Abstract
Objectives
Angiotensin II (AngII), a major product of renin-angiotensin system (RAS) has important role in induction of hypertension and antihypertensive effect of several medicinal plant was mediated by effect on this agent. Therefore, this study examined the possible effect of hydroalcoholic extract of Crocus sativus (C. sativus) on hypertension induced by AngII.
Methods
Six groups (n = 6) of rats were used as follow: 1) Control, 2) AngII (300 ng/kg), 3) Losartan (Los, 10 mg/kg) + AngII and 4–6) C. sativus extract (10, 20 & 40 mg/kg,) + AngII. The femoral artery and vein were cannulated for recording cardiovascular parameters and drugs administration, respectively. All drugs were injected intravenously (i.v). Los and all doses of C. sativus injected 10 min before AngII. Systolic blood pressure (SBP), mean arterial blood pressure (MAP) and heart rate (HR) were recorded throughout the experiment and those peak changes (Δ) were calculated and compared to control and AngII.
Results
AngII significantly increased ΔMAP, ΔSBP and ΔHR than control (P < 0. 01 to P < 0.001) and these increments were significantly attenuated by Los. All doses of C. sativus significantly reduced peak ΔMAP, ΔSBP, and ΔHR than AngII group (P < 0. 05 to P < 0.001). In addition, peak ΔMAP, ΔSBP in doses 10 and 20 were significant than Los + AngII group (P < 0.05 to P< 0.01) but in dose 40 only MAP was significant (P < 0.05). Peak ΔHR in all doses of C sativus was not significant than Los+ AngII.
Conclusion
Regarding the improving effect of the C. sativus extract on AngII induced hypertension, it seems that this ameliorating effect partly mediated through inhibition of RAS.
Keywords: angiotensin II, crocus sativus, hypertension, blood pressure, heart rate
1. Introduction
Hypertension is a highly prevalent cardiovascular risk factor for cardiovascular disease that numerous individuals around the world suffer from this condition. Hypertension shortens the life expectancy of people due to heart disease, stroke, impairment of vision and kidney disease [1].
The exact mechanisms of hypertension is unknown but suggested factors such as endothelial dysfunction, extracellular matrix alterations and activation of renin-angiotensin system (RAS) are involved [2]. The RAS plays an important vital role in physiological regulation of cardiovascular, renal and endocrine functions [3]. This system has been also shown to involve in development and maintenance of different forms of hypertension [4] so drugs that inhibit the RAS can apply useful therapeutic effects on hypertension and heart failure [5]. In addition, an extensive interaction between the RAS and other blood pressure control systems such as sympathetic nervous system [6] and baroreceptor reflexes [7] and nitric oxide (NO) has been demonstrated.
Effect of medicinal plants on RAS especially angiotensin II (AngII), main product of this system, has been reported [8]. One of these plants is Crocus sativus (C. sativus). This plant which is also known as saffron spice has several part including stigma, petal, stamen. The valuable part of this plant is stigma that possesses various beneficial components including crocin, Safranal, crocetin, anthocyanin, flavonoids, vitamins such as riboflavin and thiamine, minerals, carbohydrate, raw fibers, proteins, and fats [9]. Besides its use in food as a spice, C. sativus has been documented to have therapeutic properties [10]. C. sativus has been shown to have the beneficial effects against the disturbances of the nervous system, blood pressure, lungs, kidneys, gastrointestinal tract and endocrine gland [11]. In addition, crocetin a component of C. sativus has been claimed to has cardioprotective effects via exerting antioxidant, anti-inflammatory and anti-apoptotic effects [12]. We previously has been shown that crocin, a main product of C. sativus has ameliorative effect on hypertension induced by AngII [8]. Despite all these findings, the effect of C. sativus on RAS especially AngII has not yet been investigated. Therefore, current study was conducted to determine the effect of the hydroalcoholic extract of C. sativus on cardiovascular parameters in AngII hypertensive rats.
2. Materials and Methods
2.1. Preparation of plant extract
C. sativus was collected from saffron farms of South Khorasan Province (Esfeden city). After identifying by a botanist in the herbarium of the Ferdowsi University of Mashhad (Herbarium No: 143-0319-1), the hydroalcoholic extract was prepared. For this purpose, 10 g of stigma of plants and 400 mL of 70% aqueous alcohol solution in a Soxhlet extractor were mixed for 14 h. Then prepared extract was filtered through a 0.2 mm filter to be sterilized. Finally, the extract was concentrated to 100 mL by a rotatory evaporator and was stored at −200°C until being used. The prepared extract was dissolved in saline in order to arrive at desired doses and was then administered.
2.2. Experimental animals
In this study, 36 male wistar rats with 250 ± 20 g of weight were purchased from animal’s house of Mashhad University of Medical Sciences, the animals were housed in standard condition (light / dark cycle = 12 h and temperature = 21 ± 4°C). Working with animals was done based on approved procedures by the Animal Protection Committee of Mashhad University of Medical Sciences (Code Number 922834).
2.3. Drugs
The drugs used in current study are include: AngII and urethane purchased from Sigma, USA and losartan (Los) prepared from Darupaksh Company, Iran. The solvent of drugs was saline.
2.4. Experimental groups
The rats were assigned to 6 groups (n = 6 rats/group): (1) Control, (2) AngII, (3) Los + AngII, (4) C. sativus 10 + AngII, (5) C. sativus 20 + AngII and (6) C. sativus 40 + AngII. AngII (300 ng/kg) was intravenously (i.v) administered during 2 min. Los as AT1 blocker (10 mg/kg, i.v) and 3 doses of C. sativus extract (10, 20 and 40 mg/kg) was injected (i.v) 10 min before AngII. The doses of all drugs were selected based on a previous study [8, 13].
2.5. Experimental procedure
In this study, urethane (1.5 g/kg, i.p) was used to anesthetize the animal. After anesthesia the femoral artery and vein catheterized by a polyethylene tube 50 which was filled with heparinized saline to recording of blood pressure and drug injection, respectively. Arterial catheter connected to a blood pressure transducer and cardiovascular parameters [mean arterial pressure (MAP), systolic blood pressure (SBP) and heart rate (HR)] were recorded [14, 15] by a power lab instrument(ID instrument, Australia). After stabilization of cardiovascular parameters, In AngII group, AngII (300 mg/kg) was intravenously administered during 2 min to achieve a high blood pressure. In Los group, Los injected after 10 min AngII (300 mg/kg) injected. In three doses of C. sativus groups firstly extract was injected in separate groups after 10 min AngII (300 mg/kg) injected and cardiovascular parameters recorded [8, 13].
2.6. Statistical Analysis
The results of current study were express as mean ± SEM. The peak changes of cardiovascular parameters calculated and were statistically analyzed by one-way ANOVA followed by Tukey’s post hoc comparisons test. Value of p < 0.05 was considered as statistically significant.
3. Results
3.1. Effect of saline administration on cardiovascular parameters
To determine the effect of saline on cardiovascular parameters, cardiovascular parameters were recorded before and after saline injection (i.v). Saline administration did not significantly changed MAP (before: 113.15 ± 3.22 mmHg vs after: 108.90 ± 2.2 mmHg), SBP (before: 127.3 ± 3.42 mmHg vs after: 124.4 ± 3.32 mmHg) and HR (before: 318.67 ± 10.1 beats/min vs after: 322.56 ± 11.3 beats/min) in rats.
3.2 Effect of AngII administration on cardiovascular parameters
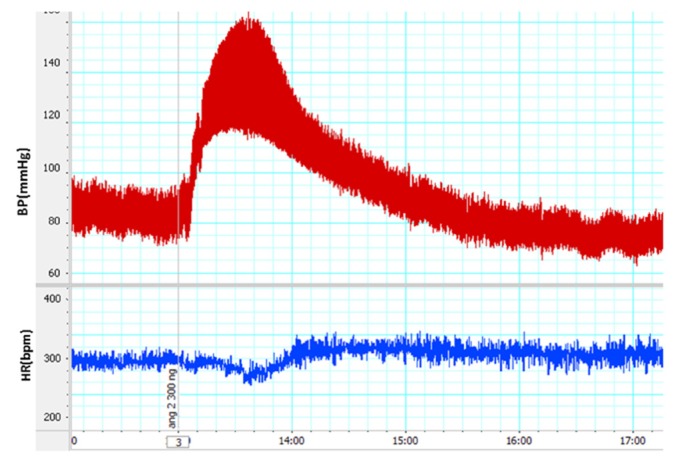
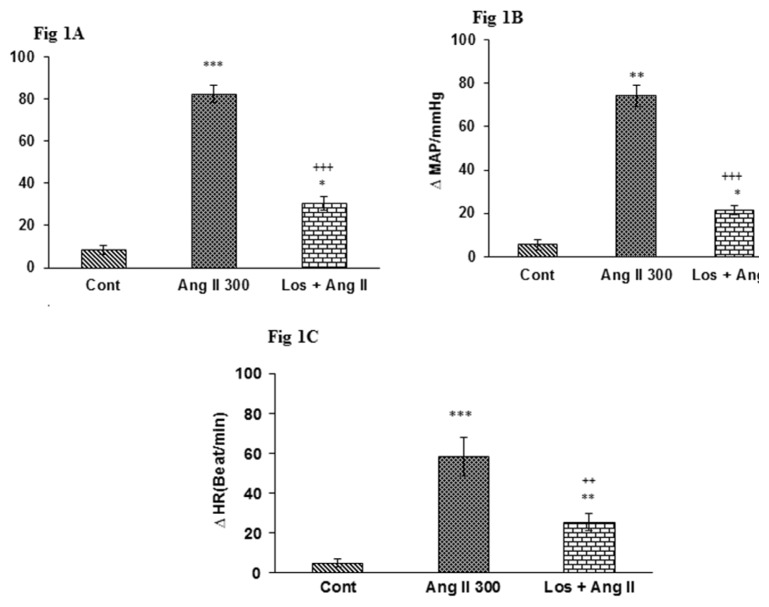
In this experiment, AngII (300 ng/kg, i.v) injected and cardiovascular parameters were recorded (Fig. 1). Injection of AngII significantly increased maximal changes of SBP, MAP and HR in AngII group than control group (P < 0.001in all parameters, Fig. 2a, b and c).
Figure 1.
A sample of cardiovascular responses recorded record after intravenous injection 300 mg/kg of AngII. Injection time marked by a vertical line
Figure 2.
Peak changes of SBP (A), MAP (B), and HR (C) after infusion 300 ng/kg of AngII and Losartan (Los; 10 mg/kg) + AngII.
Expression of Data: mean ± SEM (n = 6); Statistical analysis: One-way ANOVA with Tukey’s post hoc; *p < 0.05, **P < 0.01 and ***p < 0.001 vs control; ++p < 0.01, +++p < 0.001 vs AngII 300
MAP: mean arterial pressure; SBP: systolic blood pressure; HR: heart rate
3.3. Effect of losartan on the cardiovascular parameters in hypertension induced by AngII
Pretreatment with Los led to a significant reduction in cardiovascular responses evoked by AngII. Enhancement of MAP, SBP in AngII group significantly attenuate with respect to Los (P < 0.001; 2a and b). Injection of Los also significantly decreased tachycardia induced by AngII (P < 0.01; (Fig. 2c).
3.4. Effect of three doses of C. sativus extract on the cardiovascular parameters in hypertension induced by AngII
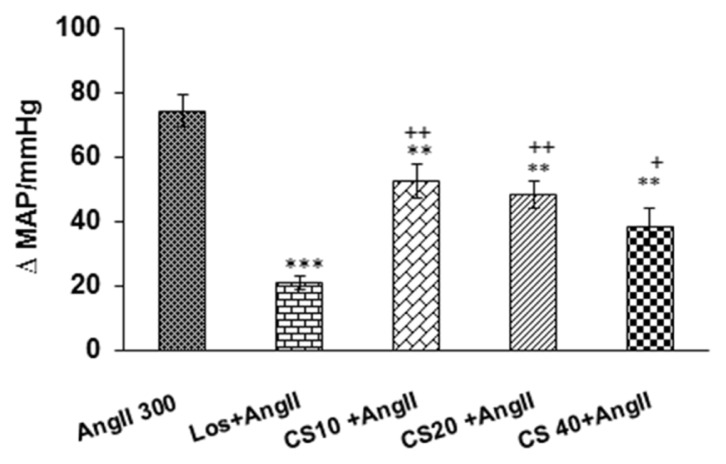
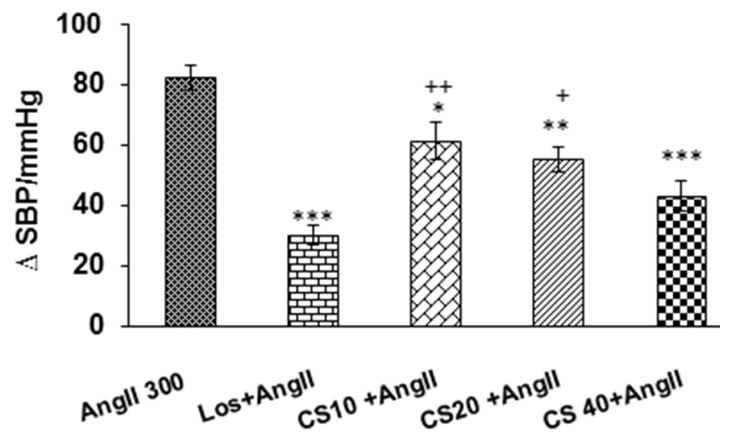
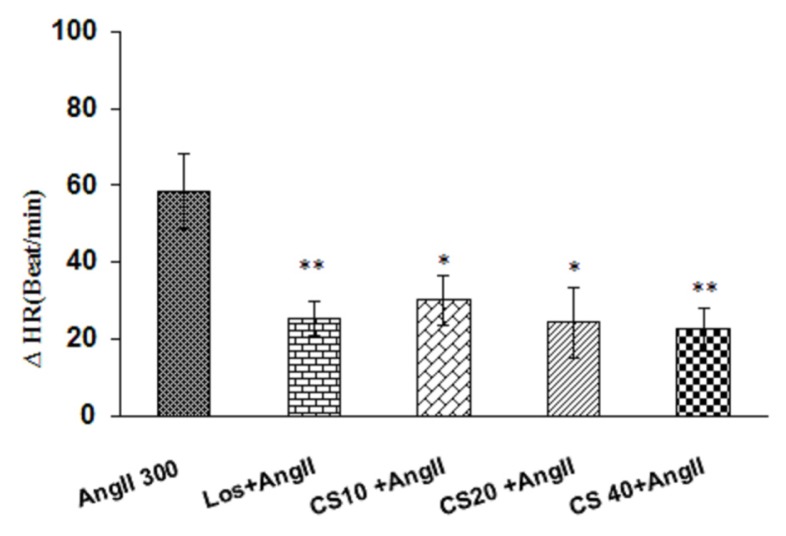
Pretreatment with the 3 doses of extract of C. sativus (10, 20 and 40 mg/kg) resulted in significant reduction cardiovascular parameters in AngII hypertensive rats. As has been shown in Fig 3 and 4, enhancement of MAP and SBP in C. sativus + AngII groups significantly decreased compared to AngII alone (P < 0.05 to P < 0.001). Tachycardia in AngII group also decreased by pretreatment of all doses of C. sativus extract (P < 0.05 and P < 0.01) (Fig. 5). Comparing all doses of C. sativus + AngII with Los+ AngII has been shown that MAP and SBP in doses10 and 20 mg/kg of C. sativus are significant than Los + AngII group but dose 40 mg/kg was not significant (Fig. 3 and Fig. 4). Changes of HR in all doses of C. sativus was not significant compared to Los + AngII (Fig 5).
Figure 3.
Comparison peak changes of MAP induced by i.v injection of 3 doses of C. sativus extract (10, 20 & 40 mg/kg) + AngII with AngII alone and Los + AngII groups. Expression of Data: mean ± SEM (n = 6). Statistical analysis: One-way ANOVA with Tukey’s post hoc;
* P < 0.05, ** P < 0.01 and *** p < 0.001 vs AngII; ++p < 0.01, +++p < 0.001 vs AngII + Los
MAP: mean arterial pressure
Figure 4.
Comparison peak changes of SBP induced by i.v injection of 3 doses of C. sativus extract (10, 20 & 40 mg/kg) + AngII with AngII alone and Los + AngII groups. Expression of Data: mean ± SEM (n = 6). Statistical analysis: One-way ANOVA with Tukey’s post hoc; *P < 0.05, **P < 0.01 and ***p < 0.001 vs AngII; +p < 0.05, ++p < 0. 01 vs AngII + Los; SBP: systolic blood pressure
Figure 5.
Comparison peak changes of HR induced by i.v injection of 3 doses of C. sativus extract (10, 20 & 40 mg/kg) + AngII with AngII alone and Los + AngII groups. Expression of Data: mean ± SEM (n = 6). Statistical analysis: One-way ANOVA with Tukey’s post hoc; *P < 0.05, **P < 0.01 vs AngII; HR: heart rate
4.Discussion
Our result showed that hydroalcoholic extract of C. sativus ameliorates cardiovascular responses by acute hypertension induced by AngII and this effect is comparable with Los.
One of the most important systems affecting blood pressure is the renin-angiotensin system (RAS). AngII as primary effector of RAS and its AT1 receptor has been shown to have several deleterious effects such as hypertension, cardiac and vascular hypertrophy, endothelial dysfunction and vasoconstriction [3]. It has been also reported that AngII by vasoconstriction, induce the release of catecholamines from the adrenal gland and increase of salt retention causing to development of hypertension. In addition, AngII could induce hypertension via activation of phospholipase C enzymes and the increase of cytoplasmic calcium concentration as well as reduction of nitric oxide (NO) [8, 16–18]. In line with these findings, in our study also administration of AngII increased blood pressure in rats [8, 19]. C. sativus which possesses several components with different properties including antitumor, anti-inflammatory, antioxidant, anti-depressant and hypolipidemic effects [11, 20], also can exert protective effects on cardiovascular system [21]. For example, the aqueous extract of C. sativus has been documented to have the hypotensive effect in normotensive and hypertensive anesthetized rats [13]. In our finding also pretreatment with the hydroalcoholic extract of C. sativus attenuate increased MAP, SBP, and HR in AngII hypertensive rats. In addition, radical scavenging effects and antioxidant properties of C. sativus have been well documented [22] 22]. The C. sativus extract has been exhibited to improve isoproterenol-induced myocardial infarction through reduction of oxidative stress [21]. C. sativus and its crocin have been proposed to inhibit lipid peroxidation and increase enzymatic and non-enzymatic antioxidant [23]. An important component of C. sativus is crocin that is a water-soluble carotenoids [24] and has effect on cardiovascular system. In recent study we show that crocin attenuate cardiovascular effect induced by AngII [8].
Because we use hydroalcoholic extract therefore this component is soluble in extract and effect of C. sativus on AngII partly medicated by this component. C. sativus also contain another important component such as Crocetin and Safranal that has beneficial effect on cardiovascular system. It has been reported that crocetin could suppress effect of AngII in vascular smooth-muscle cell [25] and Safranal attenuated cardiovascular parameters in hypertension induced by desoxycorticosterone acetate (DOCA) – salt [26]. Therefore, it is possible these components of C. sativus inhibit effect of AngII in our experiment. However, future studies are needed to confirm our opinion.
Interaction between NO and AngII in vascular also has been reported so AngII decrease NO bioavailability [27]. Because C. sativus could increase NO, we suggested that this plant by effect on NO production ameliorate effect of AngII and reduced cardiovascular effect.
In the other hand, a part of the effects of AngII on blood pressure is attributed to stimulate the overproduction of reactive species (ROS) and induction of oxidative stress by this substance [28]. Researcher has been suggested that AngII can induce vasoconstriction through enhancing the ROS concentration in smooth muscle of the vessels [29]. Furthermore, in recent studies, RAS and AngII have been shown to involve in inflammatory processes [30]. It has been suggested that using immunosuppressive drugs can protect hypertensive rats against AngII–caused renal damage [31]. In addition, the role of T cells in the pathogenesis of hypertension and vascular dysfunction induced by AngII in mice has been reported [32]. Regarding the antioxidant and anti-inflammatory effects of C. sativus and its components, it is guessed that the possible improving effect of C. sativus against oxidative stress and inflammation induced by AngII contributes in the results of the current study.
In addition, type 1 (AT1) receptor of AngII is considered as an important component of RAS [33]. The evidence reveals that AT1 receptors antagonists can have beneficial effects on the blood pressure caused by the activity of RAS [34]. Losartan is a selective AT1 receptors antagonist which relaxes muscle cell and dilates blood vessels and diminishes blood pressure [35]. Losartan has also been shown to diminish blood pressure in hypertensive rats [8]. It has been documented that losartan induces a renin-dependent hypotensive effect in the patient with essential hypertension [36]. In the current study, pretreatment with losartan restored Ang II-caused blood pressure and tachycardia in rats. Considering this finding it seems that AT1 receptors are involved in AngII effects on cardiovascular parameters in this research. Based on the results of current study, attenuating effects of C. sativus extract against enhanced blood pressure and tachycardia induced by AngII is comparable to that of losartan. Therefore, we suggested that the improving effect of the hydroalcoholic extract of C. sativus on hypertensive induced by AngII is mediated through AT1 receptors. In this study we used acute hypertension, because effect of AngII and C. sativus may be achieved in long term we proposed that effect of C. sativus evaluated in chronic model of hypertension such as Goldblatt hypertension.
In the brief, the current study data showed that the hydroalcoholic extract of C. sativus ameliorate cardiovascular effect of AngII, the main product of RAS. Therefore, we suggested that C. sativus by inhibition of RAS has a preventive effect in hypertension induce by RAS.
Acknowledgement
We would like to thank the Research Council of Mashhad University of Medical Sciences for their financial support.
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Conflict of interest
There is not any conflict of interest in this study.
References
- 1.Xie Y, Zhang W. Antihypertensive activity of Rosa rugosa Thunb. flowers: angiotensin I converting enzyme inhibitor. J Ethnopharmacol. 2012;144(3):562–566. doi: 10.1016/j.jep.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Stewart S, Gersh BJ. Hypertension: a global perspective. Circulation. 2011;123(24):2892–2896. doi: 10.1161/CIRCULATIONAHA.110.992362. [DOI] [PubMed] [Google Scholar]
- 3.Moraes PL, Kangussu LM, Silva LG, Castro CH, Santos RA, Ferreira AJ. Cardiovascular effects of small peptides of the renin angiotensin system. Physiol Rep. 2017;5(22):e13505. doi: 10.14814/phy2.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharm. 2011;11(2):180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue H, Lu Z, Tang W, Pang L, Wang G, Wong G, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev. 2018;2018(11):CD008170. doi: 10.1002/14651858.CD008170.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303(2):H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylitalo A, Airaksinen KJ, Hautanen A, Kupari M, Carson M, Virolainen J, et al. Baroreflex sensitivity and variants of the renin angiotensin system genes. J Am Coll Cardiol. 2000;35(1):194–200. doi: 10.1016/S0735-1097(99)00506-9. [DOI] [PubMed] [Google Scholar]
- 8.Shafei MN, Faramarzi A, Rad AK, Anaeigoudari A. Crocin prevents acute angiotensin II-induced hypertension in anesthetized rats. Avicenna J Phytomed. 2017;7(4):345. [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano-Díaz J, Sánchez AM, Martínez-Tomé M, Winterhalter P, Alonso GL. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J Food Compost Anal. 2013;31(1):101–108. doi: 10.1016/j.jfca.2013.03.009. [DOI] [Google Scholar]
- 10.Bhargava V. Medicinal uses and pharmacological properties of Crocus sativus linn (saffron) Int J Pharm Pharm Sci. 2011;3(Suppl 3):22–26. [Google Scholar]
- 11.José Bagur M, Alonso Salinas GL, Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M, et al. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules. 2017;23(1):30. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Sun J, Liu C, Fang C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur J Pharmacol. 2014;741:290–296. doi: 10.1016/j.ejphar.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 13.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24(7):990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 14.Shafei MN, Nasimi A. Effect of glutamate stimulation of the cuneiform nucleus on cardiovascular regulation in anesthetized rats: Role of the pontine Kolliker–Fuse nucleus. Brain Res. 2011;1385:135–143. doi: 10.1016/j.brainres.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Nasimi A, Shafei M, Alaei H. Glutamate injection into the cuneiform nucleus in rat, produces correlated single unit activities in the Kolliker-Fuse nucleus and cardiovascular responses. Neuroscience. 2012;223:439–446. doi: 10.1016/j.neuroscience.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology-Cell Physiology. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 17.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639–672. [PubMed] [Google Scholar]
- 18.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci. 2006;103(47):17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enayatfard L, Mohebbati R, Niazmand S, Hosseini M, Shafei MN. The standardized extract of Nigella sativa and its major ingredient, thymoquinone, ameliorates angiotensin II-induced hypertension in rats. J Basic Clin Physiol Pharmacol. 2018;30(1):51–58. doi: 10.1515/jbcpp-2018-0074. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava R, Ahmed H, Dixit R. Crocus sativus L.: a comprehensive review. Pharmacogn Rev. 2010;4(8):200. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehdizadeh R, Parizadeh MR, Khooei A-R, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16(1):56. [PMC free article] [PubMed] [Google Scholar]
- 22.Assimopoulou A, Sinakos Z, Papageorgiou V. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y-Q, Liu J-X, Wang J-N, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 24.Bountagkidou O, van der Klift EJ, Tsimidou MZ, Ordoudi SA, van Beek TA. An on-line high performance liquid chromatography-crocin bleaching assay for detection of antioxidants. J Chromatogr. 2012;1237:80–85. doi: 10.1016/j.chroma.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Qian Z, Wen N, Xi L. Crocetin suppresses angiotensin II-induced vascular smooth-muscle cell proliferation through inhibition of ERK1/2 activation and cell-cycle progression. J Cardiovasc Pharmacol. 2007;50(5):519–525. doi: 10.1097/FJC.0b013e31813c114e. [DOI] [PubMed] [Google Scholar]
- 26.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of safranal on systolic blood pressure in rats. Iran J Pharm Res. 2015;14(2):585. [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman IH, Zhou M-S, Raij L. Interaction between nitric oxide and angiotensin II in the endothelium: role in atherosclerosis and hypertension. J Hypertens. 2006;24:S45–S50. doi: 10.1097/01.hjh.0000220406.46246.f2. [DOI] [PubMed] [Google Scholar]
- 28.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, et al. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–515. doi: 10.1161/01.HYP.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis: part II: AT1 receptor regulation. Circulation. 2002;105(4):530–536. doi: 10.1161/hc0402.102619. [DOI] [PubMed] [Google Scholar]
- 30.Stegbauer J, Lee D-H, Seubert S, Ellrichmann G, Manzel A, Kvakan H, et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci. 2009;106(35):14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller DN, Shagdarsuren E, Park J-K, Dechend R, Mervaala E, Hampich F, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161(5):1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng F, Lin J, Lin L, Tang H. Transient prehypertensive treatment in spontaneously hypertensive rats: A comparison of losartan and amlodipine regarding long-term blood pressure, cardiac and renal protection. Int J Mol Med. 2012;30(6):1376–1386. doi: 10.3892/ijmm.2012.1153. [DOI] [PubMed] [Google Scholar]
- 34.Wang T, Lian G, Cai X, Lin Z, Xie L. Effect of prehypertensive losartan therapy on AT1R and ATRAP methylation of adipose tissue in the later life of high-fat-fed spontaneously hypertensive rats. Mol Med Report. 2018;17(1):1753–1761. doi: 10.3892/mmr.2017.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghods R, Gharouni M, Amanlou M, Sharifi N, Ghobadi A, Amin G. Effect of Onopordon acanthium L. as Add on Antihypertensive Therapy in Patients with Primary Hypertension Taking Losartan: a Pilot Study. Adv Pharm Bull. 2018;8(1):69. doi: 10.15171/apb.2018.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsunoda K, Abe K, Hagino T, Omata K, Misawa S, Imai Y, et al. Hypotensive effect of losartan, a nonpeptide angiotensin II receptor antagonist, in essential hypertension. Am J Hypertens. 1993;6(1):28–32. doi: 10.1093/ajh/6.1.28. [DOI] [PubMed] [Google Scholar]