Abstract
Some earlier studies suggested that graphene oxide (GO) sheets adopt a crumpled configuration in water, similar to the shape of a paper ball, which turns into an even more compact, collapsed form upon addition of a poor solvent due to enhanced intrasheet affinity. Although those results have been debated in studies concerning membrane configurations, they are now often used to justify the existence of folds and wrinkles in solution-processed GO-based structures. This has led to a misconception that wrinkled and crumpled features may be intrinsic to solution processing and unavoidable. Here, we connect this problem to experimental observations of the liquid crystallinity of GO dispersions, which clearly show that the sheets are neither crumpled nor collapsed, with or without poor solvent. The sheets can simply fold flat or restack to hide their surfaces from poor solvent, without incurring the energetic cost of severe deformations to crumple.
Introduction
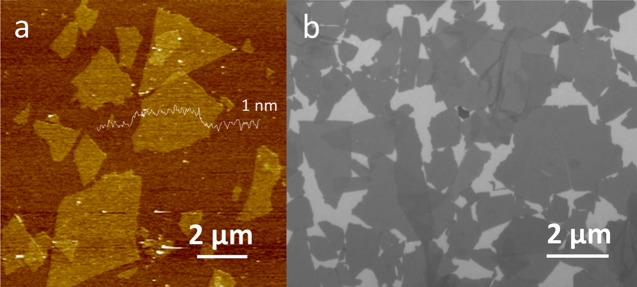
Determining the statistically likely configurations of one-dimensional polymer chains is a straightforward exercise, but the configurations of two-dimensional (2D) solid sheets, first studied decades ago,1 remain a computational and theoretical interest.2−4 Graphene oxide (GO), which used to be called graphite oxide layers, is a single-atomic-layer material made by chemical exfoliation of graphite powders.5 GO sheets can be readily made with apparent thickness of around 1 nm (Figure 1a) and lateral dimensions of micron to tens of micron scale (Figure 1b),6 and they are well dispersible in water. Therefore, they offer a convenient model system to study these questions experimentally. About two and half decades ago, light-scattering studies found that GO sheets in water are fractals with dimension of 2.5, and this was taken to mean that GO sheets adopt a crumpled conformation, akin to that of a crumpled paper ball.7,8 Adding more poor solvent, such as acetone, then further densifies the GO crumples into a collapsed configuration7 with a fractal dimension near 3. In response to the light-scattering studies, Spector et al. conducted freeze-fracture electron microscopy studies but only found some lightly wrinkled sheets, instead of crumpled structures.9 Because the samples for electron microscopy observation needed to be frozen first, there was some speculation that such a sample preparation procedure might have helped the “sloppy” GO crumples to transform into a more flattened shape.10 Both light-scattering and electron microscopy can only provide indirect results to infer the solution configuration of GO sheets, so obtaining a direct, more robust experimental evidence about GO’s configuration in a solution would help settle this debate.
Figure 1.
(a) Atomic force microscopy image showing the apparent thickness of GO single layer to be around 1 nm. (b) Fluorescence quenching microscopy23 image showing an overview of GO sheets, with lateral dimensions, defined as the diameter of the circumscribing circle, at the micron scale.
More than a decade after this question was first experimentally studied, GO has become a subject of extensive interest for alternative reasons, such as a precursor for making graphene,11,12 and as an interesting material in its own right.13 The question of the sheets’ solution configuration has now become technically relevant, especially for solution processing of graphene-based sheets, because the properties of the final materials are affected by features like wrinkles, folds, and crumples.14 Local wrinkles and folds are often observed in solution-processed GO or graphene sheets.14,15 Unfortunately, the results of the earlier studies of the solution conformation of GO sheets, represented by Wen et al.’s work,7 have been widely misapplied in graphene research, which are often used to justify the existence of folds and wrinkles in dried GO or graphene structures. This has become problematic because it has created a misconception that those deformed features in GO or graphene sheets are intrinsic to solution processing and unavoidable, discouraging many from improving processing strategies for making wrinkle-free thin films. Therefore, it has become important to revisit this old problem both to solve a basic soft matter science puzzle and address the new technological problems.
Results and Discussion
Here, we connect some known results from literature and present new experiment observations that clearly show that with or without bad solvent, GO sheets in water are neither crumpled nor collapsed. A simple but effective marker for flat versus crumpled or collapsed configurations is whether they can form a nematic liquid crystalline phase in solution. With their high aspect ratios and excellent dispersity in water, planar GO sheets should be able to align to form a nematic liquid crystalline phase over a concentration threshold predicted by Onsager theory.16,17 In recent years, Kim et al.18 and then Xu et al.19 have first demonstrated GO-based nematic liquid crystalline phases in water. They observed a strong birefringence when viewing aqueous dispersion of micron-sized GO sheets through a pair of cross-polarizers. These results already suggest that GO sheets in water do not adopt a crumpled configuration. If GO sheets were to transform into a shape like crumpled paper balls or the even denser collapsed form, the resulting particles would not have formed a nematic phase at all because their contour is largely isotropic amid the complexity of the structures.
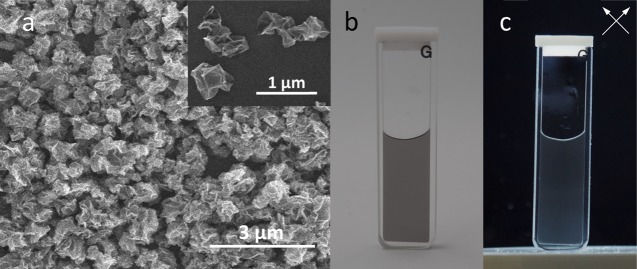
Testing the above-mentioned hypothesis linking sheet configuration and liquid crystallinity would require model colloidal particles with crumpled paper ball shape. In an earlier work, we have successfully created such particles by mechanically squeezing the GO sheets using capillary actions in evaporating aerosol droplets.20 GO can be thermally reduced during or after the aerosol processing, yielding crumpled graphene balls (Figure 2a) with around 2.5 fractal dimension.21 The crumpled morphology is highly stable during various materials processing steps involving mechanical stress or different solvents.20,22 Such morphological stability makes them the ideal model material representing the crumpled configuration under various solvent conditions. When crumpled graphene balls are dispersed in water and viewed through cross-polarizers, as expected, no liquid crystalline phase was observed (Figure 2b,c), no matter how high their concentration was.
Figure 2.
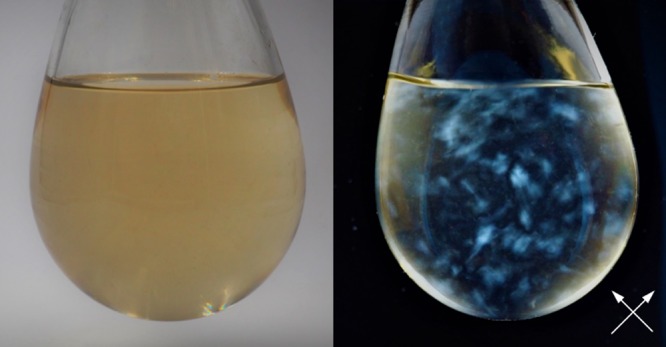
(a) Scanning electron microscopy (SEM) image showing sub-micron-sized crumpled graphene balls. (b) Photo of a dispersion of these particles in water at a concentration of 0.01 wt %, taken under ambient illumination. (c) When viewed through a pair of cross-polarizers (directions marked by white arrows), no birefringence is observed for this sample or others with concentration up to 1 wt %. Thin glass cuvettes with short optical path length (1 mm for the one shown here) are used to allow sufficient light to pass through the dispersion.
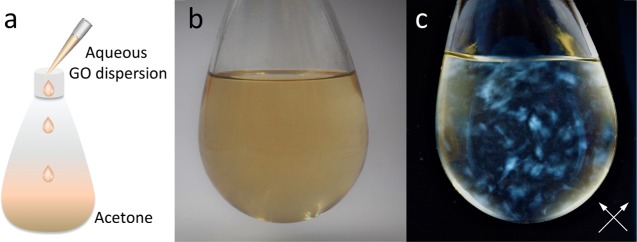
Next, we move to examine whether GO sheets would crumple under poor solvent conditions, as reported in the earlier scattering work.7 GO sheets were prepared and processed based on previously published procedure,5,23 and have similar lateral size distribution as those used in that work. GO dispersions were also prepared at the identical concentration (0.01 wt %) and solvent compositions (i.e., water and water/acetone mixture with 10 vol % of acetone), both of which were found to exhibit birefringence when viewed through cross-polarizers (Figure 3, left two vials). Then, we extended the poor solvent condition and increased the fraction of acetone from 10 to 30, 50, 70, and finally 90 vol %. Birefringence was unambiguously observed under all of these poorer solvent conditions (Figure 3). With 70 and 90 vol % of acetone, GO sheets sedimented after aging—but they did so as gels, which still remained highly birefringent, indicating high orientational ordering in the sediment that can only be achieved with anisotropically shaped units. Besides acetone, a number of other water-miscible solvents such as methanol, ethanol, 2-propanol, and ethylene glycol were also tested. Birefringence was persistently observed in GO aqueous dispersions mixed with these solvents.
Figure 3.
(a) Photo showing freshly prepared 0.01 wt % GO dispersions by mixing aqueous GO stock solution with acetone. From left to right, the volume fractions of acetone are 0, 10, 30, 50, 70, and 90 vol %. (b) All of the samples exhibit obvious birefringence when viewed through a pair of cross-polarizers (directions marked by white arrows). Strong birefringence is also observed for all of the samples after the sheets were sedimented.
To rule out the potential confining influence between the GO sheets that may prevent them from crumpling, GO solution was added dropwise to a large acetone bath over extended period of time (procedure illustrated in Figure 4a). The concentration of GO sheets in the acetone bath starts from near zero and gradually reaches 0.002 wt % by the end of the 6 h of addition (Figure 4b). The volume fraction of the poor solvent acetone started at nearly 100% and gradually reached 80 vol %. If the GO sheets were to crumple or collapse in water/acetone mixtures as concluded in the earlier scattering study,7 this slow addition experiment would have created the most favorable conditions for the sheets to do so during addition, and they would have transformed into crumpled or collapsed geometries by the end of this slow addition experiment. On the contrary, birefringent schlieren texture is still vivid when the final dispersion is gently swirled and observed through cross-polarizers (Figure 4c), indicating a liquid crystalline phase with an orientational order that is characteristic of anisotropically shaped units.
Figure 4.
(a, b) Fifty milliliter of aqueous GO dispersion was added dropwise into an acetone bath of 200 mL over 6 h, reaching a final GO concentration of 0.002 wt %. (c) The resulting dispersion still exhibits a strong birefringence when viewed through a pair of cross-polarizers (directions marked by white arrows).
In control experiments, dispersions of crumpled graphene balls did not exhibit birefringence in any of these poor solvent tests. Taken together, our observations establish that GO sheets neither crumpled nor collapsed in poor solvents. Then, why did not Wen et al. observe the characteristic dimension of 2.0 for flat GO sheets in their scattering experiments?7 Several factors could be involved. As Abraham and Goulian pointed out, scattering measurements can yield a higher-than-two fractal dimension for sheets if their thickness is not uniform, especially when there is a scaling relationship between their width and thickness.24 Such a nonuniformity may be contributed by local roughness on the sheets, but also inhomogeneous dispersion that contains agglomerated or flocculated sheets. Colloidal stability of GO sheets is quite sensitive to the presence of ionic byproducts from synthesis,18,25 which are not trivial to remove.23 Today’s researchers are well equipped with new knowledge about GO’s colloidal properties and processing methods to make high-quality single-layer dispersions. However, the samples used in the earlier scattering work7 may not be well-dispersed in water, as noted in another related work from the same group.8 This suggests that the earlier GO samples may not have been sufficiently purified, which could lead to the formation of small flocculates or aggregates of GO sheets.18 These features may not be obvious to visual observation, but they can yield fractal dimensions in light-scattering experiments.
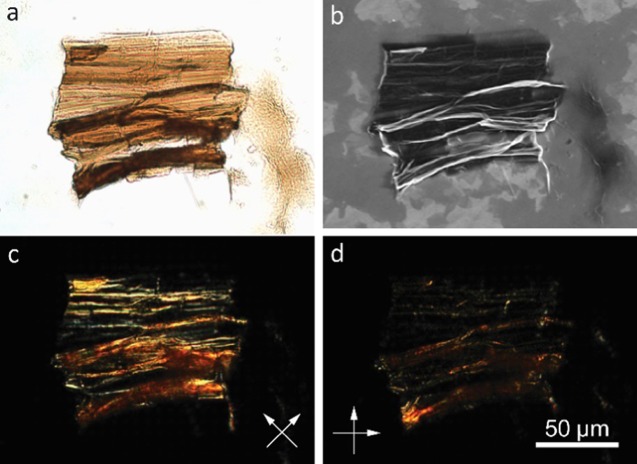
Crumpling a sheet requires substantial mechanical work to generate features like creases and vertices due to severe local deformation,24,26 yet it does a poor job of sheltering GO surfaces from solvent. Even a densely crumpled paper ball still has about half of the surface area of a planar sheet.27 However, face-to-face restacking is a much more efficient way for sheets to hide their surfaces from poor solvent, without incurring the energetic cost of those severe deformations. In all of the experiments involving high acetone concentrations, sediments of GO can be observed. But when the sedimented pieces were collected and viewed under an optical microscope equipped with polarizers, they were all found to be highly birefringent. An optical microscopy image of a piece of sediment collected from the slow addition experiment are shown in Figure 5a. The sample was dropcast onto a conductive, indium tin oxide-coated glass slide to allow SEM studies. Figure 5b is the SEM image of the same piece, which is made of restacked GO sheets. SEM also revealed many flat sheets deposited on the substrate, similar to those reported before.6 Notably, they are neither crumpled nor collapsed. Observation through cross-polarizers (Figure 5c,d) clearly shows the alignment of the sheets in the sediment is parallel to the wrinkles. Therefore, formation of flocculates and aggregates in the samples used for the earlier scattering study might be the root cause of the elevated fractal dimensions observed under the poor solvent conditions.
Figure 5.
(a) Optical and (b) corresponding SEM images of a piece of sediment obtained from slow addition of GO sheets into acetone. The sample was prepared by casting a droplet of the final dispersion containing the piece. In the SEM image (b), many flat sheets can be seen in the background that are neither crumpled nor collapsed. (c, d) Polarized optical microscopy images (polarizer directions marked by white arrows) indicate aligned microstructures along the horizontal wrinkles in this particle.
Conclusions
In conclusion, results that already existed in literature about GO liquid crystals, in conjunction with the new observations reported here, should clearly establish that GO sheets do not crumple nor collapse into morphologies with nearly isotropic contour in poor solvent conditions. Under the conditions most commonly used for solution processing of GO, they maintain an extended, sheet-like shape. When self-attraction becomes stronger, the sheets prefer to restack or fold flat, forming anisotropic platelets. Although it is not always obvious, some form of compressive stress, especially capillary action, is always present during solution processing, which can readily deform the highly flexible GO sheets28 to form extensively folded, wrinkled, or crumpled features.14,15,20 However, this should not be attributed to solvent–sheet interactions. Smooth sheets can indeed be obtained under carefully controlled dewetting conditions during solution processing.6,29 The collective organizational behavior of 2D sheets (e.g., their liquid crystallinity) is a very effective marker of their shapes, which we recommend should be included in future experimental studies of membrane configurations in solution.
Acknowledgments
The work was mainly supported by the Office of Naval Research (ONR-N000141310556 and then N000141612838), and supplemented by a seed grant from Northwestern MRSEC (NSF DMR-1121262) that provided partial support to A.R.K. C.L. thanks the Oversea Study Program of Guangzhou Elite Project to support his visit to Northwestern. The authors also thank Bernard Beckerman, Profs. Samuel I. Stupp, Yonggang Huang, Erik Luijten, and Monica Olvera de la Cruz for helpful discussions.
Author Present Address
§ Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China (J.L.).
The authors declare no competing financial interest.
References
- Kantor Y.; Kardar M.; Nelson D. R. Statistical Mechanics of Tethered Surfaces. Phys. Rev. Lett. 1986, 57, 791–794. 10.1103/PhysRevLett.57.791. [DOI] [PubMed] [Google Scholar]
- Mizuochi K.-i.; Nakanishi H.; Sakaue T. Dynamical scaling of polymerized membranes. Europhys. Lett. 2014, 107, 38003 10.1209/0295-5075/107/38003. [DOI] [Google Scholar]
- Schlüter A. D.; Payamyar P.; Öttinger H. C. How the World Changes By Going from One- to Two-Dimensional Polymers in Solution. Macromol. Rapid Commun. 2016, 37, 1638–1650. 10.1002/marc.201600425. [DOI] [PubMed] [Google Scholar]
- Knauert S. T.; Douglas J. F.; Starr F. W. Morphology and Transport Properties of Two-Dimensional Sheet Polymers. Macromolecules 2010, 43, 3438–3445. 10.1021/ma902081m. [DOI] [Google Scholar]
- Hummers W. S.; Offeman R. E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. 10.1021/ja01539a017. [DOI] [Google Scholar]
- Cote L. J.; Kim F.; Huang J. Langmuir–Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. 10.1021/ja806262m. [DOI] [PubMed] [Google Scholar]
- Wen X.; Garland C. W.; Hwa T.; Kardar M.; Kokufuta E.; Li Y.; Orkisz M.; Tanaka T. Crumpled and Collapsed Conformations In Graphite Oxide Membranes. Nature 1992, 355, 426–428. 10.1038/355426a0. [DOI] [Google Scholar]
- Hwa T.; Kokufuta E.; Tanaka T. Conformation of Graphite Oxide Membranes In Solution. Phys. Rev. A 1991, 44, R2235–R2238. 10.1103/PhysRevA.44.R2235. [DOI] [PubMed] [Google Scholar]
- Spector M. S.; Naranjo E.; Chiruvolu S.; Zasadzinski J. A. Conformations of the Tethered Membrane: Crumpling in Graphitic Oxide. Phys. Rev. Lett. 1994, 73, 2867–2870. 10.1103/PhysRevLett.73.2867. [DOI] [PubMed] [Google Scholar]
- Wiese K. J.; David F. New renormalization group results for scaling of self-avoiding tethered membranes. Nucl. Phys. B 1997, 487, 529–632. 10.1016/S0550-3213(96)00588-3. [DOI] [PubMed] [Google Scholar]
- Segal M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. 10.1038/nnano.2009.279. [DOI] [PubMed] [Google Scholar]
- Dreyer D. R.; Park S.; Bielawski C. W.; Ruoff R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. 10.1039/B917103G. [DOI] [PubMed] [Google Scholar]
- Kim J.; Cote L. J.; Huang J. Two Dimensional Soft Material: New Faces of Graphene Oxide. Acc. Chem. Res. 2012, 45, 1356–1364. 10.1021/ar300047s. [DOI] [PubMed] [Google Scholar]
- Cote L. J.; Kim J.; Zhang Z.; Sun C.; Huang J. Tunable assembly of graphene oxide surfactant sheets: wrinkles, overlaps and impacts on thin film properties. Soft Matter 2010, 6, 6096–6101. 10.1039/c0sm00667j. [DOI] [Google Scholar]
- Stankovich S.; Dikin D. A.; Dommett G. H. B.; Kohlhaas K. M.; Zimney E. J.; Stach E. A.; Piner R. D.; Nguyen S. T.; Ruoff R. S. Graphene-based composite materials. Nature 2006, 442, 282–286. 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- Forsyth P. A.; Marcelja S.; Mitchell D. J.; Ninham B. W. Onsager transition in hard plate fluid. J. Chem. Soc., Faraday Trans. 2 1977, 73, 84–88. 10.1039/F29777300084. [DOI] [Google Scholar]
- van der Kooij F. M.; Kassapidou K.; Lekkerkerker H. N. W. Liquid crystal phase transitions in suspensions of polydisperse plate-like particles. Nature 2000, 406, 868–871. 10.1038/35022535. [DOI] [PubMed] [Google Scholar]
- Kim J. E.; Han T. H.; Lee S. H.; Kim J. Y.; Ahn C. W.; Yun J. M.; Kim S. O. Graphene Oxide Liquid Crystals. Angew. Chem., Int. Ed. 2011, 50, 3043–3047. 10.1002/anie.201004692. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Gao C. Aqueous Liquid Crystals of Graphene Oxide. ACS Nano 2011, 5, 2908–2915. 10.1021/nn200069w. [DOI] [PubMed] [Google Scholar]
- Luo J.; Jang H. D.; Sun T.; Xiao L.; He Z.; Katsoulidis A. P.; Kanatzidis M. G.; Gibson J. M.; Huang J. Compression and Aggregation-Resistant Particles of Crumpled Soft Sheets. ACS Nano 2011, 5, 8943–8949. 10.1021/nn203115u. [DOI] [PubMed] [Google Scholar]
- Wang W.-N.; Jiang Y.; Biswas P. Evaporation-Induced Crumpling of Graphene Oxide Nanosheets in Aerosolized Droplets: Confinement Force Relationship. J. Phys. Chem. Lett. 2012, 3, 3228–3233. 10.1021/jz3015869. [DOI] [PubMed] [Google Scholar]
- Dou X.; Koltonow A. R.; He X.; Jang H. D.; Wang Q.; Chung Y.-W.; Huang J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 1528–1533. 10.1073/pnas.1520994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Kim J.; Huang J. Material Processing of Chemically Modified Graphene: Some Challenges and Solutions. Acc. Chem. Res. 2013, 46, 2225–2234. 10.1021/ar300180n. [DOI] [PubMed] [Google Scholar]
- Abraham F. F.; Goulian M. Diffraction From Polymerized Membranes: Flat vs. Crumpled. Europhys. Lett. 1992, 19, 293–296. 10.1209/0295-5075/19/4/008. [DOI] [Google Scholar]
- Yeh C.-N.; Raidongia K.; Shao J.; Yang Q.-H.; Huang J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. 10.1038/nchem.2145. [DOI] [PubMed] [Google Scholar]
- Cerda E.; Mahadevan L. Conical surfaces and crescent singularities in crumpled sheets. Phys. Rev. Lett. 1998, 80, 2358–2361. 10.1103/PhysRevLett.80.2358. [DOI] [Google Scholar]
- Matan K.; Williams R. B.; Witten T. A.; Nagel S. R. Crumpling a thin sheet. Phys. Rev. Lett. 2002, 88, 076101 10.1103/PhysRevLett.88.076101. [DOI] [PubMed] [Google Scholar]
- Poulin P.; Jalili R.; Neri W.; Nallet F.; Divoux T.; Colin A.; Aboutalebi S. H.; Wallace G.; Zakri C. Superflexibility of graphene oxide. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 11088–11093. 10.1073/pnas.1605121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W.; Kang D.; Kim T. H.; Lee S. G.; Byun N.; Lee D. W.; Seo B. H.; Ruoff R. S.; Shin H. S. Mosaic-like Monolayer of Graphene Oxide Sheets Decorated with Tetrabutylammonium Ions. ACS Nano 2013, 7, 8082–8088. 10.1021/nn403363s. [DOI] [PubMed] [Google Scholar]