (λex) continuously varied. The incident photon-to-current efficiency (IPCE) was calculated by the equation IPCE = JphNAhc/IFλ, where Jph is the photocurrent, NA is Avogadro constant, h is Planck constant, c is the speed of light, I is the light intensity, F is Faraday constant, and λ is the wavelength of the light. Figure 6A shows the IPCE action spectrum of the H2O2-PFC. The photocurrent rises at λex = 500 nm, which is in agreement with the absorption edge of the ms-BiVO4/FTO electrode. Also, the IPCE action spectrum well traces the absorption spectrum of the ms-BiVO4/FTO electrode in Figure 2B (Figure S4). Further, the IPCE value at λex = 400 nm reaches 6% at λex = 400 nm, which is as much as 60 times greater than the value of the prototype of H2O2-PFC using the mp-TiO2/FTO photoanode.14 Finally, the effect of light intensity (I) on the cell performance was studied for the one-compartment H2O2-PFC. Figure 6B shows the maximum power density (Pmax) as a function of I. The Pmax increases with an increase in I to reach a saturated value of 0.13 mW cm–2 at I > 1 sun (100 mW cm–2).
Figure 6.
(A) IPCE action spectrum for the two-electrode cell with the structure of BiVO4/FTO photoanode (N = 10)|deaerated electrolyte solution of 0.1 M NaClO4 with 0.1 M H2O2 (pH 3)|PB/FTO cathode under the illumination of simulated solar light (AM 1.5, 100 mW cm–2, 1 sun). (B) Maximum power density (Pmax) as a function of light intensity (I) for the H2O2-PFC.
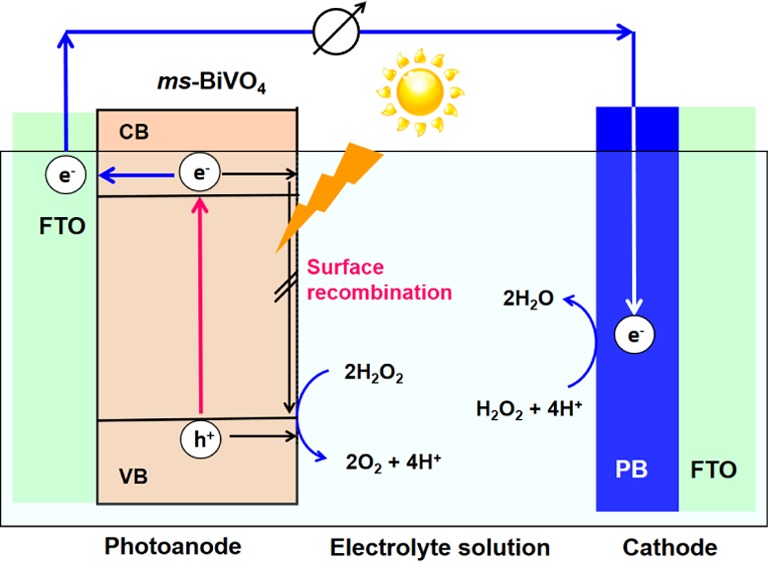
The present H2O2-PFC using ms-BiVO4/FTO photoanode is considered to operate via the mechanism explained as follows (Scheme 2). Under irradiation of simulated sunlight, the ms-BiVO4/FTO photoanode absorbs light at λ < 500 nm and the electrons in the VB are excited to the CB. The VB-holes (EVB = +2.44 V vs SHE at pH 3) can oxidize H2O2 to produce O2 with E(O2/H2O2) = +0.518 V vs SHE at pH 3 (eq 1).32 Importantly, the surface recombination of the unmodified BiVO4 photoanode can be effectively suppressed in the oxidation of H2O2, whereas it undergoes significant surface recombination in the H2O oxidation.33 Simultaneously, the CB-electrons (ECB = −0.064 V at pH 3 vs SHE) are transported to the FTO electrode, and further to the PB/FTO cathode through the external circuit, taking part in the reduction of H2O2 to H2O with E(H2O2/H2O) = +1.586 V (vs SHE at pH 3)32 (eq 2). According to this scheme, the theoretical Voc,theo can be determined to be 1.65 V from eq 3.34 As the film thickness of ms-BiVO4 increases, the light absorption is enhanced, whereas the probability of the electrons to reach the the electron-collecting FTO electrode decreases due to the recombination in the bulk. Consequently, the balance between them would determine an optimum thickness of the ms-BiVO4 film.
| 3 |
The practical power-generating efficiency of FCs (εFC) is expressed by eq 4.14
| 4 |
where εtheo is the theoretical power-generating efficiency and εv and εc are the voltage efficiency and the current efficiency, respectively.
Scheme 2. Action Mechanism Proposed for the Solar-Driven H2O2-PFC Using ms-BiVO4/FTO as the Photoanode.
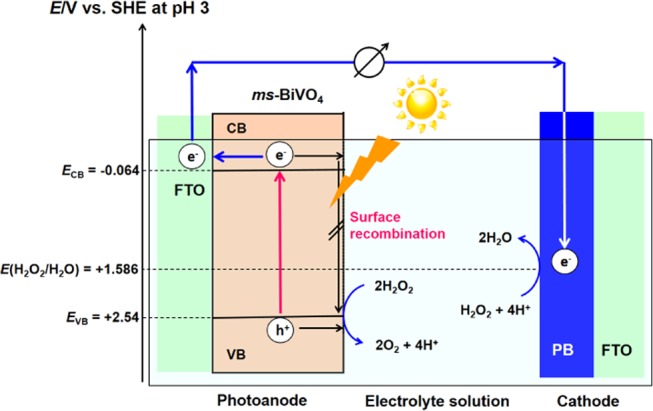
The ECB of ms-BiVO4 was cited from ref (31), and the EVB was estimated using the ECB value and the Eg of 2.60 eV.
Because the εtheo is 119%14 and the voltage efficiency (εv) is given by 0.571 (=0.61/1.068 V) for the H2O2-PFC with N = 10, the maximum εFC can be estimated to be 68% by assuming the current efficiency to be 100% in this H2O2-PFC. However, the Voc of the present cell (∼0.6 V) is much smaller than the Voc,theo value (1.65 V) for which the overpotential for H2O2 reduction at the cathode is partly responsible. Also, van de Krol and co-workers have shown that the IPCE of the PEC cell for water splitting with BiVO4/FTO as the photoanode can be drastically boosted by doping W6+ ions into BiVO4 and intervening a SnO2 layer between BiVO4 and FTO.35 Thus, there is still plenty of room for improvement in the present H2O2-PFC performances by exploring cathode materials and enhancing the electron transport in the ms-BiVO4 film and charge collection at the interface with the back-contact.
Conclusions
This study has shown that monoclinic scheelite (ms)-BiVO4 possesses the basic properties required for the photoanode of H2O2-PFC. A solar-driven one-compartment H2O2-PFC using ms-BiVO4 as the photoanode and Prussian blue as the cathode provides Jsc = 0.81 mW cm–2 and Voc = 0.61 V under illumination of simulated solar light (AM 1.5, 1 sun), and the incident photon-to-current efficiency at the excitation wavelength of 400 nm exceeds 6%. We anticipate that the performance can be further improved by reducing the losses during the electron transport in the photoanode and at the interface between the back-contact.
Experimental Section
Synthesis of ms-BiVO4 Particles
The BiVO4 particles were synthesized by the method reported in the literature.36 Bi(NO3)3·5H2O, 0.2 M, and 0.2 M NH4VO3 were completely dissolved in dilute nitric acid (500 mL, volume ratio: concn HNO3/H2O = 1:4) by gentle stirring at room temperature for 1 h. After the addition of 1.66 M urea to the solution, the mixed solution was heated at 80 °C for 8 h. Precursor particles thus prepared were harvested from the solution by centrifugal separation and then repetitively washed with purified water and vacuum dried at room temperature. The samples were then heated at 400 °C under an air atmosphere for 1 h to form the BiVO4 particles.
Preparation and Characterization of Electrodes
BiVO4/FTO electrodes were prepared by the metal–organic decomposition method.37 Mixed solution of acetic acid (15 mL) containing 0.6 mol/L Bi(NO3)3·5H2O and acetylacetone (75 mL) containing 0.04 M VO(acac)2 was spin-coated on fluorine-doped tin oxide (FTO) film-coated glass substrate (20 × 20 mm2, <10 Ω/sq) at a rotation speed of 500 rpm for 30 s at room temperature. After this process was repeatedly conducted for N cycles, the as-obtained films were heated in air at 673 K for 4h to form BiVO4/FTO. The film thickness of BiVO4/FTO was determined by cross-sectional SEM observation of the photoanode with Hitachi S-4800 Type II. The PB films were electrochemically deposited on FTO (PB/FTO) from an aqueous mixed solution of 0.02 M FeCl3·6H2O and 0.02 M K3[Fe(CN)6] according to the method described in the literature.38 Electrodeposition of the PB films on FTO was conducted by flowing a constant current of −40 μA cm–2 for 10 min. The film thickness (lPB) was estimated to be 0.94 μm. The X-ray diffraction (XRD) analysis was carried out with a Rigaku SmartLab X-ray diffractometer. Diffuse reflectance UV–vis spectra of the photoanodes were recorded on a UV-2600 spectrometer (Shimadzu) with integrating sphere unit (Shimadzu, ISR-2600Plus) at room temperature. The reflectance (R∞) of the photoanodes was measured with respect to a standard sample (BaSO4), and the Kubelka–Munk function (F(R∞)) defined by the equation of F(R∞) = (1 – R∞)2/2R∞ was plotted against the wavelength. The Raman spectroscopy was carried out with a JASCO NRS-1000 laser Raman spectrometer at room temperature. Green laser with an emission wavelength of 532 nm was used as an excitation source.
Photocatalytic Decomposition of H2O2
Deaerated aqueous solutions of 0.1 M H2O2 solutions (10 mL, pH 3) containing BiVO4 powder (10 mg, specific surface area = 0.673 m2 g–1) were irradiated by simulated sunlight without (AM 1.5, 19 mW cm–2) and with an optical cutoff filter (λ > 430 nm, AM 1.5, 15 mW cm–2) at 25 °C. The amount of O2 was determined by gas chromatography (Shimadzu, GC-8A).14 For comparison, the same experiments were carried out using anatase TiO2 particles (10 mg, specific surface area = 309 m2 g–1, ST-01, Ishihara Sangyo).
Evaluation of Photofuel Cell Performances
The photoelectrochemical (PEC) response of the photoanodes incorporated in three-electrode PEC cells was evaluated by the measurements of current (J)–electrode potential (E) curves and decay curves of photocurrent at the rest potential under the irradiation of simulated solar light (AM 1.5, 100 mW cm–2, 1 sun). Current (J)–cell voltage (Vcell) curves and power density–J curves were measured to determine the cell performance of two-electrode H2O2 PFCs. The action spectra of the incident photon-to-current conversion efficiency (IPCE) were measured by the same method as recently reported.14
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research (C) No. 15K05654, and MEXT-Supported Program for the Strategic Research Foundation at Private Universities.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01333.
Tauc plots for ms-BiVO4/FTO (Figure S1); time courses for O2 generation from water in the ms-BiVO4 particles under illumination of simulated sunlight (Figure S2); influence of O2 on the cell performances (Figure S3); IPCE action spectrum (Figure S4) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Teranishi M.; Naya S.; Tada H. Temperature- and pH-Dependences of Hydrogen Peroxide Formation from Molecular Oxygen by Gold Nanoparticle-Loaded Titanium (IV) Oxide Photocatalyst. J. Phys. Chem. C 2016, 120, 1083–1088. 10.1021/acs.jpcc.5b10626. [DOI] [Google Scholar]
- Reidl H.-J.; Pfleiderer G.. Production of Hydrogen Peroxide. U.S. Patent US2215883A1940.
- Teranishi M.; Naya S.; Tada H. In Situ Liquid-Phase Synthesis of Hydrogen Peroxide from Molecular Oxygen Using Gold Nanoparticle-Loaded Titanium (IV) Dioxide Photocatalyst. J. Am. Chem. Soc. 2010, 132, 7850–7851. 10.1021/ja102651g. [DOI] [PubMed] [Google Scholar]
- Tsukamoto D.; Shiro A.; Shiraishi Y.; Sugano Y.; Ichikawa S.; Tanaka S.; Hirai T. Photocatalytic H2O2 Production from Ethanol/O2 System Using TiO2 Loaded with Au–Ag Bimetallic Alloy Nanoparticles. ACS Catal. 2012, 2, 599–603. 10.1021/cs2006873. [DOI] [Google Scholar]
- Moon G.-H.; Kim W.; Bokare A. D.; Sung N.-E.; Choi W. Solar Production of H2O2 on Reduced Graphene Oxide–TiO2 Hybrid Photocatalysts Consisting of Earth-Abundant Elements Only. Energy Environ. Sci. 2014, 7, 4023–4028. 10.1039/C4EE02757D. [DOI] [Google Scholar]
- Shiraishi Y.; Kanazawa S.; Sugano; Tsukamoto D.; Sakamoto H.; Ichikawa S.; Hirai T. Highly Selective Production of Hydrogen Peroxide on Graphitic Carbon Nitride (g-C3N4) Photocatalyst Activated by Visible Light. ACS Catal. 2014, 4, 774–780. 10.1021/cs401208c. [DOI] [Google Scholar]
- Kaynan N.; Berke B. A.; Hazut O.; Yerushalmi R. Sustainable Photocatalytic Production of Hydrogen Peroxide from Water and Molecular Oxygen. J. Mater. Chem. A 2014, 2, 13822–13826. 10.1039/C4TA03004D. [DOI] [Google Scholar]
- Fuku K.; Sayama K. Efficient Oxidative Hydrogen Peroxide Production and Accumulation in Photoelectrochemical Water Splitting Using a Tungsten Trioxide/Bismuth Vanadate Photoanode. Chem. Commun. 2016, 52, 5406–5409. 10.1039/C6CC01605G. [DOI] [PubMed] [Google Scholar]
- Drew K.; Girishkumar G.; Vinodgopal K.; Kamat P. V. Boosting Fuel Cell Performance with a Semiconductor Photocatalyst: TiO2/Pt–Ru Hybrid Catalyst for Methanol Oxidation. J. Phys. Chem. B 2005, 109, 11851–11857. 10.1021/jp051073d. [DOI] [PubMed] [Google Scholar]
- Kaneko M.; Nemoto J.; Ueno H.; Gokan N.; Ohnuki K.; Horikawa M.; Saito R.; Shibata T. Photoelectrochemical Reaction of Biomass and Bio-related Compounds with Nanoporous TiO2 Film Photoanode and O2-Reducing Cathode. Electrochem. Commun. 2006, 8, 336–340. 10.1016/j.elecom.2005.12.004. [DOI] [Google Scholar]
- Horiuchi Y.; Toyao T.; Takeuchi M.; Matsuoka M.; Anpo M. Recent Advances in Visible-Light-Responsive Photocatalysts for Hydrogen Production and Solar Energy Conversion—from Semiconducting TiO2 to MOF/PCP Photocatalysts. Phys. Chem. Chem. Phys. 2013, 15, 13243–13253. 10.1039/c3cp51427g. [DOI] [PubMed] [Google Scholar]
- Lianos P. Review of Recent Trends in Photoelectrocatalytic Conversion of Solar Energy to Electricity and Hydrogen. Appl. Catal., B 2017, 210, 235–254. 10.1016/j.apcatb.2017.03.067. [DOI] [Google Scholar]
- Mase K.; Yoneda M.; Yamada Y.; Fukuzumi S. Seawater Usable for Production and Consumption of Hydrogen Peroxide as a Solar Fuel. Nat. Commun. 2016, 7, 11470 10.1038/ncomms11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K.; Akita A.; Kawano S.; Fujishima M.; Tada H. Hydrogen Peroxide-Photofuel Cell using TiO2 Photoanode. Electrochem. Commun. 2017, 84, 71–74. 10.1016/j.elecom.2017.10.008. [DOI] [Google Scholar]
- Fujishima A.; Zhang X.; Tryk D. A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. 10.1016/j.surfrep.2008.10.001. [DOI] [Google Scholar]
- Iyatani K.; Horiuchi Y.; Moriyasu M.; Fukumoto S.; Cho S.-H.; Takeuchi M.; Matsuoka M.; Anpo M. Development of Separate-Type Pt-Free Photofuel Cells on Visible-Light Responsive TiO2 Photoanode. J. Mater. Chem. 2012, 22, 10460–10463. 10.1039/c2jm32064a. [DOI] [Google Scholar]
- Iyatani K.; Horiuchi Y.; Fukumoto S.; Takeuchi M.; Anpo M.; Matsuoka M. Separate-Type Pt-Free Photofuel Cell Based on a Visible-Responsive TiO2 Photoanode: Effect of Hydrofluoric Acid Treatment of the Photoanode. Appl. Catal., A 2013, 458, 162–168. 10.1016/j.apcata.2013.03.039. [DOI] [Google Scholar]
- Park Y.; McDonald K. J.; Choi K.-S. Progress in Bismuth Vanadate Photoanodes for Use in Solar Water Oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. 10.1039/C2CS35260E. [DOI] [PubMed] [Google Scholar]
- Wood P.; Glasser F. P. Preparation and Properties of Pigmentary Grade BiVO4 Precipitated from Aqueous Solution. Ceram. Int. 2004, 30, 875–882. 10.1016/j.ceramint.2003.10.008. [DOI] [Google Scholar]
- Shaegh S. A. M.; Nguyen N.-T.; Ehteshami S. M. M.; Chan S. H. A Membraneless Hydrogen Peroxide Fuel Cell Using Prussian Blue as Cathode Material. Energy Environ. Sci. 2012, 5, 8225–8228. 10.1039/c2ee21806b. [DOI] [Google Scholar]
- Akita A.; Masuda T.; Fujiwara K.; Fujishima M.; Tada H. One-Compartment Hydrogen Peroxide-Photofuel Cell Using TiO2 Photoanode and Prussian Blue Cathode. J. Electrochem. Soc. 2018, 165, F300–F304. 10.1149/2.0591805jes. [DOI] [Google Scholar]
- Kudo A.; Omori K.; Kato H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. 10.1021/ja992541y. [DOI] [Google Scholar]
- Tokunaga S.; Kato H.; Kudo A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. 10.1021/cm0103390. [DOI] [Google Scholar]
- Zhang A.; Zhang J. Hydrothermal processing for obtaining of BiVO4 nanoparticles. Mater. Lett. 2009, 63, 1939–1942. 10.1016/j.matlet.2009.06.013. [DOI] [Google Scholar]
- Yu J.; Kudo A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. 10.1002/adfm.200500799. [DOI] [Google Scholar]
- Galembeck A.; Alves O. L. BiVO4 Thin Film Preparation by Metalorganic Decomposition. Thin Solid Films 2000, 365, 90–93. 10.1016/S0040-6090(99)01079-2. [DOI] [Google Scholar]
- Zhao Z.; Li Z.; Zou Z. Electronic Structure and Optical Properties of Monoclinic Clinobisvanite BiVO4. Phys. Chem. Chem. Phys. 2011, 13, 4746–4753. 10.1039/c0cp01871f. [DOI] [PubMed] [Google Scholar]
- Serpone N.; Lawless D.; Khairutdinov R. Size Effects on the Photophysical Properties [Kudo, A of Colloidal Anatase TiO2 Particles: Size Effects on the Photophysical Properties of Colloidal Anatase TiO2 Particles: Size Quantization versus Direct Transitions in This Indirect Semiconductor?. J. Phys. Chem. 1995, 99, 16646–16654. 10.1021/j100045a026. [DOI] [Google Scholar]
- Kudo A.; Miseki Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. 10.1039/B800489G. [DOI] [PubMed] [Google Scholar]
- Laursen A. B.; Man I. C.; Trinhammer O. L.; Rossmeisl J.; Dahl S. The Sabatier Principle Illustrated by Catalytic H2O2 Decomposition on Metal Surfaces. J. Chem. Educ. 2011, 88, 1711–1715. 10.1021/ed101010x. [DOI] [Google Scholar]
- Long M.; Cai W.; Kisch H. Visible Light Induced Photoelectrochemical Properties of n-BiVO4 and n-BiVO4/p-Co3O4. J. Phys. Chem. C 2008, 112, 548–554. 10.1021/jp075605x. [DOI] [Google Scholar]
- Electrochemical Society of Japan . Denki Kagaku Binran (Handbook of Electochemistry); Maruzen Publishing: Tokyo, 2000. [Google Scholar]
- Zhong D. K.; Choi S.; Gamelin D. R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co–Pi” Catalyst-Modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. 10.1021/ja207348x. [DOI] [PubMed] [Google Scholar]
- Masuda T.; Fujishima M.; Tada H. Photo-Effect on the Electromotive Force in Two-Compartment Hydrogen Peroxide-Photofuel Cell. Electrochem. Commun. 2018, 93, 31–34. 10.1016/j.elecom.2018.05.025. [DOI] [Google Scholar]
- Liang Y.; Tsubota T.; Mooij L. P. A.; van de Krol R. Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. 10.1021/jp203004v. [DOI] [Google Scholar]
- Kotani S.; Kudo A.; Nakagaki R.; Tokumura K.. Novel Synthesis Method of Visible-Light-Responsive Bismuth Vanadate Fine Powder, a Photocatalyst Consisting of the Fine Powder, and a Purification Method Using the Fine Powder Photocatalyst. Japanese Published Unexamined Application No. 2004-24936.
- Sayama K.; Nomura A.; Arai T.; Sugita T.; Abe R.; Yanagida S.; Oi T.; Iwasaki Y.; Abe Y.; Sugihara H. Photoelectrochemical Decomposition of Water into H2 and O2 on Porous BiVO4 Thin-Film Electrodes under Visible Light and Significant Effect of Ag Ion Treatment. J. Phys. Chem. B. 2006, 110, 11352–11360. 10.1021/jp057539+. [DOI] [PubMed] [Google Scholar]
- Itaya K.; Akahoshi H.; Toshima S. Electrochemistry of Prussian Blue-Modified Electrodes: An Electrochemical Preparation Method. J. Electrochem. Soc. 1982, 129, 1498–1500. 10.1149/1.2124191. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.