Abstract
Introduction: Cancer-related fatigue (CRF) is the most debilitating symptom with the greatest adverse side effect on quality of life. The etiology of this symptom is still not understood. The purpose of this study was to examine the relationship between mitochondrial gene expression, mitochondrial oxidative phosphorylation, electron transport chain complex activity, and fatigue in prostate cancer patients undergoing radiotherapy (XRT), compared to patients on active surveillance (AS).
Methods: The study used a matched case–control and repeated-measures research design. Fatigue was measured using the revised Piper Fatigue Scale from 52 patients with prostate cancer. Mitochondrial oxidative phosphorylation, electron-transport chain enzymatic activity, and BCS1L gene expression were determined using patients’ peripheral mononuclear cells. Data were collected at three time points and analyzed using repeated measures ANOVA.
Results: The fatigue score was significantly different over time between patients undergoing XRT and AS (P<0.05). Patients undergoing XRT experienced significantly increased fatigue at day 21 and day 42 of XRT (P<0.01). Downregulated mitochondrial gene (BC1, ubiquinol-cytochrome c reductase, synthesis-like, BCS1L, P<0.05) expression, decreased OXPHOS-complex III oxidation (P<0.05), and reduced activity of complex III were observed over time in patients with XRT. Moreover, increased fatigue was significantly associated with downregulated BCS1L and decreased complex III oxidation in patients undergoing XRT.
Conclusion: Our results suggest that BCS1L and complex III in mitochondrial mononuclear cells are potential biomarkers and feasible therapeutic targets for acute XRT-induced fatigue in this clinical population.
Keywords: BCS1L, gene expression, mitochondrial bioenergetics, cancer-related fatigue, prostate cancer, radiation therapy
Introduction
Prostate cancer is a highly prevalent carcinoma, the second most common malignancy, and the third leading cause of cancer mortality in the US. The American Cancer Society estimated 164,690 new diagnoses of this disease and 29,430 deaths in 2018.1 The survival rate of patients with locally advanced prostate cancer has greatly increased with advances in primary therapies. External beam radiation therapy (XRT) using conformal techniques such as intensity-modulated radiation with image-guided XRT, is a standard treatment option for patients with localized prostate cancer.2 Although XRT has increased survival rates for men with prostate cancer, it results in numerous side effects which persist even in disease-free states3,4 including distressing fatigue during treatment.
Cancer-related fatigue (CRF) is the most prevalent, debilitating, and persistent symptom experienced by patients with cancer undergoing treatment,5–8 and typically increases during XRT.9,10 CRF is pervasive, a whole-body excessive tiredness or exhaustion related to the disease or its treatment, unrelated to activity or exertion and not relieved by rest or sleep.11,12 CRF decreases compliance with cancer treatment13 and it negatively impacts other health outcomes, leading to increased depression, impaired cognitive function, increased sleep disturbance, decreased physical activity, and decreased health-related quality of life.14–17 CRF in men treated for prostate cancer has been found to increase beginning at week threeof XRT, increasing significantly by week six,9 remaining elevated at the completion of XRT,18 and lasting months to years afterward.3,9,19–21
With limited available options to manage CRF, strategies are needed to identify effective interventions. To guide the development of effective therapies for the management of CRF, identification of pathophysiological mechanism(s) and related-biomarkers associated with CRF is an essential step. Various attempts have been made to investigate multifactorial mechanisms of CRF,22–30 but the pathophysiological mechanisms of CRF remain unclear. For example, several mechanisms have been proposed to influence the individual CRF experience, including genetic factors, energy expenditure, metabolism, aerobic capacity, and the patient’s immune response to inflammation.31–33 The inflammation hypothesis has been the primary focus for investigations into the mechanism of CRF,27,34–36 but some reports have indicated no association.37–39 We have determined that a significant relationship exists between radiation-related fatigue and the differential expression of genes related to mitochondrial bioenergetics.40,41 More recently, we have shownsignificantly decreased mitochondrial oxidative phosphorylation (OXPHOS) starting at complex III associated with increased fatigue in patients receiving XRT by week three.42
We have proposed a mechanism of mitochondrial bioenergetics in CRF determining the relationship between bioenergetics and XRT-induced CRF, linking downregulated BC1 (ubiquinol-cytochrome c reductase) synthesis-like (BCS1L) and impaired OXPHOS starting at complex III as causes of CRF development.43 OXPHOS is the process where ATPis formed as a result of the transfer of electrons from nicotinamide adenine dinucleotide (reduced form) (NADH) or flavin adenine dinucleotide (reduced form) (FADH2) to oxygen (O2) by a series of electron carriers in the mitochondrial inner membrane. As defined,44 integrated mitochondrial function is measured as OXPHOS and involves transport of substrates into the mitochondria, the generation of reducing equivalents (NADH and FADH2) by specific dehydrogenases, entry into the electron transport chain (ETC), and coupling to the production of ATP.45,46 The human BCS1L gene encodes a member of the ATPases associated with diverse cellular activities (AAA) family of ATPases. The BCS1L chaperone protein inserts the Rieske iron sulfur protein into complex III of the respiratory chain in mitochondria, where the respiratory chain produces and maintains effective ATP content.47,48
The purpose of this study was to examine the relationship between the expression of mitochondrial gene BCS1L, integrated mitochondrial OXPHOS, ETC enzymatic activity, and CRF in prostate cancer patients receiving XRT and to compare these to patients undergoing active surveillance (AS), but who had not received any treatment.
Material and methods
This was a prospective and case-matched study designed to encompass repeated measurements. Patients with nonmetastatic prostate cancer scheduled to receive localized XRT or without any treatment but on AS and able to provide written informed consent were enrolled. Patients were excluded from the study if they had progressive disease causing significant fatigue; documented major psychiatric illness within five years; had uncorrected hypothyroidism or untreated anemia; took sedatives, steroids or nonsteroidal anti-inflammatory agents; or had a second malignancy or mitochondrial disease.
This study was reviewed and approved by the Institutional Review Board of the Case Comprehensive Cancer Center. The study conformed to the principles outlined in the Declaration of Helsinki. From August 2015 to December 2017 we screened a total of 153 patients; 44 were ineligible and 56 refused to participate. As a result, 53 patients were enrolled (one withdrew because of a time conflict). After obtaining written informed consent, we collected demographic information and medical history via interviews and from the medical records. Study variables included fatigue, mitochondrial bioenergetics, and BCS1L gene expression. In addition, depression was evaluated as a covariate of fatigue in this population. Peripheral blood samples and questionnaires were obtained from each participant at day 0 (before XRT, baseline), day 21 of XRT (midpoint), and day 42 of XRT (completion of XRT, endpoint).
Fatigue
Patient-reported fatigue was measured using the revised Piper Fatigue Scale (rPFS) at each time point. The rPFS combines a structured questionnaire and four open-ended questions related to fatigue management. It is a 22-item paper-pencil, self-administered questionnaire that measures CRF in multiple dimensions (behavioral, severity, sensory, cognitive/mood, and affective). This 10-point (0= none; 10= worst intensity) intensity rating scale has good reliability and validity with internal consistency ranging from 0.7 to 0.9 across four dimensions in cancer patients.49 Based on clinically meaningful cutoff scores for rPFS (0–10 severity metric), mild fatigue was conceptualized as 1–3, moderate fatigue as 4–6, and severe fatigue as 7–10.50 The rPFS can be completed in 15 minutes.
Depression
Hamilton Depression Rating Scale (HAM-D) was used to screen depressive symptoms in each subject at each time point. HAM-D is a 21-item, clinician-rated paper questionnaire with good internal reliability (α=0.81–0.98).51 The predefined cutoff score for depression is 15 in cancer patients, with higher scores indicating more symptoms of depression.52
Peripheral blood collection, mononuclear cells isolation, and sample preparation
EDTAvacutainers were used to collect 40–45 mL peripheral blood sample from each subject. The specimens were stored at room temperature, and processed within 24 hours, with a range of 20–24 hours. As we published, there is no significant difference in mononuclear cell OXPHOS between 0 and 24 hours after the blood sample was collected;44 however, there were decreased activities of complex III, I/III, and II/III between frozen samples and fresh samples. Therefore, all specimens were stored at room temperature, processed, and assayed within 24 hours for ETC and OXPHOS. The standard procedure using a published protocol with optimal conditions (eg, using LymphoprepTM [COSMO BIO, Carlsbad, CA, USA] and PBS with room temperature environment) was used to process and harvest mononuclear cell pellets from human peripheral blood samples.44 Sample preparations and methods for mitochondrial OXPHOS44,53 and ETC enzymatic activity54–56 have been published.
The mononuclear cell pellet was suspended with pre-warmed (37°C) mitochondria respiration medium (MiR05).57 We used a hemocytometer with trypan blue to count the number of live mononuclear cells. The mononuclear cells were adjusted to a final concentration of 1.5–2 million cells/mL with MiR05 for the study of OXPHOS. For the electron transport complex (ETC) assay, the mononuclear pellet was resuspended in of MSM (mannitol 220 mM, sucrose 70 mM, and MOPS 5 mM)/2 mM EDTA buffer with 7.5 μL of proteinase inhibitor (Sigma-AldrichCo., St Louis, MO, USA, Cat # P8340) and kept on ice.55,56 We determined the protein concentration in mononuclear cells using the Lowry assay58 and prepared the sample using 5% cholate and MSM/2 mM EDTA at 1 mg/mL of protein for assays except for the activity of complex IV-cytochrome c oxidase.59 A second sample for cytochrome c oxidase was prepared in MSM/2 mM EDTA at 1 mg/mL of protein without cholate.60,61
Measurement of integrated mitochondrial function using high-resolution respirometry
Integrated mitochondrial function was measured as OXPHOS following two published substrate-uncoupler-inhibitor protocols44,53,57 with a high-resolution respirometer, Oxygraph-2 K using DatLab 4 software (Oroboros Instruments, Innsbruck, Austria). Two different substrate-uncoupler-inhibitor protocols were used to determine flux through complex I, II, III, and IV, and fatty acid oxidation.53,57 We collected the incubation mixture with cells at the conclusion of each experiment to determine citrate synthase as a mitochondria marker.
Measurement of electron transport complex activity
To analyze the ETC complex activity in fresh isolated mononuclear cells, we followed established protocols46,54,55 using spectrophotometry. A total of nine assays were performed. Rotenone-sensitive NADH cytochrome c reductase was used to measure the linked activity of complex I and III. Antimycin A-sensitive succinate cytochrome c reductase was used to measure the linked activity of complexes II and III. Succinate dehydrogenase (SDH) was used to measure the first two subunits (A and B) of complex II and served as a membrane-bonded mitochondrial marker enzyme. Complex II activity was measured as the thenoyltrifluoroacetone (TTFA)-sensitive succinate-2,6 dichlorophenol-indophenol (DCPIP) reductase and total complex II determined with coenzyme Q1 added.55 Antimycin A-sensitive decylubiquinol cytochrome c reductase (complex III) was utilized to determine the activity of complex III. Cytochrome c oxidase (complex IV) activity was measured as the first-order rate constant. In addition to SDH, we measured citrate synthase activity as the other mitochondrial marker, as well as the activity of lactate dehydrogenase (LDH), a cytoplasmic marker.
BCS1L gene expression
Total RNA (100–120 ng) was extracted from buffy coat mononuclear cells collected from peripheral blood. After ensuring adequate quality and quantity, RNA was converted to cDNA following a standard protocol to minimize nonbiological technical bias.41 To measure the changes in gene expression of BCS1L, real-time polymerase chain reaction (PCR) was performed using TaqMan Chemistry from Applied Biosystems. Expression values were assessed by monitoring in real time the PCR cycle in which a minimum level of product is produced, ie, crosses a threshold, the cycle threshold (Ct) value. The lower the Ct value, the higher the gene expression level. Three housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH); β-actin, ACTB); hypoxanthine phosphoribosyltransferase 1, HPRT1) were used as endogenous controls (ECs) to adjust for the input RNA; expression levels were reported as delta Cts between the gene of interest and the EC.
Statistical analysisDescriptive statistics were calculated for the mean, standard deviation, standard error of the mean (SEM), and range for the participants’ demographic, clinical characteristics, and mitochondrial OXPHOS. The study purpose was to examine the relationship between mitochondrial gene expression, mitochondrial oxidative phosphorylation (OXPHOS), electron transport chain ETC complex (ETC) activity, and fatigue symptoms in prostate cancer patients undergoing radiation therapy (XRT), compared to patients on active surveillance (AS). Repeated measures ANOVA was used to compare the mean differences of fatigue score, mitochondrial OXPHOS, ETC activity, and BCS1L gene expression between patients undergoing XRT and AS at each time point and to measure changes in study variables (fatigue severity, OXPHOS, ETC activity, and BCS1L gene expression) over time. We used repeated measures ANOVA to understand the mean differences in relevant variables between the XRT and AS groups over time and the Bonferroni correction was used for the multiple comparisons for each dependent variable. To compare the mean differences in fatigue scores, OXPHOS, ETC activity, and BCS1L gene expression between time points within groups, Paired t-testss were used to better understand where the differences were for simplicity. After that, Pearson correlations were used to analyze the associations between study variables at each time point and changes in midpoint and endpoint from baseline. Power analysis was employed to determine that a sample size of 25 would achieve 80% power with the detection of differences in fatigue score, BCS1L gene expression, and mitochondrial OXPHOS within and between subjects. All statistical analysis and graphics were conducted using SPSS 25.0 (IBM Corporation, Armonk, NY, USA), and R 3.0.0 for Windows. The mitochondrial OXPHOS and ETC experiments were consistently performed in the morning by the same investigator and technician. The OXPHOS data were normalized to live cell number with the non-mitochondrial respiration subtracted. The ETC data were normalized to the protein concentration of mononuclear cells. Data are expressed as means ±SEM standard error of the mean of 52 patients for demographic and clinical characteristics, of 48 for OXPHOS and ETC, of 46 for gene expression. Because of biological contamination in the O2K respirometer chamber, low yield of blood sample, and technician absence, we were not able to perform OXPHOS and ETC for six6 data points from the total of 156 data collection points.
Results
A total of 52 patients diagnosed with localized prostate cancer were enrolled in this study (35 patients who received XRT and 17 age-, gender-matched patients on AS as controls). Table 1 describes the demographic and clinical characteristics of the study sample. 70% of subjects (N=36/52) were Caucasian with a mean age of 67.7 years (±1.4) among the XRT subjects and 63.7 (±1.7) years for the matched controls. Most of the subjects (71%, N=37/52) were married and 51% of participants (N=27/52) were employed full-time.
Table 1.
Demographic and clinical characteristics of sample (N=52)
| Variables | Participants | P-value | |
|---|---|---|---|
| XRT subjects (N=35) | Controls (N=17) | ||
| Mean (± SEM) or N (%) | Mean (± SEM) or N (%) | ||
| Mean age, years | 67.7 (±1.4) | 63.7 (±1.7) | 0.09 |
| Ethnicity: | |||
| Caucasian | 21 (60%) | 15 (88%) | |
| African-American | 14 (40%) | 1 (6%) | |
| Asian | 1 (6%) | ||
| Marital status | |||
| Married | 22 (63%) | 15 (88%) | |
| Widowed | 6 (17%) | ||
| Single | 3 (9%) | ||
| Divorced/separated | 4 (11%) | 2 (12%) | |
| Employment status | |||
| Employed | 14 (40%) | 13 (76%) | |
| Retired | 19 (54%) | 4 (24%) | |
| Disabled | 2 (6%) | ||
| T-stage | |||
| T1c | 31 (88%) | 16 (94%) | |
| T2a-c | 2 (6%) | 1 (6%) | |
| T3a | 2 (6%) | ||
| Gleason Score | |||
| 7 Jun | 21 (60%) | 16 (94%) | |
| 10 Aug | 14 (40%) | 1 (6%) | |
| Karnofsky performance scale | 88.6 (±0.8) | 90.0 (±0.8) | 0.62 |
| PSA (ng/mL) | |||
| Baseline | 12.6 (±2.12) | 4.7 (±0.6) | 0.02 |
| Endpoint | 0.7 (±0.95) | 4.2 (±0.6) | 0.01 |
| Hemoglobin (mg/dL) | 0.46 | ||
| Baseline | 14.4 (±0.3) | 15.3 (±0.7) | |
| Endpoint | 13.9 (±0.4) | ||
| Hematocrit (%) | 0.22 | ||
| Baseline | 43.4 (±0.8) | 45.6 (±1.7) | |
| Endpoint | 41.1 (±0.9) | ||
| Depression (HAM-D) | |||
| Baseline | 1.3 (±0.3) | 0.5 (±0.3) | 0.12 |
| Endpoint | 1.2 (±0.3) | 0.2 (±0.1) | 0.09 |
| Total EBRT dosage (Gy) | |||
| 79.2–81 | 24 (68%) | None | |
| 100 | 11 (32%) | ||
Notes: Controls are men with nonmetastatic prostate cancer and not receiving any treatment are placed in active surveillance.
Abbreviations: SEM, standard error of the mean; PSA, prostate- specific antigen; HAM-D, Hamilton Depression Rating Scale; XRT, external beam radiation therapy.
Most of the subjects (88%, N=31/35 in XRT group, 94%, N=16/17 in AS group) had a clinical stage T1c with a Gleason score of 6 or 7. The clinical T1c stage indicates that the tumor was not palpable and found only through a needle biopsy, with moderate or intermediate differentiation with an elevated prostate-specific antigen (PSA) level.1 The mean Karnofsky performance score was 89 for patients with XRT and 90 for the AS group, indicating participants were able to carry out normal activities with only minor signs or symptoms of disease. There was a significant difference in PSA levels between patients with XRT and AS at baseline and endpoint. There was no difference in baseline hemoglobin (14.4±0.3 for XRT group, 15.3±0.7 for AS group) and hematocrit (43.4±0.8 for XRT group, 45.6±1.7 for AS group). None of the 52 participants reached the cutoff score for clinical depression.62 Eleven (32%) of the 35 XRT subjects received a total dose of 100 Gy of XRT, while the rest received a 80 Gy.
Fatigue
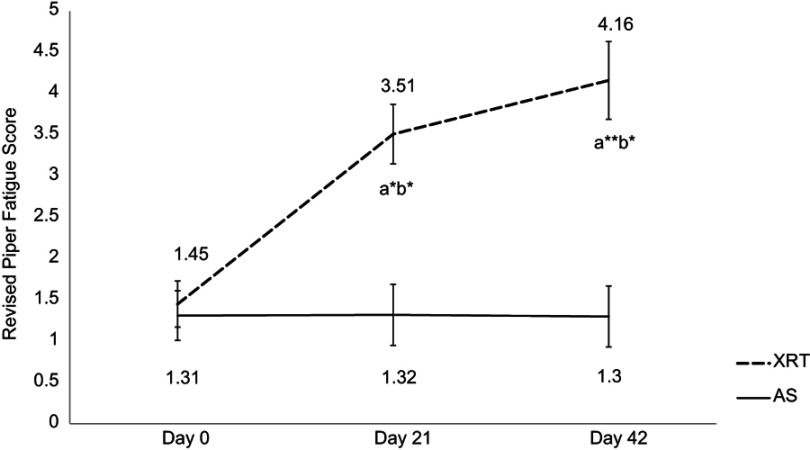
The mean r-PFS score at day 0/baseline for XRT subjects (1.45±0.29) and controls (1.31±0.29) was not significantly different (P=0.34). There was no significant change in fatigue over time in patients undergoing AS. Compared to the AS group, the fatigue score for XRT subjects worsened significantly at day 21/midpoint (3.51±0.35, P<0.05) and trended towards significance at the completion/day 42 of XRT (4.16±0.43, P<0.01), as shown in Figure 1. There was a significant difference in fatigue scores at the midpoint and endpoint between XRT and AS groups (P<0.05).
Figure 1.
Changes in fatigue score between patients undergoing radiation therapy (N=35) and active surveillance (N=17). Fatigue was measured by the revised Piper Fatigue Scale (rPFS).
Notes: X-axis indicates time points: day 0=prior XRT; day 21=day 21 of XRT, midpoint; day 42=completion of XRT, endpoint. Y-axis represents revised Piper Fatigue score. Fatigue score was significantly increased at day 21 (t=2.06, P=0.03) and day 42 (t=2.71, P=0.002) in patients with XRT; additionally, there was a significant difference in fatigue scores between XRT and AS groups over time. aRefers to a significant difference compared to day 0 time point of the same group. bRefers to a significant difference compared to the AS group at the same time point. *P<0.05; **P<0.01. a*b*Represents a significant change in fatigue score (P<0.05) in patients receiving XRT and a significant difference in fatigue score (P<0.05) between XRT and AS groups. a**b*Represents a significant change in fatigue score (P<0.01) in patients receiving XRT, and a significant difference in fatigue score (P<0.05) between XRT and AS groups.
Abbreviations: XRT, external beam radiation therapy; AS, active surveillance.
Mitochondrial OXPHOS
Integrated mitochondrial function measured as OXPHOS in peripheral mononuclear cells from patients undergoing XRT and AS at each time point is provided in Table 2. Intact cellular respiration was measured after air calibration and not changed following the addition of malate and pyruvate (protocol 1), or malate and palmitoylcarnitine (protocol 2) were not different in either group at any timepoint. To access mitochondria, digitonin was added to permeabilize the plasma membrane without interrupting the integrity of the mitochondrial outer membrane. The digitonin-permeabilized rate, referred to as the leak rate by Pesta and Gnaiger,57 was not different at any timepoint in either group. OXPHOS-complex I-linked oxidation was measured as ADP-stimulated respiration in the presence of malate, pyruvate, and glutamate. There was no difference in OXPHOS-complex I-linked oxidation in either group at any timepoint. We measured OXPHOS-complex I + II-linked oxidation in the presence of malate, pyruvate, glutamate, and succinate. Compared to day 0, OXPHOS-complex I+II-linked oxidation was significantly decreased at day 21 (16%, ↓2.26 pmol O2/sec/106 cells, P=0.042) and day 42 (8%, ↓2.2 pmol O2/sec/106 cells, P=0.045) in patients undergoing XRT. There was no significant change in mononuclear cell mitochondrial OXPHOS over time in patients undergoing AS. (Table 2)
Table 2.
Integrated mitochondrial function in peripheral blood mononuclear cells of patients with prostate cancer
| Variables | Prostate cancer patients with radiation therapy or on active surveillance | |||||
|---|---|---|---|---|---|---|
| Baseline/day 0 | Midpoint/day 21 | Endpoint/day 42 | ||||
| XRT (N=33) |
AS (N=17) |
XRT (N=34) |
AS (N=17) |
XRT (N=35) |
AS (N=17) |
|
| Cell number (106/mL) | 1.8±0.19 | 2.0±0.26 | 1.5±0.33 | 2.1±0.26 | 1.6±0.48 | 1.9±0.39 |
| Protein concentration (mg/mL) | 0.51±0.25 | 0.49±0.32 | 0.50±0.18 | 0.50±0.28 | 0.55±0.22 | 0.51±0.39 |
| Protocol 1 | (pmol O2/sec/106 cells) Mean ± SEM | |||||
| Intact cell | 3.55±0.23 | 3.54±0.29 | 3.61±0.21 | 4.06±0.32 | 3.31±0.18 | 3.75±0.34 |
| Intact cell (malate+pyruvate) | 4.49±0.26 | 4.38±0.32 | 4.51±0.29 | 4.84±0.36 | 4.41±0.26 | 4.63±0.31 |
| Leak (digitonin) | 1.24±0.20 | 1.02±0.23 | 1.26±0.25 | 1.70±0.46 | 1.18±0.19 | 0.83±0.21 |
| OXPHOS (complex I-linked) | 4.67±0.35 | 4.59±0.77 | 4.51±0.44 | 5.12±0.39 | 5.19±0.45 | 6.04±0.58 |
| OXPHOS (complex I+II-linked) | 14.21±0.76 | 13.16±1.21 | a*11.95±0.7 | 13.57±1.15 | a*b*12.29±0.9 | 15.15±1.35 |
| Uncoupled CI+II-linked, maximal oxidative capacity (uncoupler) | 15.45±0.89 | 14.23±1.13 | a*13.25±1.0 | 14.25±1.24 | b*13.85±0.9 | 16.11±1.44 |
| Uncoupled complex I | 4.96±0.46 | 5.11±0.59 | 5.01±0.77 | 4.82±0.71 | 4.91±0.47 | 6.03±0.59 |
| Uncoupled complex II | 8.74±0.64 | 8.06±0.93 | a*6.17±0.53 | 8.18±0.84 | 7.15±0.66 | 8.86±1.57 |
| Uncoupled complex IV | 229.5±15.9 | 247.9±20.8 | 245.7±13.5 | 251.1±30.9 | 220.6±10.8 | 272.9±17.6 |
| Citrate synthase | 3.58±0.34 | 2.98±0.59 | 3.31±0.29 | 3.27±0.64 | 4.09±0.63 | 2.95±0.47 |
| Acceptor control ratio | 4.53±0.58 | 5.05±0.72 | 4.12±0.64 | 5.36±1.50 | 4.68±0.51 | 5.41±1.02 |
| Uncoupled control ratio | 1.09±0.25 | 1.10±0.43 | 1.15±0.62 | 1.06±0.03 | 1.07±0.25 | 1.03±0.34 |
| Protocol 2 | ||||||
| Intact cell | 3.36±0.21 | 3.72±0.27 | 3.52±0.32 | 3.53±0.31 | 3.19±0.21 | 3.65±0.29 |
| Intact cell (malate+palmitoylcarnitine) | 3.95±0.25 | 4.17±0.23 | 4.29±0.41 | 4.13±0.25 | 3.97±0.21 | 4.43±0.33 |
| Leak (digitonin) | 1.20±0.21 | 1.15±0.22 | 1.70±0.42 | 1.37±0.41 | 1.33±0.18 | 1.23±0.32 |
| OXPHOS (fatty acid oxidation) | 3.76±0.29 | 3.31±0.27 | 3.84±0.33 | 3.35±0.37 | 3.64±0.23 | 3.46±0.23 |
| OXPHOS (coupled complex III) | 21.43±1.03 | 19.46±0.84 | a*19.05±0.84 | 19.31±1.91 | a*b*19.01±1.18 | 21.26±1.75 |
| Uncoupled complex III (uncoupler) | 20.26±1.12 | 18.36±1.39 | a*17.74±1.04 | 18.46±1.52 | a*18.44±1.38 | 19.95±1.52 |
| Citrate synthase | 4.91±0.55 | 2.60±0.41 | 4.95±0.58 | 2.97±0.47 | 3.97±0.54 | 2.79±0.39 |
| Acceptor control ratio | 4.24±0.68 | 4.36±0.50 | b*3.65±0.63 | 6.31±1.75 | 3.94±0.49 | 5.21±0.79 |
| Uncoupled control ratio | 0.96±0.22 | 0.95±0.25 | 0.93±0.31 | 0.90±0.34 | 0.98±0.02 | 0.94±0.32 |
Notes: Data are subtracted from non-mitochondrial respiration (antimycin A respiration for protocol 1, rotenone and antimycin A respiration for protocol 2). Data are presented as mean ± standard error. Integrated mitochondrial function was measured as oxidative phosphorylation. aSignificant difference compared to day 0 time point of the same group. bSignificant difference compared to the AS group at same time point. *P<0.05.
Abbreviations: XRT, external radiation therapy; AS, active surveillance; SEM, standard error of the mean; OXPHOS, oxidative phosphorylation; CI, complex I; CII, complex II; CIII, complex III; CIV, complex IV.
To assess maximal oxidative capacity, the uncoupler, carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP), was added and uncoupled-complex I+II-linked oxidation was measured. At day 21 of XRT, the uncoupled-complex I + II oxidation was significantly increased 15% (↑ 1.3 pmol O2/sec/106 cells, P=0.045) after the addition of FCCP, indicating a small reserve capacity in patients with XRT. Compared to day 0, uncoupled complex I + II oxidation was significantly decreased at day 21 (11%, ↓ 2.26 pmol O2/sec/106 cells, P=0.045) and day 42 (9%, ↓1. 6 pmol O2/sec/106 cells, P=0.048) in patients receiving XRT (Table 2). Uncoupled complex II oxidation was measured following the addition of rotenone, a complex I inhibitor. The rotenone-insensitive rate was considered uncoupled complex II activity; there was significantly decreased uncoupled complex II oxidation at day 21 (28%, ↓2.57 pmol O2/sec/106 cells, P=0.045) in patients receiving XRT. Uncoupled complex I was the difference seen between the oxidation of uncoupled complex I+II and uncoupled complex II, and it was not different at any timepoint in either group. The antimycin A-insensitive rate represented nonmitochondrial respiration57 and was subtracted from all data. To measure uncoupled complex IV, ascorbate and tetramethyl-p-phenylenediamine (TMPD) were added followed by the inhibitor, sodium azide. The azide-sensitive uncoupled complex IV oxidation was not significantly different in either group at any timepoint, as shown in Table 2.
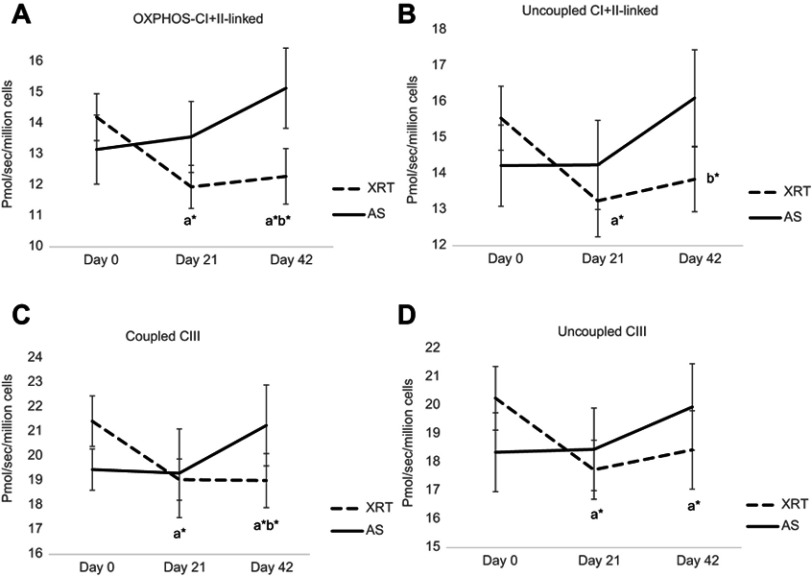
In protocol 2, we measured the oxidation of fatty acid and complex III. With the addition of palmitoylcarnitine, there was no change in fatty acid oxidation in either group at any timepoint (Table 2). The coupled complex III oxidation was measured as ADP-stimulated respiration with a reduced CoQ analog-duroquinol. With the addition of an uncoupler, the oxidation rate was not increased compared to coupled complex III in either group at any timepoint, indicating there was no reserve capacity of complex III in mononuclear cells mitochondria. Compared to day 0, coupled complex III oxidation was significantly decreased at day 21 (10%, ↓2.38 pmol O2/sec/106 cells, P=0.044) and day 42 (11%, ↓2.42 pmol O2/sec/106 cells, P=0.041) in patients undergoing XRT. Uncoupled complex III oxidation was measured with the addition of FCCP. Compared to day 0, uncoupled complex III oxidation was significantly decreased at day 21 (12%, ↓2.52 pmol O2/sec/106 cells, P=0.042) and day 42 (10%, ↓1.82 pmol O2/sec/106 cells, P=0.047) in mononuclear cell mitochondria in patients receiving XRT (Table 2). Figure 2 shows a significant difference in OXPHOS over time between XRT and AS groups, including the oxidation of OXPHOS complex I + II-linked, uncoupled complex I + II oxidation, and coupled complex III at day 42 (Figure 2).
Figure 2.
Changes in mitochondrial oxidative phosphorylation (OXPHOS, complex I+II linked, uncoupled complex I+II-linked, coupled complex III, and uncoupled complex III) over time between patients with radiation therapy (N=33) and active surveillance (N=15). 26pp
Notes: X-axis indicates time points and Y-axis represents cell respiration rate (pmol O2/sec/106 cells). A: OXPHOS-complex I+II linked oxidation was measured as ADP-stimulated respiration with malate, pyruvate, glutamate, and succinate as substrates, reflecting the convergence of complex I and II. B: Uncoupled complex I+II was obtained with addition of the uncoupler, FCCP representing maximal oxidative capacity. C: Coupled complex III rate was measured as the ADP-stimulated respiration with DHQ, a substrate for complex III. D: Uncoupled complex III was determined after the addition of the uncoupler, FCCP. aRefers to a significant difference compared to day 0 time point of the same group. bRefers to a significant difference compared to the AS group at the same time point. *P<0.05; **P<0.01. a*b*Represents a significant change in fatigue score (P<0.05) in patients receiving XRT and a significant difference in fatigue score (P<0.05) between XRT and AS groups.
Abbreviations: OXPHOS, oxidative phosphorylation; XRT, external beam radiation therapy; AS, active surveillance; FCCP, carbonylcyanide-p-trifluoromethoxyphenylhydrazone; DHQ, duroquinol.
Table 2 also displays the acceptor control ratio (ACR) and the uncoupled control ratio (UCR). The ACR provides an estimate of the coupling of mitochondrial oxidation to the production of ATP (OXPHOS). It was calculated as the ADP-stimulated OXPHOS of malate + pyruvate + ADP oxidation divided by the leak rate (digtonin-permeabilized respiration). The UCR is the maximum oxidative capacity (evoked by FCCP) divided by the ADP-stimulated OXPHOS linked complex I through V of malate + pyruvate + glutamate + succinate. The UCR provides an estimate of the respiratory capacity to the rate of oxidative phosphorylation. The ACR was decreased over time in patients undergoing XRT, compared to AS group. There was a significant difference (t=2.66, P=0.03) in ACR at day 21 (in protocol 2) between the XRT (3.65±0.63) and the AS (6.31±1.75) group, indicating the production of ADP at day 21 in patients undergoing XRT is less than it is in patients undergoing AS. There was no difference in UCR in either group at any time point, and the value was around 1.1 (protocol 1) and 0.9 (protocol 2), indicating the oxidative capacity is basically equal to OXPHOS capacity (Table 2).
ETC enzymatic activity
The analysis of ETC enzymatic activity from mononuclear cell mitochondria for patients with XRT and AS at day 0/baseline, day 21/midpoint, and day 42/endpoint is presented in Table 3. There was no significant difference in ETC between XRT and AS groups at baseline. There was no significant difference in mononuclear cell mitochondrial ETC data in patients undergoing AS at any time point (Table 3).
Table 3.
Electron-transport chain enzymatic activity in peripheral blood mononuclear cells
| Variables | Prostate cancer patients with radiation therapy or on active surveillance | |||||
|---|---|---|---|---|---|---|
| Baseline/day 0 | Midpoint/day 21 | Endpoint/day 42 | ||||
|
XRT (N=35) |
AS (N=17) |
XRT (N=34) |
AS (N=15) |
XRT (N=35) |
AS (N=17) |
|
| Protein concentrations (mg/mL) | 4.29±0.41 | 3.87±0.29 | 4.33±0.34 | 3.94±0.25 | 4.24±0.29 | 4.59±0.35 |
| nmol/min/mg protein | Mean ± SEM | |||||
| Rotenone-sensitive CI/III | 13.7±0.89 | 12.93±1.27 | 12.35±0.88 | 13.75±1.42 | 12.85±0. 98 | 12.39±0.74 |
| Antimycin A-sensitive CII/III | 10.3±0.63 | 11.18±0.92 | 9.69±0.55 | 11.24±0.82 | a*b*8.92±0.59 | 11.01±0.85 |
| Succinate dehydrogenase (SDH) | 4.19±0.32 | 3.69±0.45 | 3.89±0.25 | 4.66±0.46 | 3.38±0.35 | 3.96±0.36 |
| TTFA-sensitive CII | 2.01±0.14 | 1.41±0.19 | 1.79±0.13 | 2.04±0.19 | 1.41±0.14 | 1.88±0.19 |
| TTFA-sensitive CII+Q1 | 9.28±0.58 | 8.39±0.89 | a*8.37±0.43 | 10.18±0.62 | a*7.65±0.51 | 9.09±0.56 |
| Antimycin A-sensitive CIII | 42.44±3.07 | 43.56±2.83 | a*b*39.8±1.62 | 45.63±2.78 | a*b*40.66±1.79 | 45.71±2.85 |
| Cytochrom C Oxidase CIV (k−1/mg protein) |
1.56±0.98 | 1.59±0.16 | 1.81±0.13 | 1.91±0.15 | 1.51±0.96 | 1.76±0.15 |
| Mitochondrial marker Citrate synthase |
52.63±2.69 | 50.89±4.68 | 50.98±2.48 | 52.08±4.34 | a*b*48.95±2.75 | 52.43±5.48 |
| Cytoplasmic marker lactate dehydrogenase (LDH) |
502.1±26.1 | 568.1±43.7 | 514.1±29.2 | 591.7±30.1 | 557.7±29.1 | 580.8±42.5 |
Notes: Data are presented as mean ± standard error. aSignificant difference compared to day 0 time point of the same group; bSignificant difference compared to the AS group at same time point. *P<0.05.
Abbreviations: SEM, standard error of the mean; Rotenone-sensitive CI/III, rotenone-sensitive linked activity of complex I and III; antimycin A-sensitive CII/III, antimycin A-sensitive linked activity of complex II and III; SDH, succinate dehydrogenase; TTFA-sensitive CII, complex II activity as the thenoyltrifluoroacetone (TTFA)-sensitive without CoQ1 or with CoQ1; antimycin A-sensitive CIII, complex III activity; cytochrome c oxidase; CIV, cytochrome c oxidase, complex IV activity; LDH, lactate dehydrogenase.
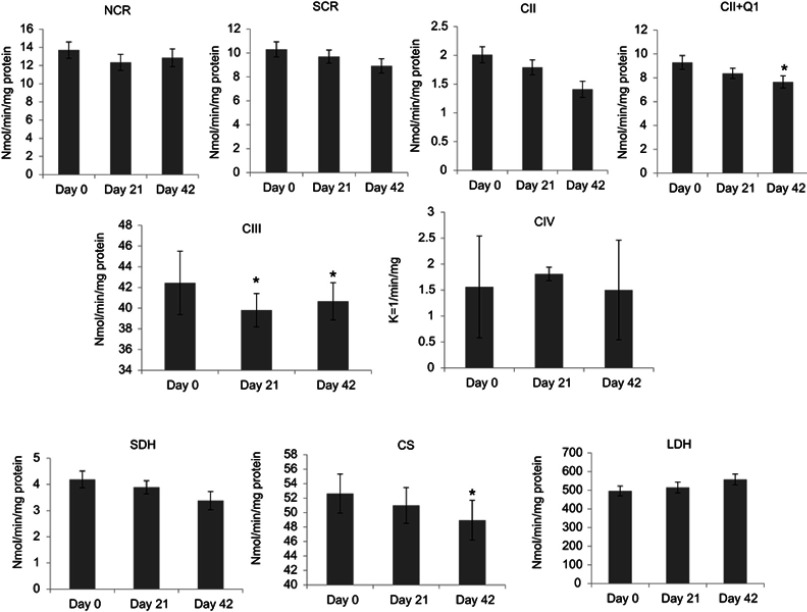
Compared to day 0, complex III activity was significantly decreased (6%, ↓2.64 nmol/min/mg protein, P=0.044) at day 21 in patients receiving XRT. At day 42, there were significantly decreased linked activity of complex II/III (11%, ↓1.38 nmol/min/mg protein, P=0.046), activity of complex II + Q1 (17.5%, ↓1.63 nmol/min/mg protein, P=0.045), complex III activity (5%, ↓1.78 nmol/min/mg protein, P=0.048), and decreased citrate synthase (6%, ↓3.68 nmol/min/mg protein, P=0.041) in mononuclear cell mitochondria in patients undergoing XRT, compared to day 0. Decreased ETC enzymatic activity in mononuclear cells mitochondria of patients undergoing XRT over time are presented in Figure 3.
Figure 3.
Changes in electron-transport chain complex enzymatic activity in mononuclear cells in patients with radiation therapy (N=33).
Notes: CII was measured as TTFA-sensitive DCPIP reductase; total complex II determined with coenzyme Q1 added (CII+Q1); CIII, measured as antimycin A-sensitive decylubiquinol cytochrome c reductase; CS is mitochondrial marker; SDH was used to measure the first 2 subunits of complex II and served as a membrane-bonded mitochondrial marker enzyme; LDH as a cytoplasm marker. *P<0.05.
Abbreviations: NADH, nicotinamide adenine dinucleotide; NCR, rotenone-sensitive NADH cytochrome c reductase; SCR, antimycin A-sensitive succinate cytochrome c reductase; CII, complex II activity; TTFA, thenoyltrifluoroacetone; DCPIP, dichlorophenol-indophenol; CII+Q1, complex II with coenzyme Q1; CIII, complex III activity, measured as antimycin A-sensitive decylubiquinol cytochrome c reductase; CIV, complex IV (cytochrome c oxidase) activity; CS, citrate synthase, mitochondrial marker; SDH, succinate dehydrogenase; LDH, lactate dehydrogenase.
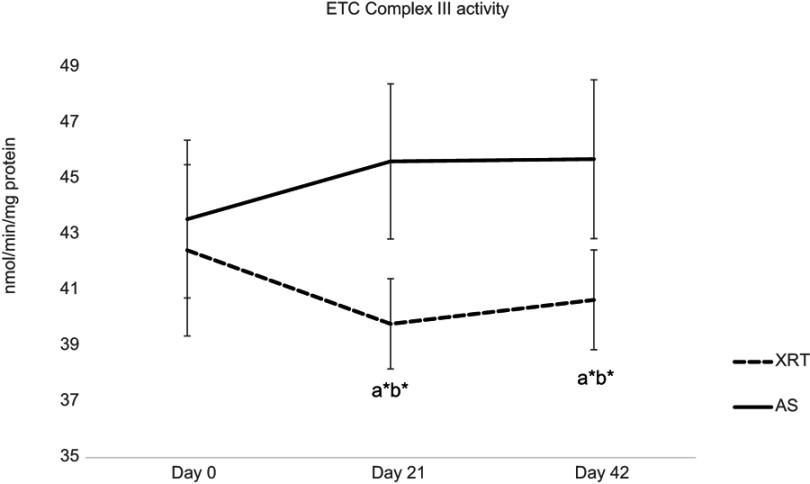
Compared to the AS group, the activity of complex II + Q1 (18%, ↓1.81 nmol/min/mg protein, P=0.058), complex III (13%, ↓5.05 nmol/min/mg protein, P=0.041), and lactate dehydrogenase (13%, ↓77.6 nmol/min/mg protein, P=0.065) were lower in mononuclear cells at day 21 in patients undergoing XRT. At day 42, there was significantly lower activity of complex II/III (19%, ↓2.09 nmol/min/mg protein, P=0.04), complex II + Q1 (15%, ↓1.44 nmol/min/mg protein, P=0.045), complex III activity (11%, ↓5.05 nmol/min/mg protein, P=0.04), and citrate synthase (7%, ↓3.48 nmol/min/mg protein, P=0.047) in mononuclear cells from patients undergoing XRT, compared to AS group (Table 3). Complex IV activity was not different in either group at any timepoint. Therefore, complex III activity was significantly decreased (t=1.78–2.64, P=0.045) over time in patients undergoing XRT, and significantly different from that of AS patients (t=5.05–5.83, P=0.041, Figure 4).
Figure 4.
Changes in ETC complex III between patients with radiation therapy (N=33) and active surveillance (N=15).
Notes:Y-axis represents cell enzyme activity (nmol/min/mg protein). ETC CIII enzymatic activity, which was measured as antimycin A-sensitive decylubiquinol cytochrome c reductase. ETC CIII activity was significantly decreased at day 21 (t=−2.64, P=0.044) and day 42 (t=−1.78, P=0.047) in patients with XRT; additionally, there was a significant difference in CIII activity between XRT and AS groups over time. aRefers to a significant difference compared to day 0 time point of the same group. bRefers to a significant difference compared to the AS group at the same time point. *P<0.05.
Abbreviations: ETC, electron transport chain; CIII, complex III activity; XRT, external beam radiation; AS, active surveillance.
Expression of BCS1L gene
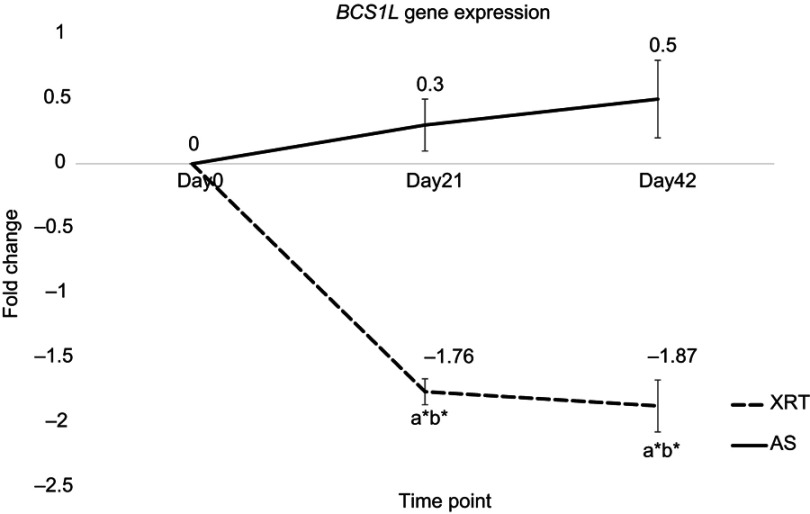
There was no significant change in the expression of BCS1L gene in patients with AS over time. In peripheral mononuclear cells, the expression of BCS1L gene was significantly downregulated in patients undergoing XRT at day 21 (fold change =−1.76, P=0.03) and day 42 (fold change =−1.87, P=0.02) compared to day 0 (Figure 5). Additionally, significantly different BCS1L gene expression in mononuclear cells was observed between XRT and AS groups at day 21 and day 42, as presented in Figure 5.
Figure 5.
Differences in gene expression of BCS1L at day 21 and day 42 between patients undergoing radiation therapy (XRT, N=33) and active surveillance (AS, N=13).
Notes: Left figure indicates the expression of BCS1L gene. Y-axis represents expression values assayed by monitoring in real-time polymerase chain reaction calculated as fold change. The expression of BCS1L was significant different between patients undergoing XRT and AS (P<0.05). Furthermore, BCS1L was significantly downregulated at day 21 (fold change=−1.76, P=0.03) and day 42 (fold change=−1.87, P=0.02) in XRT patients compared to day 0. aRefers to a significant difference compared to day 0 time point of the same group. bRefers to a significant difference compared to the AS group at the same time point. *P<0.05.
Abbreviations: BCS1L, BC1 (ubiquinol-cytochrome c reductase) synthesis-like; XRT, external beam radiation therapy; AS, active surveillance.
Increased fatigue was significantly associate with down-regulated BCS1L gene at midpoint (r=−0.18, P=0.045) and endpoint (r=−0.31, P=0.039), decreased OXPHOS-complex III oxidation at midpoint (r=−0.12, P=0.044), and decreased ETC-complex III activity at endpoint (r=−0.09, P=0.047) in patients undergoing XRT. In addition, downregulated BCS1L was associated with decreased OXPHOS-complex III oxidation (r=0.38, P=0.054) and decreased activity of complex III (r=0.29, P=0.058). There was no significant association between fatigue, BCS1L, and mitochondrial function at any time point in patients undergoing AS.
Discussion
Our major findings include: (1) a significant difference in fatigue scores at the midpoint and endpoint between XRT and AS groups; (2) compared to AS group, there is a significant difference in changes of OXPHOS, ETC activity, and BCS1L gene expression over time in patients receiving XRT; (3) there are significantly decreased OXPHOS-complex III oxidation, decreased activity of complex III, and downregulated BCS1L gene expression in peripheral mononuclear cells at midpoint and endpoint in patients receiving XRT, compared to their baseline; and (4) worsened fatigue symptoms are associated with decreased complex III oxidation and downregulation of BCS1L. This evidence is the first to our knowledge to describe the association between the expression of the mitochondrial gene, BCS1L, integrative mitochondrial function, ETC enzymatic activity, and patient-reported fatigue in men receiving XRT for prostate cancer, compared to patients with AS.
Our findings confirm our earlier report on a defect in OXPHOS complex III activity at week three of XRT,42 the defect in oxidation starting at complex III affected upstream complex II oxidation, but not complex I or fatty acid oxidation. There was no change in oxidation for both coupled and uncoupled complex I over time, and uncoupled complex IV oxidation rate was not different at day 21 and day 42 compared to day 0. Therefore, we propose that the activity of complex I limited both uncoupled complex I oxidation and fatty acid oxidation and these rates are not affected by the defect in complex III.
To determine whether OXPHOS was limited by the oxidation component or the phosphorylation component, we uncoupled oxidation from phosphorylation additionally, the uncoupled oxidation rate represents the reserve capacity. For example, coupled complex I-linked activity was identical to uncoupled complex I activity, a reflection of the fact that the limiting step for OXPHOS starting at complex I was oxidation. Uncoupled complex II oxidation was increased compared to coupled complex II-linked activity, indicating that the limiting step in OXPHOS starting at complex II was phosphorylation (Table 2). For complex III, there was no significant difference in oxidation rates between coupled and uncoupled respiration at each time point in patients undergoing XRT, showing no reserve capacity in peripheral blood mononuclear cell mitochondria in patients with prostate cancer. This observation indicates that the limiting step for OXPHOS starting at complex III was oxidation. The UCR is a reflection of the ratio of the respiratory capacity of mitochondria to the rate of oxidative phosphorylation.53,57 There was little difference in the maximum oxidative capacity because the UCR was close to 1 (1.1 in protocol 1 and 0.96 in protocol 2).
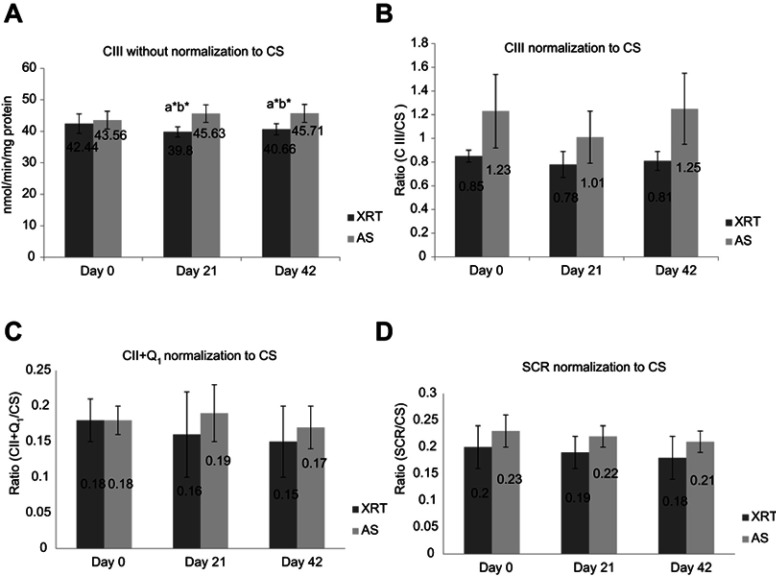
Mitochondrial OXPHOS provides screening of systemic mitochondrial dysfunction; ETC enzymatic activity further assists in identifying the specific complex defects.55,63 In support of a defect in OXPHOS complex III oxidation, the activity of ETC complex III enzyme in mononuclear cells mitochondria was significantly decreased at day 21 and day 42 in patients undergoing XRT compared to day 0. There was no change in ETC complex IV activity in patients undergoing XRT over time (Figure 3), consistent with no change in uncoupled complex IV oxidation and a defect in complex III oxidation (Figure 2) in mononuclear cells of XRT patients. Citrate synthase activity, a marker of mitochondrial content, was significantly decreased at the completion of XRT (day 42), suggesting that mitochondrial content was suppressed by repeated daily irradiation. To determine if decreased activity of complex III, II/III, and complex II+Q1 are associated with the number of mitochondria, we compared the difference in complex III activity with and without the normalization of citrate synthase (Figure 6A and B), and succinate cytochrome c reductase (SCR) (Figure 6C) and complex II + Q1 SCR (Figure 6D) normalized to CS. After normalization to citrate synthase, there is a trend for the ratio of complex III activity to be decreased, but the P-value (P=0.056–0.059) does not reach significance. A discrepancy of citrate synthase activity was observed between samples from the O2K chambers (cell suspension, mU/106 cells) and the ETC assay (mononuclear cell pellet, mU/mg protein), although the protein concentration was not significantly different (P>0.05) at any time point in either the OXPHOS assay (Table 2) or the ETC assay (Table 3).
Figure 6.
ETC complex III activity without and with normalization of citrate synthase (A and B) and CII+Q1 (C) and SCR (D) with normalization of citrate synthase between patients undergoing XRT (N=35) and AS (N=15). (A) CIII without normalization of CS; (B) CIII with normalization of CS; (C) CII+Q1 with normalization of CS; (D) SCR with normalization of CS.
Notes:aSignificant difference compared to day 0 time point of the same group. bSignificant difference compared to the AS group at the same time point. *P<0.05. CIII, measured as antimycin A-sensitive decylubiquinol cytochrome c reductase; CII, measured as the TTFA-sensitive succinate-2,6 DCPIP reductase and total CII determined with coenzyme Q added (CII+Q1); SCR, antimycin A-sensitive succinate cytochrome c reductase was used to measure the linked activity of CII and CIII.
Abbreviations: CIII, complex III activity; CS, citrate synthase; CII, complex II activity; TTFA, thenoyltrifluoroacetone; DCPIP, dichlorophenol-indophenol; CII+Q1, complex II with coenzyme Q1; SCR, succinate cytochrome c reductase; XRT, patients undergoing radiation therapy; AS, patients undergoing active surveillance.
Consistent with our previous finding,21,41 the expressions of BCS1L was decreased in peripheral mononuclear cells at day 21 and remained downregulated at the end of XRT in patients with prostate cancer. BCS1L gene encodes protein that is a chaperone for the Rieske iron-sulfur protein into complex III; genetically when BCS1L gene is downregulated that is associated with a defect in complex III.47 Decreased BCS1L protein has been shown to lead to decreased incorporation of the Rieske iron-sulfur protein into complex III in Escherichia coliyeast, and human cells, resulting in decreased complex III activity.47,64,65 A defect in complex III leads to a functional deficit in the respiratory chain and impairs ATP production.66 Decreased OXPHOS complex III activity and downregulation of BCS1L expression were significantly associated with increased fatigue in patients undergoing XRT for prostate cancer.
The trajectory of CRF reported by patients undergoing XRT is consistent with our previous findings21,41 and with other studies.19,20,67 This progression suggests that the pathophysiological mitochondrial mechanism related to daily irradiation is likely involved in the worsening of CRF. Therefore, we suggest that prevention through implementing interventions for XRT-associated fatigue should start early.
Limitations
This study was conducted in a tertiary research setting with a selected patient population; therefore, the results may not apply generally. These hypothesis-testing findings demonstrate an association of bioenergetics gene, integrated mitochondrial function, ETC enzymatic activity, and fatigue intensification during XRT in patients with prostate cancer, but do not prove causation. In addition, the variability of specific peripheral mononuclear cell populations may influence OXPHOS and ETC activity. Another limitation of this study is the lack of measurement of variables that might affect CRF such as cognitive function, sleep disturbance, and physical activity.
Conclusion
Our findings provide evidence that XRT triggers genetic and cellular instability, specifically, downregulated BCS1Lgene, leading to a defect in complex III activity of mitochondrial OXPHOS, which is associated with worsen fatigue experienced by patients with prostate cancer undergoing XRT. We suggest that mononuclear cells BCS1L and complex III are potential biomarkers for CRF. Moreover, BCS1L and complex III are potential targets for pharmacological and, in particular, nutraceutical interventions. For example, administration of coenzyme Q and ascorbate to bypass complex III might improve radiation-induced fatigue.
Acknowledgments
This study is supported by the National Institute of Nursing Research, National Institutes of Health (K01 NR015246, 2015-2018); the Oncology Nursing Society Foundation, Pittsburg, PA (RES125833, 2014–2015); and the Clinical and Translational Science Collaborative Core Utilization Pilot Grant and the Center for Mitochondrial Disease, Case Western Reserve University. The patients generously participated in the study and this is greatly appreciated. The authors appreciated the editorial assistance by Bernard Tandler, PhD.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Cancer Society. Cancer facts & figures 2019-American Cancer Society; 2019. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed February16, 2019.
- 2.Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V. Prostate cancer: ESMO consensus conference guidelines 2012. Ann Oncol, 2013;24(5):1141–1162 doi: 10.1093/annonc/mds624 [DOI] [PubMed] [Google Scholar]
- 3.Langston B, Armes J, Levy A, Tidey E, Ream E. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer. 2013;21(6):1761–1771. [DOI] [PubMed] [Google Scholar]
- 4.Brix C, Schleussner C, Fuller J, Rohrig B, Strauss B. [Fatigue and its determinants in radio-oncology]. Psychother Psychosom Med Psychol. 2009;59(2):42–49. doi: 10.1055/s-2008-1067341 [DOI] [PubMed] [Google Scholar]
- 5.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivor. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XS. Pathophysiology of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 Suppl):11–20. doi: 10.1188/08.CJON.S2.11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minton O, Berger A, Barsevick A, et al. Cancer‐related fatigue and its impact on functioning. Cancer. 2013;119(S11):2124–2130. doi: 10.1002/cncr.28058 [DOI] [PubMed] [Google Scholar]
- 8.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danjoux C, Gardner S, Fitch M. Prospective evaluation of fatigue during a course of curative radiotherapy for localised prostate cancer. Support Care Cancer. 2007;15(10):1169–1176. doi: 10.1007/s00520-007-0229-8 [DOI] [PubMed] [Google Scholar]
- 10.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol. 2015;1(7):931–941. doi: 10.1001/jamaoncol.2015.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. Cancer-Related Fatigue. National Compr Cancer Network Pract Guide Oncol. 2018;1;1–64. [Google Scholar]
- 12.Piper BF, Cella D. Cancer-related fatigue: definitions and clinical subtypes. J Natl Compr Canc Netw. 2010;8(8):958–966. [DOI] [PubMed] [Google Scholar]
- 13.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4 [DOI] [PubMed] [Google Scholar]
- 14.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33(1):E18–E26. doi: 10.1188/06.ONF.E18-E26 [DOI] [PubMed] [Google Scholar]
- 15.Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw. 2008;6(1):3–13. [DOI] [PubMed] [Google Scholar]
- 16.Monga U, Kerrigan AJ, Thornby J, Monga TN. Prospective study of fatigue in localized prostate cancer patients undergoing radiotherapy. Radiat Oncol Investig. 1999;7(3):178–185. doi: [DOI] [PubMed] [Google Scholar]
- 17.Pinto BM, Dunsiger S, Waldemore M. Physical activity and psychosocial benefits among breast cancer patients. Psycho-Oncol. 2013;22(10):2193–2199. doi: 10.1002/pon.3272 [DOI] [PubMed] [Google Scholar]
- 18.Feng LR, Wolff BS, Lukkahatai NP, Espina AB, Saligan LN. Exploratory investigation of early biomarkers for chronic fatigue in prostate cancer patients following radiation therapy. Cancer Nurs. 2017;40(3):184–193. doi: 10.1097/NCC.0000000000000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conaglen HM, de Jong D, Hartopeanu C, Conaglen JV, Tyrie LK. The effect of high dose rate brachytherapy in combination with external beam radiotherapy on men’s health-related quality of life and sexual function over a 2 year time span. Clin Oncol (R Coll Radiol). 2013;25(3):197–204. doi: 10.1016/j.clon.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 20.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35(6):632–643. doi: 10.1016/j.jpainsymman.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao C-P, Wang D, Kaushal A, Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs. 2013;36(3):189–197. doi: 10.1097/NCC.0b013e318263f514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St Pierre BA, Kasper CE, Lindsey AM. Fatigue mechanisms in patients with cancer: effects of tumor necrosis factor and exercise on skeletal muscle. Oncol Nurs Forum. 1992;19(3):419–425. [PubMed] [Google Scholar]
- 23.Monga U, Jaweed M, Kerrigan AJ, et al. Neuromuscular fatigue in prostate cancer patients undergoing radiation therapy. Arch Phys Med Rehabil. 1997;78(9):961–966. [DOI] [PubMed] [Google Scholar]
- 24.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22 [DOI] [PubMed] [Google Scholar]
- 25.Yavuzsen T, Davis MP, Ranganathan VK, et al. Cancer-related fatigue: central or peripheral? J Pain Symptom Manage. 2009;38(4):587–596. doi: 10.1016/j.jpainsymman.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Saligan LN, Hsiao CP, Wang D, et al. Upregulation of alpha-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain Behav Immun. 2013;27(1):63–70. doi: 10.1016/j.bbi.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orre IJ, Reinertsen KV, Aukrust P, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71(3):136–141. doi: 10.1016/j.jpsychores.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 30.Okunieff P, Chen Y, Maguire DJ, Huser AK. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27(3):363–374. doi: 10.1007/s10555-008-9138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voss JG, Dodd M, Portillo C, Holzemer W. Theories of fatigue: application in HIV/AIDS. J Assoc Nurses AIDS Care. 2006;17(1):37–50. doi: 10.1016/j.jana.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Wang X. Pathophysiology of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 Suppl):11–20. doi: 10.1188/08.CJON.S2.11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26(5):699–705. doi: 10.1016/j.bbi.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol. 2005;69(1):85–96. doi: 10.1016/j.biopsycho.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Geinitz H, Zimmermann FB, Stoll P, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):691–698. doi: 10.1016/S0360-3016(01)01657-1 [DOI] [PubMed] [Google Scholar]
- 38.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. [DOI] [PubMed] [Google Scholar]
- 39.Reinertsen KV, Grenaker Alnaes GI, Landmark-Høyvik H, et al. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav Immun. 2011;25(7):1376–1383. doi: 10.1016/j.bbi.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 40.Hsiao CP, Wang D, Kaushal A, Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nur. 2012;36(3):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsiao C-P, Wang D, Kaushal A, Chen M-K SL. Differential expression of genes related to mitochondrial biogenesis and bioenergetics in fatigued prostate cancer men receiving external beam radiation therapy. J Pain Symptom Manage. 2014;48(6):1080–1090. doi: 10.1016/j.jpainsymman.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiao C-P, Chen M-K, Daly B, Hoppel C. Integrated mitochondrial function and cancer-related fatigue in men with prostate cancer undergoing radiation therapy. Cancer Manag Res. 2018;10:6367–6377. doi: 10.2147/CMAR.S185706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao C-P, Daly B, Hoppel C. Association between mitochondrial bioenergetics and radiation-related fatigue: a possible mechanism and novel target. Archives in Cancer Research. 2015;3(2):14. doi: 10.21767/2254-6081.100014 [DOI] [Google Scholar]
- 44.Hsiao CP, Hoppel C. Analyzing mitochondrial function in human peripheral blood mononuclear cells. Anal Biochem. 2018;549:12–20. doi: 10.1016/j.ab.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis RE, Williams M. Mitochondrial function and dysfunction: an update. J Pharmacol Exp Ther. 2012;342(3):598–607. doi: 10.1124/jpet.112.192104 [DOI] [PubMed] [Google Scholar]
- 46.Puchowicz MA, Varnes ME, Cohen BH, Friedman NR, Kerr DS, Hoppel CL. Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria–case studies. Mitochondrion. 2004;4(5–6):377–385. doi: 10.1016/j.mito.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 47.Hinson JT, Fantin VR, Schönberger J, et al. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N Engl J Med. 2007;356(8):809–819. doi: 10.1056/NEJMoa055262 [DOI] [PubMed] [Google Scholar]
- 48.Mandelker L. Introduction to oxidative stress and mitochondrial dysfunction. Vet Clin North Am Small Anim Pract. 2008;38(1):1–30. doi: 10.1016/j.cvsm.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 49.Piper BF. Piper fatigue scale available for clinical testing. Oncol Nurs Forum. 1990;17(5):661–662. [PubMed] [Google Scholar]
- 50.Stover AM, Reeve BB, Piper BF, et al. Deriving clinically meaningful cut-scores for fatigue in a cohort of breast cancer survivors: a Health, Eating, Activity, and Lifestyle (HEAL) Study. Qual Life Res. 2013;22(9):2279–2292. doi: 10.1007/s11136-013-0360-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ. A randomized, placebo-controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(5):528–535. doi: 10.1001/archotol.134.5.528 [DOI] [PubMed] [Google Scholar]
- 53.Ye F, Hoppel CL. Measuring oxidative phosphorylation in human skin fibroblasts. Anal Biochem. 2013;437(1):52–58. doi: 10.1016/j.ab.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 54.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80(1):71–77. doi: 10.1172/JCI113066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krahenbuhl S, Chang M, Brass EP, Hoppel CL. Decreased activities of ubiquinol: ferricytochromec oxidoreductase (complex III) and ferrocytochrome c: oxygenoxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem. 1991;266(31):20998–21003. [PubMed] [Google Scholar]
- 56.Krähenbühl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230(2):177–187. doi: 10.1016/0009-8981(94)90270-4 [DOI] [PubMed] [Google Scholar]
- 57.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3 [DOI] [PubMed] [Google Scholar]
- 58.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 59.Hoppel C, Cooper C. An improved procedure for preparation of inner membrane vesicles from rat liver mitochondria by treatment with digitonin. Arch Biochem Biophys. 1969;135(1):173–183. [DOI] [PubMed] [Google Scholar]
- 60.Krahenbuhl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230(2):177–187. [DOI] [PubMed] [Google Scholar]
- 61.Slater EC. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochem J. 1949;44(3):305. doi: 10.1042/bj0440305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazure KE, Lydiatt WM, Denman D, Burke WJ. Association between depression and survival or disease recurrence in patients with head and neck cancer enrolled in a depression prevention trial. Head Neck. 2009;31(7):888–892. doi: 10.1002/hed.v31:7 [DOI] [PubMed] [Google Scholar]
- 63.Puchowicz MA, Varnes ME, Cohen BH, Friedman NR, Kerr DS, Hoppel CL. Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria—case studies. Mitochondrion. 2004;4(5):377–385. doi: 10.1016/j.mito.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 64.Borisov VB, Liebl U, Rappaport F, et al. Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: a photoselection study using femtosecond spectroscopy. Biochemistry. 2002;41(5):1654–1662. [DOI] [PubMed] [Google Scholar]
- 65.Meunier B, Fisher N, Ransac S, Mazat JP, Brasseur G. Respiratory complex III dysfunction in humans and the use of yeast as a model organism to study mitochondrial myopathy and associated diseases. Biochim Biophys Acta. 2013;1827(11–12):1346–1361. doi: 10.1016/j.bbabio.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 66.Hinson JT, Fantin VR, Schonberger J, et al. Missense mutations in the BCS1L gene as a cause of the Bjornstad syndrome. N Engl J Med. 2007;356(8):809–819. doi: 10.1056/NEJMoa055262 [DOI] [PubMed] [Google Scholar]
- 67.Feng L, Chen M-K, Lukkahatai N, et al. Clinical predictors of fatigue in men with non-metastatic prostate cancer receiving external beam radiation therapy. Clin J Oncol Nurs. 2015;19(6):744–750. doi: 10.1188/15.CJON.744-750 [DOI] [PubMed] [Google Scholar]