Abstract

A Rh(III)-catalyzed C–H activation/cyclization cascade reaction is described. The reaction involves cyclic 2-diazo-1,3-diketones and N-arylamides, and it proceeds via an intermolecular C–C bond formation and subsequent intramolecular C–N bond formation. A variety of N-acyl-2,3-dihydro-1H-carbazol-4(9H)-ones were obtained under mild conditions in good to excellent yields (65–90%). Key features of this strategy include high-efficiency, operational simplicity, scalability, and broad functional-group tolerance. In addition, H2O and N2 are the only byproducts. Carbazole derivatives with free NH groups can be easily obtained through N-deprotection reactions.
Introduction
Dihydrocarbazole scaffolds are the most important building blocks in total synthesis of many natural products and biologically active compounds1,2 and fluorescent probes.3 In addition, they are widely used in organic luminescent materials because of their conjugated coplanar structures.4 Because of their excellent physical and chemical properties, a variety of efficient and accurate methods for the synthesis of dihydrocarbazole and their derivates have been reported.5 Traditionally, dihydrocarbazole scaffolds are constructed via the well-known Fischer-indole synthesis,6 reductive cylization,7 intramolecular arylation,8 oxidative cylization9 mainly using indoles,10 anilines,11 or nitrobenzenes12 as starting materials. The development of more efficient and general methods for the synthesis of dihydrocarbazole compounds remains an attractive challenge.
Diazo compounds are important and versatile building blocks in organic synthesis and have been widely studied.13 The transition-metal-catalyzed transformations of diazo compounds to carbenoids and their diversified reactivities have been well-developed.14 In addition, Cp*Rh(III)-catalyzed C–H activation/cyclization has also emerged as a versatile and step-economic approach for building diverse carbocycles and heterocycles via the formation of carbon–carbon and carbon–heteroatom bonds.15 Recently, cyclic 2-diazo-1,3-diketones, having higher stability and dipole moments, are important building blocks in synthetic organic chemistry. Several new reactions of cyclic 2-diazo-1,3-diketones, including Wolff rearrangement,16 1,3-dipolar cycloadditions,17 and other reactions,18 have been developed. Previously, we described Rh-catalyzed reactions of cyclic 2-diazo-1,3-diketones with aryl isothiocyanates, benzoic acids, and isocyanides for the synthesis of 2-arylimino-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-ones, isocoumarins, and 2-hydroxy-6-oxocyclohex-1-enecarboxamides.19 In a continuation of our exploration of transformations of diazo compounds and the synthesis of heterocycles, we herein report a convenient method for the synthesis of carbazole derivatives via a Rh(III)-catalyzed C–H bond functionalization/intramolecular cyclization with cyclic 2-diazo-1,3-diketones and arylamides (Scheme 1).
Scheme 1. Rh(III)-Catalyst C–H Functionalization for the Synthesis of Dihydrocarbazole Derivatives.

Results and Discussion
The reaction was initially tested with 2-diazo-5,5-dimethylcyclohexane-1,3-dione (1e) and acetanilide (2a) as model substrates to optimize the reaction conditions (Table 1). The desired product, 3ea, was generated in 81% yield when the reaction was carried out with [Cp*RhCl2]2 (1.0 mol %) and AgNTf2 (10 mol %) in 1,2-dichloroethane (DCE) for 12 h (Table 1, entry 4). The structure of 3ea was unequivocally determined by X-ray diffraction measurements (see the Supporting Information). The yield of 3ea decreased sharply to 49% when the loading of AgNTf2 was decreased to 5 mol % (Table 1, entry 11). The results were not improved by increasing the AgNTf2 loading to 20 mol % (Table 1, entry 12). A screening of Rh-catalysts revealed that Rh(PPh3)3Cl and Rh2(OAc)4 were ineffective in the reaction, and only trace amounts of the product were detected in each case (Table 1, entries 1, 4). Other transition-metal catalysts were also investigated, and all led to inferior results (Table 1, entry 3, and Table S1 in the Supporting Information). [Cp*RhCl2]2 was found to be the best catalyst (Table 1, entries 13 and 14). Then, the effect of solvents was tested. The yield of the reaction is relatively low in MeOH, EtOH, toluene, DCM (dichloromethane), TFEA (2,2,2-trifluoroethanol) compared to that of DCE (Table 1, entries 6–10). Lower temperatures led to lower yields (Table 1, entries 15–18). Thus, the conditions used in entry 3 were selected as the optimized conditions for the reaction.
Table 1. Optimization of Reaction Conditionsa.

| entry | catalyst | additive | solvent | temp (°C) | yield (%)b |
|---|---|---|---|---|---|
| 1 | Rh(PPh3)3Cl | AgNTf2 | DCE | reflux | trace |
| 2 | Rh2(OAc)4 | AgNTf2 | DCE | reflux | trace |
| 3 | [Cp*Co(CO)I2] | AgNTf2 | DCE | reflux | trace |
| 4 | [Cp*RhCl2]2 | AgNTf2 | DCE | reflux | 81 |
| 5 | AgNTf2 | DCE | reflux | trace | |
| 6 | [Cp*RhCl2]2 | AgNTf2 | MeOH | reflux | 31 |
| 7 | [Cp*RhCl2]2 | AgNTf2 | EtOH | reflux | 35 |
| 8 | [Cp*RhCl2]2 | AgNTf2 | toluene | 100 | 56 |
| 9 | [Cp*RhCl2]2 | AgNTf2 | DCM | reflux | 51 |
| 10 | [Cp*RhCl2]2 | AgNTf2 | TFEA | reflux | 28 |
| 11c | [Cp*RhCl2]2 | AgNTf2 | DCE | reflux | 49 |
| 12d | [Cp*RhCl2]2 | AgNTf2 | DCE | reflux | 75 |
| 13e | [Cp*RhCl2]2 | AgNTf2 | DCE | reflux | 46 |
| 14f | [Cp*RhCl2]2 | AgNTf2 | DCE | reflux | 78 |
| 15 | [Cp*RhCl2]2 | AgNTf2 | DCE | rt | trace |
| 16 | [Cp*RhCl2]2 | AgNTf2 | DCE | 40 | 18 |
| 17 | [Cp*RhCl2]2 | AgNTf2 | DCE | 60 | 32 |
| 18 | [Cp*RhCl2]2 | AgNTf2 | DCE | 80 | 65 |
Reaction conditions: 2-diazo-5,5-dimethylcyclohexane-1,3-dione 1e (0.5 mmol), acetanilide 2a (0.5 mmol), solvent (3 mL), and catalyst (1.0 mol %) for 12 h.
Isolated yields.
5 mol % of additive was used.
20 mol % of additive was used.
0.5 mol % of catalyst was used.
2 mol % of catalyst was used.
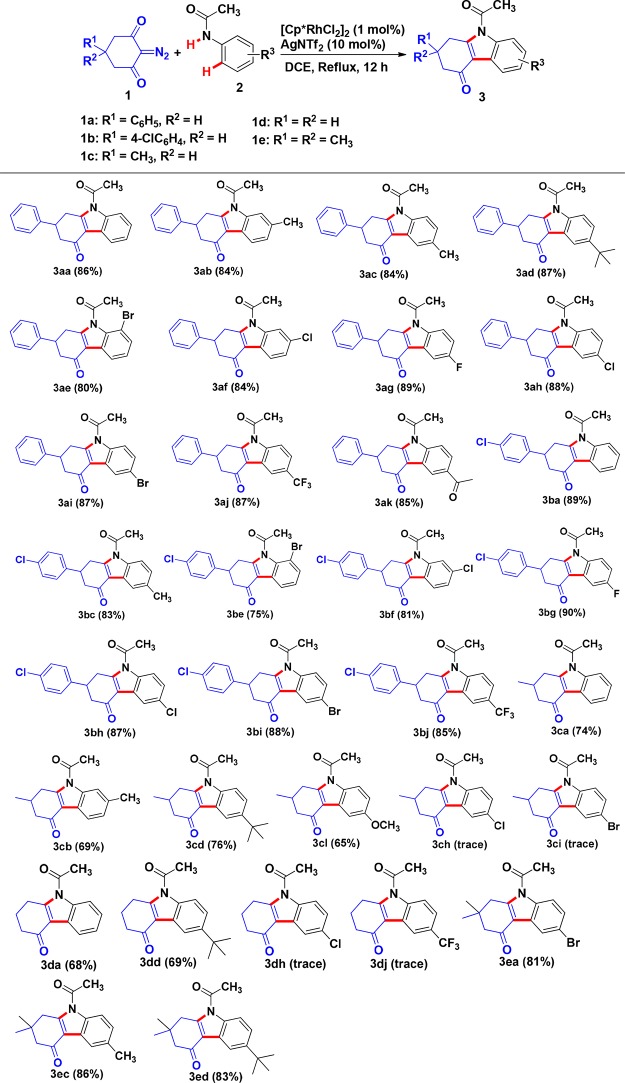
With the optimized conditions in hand, we set out to explore the generality of this reaction (Table 2). Various cyclic 2-diazo-1,3-diketones and N-arylacetamides were tested, and the results are summarized in Table 2. A series of carbazole derivatives were obtained in good to excellent yields (65–90%). For N-arylacetamides 2, both electron-donating and electron-withdrawing groups on the N-aryl ring of the acetamides were well-tolerated, and the corresponding carbazoles were generated in appreciable yields. Notably, functional groups such as chloro, bromo, fluoro, methyl, methoxyl, tert-butyl, trifluoromethyl, and acetyl on the N-aryl ring of the acetamides were well-tolerated, and the steric hindrance and electronic effects did not noticeably influence the transformation. The scope of the substituents on the cyclic 2-diazo-1,3-diketones was then examined. Generally, the reaction using substrates bearing alkyl (e.g., CH3) and aryl (e.g., phenyl or 4-ClC6H4) groups for R1 proceeded smoothly, and the desired carbazole derivatives were synthesized in good to excellent yields (65–90%). As for cyclic 2-diazo-1,3-diketones bearing H and alkyl groups (1c, 1d, and 1e), when N-arylacetamides 2 bearing withdrawing-electronic groups (e.g., −Cl, −Br, and −CF3) were used, the reaction was almost completely suppressed illustrating that the electronic effect of the aromatic ring bearing withdrawing-electronic group may reduce the activity of the C–H bond of the aromatic ring.
Table 2. Rh(III)-Catalyzed Reactions of Various Cyclic 2-Diazo-1,3-diketones and N-Arylacetamidesa,b.
Reaction conditions: cyclic 2-dizao-1,3-diketone 1 (0.5 mmol), N-arylacetamides 2 (0.5 mmol), [Cp*RhCl2]2 (1 mol %), and AgNTf2 (10 mol %) in DCE (3 mL) were stirred at reflux for 12 h.
Isolated yield.
Furthermore, we carried out a gram-scale reaction of 2-diazo-5,5-dimethylcyclohexane-1,3-dione (1e, 10 mmol) and acetanilide (2a, 10 mmol) under the standard conditions, and the desired product, 3ea, was isolated in 78% (1.99 g) yield (Scheme 2). This demonstrated the applicability of this method as a useful tool in practical synthetic contexts.
Scheme 2. Gram-Scale Synthesis of This Method.

Subsequently, the scope of N-acylanilines was investigated, and the results are summarized in Table 3. Various N-acylanilines bearing alkyl (2m), benzyl (2n) and cyclohexyl (2o) groups could react with 2-diazo-5,5-dimethylcyclohexane-1,3-dione (1e) to give the desired products 3em, 3en, and 3eo in 86, 78, and 81% yields, respectively (Table 3, entries 4–6). In addition, we have also explored the generality of our reaction using other cyclic 2-diazo-1,3-diketones as substrates. The reactions of 1a with 2m and 1c with 2m and 2n under the above reaction conditions afforded good yields (86, 76, and 73%, respectively) of the corresponding products (3am, 3cm, and 3cn, respectively) (Table 3, entries 1–3). These examples prove the generality of this method.
Table 3. Rh(III)-Catalyzed Reactions of Various Cyclic 2-Diazo-1,3-diketones and N-Acylanilinesa,b.

Reaction conditions: cyclic 2-dizao-1,3-diketone 1 (0.5 mmol), arylamide 2 (0.5 mmol), [Cp*RhCl2]2 (1 mol %), and AgNTf2 (10 mol %) in DCE (3 mL) were stirred at reflux for 12 h.
Isolated yield.
Because of the prevalence of carbazoles with a free NH group in the cores of a large number of natural products and privileged heterocyclic compounds possessing significant biological activities, we tested N-deprotection reactions on a few representative compounds (3ea and 3en) as shown in Scheme 3. The transformations of 3ea and 3en can easily be accomplished using NaOH in EtOH at room temperature for 10 min, and the corresponding free NH product, 4ea, was obtained in excellent yield (95%).
Scheme 3. N-Deprotection Reactions.
Furthermore, the mechanism of this reaction system was examined by the deuterium experiments. The reaction of 1e with the same amounts of both 2a and 2a-d5 was explored under the standard conditions for 2 h. A significant kinetic isotope effect (KIE) value of 2.5 was measured based on 1H NMR analysis (Scheme 4 eq 1). Then, separate reactions of 1e with either 2a or 2a-d5 were performed in parallel, and a similar KIE value of 2.0 was obtained (Scheme 4 eq 2). These results suggested that C–H cleavage may be involved in the rate-determining step.20
Scheme 4. The Deuterium Experiments.
Based on the experimental results and literature reports,19a,21 a plausible mechanism was proposed and is shown in Scheme 5. First, treatment of [Cp*RhCl2]2 with AgNTf2 generated the active catalytic species A, which was followed by C–H bond activation of arylamide 2 with the Rh(III) to generate rhodacycle B. Coordination and subsequent migratory insertion afforded intermediate D, which then regenerated catalytic species A and released intermediate E. Tautomerization of intermediate E generates enol intermediate F in situ, which then cyclizes via the elimination of water to generate final product 3.
Scheme 5. Proposed Mechanism for the Rh(III)-Catalyzed C–H Activation/Cyclization of Cyclic 2-Diazo-1,3-diketones and N-Arylamides.
Conclusion
In summary, we have developed an efficient route to dihydrocarbazole derivatives from easily available cyclic diazo-1,3-diketones and N-arylamides. The process involves C–H activation/intramolecular cyclization and proceeded smoothly with broad functional group tolerance and high atom efficiency. Dihydrocarbazole with free NH groups could be easily obtained under mild conditions.
Experimental Section
General Remarks
Unless otherwise specified, all reagents and starting materials were purchased from commercial sources and used as received, and the solvents were purified and dried using standard procedures. The chromatography solvents were of technical grade and distilled prior to use. Flash chromatography was performed using 200–300 mesh silica gel with the indicated solvent system according to standard techniques. The 1H and 13C NMR spectra were recorded using 300 MHz spectrometers unless otherwise specified. The chemical shifts (δ) in parts per million are reported relative to the residual signals of chloroform (7.26 ppm for 1H and 77.16 ppm for 13C), and all 13C NMR spectra that were recorded with broadband proton decoupling are denoted 13C{1H} NMR. The multiplicities are described as s (singlet), d (doublet), t (triplet), q (quartet), or m (multiplet), and the coupling constants (J) are reported in hertz. The HRMS analyses with a quadrupole time-of-flight mass spectrometer yielded ion mass/charge (m/z) ratios in atomic mass units. The IR spectra were measured as dry pellets (KBr), and the peaks are reported in terms of the wavenumber (cm–1).
General Procedure for the Synthesis of the Dihydrocarbazole Derivatives 3
A mixture of cyclic 2-diazo-1,3-diketone 1 (0.5 mmol), N-arylamide 2 (0.5 mmol), [Cp*RhCl2]2 (0.005 mmol), and AgNTf2 (0.05 mmol) in DCE (3 mL) was heated to reflux in an oil bath for 12 h. After the reaction was complete (as determined by TLC), the reaction mixture was cooled to room temperature, extracted with CH2Cl2 (3 × 10 mL), and washed with brine. The organic layers were combined, dried over Na2SO4, and filtered, and then the solvent was evaporated under vacuum. The residue was purified using flash column chromatography with silica gel (200–300 mesh) using ethyl acetate and petroleum ether (1:8–1:10, v/v) as the elution solvent to give the desired product 3.
9-Acetyl-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3aa)
Petroleum ether/ethyl acetate 8:1; yield 86% (130 mg, 0.43 mmol); white solid; mp 149–151 °C; 1H NMR (300 MHz, CDCl3): δ 8.36 (d, J = 8.7 Hz, 1H), 7.85 (d, J = 8.7 Hz, 1H), 7.38–7.30 (m, 7H), 3.67–3.50 (m, 2H), 3.37–3.28 (m, 1H), 2.95–2.84 (m, 2H), 2.77 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.4, 170.3, 150.7, 142.7, 135.7, 127.2, 126.9, 126.0, 125.2, 124.7, 122.0, 117.5, 114.3, 44.6, 42.0, 34.3, 27.5; IR (KBr) ν: 3412, 1714, 1692, 1603, 1548, 1484, 1457, 1400, 1361, 1299, 1274, 1169, 1142, 1069, 1017, 959, 907, 766, 749, 695 cm–1; HRMS (APCI): calcd for [C20H17NO2 + H]+, 304.1332; found, 304.1334.
9-Acetyl-7-methyl-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ab)
Petroleum ether/ethyl acetate 8:1; yield 84% (133 mg, 0.42 mmol); white solid; mp 168–170 °C; 1H NMR (300 MHz, CDCl3): δ 8.22 (d, J = 7.8 Hz, 1H), 7.69 (s, 1H), 7.40–7.29 (m, 5H), 7.21 (d, J = 7.8 Hz, 1H), 3.65–3.52 (m, 2H), 3.35–3.29 (m, 1H), 2.96–2.83 (m, 2H), 2.77 (s, 3H), 2.50 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.4, 170.4, 150.1, 142.7, 136.2, 135.3, 128.8, 127.2, 126.9, 126.1, 123.6, 121.6, 114.7, 44.6, 42.1, 34.3, 27.5, 22.2; IR (KBr) ν: 3458, 1721, 1648, 1637, 1600, 1557, 1470, 1395, 1356, 1341, 1261, 1208, 1131, 1062, 1028, 793, 761, 702 cm–1; HRMS (APCI): calcd for [C21H19NO2 + H]+, 318.1489; found, 318.1488.
9-Acetyl-6-methyl-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ac)
Petroleum ether/ethyl acetate 8:1; yield 84% (133 mg, 0.42 mmol); white solid; mp 193–195 °C; 1H NMR (300 MHz, CDCl3): δ 8.16 (s, 1H), 7.70 (d, J = 8.7 Hz, 1H), 7.40–7.29 (m, 5H), 7.17 (d, J = 8.4 Hz, 1H), 3.67–3.50 (m, 2H), 3.36–3.26 (m, 1H), 2.94–2.82 (m, 2H), 2.75 (s, 3H), 2.47 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.6, 170.2, 150.9, 142.8, 134.6, 133.9, 128.8, 127.2, 126.9, 126.4, 126.2, 122.0, 117.3, 114.0, 44.6, 41.9, 34.3, 27.4, 21.2; IR (KBr) ν: 3426, 1721, 1653, 1637, 1550, 1482, 1468, 1395, 1359, 1261, 1211, 1192, 1124, 1062, 889, 796, 759 cm–1; HRMS (APCI): calcd for [C21H19NO2 + H]+, 318.1489; found, 318.1486.
9-Acetyl-6-(tert-butyl)-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ad)
Petroleum ether/ethyl acetate 8:1; yield 87% (156 mg, 0.43 mmol); white solid; mp 192–194 °C; 1H NMR (300 MHz, CDCl3): δ 8.40 (s, 1H), 7.75 (d, J = 8.7 Hz, 1H), 7.44–7.29 (m, 6H), 3.67–3.50 (m, 2H), 3.37–3.28 (m, 1H), 2.95–2.83 (m, 2H), 2.75 (s, 3H), 1.41 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 194.6, 170.2, 151.0, 148.1, 142.8, 133.7, 128.8, 127.2, 126.9, 126.1, 122.9, 118.4, 117.6, 113.8, 44.7, 41.9, 34.8, 34.3, 31.6, 27.4; IR (KBr) ν: 3462, 1719, 1669, 1605, 1555, 1480, 1459, 1395, 1366, 1299, 1272, 1181, 1078, 1010, 825, 764, 691 cm–1; HRMS (APCI): calcd for [C24H25NO2 + H]+, 360.1958; found, 360.1960.
9-Acetyl-8-bromo-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ae)
Petroleum ether/ethyl acetate 8:1; yield 80% (152 mg, 0.40 mmol); white solid; mp 128–130 °C; 1H NMR (300 MHz, CDCl3): δ 8.30 (d, J = 7.8 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.45–7.22 (m, 6H), 3.64–3.58 (m, 1H), 3.36–3.12 (m, 2H), 2.90–2.86 (m, 2H), 2.70 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.7, 172.8, 150.9, 142.7, 134.8, 129.5, 129.4, 129.3, 128.5, 128.3, 127.7, 127.2, 126.1, 115.6, 106.3, 45.3, 41.9, 31.7, 30.6; IR (KBr) ν: 3444, 1730, 1662, 1557, 1546, 1466, 1434, 1391, 1356, 1263, 1163, 1119, 786, 764, 697 cm–1; HRMS (APCI): calcd for [C20H16BrNO2 + H]+, 382.0437; found, 382.0440.
9-Acetyl-7-chloro-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3af)
Petroleum ether/ethyl acetate 8:1; yield 84% (141 mg, 0.42 mmol); white solid; mp 180–182 °C; 1H NMR (300 MHz, CDCl3): δ 8.26 (d, J = 8.4 Hz, 1H), 7.94 (s, 1H), 7.39–7.31 (m, 6H), 3.64–3.54 (m, 2H), 3.40–3.30 (m, 1H), 2.98–2.85 (m, 2H), 2.76 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.1, 169.8, 150.6, 142.4, 136.2, 131.2, 128.9, 127.4, 126.8, 125.2, 124.3, 122.6, 117.3, 115.0, 44.5, 42.0, 34.4, 27.4; IR (KBr) ν: 3467, 1712, 1662, 1607, 1550, 1466, 1366, 1288, 1270, 1197, 1126, 1033, 996, 798 cm–1; HRMS (APCI): calcd for [C21H16ClNO2 + H]+, 372.1206; found, 372.1207.
9-Acetyl-6-fluoro-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ag)
Petroleum ether/ethyl acetate 8:1; yield 89% (143 mg, 0.45 mmol); white solid; mp 109–111 °C; 1H NMR (300 MHz, CDCl3): δ 8.01–7.99 (m, 1H), 7.88–7.84 (m, 1H), 7.41–7.31 (m, 5H), 7.09–7.03 (m, 1H), 3.64–6.52 (m, 2H), 3.38–3.28 (m, 1H), 2.94–2.82 (m, 2H), 2.74 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.0, 169.9, 160.2 (d, JC–F = 240.7 Hz), 142.4, 132.2, 128.9, 127.4, 126.8, 115.7 (d, JC–F = 9.0 Hz), 112.9 (d, JC–F = 24.7 Hz), 107.7 (d, JC–F = 24.7 Hz), 44.4, 42.0, 34.4, 27.3; IR (KBr) ν: 3312, 1719, 1667, 1635, 1548, 1457, 1398, 1366, 1295, 1254, 1133, 1046, 1005, 773, 700 cm–1; HRMS (APCI): calcd for [C20H16FNO2 + H]+, 322.1238; found, 322.1242.
9-Acetyl-6-chloro-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ah)
Petroleum ether/ethyl acetate 8:1; yield 88% (148 mg, 0.44 mmol); white solid; mp 144–146 °C; 1H NMR (300 MHz, CDCl3): δ 8.23 (s, 1H), 7.77 (d, J = 9.0 Hz, 1H), 7.41–7.31 (m, 6H), 7.23 (d, J = 9.0 Hz, 1H), 3.57–3.51 (m, 2H), 3.30–3.20 (m, 1H), 2.88–2.79 (m, 2H), 2.68 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.1, 170.3, 150.7, 141.2, 134.6, 133.8, 132.9, 128.9, 128.3, 126.4, 126.1, 122.0, 117.2, 113.9, 44.5, 41.2, 34.1, 27.4, 21.2; IR (KBr) ν: 3425, 1725, 1680, 1636, 1525, 1495, 1458, 1383, 1341, 1296, 1252, 1011, 817, 785, 756, 641 cm–1; HRMS (APCI): calcd for [C20H16ClNO2 + H]+, 338.0942; found, 338.0937.
9-Acetyl-6-bromo-2-phenyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ai)
Petroleum ether/ethyl acetate 8:1; yield 87% (166 mg, 0.43 mmol); white solid; mp 145–147 °C; 1H NMR (300 MHz, CDCl3): δ 8.42 (s, 1H), 7.74 (d, J = 8.7 Hz, 1H), 7.39–7.31 (m, 6H), 3.59–3.50 (m, 2H), 3.33–3.23 (m, 1H), 2.91–2.79 (m, 2H), 2.71 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.9, 169.8, 151.0, 142.4, 134.5, 128.9, 128.0, 127.4, 126.9, 124.3, 118.2, 116.8, 116.1, 44.4, 41.9, 34.3, 27.4; IR (KBr) ν: 3449, 1735, 1664, 1616, 1555, 1480, 1332, 1242, 1195, 1151, 1028, 777, 759, 691 cm–1; HRMS (APCI): calcd for [C20H16BrNO2 + H ]+, 382.0437; found, 382.0442.
9-Acetyl-2-phenyl-6-(trifluoromethyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3aj)
Petroleum ether/ethyl acetate 8:1; yield 87% (161 mg, 0.44 mmol); white solid; mp 128–130 °C; 1H NMR (300 MHz, CDCl3): δ 8.62 (s, 1H), 8.00 (d, J = 8.7 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.39–7.31 (m, 5H), 3.66–3.54 (m, 2H), 3.41–3.31 (m, 1H), 2.97–2.85 (m, 2H), 2.78 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.9, 169.9, 151.6, 142.3, 137.4, 129.0, 126.8, 125.7, 122.1 (q, JC–F = 3.7 Hz), 119.3 (q, JC–F = 3.7 Hz), 117.3, 114.9, 44.4, 41.9, 34.3, 27.4; IR (KBr) ν: 3439, 1721, 1662, 1548, 1491, 1452, 1409, 1366, 1338, 1284, 1182, 1014, 839, 773, 707 cm–1; HRMS (APCI): calcd for [C21H16F3NO2 + H]+, 372.1206; found, 372.1207.
1,1′-(4-Oxo-2-phenyl-3,4-dihydro-1H-carbazole-6,9(2H)-diyl)diethanone (3ak)
Petroleum ether/ethyl acetate 8:1; yield 85% (146 mg, 0.43 mmol); white solid; mp 201–203 °C; 1H NMR (300 MHz, CDCl3): δ 8.92 (s, 1H), 8.05 (d, J = 8.7 Hz, 1H), 7.94 (d, J = 9.0 Hz, 1H), 7.42–7.39 (m, 6H), 3.71–3.57 (m, 2H), 3.45–3.36 (m, 1H), 2.97–2.89 (m, 2H), 2.83 (s, 3H), 2.72 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 197.9, 194.2, 170.0, 151.8, 142.4, 138.2, 133.8, 128.9, 127.4, 126.8, 125.8, 125.1, 123.1, 117.6, 114.4, 44.5, 41.9, 34.3, 27.5, 26.8; IR (KBr) ν: 3458, 1740, 1667, 1612, 1557, 1480, 1363, 1318, 1245, 1151, 1028, 989, 784, 759, 695, 531 cm–1; HRMS (APCI): calcd for [C22H19NO3 + H]+, 346.1438; found, 346.1438.
9-Acetyl-2-(4-chlorophenyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3ba)
Petroleum ether/ethyl acetate 8:1; yield 89% (150 mg, 0.45 mmol); white solid; mp 171–173 °C; 1H NMR (300 MHz, CDCl3): δ 8.36 (d, J = 8.7 Hz, 1H), 7.81 (d, J = 9.0 Hz, 1H), 7.39–7.33 (m, 4H), 7.27–7.25 (m, 2H), 3.70–3.62 (m, 1H), 3.59–3.51 (m, 1H), 3.38–3.29 (m, 1H), 2.93–2.83 (m, 2H), 2.80 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.0, 170.3, 150.6, 141.1, 135.6, 132.9, 129.0, 128.2, 125.9, 125.3, 124.8, 122.1, 117.5, 114.1, 44.5, 41.3, 34.1, 27.5; IR (KBr) ν: 3417, 1714, 1662, 1559, 1493, 1457, 1365, 1334, 1270, 1206, 1181, 1062, 992, 827, 743, 606, 588 cm–1; HRMS (APCI): calcd for [C20H16ClNO2 + H]+, 338.0942; found, 338.0943.
9-Acetyl-2-(4-chlorophenyl)-6-methyl-2,3-dihydro-1H-carbazol-4(9H)-one (3bc)
Petroleum ether/ethyl acetate 8:1; yield 83% (146 mg, 0.42 mmol); white solid; mp 202–204 °C; 1H NMR (300 MHz, CDCl3): δ 8.15 (s, 1H), 7.66 (d, J = 8.4 Hz, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.25–7.18 (m, 2H), 7.16 (d, J = 8.4 Hz, 2H), 3.63–3.49 (m, 2H), 3.31–3.21 (m, 1H), 2.87–2.78 (m, 2H), 2.75 (s, 3H), 2.46 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 194.5, 170.6, 151.1, 141.5, 135.1, 134.2, 133.2, 129.3, 128.6, 126.8, 122.4, 117.7, 114.1, 44.9, 41.6, 34.5, 27.8, 21.5; IR (KBr) ν: 3444, 1710, 1664, 1607, 1550, 1493, 1455, 1398, 1343, 1293, 1220, 1005, 832, 805, 638 cm–1; HRMS (APCI): calcd for [C21H18ClNO2 + H]+, 352.1099; found, 352.1096.
9-Acetyl-8-bromo-2-(4-chlorophenyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3be)
Petroleum ether/ethyl acetate 8:1; yield 75% (156 mg, 0.43 mmol); white solid; mp 172–174 °C; 1H NMR (300 MHz, CDCl3): δ 8.28 (d, J = 7.8 Hz, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.1 Hz, 2H), 7.26–7.23 (m, 3H), 3.63–3.56 (m, 1H), 3.34–3.27 (m, 1H), 3.18–3.08 (m, 1H) 2.85–2.82 (m, 2H), 2.70 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 192.8, 172.4, 150.2, 140.7, 134.4, 133.1, 129.1, 129.0, 128.4, 128.1, 125.9, 121.2, 106.0, 44.8, 40.9, 31.1, 30.2; IR (KBr) ν: 3423, 1726, 1649, 1566, 1535, 1456, 1422, 1391, 1346, 1263, 1163, 1119, 788, 756, 701 cm–1; HRMS (APCI): calcd for [C20H15BrClNO2 + H]+, 416.0047; found, 416.0043.
9-Acetyl-7-chloro-2-(4-chlorophenyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3bf)
Petroleum ether/ethyl acetate 8:1; yield 81% (150 mg, 0.41 mmol); white solid; mp 165–167 °C; 1H NMR (300 MHz, CDCl3): δ 8.23 (d, J = 8.4 Hz, 1H), 7.87 (s, 1H), 7.34 (d, J = 8.1 Hz, 3H), 7.25 (d, J = 8.1 Hz, 2H), 3.63–3.50 (m, 2H), 3.34–3.24 (m, 1H), 2.90–2.79 (m, 2H), 2.76 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.6, 169.8, 150.5, 140.9, 136.1, 133.1, 131.3, 129.0, 128.2, 125.3, 122.6, 117.2, 114.8, 44.3, 41.3, 34.2, 27.4; IR (KBr) ν: 3435, 1721, 1673, 1546, 1493, 1470, 1409, 1368, 1331, 1274, 1169, 1012, 821, 613 cm–1; HRMS (APCI): calcd for [C20H15ClNO2 + H]+, 372.0553; found, 372.0553.
9-Acetyl-2-(4-chlorophenyl)-6-fluoro-2,3-dihydro-1H-carbazol-4(9H)-one (3bg)
Petroleum ether/ethyl acetate 8:1; yield 90% (160 mg, 0.45 mmol); white solid; mp 137–139 °C; 1H NMR (300 MHz, CDCl3): δ 8.04–8.01 (m, 1H), 7.85–7.81 (m, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.27–7.26 (m, 3H), 7.13–7.09 (m, 1H), 3.68–3.52 (m, 2H), 3.38–3.29 (m, 1H), 2.94–2.82 (m, 2H), 2.78 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.6, 169.9, 160.3 (d, JC–F = 242.25 Hz), 151.3, 140.9, 133.1, 132.1, 129.0, 128.2, 127.1 (d, JC–F = 10.5 Hz), 115.5 (d, JC–F = 9.0 Hz), 113.0 (d, JC–F = 25.5 Hz), 107.9 (d, JC–F = 24.5 Hz), 44.3, 41.3, 34.3, 27.4; IR (KBr) ν: 3458, 1719, 1664, 1637, 1553, 1500, 1466, 1441, 1395, 1366, 1279, 1258, 1197, 1131, 1028, 987, 832, 800, 786 cm–1; HRMS (APCI): calcd for [C21H15FClNO2 + H]+, 356.0848; found, 356.0850.
9-Acetyl-6-chloro-2-(4-chlorophenyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3bh)
Petroleum ether/ethyl acetate 8:1; yield 87% (162 mg, 0.44 mmol); white solid; mp 177–179 °C; 1H NMR (300 MHz, CDCl3): δ 8.34 (s, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.36–7.31 (m, 3H), 7.27–7.24 (m, 2H), 3.67–3.51 (m, 2H), 3.37–3.28 (m, 1H), 2.93–2.82 (m, 2H), 2.78 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.9, 169.8, 151.1, 142.4, 134.2, 130.4, 128.9, 127.4, 126.9, 125.2, 121.2, 116.7, 115.8, 44.3, 41.9, 34.3, 27.3; IR (KBr) ν: 3412, 1717, 1660, 1553, 1496, 1448, 1393, 1361, 1336, 1277, 1261, 1133, 1069, 989, 834, 800, 668, 599 cm–1; HRMS (APCI): calcd for [C20H15Cl2NO2 + H]+, 372.0553; found, 372.0550.
9-Acetyl-6-bromo-2-(4-chlorophenyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3bi)
Petroleum ether/ethyl acetate 8:1; yield 88% (183 mg, 0.44 mmol); white solid; mp 170–172 °C; 1H NMR (300 MHz, CDCl3): δ 8.48 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.35 (d, J = 8.7 Hz, 2H), 7.26 (d, J = 7.8 Hz, 2H), 3.66–3.51 (m, 2H), 3.36–3.27 (m, 1H), 2.91–2.81 (m, 2H), 2.77 (s, 3H); 13C NMR (75 MHz CDCl3): δ 193.5, 169.8, 150.9, 140.8, 134.5, 133.1, 129.1, 128.2, 128.2, 127.4, 124.6, 118.4, 116.8, 115.8, 44.3, 41.3, 34.2, 27.4; IR (KBr) ν: 3444, 1717, 1664, 1653, 1557, 1496, 1468, 1366, 1336, 1277, 1199, 1135, 992, 830, 796, 606 cm–1; HRMS (APCI): calcd for [C20H15BrClNO2 + H]+, 416.0047; found, 416.0042.
9-Acetyl-2-(4-chlorophenyl)-6-(trifluoromethyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3bj)
Petroleum ether/ethyl acetate 8:1; yield 85% (172 mg, 0.43 mmol); white solid; mp 146–148 °C; 1H NMR (300 MHz, CDCl3): δ 8.61 (s, 1H), 7.96 (d, J = 8.7 Hz, 1H), 7.59 (d, J = 8.7 Hz, 1H), 7.34 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 3.67–3.54 (m, 2H), 3.38–3.29 (m, 1H), 2.92–2.82 (m, 2 H), 2.80 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.5, 169.9, 151.5, 140.7, 137.3, 133.1, 129.1, 128.2, 126.9, 126.1, 125.7, 122.1 (q, JC–F = 3.7 Hz), 119.4 (q, JC–F = 3.7 Hz), 117.2, 114.7, 44.3, 41.3, 34.1, 27.4; IR (KBr) ν: 3403, 1828, 1716, 1667, 1557, 1493, 1482, 1461, 1366, 1325, 1274, 1178, 1115, 1060, 1001, 825, 725 cm–1; HRMS (APCI): calcd for [C21H15ClF3NO2 + H]+, 405.0816; found, 405.0815.
9-Acetyl-2-methyl-2,3,9,9a-tetrahydro-1H-carbazol-4(4aH)-one (3ca)
Petroleum ether/ethyl acetate 8:1; yield 74% (120 mg, 0.37 mmol); white solid; mp 109–111 °C; 1H NMR (300 MHz, CDCl3): δ 8.34 (d, J = 8.7 Hz, 1H), 7.84 (d, J = 9.0 Hz, 1H), 7.37–7.34 (m, 2H), 3.49–3.42 (m, 1H), 2.96–2.87 (m, 1H), 2.82 (s, 3H), 2.66–2.61 (m, 1H), 2.42–2.33 (m, 2H), 1.23 (d, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 195.8, 170.8, 151.7, 136.0, 126.5, 125.4, 125.0, 122.4, 117.8, 114.6, 46.5, 34.8, 31.7, 27.9, 21.7; IR (KBr) ν: 3459, 1721, 1664, 1651, 1598, 1559, 1482, 1434, 1363, 1284, 1213, 1181, 1001, 996, 773, 739, 588 cm–1; HRMS (APCI): calcd for [C15H15NO2 + H]+, 242.1176; found, 242.1172.
9-Acetyl-2,7-dimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (3cb)
Petroleum ether/ethyl acetate 8:1; yield 69% (88 mg, 0.35 mmol); white solid; mp 112–114 °C; 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.1 Hz, 1H), 7.65 (s, 1H), 7.18 (d, J = 7.8 Hz, 1H), 3.44–3.36 (m, 1H), 2.91–2.82 (m, 1H), 2.79 (s, 3H), 2.62–2.57 (m, 1H), 2.48 (s, 3H). 2.38–2.29 (m, 2H), 1.21 (d, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 195.4, 170.4, 150.7, 136.1, 135.0, 125.9, 123.7, 121.5, 117.4, 114.6, 46.0, 34.4, 31.4, 27.5, 22.1, 21.3; IR (KBr) ν: 3439, 1717, 1665, 1660, 1562, 1548, 1493, 1416, 1363, 1334, 1281, 1201, 1191, 830, 818, 645 cm–1, HRMS (APCI): calcd for [C16H17NO2 + H]+, 256.1332; found, 256.1336.
9-Acetyl-6-(tert-butyl)-2-methyl-2,3-dihydro-1H-carbazol-4(9H)-one (3cd)
Petroleum ether/ethyl acetate 8:1; yield 76% (113 mg, 0.38 mmol); white solid; mp 73–75 °C; 1H NMR (300 MHz, CDCl3): δ 8.35 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 3.44–3.37 (m, 1H), 2.88–2.82 (m, 1H), 2.77 (s, 3H), 2.61–2.56 (m, 1H), 2.36–2.27 (m, 2H), 1.39 (s, 9H), 1.19 (d, J = 6.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 195.5, 170.3, 151.5, 148.0, 133.7, 126.1, 122.7, 118.3, 117.4, 113.8, 46.1, 34.7, 34.4, 31.6, 27.4, 21.2; IR (KBr) ν: 3444, 1717, 1655, 1553, 1468, 1363, 1297, 1258, 1190, 1144, 1008, 903, 825, 622 cm–1; HRMS (APCI): calcd for [C20H25NO2 + H]+, 312.1958; found, 312.1956.
9-Acetyl-6-methoxy-2-methyl-2,3-dihydro-1H-carbazol-4(9H)-one (3cl)
Petroleum ether/ethyl acetate 8:1; yield 65% (88 mg, 0.32 mmol); white solid; mp 168–170 °C; 1H NMR (300 MHz, CDCl3): δ 7.82 (s, 1H), 7.74 (d, J = 9.0 Hz, 1H), 6.93 (d, J = 9.0 Hz, 1H), 3.89 (s, 3H), 3.46–3.39 (m, 1H), 2.92–2.82 (m, 1H), 2.77 (s, 3H), 2.63–2.59 (m, 1H), 2.39–2.30 (m, 2H), 1.22 (d, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 195.9, 170.5, 157.6, 151.9, 130.5, 127.6, 117.7, 115.6, 114.4, 104.3, 56.1, 46.4, 34.9, 31.7, 27.7, 21.6; IR (KBr) ν: 3444, 1733, 1712, 1662, 1598, 1549, 1464, 1441, 1356, 1281, 1167, 1053, 1003, 880, 764, 691 cm–1; HRMS (APCI): calcd for [C16H17NO3 + H]+, 271.1281; found, 272.1279.
9-Acetyl-2,3-dihydro-1H-carbazol-4(9H)-one (3da)
Petroleum ether/ethyl acetate 6:1; yield 68% (77 mg, 0.34 mmol); white solid; mp 116–118 °C; 1H NMR (300 MHz, CDCl3): δ 8.34 (d, J = 8.7 Hz, 1H), 7.85 (d, J = 8.7 Hz, 1H), 7.37–7.34 (m, 2H), 3.31 (t, J = 6.3 Hz, 2H), 2.81 (s, 3H), 2.61 (t, J = 6.3 Hz, 2H), 2.29–2.21 (m, 2H); 13C NMR (75 MHz, CDCl3): δ 195.7, 170.4, 151.7, 135.4, 126.2, 125.0, 124.7, 122.1, 117.6, 114.2, 37.8, 27.5, 26.3, 23.5; IR (KBr) ν: 3439, 1721, 1655, 1646, 1563, 1553, 1482, 1411, 1361, 1281, 1174, 1035, 939, 900, 764, 748 cm–1; HRMS (APCI): calcd for [C14H13NO2 + H]+, 228.1019; found, 228.1023.
9-Acetyl-6-(tert-butyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3dd)
Petroleum ether/ethyl acetate 6:1; yield 69% (97 mg, 0.35 mmol); white solid; mp 110–112 °C; 1H NMR (300 MHz, CDCl3): δ 8.36 (s, 1H), 7.75 (d, J = 8.7 Hz, 1H), 7.40 (d, J = 8.7 Hz, 1H), 3.29 (t, J = 6.0 Hz, 2H), 2.79 (s, 3H), 2.59 (t, J = 6.0 Hz, 2H), 2.26–2.18 (m, 2H), 1.39 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 195.8, 170.3, 152.0, 148.0, 133.5, 126.3, 122.6, 118.4, 113.7, 37.9, 31.6, 27.4, 26.4, 23.5; IR (KBr) ν: 3426, 1721, 1662, 1651, 1550, 1477, 1461, 1400, 1363, 1286, 1174, 1031, 998, 905, 827, 611 cm–1; HRMS (APCI): calcd for [C18H21NO2 + H]+, 284.1645; found, 284.1647.
9-Acetyl-2,2-dimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ea)
Petroleum ether/ethyl acetate 8:1; yield 81% (103 mg, 0.41 mmol); white solid; mp 132–134 °C; 1H NMR (300 MHz, CDCl3): δ 8.28 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 8.7 Hz, 1H), 7.33–7.30 (m, 2H), 3.11 (s, 2H), 2.76 (s, 3H), 2.41 (s, 2H), 1.13 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 195.5, 170.8, 150.7, 136.1, 126.4, 125.3, 124.9, 122.2, 116.9, 114.7, 52.0, 40.6, 35.5, 29.0, 27.9; IR (KBr) ν: 3403, 1724, 1709, 1651, 1621, 1557, 1484, 1443, 1352, 1295, 1272, 1176, 1140, 1071, 1010, 939, 768, 752, 679, 654 cm–1; HRMS (APCI): calcd for [C16H17NO2 + H]+, 256.1332; found, 256.1331.
9-Acetyl-2,2,6-trimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ec)
Petroleum ether/ethyl acetate 8:1; yield 83% (111 mg, 0.42 mmol); white solid; mp 120–122 °C; 1H NMR (300 MHz, CDCl3): δ 8.13 (s, 1H), 7.69 (d, J = 8.7 Hz, 1H), 7.16 (d, J = 8.4 Hz, 1H), 3.17 (s, 2H), 2.80 (s, 2H), 2.47 (s, 3H), 1.17 (s, 6H) m; 13C NMR (75 MHz, CDCl3): δ 195.7, 170.8, 151.0, 134.8, 134.3, 126.6, 126.5, 122.3, 116.8, 114.3, 52.1, 40.7, 35.5, 27.9, 21.5; IR (KBr) ν: 3467, 1712, 1662, 1553, 1459, 1402, 1366, 1345, 1299, 1190, 1069, 998, 818, 631 cm–1; HRMS (APCI): calcd for [C17H19NO2 + H]+, 270.1489; found, 270.1485.
9-Acetyl-6-(tert-butyl)-2,2-dimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (3ed)
Petroleum ether/ethyl acetate 8:1; yield 86% (134 mg, 0.43 mmol); white solid; mp 73–75 °C; 1H NMR (300 MHz, CDCl3): δ 8.35 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.41 (d, J = 8.7 Hz, 1H), 3.18 (s, 1H), 2.80 (s, 3H), 2.47 (s, 2H), 1.39 (s, 9H), 1.17 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 195.4, 170.4, 150.7, 148.0, 133.7, 126.1, 122.6, 118.3, 116.7, 113.7, 51.8, 40.3, 35.1, 34.7, 31.6, 28.6, 27.5; IR (KBr) ν: 3444, 1717, 1655, 1553, 1468, 1363, 1297, 1258, 1190, 1144, 1008, 903, 825, 622 cm–1; HRMS (APCI): calcd for [C20H25NO2 + H]+, 312.1958; found, 312.1956.
2-Phenyl-9-propionyl-2,3-dihydro-1H-carbazol-4(9H)-one (3am)
Petroleum ether/ethyl acetate 8:1; yield 86% (136 mg, 0.43 mmol); white solid; mp 158–160 °C; 1H NMR (300 MHz, CDCl3): δ 8.43–8.29 (m, 1H), 7.86–7.84 (m, 1H), 7.44–7.26 (m, 7H), 3.71–3.54 (m, 2H), 3.45–3.36 (m, 1H), 3.08 (q, J = 6.3 Hz, 2H), 2.99–2.82 (m, 2H), 1.36 (t, J = 6.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 194.4, 174.4, 150.9, 142.7, 135.5, 128.8, 127.2, 126.9, 126.0, 125.1, 124.6, 122.1, 114.4, 44.6, 42.0, 34.5, 32.7, 8.9; IR (KBr) ν: 3458, 1730, 1653, 1637, 1600, 1548, 1484, 1450, 1413, 1345, 1249, 1135, 1053, 955, 880, 818, 759, 701 cm–1; HRMS (APCI): calcd for [C21H19NO2 + H]+, 318.1489; found, 318.1487.
2-Methyl-9-propionyl-2,3,9,9a-tetrahydro-1H-carbazol-4(4aH)-one (3cm)
Petroleum ether/ethyl acetate 8:1; yield 76% (97 mg, 0.38 mmol); white solid; mp 128–130 °C; 1H NMR (300 MHz, CDCl3): δ 8.33 (d, J = 8.7 Hz, 1H), 7.83 (d, J = 8.7 Hz, 1H), 7.35–7.33 (m, 2H), 3.49–3.41 (m, 1H), 3.09 (q, J = 6.9 Hz, 2H), 2.96–2.87 (m, 1H), 2.64–2.60 (m, 1H), 2.40–2.31 (m, 2H), 1.38 (t, J = 6.9 Hz, 3H), 1.22 (d, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 195.4, 174.5, 151.5, 135.4, 126.1, 124.9, 124.5, 121.9, 117.2, 114.3, 46.1, 34.6, 32.7, 31.3, 21.3, 8.9; IR (KBr) ν: 3449, 1730, 1651, 1639, 1600, 1559, 1548, 1484, 1450, 1407, 1345, 1254, 1138, 1051, 1017, 953, 882, 825, 759, 702, 536 cm–1; HRMS (APCI): calcd for [C16H17NO2 + H]+, 256.1332; found, 256.1333.
2-Methyl-9-(2-phenylacetyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3cn)
Petroleum ether/ethyl acetate 8:1; yield 73% (115 mg, 0.36 mmol); white solid; mp 142–144 °C; 1H NMR (300 MHz, CDCl3): δ 8.34 (d, J = 7.5 Hz, 1H), 7.87 (d, J = 7.5 Hz, 1H), 7.40–7.26 (m, 7H), 4.41 (m, 2H), 3.45–3.38 (m, 1H), 2.94–2.85 (m, 1H), 2.64–2.60 (m, 1H), 2.40–2.31 (m, 2H), 1.18 (d, J = 5.7 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 195.4, 171.8, 151.5, 135.4, 132.6, 129.4, 128.9, 127.7, 126.2, 125.1, 124.7, 122.1, 117.5, 114.2, 46.1, 45.4, 34.4, 31.3, 21.2; IR (KBr) ν: 3408, 1719, 1653, 1605, 1555, 1484, 1445, 1416, 1350, 1325, 1264, 1142, 1097, 1053, 939, 766, 702 cm–1; HRMS (APCI): calcd for [C22H21NO2 + H]+, 332.1645; found, 332.1642.
2,2-Dimethyl-9-propionyl-2,3-dihydro-1H-carbazol-4(9H)-one (3em)
Petroleum ether/ethyl acetate 8:1; yield 86% (115.81 mg, 0.43 mmol); white solid; mp 94–96 °C; 1H NMR (300 MHz, CDCl3): δ 8.32 (d, J = 8.7 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.35–7.33 (m, 2H), 3.19 (s, 2H), 3.09 (q, J = 6.9 Hz, 2H), 2.47 (s, 2H), 1.38 (t, J = 6.9 Hz, 3H), 1.16 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 195.3, 174.6, 150.6, 135.5, 126.1, 124.8, 124.5, 122.0, 116.5, 114.3, 51.7, 40.4, 32.8, 28.6, 8.8; IR (KBr) ν: 3408, 1728, 1712, 1661, 1557, 1484, 1450, 1416, 1402, 1354, 1314, 1293, 1272, 1179, 1069, 1008, 766, 754, 677, 672 cm–1; HRMS (APCI): calcd for [C17H19NO2 + H]+, 270.1489; found, 270.1484.
2,2-Dimethyl-9-(2-phenylacetyl)-2,3-dihydro-1H-carbazol-4(9H)-one (3en)
Petroleum ether/ethyl acetate 8:1; yield 78% (129 mg, 0.39 mmol); white solid; mp 165–167 °C; 1H NMR (300 MHz, CDCl3): δ 8.35 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 8.4 Hz, 1H), 7.38–7.35 (m, 5H), 7.29–7.26 (m, 2H), 4.42 (s, 2H), 3.15 (s, 2H), 2.47 (s, 2H), 1.13 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 195.1, 171.9, 150.6, 135.5, 132.6, 129.3, 128.9, 127.7, 126.2, 125.0, 124.7, 122.1, 116.6, 114.2, 51.7, 45.4, 40.1, 35.1, 28.5, 28.0; IR (KBr) ν: 3411, 1715, 1661, 1609, 1573, 1456, 1445, 1421, 1369, 1386, 1264, 1142, 1097, 1053, 10025, 768, 705 cm–1; HRMS (APCI): calcd for [C21H25NO2 + H]+, 324.1958; found, 342.1961.
9-(Cyclohexanecarbonyl)-2,2-dimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (3eo)
Petroleum ether/ethyl acetate 8:1; yield 81% (131 mg, 0.41 mmol); white solid; mp 88–90 °C; 1H NMR (300 MHz, CDCl3): δ 8.31 (d, J = 8.7 Hz, 1H), 7.63 (d, J = 8.7 Hz, 1H), 7.35–7.26 (m, 2H), 3.28–3.22 (m, 1H), 3.08 (s, 2H), 2.47 (s, 2H) 2.05–2.01 (m, 2H), 1.90–1.88 (m, 3H), 1.78–7.65 (m, 3H), 1.46–1.35 (m, 3H), 1.15 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 195.0, 177.6, 150.5, 135.4, 126.0, 124.7, 124.3, 122.0, 115.9, 113.3, 51.8, 46.0, 39.7, 35.2, 29.3, 28.6, 25.5, 25.4; IR (KBr) ν: 3408, 1717, 1655, 1603, 1553, 1484, 1354, 1347, 1256, 1144, 1126, 1087, 998, 750, 593 cm–1; HRMS (APCI): calcd for [C21H25NO2 + H]+, 324.1958; found, 324.1961.
2,2-Dimethyl-2,3-dihydro-1H-carbazol-4(9H)-one (4ea)
Petroleum ether/ethyl acetate 6:1; yield 95% (101 mg, 0.48 mmol); white solid; mp 94–96 °C; 1H NMR (300 MHz, CDCl3): δ 9.73 (s, 1H), 8.20 (d, J = 8.1 Hz, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.24–7.21 (m, 2H), 2.83 (s, 2H), 2.47 (s, 2H), 1.14 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 194.1, 151.1, 136.2, 124.6, 123.1, 122.4, 121.1, 111.3, 52.2, 37.3, 35.7, 28.6; IR (KBr) ν: 3421, 1728, 1707, 1655, 1623, 1555, 1480, 1448, 1420, 1351, 1341, 1181, 1069, 1021, 998, 941, 769, 754, 675 cm–1; HRMS (APCI): calcd for [C14H17NO + H]+, 215.1323; found, 215.1325.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 21772001, 21372008), the Natural Science Foundation of Education Administration of Anhui Province (no. KJ2016A267), and the Special and Excellent Research Fund of Anhui Normal University.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01637.
The authors declare no competing financial interest.
Supplementary Material
References
- a Hu L.; Li Z.-r.; Li Y.; Qu J.; Ling Y.-H.; Jiang J.-d.; Boykin D. W. Synthesis and structure–activity relationships of carbazole sulfonamides as a novel class of antimitotic agents against solid tumors. J. Med. Chem. 2006, 49, 6273–6282. 10.1021/jm060546h. [DOI] [PubMed] [Google Scholar]; b Schmidt A. W.; Reddy K. R.; Knölker H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]; c Sekhar B. C.; Ramadas S. R.; Ramana D. V. β-Halovinylaldehydes-as versatile reactive intermediates in the syntheses of condensed fused ring polycyclic heterocycles. Heterocycles 2000, 52, 941–977. 10.3987/rev-99-527. [DOI] [Google Scholar]
- Wang T.; Mäser P.; Picard D. Inhibition of plasmodium falciparum Hsp90 contributes to the antimalarial activities of aminoalcohol-carbazoles. J. Med. Chem. 2016, 59, 6344–6352. 10.1021/acs.jmedchem.6b00591. [DOI] [PubMed] [Google Scholar]
- a Liu Y.; Meng F.; He L.; Yu X.; Lin W. Fluorescence behavior of a unique two-photon fluorescent probe in aggregate and solution states and highly sensitive detection of RNA in water solution and living systems. Chem. Commun. 2016, 52, 8838–8841. 10.1039/c6cc03746a. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Chi L.; Ji S.; Wu Y.; Song P.; Han K.; Guo H.; James T. D.; Zhao J. Rational design of d-PeT phenylethynylated-carbazole monoboronic acid fluorescent sensors for the selective detection of α-hydroxyl carboxylic acids and monosaccharides. J. Am. Chem. Soc. 2009, 131, 17452–17463. 10.1021/ja9060646. [DOI] [PubMed] [Google Scholar]; c Zhang X.; Wu Y.; Ji S.; Guo H.; Song P.; Han K.; Wu W.; James T. D.; Zhao J. Effect of the electron donor/acceptor orientation on the fluorescence transduction efficiency of the d-PET effect of carbazole-based fluorescent boronic acid sensors. J. Org. Chem. 2010, 75, 2578–2588. 10.1021/jo100119y. [DOI] [PubMed] [Google Scholar]; d Wu Y.; Guo H.; James T. D.; Zhao J. Enantioselective recognition of mandelic acid by a 3,6-dithiophen-2-yl-9H-carbazole-based chiral fluorescent bisboronic acid sensor. J. Org. Chem. 2011, 76, 5685–5695. 10.1021/jo200675j. [DOI] [PubMed] [Google Scholar]
- a Yang X.; Zhou G.; Wong W.-Y. Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices. Chem. Soc. Rev. 2015, 44, 8484–8575. 10.1039/c5cs00424a. [DOI] [PubMed] [Google Scholar]; b Albrecht K.; Matsuoka K.; Yokoyama D.; Sakai Y.; Nakayama A.; Fujita K.; Yamamoto K. Thermally activated delayed fluorescence OLEDs with fully solution processed organic layers exhibiting nearly 10% external quantum efficiency. Chem. Commun. 2017, 53, 2439–2442. 10.1039/c6cc09275f. [DOI] [PubMed] [Google Scholar]; c Thomas K. R. J.; Lin J.; Tao Y.-T.; Ko C.-W. Light-emitting carbazole derivatives: potential electroluminescent materials. J. Am. Chem. Soc. 2001, 123, 9404–9411. 10.1021/ja010819s. [DOI] [PubMed] [Google Scholar]
- a Knölker H.-J.; Reddy K. R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]; b Konkol L. C.; Guo F.; Sarjeant A. A.; Thomson R. J. Enantioselective total synthesis and studies into the configurational stability of Bismurrayaquinone A. Angew. Chem., Int. Ed. 2011, 50, 9931–9934. 10.1002/anie.201104726. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Dhara K.; Mandal T.; Das J.; Dash J. Synthesis of carbazole alkaloids by ring-closing metathesis and ring rearrangement-aromatization. Angew. Chem., Int. Ed. 2015, 54, 15831–15835. 10.1002/anie.201508746. [DOI] [PubMed] [Google Scholar]; d Alt I. T.; Plietker B. Iron-catalyzed intramolecular C(sp2)–H amination. Angew. Chem., Int. Ed. 2016, 55, 1519–1522. 10.1002/anie.201510045. [DOI] [PubMed] [Google Scholar]; e Noji T.; Fujiwara H.; Okano K.; Tokuyama H. Synthesis of substituted indoline and carbazole by benzyne-mediated cyclization–functionalization. Org. Lett. 2013, 15, 1946–1949. 10.1021/ol400597f. [DOI] [PubMed] [Google Scholar]; f Ueno A.; Kitawaki T.; Chida N. Total synthesis of (±)-Murrayazoline. Org. Lett. 2008, 10, 1999–2002. 10.1021/ol800602v. [DOI] [PubMed] [Google Scholar]
- a Li X.; Vince R. Conformationally restrained carbazolone-containing α,γ-diketo acids as inhibitors of HIV integrase. Bioorg. Med. Chem. 2006, 14, 2942–2955. 10.1016/j.bmc.2005.12.013. [DOI] [PubMed] [Google Scholar]; b Kudzma L. V. Synthesis of substituted indoles and carbazoles from 2-fluorophenyl imines. Synthesis 2003, 11, 1661–1666. 10.1055/s-2003-40880. [DOI] [Google Scholar]
- a Janreddy D.; Kavala V.; Bosco J. W. J.; Kuo C.-W.; Yao C.-F. An easy access to carbazolones and 2,3-disubstituted indoles. Eur. J. Org. Chem. 2011, 2011, 2360–2365. 10.1002/ejoc.201001357. [DOI] [Google Scholar]; b Smitrovich J. H.; Davies I. W. Catalytic C–H functionalization driven by CO as a stoichiometric reductant: application to carbazole synthesis. Org. Lett. 2004, 6, 533–535. 10.1021/ol036294l. [DOI] [PubMed] [Google Scholar]
- a Willis M. C.; Brace G. N.; Holmes I. P. Palladium-catalyzed tandem alkenyl and aryl C–N bond formation: a cascade N-annulation route to 1-functionalized indoles. Angew. Chem., Int. Ed. 2005, 44, 403–406. 10.1002/anie.200461598. [DOI] [PubMed] [Google Scholar]; b Jiang Y.; Yan S.; Wu H.; Wu N. A mild and effective method to synthesize carbazolones by CuI/L-Proline-catalyzed intramolecular arylation. Synlett 2007, 17, 2699–2702. 10.1055/s-2007-991079. [DOI] [Google Scholar]; c Alberico D.; Scott M. E.; Lautens M. Aryl–aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- a Witulski B.; Alayrac C.; Tevzadze-Saeftel L. Palladium-catalyzed synthesis of 2-aminoindoles by a heteroanulation reaction. Angew. Chem., Int. Ed. 2003, 42, 4257–4260. 10.1002/anie.200351977. [DOI] [PubMed] [Google Scholar]; b Würtz S.; Rakshit S.; Neumann J. J.; Dröge T.; Glorius F. Palladium-catalyzed oxidative cyclization of N-aryl enamines: from anilines to indoles. Angew. Chem., Int. Ed. 2008, 47, 7230–7233. 10.1002/anie.200802482. [DOI] [PubMed] [Google Scholar]; c Maiti S.; Mal P. Dehydrogenative aromatic ring fusion for carbazole synthesis via C–C/C–N bond formation and alkyl migration. Org. Lett. 2017, 19, 2454–2457. 10.1021/acs.orglett.7b01117. [DOI] [PubMed] [Google Scholar]; d Iida H.; Yuasa Y.; Kibayashi C. Intramolecular cyclization of enaminones involving arylpalladium complexes. Synthesis of carbazoles. J. Org. Chem. 1980, 45, 2938–2942. 10.1021/jo01303a003. [DOI] [Google Scholar]
- a Jaiswal P. K.; Biswas S.; Singh S.; Samanta S. An organocatalytic highly efficient approach to the direct synthesis of substituted carbazoles in water. Org. Biomol. Chem. 2013, 11, 8410–8418. 10.1039/c3ob42034e. [DOI] [PubMed] [Google Scholar]; b Zheng X.; Lv L.; Lu S.; Wang W.; Li Z. Benzannulation of indoles to carbazoles and its applications for syntheses of carbazole alkaloids. Org. Lett. 2014, 16, 5156–5159. 10.1021/ol5025053. [DOI] [PubMed] [Google Scholar]; c Paul K.; Bera K.; Jalal S.; Sarkar S.; Jana U. Fe-catalyzed novel domino isomerization/cyclodehydration of substituted 2-[(Indoline-3-ylidene)(methyl)]benzaldehyde derivatives: an efficient approach toward benzo[b]carbazole derivatives. Org. Lett. 2014, 16, 2166–2169. 10.1021/ol500505k. [DOI] [PubMed] [Google Scholar]; d Zhou L.; Xu B.; Zhang J. Metal-free dehydrogenative Diels-Alder reactions of 2-methyl-3-alkylindoles with dienophiles: rapid access to tetrahydrocarbazoles, carbazoles, and heteroacenes. Angew. Chem., Int. Ed. 2015, 54, 9092–9096. 10.1002/anie.201503549. [DOI] [PubMed] [Google Scholar]; e Liu Y.; Guo Y.; Ji F.; Gao D.; Song C.; Chang J. Divergent syntheses of carbazole alkaloids clausenapin, indizoline, claulansine M, and clausenaline D. J. Org. Chem. 2016, 81, 4310–4315. 10.1021/acs.joc.6b00729. [DOI] [PubMed] [Google Scholar]; f Chen S.; Li Y.; Ni P.; Huang H.; Deng G.-J. Indole-to-carbazole strategy for the synthesis of substituted carbazoles under metal-free conditions. Org. Lett. 2016, 18, 5384–5387. 10.1021/acs.orglett.6b02762. [DOI] [PubMed] [Google Scholar]
- a Jiao L.; Zhang Z.-X.; Chen S.-C. Asymmetric total synthesis of (+)-Minfiensine by an asymmetric cascade cyclization strategy. Synlett 2017, 28, 2199–2204. 10.1055/s-0036-1589075. [DOI] [PubMed] [Google Scholar]; b Jordan-Hore J. A.; Johansson C. C. C.; Gulias M.; Beck E. M.; Gaunt M. J. Oxidative Pd(II)-catalyzed C–H bond amination to carbazole at ambient temperature. J. Am. Chem. Soc. 2008, 130, 16184–16186. 10.1021/ja806543s. [DOI] [PubMed] [Google Scholar]; c Xu D.-Q.; Wu J.; Luo S.-P.; Zhang J.-X.; Wu J.-Y.; Du X.-H.; Xu Z.-Y. Fischer indole synthesis catalyzed by novel SO3H-functionalized ionic liquids in water. Green Chem. 2009, 11, 1239–1246. 10.1039/b901010f. [DOI] [Google Scholar]; d Li J.-H.; Weng B.; Liu R. An improved method for the synthesis of carbazolones by palladium/copper-catalyzed intramolecular annulation of N-arylenaminones. Synthesis 2010, 17, 2926–2930. 10.1055/s-0030-1258141. [DOI] [Google Scholar]; e Jash M.; Das B.; Chowdhury C. One-pot access to benzo[a]carbazoles via palladium(II)-catalyzed hetero- and carboannulations. J. Org. Chem. 2016, 81, 10987–10999. 10.1021/acs.joc.6b02022. [DOI] [PubMed] [Google Scholar]
- a Scott T. L.; Söderberg B. C. G. Novel palladium-catalyzed synthesis of 1,2-dihydro-4(3H)-carbazolones. Tetrahedron Lett. 2002, 43, 1621–1624. 10.1016/s0040-4039(02)00072-2. [DOI] [Google Scholar]; b Scott T. L.; Söderberg B. C. G. Palladium-catalyzed synthesis of 1,2-dihydro-4(3H)-carbazolones. Formal total synthesis of murrayaquinone A. Tetrahedron 2003, 59, 6323–6332. 10.1016/s0040-4020(03)00976-1. [DOI] [Google Scholar]; c Scott T. L.; Burke N.; Carrero-Martínez G.; Söderberg B. C. G. Synthesis of 1,2,3,4-tetrahydrocarbazoles and related tricyclic indoles. Tetrahedron 2007, 63, 1183–1190. 10.1016/j.tet.2006.11.052. [DOI] [Google Scholar]; d Yan Q.; Gin E.; Wasinska-Kalwa M.; Banwell M. G.; Carr P. D. A Palladium-catalyzed Ullmann cross-coupling/reductive cyclization route to the carbazole natural products 3-methyl-9H-carbazole, Glycoborine, Glycozoline, Clauszoline K, Mukonine, and Karapinchamine A. J. Org. Chem. 2017, 82, 4148–4159. 10.1021/acs.joc.7b00044. [DOI] [PubMed] [Google Scholar]
- a Loy N. S. Y.; Kim S.; Park C.-M. Synthesis of unsymmetrical pyrazines based on α-diazo oxime ethers. Org. Lett. 2015, 17, 395–397. 10.1021/ol5034173. [DOI] [PubMed] [Google Scholar]; b Keipour H.; Ollevier T. Iron-catalyzed carbene insertion reactions of α-diazoesters into Si–H bonds. Org. Lett. 2017, 19, 5736–5739. 10.1021/acs.orglett.7b02488. [DOI] [PubMed] [Google Scholar]; c Qiu H.; Srinivas H. D.; Zavalij P. Y.; Doyle M. P. Unprecedented intramolecular [4 + 2] cycloaddition between a 1,3-diene and a diazo ester. J. Am. Chem. Soc. 2016, 128, 1808–1811. 10.1021/jacs.5b12877. [DOI] [PubMed] [Google Scholar]; e Fructos M. R.; Díaz-Requejo M. M.; Pérez P. J. Gold and diazo reagents: a fruitful tool for developing molecular complexity. Chem. Commun. 2016, 52, 7326–7355. 10.1039/c6cc01958g. [DOI] [PubMed] [Google Scholar]; f Xu X.; Deng Y.; Yim D. N.; Zavalij P. Y.; Doyle M. P. Enantioselective cis-β-lactam synthesis by intramolecular C–H functionalization from enoldiazoacetamides and derivative donor–acceptor cyclopropenes. Chem. Sci. 2015, 6, 2196–2201. 10.1039/c4sc03991b. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Best D.; Jean M.; van de Weghe P. Modular synthesis of arylacetic acid esters, thioesters, and amides from aryl ethers via Rh(II)-catalyzed diazo arylation. J. Org. Chem. 2016, 81, 7760–7770. 10.1021/acs.joc.6b01426. [DOI] [PubMed] [Google Scholar]; h Zhu R.; Cheng G.; Jia C.; Xue L.; Cui X. Access to C4-functionalized quinolines via copper-catalyzed tandem annulation of alkynyl imines with diazo compounds. J. Org. Chem. 2016, 81, 7539–7544. 10.1021/acs.joc.6b01227. [DOI] [PubMed] [Google Scholar]; i Ravi M.; Allu S.; Swamy K. C. K. Rhodium(III)-catalyzed ortho-alkylation of phenoxy substrates with diazo compounds via C–H activation: a case of decarboxylative pyrimidine/pyridine migratory cyclization rather than removal of pyrimidine/pyridine directing group. J. Org. Chem. 2017, 82, 2355–2363. 10.1021/acs.joc.6b02693. [DOI] [PubMed] [Google Scholar]; j Du F.; Zhou J.; Peng Y. Asymmetric reaction of α-diazomethylphosphonates with α-ketoesters to access optically active α-diazo-β-hydroxyphosphonate derivatives. Org. Lett. 2017, 19, 1310–1313. 10.1021/acs.orglett.7b00128. [DOI] [PubMed] [Google Scholar]
- a Ye T.; Mckervey M. A. Organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 1994, 94, 1091–1160. 10.1021/cr00028a010. [DOI] [PubMed] [Google Scholar]; b Davies H. M. L.; Hedley S. J. Intermolecular reactions of electron-rich heterocycles with copper and rhodium carbenoids. Chem. Soc. Rev. 2007, 36, 1109–1119. 10.1039/b607983k. [DOI] [PubMed] [Google Scholar]; c Davies H. M. L.; Denton J. R. Application of donor/acceptor-carbenoids to the synthesis of natural products. Chem. Soc. Rev. 2009, 38, 3061–3071. 10.1039/b901170f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ford A.; Miel H.; Ring A.; Slattery C. N.; Maguire A. R.; Mckervey M. A. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015, 115, 9981–10080. 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]; e Marinozzi M.; Pertusati F.; Serpi M. λ5-Phosphorus-containing α-diazo compounds: A valuable tool for accessing phosphorus-functionalized molecules. Chem. Rev. 2016, 116, 13991–14055. 10.1021/acs.chemrev.6b00373. [DOI] [PubMed] [Google Scholar]; f Sharma S.; Han S. H.; Han S.; Ji W.; Oh J.; Lee S.-Y.; Oh J. S.; Jung Y. H.; Kim I. S. Rh(III)-catalyzed direct coupling of azobenzenes with α-diazo esters: facile synthesis of cinnolin-3(2H)-ones. Org. Lett. 2015, 17, 2852–2855. 10.1021/acs.orglett.5b01298. [DOI] [PubMed] [Google Scholar]; g Choi M.; Park J.; Mishra N. K.; Lee S.-Y.; Kim J. H.; Jeong K. M.; Lee J.; Jung Y. H.; Kim I. S. Rh(III)-catalyzed C–H alkylation of 2-arylbenzothiazoles with α-diazo esters. Tetrahedron Lett. 2015, 56, 4678–4682. 10.1016/j.tetlet.2015.06.036. [DOI] [Google Scholar]; h Han S. H.; Mishra N. K.; Kim I. S. Rh(III)-catalyzed ortho-alkylation of N-benzyltriflamides with diazo compounds. Bull. Korean Chem. Soc. 2015, 36, 2823–2828. 10.1002/bkcs.10574. [DOI] [Google Scholar]
- For reviews on Rh(III) catalyzed C-H activation, see; a Satoh T.; Miura M. Oxidative coupling of aromatic substrates with alkynes and alkenes under rhodium catalysis. Chem.—Eur. J. 2010, 16, 11212–11222. 10.1002/chem.201001363. [DOI] [PubMed] [Google Scholar]; b Wencel-Delord J.; Dröge T.; Liu F.; Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]; c Colby D. A.; Tsai A. S.; Bergman R. G.; Ellman J. A. Rhodium catalyzed chelation-assisted C–H bond functionalization reactions. Acc. Chem. Res. 2012, 45, 814–825. 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Song G.; Wang F.; Li X. C–C, C–O and C–N bond formation via rhodium(III)-catalyzed oxidative C–H activation. Chem. Soc. Rev. 2012, 41, 3651–3678. 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; e Kuhl N.; Schröder N.; Glorius F. Formal SN-type reactions in rhodium(III)-catalyzed C–H bond activation. Adv. Synth. Catal. 2014, 356, 1443–1460. 10.1002/adsc.201400197. [DOI] [Google Scholar]; f Shi L.; Yu K.; Wang B. Regioselective synthesis of multisubstituted isoquinolones and pyridones via rh(III)-catalyzed annulation reactions. Chem. Commun. 2015, 51, 17277–17280. 10.1039/c5cc05977a. [DOI] [PubMed] [Google Scholar]; g Cheng Y.; Bolm C. Regioselective syntheses of 1,2-benzothiazines by rhodium-catalyzed annulation reactions. Angew. Chem., Int. Ed. 2015, 54, 12349–12352. 10.1002/anie.201501583. [DOI] [PubMed] [Google Scholar]; h Patel P.; Borah G. Direct access to indoles by IrIII-catalyzed C-H functionalization of acetanilides with diazo compounds. Eur. J. Org. Chem. 2017, 2017, 2272–2279. 10.1002/ejoc.201700095. [DOI] [Google Scholar]
- a Presset M.; Coquerel Y.; Rodriguez J. Microwave-assisted wolff rearrangement of cyclic 2-diazo-1,3-diketones: an eco-compatible route to α-carbonylated cycloalkanones. J. Org. Chem. 2009, 74, 415–418. 10.1021/jo8021567. [DOI] [PubMed] [Google Scholar]; b Presset M.; Coquerel Y.; Rodriguez J. Microwave-assisted domino and multicomponent reactions with cyclic acylketenes: expeditious syntheses of oxazinones and oxazindiones. Org. Lett. 2009, 11, 5706–5709. 10.1021/ol9024056. [DOI] [PubMed] [Google Scholar]; c Galvez J.; Castillo J.-C.; Quiroga J.; Rajzmann M.; Rodriguez J.; Coquerel Y. Divergent chemo-, regio-, and diastereoselective normal electron-demand povarov-type reactions with α-oxo-ketene dienophiles. Org. Lett. 2014, 16, 4126–4129. 10.1021/ol5018245. [DOI] [PubMed] [Google Scholar]; d Castillo J.-C.; Presset M.; Abonia R.; Coquerel Y.; Rodriguez J. Microwave-assisted domino benzannulation of α-oxo ketenes: preparation of 1,3-dihydro-2H-1,5-benzodiazepin-2-ones. Eur. J. Org. Chem. 2012, 2012, 2338–2345. 10.1002/ejoc.201200093. [DOI] [Google Scholar]; e Mohanan K.; Presset M.; Mailhol D.; Coquerel Y.; Rodriguez J. Catalyst- and halogen-free regioselective Friedel-Crafts α-ketoacylations. Chem.—Eur. J. 2012, 18, 9217–9220. 10.1002/chem.201201027. [DOI] [PubMed] [Google Scholar]; f Boddaert T.; Coquerel Y.; Rodriguez J. Combination of rearrangement with metallic and organic catalyses—a step- and atom-economical approach to α-spiroactones and -lactams. Eur. J. Org. Chem. 2011, 2011, 5061–5070. 10.1002/ejoc.201100734. [DOI] [Google Scholar]; g Boddaert T.; Coquerel Y.; Rodriguez J. Expeditious divergent synthetic approach to polycyclic terpene-like molecules. Chem.—Eur. J. 2011, 17, 2048–2051. 10.1002/chem.201003261. [DOI] [PubMed] [Google Scholar]; h Presset M.; Coquerel Y.; Rodriguez J. Periselectivity switch of acylketenes in cycloadditions with 1-azadienes: microwave-assisted diastereoselective domino three-component synthesis of α-spiro-δ-lactams. Org. Lett. 2010, 12, 4212–4215. 10.1021/ol101938r. [DOI] [PubMed] [Google Scholar]; i Presset M.; Mohanan K.; Hamann M.; Coquerel Y.; Rodriguez J. 1,3-Dipolar cycloaddition of hydrazones with α-oxo-ketenes: a three-component stereoselective entry to pyrazolidinones and an original class of spirooxindoles. Org. Lett. 2011, 13, 4124–4127. 10.1021/ol2016669. [DOI] [PubMed] [Google Scholar]
- a Xia L.; Lee Y. R. Regioselective synthesis of highly functionalized furans through the RuII-catalyzed [3+2] cycloaddition of diazodicarbonyl compounds. Eur. J. Org. Chem. 2014, 2014, 3430–3442. 10.1002/ejoc.201402067. [DOI] [Google Scholar]; b Du T.; Du F.; Ning Y.; Peng Y. Organocatalytic enantioselective 1,3-dipolar cycloadditions between Seyferth–Gilbert reagent and isatylidene malononitriles: synthesis of chiral spiro-phosphonylpyrazoline-oxindoles. Org. Lett. 2015, 17, 1308–1311. 10.1021/acs.orglett.5b00311. [DOI] [PubMed] [Google Scholar]
- a Castillo J.-C.; Quiroga J.; Rodriguez J.; Coquerel Y. Time-efficient synthesis of pyrido[2,3-d]pyrimidinones via α-oxoketenes. Eur. J. Org. Chem. 2016, 2016, 1994–1999. 10.1002/ejoc.201600171. [DOI] [Google Scholar]; b Shi J.; Zhou J.; Yan Y.; Jia J.; Liu X.; Song H.; Xu H. E.; Yi W. One-pot cascade synthesis of N-methoxyisoquinolinediones via Rh(III)-catalyzed carbenoid insertion C–H activation/cyclization. Chem. Commun. 2015, 51, 668–671. 10.1039/c4cc08407a. [DOI] [PubMed] [Google Scholar]; c Dudognon Y.; Presset M.; Rodriguez J.; Coquerel Y.; Bugaut X.; Constantieux T. Addition of silylated nucleophiles to α-oxoketenes. Chem. Commun. 2016, 52, 3010–3013. 10.1039/c5cc10217k. [DOI] [PubMed] [Google Scholar]
- a Yang C.; He X.; Zhang L.; Han G.; Zuo Y.; Shang Y. Synthesis of isocoumarins from cyclic 2-diazo-1,3-diketones and benzoic acids via Rh(III)-Catalyzed C–H activation and esterification. J. Org. Chem. 2017, 82, 2081–2088. 10.1021/acs.joc.6b02906. [DOI] [PubMed] [Google Scholar]; b He X.; Zhou Y.; Shang Y.; Yang C.; Zuo Y. Synthesis of 2-arylimino-6,7-dihydrobenzo[d][1,3]oxathiol-4(5H)-ones via Rh2(OAc)4-catalyzed reactions of cyclic 2-diazo-1,3-diketones with aryl isothiocyanates. ACS Omega 2016, 1, 1277–1283. 10.1021/acsomega.6b00295. [DOI] [PMC free article] [PubMed] [Google Scholar]; c He X.; Yu Z.; Zuo Y.; Yang C.; Shang Y. Direct carboxamidation of cyclic 2-diazo-1,3-diketones by Rh2(OAc)4-catalyzed isocyanide insertion–hydrolysis. Org. Biomol. Chem. 2017, 15, 7127–7130. 10.1039/c7ob01801k. [DOI] [PubMed] [Google Scholar]
- Li X. G.; Sun M.; Liu K.; Jin Q.; Liu P. N. Rh(III)-catalyzed C–H activation/cyclization of benzamides and diazo compounds to form isocoumarins and α-pyrones. Chem. Commun. 2015, 51, 2380–2383. 10.1039/c4cc09314c. [DOI] [PubMed] [Google Scholar]
- a Patel P.; Borah G. Synthesis of oxindole from acetanilide via Ir(III)-catalyzed C–H carbenoid functionalization. Chem. Commun. 2017, 53, 443–446. 10.1039/c6cc08788d. [DOI] [PubMed] [Google Scholar]; b Mishra N. K.; Choi M.; Jo H.; Oh Y.; Sharma S.; Han S. H.; Jeong T.; Han S.; Lee S.-Y.; Kim I. S. Direct C–H alkylation and indole formation of anilines with diazo compounds under rhodium catalysis. Chem. Commun. 2015, 51, 17229–17232. 10.1039/c5cc07767b. [DOI] [PubMed] [Google Scholar]; c Li X. G.; Sun M.; Jin Q.; Liu K.; Liu P. N. Access to isoquinolines and isoquinolin-3-ols via Rh(III)-catalyzed coupling/cyclization cascade reaction of arylimidates and diazo Compounds. J. Org. Chem. 2016, 81, 3901–3910. 10.1021/acs.joc.6b00264. [DOI] [PubMed] [Google Scholar]; d Liang Y.; Yu B.; Li B.; Xu S.; Song H.; Wang B. Rh(III)-catalyzed synthesis of 1-aminoindole derivatives from 2-acetyl-1-arylhydrazines and diazo compounds in water. Chem. Commun. 2014, 50, 6130–6133. 10.1039/c4cc01520g. [DOI] [PubMed] [Google Scholar]; e Wang Q.; Li X. Rhodium/copper-cocatalyzed annulation of benzylamines with diazo compounds: access to fused isoquinolines. Org. Chem. Front. 2016, 3, 1159–1162. 10.1039/c6qo00287k. [DOI] [Google Scholar]; f Sun P.; Wu Y.; Huang Y.; Wu X.; Xu J.; Yao H.; Lin A. Rh(III)-catalyzed redox-neutral annulation of azo and diazo compounds: one-step access to cinnolines. Org. Chem. Front. 2016, 3, 91–95. 10.1039/c5qo00331h. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.