Abstract
Purpose
The published data indicate that the irradiation of the subventricular zone (SVZ) might play a role in the treatment of patients with glioblastoma (GBM). We aimed to determine whether radiation treatment doses (high vs low) applied to the SVZ can lead to an increase in progression free survival (PFS) and overall survival (OS).
Patients and methods
We undertook a systematic review and meta-analysis according to the PICOS research criteria of patients with glioblastoma which received high doses compared to low doses in order to determine if they have a better survival in observational and experimental studies.
Results
Our survey of the literature yielded 2573 unique records. After screening, 17 were assessed for eligibility, and in the end 8 were included in the qualitative and 4 in the quantitative analysis. Subjects who received higher doses of ipsilateral SVZ (iSVZ) irradiation had a statistically significant better PFS than those receiving lower doses (HR 0.58 [95% CI 0.42–0.82], p=0.002). Subjects receiving higher doses of contralateral SVZ (cSVZ) irradiation did not have a statistically significant better PFS than those receiving lower doses (HR =0.89 [95% CI 0.35–2.26], p=0.81). Also for OS the subjects receiving higher doses to the iSVZ did not have a statistically significant better survival than those receiving lower doses (HR =0.75 [95% CI 0.51–1.11], p=0.15).
Conclusion
The data indicate a possible involvement of the SVZ in the onset and progression of the GBM, as well as a possible role of the SVZ in radiation therapy.
Keywords: glioblastoma, radiation therapy, neural stem cells, survival
Introduction
The WHO 2016 classification ranks GBM as a grade IV tumor, survival at 5 years being extremely rare despite the multimodal treatment administered nowadays.28 The histopathological criteria for GBM are a diffuse growth pattern accompanied by brisk mitotic activity, necrosis, and microvascular proliferation.43 Alongside the intrinsic features of the tumor, the interaction between the GBM and the surrounding normal brain structures, such as the SVZ, is currently taken into consideration in the attempt to understand the onset, progression, and resistance to treatment.13,30,41
The SVZ is an area 3–5 mm thick covering the wall of the lateral ventricles. Especially important during the development of the brain, in adulthood it contains NSC (neural stem cells) and provides a particular environment (the niche) which these cells need in order to preserve their biological properties.26,35,45
The importance of the interaction between GBM and the SVZ has been highlighted, as the tumors coming into contact with the area that contains NSC have a worse prognosis than those located at a distance.2,3,11,17,25,36 The subject needs more research because there are also studies that could not confirm the prognostic role of SVZ invasion by GBM.15,16
Given the diffuse extension of tumor cells in GBM, the significant migration potential of the NSC from the SVZ to the pathological areas (including tumor lesions), and the possibility of communication via the cerebrospinal fluid secreted by the choroid plexus (CP), questions have been asked about a possible long-distance interaction between GBM and the ipsi- and the contralateral SVZ.4,14,23,27
Thus, a number of studies have ascertained the existence of a correlation between the irradiation of the SVZ (ipsi- and contralateral), the administered doses, and survival.6,7,11,19 Nevertheless, not all of the published studies have reached the same conclusions, as other authors failed to identify an increase in survival in the patients subjected to a radiotherapy protocol that also targeted the SVZ.8,20
Considering the importance of the topic and the divergent conclusions in the literature, in the present study we performed a systematic review with meta-analysis of the observational and experimental studies done on patient populations histopathologically diagnosed with GBM, which compare the doses of radiotherapy administered to the iSVZ and cSVZ and their effect upon survival.
Materials and methods
We undertook a systematic review and meta-analysis taking into account the PRISMA guideline.34
Eligibility criteria
The PICO research question was: In patients with glioblastoma (Population), do high doses of radiotherapy (Intervention) compared to low doses of radiotherapy (Comparison intervention) offer better progression-free survival or overall survival (Outcome), in observational and experimental studies (Study design)? We considered all published original articles published till December 2018, in any language.
Information sources
We undertook a comprehensive research in seven bibliographic databases (Pubmed, EMBASE, Cochrane CENTRAL, Web of Science (Science Citation Index Expanded (SCI-EXPANDED) −1975-present, Emerging Sources Citation Index (ESCI) –2015-present, Conference Proceedings Citation Index- Science (CPCI-S) - 1990-present, SCOPUS, Proquest, and LILACS). A further search was done in the WHO International Clinical Trials Registry Platform, and Clinical Trials.
Search
We used customized search strategies for each search engine. The search strategy included: glioblastoma, radiotherapy, radiation, cerebral/lateral ventricles, periventricular, subventricular, subependimal, stem cells, progenitor cells. The full search strategy for the Pubmed database is presented in the supplementary file. We limited the results to human subjects, articles, letters to the editor, meeting abstracts, proceedings papers, and reviews. The reference lists of the useful articles were screened to find further papers to include in the research. The search results were combined in EndNote online (Clarivate Analytics, Philadelphia, United States), and duplicates were removed. The selection of eligible articles and their quality assessment were performed in Mendeley (Elsevier, Amsterdam, Netherlands).
Study selection
Two authors independently proceeded to read all the titles and abstracts resulted after the search endeavor to select includible papers. Differences in opinion were solved by discussion.
Data collection process
Two authors independently extracted data from papers using a form in Microsoft Excel. The forms were confronted and differences were corrected by rechecking the papers.
Data items
The collected data were: study design, number of subjects, radiotherapy dose cut-off, mean SVZ volume and dose (ipsilateral, contralateral, and bilateral), progression free survival, overall survival (follow-up, hazard ratio in univariate and multivariate analysis, CI, p-value), confounders that were adjusted in multivariate analyses.
Risk of bias in individual studies
All the included studies were assessed for the presence of bias using the Newcastle - Ottawa Quality Assessment Scale (NOS), independently by two authors.44 Divergences in assessment were solved by discussion.
Summary measures
The principal summary measure was hazard ratio obtained in multivariate analysis, comparing the high with the low dose radiation regimes, for progression free survival and for overall survival.
Synthesis of results
We performed a meta-analysis using the fixed and random effects models (the last model was chosen in case of important heterogeneity) to obtain the final pooled results in case we found at least two studies offering the principal summary measure. The corresponding summary measure with a 95% confidence interval and p-value was computed. The heterogeneity of the results was assessed using the inconsistency index (I2), and the Q test was performed for heterogeneity.
Risk of bias across studies
A screening for publication bias was not performed using a funnel plot or formal tests, since the number of studies was too small for an adequate assessment.
Additional analyses
For all statistical tests used, the significance level alpha was 0.05, and the two tailed p value was computed. All statistical analyses were performed in R environment for statistical computing and graphics (R Foundation for Statistical Computing, Vienna, Austria), version 3.4.3 [R Core team. Vienna, Austria].
Results
Study selection
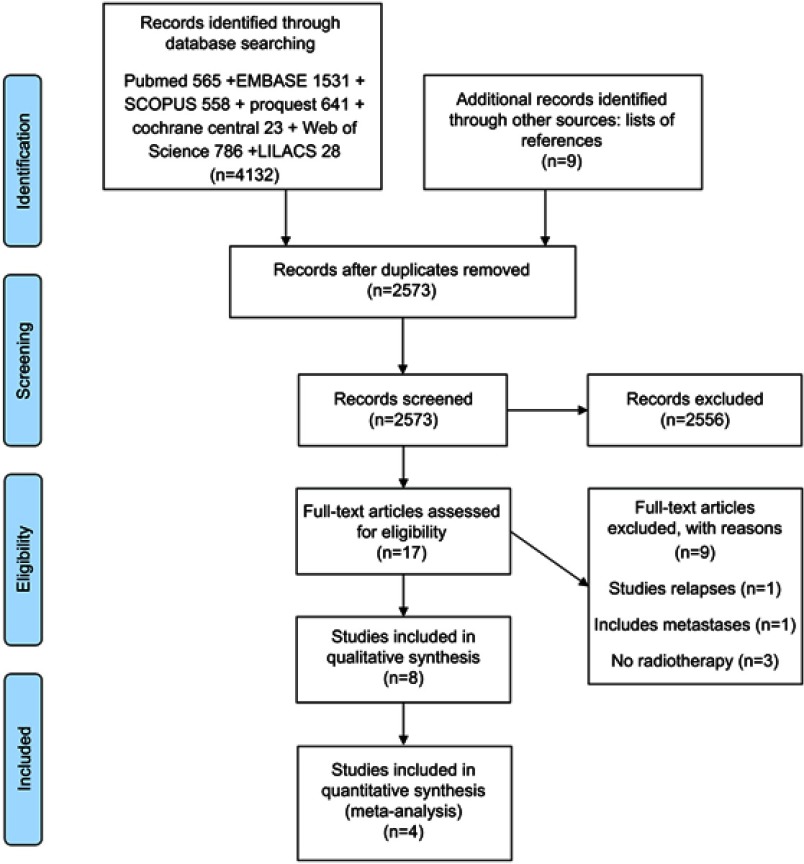
The search for papers yielded 2573 unique records. During screening we excluded papers for the following reasons: studies not on the topic, studies that didn’t compare high dose with low dose irradiation, studies that didn’t target the SVZ zone, studies that didn’t use irradiation, other gliomas, case reports, abstracts of congress presentations, reviews. After screening 17 were assessed for eligibility, and in the end 8 were included in the qualitative and 4 in the quantitative analysis (Figure 1). Four studies were excluded from the quantitative analysis for the following reasons: Gupta et al,33 2012 – was excluded for having continuous dose, not in the binary form, high dose vs. low dose, while all the other studies had it in binary format. Kusumawidjaja et al,20 2014 – was excluded for having irradiation doses that were not comparable with the other studies (70 dose escalated vs 60 conventional, and no dose below 60 – while all the others compared high doses with doses below 60). Evers et al,10 2010, and Khalifa et al,19 2016 – were excluded due to the fact that they didn’t provide the HR needed for the analysis.
Figure 1.
Flowchart of the results of the literature search.
Study characteristics and risk of bias
The study characteristics and the NOS score assessment for their risk of bias are presented in Table 1. The total number of subjects included in the meta-analysis (from the four selected studies) was 328. The high dose group had 97 (29.6%) of the subjects, while the low-dose group had 231 (70.4%) of the subjects.
Table 2.
Multivariate analyses results found in the studies
| Study | High dose group | Multivariate analysis | Confounder analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | PFS HR (95% CI) | p-value | OS HR (95% CI) | p-value | Age | Gender | Extent of resection | Tumor location | SVZ dose | RPA class | KPS | MGMT promoter status | |
| Lee et al., 2013 2222 | iSVZ >59.4 vs. <59.4 | 0.45 (0.25–0.82) | 0.009 | 0.65 (0.35–1.21) | 0.177 | yes | yes | yes | |||||
| Elicin et al., 2014 88 | cSVZ>59.2 | 1.72 (0.80–3.53) | 0.161 | 1.49 (0.72–2.88) | 0.268 | yes | |||||||
| Adeberg et al., 2014b 33 | iSVZ>40 | 0.52 (0.26–1.03) | 0.06 | yes | yes | yes | yes | ||||||
| cSVZ>30 | 0.45 (0.20–0.98) | 0.04 | yes | ||||||||||
| Gupta et al., 2012 3333 | iSVZ continuous | 0.91 (0.80–1.03) | 0.116 | 0.87 (0.77–0.98) | 0.025 | yes | yes | yes | yes | yes | yes | ||
| cSVZ continuous | 0.96 (0.71–1.30) | 0.797 | 0.95 (0.72–1.26) | 0.736 | |||||||||
| bSVZ continuous | 1.06 (0.97–1.15) | 0.187 | 1.08 (0.97–1.19) | 0.162 | |||||||||
| Chen et al., 2013 77 | iSVZ≥40 | 0.749 (0.453–1.24) | 0.259 | 0.827 (0.502–1.36) | 0.455 | yes | yes | yes | |||||
| 0.385 (0.165–0.901) | 0.028 | 0.385 (0.165–0.895) | 0.027 | ||||||||||
| >0.05 | >0.05 | ||||||||||||
Abbreviations: GBM, glioblastoma multiforme; SVZ, subvnetricular zone; iSVZ, cSVZ, bSVZ, ipsilateral, contralateral, bilateral subventricular zone; IL, ipsilateral; CL, contralateral; BL, bilateral; NSC, neural stem cells; CP, choroid plexus; GTR, gross total resection; NOS, Newcastle-Ottawa Scale (article quality assessment scale); PFS, progression free survival; OS, overall survival; HR, hazard ratio; RPA class, recursive partitioning analysis class; KPS, Karnofsky performance score; MGMT, O(6)-methylguanine-DNA methyltransferase; IQR, interquartile range; CI, confidence interval; NR, non reported.
Table 1.
The study characteristics and the NOS score assessment for their risk of bias
| Mean SVZ volume cc | Mean SVZ dose (Gy) | Progression free survival | Overall survival | Study quality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study (Author, year) | Number of patients | High dose group | IL | CL | BL | IL | CL | BL | Median (IQR)[range], months | HR univariate (95% CI) | p-value | Median (IQR)[range] {95%CI}, months |
HR univariate (95% CI) | p-value | NOS |
| Lee et al., 2013 2222 | 173 | iSVZ >59.4 vs. <59.4 (n:21/152) | 4.3±1.3 | 5.0±1.6 | 49.2 | 35.2 | 60.1 | 10.4 [0.1–71.3] | 0.56 (0.32–0.98) | 0.042 | 19.6 [4.4–104.0] | 0.67 (0.38–1.19) | 0.173 | 9 | |
| bSVZ >43 vs <43 | 0.74 (0.51–1.06) | 0.103 | |||||||||||||
| Kusuma-widjaja et al., 2014 2020 | 72 | iSVZ - 70 dose escalated vs 60 conventional | 60.6 (33.4–69.8) | 39.5 (19.4–61.2) | 49.1 (28.3–64.3) | 7.1 {5.6–9.6} vs. 11.1 (6.0–24.6) | 0.95 (0.9–1) | 0.052 | 15.2 {11–18.6} vs. 18.4 {12.5–31.4} | 1.03 (0.97–1.10) | 0.352 | 5.5 | |||
| cSVZ, bSVZ | >0.05 | >0.05 | |||||||||||||
| Evers et al., 2010 1111 | 55 | >43 | 5.05 | 46±15.5 | 41±16.1 | 15 vs. 7.2 | 0.03 | 7 | |||||||
| Khalifa et al., 2016 1919 | 43 | bSVZ >40Gy | 5 (3.4–11) | 5.5 (3.4–9.6) | 10.6 (6.8–20.6) | 51.3 (17.9–61.4) | 15.4 (1.4–48.7) bSVZ | 35 (10.8–51.8) | 6.5 {4.4–9.3} | 22.7{14.5–26.2} | 8 | ||||
| Elicin et al., 2014 88 | 60 | cSVZ>59.2 (n:14/46) | 5.2±2.4 | 6.4±2.3 | 11.6±4.2 | 58.8±6.5 | 44.9±15.9 | 51.9±10.4 | all 9.5 (95 % CI 7.7–11.1), 10.37 {95 % CI 8.37–13.53} vs 7.1 {95 % CI 3.5–8.97} | 2.42 (1.18–4.71) | 0.018 | 19.27 (95 % CI 12.77–25.23) | 4.83 (1.71–13.97) | 0.004 | 7 |
| Adeberg et al., 2014b 33 | 65 | iSVZ>40 (n: 31/23) | 14.05mL [8.41–22.80 mL] | 14.50mL [8.68–23.80 mL] | 40.67Gy [14.84–56.87Gy] | 20.86Gy [4.10–45.07Gy] | 7.1 [1.6–52.4] | 0.40 (0.24–0.78) | 0.043 | 20.8 [4.3–53.8] | 0.65 (0.34–1.24) | 0.1 | 9 | ||
| cSVZ>30 | 0.44 (0.21–0.92) | 0.03 | 1.53 (0.36–6.43) | 0.56 | |||||||||||
| Gupta et al., 2012 3333 | 40 | iSVZ≥58 | 5.6±2.5 | 6.4±3 | 58.7 | 53.6 | 56.2 | 11 {8.9–13.0}, 10 vs. 11 | 0.92 | 17 {11.6–22.4}, 17 vs. 15 | 0.95 | 9 | |||
| iSVZ continuous | |||||||||||||||
| cSVZ≥58 | 10 vs. NR | 0.02 | 14 vs. NR | 0.05 | |||||||||||
| cSVZ continuous | |||||||||||||||
| bSVZ continuous | |||||||||||||||
| bSVZ≥58 | 10 vs. 14 | 0.06 | 14 vs. NR | 0.22 | |||||||||||
| i,c,bSVZ>43 | >0.05 | >0.05 | |||||||||||||
| i,c,bSVZ>50 | >0.05 | >0.05 | |||||||||||||
| Chen et al., 2013 77 | 116 | iSVZ≥40 (n:31/10) | 7.05 [2.99–14.2] | 7.91 [4.18–14.6] | 14.76[5.37–28.3] | 48.7[1.96–60] | 34.4[1.59–60] | 41.5 [1.77–60] | 0.824 (0.506–1.34) | 0.434 | 0.926 (0.570–1.50) | 0.754 | 9 | ||
| iSVZ≥40 gross total resection only | 0.471 (0.209–1.06) | 0.07 | 17.5 vs. 15.6 | 0.607 (0.280–1.32) | 0.207 | ||||||||||
| c,bSVZ≥40 | |||||||||||||||
Notes: N. – number of subjects per study; n – number of subjects per group; iSVZ, cSVZ, bSVZ – ipsilateral, contralateral, bilateral subventricular zone; IL – ipsilateral; CL – contralateral; BL – bilateral; continuous data is presented as mean ± one standard deviation, or median (IQR – interquartile range), or [range], or {95% CI – confidence interval}; NOS - Newcastle-Ottawa Scale (article quality assessment scale); NR – non reported; HR – hazard ratio.
Results of individual studies
The results of the studies are presented in Table 2 (multivariate analyses) and in Figures 2–4 (Forest plot)
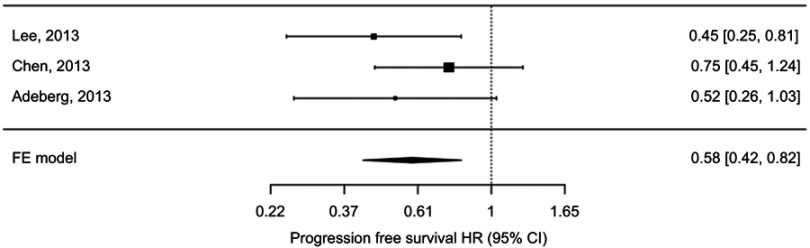
Figure 2.
Fixed effects meta-analysis of progression free survival hazard ratio (HR) comparing high v low ipsilateral SVZ irradiation doses.
Figure 4.
Fixed effects meta-analysis of overall survival hazard ratio (HR) comparing high vs low ipsilateral SVZ irradiation doses.
Synthesis of results
Progression free survival
Ipsilateral SVZ irradiation
The pooled HR for progression free survival of high versus low irradiation dose of ipsilateral SVZ was 0.58 (95% CI 0.42–0.82), p=0.002, using the fixed effects model (test for heterogeneity p-value=0.41; I2=~0%) (Figure 2). Subjects receiving higher doses of ipsilateral SVZ irradiation had statistically significant better PFS than those receiving lower doses.
Contralateral SVZ irradiation
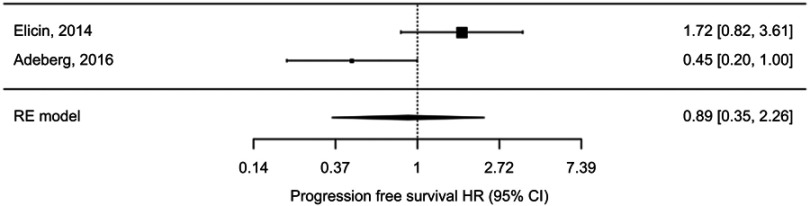
The pooled HR for progression free survival of high versus low irradiation dose of contralateral SVZ was 0.89 (95% CI 0.35–2.26), p=0.81, using the random effects model (test for heterogeneity p-value=0.02, I2=65.7%) (Figure 3). Subjects receiving higher doses of contralateral SVZ irradiation did not have statistically significant better PFS than those receiving lower doses.
Figure 3.
Random effects meta-analysis of progression free survival hazard ratio (HR) comparing high vs low contralateral SVZ irradiation doses.
Overall survival
Ipsilateral SVZ irradiation
The pooled HR for overall survival of high versus low irradiation dose of ipsilateral SVZ was 0.75 (95% CI 0.51–1.11), p=0.15, using the fixed effects model (test for heterogeneity p-value=0.55; I2=~0%). (Figure 4). Subjects receiving higher doses of ipsilateral SVZ irradiation did not have statistically significant better overall survival than those receiving lower doses.
Contralateral SVZ irradiation
We couldn’t find at least two studies that presented the contralateral SVZ irradiation HR, so a meta-analysis for this outcome was not possible. The only study that presented such a result was Gupta 2012, but it was for the continuous variable indicating the dosage, not for the comparison between high and low dosage.
Risk of bias across studies
The quality of the studies assessed using the NOS was adequate. Some studies had a lower NOS score due to the fact they lacked confounder adjustment, or the source of the patients in the two groups was not always the same hospital.
Clinical trials
The search in all databases provided only observational studies. We found two ongoing clinical trials, NCT02177578 and NCT02039778, in the experimental studies databases, but no finished ones.
Discussion
In the present study we undertook a systematic review and meta-analysis on a population of patients with GBM treated by multimodal treatment. We focused on RT treatment and compared high doses to low doses administered on iSVZ and cSVZ in order to determine if the patients with high doses have a better survival.
Studies description
According to Lee et al, a dose >59.4 Gy administered on the iSVZ led to increased PFS, but without any increase in OS, in patients with GBM in both univariate and multivariate models. Although a certain improvement in OS has been identified, this trend is not statistically significant.22
The study by Evers et al. draws on previously published data which indicate an increase in PFS in patients with grade III and IV gliomas who received a dose of 43 Gy in the bilateral SVZ.11 This dose is likely to increase the PFS in patients with grade III tumors, but not in those with grade IV tumors. This demonstrates the importance of dosage in the treatment of GBM. Chen et al. identified an increase in both PFS and OS in GBM patients who received a >40Gy dose on the iSVZ. However, this is an independent predictor only for patients with gross total resection (GTR).6,7 Consequently, the residual tumor mass can be more important in patient relapse than the NSC located in the SVZ.30
Adeberg found an increase in PFS in patients who received a >40Gy dose on the iSVZ and >30Gy on the cSVZ, respectively. These results could also be ascribed to the differences in contoured SVZ volume, as Adeberg took into account the anterior, superior, and inferior aspects, as opposed to other authors, who only looked at the anterior aspects of the SVZ. Another observation of this study is that higher doses (>30 Gy) administered on the cSVZ entail higher total bilateral doses, with a life-limiting side effect. This could also account for the results of our own study, which highlighted an improved PFS only for the iSVZ. No relation could be identified between the OS and the dose volume parameters. Furthermore, the irradiation of the subgranular layer of the dentate gyrus, a secondary location that could offer an suitable micro-environment to stem cells, had no effect on both PFS and OS.3,38
The study by Elicin included in this meta-analysis provides data that come to contradict those in the literature, showing diminished survival, albeit non statistically significant.8 Given the heterogeneous nature of the data published in the literature, it is difficult to find studies with similar results.
Strengths and weaknesses
The studies included in our meta-analysis sought to mitigate the inherent deficiencies of the analyses conducted on retrospective series of patients. The selected populations were relatively homogeneous, with lengthy follow-ups, and the multivariate analyses factored in the extent of resection, age, and the KPS score. The variables chosen to be adjusted in the multivariate analyses were also heterogeneous between studies. As with all observational studies, residual confounding remains a possible source of bias. The risk of bias across individual studies assessed using the NOS was low, their quality being adequate. The systematic approach to the search of the literature, in numerous databases, and with a rigorous search strategy, limits the risk of incomplete retrieval of relevant literature. Since the number of retrieved studies was small, the assessment of publication bias through any method is not reliable.
However, there are differences between studies and between the patients included in the same study, when it comes to the doses applied on the SVZ and to the variation in shape/volume of the contoured SVZ. The cutoff used for identifying the high-dose and low-dose groups was different between the analyzed studies, some using 59.4 Gy (Lee et al), others 40 Gy (Adeberg et al and Chen et al). This induces an overlap, increases the heterogeneity of the studies and limits the conclusions. However it is interesting to see that regarding ipsilateral SVZ irradiation progression free survival, if the cutoff is high (59.4, for Lee et al), their result is larger than in studies where the cutoff is lower (40 Gy for Adeberg et al and Chen et al) - Figure 2. This seems to suggest that higher cutoffs increase the survival more than lower cutoffs. Moreover this overlap would more likely decrease the likelihood of finding statistically significant results, and our study found a statistically significant difference even with this overlap, which seems to sustain our conclusion for progression free survival. The same tendency might be seen regarding the overall survival where the higher-dose group (Lee et al) was better placed than the lower-dose group (Chen at al), but here the results were not statistically significant. For the contralateral SVZ irradiation both studies used the lower cutoff value, thus the comparison is not influenced.
Tumor volume might also play a role in survival for GBM patients, but our meta-analysis could not take this into account since individual studies didn’t control for it in multivariable analyses.
It is important to note that only the study of Chen at al7 used intensity modulated radiotherapy (IMRT), whereas all others tridimesional conformal radiotherapy (3DCRT). IMRT provides dosimetric advantages in both target volumes coverage and sparing of healthy neighboring structures. Considering complex shape of the SVZ, its’ very small volume (4–5 cc), IMRT would be preferable. The accuracy of delineating the ipsi and contralateral SVZ can induce significant biases if not rigorously predefined (for example 4 mm, not 3 to 5 mm next to the lateral ventricles) and not centrally reviewed by an experienced neuro-radiologist, generating – at such small volumes- significant differences in dose-volume histograms. As target structures, we would favor a PTV expansion of 3 mm of both iSVZ and cSVZ and - derived from this systematic review - a “prescription dose” of 43 Gy/30fr. Hippocampus delineation would be also of interest, as well as its’ dosimetric analysis correlated with neuro-cognitive function for potential longer-term survivors.
As with any systematic review, the data should be interpreted with care. Since we have only secondary data, and not individual patient data, it is difficult to conclude that longer progression free survival is independent of all important prognostic factors. The heterogeneity of the treatment regimen and even molecular biology also take a toll on the results. The multivariate analyses of each study included in the meta-analysis had a different selection of confounders and methods. Thus confounders in the meta-analysis are taken into account in a heterogeneous way.
Scientifically, to the best of our knowledge this is the first review with meta-analysis on the topic. The present work represents an evidence-based improvement in knowledge regarding the implications of subventricular zone irradiation in patients with glioblastoma. Future work can be built upon this current state of knowledge, in order to shed more light on the topic. The possible role of the subventricular zone in the progression of GBM might change the clinical treatment paradigm for this deadly disease. This will be a shift from the focus only on the lesion towards a more complex approach that takes into account the biology of other brain structures (SVZ and CP) and their interaction with GBM.
Study outcome
Our systematic review and meta-analysis indicate that there is a statistically significant difference in PFS between the GBM patients who received high vs. low radiotherapy doses in iSVZ. Normally, this gain should mean a higher OS, but this could not be confirmed by the present study. For cSVZ, no difference in PFS between high dose patients and low dose patients could be identified. This could be explained by the fact that in the patients whose cSVZ was also irradiated, the tumor was in a more advanced stage at the time of the radiotherapy, and the irradiation of this zone came as a consequence of the adequate coverage of the entire volume.
The location of the tumor is also to be taken into account. Studies conducted on patients whose tumors come into contact with the SVZ have indicated decreased OS and PFS.15,32 This could be explained by the fact that the NSC located in the SVZ can increase the aggressiveness of the tumor.24,31 It would be interesting to determine whether there is a “dialog” between the SVZ and the tumors located at a distance from it, and to identify the cellular and molecular mechanisms involved.21,37 Animal models have indicated that the NSC show tropism for gliomas, but in humans the role of NSC remains to be determined.1 Considering the dependence of stem cells on their microenvironment, one element than could be taken into consideration is the interaction between NCS and tumor stem cells and SVZ structures, such as the CP.9,18,23,42 According to our own observations, CPs show changes in volume and aspect in GBM patients, suggesting a possible change in their activity during the oncogenesis. A number of studies have indicated the importance of the CP in the morphogenesis and the onset of neurodegenerative diseases.10,18,29 Given the microenvironment they create as part of the SVZ, the CPs may play a part in the biology of the NSC and in their interaction with the tumor processes of the central nervous system.5,12,39,40 An investigation of the normal and the pathological biology of the SVZ, and of the CP as part of the latter, could provide new information likely to increase the effectiveness of the current therapeutic methods, and even lead to new treatments confirming our preliminary observations on CP morphology.
Insight for future research
Finally, until fundamental research and the ongoing clinical trials (NCT02177578 and NCT02039778) provide us with new data that could make possible the application of these preliminary findings in the actual clinical practice, a randomized prospective trial remains necessary. This trial should include as uniform a population as possible, receiving the same doses (iSVZ, cSVZ,), on the same tumor volumes. The location of the tumor, the GTR, the age, the status of the MGMT gene promoter, and the administered medication should also be factored in.
Conclusion
Our systematic review and meta-analysis indicate that there was a statistically significant difference in PFS between the GBM patients who received high vs. low radiotherapy doses in iSVZ, but we couldn’t find a similar result regarding overall survival. Although the data published so far do not lead to a firm conclusion, they nevertheless open new perspectives on the mechanisms involved in the onset and progression of GBM, regarding a possible involvement of the SVZ in the progression of the GBM, as well as a possible role of the SVZ in radiation therapy. Integrating functional MRI, PET and/or fluorescence imaging with tracers coupled to monoclonal antibodies against NSC (like CD133) are the most promising modalities for clinical application of CSCs detection and accurate target delineation and dose-histogram analysis.
Acknowledgment
No funding was received for this work.
Authors’ contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
The Pubmed search strategy was: (Glioblastoma[MeSH Terms] OR Glioblastoma[All Fields] OR Glioblastomas[All Fields] OR GBM[All Fields] OR ((glioma[MeSH Terms] OR glioma[All Fields] OR gliomas[All Fields] OR Astrocytoma[MeSH Terms] OR Astrocytoma [All Fields] OR Astrocytomas [All Fields]) AND (“Grade IV”[All Fields]))) AND (radiotherapy[MeSH Terms] OR radiotherapy[All Fields] OR radiotherapies[All Fields] OR Radiation[All Fields] OR Radiations[All Fields] OR Irradiation[All Fields] OR Irradiations[All Fields] OR irradiate[All Fields] OR irradiated[All Fields]) AND (Cerebral Ventricles[MeSH Terms] OR ((Cerebrum[MeSH Terms] OR Cerebral[All Fields] OR Brain[MeSH Terms] OR brain[All Fields]) AND (Ventricle[All Fields] OR Ventricles[All Fields] OR peri-ventricular[All Fields] OR periventricular[All Fields] OR lateral ventricle[All Fields] OR lateral ventricles[All Fields] OR subventricular[All Fields] OR subventricular zone[All Fields] OR subventricular zones[All Fields] OR SVZ OR ependyma[MeSH Terms] OR ependyma[All Fields] OR ependymas[All Fields] OR subependymal[All Fields] OR Stem Cells[MeSH Terms] OR Stem Cell[All Fields] OR Stem Cells[All Fields] OR Progenitor Cell[All Fields] OR Progenitor Cells[All Fields]))) AND “humans”[MeSH Terms].
References
- 1.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeberg S, Bostel T, König L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol. 2014;9:95. doi: 10.1186/1748-717X-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeberg S, König L, Bostel T, et al. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. Int J Radiat Oncol Biol Phys. 2014;90:886–893. doi: 10.1016/j.ijrobp.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 4.Bagó JR, Sheets KT, Hingtgen SD. Neural stem cell therapy for cancer. Methods. 2016;99:37–43. doi: 10.1016/j.ymeth.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch K, Deczkowska A, David E, et al. Aging-induced type I interferon signaling at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1255826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Quinones-Hinojosa A, Ford E, et al. Increased radiation dose to the SVZ improves survival in patients with GBM. Int J Radiat Oncol Biol Phys. 2012;84:S8. doi: 10.1016/j.ijrobp.2012.07.027 [DOI] [Google Scholar]
- 7.Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;15:616–622. doi: 10.1016/j.ijrobp.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elicin O, Inac E, Uzel EK, Karacam S, Uzel OE. Relationship between survival and increased radiation dose to subventricular zone in glioblastoma is controversial. J Neurooncol. 2014;118:413–419. doi: 10.1007/s11060-014-1424-3 [DOI] [PubMed] [Google Scholar]
- 9.Emerich DF, Skinner SJM, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. BioEssays. 2005;27:262–274. doi: 10.1002/(ISSN)1521-1878 [DOI] [PubMed] [Google Scholar]
- 10.Emerich DF, Schneider P, Bintz B, Hudak J, Thanos CG. Aging reduces the neuroprotective capacity, VEGF secretion, and metabolic activity of rat choroid plexus epithelial cells. Cell Transplant. 2007;16:697–705. doi: 10.3727/000000007783465145 [DOI] [PubMed] [Google Scholar]
- 11.Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcão AM. The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci. 2012;6:1–8. doi: 10.3389/fncel.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gollapalli K, Ghantasala S, Kumar S, et al. Subventricular zone involvement in glioblastoma-a proteomic evaluation and clinicoradiological correlation. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-01202-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grégoire C-A, Goldenstein BL, Floriddia EM, Barnabé-Heider F, Fernandes KJL. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63:1469–1482. doi: 10.1002/glia.22851 [DOI] [PubMed] [Google Scholar]
- 15.Han S, Li X, Qiu B, et al. Can lateral ventricle contact predict the ontogeny and prognosis of glioblastoma? J Neurooncol. 2015;124:45–55. doi: 10.1007/s11060-015-1858-2 [DOI] [PubMed] [Google Scholar]
- 16.Ho J, Ondos J, Ning H, et al. Chemoirradiation for glioblastoma multiforme: the national cancer institute experience. PLoS One. 2013;8(8):e70745. doi: 10.1371/journal.pone.0070745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15:91–96. doi: 10.1093/neuonc/nos268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur C, Rathnasamy G, Ling EA. The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol. 2016;75:198–213. doi: 10.1093/jnen/nlv030 [DOI] [PubMed] [Google Scholar]
- 19.Khalifa J, Tensaouti F, Lusque A, et al. Subventricular zones: new key targets for glioblastoma treatment. Radiother Oncol. 2016;119:S302–S303. doi: 10.1016/S0167-8140(16)31897-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusumawidjaja G, Gan P, Tan D, et al. Dose-escalated intensity modulated radiation therapy and increased radiation doses to subventricular zones in treatment outcomes of patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2014;90:S289–S290. doi: 10.1016/j.ijrobp.2014.05.980 [DOI] [Google Scholar]
- 21.Lee C, Hu J, Ralls S, et al. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One. 2012;7:e50501. doi: 10.1371/journal.pone.0050501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P, Eppinga W, Lagerwaard F, et al. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: A pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86:609–615. doi: 10.1016/j.ijrobp.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 23.Lehtinen MK, Bjornsson CS, Dymecki SM, et al. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci. 2013;33:17553–17559. doi: 10.1523/JNEUROSCI.3846-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang THK, Kuo SH, Wang CW, et al. Adverse prognosis and distinct progression patterns after concurrent chemoradiotherapy for glioblastoma with synchronous subventricular zone and corpus callosum invasion. Radiother Oncol. 2016;118:16–23. doi: 10.1016/j.radonc.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 25.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–429. doi: 10.1215/15228517-2007-023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim DA, Alvarez-Buylla A. The adult Ventricular – Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a01882. doi: 10.1101/cshperspect.a018820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin R, Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015;1628:327–342. doi: 10.1016/j.brainres.2015.04.029 [DOI] [PubMed] [Google Scholar]
- 28.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 29.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–457. doi: 10.1038/nrn3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques-Torrejon MA, Gangoso E, Pollard SM. Modelling glioblastoma tumour-host cell interactions using adult brain organotypic slice co-culture. Dis Model Mech. 2018;11:dmm031435. doi: 10.1242/dmm.031435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mistry AM, Dewan MC, White-Dzuro, et al. survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol. 2017;132:341–349. doi: 10.1007/s11060-017-2374-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mistry AM, Hale AT, Chambless LB, Weaver KD, Thompson RC, Ihrie RA. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol. 2017;131:125–133. doi: 10.1007/s11060-016-2278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta T, Nair V, Paul SN, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012;109:195–203. doi: 10.1007/s11060-012-0887-3 [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega JA, Memi F, Radonjic N, et al. The subventricular zone: a key player in human neocortical development. Neuroscientist. 2018;24:156–170. doi: 10.1177/1073858417691009 [DOI] [PubMed] [Google Scholar]
- 36.Parsa AT, Wachhorst S, Lamborn KR, et al. significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg. 2005;102:622–628. doi: 10.3171/jns.2005.102.4.0622 [DOI] [PubMed] [Google Scholar]
- 37.Qin EY, Cooper DD, Abbott KL, et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170:845–859. doi: 10.1016/j.cell.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo AE, Yu J, Suh J, et al. Investigating the relationship between radiation dose to neural stem cell niches and survival in GBM. Int J Radiat Oncol Biol Phys. 2014;90:S283–S284. doi: 10.1016/j.ijrobp.2014.05.965 [DOI] [Google Scholar]
- 39.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. doi: 10.1016/j.stem.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 40.Strominger I, Elyahu Y, Berner O, et al. The choroid plexus functions as a niche for T-cell stimulation within the central nervous system. Front Immunol. 2018;9:1066. doi: 10.3389/fimmu.2018.01066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallard A, Espenel S, Guy J-B, et al. Targeting stem cells by radiation: from the biological angle to clinical aspects. World J Stem Cells. 2016;8:243–250. doi: 10.4252/wjsc.v8.i8.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandenbroucke RE. A hidden epithelial barrier in the brain with a central role in regulating brain homeostasis: implications for aging. Ann Am Thorac Soc. 2016;13:S407–S410. doi: 10.1513/AnnalsATS.201609-676AW [DOI] [PubMed] [Google Scholar]
- 43.Weller M, van Den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30072-4 [DOI] [PubMed] [Google Scholar]
- 44.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst. 2018. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 45.Xu W, Lakshman N, Morshead CM. Building a central nervous system: the neural stem cell lineage revealed. Neurogenesis. 2017;4:e1300037. doi: 10.1080/23262133.2017.1300037 [DOI] [PMC free article] [PubMed] [Google Scholar]