Abstract

The distribution of 12 environmental contaminants or metabolites with diverse polarities (2,2′,4,4′,5-pentabromodiphenyl ether; bisphenol A; estrone; glyphosate; β-hexabromocyclododecane; imidacloprid; 2,3′,4,4′,5-pentachlorobiphenyl; 3′-methylsulfone 2,2′,4,5,5′-pentachlorobiphenyl; 1,2,7,8-tetrachlorodibenzo-p-dioxin; 2-hydroxy-1,3,7,8-tetrachlorodibenzo-p-dioxin; tetrabromobisphenol A; and triclocarban) among skim milk, fat, curd, whey, whey retentate, and whey permeate was characterized. Analysis of these compounds along with 15 drugs previously studied provided a robust linear model predicting the distribution between skim and fat and the chemical’s lipophilicity (log P, r2 = 0.71; log D, r2 = 0.79). Similarly, distribution between curd and whey was correlated with lipophilicity (log P, r2 = 0.63; log D, r2 = 0.73). Phenolic compounds had less predictable distribution patterns based on their lipophilicities. Within the whey fraction, chemicals with greater lipophilicity are associated with whey proteins more than hydrophilic chemicals. The resultant model could help predict the potential distribution of chemical contaminants among milk products in cow milk, if present.
Introduction
Pesticides or environmental contaminants of milk or milk products may occur after the consumption of contaminated feed, exposure to contaminated facilities, and/or contamination during milk handling, transport, and/or processing. Reported levels of pesticides and/or persistent organic pollutants in milk vary depending on geographic region, year of survey, and chemical classes analyzed.1−6 The distribution of a chemical among a variety of milk products is dependent on its physicochemical properties, the milk processing methods used, and the final milk products produced.
Previously, we tested the hypothesis that drug distribution among milk fractions could be predicted based on lipophilicity (log D, distribution coefficients or log P, partition coefficients).7−9 A mixed linear model was proposed for the distribution between milk fat and skim milk based on the study of seven veterinary drugs (Table S1).7 The seven drugs included four antibiotics [erythromycin (ERY), penicillin G (PENG), oxytetracycline (OTET), and sulfadimethoxine (SDMX)], two anthelmintics [ivermectin (IVR) and thiabendazole (THIA)], and one analgesic [ketoprofen (KETO)]. A linear model of the same drugs was also described for the distribution between curd and whey, as well as whey protein associations.8 The model was then expanded to include four additional antibiotics [ciprofloxacin (CIPR), clarithromycin (CLA), thiamphenicol (TAP), and phenylbutazone (PBZ)], three analgesics/antipyretics [acetylsalicylic acid (ASP)/salicylic acid, acetaminophen (TYL), and flunixin (FNX)], and one anthelmintic [praziquantel (PZQ)] (Table S2).9 The distribution model fit (r2) decreased when the data set was expanded from 7 to 15 drugs;9 however, modeling based on log D values still provided a better fit than those based on log P.
In this report, we consider 12 environmental contaminants to better understand the possible impacts of a broader range of log D and log P values on xenobiotic distribution in milk. Specifically, we studied the herbicide glyphosate (GLY); an insecticide, imidacloprid (IMI); a common component of plastics and canned food liners, bisphenol A (BPA); an antibacterial, triclocarban (TCC); a steroid hormone, estrone (E1); several brominated flame retardants, β-hexabromocyclododecane (β-HBCD), tetrabromobisphenol A (TBBPA), and 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99); and a number of persistent organic pollutants and their metabolites [1,2,7,8-tetrachlorodibenzo-p-dioxin (1,2,7,8-TCDD); 2-hydroxy-1,3,7,8-tetrachlorodibenzo-p-dioxin (2-OH-1378-TCDD); 2,3′,4,4′,5-pentachlorobiphenyl (PCB-118); and 3′-methylsulfone-2,2′,4,5,5′-pentachlorobiphenyl (3′-MeSO2-PCB-101)]. To the best of our knowledge, the fate/distribution of these chemicals in milk processing has not been reported. The chemicals studied in this report expands the polarity range of the previous studies (now spanning log D from −4.2 to 7.3) and fills in the knowledge gap for chemicals with log D values between 3.4 and 6.6. Data obtained from this study have been combined with data from our previous reports,7−9 allowing us to expand distribution modeling to include a total of 27 chemicals.
Results and Discussion
Chemical Distribution from Whole Milk into Milk Fat and Skim Milk
Milk partitioning into lipid was highly reproducible, with typical coefficient of variance (CV) values of ≤5%; exception was GLY with CV up to 19% (Tables S5–S16). The high CV of GLY was due to its low partitioning into milk fat (Table S5). Similarly, CV of partitioning into skim milk was ≤5%; exceptions were BDE, β-HBCD, 3′-MeSO2-PCB-101, PCB, and TCC because of low amounts in the skim milk. Recoveries (sum of total radioactivity in skim milk and milk fat) were >90%, ranging from ∼91% (for chemicals with log D ≥ 6.7) up to 100% for GLY (Figure 1 and Tables S5–S16). Distribution of chemical residues was not dose-dependent over the range of doses used (linear regression slope P > 0.05), suggesting that a chemical’s distribution between skim milk and milk fat would be constant regardless of the concentration. In the absence of overt physiologic effects such as toxicity or effects on blood flow to the mammary gland, such results suggest that whole milk composition (i.e., across species or breed types) would influence a chemical’s presence in milk to a greater extent than the dose received.
Figure 1.
Chemical distribution and relative concentration ratios from whole milk into skim milk and milk fat fractions. Bars represent percent mean of all concentrations (n = 3 concentrations, 3 replicates per concentration, replicate exceptions are n = 2 replicates each for 1278-TCDD 20 and 200 nM and n = 2 replicates for BDE-99 2000 nM) ± SD of the three dose means based on disintegrations per minute (dpm) of skim milk and milk fat fractions compared to whole milk dpm. Values on graph represent the mean ratio of the drug concentration in the fraction (milk fat or skim milk) to the initial drug concentration in whole milk ± SD of means between doses (n = 3 mean dose ratios). Sum of stack plot represents total chemical recovery. log D values given for each compound at bottom of plot.
For the 12 chemicals tested, distribution into milk fat ranged from <3% (0.95% for GLY and 2.5% for IMI) to >80% of the total amount added (3′-MeSO2-PCB-101, TCC, PCB-118, β-HBCD, 1278-TCDD, and BDE-99). Intermediate distributions into milk fat occurred for phenolic compounds (BPA, 39%; TBBPA, 46%; 2-OH-1378-TCDD, 54%; and E1, 74%) (Tables S5–S16, Figure 1).
As would be anticipated, the data indicated that nonpolar chemicals concentrate into high lipid milk fractions. The concentration ratios in milk fat relative to whole milk for moderately polar phenolic compounds were about 10 (BPA, 8.2; TBBPA, 10.5; 2-OH-1378-TCDD, 11.2; and E1, 15.8) and were ∼18–20 for highly nonpolar persistent environmental contaminants (BDE-99, β-HBCD, 3′-MeSO2-PCB-101, PCB-118, TCC, and 1278-TCDD; Figure 1). Also as expected, polar chemicals partitioned to a large degree into skim milk, resulting in milk fat/whole milk concentration ratios of <1 (GLY was 0.2, and IMI was 0.5; Figure 1). For the phenolic compound BPA, substitution of four phenyl hydrogens with bromines to form TBBPA (Table 1) increased lipophilicity (log D = 3.60 vs 6.69) and was reflected by TBBPA’s milk fat/whole milk concentration ratio of 10.5 compared to that of 8.2 for BPA (Figure 1). Hydroxylation of a molecule decreases its relative lipophilicity with respect to its nonhydroxylated analogue, as is commonly observed during oxidative metabolism. Although 1278-TCDD and 2-OH-1378-TCDD have very similar log D values (6.15 and 6.22, respectively) hydroxylation resulted in reduced lipid solubility and a ∼30% reduction in milk fat distribution. However, the addition of a more polar functional group onto a pentachloro biphenyl molecule to form 3′-MeSO2-PCB-101 did not shift the milk fat distribution pattern when compared to PCB-118. One possible explanation may be due to the change of chlorine substitution pattern.
Table 1. Drug Structures and Physicochemical Properties.
Compound radioactively labeled with a directed label and specified on the structure with a red asterisk. An asterisk within a ring indicates a uniform label on the ring. Exceptions: IMI and β-HBCD carbon labels are unknown.
SAs were adjusted depending on dose, as indicated. Values in parentheses are nominal concentrations for initial fortification.
Average log P calculated from literature log P values accessed from www.chemspider.com, www.drugbank.ca, www.ebi.ac.uk/chembl/, and pubchem.ncbi.nlm.nih.gov/ on 7/14/2017 using the predicted and experimental values were available.
Values for log D at pH 6.8 were calculated using log P values from above sources and pKa’s from www.drugbank.ca, www.ebi.ac.uk/chembl/, www.druginfosys.com, pubchem.ncbi.nlm.nih.gov/, Johansson and Anlér26 accessed on 7/14/2017.
Although literature describing the milk partitioning of the exact compounds studied here has not been found, there are several relevant studies available for comparison. For example, Jensen and Hummel15 administered 2,4,5-trichlorophenoxyacetic acid containing 2,3,7,8-TCDD to lactating dairy cows and found that 2,3,7,8-TCDD residues in cream exceeded those in milk by a factor of about 10. Although this is much lower than our reported ratio of ∼19 for 1,2,7,8-TCDD (Figure 1), the difference could originate from the “medium heavy cream” used in the Jensen and Hummel study15 which would have a fat content <36%. On the basis of our previous reports by Hakk et al.7 and Lupton et al.,9 our milk fat had an average fat content of 82%. Regardless, our data confirmed those of Jensen and Hummel15 in that the majority of dioxin residues would be associated with milk fat.
Compounds with a log D or P value of about 6 consistently concentrated in milk fat (or cream as cited in references). Concentrations of dichlorodiphenyltrichloroethane (DDT, Table S4) (log D 6.22 and log P = 5.92) in raw whole milk (5% lipid), skim milk, and cream (70% lipid) were reported as 7.5, 0.2, and 67.2 ppm, respectively, with a cream/whole milk ratio of 9.0.16 Pasteurization produced a slight increase of the cream/whole milk distribution ratio, as pasteurized whole milk contained 6.0 ppm and cream contained 70.2 ppm DDT resulting in a cream/whole milk ratio of 12.16 Langlois et al.17 reported the identical ratio of cream/whole milk for DDT in spite of a fat content for cream of only 37%. Relative to the Mann16 and Langois et al.17 reports, higher milk fat/whole milk concentration ratios were found in this study for compounds having log P = ∼6 (TCC, log P = 5.39, ratio 17.6; 1278-TCDD, log P = 6.22, ratio 18.7; PCB-118, log P = 6.78, ratio 19.5; Figure 1), which is also consistent with IVR (log P = 6.61, ratio 18.4) as reported by Hakk et al.7 The exception was 2-OH-1378-TCDD (log P = 6.15) which had a milk fat/whole milk concentration ratio of 11.2 in this study (Figure 1). These lower concentration ratios reported in the literature versus the current findings may be a reflection of differences in composition of the milk fat prepared here and the cream prepared in the cited reports.
A compound with a log P value similar to that of BPA (log P = 3.60) is the organophosphate cruformate (log P = 3.33, Table S4), which was fed to cows.18 Similar to BPA, which concentrated eightfold in fat relative to whole milk, cruformate concentrated about fivefold into cream.18 If values were adjusted to reflect lipid mass yield (15% of whole milk in their study, 10% in ours) the fivefold concentration would increase to ∼7.6-fold, in close agreement with the eightfold concentration found for BPA. For fenthion (log P = 3.21, Table S4), an organothiophosphate insecticide, the concentration ratio of fat/whole milk was ∼5, with 80–90% of the fenthion found in the fat fractions.19 In the current work, the E1 (log P = 3.62) milk fat/whole milk concentration ratio was ∼16 and 3′-MeSO2-PCB (log P = 4.62, calculated) was ∼18. Thus, the present results and those of O’Keeffe et al.18,19 suggested that factors in addition to log P also govern chemical disposition in milk.
Similar to the studies done by Hakk et al.7 and Lupton et al.,9 GLY and IMI (this study) distributed predominantly into the skim milk; thus, the concentration ratio between skim milk/whole milk was ∼1, whereas the ratio of milk fat/whole milk was ∼0.2 (Figure 1). Hakk et al.7 observed similar distributions for compounds with low log D values, for example, OTET, PENG, and ERY, as did Lupton et al.9 for ASP, CIPR, TAP, and TYL despite the diversity of chemical structures.
Using literature values of log P and pKa for each chemical (Tables 1, S1, and S2), mean and standard deviation (SD) log D values were calculated for ionizable compounds.14 Relationships between log D or log P values and log [milk fat]/[skim milk] distributions, including 99% confidence interval (CI) and prediction interval, are shown in Figure 2A (log D) and 2B (log P). There are apparent uncertainties with respect to log D or log P for many of the studied compounds (Figure 2A,B). In general, distribution uncertainties with regard to log D or log P are much greater than the error associated with measurements of milk fat or skim partitioning. By combining the log [milk fat]/[skim milk] data of the current set with results obtained from those of Hakk et al.7 and Lupton et al.,9 the linear regression with log D had a regression coefficient of 0.79 and with log P, the resulting linear regression had an r2 = 0.71 (Figure 2A,B). The slightly better regression using log D data reinforces the conclusions of Hakk et al.7 and Lupton et al.9 that log D was a better predictor of the distribution between milk fat and skim milk than log P. Nevertheless, Figure 2A indicates that based on the 99% CI for log D, numerous outliers were present when all 27 compounds were modeled. Outliers with respect to the 99% CI for the log D plot (Figure 2A) included ERY, FNX, TAP, TBBPA, 2-OH-1378-TCDD, and TYL, compounds which distributed more toward skim than predicted. 2-OH-1378-TCDD likely would fall within the 99% CI based on the SD of the calculated log D. Conversely, E1, 3′-MeSO2-PCB-101, OTET, PBZ, and PZQ distributed more toward milk fat than predicted. Overall, the greatest limitation to predicting the behavior of any one chemical contaminant in milk seems to be the uncertainty associated with literature log P and pKa values used to calculate log D values in the model derivation.
Figure 2.

Regression analyses of log[chemical]milk fat/[chemical]skim milk (log F/S) vs log D and log P (pH 6.8). Plot A is the regression analysis of log F/S vs log D. Plot B is the regression analysis of log F/S vs log P. Error bars on the log D and log P for the chemicals reflect the variability of values reported in the literature. Compounds outside the 99% CI but within 99% of the prediction interval are labeled. Regressions are based on data from 27 chemicals. Red dots are chemicals of the current study, whereas black dots are chemicals published in Hakk et al.7 and Lupton et al.9
Slopes of the linear log D and log P models were not 1, but 0.33 and 0.39, respectively (Figure 2). There was no reason to expect a 1:1 relationship between log D or P values of a chemical and its distribution between milk fat and skim milk. The lower slopes do indicate modeled chemicals that typically distribute to a greater extent into skim milk than merely reflected by their log D or P values. Distribution data were not affected by the presence of degradates because none were detected by thin-layer chromatography (TLC) (Table S3). The model slopes highlight the differences between the simple, ideal, octanol/water partition system and the complex milk matrix which consists of water, lipid, protein, sugar, minerals, and micelles. We hypothesize that the presence of these additional milk components could account for the enhanced distribution into skim milk. For instance, milk proteins (casein, β-lactoglobulin, and lactalbumin) enhanced the solubilization of DDT in water.20
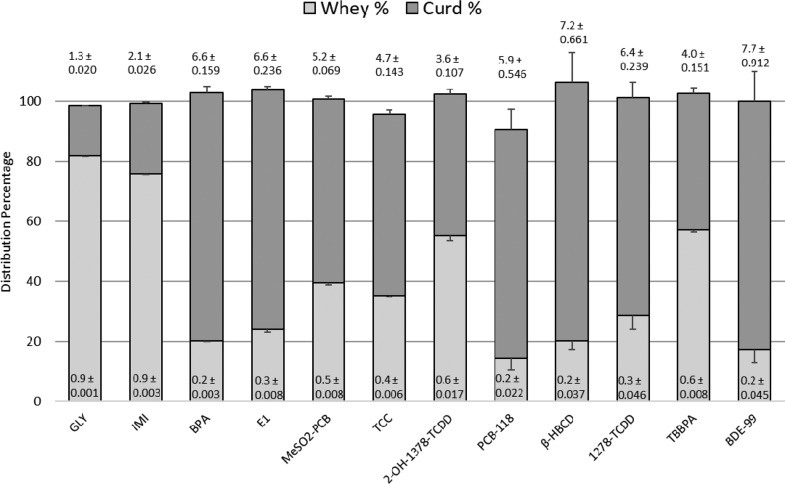
Chemical Distribution from Skim Milk into Curd and Whey
Recoveries of radioactivity across tested chemicals were ≥95% (sum of whey and curd), with the highest mean recovery (106.5%) occurring for β-HBCD and the lowest recovery occurring for PCB-118 (90.7%). The CVs for within dose replicates in whey and curd were generally <4% for the majority of chemicals tested; however, the CVs for the most lipophilic persistent organic pollutants, that is, 1278-TCDD, BDE-99, β-HBCD, 3′-MeSO2-PCB-101, and PCB-118, were considerably higher, exceeding 3% for whey (range 3.9–16.0%) and 4% for curd (range 4.3–10.0%; Figure 3 and Tables S17–S28). Higher CVs for these lipophilic chemicals in whey are to be expected, especially at lower concentrations, because of the small percentage of each compound that distributed into whey. Chemical distributions were generally not dose-dependent for 0% moisture curd/whey ratios across the starting concentrations present in skim milk, although a dose dependency was apparent for BDE-99 (p < 0.05). An ∼8% increase in association with the curd fraction was measured with BDE-99 with each 10-fold increase in dose, that is, from 73% to 80% to 92%, respectively. Initial concentrations in skim milk were 1.7, 13, and 204 nM (Table S28).
Figure 3.
Drug distribution and relative concentration ratios from skim milk into whey and curd fractions. Bars represent percent mean of all concentrations (n = 3 concentrations; n = 3 replicates per concentration, replicate exceptions are n = 2 replicates each for PCB-118 50 and 200 nM, n = 2 replicates each for β-HBCD 200 and 500 nM, n = 2 replicates each for 1278-TCDD 20 and 200 nM, and n = 2 replicates for BDE-99 20 nM) ± SD of all three dose mean percentages based on dpm of whey and curd (at 70% moisture) fractions compared to fortified skim milk dpm. Numerical values on the graph represent the mean ratio (n = 3) of the drug concentration in the fraction (curd or whey) to the initial drug concentration in skim milk ± SD. BDE-99 distribution was dose-dependent (P < 0.05). Sum of stacked plots represents total, unadjusted drug recovery values.
For the 12 compounds tested in the current study, chemicals retained in the curd fraction ranged from approximately 16.5% for GLY to 86% for β-HBCD when related to residual chemical in the skim milk of phase 1 (Tables S17–S28). Distribution into curd was largely proportional to a chemical’s lipophilicity. Of the most lipophilic compounds tested, ∼ 80% of chemical was distributed into curd (1278-TCDD, BDE-99, β-HBCD, and PCB-118). Compounds having moderate lipophilicity, that is, TBBPA, 3′-MeSO2-PCB-101, 2-OH-1378-TCDD, and TCC, were more evenly distributed into both curd (40–60%) and whey (35–60%). Highly polar compounds had the lowest affinity for curd, for example, GLY (16.5%) followed by IMI (23.7%; Figure 3, Tables S17–S28).
When curd data (normally 70% moisture) were expressed on a dry matter basis, the concentration ratios of 0% moisture curd to whey (Tables S17–S28) were >100 for the most lipophilic compounds, that is, 1278-TCDD (115), BDE-99 (327), β-HBCD (152), and PCB-118 (136), and for two of the phenolics, BPA (111) and E1 (104). Other phenolic compounds, that is, TBBPA and 2-OH-1378-TCDD, had much lower concentration ratios of 32 and 18, respectively, whereas 3′-MeSO2-PCB-101 (56) and TCC (46) were also lower than the most lipophilic compounds. The 0% moisture curd/whey concentration ratios for the most polar compounds ranged from 9.2 for IMI to 2.5 for GLY (Tables S17–S28).
Results for TBBPA were unexpected based on its structural similarity to BPA. The fire-retardant TBBPA is identical in the base structure to the plasticizer BPA with the exception that the 4-ortho hydrogens, with respect to the phenolic hydroxyls, are replaced by bromines. Bromination of the ortho-protons enhanced lipophilicity (log P) of TBBPA compared to BPA. In the 0% moisture curd/whey, however, the concentration ratio decreased from 111 for BPA to 32 for TBBPA (Tables S17–S28). Based solely on lipophilicity (log P), the curd/whey concentration ratio would have been expected to increase for TBBPA relative to BPA. One possibility for the lower concentration ratio for TBBPA is that the much larger atomic radius of bromine (compared to hydrogen) resulted in steric hindrances for potential casein–TBBPA interactions.
Hydroxylation and methylsulfonation of chemicals altered distribution patterns in milk. Aromatic hydroxylation decreased lipophilicity slightly and thus increased distribution into skim milk for phase 1 and into whey for phase 2. For example, 2-OH-1378-TCDD had a greater distribution into the whey (∼30% greater) compared to 1278-TCDD. Comparison of PCB-118 and 3′-MeSO2-PCB-101 also indicated that a methyl sulfone group decreased lipophilicity (log D 6.38 vs 4.62, respectively) and increased (>25%) distribution into whey. Despite a different chlorine substitution pattern between this pair of chemicals, the presence or absence of a methyl sulfone functional group likely plays a more important role in determining the effect on curd versus whey distribution. The full nature of this partitioning difference is undoubtedly based on more than hydrophobic interaction, for example, possible chemical/protein interactions or sequestration.
Published reports related to the partitioning of chemicals tested in this study into whey and curd are scant, but structures and characteristics of chemicals cited for comparison are provided in Table S4. For example, concentrations of the aromatic, chlorinated insecticide DDT (log D = 6.22 and log P = 5.92) were greater in cheddar cheese than in whey after milk processing, with cheese and whey concentrations of 47 and 0.5 ppm, respectively.16 Similarly, Swiss-type cheese made from milk produced by dairy cows fed DDT contained ∼8 times the original DDT concentration of whole milk, though DDT was not reported in whey.17 In other studies, however, DDT was unstable during processing and 27–53% of the starting DDT degraded to DDE and DDD21 during the manufacturing of cheese. While DDT was not identified in whey at the dipping stage, it was measured in the whey pressed from curd.22 Whey produced during the processing of raw whole milk had levels of DDE and DDD that increased twofold when measured at acidification, and concentrations were the same in the cheese product.22 Similar concentrations of DDT were reported for whole milk and cheddar or Monterey cheese, indicating some net loss of DDT, as total cheese mass would be less than the original milk mass. No changes in DDT concentration were observed during storage.
Lipophilic compounds in this study concentrated in the curd to a greater extent than whey, but the lipophilic pesticide lindane (log P = 3.99), a cyclo-chlorinated structure with similarities to β-HBCD, did not concentrate in cheese or yogurt (produced from curd) made from contaminated raw milk.23 The authors attributed the lack of concentration to heat treatment during pasteurization which resulted in phenolic metabolite formation. Pasteurization resulted in a 65–73% reduction in lindane, with more losses during refrigeration of yogurt (1.4–8% over 3 days) and cheeses (36.7% in Ras cheese during 6 months in storage). Although the effects of pasteurization and storage were not investigated in the current study, similar losses in β-HBCD might occur. Contrary to Abou-Arab,23 Langlois et al.17 found that lindane concentration in curd (4.3 ppm) was approximately 12 times that measured in whey (0.34 ppm). In a second study, Langlois et al.24 determined that curd concentrations of endrin (log D and P = 4.9) were about eight times those in whole milk (5.48 vs 0.7 ppm), whereas whey concentration was only 0.06 ppm (curd/whey concentration ratio = 91). Surprisingly, heptachlor (log P = 5.46), with higher lipophilicity than endrin, was present in whey (0.17 ppm) at approximately 1/20 the concentration measured in curd (3.77 ppm) (curd/whey concentration ratio = 22).24 Cruformate (log P = 3.33), which has a log P similar to BPA (3.60) and E1 (3.62), had a dose-dependent distribution. At a starting milk concentration of 0.07 ppm, cruformate was 22 times more concentrated in curd than that in whey (0.43 vs 0.02 ppm, respectively), but with a starting milk concentration of 0.16 ppm, the curd/whey concentration ratio was 31 (0.92 and 0.03 ppm, respectively) similar to that of BPA (29) and E1 (24).18
Hydrophilic compounds distributed more evenly between curd and whey. For example, the curd/whey concentration ratio for GLY (log D = −4.24) was 1.4 and for IMI (log D = −0.38) was 2.4, similar to SDMX (ratio 3.2), PENG (ratio 1.2), OTET (ratio 1.4), ERY (ratio 2.4), and KETO (ratio 2.4) as previously reported.8 Given the diversity of chemical structures tested, the log D value of hydrophilic compounds does provide some predictive measure for curd and whey distribution. Similarly, TAP and TYL possessed fairly low curd/whey concentration ratios, that is, 1.3 and 1.5, respectively.9
Figure 4A (log D) and 4B (log P) shows the relationships between log D or log P values and log[0% moisture curd]/[whey] concentration ratios, including 99% CI and prediction interval. By combining the log[0% moisture curd]/[whey] data of the current set with those of Shappell et al.8 and Lupton et al.,9 the regression with log D had an r2 = 0.73, whereas the log P regression had an r2 = 0.63 (Figure 4A,B). The higher regression coefficient obtained using log D data reinforces the previous conclusion that log D is a better predictor of the distribution between curd and whey than log P.8,9 On the basis of the 99% CIs for the log P regression, numerous outliers were present when all 27 compounds were modeled. Outliers for the curve fit on a log D basis (Figure 4A) included ASP, ERY, 2-OH-1378-TCDD, TAP, and TBBPA compounds which distributed more toward whey than predicted. Conversely, BPA, CIPR, E1, and PBZ distributed more toward curd than predicted. In the log P model (Figure 4B), four additional chemicals (CLA, KETO, FNX, and PENG) fell outside of the 99% CIs.
Figure 4.

Regression analyses of log[chemical]0%moisture curd/[chemical]whey (log mC/W) vs log D and log P (pH 6.8). Plot A is the regression analysis of log mC/W vs log D. Plot B is the regression analysis of log mC/W vs log P. Error bars on the log D and log P for the chemicals reflect the variability of values reported in the literature. Compounds in between the 99% CI and 99% of the prediction interval are labeled. Red dots are chemicals of the current study, whereas black dots are chemicals published in Shappell et al.8 and Lupton et al.9 Regressions are based on data from 27 chemicals.
Chemical Distribution from Whey into Retentate and Permeate
In order to assess the percent of drug associated with the whey proteins, ultrafiltration in conjunction with centrifugation was performed (phase 3, Figure 5). The expected volume of retentate was 33% of the applied sample volume based on centrifugation time and speed, with the actual measured mean for all compounds being 37 ± 3.3%. Mean recovery of radioactivity across all compounds was 100 ± 4.5%. Mean nonspecific binding of compounds to filters ranged from 0.2% for GLY to 22.5% for E1. Compounds with >3% filter binding include PCB-118 (6.4%), BDE-99 (7.1%), β-HBCD (8.2%), BPA (13.4%), and E1 (22.5%) (Tables S29–S40). Although compounds with high log D values could be expected to be “sticky” in the aqueous medium, four compounds with high log D values [TCC (log D = 5.39), 2-OH-1378-TCDD (log D = 6.15), 1278-TCDD (log D = 6.22), and TBBPA (log D = 6.69)] had filter binding of ≤2.4%.
Figure 5.
Scheme of milk partitioning processes that yielded cream and milk fat from whole milk (phase 1) curd and whey from skim milk (phase 2) and retentate and permeate from whey (phase 3).
The associations of the 12 xenobiotics with whey protein, as determined by the percentage of compound measured in the retentate, revealed three groupings (Figure 6). The first was represented by GLY and IMI that have negative log D values (−4.24 and −0.38, respectively), where there was essentially no association with the whey protein (<5%) occurred (Tables S29 and S30). The second grouping was composed of BPA, E1, 3′-MeSO2-PCB-101, 2-OH-1378-TCDD, and 1278-TCDD, which had moderate associations with whey protein, ranging from 33 to 76% (Tables S31–S33, S35, and S38). Similar to our findings of ∼64% association of E1 with whey protein, Wolford and Argoudelis25 reported 48 and 53% of 17β-estradiol and E1, respectively, associated with whey protein. The third grouping was composed of those compounds that were almost totally associated with retentate whey proteins (84–98%, one outlier of 107% for PCB-118 due extremely low starting radiocarbon in the whey). Chemicals in this grouping included BDE-99, β-HBCD, PCB-118, TBBPA, and TCC (Tables S34, S36, S37, S39, and S40). If present in whey, these compounds would concentrate in whey-derived protein products.
Figure 6.
Drug distribution from whey into permeate, retentate, and filter fractions. Bars represent percent mean of all concentrations (n = 3 concentrations, concentration exceptions are PCB-118 and β-HBCD n = 2 concentrations; n = 3 replicates per concentration, replicate exceptions n = 2 replicates for TCC 20 nM, n = 2 replicates each for 1278-TCDD 200 and 2000 nM, n = 2 replicates for BDE-99 200 nM) ± SD of all three dose mean percentages based on dpm of permeate and retentate fractions compared to fortified whey dpm. Horizontal lines on each bar represent the actual retentate and permeate volume percentage after centrifugation. Sum of stacked plots represents total, unadjusted drug recovery values.
The percent of whole milk dose associated with either casein or whey proteins is reported in Table 2. About 25% of TBBPA and 2-OH-1378-TCDD from whole milk distributed to whey, yet ∼90 and 70% (TBBPA and 2-OH-1378-TCDD, respectively) of that were associated with whey protein.
Table 2. Compound Associated with Casein or Whey Protein (nmol/mg Protein and Percent Association Based on Whole Milk).
| compound | nominal conc. of whole milka (actual) nM | nmol/mg casein proteinb | nmol/mg whey proteinc | conc. in casein/conc. in whey protein | mean % casein association based on whole milkd | mean % whey association based on whole milkd |
|---|---|---|---|---|---|---|
| GLY | 20 (22) | 0.08 | 0.15 | 0.53 | 7.92 | 3.68 |
| 200 (217) | 0.71 | 1.40 | 0.51 | |||
| 2000 (2059) | 6.52 | 14.09 | 0.46 | |||
| IMI | 20 (20) | 0.21 | 0.11 | 1.91 | 15.43 | 3.19 |
| 200 (201) | 2.20 | 1.13 | 1.95 | |||
| 2000 (2066) | 22.33 | 12.29 | 1.82 | |||
| BPA | 20 (22) | 0.44 | 0.23 | 1.91 | 45.76 | 6.68 |
| 200 (216) | 4.54 | 2.25 | 2.02 | |||
| 2000 (1992) | 42.43 | 20.88 | 2.03 | |||
| E1 | 20 (20) | 0.21 | 0.10 | 2.10 | 17.86 | 3.52 |
| 200 (200) | 1.66 | 0.96 | 1.73 | |||
| 2000 (1796) | 15.89 | 8.70 | 1.83 | |||
| 3-MeSO2-PCB-101 | 20 (24) | 0.06 | 0.04 | 1.50 | 4.25 | 0.99 |
| 100 (70) | 0.18 | 0.12 | 1.50 | |||
| 500(628) | 1.60 | 1.08 | 1.48 | |||
| TCC | 20 (19) | 0.05 | 0.10 | 0.50 | 5.92 | 3.62 |
| 200 (193) | 0.54 | 0.95 | 0.57 | |||
| 2000 (1938) | 5.68 | 9.72 | 0.58 | |||
| 2-OH-1378-TCDD | 20 (22) | 0.16 | 0.64 | 0.25 | 17.85 | 16.79 |
| 100 (107) | 0.77 | 3.26 | 0.24 | |||
| 500 (556) | 4.71 | 16.16 | 0.29 | |||
| PCB-118 | 50 (60) | 0.07 | <LOQe | 3.42 | 0.70 | |
| 200 (221) | 0.35 | 0.24 | 1.46 | |||
| 2000 (2203) | 2.90 | 2.37 | 1.22 | |||
| β-HBCD | 200 (229) | 0.38 | <LOQe | 2.95 | 0.59 | |
| 500(656) | 1.09 | 0.59 | 1.85 | |||
| 2000 (2067) | 3.21 | 2.11 | 1.52 | |||
| 1278-TCDD | 20 (27) | 0.11 | 0.06 | 1.83 | 4.14 | 1.29 |
| 200 (184) | 0.46 | 0.34 | 1.35 | |||
| 2000 (1784) | 5.07 | 3.58 | 1.42 | |||
| TBBPA | 20 | 0.27 | 0.79 | 0.34 | 18.01 | 22.96 |
| 200 (234) | 3.53 | 9.36 | 0.38 | |||
| 2000 (1815) | 21.63 | 76.47 | 0.28 | |||
| BDE-99 | 20 (20) | 0.12 | 0.03 | 4.0 | 6.66 | 1.20 |
| 200 (178) | 1.05 | 0.38 | 2.76f | |||
| 2000 (1890) | 18.33 | 3.35 | 5.47 |
SA of some compounds required different doses, as indicated by bold text. Each fortified level contains three replicates.
These data were derived from phase 2 data and have whey associated drug subtracted, using “0% moisture curd” as described in text.
These data were derived from phase 3 data as described in the text.
Mean of all doses.
Less than limit of quantitation (<LOQ). LOQ for PCB-118 is 1.92 nmol/L and for β-HBCD was 9.87 nmol/L.
Inconsistent with other doses. No explanation.
Chemical Concentration Based on Protein Mass for Casein and Whey Proteins
Using 0% moisture curd data from phase 2, the amount of chemical associated with caseins was calculated based on proteins present in curd and largely result from agglutination of casein (Table 2). Similarly, using phase 3 data, the amount of chemical associated with whey proteins can be calculated (Table 2). Chemical saturation of casein or whey protein was not observed because the mass of chemical per milligram protein increased as the concentration increased. In some instances, the initial expected fortification concentrations in whole milk differed from measured concentrations, as seen with 3′-MeSO2-PCB-101 and β-HBCD. Whey protein association values for the lowest dose of BDE-99 are questionable because the starting skim milk contained <2 nM and whey 0.3 nM. However, confidence in casein/whey protein association results is enhanced by the agreement found across doses (Table 2), exception was BDE-99, where ratios ranged from 2.8 to 5.5.
For the majority of chemicals tested (BDE-99, BPA, E1, β-HBCD, IMI, 3′-MeSO2-PCB-101, PCB-118, and 1278-TCDD), the association with caseins was greater than that for whey proteins (ratio > 1, Table 2). The importance of methodology is evident when comparing our findings to those of Wolford and Argoudelis25 that used equilibrium dialysis with E1 and the slightly more hydrophilic compound E2. They reported that E1 and E2 were largely (>84%) bound to protein when incubated in skim milk, and >50% of the bound estrogens was associated with whey proteins. These data are in contrast to our findings for E1, in which the association (nmol/mg protein) ratio was approximately 2 for casein/whey. The difference between the results of the two studies was most likely the precipitation of curd caseins in the present work versus the presence of soluble caseins used for dialysis by Wolford and Argoudelis25 (1979).
Other chemicals that preferentially associated with caseins relative to whey protein (ratio > 1) include THIA (2.5), IVR (2.0),8 TYL (1.4), CIPR (2.0), and PZQ (1.5).9 Although the current work used a majority of chemicals with log D greater than 3.4, our previous reports described only one such chemical (IVR). The casein/whey protein association ratio of IVR was more similar to BPA (2.0), E1 (1.9), and IMI (1.9) (Table 2).
In spite of higher distribution of GLY into whey than curd (Figure 3), there was in fact very little preferential retention of GLY associated with whey protein (Figure 6). Similarly, TCC, 2-OH-1378-TCDD, and TBBPA also had casein/whey protein ratios <1. Although most of the total TCC dose was partitioned with milk fat (mean 85%), the remainder distributed almost equally between whey and 0% moisture curd (57% curd, Table S22). TCC remaining in the whey was concentrated almost exclusively in the retentate (98%) during ultracentrifugation (Table S34). The log D values of 2-OH-1378-TCDD (6.15) and TBBPA (6.69) did not predict the respective mean casein/whey protein ratios of 0.26 and 0.33. Both chemicals also distributed to a lesser extent than predicted into milk fat. The common feature of both compounds is a hydroxyl moiety between two halogens (chlorines for 2-OH-1378-TCDD and bromines for TBBPA).
Previously studied chemicals that had higher association for whey proteins versus caseins were PENG (casein/whey ratio = 0.2), ERY (0.5), KETO (0.4), SDMX (0.8);8 TAP (0.5), CLA (0.4), and FNX (0.25).9 Although the distribution between lipid and aqueous phases was markedly dependent on the property of proteins, namely lipophilicity, small-molecule binding to proteins seems to be more dependent on specific functional groups within the protein. Identifying the specific functional groups and binding domains that can associate with studied chemicals within a plethora of whey and casein proteins lies outside the scope of the present research.
Relation to Consumer Products
To determine how the distributions of these compounds, if detected in whole milk, related to consumer products, the percent distributions into milk fat, curd, retentate, and permeate were calculated in relation to the starting concentration in whole milk. Figure 7 includes the experimentally derived percentages of each compound in high-fat products which would include butter, cream, and cheese; low-fat products would include skim milk, low fat cheese, yogurt, and low-fat derived whey protein products such as whey protein powders and baby formulas. Comparable to compounds previously tested,8,9 higher log D compounds (i.e., E1, 3′-MeSO2-PCB-101, TCC, PCB-118, β-HBCD, 1278-TCDD, and BDE-99) generally distributed to high-fat products such as butter and cream. High-fat products that contain protein (i.e., cheese) will concentrate both mid- to high-range log D molecules such as BPA, 2-OH-1378-TCDD, and TBBPA along with the higher log D compounds. Two compounds with low log D’s, that is, GLY and IMI, will primarily distribute into aqueous products, such as skim milk and whey.
Figure 7.
Normalized percentages of chemicals calculated from whole milk to be in the milk end products of milk fat, curd, permeate, and retentate based on data generated from the current studies as well as those reported in Hakk et al.,7 Shappell et al.,8 and Lupton et al.9 The PZQ bar has additional information on which milk end products comprise whole milk, skim milk, curd, low-fat curd, and whey, as a guide to where drug may partition during commercial milk processing. For percentage of chemical associated with whey protein see supplemental information tables S29−S40.
Determining where a compound would concentrate in consumer products will also depend on the processing steps involved and what specific end product is being manufactured. For example, whole milk processed into skim milk and cream would generally have compounds with high log D values concentrated in butter and cream, whereas compounds with low log D values will be in skim milk. Compounds with mid-range log D values will be split between the higher fat products and skim milk. However, if whole milk is processed directly into cheese, then the mid-range and high-range log D value compounds will mainly concentrate in the cheese.
Conclusions
The partitioning of 12 environmental contaminants or metabolites into milk fractions was assessed. Partitioning between milk fat and skim milk and between 0% moisture curd and whey was usually governed by the compound’s lipophilicity. If a chemical was found in whey, the more nonpolar the compound the more likely it would be found in whey protein products. Phenolic compounds were the main chemicals that fell outside of the 99% CIs of the models’ regression analyses. These models provide a tool using log D as the primary chemical property to predict the distribution of chemicals into various milk products.
Experimental Section
Safety
Nuclear Regulatory Commission and USDA regulations were followed for handling all radiolabeled chemicals.
Selection of Drugs and Concentrations
Chemicals selected for study had to be potential environmental contaminants, encompass a wide range of lipophilicities, and be available with radiolabel (3H or 14C) incorporation. The chemicals selected had a log P range of −3.3 to 7.3. Chemical structures, site of radiolabel, specific activity (SA), and physio-chemical properties are provided in Table 1.
To detect potential concentration-dependent distribution, chemical concentrations spanning 3 orders of magnitude (i.e., 20–2000 nM) were generally used. The lowest concentration (usually 20 nM) was typically relevant to possible contamination scenarios with sufficient activity to allow radiochemical detection. Higher concentrations were used to determine whether concentration influenced overall xenobiotic distribution. In some instances, concentrations were adjusted because of limited solubility or if the SA of the radiolabeled compound was inadequate for the sensitivity of the analysis (Table 1). As a result of adding unlabeled chemical (typically 9:1 parts) for the highest dose, SA was lowered, relative to low concentration.
Chemicals, Supplies, and Equipments
Raw (unpasteurized, nonhomogenized) cow milk was obtained from the bulk milk tank located at the North Dakota State University (Fargo, ND) Dairy farm within 48 h of milking. Nonradiolabeled chemicals and solvents were obtained from Sigma-Aldrich (St. Louis, MO), U.S. Pharmacopeia (Rockville, MD), or other common vendors. Radiolabeled E1, GLY, PCB-118, and β-HBCD were procured through American Radiolabeled Chemicals, Inc. (ARC, St. Louis, MO). A mixture of the β- and γ-diastereoisomers of [14C]-HBCD was identified in the ARC product. Flash chromatography on a silica gel column eluted with hexane containing increasing amounts of methylene chloride (0–50%) was used to isolate [14C]-β-HBCD. [14C]-BPA and [14C]-TCC were purchased from Moravek Inc. (Brea, CA). [14C]-IMI was a gift from Bayer Crop Science (Research Triangle Park, NC). [UL-7,8-ring 14C]-1278-TCDD was purchased from ChemSyn Science Laboratories (Lenexa, KS). [14C]-2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) was synthesized using published methods.10 2-OH-1378-TCDD was prepared in-house from [UL-7,8-ring 14C]-1278-TCDD by in vitro oxidation with human CYP1A1R Baculosomes (Cypex Ltd., Dundee, UK) and a glucose-6-phosphate dehydrogenase regenerating system according to manufacturer’s instructions. [14C]-2,2-bis(4-hydroxy-3,5-dibromophenyl)propane (TBBPA) was synthesized by brominating bis[14C]-phenol A with 4.2 equivalents of bromine in 1:1 methanol/water; bis[14C]-phenol A was prepared in-house from [UL-14]-phenol (2.0 mCi, 25 mCi/mmol) and acetone according to a published method.11 3′-[14C]-MeSO2-PCB-101 was synthesized de novo by Cadogan coupling as described in Haraguchi et al.12 using sodium [14C]-methyl thiolate for label introduction.
Silica gel plates were purchased from Analtech (Newark, DE). Scintillation cocktails were purchased from MP Biochemicals, LLC, (Ecolite; Solon, OH) or PerkinElmer (Waltham, MA; Carbosorb, and Permafluor). Amicon Ultra-15 centrifugal filters were purchased from Millipore (Billerica, MA). An Allegra X-14R centrifuge was obtained from Beckman-Coulter (Brea, CA). Liquid milk product fractions were mixed with scintillation fluid and assayed using a Tri-Carb 1900 liquid scintillation counter (LSC, Packard, Meriden, CT). Solid milk product samples were combusted using a Packard model 307 tissue oxidizer (Meriden, CT), trapped into Carbosorb, diluted with Permafluor, and then assayed by LSC. Sample purity was assessed by TLC and radioassay using a Bioscan AR-2000 Imaging Scanner for TLC (Washington, DC).
Determination of Chemical Purity and Confirmation of Test Article Stability
TLC analyses were used to assess chemical purities before and after the experiments, although for GLY, high-performance liquid chromatography instead of TLC was employed. Initial analyses were used to evaluate dose purity, whereas postincubation analyses were used to evaluate whether chemical degradation occurred during milk processing. TLC conditions and results are included in Table S3. GLY radiochemical purity (98.0 ± 0.4%, n = 4) was determined based on Nagatomi et al.13 using a Waters 2695 HPLC, a radiometric detector (Packard LFA 515TR, PerkinElmer, Waltham, MA), and a Dionex IonPac AS 12 column (4 × 200 mm, 9 μm, Dionex Company, Sunnyvale, CA). The mobile phase was isocratic 0.2% aqueous formic acid/acetonitrile (5/95 v/v), and the flow rate was 1 mL/min.
Milk Processing and Radiochemical Analysis
The milk processing experiments consisted of three sequential phases. Specific details pertaining to preparation of phases are reported in Hakk et al.7 and Shappell et al.8 Briefly, 12 tubes of raw milk (50 mL) were pasteurized at 63 °C for 30 min. Triplicate tubes were fortified with each level of radiolabeled chemicals using three working solutions or with the appropriate solvent for blank milk, as described in Table 2. In phase 1, the fortified, pasteurized, whole milk samples were separated into milk fat and skim milk by centrifugation after equilibration; the partitioning of chemical between these phases was then determined by radiochemical detection methods. In phase 2, the skim milk originating from phase 1 was partitioned into curd and whey (enzymatically with rennet) and the distribution of the target chemical between these phases determined by radiochemical detection. In phase 3, the residual whey (15 mL) from phase 2 was separated into a protein-enriched fraction (>10 kD), retentate (∼5 mL) and permeate (∼10 mL) fractions using ultracentrifuge filters. To determine if degradation occurred during processing, milk fat, curd, and whey from the highest dose concentration were extracted and analyzed by TLC side by side with radiolabeled standards with the exception of GLY because no satisfactory TLC method was found. The main difference in the current study compared to the cited research7−9 was that here the radiolabeled compounds were fortified only once into whole milk and not anew at the beginning of each phase (Figure 5), resulting in lower initial chemical concentrations in skim and whey fractions.
Calculation of Chemical Associated with Casein and Whey Protein
The percentage of chemical associated with whey proteins was calculated according to Shappell et al.8 Briefly, the amount of free chemical measured in permeate (calculated by concentration and volume) was subtracted from the total amount of chemical present in retentate. The difference was assumed to be the amount of chemical associated with whey protein. Residual radioactivity on ultrafilters (measured by combustion analysis) was considered nonspecific binding and was subtracted from the fortified whey results; however, radioactivity present in filter washes was included with retentate radioactivity. Averaged Kjeldahl protein concentrations in curd from Shappell et al.8 and Lupton et al.9 and the resultant 0% moisture curd radioactivity (see below) along with its SA were used to calculate nanomole per milligram casein protein association. Similarly, averaged Kjeldahl protein concentration in retentate from Shappell et al.8 and Lupton et al.9 and the protein associated radioactivity and its SA in retentate was used to calculate nanomole per milligram whey protein association.
Statistical Analyses
Standard statistical methods were used to calculate means and variability and make inferences with respect to the significance of differences between means. Linear regression was used to assess dose dependence of the observed drug distribution log ratio of [chemical]milk fat/[chemical]skim milk or 0% moisture [chemical]curd/[chemical]whey. Dose dependency was based on instances when the slope differed (P < 0.05) from zero. Because curd is 70% moisture and contains a small quantity of entrained whey, a 0% moisture curd radioactivity value was calculated by subtracting entrained whey-associated radioactivity (calculated based on the percent moisture) from curd. The value representing entrained whey was added back to the whey fraction.
Coefficient of variation with respect to measured partition values across doses was typically much less than 10%, whereas literature values for log P for a given chemical could sometimes differ by an order of magnitude or greater. Therefore, distribution data were modeled using mean log P values ± SD for each chemical. Mean log P values were calculated from predicted and measured entries included in Chemspider, DrugBank, ChemBL, and Pubchem databases. For 3′-MeSO2-PCB-101, the log P value was derived from using conversion of chlorocyclohexatriene into p-chlorophenyl methyl sulfone as a model, which has log differences of 1.76. By using PCB-101 log P of 6.38 and subtracting 1.76, the log P of 3′-MeSO2-PCB-101 was derived as 4.62. Log D values were calculated as described by Scherrer and Howard14 using a pH of 6.8 (reflecting the pH of milk); to obtain a theoretical range of log D values for each compound, the range of log P values derived from the above sources was used in conjunction with the range of pKa values obtained from the same sources; log D values were averaged and SDs calculated. Relationships between the log distribution ratios and lipophilicity (log D and log P) were performed using linear function and included the 99% CI and prediction interval by GraphPad Prism Version 7.03 (GraphPad Software, La Jolla, CA).
Acknowledgments
The authors would like to acknowledge Dee Ellig, Lindsey Fransen, Jason Holthusen, Amy McGarvey, Jason Neumann, Colleen Pfaff, and Michael Woodworth from the ARS Red River Valley Agricultural Research Center for their technical assistance. We would like to thank Todd Molden and Thomas Brown for milk collection from the NDSU dairy barn. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture (USDA) or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable. The USDA is an equal opportunity employer.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00762.
TLC conditions, data table summaries for individual drugs for phases 1, 2, and 3, and tables of compound structures for previous compounds and discussion compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rocha D. A. M.; Torres J. P. M.; Reichel K.; Novotny E. H.; Estrella L. F.; Medeiros R. O.; Netto A. D. P. Determination of polychlorinated dibenzo- p -dioxins and dibenzofurans (PCDD/Fs) in Brazilian cow milk. Sci. Total Environ. 2016, 572, 177–184. 10.1016/j.scitotenv.2016.07.179. [DOI] [PubMed] [Google Scholar]
- Avancini R. M.; Silva I. S.; Rosa A. C. S.; Sarcinelli P. d. N.; de Mesquita S. A. Organochlorine compounds in bovine milk from the state of Mato Grosso do Sul - Brazil. Chemosphere 2013, 90, 2408–2413. 10.1016/j.chemosphere.2012.10.069. [DOI] [PubMed] [Google Scholar]
- Perez J. J.; León S. V.; Gutiérrez R.; López Y.; Faure R.; Escobar A. Polychlorinated biphenyls (PCBs) residues in milk from an agroindustrial zone of Tuxpan, Veracruz, Mexico. Chemosphere 2012, 89, 404–408. 10.1016/j.chemosphere.2012.05.055. [DOI] [PubMed] [Google Scholar]
- Esposito M.; Cavallo S.; Serpe F. P.; D’Ambrosio R.; Gallo P.; Colarusso G.; Pellicanò R.; Baldi L.; Guarino A.; Serpe L. Levels and congener profiles of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and dioxin-like polychlorinated biphenyls in cow’s milk collected in Campania, Italy. Chemosphere 2009, 77, 1212–1216. 10.1016/j.chemosphere.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Schettino B.; Gutiérrez R.; Ortiz R.; Vega S.; Urban G.; Ramírez A. Residues of legacy organochlorine contaminants in the milk of Alpine and Saanen goats from the central region of Mexico. Bull. Environ. Contam. Toxicol. 2013, 91, 154–159. 10.1007/s00128-013-1005-8. [DOI] [PubMed] [Google Scholar]
- Pagliuca G.; Serraino A.; Gazzotti T.; Zironi E.; Borsari A.; Rosmini R. Organophosphorus pesticides residues in Italian raw milk. J. Dairy Res. 2006, 73, 340–344. 10.1017/s0022029906001695. [DOI] [PubMed] [Google Scholar]
- Hakk H.; Shappell N. W.; Lupton S. J.; Shelver W. L.; Fanaselle W.; Oryang D.; Yeung C. Y.; Hoelzer K.; Ma Y.; Gaalswyk D.; Pouillot R.; Van Doren J. M. Distribution of Animal Drugs between Skim Milk and Milk Fat Fractions in Spiked Whole Milk: Understanding the Potential Impact on Commercial Milk Products. J. Agric. Food Chem. 2016, 64, 326–335. 10.1021/acs.jafc.5b04726. [DOI] [PubMed] [Google Scholar]
- Shappell N. W.; Shelver W. L.; Lupton S. J.; Fanaselle W.; Van Doren J. M.; Hakk H. Distribution of animal drugs among curd, whey, and milk protein fractions in spiked skim milk and whey. J. Agric. Food Chem. 2017, 65, 938–949. 10.1021/acs.jafc.6b04258. [DOI] [PubMed] [Google Scholar]
- Lupton S. J.; Shappell N. W.; Shelver W. L.; Hakk H. Distribution of Spiked Drugs between Milk Fat, Skim Milk, Whey, Curd, and Milk Protein Fractions: Expansion of Partitioning Models. J. Agric. Food Chem. 2018, 66, 306–314. 10.1021/acs.jafc.7b04463. [DOI] [PubMed] [Google Scholar]
- Jensen D. J.; Hummel R. A. Secretion of TCDD in milk and cream following the feeding of TCDD to lactating dairy cows. Bull. Environ. Contam. Toxicol. 1982, 29, 440–446. 10.1007/bf01605609. [DOI] [PubMed] [Google Scholar]
- Johansson M.; Anlér E.-L. Gas chromatographic analysis of flunixin in equine urine after extractive methylation. J. Chromatogr. 1988, 427, 55–66. 10.1016/0378-4347(88)80104-x. [DOI] [PubMed] [Google Scholar]
- Mann H. D.; Carter R. H. The DDT content of milk products. J. Milk Food Technol. 1950, 13, 340–341. [Google Scholar]
- Langlois B. E.; Liska B. J.; Hill D. L. The effects of processing and storage of dairy products on chlorinated insecticide residue. I. DDT and lindane. J. Milk Food Technol. 1964, 27, 264–267. [Google Scholar]
- O’Keeffe M.; Eades J. F.; Strickland K. L.; Harrington D. Crufomate residues in milk and milk products following treatment of dairy cows for Warble-fly. J. Sci. Food Agric. 1982, 33, 355–360. 10.1002/jsfa.2740330409. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M.; Eades J. F.; Strickland K. L. Fenthion residues in milk and milk products following treatment of dairy cows for Warble-fly. J. Sci. Food Agric. 1983, 34, 192–197. 10.1002/jsfa.2740340213. [DOI] [PubMed] [Google Scholar]
- Scherrer R. A.; Howard S. M. Use of distribution coefficients in quantitative structure-activity relations. J. Med. Chem. 1977, 20, 53–58. 10.1021/jm00211a010. [DOI] [PubMed] [Google Scholar]
- Hugunin A. G.; Bradley R. L. Jr. Distribution of organochlorine pesticides among some milk components. J. Dairy Sci. 1971, 54, 355–359. 10.3168/jds.s0022-0302(71)85843-5. [DOI] [PubMed] [Google Scholar]
- Liska B. J.Effects of processing on residues in foods. Proceedings of 25th Semi-Annual Meeting; AFMA Nutrition Council, American Feed Manufacturers’ Association, Chicago, 1965; Vol. 35.
- Montoure J. E.; Muldoon P. J. Distribution and stability of DDE, DDD, and DDT in Monterey and Cheddar cheese during manufacture and storage. J. Dairy Sci. 1967, 51, 858–862. 10.3168/jds.s0022-0302(68)87093-6. [DOI] [PubMed] [Google Scholar]
- Abou-Arab A. A. K. Effects of processing and storage of dairy products on lindane residues and metabolites. Food Chem. 1999, 64, 467–473. 10.1016/s0308-8146(98)00126-5. [DOI] [Google Scholar]
- Langlois B. E.; Liska B. J.; Hill D. L. The effects of processing and storage of dairy products on chlorinated insecticide residue. II. Endrin, dieldrin, and heptachlor. J. Milk Food Technol. 1965, 28, 9–11. [Google Scholar]
- Wolford S. T.; Argoudelis C. J. Measurement of Estrogens in Cow’s Milk, Human Milk, and Dairy Products. J. Dairy Sci. 1979, 62, 1458–1463. 10.3168/jds.s0022-0302(79)83446-3. [DOI] [PubMed] [Google Scholar]
- Örn U.; Eriksson L.; Jakobsson E.; Bergman Å.; Samuelsen E. J.; Robinson W. T.; Undheim K.; Rosendahl C. N.; Haugg M.; Trabesinger-Rüf N.; Weinhold E. G. Synthesis and Characterization of Polybrominated Diphenyl Ethers -- Unlabelled and Radiolabelled Tetra-, Penta- and Hexabromodiphenyl Ethers. Acta Chem. Scand. 1996, 50, 802–807. 10.3891/acta.chem.scand.50-0802. [DOI] [Google Scholar]
- Susán A. B.; Ebert D. A.; Duncan W. P. Synthesis of 14C-labeled flame retardants. 14C-labeled-tetrabromophthalic anhydride and tetrabromobisphenol-A. J. Label. Compd. Radiopharm. 1979, 16, 579–589. 10.1002/jlcr.2580160410. [DOI] [Google Scholar]
- Haraguchi K.; Kuroki H.; Masuda Y. Synthesis and characterization of tissue-retainable methylsulfonyl polychlorinated biphenyl isomers. J. Agric. Food Chem. 1987, 35, 178–182. 10.1021/jf00074a003. [DOI] [Google Scholar]
- Nagatomi Y.; Yoshioka T.; Yanagisawa M.; Uyama A.; Mochizuki N. Simultaneous LC-MS/MS Analysis of Glyphosate, Glufosinate, and Their Metabolic Products in Beer, Barley Tea, and Their Ingredients. Biosci., Biotechnol., Biochem. 2013, 77, 2218–2221. 10.1271/bbb.130433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.