Abstract
Quantitative understanding of reactivity and stability for a chemical species is fundamental to chemistry. The concept has undergone many changes and additions throughout the history of chemistry, stemming from the ideas such as Lewis acids and bases. For a given complexing ligand (Lewis base) and a group of isovalent metal cations (Lewis acids), the stability constants of metal–ligand (ML) complexes can simply correlate to the known properties of metal ions [ionic radii (rMn+), Gibbs free energy of formation (ΔG°f,Mn+), and solvation energy (ΔG°s,Mn+)] by 2.303RT log KML = (α*MLΔG°f,Mn+ – β*MLrMn+ + γ*MLΔG°s,Mn+ – δ*ML), where the coefficients (α*ML, β*ML, γ*ML, and intercept δ*ML) are determined by fitting the equation to the existing experimental data. Coefficients β*ML and γ*ML have the same sign and are in a linear relationship through the origin. Gibbs free energies of formation of cations (ΔG°f,Mn+) are found to be natural indices for the softness or hardness of metal cations, with positive values corresponding to soft acids and negative values to hard acids. The coefficient α*ML is an index for the softness or hardness of a complexing ligand. Proton (H+) with the softness index of zero is a unique acid that has strong interactions with both soft and hard bases. The stability energy resulting from the acid–base interactions is determined by the term α*MLΔG°f,Mn+; a positive product of α*ML and ΔG°f,Mn+ indicates that the acid–base interaction between the metal cation and the complexing ligand stabilizes the complex. The terms β*MLrMn+ and γ*MLΔG°s,Mn+, which are related to ionic radii of metal cations, represent the steric and solvation effects of the cations. The new softness indices proposed here will help to understand the interactions of ligands (Lewis bases) with metal cations (Lewis acids) and provide guidelines for engineering materials with desired chemical reactivity and selectivity. The new correlation can also enhance our ability for predicting the speciation, mobility, and toxicity of heavy metals in the earth environments and biological systems.
Introduction
Many chemical reactions can be generalized as Lewis acid–base reactions in which the Lewis acid is an electron acceptor and the Lewis base is an electron donor.1,2 If Lewis acids are metal cations, the Lewis bases are called ligands. A metal–ligand complex formation can be generalized as
| 1 |
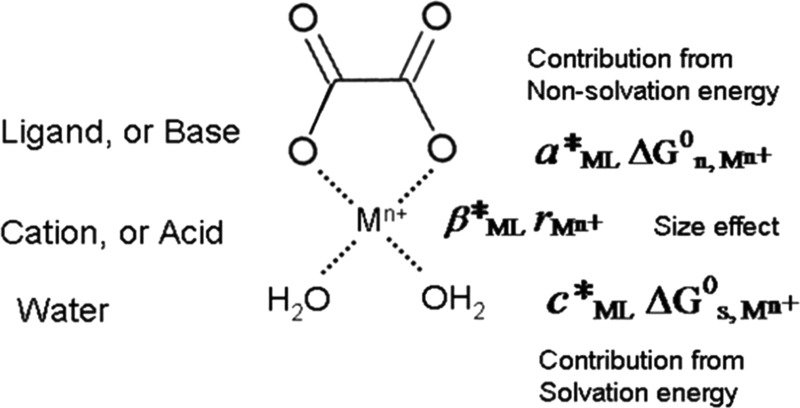
where M is a Lewis acid that can accept electrons and L is a Lewis base that can donate electrons (Figure 1). Stability of the Lewis acid–base complex was tackled by pioneers such as Williams and Irving, who proposed that stability of transition metal–ligand complexes were related to ionic radii and the second ionization potentials of the metals concerned (the Irving–Williams order).3 Much progress was made with the development of hard/soft acids/bases (HSABs) principle by Pearson1,2 and its explanation for observed stabilities in Lewis acid/base reactions. Later, Klopman tried to quantify the HSAB principle by using terms from ionic interaction (contribution from charge-controlled reaction) and covalent interaction (contribution from frontier molecular orbital) through a generalization of Fukuis frontier orbital’s density.4 Hancock and Martell5 summarized empirical parameters for estimating stability constants of some acid–base compound ligands based on HSAB principle by considering contributions from ionic interactions (EA·EB), covalent bonding (CA·CB), and size effects (DA·DB) using an equation
| 2 |
Parameters E and C are the tendency of acid A and base B to undergo ionic and covalent bonding, respectively. The method is very limited in predicting stabilities of metal complexes.
Figure 1.
Diagram schematically showing metal–ligand (ML) complex in water and different energy contributions to the formation of an aqueous metal complex.
Parr and Pearson used the density functional theory (DFT) to calculate the absolute hardness of Lewis acids and bases.6 The absolute hardness (η) is the second derivative of the energy with respect to the number of electrons with a fixed external potential analogous to the absolute electronegativity. The absolute softness is reciprocal value of hardness (1/η).7 The hardness/softness concept was supported by the DFT calculations.8−10 However, the absolute hardness of acids and bases cannot be directly used to calculate the stability constants of acid–base complexes. Furthermore, there are still some exceptions to the definition for acids such as H+ and Be2+.
Results and Discussion
We here report a linear free energy relationship that correlates the stability constants of metal–ligand (ML) complexes to the known properties of metal ions including ionic radius (rMn+), Gibbs free energy of formation (ΔG°f,Mn+), and solvation energy (ΔG°s,Mn+). In this correlation, Gibbs free energy of formation (ΔG°f,Mn+) of a cation is broken into two parts—the solvation energy (ΔG°s,Mn+) and the nonsolvation energy (ΔG°n,Mn+) (see the list of Symbols Used for details)
| 3 |
The solvation energy of a cation can be calculated with Born equation
| 4 |
where ε is the dielectric constant of water (78.47 at 25 °C). ΔG°s,Mn+ is negative. The parameter ωMn+ is the Born solvation coefficient of the cation, which can be further related to the radius of the cation (rMn+).11−13 The parameter ωMn+ is the Born solvation coefficients for aqueous cations and can be calculated by
| 4a |
In eq 4a, ωabsH+ is the absolute Born solvation coefficient of H+ (53.87 kcal/mol) and ωabsMn+ is the absolute Born solvation coefficient of the cations with the effective electrostatic radii of aqueous ions (re,Mn+). They can be calculated by
| 4b |
| 4c |
The solvation energies of divalent and trivalent cations have been calculated and tabulated (Table 1)12,13 using ionic radii provided in refs (14−16).
Table 1. Ionic Radii, Solvation Energies, Gibbs Free Energy of Formation of Divalent Cations (or, Natural Indices for Lewis Acid Softness), and Stability Constants for Some Metal–Ligand Complexes Familiesa,b,c.
| log KML |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M2+ | rM2+ (Å) | ΔGs,M2+ (aq) | ΔGf,M2+ (aq) | acetate (exp.) | acetate (calcd ±0.25) | oxalate (exp.) | oxalate (calcd ±0.32) | ADP (exp.) | ADP (calcd ±0.19) | H-ADP (exp.) | H-ADP (calcd ±0.18) | sulfuric acid (exp.) | sulfuric acid (calcd ±0.16) |
| Pt | 0.80 | –141.87 | 54.80 | 2.08 | 9.34 | 5.80 | 3.39 | 2.02 | |||||
| Pd | 0.80 | –141.87 | 42.49 | 1.97 | 8.97 | 5.57 | 3.54 | 2.04 | |||||
| Hg | 1.02 | –119.71 | 39.36 | 2.60 | 8.48 | 5.84 | 3.13 | 2.11 | |||||
| Cu | 0.73 | –144.83 | 15.55 | 2.22 | 1.91 | 6.23 | 6.00 | 5.90 | 5.28 | 3.16 | 3.57 | 2.36 | 2.31 |
| Pb | 1.18 | –107.89 | –5.79 | 2.68 | 2.30 | 9.26 | 4.92 | 3.78 | 2.75 | 2.01 | |||
| Sn | 1.11 | –112.91 | –6.63 | 2.27 | 8.22 | 4.96 | 3.72 | 2.11 | |||||
| Ni | 0.70 | –147.75 | –10.90 | 1.43 | 1.60 | 5.16 | 5.21 | 4.50 | 4.75 | 4.24 | 3.93 | 2.32 | 2.36 |
| Co | 0.74 | –144.35 | –13.00 | 1.46 | 1.68 | 4.72 | 5.16 | 4.20 | 4.76 | 4.25 | 3.90 | 2.36 | 2.37 |
| Cd | 0.95 | –125.31 | –18.57 | 1.93 | 2.04 | 6.07 | 4.78 | 3.80 | 2.46 | 2.29 | |||
| Fe | 0.77 | –141.04 | –21.87 | 1.40 | 1.69 | 4.95 | 4.63 | 3.95 | 2.20 | 2.38 | |||
| Zn | 0.75 | –143.30 | –35.17 | 1.57 | 1.52 | 4.87 | 4.45 | 4.28 | 4.37 | 4.20 | 4.13 | 2.38 | 2.42 |
| Mn | 0.82 | –136.46 | –55.20 | 1.40 | 1.51 | 3.95 | 4.12 | 4.05 | 4.06 | 4.17 | 4.29 | 2.26 | 2.44 |
| Be | 0.45 | –175.02 | –89.80 | –0.01 | (4.08) | 4.49 | 2.77 | 5.58 | 1.95 | 2.33 | |||
| Mg | 0.72 | –145.80 | –108.83 | 1.27 | 0.82 | 2.30 | 3.17 | 2.98 | 4.91 | 5.05 | 2.23 | 2.56 | |
| Eu | 1.17 | –108.59 | –129.10 | 1.25 | 5.43 | 2.66 | 5.21 | 2.28 | |||||
| Ca | 1.00 | –121.28 | –132.12 | 1.18 | 1.13 | 3.19 | 3.16 | 2.69 | 5.16 | 5.13 | 2.31 | 2.49 | |
| Ba | 1.36 | –95.99 | –132.73 | 1.07 | 1.19 | 8.65 | 2.36 | 2.38 | 5.52 | 5.55 | 2.70 | 1.97 | |
| Sr | 1.16 | –109.30 | –133.72 | 1.14 | 1.21 | 5.15 | 2.54 | 2.58 | 5.43 | 5.26 | 2.55 | 2.30 | |
| Ra | 1.39 | –94.14 | –134.20 | 1.16 | 9.21 | 2.31 | 5.63 | 1.91 | |||||
| UO2 | 0.75 | –142.54 | –227.70 | –0.10 | –1.21 | 0.83 | 6.39 | 2.81 | |||||
ADP = adenosine-5′-diphosphate; H-ADP = protonated ADP that forms metal complexes in M–HL form.
Radii of the cations are from ref (15). The values of log K metal complexes are from ref (17). All of the values were determined at standard state, except for ADP and H-ADP in ionic strength of 0.1. The stability constant (log K = 4.08) for Be2+–oxalate was determined in ionic strength of 0.1 at 20 °C. The predicted value should be very close to its real value in ionic strength of 0.
For a given complexing ligand (Lewis base) and a group of isovalent metal cations (Lewis acids), our correlation assumes that the total free energy of a metal–ligand complex can be written as a linear combination of three main contributors (Figure 1): (1) the interaction between the cation and the ligand, which is characterized by the nonsolvation energy (ΔG°n,Mn+), (2) the interaction between the cation and the surrounding water, which is characterized by the solvation energy (ΔG°s,Mn+), and (3) the size (rMn+) effect of the cation on coordination environments (the steric effect)
| 5 |
The intercept bML is related to the strength of the complexing ligand L. Because ΔG°f,ML dominates other terms, direct use of eq 5 for correlation may falsely lead to a high correlation coefficient between the quantities on the two sides of the equation. Therefore, it is preferable to use stability constants of metal complexes (log KML) for correlation. The log KML can be expressed in terms of the free energy of reactants (Mn+ and L) and products (ML)
| 6 |
where ΔG°f,L is Gibbs free energy of formation of a ligand L. Substituting eq 5 into eq 6, we obtain
| 7 |
where δ*ML = bML – ΔG°f,L. Finally, we have
| 8 |
where α*ML = (1 – aML) and γ*ML = (aML – cML).
The coefficients (α*ML, β*ML, γ*ML) and intercept δ*ML can be calculated by fitting eq 8 to existing experimental data for each ligand.
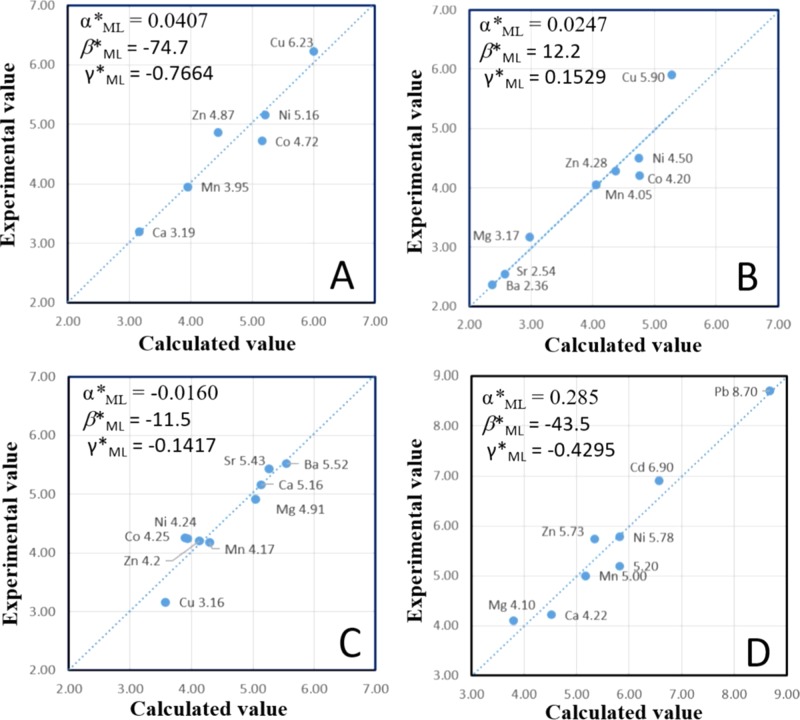
A large body of stability constants and Gibbs free energies of formation for metal–ligand complexes have been documented.16−18Equation 8 closely fits the existing experimental data for all of the metal complex families, even very complicated M–humate complexes (Figure 2D) (detail values for M–humate and M–fulvic acid complexes are listed in Supporting Information). Some selected metal-complex families are listed in Table 1. Figure 2 illustrates the differences between the experimental and the calculated values using eq 8. The discrepancies between the calculated and experimental data are within ±0.7 kcal/mol (or ∼0.5 log unit) for the majority of the data, especially for well-studied ligands. Previously proposed linear free energy relationship based on metals binding to one ligand (e.g., OH–) and metals binding to other ligand.20 The difference between the experimentally measured value and predicted value is also large (∼1–2 log units).20
Figure 2.
Diagrams showing the differences between experimental (vertical axis) and calculated log K values (horizontal axis) for four M2+–ligand families: M2+–oxalate (A), M2+–ADP (B), protonated ADP, or M2+–H-ADP (C), and complicated M2+–humate complexes (D), respectively.
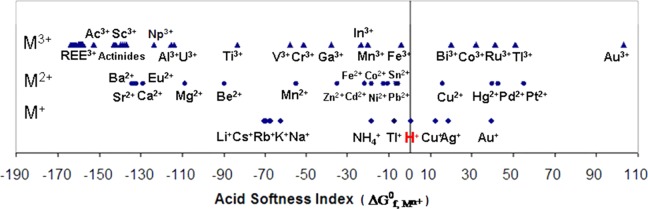
Interestingly, as shown in Figure 3, Gibbs free energies of formation of cations (ΔG°f,Mn+) are natural indices for the hardness/softness of metal cations (Lewis acids). Cations with positive ΔG°f,Mn+ values are soft acids, and those with negative ΔG°f,Mn+ values are hard acids (Figure 3). Cations with a high positive ΔG°f,Mn+ values tend not to lose electrons but to share them: softness is coincidentally related to the tendency of a complex to form covalent bonds. The new index is better than the empirical parameter Δβ (=log β0MF – log β0MCl) used for defining cation types like (a)-type, (b)-type, and borderline cations.21 Previously proposed criteria can qualitatively categorize metal cations into three groups, hard acids (or type A metal cations), borderline (or intermediate cations), and hard acids (or B type metal cations).1,21 The product of the term α*MLΔG°f,Mn+ is related to the stability of a metal–ligand complex. Both positive and both negative values of α*ML and ΔG°f,Mn+ will result in a positive product and hence stabilize the metal–ligand complex. It is then logical to postulate that the coefficient α*ML is an index for the hardness/softness of ligands (Lewis bases). Positive α*ML values indicate soft bases, and the negative α*ML values indicate hard bases.
Figure 3.
Gibbs free energy of formations (G°f,Mn+) as natural indices for the softness of Lewis acids. The values of ΔGf of the cations are taken from refs (12, 16, 18), except for Bi3+ from ref (22), Ti3+ from ref (23), Pu3+, Np3+, and Am3+ from refs (13, 24), and Pt2+, Pd2+ from ref (19). See Table 1 for detail values of divalent cations.
Table 2 lists the softness of some bases according to their α*ML values. Hard bases (e.g., F– with α*ML = −0.0101) have lower α*ML values than that of soft bases (e.g., ethylenediaminetetraacetate (EDTA) with α*ML = 0.0931) in divalent metal-complex families. For a ligand with coefficient α*ML close to 0 (e.g., Cl– with α*ML = 0.0036), the relative selectivity for metal cations is mainly determined by the ionic radii of the cations. If both coefficients α*ML and γ*ML are close to 0 (e.g., formic acid, and SO42), the ligand will have no selectivity for metal cations. In general, the overall stability constants are determined by both the ionic radii of metal cations and the softness of cations (acids) and ligands (bases). The new relationship can quantitatively explain the Irving–Williams order. The coefficient δ*ML relates to the complexing strength of a ligand with metals. A large negative δ*ML value indicates a strong chelating ability of the ligand if other coefficients are same. Both coefficients β*ML and γ*ML have the same sign (Table 2). Positive β*ML or γ*ML values indicate that increasing cation size will lower the stability of complex in a ML family (e.g., M2+–EDTA family, see Table 2), whereas negative β*ML or γ*ML values indicate that increasing cation size will increase the stability of metal complex in a ML family (e.g., Pb2+ in M2+–humate family with α*ML = 0.0285, see Figure 2D, Table S1, and Table 2). Some bases (like phosphoric acids) and diazoles have very low or zero values of β*ML and γ*ML (Table 2). This indicates that the size effect from the cations is diminished for such kinds of bases. It is proposed that the lesser hydrophilic (or more hydrophobic) nature of the bases causes this phenomenon.
Table 2. Summary of Regression Coefficientsa,c,d.
| bases, L | α*ML | β*ML | γ*ML | δ*ML or dML | Ib |
|---|---|---|---|---|---|
| hydrogen cyanide, ML | 0.5876 | 343.4 | 3.277 | –772.5 | 0 |
| sulfide, ML | 0.4390 | 195.56 | 2.3997 | –528.08 | 0 |
| ethylenediamine, ML | 0.1458 | 24.7 | 0.2246 | –62.39 | 0.1 |
| protein of hTFf, ML | 0.1368 | 95.5 | 0.8632 | −209.46 | 0 |
| histidine, ML2 | 0.1337 | –22.5 | –0.4334 | 58.62 | 0.1 |
| cysteine, ML | 0.1193 | –74.7 | –0.8502 | 163.26 | 0.1 |
| methylenephosphonic acid, ML | 0.1172 | 0 | 0 | –23.29 | 0.1 |
| glycine, ML | 0.1072 | 55 | 0.6116 | –138.5 | 0.1 |
| EDTA, ML | 0.0931 | 33.5 | 0.4074 | –107.54 | 0.1 |
| 1,3-diazole, ML2 | 0.0794 | 0 | 0 | –8.45 | 0.16 |
| O2–, ML | 0.0789 | –163.3 | –1.9486 | 368.91e | 0 |
| ammonia, ML2 | 0.0758 | 295.8 | 3.2876 | –700.64 | 0.1 |
| methionine, ML2 | 0.0712 | –29.9 | –0.4147 | 73.81 | 0.1 |
| histidine, ML | 0.0694 | –22.3 | –0.3368 | 53.97 | 0.1 |
| pyrindine, ML | 0.0652 | 15.7 | 0.206 | –52.02 | 0.1 |
| ammonia, ML | 0.0547 | 130 | 1.4563 | –310.45 | 0.1 |
| aspartic acid, ML | 0.0466 | –25.1 | –0.3329 | 57.93 | 0.1 |
| OH–, ML | 0.0440 | –73.6 | –0.8944 | 138.75 | 0 |
| oxalic acid, ML | 0.0407 | –74.7 | –0.7664 | 157.96 | 0 |
| glutamic acid, ML | 0.0315 | –52.33 | –0.7216 | 135.07 | 0.1 |
| methionine, ML | 0.0291 | –102.8 | –1.2322 | 252.87 | 0.1 |
| humic acid, ML | 0.0285 | –43.5 | –0.4295 | 85.66 | 0 |
| citric acid, ML | 0.0257 | –14.9 | –0.2632 | 41.57 | 0.1 |
| ADP, ML | 0.0247 | 12.2 | 0.1529 | –37.78 | 0.1 |
| glycylglycine, ML | 0.0221 | –36.1 | –0.4663 | 88.25 | 0 |
| phthalic acid, ML | 0.0196 | –30.8 | –0.39 | 75.24 | 0.1 |
| dimethylmalonic acid, ML | 0.0190 | 5.3 | 0.0781 | –19.07 | 0.1 |
| malonic acid | 0.0180 | –15 | –0.2059 | 34.89 | 0.1 |
| 1,3-diazole, ML | 0.0180 | 0 | 0 | –4.65 | 0.16 |
| ATP, ML | 0.0173 | 11.4 | 0.1374 | –35.47 | 0.1 |
| lactic acid, ML | 0.0145 | 3.2 | 0.0392 | –11.27 | 0.1 |
| fulvic acid, ML | 0.0132 | 9.8 | 0.1115 | –28.91 | 0 |
| adenosine 5′-monophosphate (AMP-5′), ML | 0.0119 | 0.9 | 0.0114 | –6.29 | 0.1 |
| acetic acid, ML | 0.0115 | 8.6 | 0.1261 | –26.95 | 0.1 |
| adenosine 2′-monophosphate (AMP-2′), ML | 0.0111 | 2.5 | 0.0318 | –10.26 | 0.1 |
| salicyclic acid, ML | 0.0110 | 26.6 | 0.2106 | –59.41 | 0.1 |
| Cl, ML2 | 0.0105 | 56 | 0.7511 | –213.83e | 0 |
| adenosine 3′-monophosphate (AMP-3′), ML | 0.0100 | 0.9 | 0.0105 | –5.9 | 0.1 |
| Cl, ML | 0.0036 | 49.5 | 0.6505 | –161.63e | 0 |
| formic acid, ML | 0.0018 | 0 | 0 | –2.15 | 0.1 |
| sulfuric acid, ML | –0.0028 | 7.4 | 0.0774 | –19.81 | 0 |
| ATP, MHL | –0.0090 | –8.7 | –0.1025 | 15.79 | 0.1 |
| F, ML | –0.0101 | 14.3 | 0.1615 | –102.72e | 0 |
| ADP, MHL | –0.0160 | –11.5 | –0.1417 | 23.8 | 0.1 |
| methylenephosphonic acid, MHL | –0.0235 | 0 | 0 | –11.57 | 0.1 |
| F, ML2 | –0.0517 | –69.1 | –0.7212 | 20.38e | 0 |
| CO3, ML | –0.0808 | –16.2 | –0.2219 | –77.08 | 0 |
| inosine-5′-dihydrogenphosphase | –0.1198 | 0 | 0 | –6.03 | 0.1 |
| iodic acid, ML | –0.1198 | –79.1 | –1.1107 | 228.99 | 0.1 |
The coefficient α*ML is the natural indices for the chemical softness of the ligands or Lewis bases.
I: ionic strength.
The values of log K metal complexes are from ref (17), except for M–Cl, M–Cl2, M–F, M–F2, and M–O2– complexes from refs (16, 18). M–OH complexes are from reference17 except for Hg because of large discrepancy and no Hg values were suggested in references.16,18
M–humates data are from refs (21) and (25). A Cu(II)–humate value is not used because it is pH dependent and related to the formation of polynuclear complexes (i.e., both ML and ML2 complexes).26 The data for M–fulvic acid complexes are from Schnitzer and colleagues.27−29
The value is dML for the Gibbs free energy of formation.
Protein of human serum transferrin.
According to Table 2, EDTA is one of the strongest chelating agents for divalent metals. The softness of bases follows the order of cyanide > sulfide (S2–) > ethylenediamine > cysteine > glycine > EDTA > O2– > histidine > ammonia > OH– > carboxylic acids > Cl– > sulfuric acid > F– > CO32– > iodic acid. Based on the obtained softness indices (Table 2), increasing the number of soft bases (like Cl, histidine, or ammonia) bonded to metal cations generally results in an even softer ligand, that is, Cl2 in M–L2 is softer than Cl in M–L. Increasing the number of hard bases (like F) bonded to metal cations generally results in an even harder ligand, that is, F2 in M–L2 is harder than F in M–L (Table 2). Softness also changes based on the arrangement of atoms in isomers. For instance, andenosine monophosphate (AMP-5′) is the precursor to ADP, and the biologically important AMP has two other isomers: AMP-2′ (α*ML = 0.0111) and AMP-3′ (α*ML = 0.0100). AMP-5′ is the softest (α*ML = 0.0119) among the three isomers (Table 2). The new softness indices for Lewis bases can serve as a guideline for designing new functional materials or even engineered proteins with chemical selectivity because each individual functional group (like amine, carboxylic group, carbonate, and phosphate) has its own softness value.
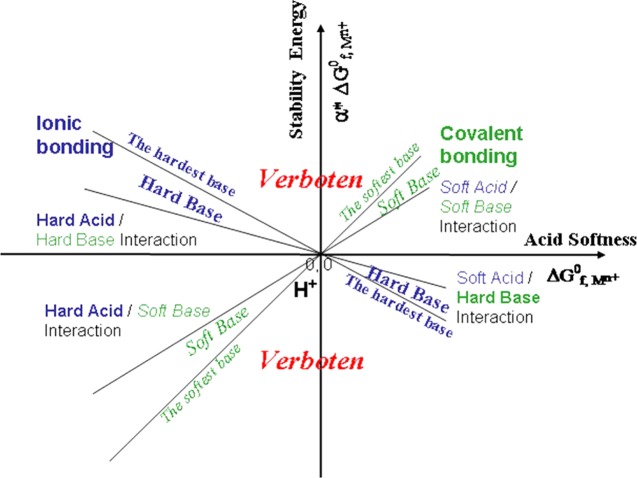
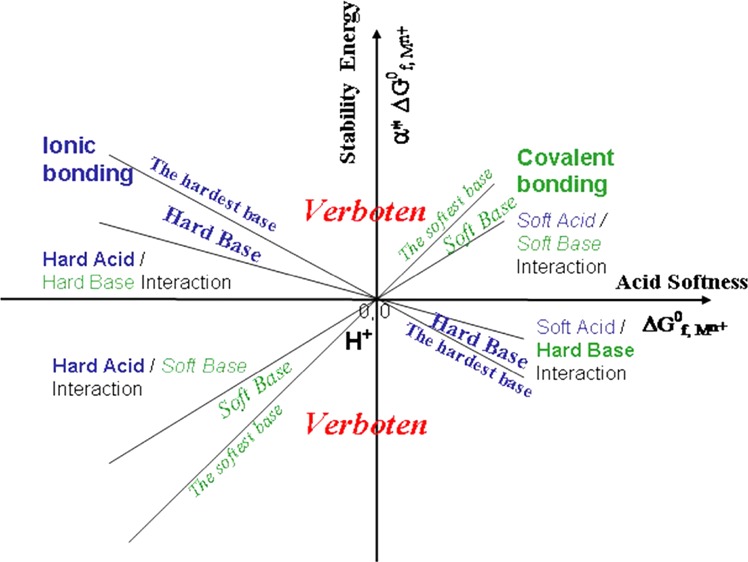
Unlike the hardness parameters (η) proposed by Parr and Pearson,6 the new softness (or hardness) indices have positive and negative signs, which clearly indicate the reaction tendencies between Lewis acids and Lewis bases. The product of the acid softness (ΔG°f,Mn+) and base softness (α*ML) or the term of α*MLΔG°f,Mn+ corresponds to the acid–base interaction energy. By plotting the α*MLΔG°f,Mn+ term against the Lewis acid softness ΔG°f,Mn+, we can clearly see that the stability energies due to the acid–base interactions follow lines with different slopes (α*ML) (Figure 4). Soft bases follow lines with positive slopes, and hard bases follow lines with negative slopes. The areas labeled “verboten” are beyond the softness of the softest base and the hardness of the hardest base. The four quadrants in the plot correspond to soft acid–soft base interaction (quadrant I), hard acid–hard base interaction (quadrant II), hard acid–soft base interaction (quadrant III), and soft acid–hard base interaction (quadrant IV). The acid–base interaction energy not only quantitatively explains Pearson’s statement of “hard acids prefer to associate with hard bases, and soft acids prefer to associate with soft bases”,2,30 but also accounts for all other reactions of varying hardness/softness.
Figure 4.
A diagram illustrating Lewis acid–base interaction, or acid–base stability energies in term of α*ΔG°f,Mn+ vs ΔG°f,Mn+ plot. The proton (H+) is at the unique position of the origin. The softness of a base (ligand) is gauged with the slope of α*ΔG°f,Mn+ vs ΔG°f,Mn+ with a positive slope for a soft base. A positive α*ΔG°f,Mn+ value indicates a preferential complexation between the acid and the base.
Hydrogen has puzzled scientists in the past in their explanations of both its role as a hard or a soft cation, and its effect on bases/ligands. Previous HSAB models were not able to qualitatively explain its behavior, much less quantify it. To briefly illustrate, H+ can bond to F–, which is very hard. H+ is also known to bond to S2–, a soft base, forming HS– or H2S. So, hydrogen as a cation exhibits both hard and soft behaviors depending on the ligand. On an arbitrary scale, such a quantification is quite impossible, as H+ would have to appear twice—two contradicting facts. Our equation and new softness indices appropriately explain hydrogen’s behavior by positioning its softness at 0. This point is equally positive as it is negative, which allows it to react with both hard and soft bases, and forms stable bonds.
The second unique feature of hydrogen is its effect on ligands, or more specifically, what happens when a ligand is protonated. It is observed that protonated ligands become less soft with the addition of protons, such as ML complexes and MHL complexes for ATP, ADP, and methylenephosphonic acid (Table 2). In the M–ADP family, the Cu–ADP complex is the strongest. The order of binding strength is reversed in the M–H-ADP family (Figure 2) because protonated ADP (H-ADP) becomes a hard base (Table 2), and its coefficients β*ML and γ*ML become negative. OH– may be considered as protonated O2– in this regard. This phenomenon is difficult to explain, but is nevertheless observable. We currently believe that hydrogen changes the covalency of a bond between cation and ligand. For instance, in an ideal covalent metal–ligand complex, the electrons in a neutral position receive equal forces from both nuclei. If a proton with a high electron affinity is added to a ligand, the electron in the neutral position moves toward the protonated side, “pinching off” the bond. The compound is now less covalent, which implies that it is less soft than its deprotonated base.
Previous models also had trouble explaining the behavior of beryllium (Be2+). Its family exhibits ionic properties (a general indicator of a hardness) when it reacts with Cl–. According to the previous charge over radius rule, Be2+, which is both small and highly charged, should be more ionic and thus harder than other divalent cations in the alkali earth group. BeCl2, however, is more covalent than others in its family, as it is observed to be soluble in organic solvents, and the molten material is a poor conductor.31 The reason as explained is that because Be2+ is so small and also highly charged, it is capable of pulling enough electrons toward it to form a quasi-covalent structure. Charge over radius could not be extended to calculate the hardness of Be2+, and likewise rules devised around the Be2+ case could not be used to define other Lewis acids. Based on Pearson–Klopman HSAB model, Be2+ is considered an anomaly and exception. As we have found, the key to softness or hardness is not solely charge over radius, but the Gibbs free energy of formation, ΔG°f,Mn+. Be2+ has the highest ΔG°f,Mn+ value among the alkali earth elements (Table 1); therefore, it is softer than all of the other divalent cations in the alkali earth group. However, the charge/radius cannot be ignored. In our equation, the terms of β*MLrMn+ (steric effect) and γ*MLΔG°s,Mn+ (solvation effect) still account for this. Ignoring the size effect terms can cause major discrepancies when cations are very small (e.g., Be2+) because the solvation energy is proportional to the reciprocal value of ionic radius.11 Our equation produces the results for Be2+–ligand complexes that are difficult to be measured, consistent with the experimental data (Tables 1 and S1, Figure S1).
If we plot the values of β*ML against γ*ML, then a linear relationship between β*ML and γ*ML values is displayed (Figure 5)
| 9 |
The straight line passes through the origin (Figure 5), which indicates that β*ML and γ*ML are not independent. The value of slope is about 81.5 kcal/Å. The slope applies to all of the divalent metal–ligand complexes in a given solvent, water in this case. The slope (or “charge-solvent parameter”) corresponds to the strength of the coordination field around the cation in a given solvent. Equation 9 for divalent cations may be modified as
| 10a |
or
| 10b |
The new equation will help us better understand the hardness/softness of Lewis acids and bases. It can also predict the unknown thermodynamic data based on a limited number of the existing measurements, and this equation can also be used to check the internal consistency of the thermodynamic databases. It is essential to have reliable data for metal–ligand complexes to evaluate the impact of pollutants on water, soils, and biological systems, as metal complexation with various inorganic or organic ligands in aqueous solutions directly controls the solubility, sorption, and toxicity of metals including radionuclides in natural environments. The effectiveness of these calculations heavily depends on the completeness and quality of the thermodynamic databases on which the calculations are based.32,33 The equation can also be used to predict M3+–ligand bindings and metal–protein bindings, which, in turn, can be used in the future for designing metal-based anticancer drugs like Ru3+ compounds34,35 and microbe–mineral interactions in natural environments.36,37
Figure 5.
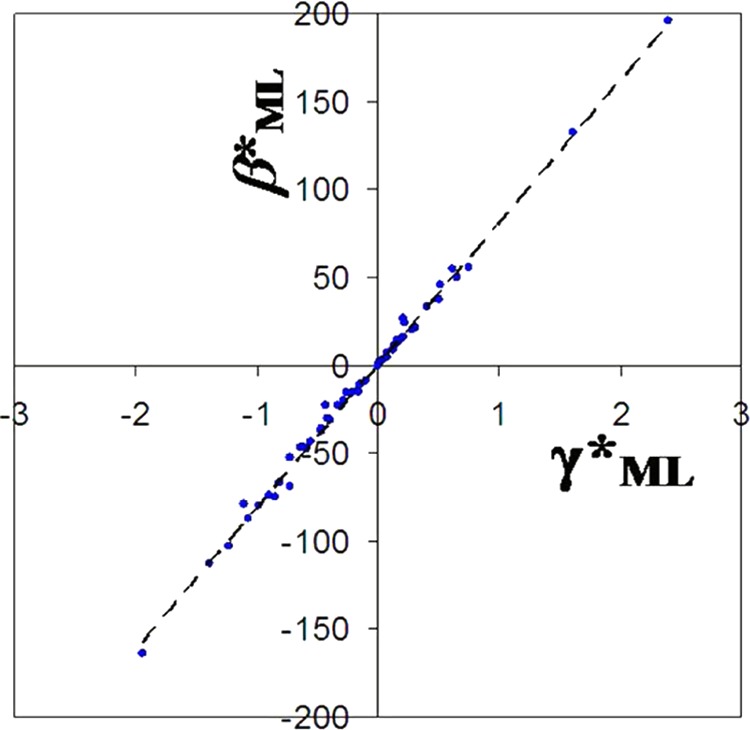
Diagram showing a linear relationship between the fitting parameters β*ML and γ*M based on 80 metal–ligand complex families with β*ML < 200 kcal/Å. A slope of 81.5 kcal/Å is a charge-solvent parameter for solvent water.
Acknowledgments
This work is supported by the NASA Astrobiology Institute under grant N07-5489. Authors thank Mr. Franklin Hobbs and two anonymous reviewers for their comments.
Glossary
Symbols Used
- rMn+
Ionic radii of metal cations with charge +n, experimental quantity
- ΔG°f,Mn+
Gibbs free energy of formation (softness) of cations, experimental quantity
- ΔG°s,Mn+
Solvation energy of cations, calculated using Born solvation theory eq 4
- ΔG°n,Mn+
Non-solvation energy of cations, a quantity defined by eqs 3 and 4 in term of experimental quantity of ΔG°f,Mn+
- α*ML
Softness of ligand or Lewis base, a fitting coefficient obtained by fitting eq 8
- β*ML
A fitting coefficient obtained by fitting eq 8
- γ*ML
A fitting coefficient obtained by fitting eq 8
- δ*ML
A fitting coefficient obtained by fitting eq 8
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01039.
Ionic radii, solvation energies, Gibbs free energy of formation of divalent cations, and stability constants for M2+–humic acid, M2+–fulvic acid, M2+–ATP, and M2+–H-ATP complexes (Table S1); plots showing experimental values versus calculated values for M–ATP and M–H-ATP complexes (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pearson R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. 10.1021/ja00905a001. [DOI] [Google Scholar]
- Pearson R. G. Acids and bases. Science 1966, 151, 172–177. 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- Irving H.; Williams R. J. P. Order of stability of metal complexes. Nature 1948, 162, 746–747. 10.1038/162746a0. [DOI] [Google Scholar]
- Klopman G. Chemical reactivity and concept of charge- and frontier-controlled reactions. J. Am. Chem. Soc. 1968, 90, 223–234. 10.1021/ja01004a002. [DOI] [Google Scholar]
- Hancock R. D.; Martell A. E. Lewis acid-base behavior in aqueous solution: some implications for metal ions in biology. Adv. Inorg. Chem. 1995, 42, 89–146. 10.1016/s0898-8838(08)60052-5. [DOI] [Google Scholar]
- Parr R. G.; Pearson R. G. Absolute hardness – companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. 10.1021/ja00364a005. [DOI] [Google Scholar]
- Pearson R. G. Chemical hardness and the electronic chemical-potential. Inorg. Chim. Acta 1992, 198–200, 781–786. 10.1016/S0020-1693(00)92423-X. [DOI] [Google Scholar]
- Ayers P. W.; Parr R. G.; Pearson R. G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107 10.1063/1.2196882. [DOI] [PubMed] [Google Scholar]
- Ayers P. W. The physical basis of the hard/soft acid/base principle. Faraday Discuss. 2007, 135, 161–190. 10.1039/B606877D. [DOI] [PubMed] [Google Scholar]
- Cohen M. H.; Wasserman A. On the foundations of chemical reactivity theory. J. Phys. Chem. A 2007, 111, 2229–2242. 10.1021/jp066449h. [DOI] [PubMed] [Google Scholar]
- Bockris J. O. M.; Reddy A. K. N.. Ionic Liquids. Modern Electrochemistry: An Introduction to an Interdisciplinary Research; Plenum Press: New York, 1970; p 622. [Google Scholar]
- Sverjensky D. A.; Molling P. A. A linear free-energy relationship for crystalline solids and aqueous ions. Nature 1992, 356, 231–234. 10.1038/356231a0. [DOI] [Google Scholar]
- Wang Y.; Xu H. Prediction of trace metal partitioning between minerals and aqueous solutions: a linear free energy correlation approach. Geochim. Cosmochim. Acta 2001, 65, 1529–1543. 10.1016/S0016-7037(01)00551-8. [DOI] [Google Scholar]
- Shannon R. D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr., Sect. A: Found. Adv. 1976, 32, 751–767. 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Shannon R. D.; Prewitt C. T. Effective ionic radii in oxides and fluorides. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. 10.1107/S0567740869003220. [DOI] [Google Scholar]
- Shock E. L.; Helgeson H. C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 kb and 1000 °C. Geochim. Cosmochim. Acta 1988, 52, 2009–2036. 10.1016/0016-7037(88)90181-0. [DOI] [Google Scholar]
- Martell A. E.; Smith R. M.. Critical Stability Constants; Plenum Press: New York, 1974–1989; p 469, 416, 511, 257, 604, 664. [Google Scholar]
- Shock E. L.; Sassani D. C.; Willis M.; Sverjensky D. A. Inorganic species in geologic fluids: Correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim. Cosmochim. Acta 1997, 61, 907–950. 10.1016/S0016-7037(96)00339-0. [DOI] [PubMed] [Google Scholar]
- Pourbaix M.Atlas of Electrochemical Equilibria in Aqueous Solutions; Ceblcor: Brussels, 1974; p 644. [Google Scholar]
- Li H.; Sadler P. J.; Sun H. Rationalization of the Strength of Metal Binding to Human Serum Transferrin. Eur. J. Biochem. 1996, 242, 387–393. 10.1111/j.1432-1033.1996.0387r.x. [DOI] [PubMed] [Google Scholar]
- Turner D. R.; Whitefield M.; Dickson A. G. The equilibrium speciation of dissolved components in freshwater and sea water at 25 °C and 1 atm pressure. Geochim. Cosmochim. Acta 1981, 45, 855–881. 10.1016/0016-7037(81)90115-0. [DOI] [Google Scholar]
- Wagman D. D.; Evans W. H.; Parker V. B.; Schumm R. H.; Halow I.; Bailey S. M.; Churney K. L.; Buttall R. L. The NBS tables of chemical thermodynamic properties. J. Phys. Chem. Ref. Data 1982, 11, 392. [Google Scholar]
- Latimer W. M.The Oxidation States of the Elements and Their Potentials in Aqueous Solutions, 2nd ed.; Englewood C., Ed.; Princeton Hall: New Jersey, 1959; p 392. [Google Scholar]
- Brookins D. G.Eh-pH Diagrams for Geochemistry; Springer-Verlag: Berlin, 1988; p 176. [Google Scholar]
- Stevenson F. J. Stability-constants of Cu2+, Pb2+, and Cd2+ complexes with humic acids. Soil Sci. Soc. Am. J. 1976, 40, 665–672. 10.2136/sssaj1976.03615995004000050021x. [DOI] [Google Scholar]
- Stevenson F. J.; Fitch A.; Brar M. S. Stability-constants of Cu(II)-humate complexes – comparison of select models. Soil Sci. 1993, 155, 77–91. 10.1097/00010694-199302000-00001. [DOI] [Google Scholar]
- Schnitzer M.; Hansen E. H. Organo-Metallic Interactions in Soils: 8. An evaluation of methods for determination of stability constants of metal-fulvic acid complexes. Soil Sci. 1970, 109, 333–340. 10.1097/00010694-197006000-00001. [DOI] [Google Scholar]
- Schnitzer M.; Skinner S. I. M. Organo-Metallic Interactions in Soils: 5. Stability constants of Cu++-, Fe++-, and Zn++-fulvic acid complexes. Soil Sci. 1966, 102, 361–365. 10.1097/00010694-196612000-00002. [DOI] [Google Scholar]
- Schnitzer M.; Skinner S. I. M. Organo-Metallic Interactions in Soils: 7. Stability constants of Pb++-, Ni++-, Mn++-, Co++-, Ca++-, and Mg++-fulvic acid complexes. Soil Sci. 1967, 103, 247–252. 10.1097/00010694-196704000-00004. [DOI] [Google Scholar]
- Pearson R. G.Chemical Hardness; Wiley-VCH: Weinhein, 1997; p 198. [Google Scholar]
- Akdeniz Z.; Tosi M. Correlation between entropy and volume of melting in halide salts. Proc. R. Soc. A 1992, 437, 85–96. 10.1098/rspa.1992.0048. [DOI] [Google Scholar]
- Bethke C. M.Geochemical Reaction Modeling: Concepts and Applications; Oxford University Press: Oxford, 1996; p 543. [Google Scholar]
- Brantley S. L. Why geochemists never agree: it’s all about the data!. Elements 2007, 3, 432–433. [Google Scholar]
- Casini A.; Mastrobuoni G.; Terenghi M.; Gabbiani C.; Monzani E.; Moneti G.; Casella L.; Messori L. Ruthenium anticancer drugs and proteins: a study of the interactions of the ruthenium(III) complex imidazolium trans-[tetrachloro(dimethyl sulfoxide)(imidazole)ruthenate(III)] with hen egg white lysozyme and horse heart cytochrome c. J. Biol. Inorg. Chem. 2007, 12, 1107–1117. 10.1007/s00775-007-0280-4. [DOI] [PubMed] [Google Scholar]
- Bergamo A.; Sava G. Ruthenium complexes can target determinants of tumour malignancy. Dalton Trans. 2007, 1267–1272. 10.1039/b617769g. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J.; Doyle R. J.. Metal Ions and Bacteria; John Wiley & Sons: New York, 1989; p 461. [Google Scholar]
- Xiong Y.; Shi L.; Chen B.; Mayer M. U.; Lower B. H.; Londer Y.; Bose S.; Hochella M. F.; Fredrickson J. K.; Squier T. C. High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA. J. Am. Chem. Soc. 2006, 128, 13978–13979. 10.1021/ja063526d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.