Abstract
Rationale
Although psilocybin and dextromethorphan (DXM) are hallucinogens, they have different receptor mechanisms of action and have not been directly compared.
Objective
This study compared subjective, behavioral and physiological effects of psilocybin and dextromethorphan under conditions that minimized expectancy effects.
Methods
Single, acute oral doses of psilocybin (10, 20, 30 mg/70kg), DXM (400 mg/70kg), and placebo were administered under double-blind conditions to 20 healthy participants with histories of hallucinogen use. Instructions to participants and staff minimized expectancy effects. Various subjective, behavioral, and physiological effects were assessed after drug administration.
Results
High doses of both drugs produced similar increases in participant ratings of peak overall drug effect strength, with similar times to maximal effect and time-course. Psilocybin produced orderly dose-related increases on most participant-rated subjective measures previously shown sensitive to hallucinogens. DXM produced increases on most of these same measures. However the high dose of psilocybin produced significantly greater and more diverse visual effects than DXM including greater movement and more frequent, brighter, distinctive, and complex (including textured and kaleidoscopic) images and visions. Compared to DXM, psilocybin also produced significantly greater mystical-type and psychologically insightful experiences, and greater absorption in music. In contrast, DXM produced larger effects than psilocybin on measures of disembodiment, nausea/emesis, and lightheadedness. Both drugs increased systolic blood pressure, heart rate, and pupil dilation, and decreased psychomotor performance and balance.
Conclusions
Psilocybin and DXM produced similar profiles of subjective experiences, with psilocybin producing relatively greater visual, mystical-type, insightful, and musical experiences, and DXM producing greater disembodiment.
Keywords: Psilocybin, Dextromethorphan, Hallucinogen, Psychedelic, Subjective experience, Mystical experience, Insightful experience, Humans
Introduction
Classic and atypical hallucinogens are a chemically and mechanistically diverse group of compounds that produce unique changes in thoughts, perceptions, and emotions, often including alterations in the perception of reality and meaning (MacLean et al. 2015). In the general population, use of hallucinogens, as a broad class has been relatively stable over the past decade, with lifetime use of 18.6% among young adults in 2015 (SAMHSA 2015, 2016).
Psilocybin and psilocin are the principal psychoactive components in Psilocybe mushrooms (Tyls et al. 2014). After oral or intravenous administration, psilocybin is rapidly metabolized to psilocin (Hasler et al. 1997; Brown et al. 2017). Similar to other classic hallucinogens (d-lysergic acid [LSD], mescaline, and N,N-dimethyltryptamine [DMT]), psilocin effects are primarily mediated via serotonin (5-HT) receptors, predominantly the 5-HT2A receptor as well as 5-HT1A and 5-HT 2C receptors (Nichols 2016; Rickli et al. 2016). Extensive clinical studies dating back to the 1950s have characterized the acute subjective, cognitive, and physiological effects of psilocybin (Tyls et al. 2014; Passie et al. 2002; Vollenweider et al. 1998; Halser et al. 2004; Griffiths et al. 2006, 2011).
Dextromethorphan (DXM) and its metabolite dextrorphan are noncompetitive NMDA (N-methyl-D-aspartic acid) receptor antagonists with other diverse pharmacological actions including interactions at serotonin transporters, 5-HT1B/1D receptors, noradrenaline transporters, and sigma-1 receptors (Nguyen et al. 2016; Taylor et al. 2016). DXM is widely available as an over-the-counter medication commonly used as a cough suppressant at doses of about 30 mg (e.g. Robitussin®). However, DXM is also sometimes used at high doses (e.g., ≥300 mg) as an atypical hallucinogen (Banken and Foster 2008; Morris and Wallach 2014) similar to the more commonly known dissociative anesthetics ketamine and phencyclidine, which are also NMDA antagonists.
Recent studies suggest that similarities in the perceptual, cognitive, and mood altering effects of classic hallucinogens and dissociative anesthetic hallucinogens may involve underlying common mechanisms of action including interactions of serotonergic and glutamatergic neurotransmitter systems (Aghajanian and Marek 1999; Fantegrossi et al. 2008; Nichols 2004; Vollenweider and Kometer 2010). For example, preclinical research suggests that group II metabotropic glutamate receptors (mGluR2/3) may be potential indirect target sites for mediating hallucinogenic effects for both classic and dissociative anesthetic hallucinogens, where mGlurR2/3 antagonists potentiate such effects (Gonzalez-Maeso et al. 2007, 2008; Delille et al. 2012; Moreno et al. 2011; Carbonaro et al. 2015; Fribourg et al. 2011; Winter et al. 2004, Winter 2009).
Consistent with a possible shared mechanism of action and with the classification of psilocybin and DXM as hallucinogens, a recent laboratory study (Reissig et al. 2012) showed that high doses of DXM in the range commonly producing hallucinogenic effects (≥400 mg/70 kg) produced a profile of subjective effects similar to those produced by psilocybin in other studies (Griffiths et al. 2006, 2011). Although no studies have directly compared the subjective experiences of psilocybin and DXM, one double-blind study comparing intravenous DMT and ketamine (Gouzoulis-Mayfrank et al. 2005) and several non-blinded comparisons between oral or intravenous psilocybin and intravenous ketamine (Studerus et al. 2012; Schmidt et al. 2013; Schartner et al. 2017) suggest greater visual effects after the classic hallucinogens than after ketamine, but greater experiences of disembodiment or catatonia-like signs after ketamine.
The aim of the present study was to directly compare the effects of psilocybin and DXM in the same participants. Single acute doses of DXM (400 mg/70 kg), psilocybin (10, 20, and 30 mg/70 kg), and placebo were administered to 20 hallucinogen-experienced volunteers under double-blind conditions. A range of participant-rated, behavioral, and physiological outcome measures were examined with particular attention given to assessing measures reflecting alterations in subjective experience. Because the effects of hallucinogens are strongly influenced by participant expectations (Griffiths et al. 2006; Metzner et al.1965; Preller and Vollenweider 2016) procedures and instructions to participants and staff were designed to minimize such effects. To reduce variability of results that might occur because of differing histories of drug use, only participants with histories of use of both classic hallucinogens and dissociative hallucinogens were enrolled.
Methods
Participants
The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. Participants gave their written informed consent before beginning the study procedures and were paid for their participation. Thirty-nine potential participants were screened. One dropped out of the study for personal reasons after completing the first session. The 20 participants who completed the study (11 females) had a mean age of 28.5 years (range = 22–43 years). All were medically and psychologically healthy and had a history of psychedelic drug use, both use of classic hallucinogens (mean = 60.9 uses; range 16–183) and dissociative anesthetic hallucinogens (mean = 19.0; range = 1–154). Nineteen participants were Caucasian (95%) and one was Asian American. Half of the participants had a bachelor’s degree or higher, 20% had an Associate’s degree, and 30% had a high school diploma as their highest level of education. The majority of participants (65%) were never married, 20% were married or living with their partner, and 15% were divorced or separated.
Individuals were excluded from participation if they had a history of substance dependence according to DSM-IV-TR criteria (excluding nicotine and caffeine), were pregnant or nursing, had a current significant medical condition. A detailed psychiatric history was taken during the screening interview to exclude individuals with a personal or immediate family history of schizophrenia, bipolar affective disorder, delusional disorder, paranoid disorder, or schizoaffective disorder.
General procedures
Throughout the study, general safety guidelines applicable to the study of high doses classic hallucinogens were observed (Johnson et al. 2008). Sessions took place in an esthetic living room-like environment. For most of the time during the sessions, participants were instructed to lie down on a couch while wearing an eyeshade and headphones through which a program of classical and world music was played. Participants were encouraged to focus their attention on their inner experiences while not engaged in experimental tasks.
After completing screening, eligible individuals participated in five experimental sessions lasting about 7 h each and, a final follow-up session. Sessions were separated by at least 48 h, but usually by about a week.
To minimize expectancy effects, participants were informed both verbally and in the consent form that during the study, they could receive placebo or doses of 38 psychoactive drugs from a variety of drug classes. Psilocybin and DXM were among the drugs listed. Participants were told that in at least one session they would receive a hallucinogen from the list, either a classic hallucinogen or a dissociative anesthetic hallucinogen. Staff monitoring drug sessions received identical instructions, with the only exception being that one of the 10 monitors was not blind to the drugs received during the experimental design; however, this monitor remained blinded to the order of drug conditions.
The participant met with their session monitors on two occasions before the first drug session (about 8 h total meeting time). The purpose of the meetings was to develop rapport and trust with participants and minimize the risk of adverse drug reactions (Johnson et al. 2008). During these meetings, participants also practiced the experimental tasks. Participants also met with their session monitors one to two days after each experimental session either in-person or over the telephone, as well as a one-week follow-up (in-person) after the fifth session.
Participants were instructed that on experimental session mornings, they should consume low-fat breakfast and their usual amount of caffeine before arriving at the laboratory. They were told to refrain from using any drugs other than non-psychoactive non-prescription analgesic, tobacco, and caffeinated products while enrolled in the study. On each session before drug administration, all participants’ urine was tested for a panel of commonly abused drugs, and female participants’ urine was tested for pregnancy. Negative results were required to proceed.
Various measures were assessed before capsule administration, repeatedly after administration, and about 7 h after capsule administration when acute drug effects had resolved, as described below.
During the study, participants received psilocybin (10, 20, and 30 mg/70 kg), dextromethorphan HBr (400 mg/70 kg expressed as the base), and placebo (lactose or microcrystalline cellulose) using a within-subject, crossover design. The sequence of drug conditions was balanced across participants and the assignment to sequence was randomized. At the one-month follow-up session, participants received 25 mg/70kg DXM to assess metabolism. Drug and placebo doses were prepared in identically appearing opaque, size 0 gelatin capsules, with lactose or microcrystalline cellulose as the inactive capsule filler. On each session, two capsules were administered with approximately 100 ml water.
Measures assessed during the session
Blood pressure, heart rate, and pupil diameter
Blood pressure and heart rate were assessed with a blood pressure cuff place on the arm (Non-Invasive Patient Monitor Model 507E; Criticare Systems, Inc., Waukesha, WI, USA). These assessments occurred approximately ten minutes before and 30, 60, 90, 120, 180, 240, 300, and 360 minutes after capsule administration. Pupil diameter was measured using a pupilometer (VIP-200 Pupilometer, Neuroptics Inc., Irvine, CA Duke et al. 2011) at 120, 240, and 360 minutes after capsule administration.
Monitor Rating Questionnaire
At the same time points at which the physiological measures were obtained, the two session monitors completed the Monitor Rating Questionnaire, which involved rating or scoring several dimensions of the participant’s behavior or mood. The dimensions that are expressed as peak scores in Table 1, were rated on a five-point scale from 0 to 4. Data were the mean of the two monitors rating at each time point.
Table 1.
Peak physiological effects, behavioral effects, and monitor ratings assessed throughout the session
| Rated description | Placebo | Psilocybin dose (mg/70 kg) | Dextromethorphan dose (mg/70 kg) | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 400 | |
| Physiological Measures (peak effects) | |||||
| Systolic blood pressure (mm Hg) | 124.70 (2.81) | 138.05 (4.18)a | 142.05 (3.11)a | 140.45 (3.74)a | 143.70 (3.58) |
| Diastolic blood pressure (mm Hg) | 78.65 (2.36) | 79.90 (2.54) | 84.75 (2.04) | 86.65 (2.35) | 85.30 (2.03) |
| Heart rate (beats per minute) | 82.25 (2.4) | 91.5 (3.3)*,a | 90.7 (2.8)*,a | 94.2 (3.6)a | 103.2 (3.9) |
| Pupil diameter (mm) | 5.78 (0.20) | 6.79 (0.19)a | 6.98 (0.17)*,a | 7.05 (0.22)*,a | 6.43 (0.19) |
| Behavioral Tasks (peak minimum effect; min value=0) | |||||
| Circular Lights (number completed) | 76.25 (1.67) | 68.90 (2.56)*,a | 60.95 (3.85)*,a,b | 55.85 (3.83)b | 50.80 (4.72) |
| Balance Task (seconds) | 39.50 (4.89) | 30.35 (4.65)* | 20.85 (4.09)* | 22.40 (4.94)* | 4.40 (1.05) |
| Monitor Ratings (peak effects; max score=4) | |||||
| Overall drug effect | 0.50 (0.12) | 2.30 (0.11 )*,a | 3.00 (0.12)b | 3.30 (0.13)b | 3.12 (0.14) |
| Restless/fidgety | 0.30 (0.08) | 0.70 (0.19)a | 0.85 (0.17)a | 1.08 (0.30)a | 1.20 (0.18) |
| Peace/harmony | 0.68 (0.12) | 1.68 (0.17)a | 1.85 (0.20)a | 2.13 (0.20)a | 1.68 (0.19) |
| Joy/Intense happiness | 0.28 (0.08) | 1.28 (0.19)a | 1.90 (0.21)b | 2.38 (0.22)*,b | 1.75 (0.26) |
| Nausea/vomiting | 0.13 (0.06) | 0.80 (0.14)*,a | 0.73 (0.15)*,a | 1.23 (0.23)*,a | 2.50 (0.27) |
| Yawning | 1.18 (0.19) | 1.75 (0.25)*,a | 1.88 (0.38)*,a | 2.30 (0.33)*,a | 0.70 (0.22) |
| Tearing/crying | 0.05 (0.05) | 0.55 (0.19)a | 1.08 (0.27)*,a | 0.85 (0.28)*,a | 0.10 (0.06) |
| Deep relaxation/drowsiness | 2.40 (0.21) | 2.03 (0.14) | 2.05 (0.15) | 2.13 (0.15) | 2.20 (0.17) |
| Unresponsive to questions | 0.15 (0.13) | 0.00 (0.00) | 0.13 (0.06) | 0.23 (0.12) | 0.25 (0.11) |
| Anxiety or fearfulness | 0.18 (0.07) | 0.25 (0.09) | 0.53 (0.17) | 0.55 (0.15) | 0.60 (0.12) |
| Systemized delusions of reference/paranoid thinking | 0.00 (0.00) | 0.03 (0.03) | 0.08 (0.04) | 0.13 (0.07) | 0.10 (0.06) |
Data are mean scores with 1 SEM shown in parentheses (N= 20); within a row, bold font indicates significant difference from 0 mg/70 kg; for psilocybin doses, values not sharing a common letter are significantly different (Fisher’s LSD p < 0.05)
indicates significant difference from 400 mg/70 kg dextromethorphan
Circular Lights and Balance
These behavioral tasks were completed before capsule administration and at 120, 240, and 360 min after administration. The Circular Lights task is a hand-eye coordination task (Mumford et al. 1995). The score was the number of correct presses (i.e., lights extinguished) in 60 s. The balance task (Carter et al. 2006) involved balancing on one foot with eyes closed. The score was the number of seconds summed across both feet (60 s total).
Subjective Effects Questionnaire
Participants completed this questionnaire before capsule administration and 60, 120, 180, 240, 300, 360, and approximately 480 minutes after capsule administration. It consisted of 19 items (e.g., “overall drug effect”, “light-headed/dizzy”). Participants were instructed to rate how they felt at the current time on a scale from 0 (none) to 10 (strongest imaginable).
Measures assessed at the end of the session, approximately 7 hours after capsule administration
Drug Effect Intensity Rating
Participants were asked to rate the overall drug effect (at peak intensity). This item was rated on a five point scale: 0 = not at all; 1 = slightly; 2 = moderately; 3 = very much; 4 = extremely.
Altered States of Consciousness (5D-ASC)
This questionnaire assesses drug- and non-drug altered states of consciousness (Dittrich 1998). Ninety-four items were rated using a visual analogue scale from 0 – 100. Eleven subscales (Studerus et al. 2010, with English translation by Hasler and Cahn), expressed as a percent of maximum possible score, were scored.
States of consciousness questionnaire
This 100-item questionnaire assessing possible hallucinogen experience content (Griffiths et al. 2006). Thirty items comprise the Mystical Experience Questionnaire (MEQ30), which were shown sensitive to several classic hallucinogens including psilocybin (MacLean et al. 2012; Barrett et al. 2015; Barrett and Griffiths 2017). These items assess four domains of mystical experiences: Mystical, Positive mood, Transcendence of time and space, and Ineffability. Data on each scale were expressed as a percentage of the maximum possible score. As in previous studies (Griffiths et al. 2006), criteria for designating a volunteer as having had a “complete” mystical experience were that scores on each of the scales had to be at least 60%. Because visual effects and the emotional significance of music are commonly reported to occur after psilocybin, 3 individual items were also analyzed separately (see Table 5). The remaining 67 items served as distracters.
Table 5.
Participant ratings of 15 specific visual effects items and one musical engagement item assessed 7 hours after drug administration
| Item Description# | Placebo | Psilocybin dose (mg/ 70 kg) | Dextromethorphan dose (mg/ 70 kg) | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 400 | |
| Visual effects | 2.50 (1.72) | 52.50 (5.71)a | 66.25 (5.52)*,a,b | 80.00 (5.00)*,b | 43.75 (6.25) |
| Room looks different | 0.00 (0.00) | 27.50 (5.71)a | 48.75 (6.14)*,b | 66.25 (5.52)*,c | 26.25 (5.87) |
| Room overlaid with visual patterns | 0.00 (0.00) | 18.75 (5.70)a | 33.75 (7.32)*,b | 55.00 (6.18)*,c | 6.25 (2.48) |
| Visual images, visions, hallucinations | 1.25 (1.25) | 36.25 (6.90)a | 67.50 (6.56)*,b | 71.25 (7.32)*,b | 25.00 (7.69) |
| Kaleidoscopic nature of images, visions, hallucinations | 1.25 (1.25) | 35.00 (6.39)*,a | 63.75 (7.36)*,b | 62.50 (7.80)*,b | 10.00 (5.26) |
| Difference in brightness of visions | 0.00 (0.00) | 21.25 (6.09)a | 43.75 (6.51)*,b | 51.25 (8.21)*,b | 22.50 (5.99) |
| Movement w/in visions, hallucinations | 1.25 (1.25) | 42.50 (6.31)a | 70.00 (4.29)*,b | 66.25 (7.54)*,b | 43.75 (7.00) |
| Change in brightness of objects in room | 1.25 (1.25) | 28.75 (6.35)a | 41.25 (7.09)a,b | 56.25 (8.09)*,b | 25.00 (6.02) |
| Change in visual distinctiveness | 0.00 (0.00) | 17.50 (3.67)a | 32.50 (5.76)b | 51.25 (6.90)*,c | 23.75 (6.14) |
| With eyes open visual field vibrating or jiggling | 0.00 (0.00) | 15.00 (4.59)a | 28.75 (6.09)a,b | 45.00 (8.23)b | 30.00 (7.61) |
| Visual synesthesia | 0.00 (0.00) | 13.75 (4.96)a | 27.50 (7.67)a | 25.00 (8.51)a | 12.50 (5.59) |
| White light | 1.25 (1.25) | 13.75 (3.84)a | 17.50 (6.04)a | 20.00 (6.18)a | 26.25 (6.14) |
| Visions of brilliant white light | 2.00 (2.00) | 14.00 (4.13)a | 17.00 (6.53)a,b | 24.00 (5.35)b | 24.00 (5.54) |
| Visions of abstract geometric colored lines | 1.00 (1.00) | 47.00 (6.03)a | 60.00 (5.98)*,a,b | 74.00 (4.13)*,b | 41.00 (7.03) |
| Increase in the beauty and significance of music | 16.00 (4.72) | 51.00 (4.92)a | 62.00 (5.21)*,a,b | 67.00 (5.29)*,b | 41.00 (5.33) |
Data are mean scores with 1 SEM shown in parentheses (N= 20); within a row, bold font indicates significant difference from 0 mg/70 kg; for psilocybin doses, values not sharing a common letter are significantly different (Fisher’s LSD p < 0.05)
indicates significant difference from 400 mg/70 kg dextromethorphan
Maximum score for each item questionnaire was 100; the first 12 items are from the Hallucinogens Rating Scale and the last 3 items are from the States of Consciousness Questionnaire.
Mysticism scale
This 32-item questionnaire which was developed to assess naturally occurring primary mystical experiences has been extensively studied, shows cross-cultural generalizability (Hood et al. 2009), and has previously been shown sensitive to psilocybin (Griffiths et al. 2006) and DXM (Reissig et al. 2012). Items were rated on a nine-point scale. Participants were instructed to complete the questionnaire with reference to their experiences since they received the capsules that morning.
Psychological Insight Questionnaire
This 37-item questionnaire (available upon request) is comprised of questions that probe psychological insights into emotions, beliefs, memories, and relationships that the participant may have had during the drug experience.
Challenging Experience Questionnaire
This 27-item questionnaire is comprised of items from the HRS, SOCQ, and 5D-ASC relating to challenging experiences of hallucinogen experiences. Seven subscales and a total score were expressed as percentage of maximum possible scores (Barrett et al. 2016).
Hallucinogen rating scale (HRS)
This questionnaire consists of six subscales assessing hallucinogen effects (intensity, somaesthesia, affect, perception, cognition, and volition) (Strassman et al. 1994). Both psilocybin and DXM have been shown to produce dose-related increases on all six subscales of the HRS (Griffiths et al. 2011; Reissig et al. 2012). Because salient visual effects are commonly reported to occur after psilocybin, 12 individual HRS items describing various visual effects were also analyzed separately.
Pharmacological class questionnaire
This questionnaire, modified from Reissig et al. (2012), listed descriptive titles and examples of 14 classes of psychoactive drugs (see Online Supplementary Table 1 for details). Participants were first instructed to choose the single drug class that most closely represented the drug effect that they experienced during the session. Then participants completed a series of visual analog scales rating how similar that day’s drug effect was to specific drugs from the previously identified drug classes. For example, participants were asked to rate how much did today’s drug effect felt like a classic hallucinogen (e.g., LSD, psilocybin, ayahuasca, mescaline, etc.). Participants were required to click a location along a 100-point line anchored on opposite ends with the labels “no, not at all” and “yes, very much.”
Other measures assessed after completion of all drug sessions
Phenotyping of DXM metabolism
Approximately 1 month after the final drug/placebo session, participants returned to the research facility for an 8-h session to permit phenotyping of CYP2D6 metabolizer status. A very low, oral dose of DXM was administered (25 mg/70 kg), and an 8-h total urine collection was performed according to previously described procedures (Schmid et al. 1985) to identify poor metabolizers of DXM (Vengurlekar et al. 2002).
Other measures
Several cognitive performance and abuse liability measures were also assessed during and after sessions. These results will be reported separately.
Data Analysis
Circular Lights and Balance tasks were scored as “0” if a volunteer was too impaired to complete the task
For time-course data, planned comparison t tests were conducted between placebo and active doses at each time point. Data were analyzed with IBM SPSS Statistics (IBM Corporation, Armonk, NY). Repeated measures ANOVAs were used. For Subjective Effects Questionnaire items, monitor ratings, cardiovascular, and pupil diameter measures, peak effects were defined as the maximum value observed after drug administration for each participant. For Circular Lights and Balance, peak effects were defined as the minimum value after drug administration. For all the peak effect and end of session measures, Fisher’s LSD post hoc tests were used to compare drug conditions. Statistical tests were considered significant at p≤0.05. Rates of endorsements for the Pharmacological Class Questionnaire were analyzed. For analysis of dichotomous responses for the occurrence of complete mystical experiences and emesis and for endorsement of various drug or drug class options on the Pharmacological Class Questionnaire, Cochran’s Q, a non-parametric, binary repeated measures test, was conducted with a factor of Drug Condition (placebo, 10, 20, and 30 mg/70 kg psilocybin, and DXM). Planned comparisons among placebo, 30 mg/70 kg psilocybin and DXM were conducted using McNemar’s test.
One of the 10 guides was not blind to the range of possible drug conditions in the study. Therefore, the analyses of the Monitor Rating Questionnaire described above was repeated excluding ratings from this monitor. Because there were only a few minor differences in the statistical significance, the data presented are from all monitor ratings.
Results
Time-course of drug effects
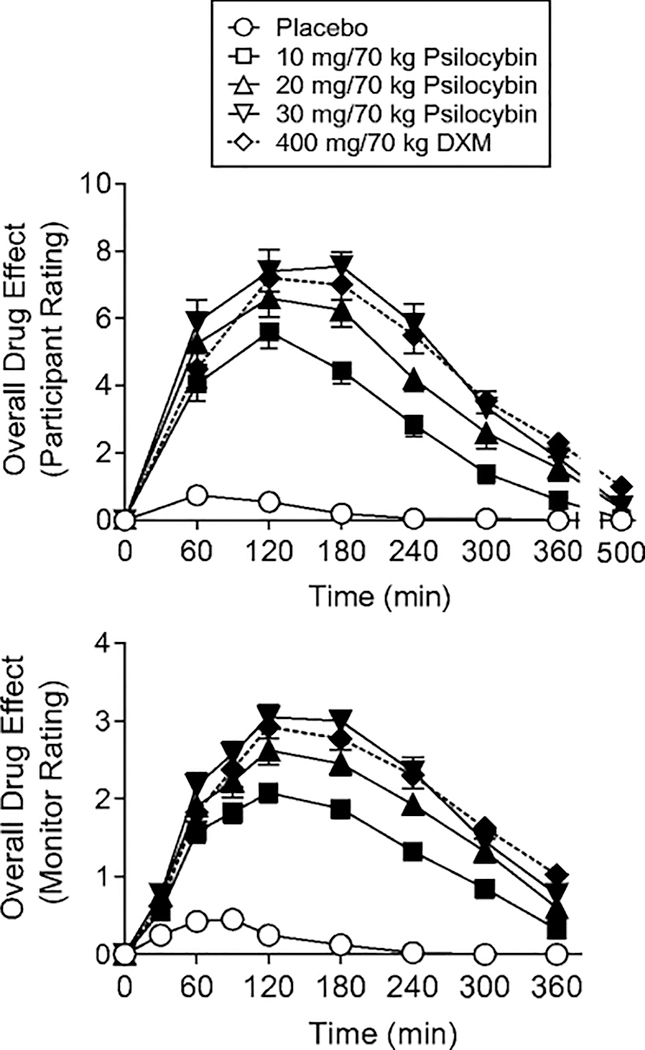
Psilocybin produced orderly dose- and time-related effects. The 400 mg/70 kg DXM also produced orderly time-dependent effects. Figure 1 shows illustrative time-course data for participant and monitor ratings of drug effect. On most subjective, behavioral, and physiological measures, the time course of DXM was similar to that of the high psilocybin doses, with an exception that DXM produced greater and longer lasting effects on the Balance Task. At doses that produced significant effects, the effects were generally significant by the 2-h time point, with maximal effects occurring at 2 to 4 h, and effects decreasing at the 6-h time point.
Fig. 1.
Time-course of effects of placebo, psilocybin (10, 20, and 30 mg/70 kg) and dextromethorphan (DXM, 400 mg/70 kg) on overall drug effect assessed repeatedly across the session. Y-axes: participant-rated overall drug effects on a 10-point scale; monitor ratings of overall drug effect on a four-point scale. X-axes: time after drug administration in minutes. Data points show means (N= 20), brackets show 1 SEM. Filled symbols indicate values that are significantly different from the corresponding placebo value at the same time point (p<0.05, planned comparisons).
Measures assessed during the session
Blood pressure, heart rate, and pupil diameter
Peak maximum effects on physiological measures are shown in Table 1. DXM and all three doses of psilocybin produced significant increases in systolic blood pressure, heart rate, and pupil diameter, but not diastolic blood pressure.
Circular Lights and Balance
Both DXM and psilocybin decreased peak performance on the Circular Lights and Balance Tasks (Table 1), with psilocybin generally producing dose-related effects. DXM produced significantly larger decreases than all doses of psilocybin on the Balance Task.
Peak monitor ratings
On those ratings affected by psilocybin, effects generally increased with dose (Table 1). Both psilocybin and DXM increased peak monitor ratings of overall drug effect, restlessness/fidgety, peace/harmony, joy/intense happiness, and nausea/vomiting, with the high dose of psilocybin producing significantly greater effects than DXM on joy/intense happiness, and DXM producing greater effects than all doses of psilocybin on nausea/vomiting. Psilocybin, but not DXM produced an increase in yawning and tearing/crying compared to placebo, with significant differences between the high doses of psilocybin vs. DXM. There were no significant effects of either psilocybin or DXM on the other monitor rated dimensions (Table 1). Compared to placebo, both psilocybin and DXM decreased the total minutes of sleep during the session, with no differences between psilocybin doses and DXM (28.5 minutes after placebo vs. a mean of 2.1 minutes of sleep after the four drug conditions).
Peak participant ratings of subjective effects and somatic symptoms
With the single exception of ratings of physically comfortable, both drugs increased peak participant ratings (Table 2). Psilocybin effects generally increased with dose. DXM was not significantly different from the high dose of psilocybin, with the exceptions that psilocybin ratings were higher for visual effects and absorption in listening to the music, while DXM ratings were higher for light-headed/dizzy.
Table 2.
Peak participant of subjective and somatic ratings assessed throughout the session
| Rated description | Placebo | Psilocybin dose (mg/70 kg) | Dextromethorphan dose (mg/70 kg) | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 400 | |
| Participant Ratings (peak effects; max score=10) | |||||
| Subjective Effects | |||||
| Overall drug effect | 0.80 (0.24) | 5.90 (0.48)*a | 7.75 (0.42)b | 8.40 (0.37)b | 7.90 (0.38) |
| Distance from normal reality | 0.90 (0.27) | 4.75 (0.56)*,a | 7.25 (0.41)b | 7.80 (0.46)b | 7.55 (0.39) |
| Sense of pure being and pure awareness† | 1.45 (0.44) | 4.95 (0.59)a | 6.45 (0.63)a,b | 6.85 (0.71)a | 6.15 (0.61) |
| Fusion of personal self into a larger whole† | 1.45 (0.44) | 4.15 (0.67)*,a | 6.35 (0.70)b | 7.85 (0.58)b | 6.40 (0.62) |
| Sense of reverence or sacredness† | 1.65 (0.39) | 4.70 (0.63)a | 6.20 (0.82)a,b | 6.80 (0.71)b | 5.85 (0.74) |
| Timelessness† | 1.10 (0.35) | 4.45 (0.66)*,a | 6.85 (0.67)b | 7.90 (0.44)b | 6.50 (0.60) |
| Ineffability† | 0.95 (0.34) | 4.40 (0.60)*,a | 7.45 (0.61)b | 7.75 (0.62)b | 7.70 (0.50) |
| Visual Effects | 0.30 (0.16) | 5.10 (0.55)a | 7.45 (0.52)*,b | 8.50 (0.40)*,b | 6.05 (0.69) |
| To what degree are you absorbed in listening to the music | 5.65 (0.49) | 7.40 (0.42)a | 8.50 (0.46)a,b | 9.15 (0.32)*,b | 7.65 (0.60) |
| Somatic Symptoms | |||||
| Physically comfortable | 8.15 (0.38) | 7.90 (0.35) | 8.35 (0.34) | 8.70 (0.25) | 8.85 (0.31) |
| Numbness/tingling | 0.20 (0.16) | 2.20 (0.47)a | 2.95 (0.61)a | 3.30 (0.74)a | 3.60 (0.73) |
| Light-headedness/dizzy | 0.20 (0.17) | 1.55 (0.52)*,a | 1.90 (0.50)*,a | 1.90 (0.61 )*,a | 5.50 (0.75) |
| Queasy/sick to stomach | 0.25 (0.10) | 1.95 (0.48)*,a | 2.35 (0.53)*,a | 3.15 (0.64)a | 3.90 (0.58) |
| Hot/flushed | 0.45 (0.22) | 1.70 (0.45)*,a | 3.15 (0.48)b | 3.15 (0.57)b | 3.65 (0.55) |
| Cold/chills | 0.25 (0.18) | 2.90 (0.60)a | 2.15 (0.42)a | 3.10 (0.60) a | 2.70 (0.57) |
Data are mean scores with 1 SEM shown in parentheses (N= 20); within a row, bold font indicates significant difference from 0 mg/70 kg; for psilocybin doses, values not sharing a common letter are significantly different (Fisher’s LSD p < 0.05)
indicates significant difference from 400 mg/70 kg dextromethorphan
Items commonly associated with mystical experiences
Nausea/emesis during the session
No participant vomited after receiving placebo, 10, or 20 mg/70 kg psilocybin. Two of the 20 participants (10%) vomited after receiving 30 mg/70 kg psilocybin, and 11 (55%) vomited after receiving 400 mg/ 70 kg DXM. The incidence of emesis after DXM was significantly higher than both placebo (p = .001) and the high dose of psilocybin (p = .022). Monitor and participant ratings of nausea/vomiting and queasy/sick to stomach, respectively, were significantly higher after DXM than all doses of psilocybin, with one exception (30 mg/70 kg, participant rating)(Tables 1 and 2). Although incomplete drug absorption after vomiting cannot be ruled out, vomiting typically occurred 90 min or longer after capsule administration, making it unlikely that significant amounts of DXM or psilocybin were purged before being absorbed. Of the participants that did vomit, none vomited on more than one drug administration session.
Participant rated measures assessed 7 h after drug administration
Tables 3, 4, and 5 show participant ratings measures on seven questionnaires and 15 specific items completed 7 h after drug administration. These results generally show significant and orderly dose-related increases after psilocybin and significant increases after DXM. Ratings of drug effect intensity (Table 3) did not differ between DXM and the high doses of psilocybin suggesting that the overall perceived strength of drug effects were similar. Likewise, DXM did not differ from the high doses of psilocybin on measure of impaired cognition and control, anxiety, elementary imagery, audiovisual synesthesia, and the total score and most of the subscales of the Challenging Experience Questionnaire.
Table 3.
Participant ratings on seven questionnaires assessed 7 hours after drug administration
| Questionnaire and Subscale Description | Placebo | Psilocybin dose (mg/70 kg) | Dextromethorphan dose (mg/70 kg) | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 400 | |
| Drug Effect Intensity Rating | |||||
| Overall drug effect (max score=4) | 0.35 (0.11) | 2.05 (0.14)*,a | 2.80 (0.19)b | 3.15 (0.15)b | 2.85 (0.15) |
| 5D-ASC questionnaire (max score=100) | |||||
| Unity† | 1.00 (0.40) | 23.01 (5.07)a | 35.18 (7.45)a,b | 49.52 (6.34)*,b | 21.06 (4.54) |
| Spiritual experience† | 2.40 (0.93) | 34.42 (6.09)a | 47.77 (6.62)*,a,b | 60.37 (6.09)*,b | 26.87 (6.60) |
| Blissful state* | 1.97 (0.72) | 31.28 (6.20)a | 46.00 (7.43)*,a,b | 56.87 (6.22)*,b | 24.38 (4.84) |
| Insightfulness | 0.93 (0.62) | 32.92 (5.61)a | 44.97 (6.71)*,a | 45.82 (6.19)*,a | 21.77 (5.87) |
| Disembodiment | 0.93 (0.76) | 15.80 (5.45)*,a | 30.45 (6.69)*,a | 26.87 (6.70)*,a | 48.35 (6.69) |
| Impaired cognition and control | 0.26 (0.12) | 4.81 (1.34)*,a | 13.04 (2.88)b | 13.99 (4.14)b | 15.71 (2.43) |
| Anxiety | 0.20 (0.14) | 4.18 (1.19)*,a | 9.62 (2.38)a,b | 16.29 (3.63)b | 11.78 (3.35) |
| Complex imagery | 2.72 (2.04) | 38.00 (7.89)a | 55.27 (6.91)*,a | 54.4 (7.11 )*,a | 31.98 (6.83) |
| Elementary imagery | 2.33 (1.32) | 36.85 (7.43)a | 48.25 (6.57)a,b | 55.02 (7.14)a | 38.22 (7.33) |
| Audiovisual synesthesia | 0.13 (0.13) | 29.83 (8.31)a | 36.22 (7.54)a | 33.47 (8.31)a | 21.78 (6.72) |
| Changed meaning of precepts | 0.52 (0.37) | 10.05 (3.07)a | 18.30 (3.09)*,b | 21.18 (3.97)*,b | 8.73 (2.59) |
| Hallucinogen Rating Scale (HRS) | |||||
| Intensity (max score=4.25) | 0.43 (0.13) | 2.22 (0.12)a | 2.68 (0.10)b | 3.00 (0.08)*,c | 2.53 (0.13) |
| Somaesthesia (max score=4) | 0.13 (0.04) | 1.03 (0.12)*,a | 1.38 (0.13)b | 1.38 (0.13)b | 1.51 (0.11) |
| Affect (max score=4) | 0.48 (0.05) | 1.31 (0.12)a | 1.79 (0.14)*,b | 1.92 (0.13)*,b | 1.05 (0.09) |
| Perception (max score=4) | 0.04 (0.02) | 1.15 (0.11)a | 1.74 (0.14)*,b | 2.04 (0.16)*,b | 1.08 (0.12) |
| Cognition (max score=4) | 0.20 (0.06) | 1.26 (0.15)a | 1.78 (0.18)*,b | 2.11 (0.14)*,b | 1.16 (0.12) |
| Volition (max score=4) | 1.15 (0.16) | 1.42 (0.13)*,a | 1.60 (0.10)ab | 1.81 (0.14)b | 1.88 (0.13) |
Data are mean scores with 1 SEM shown in parentheses (N= 20); within a row, bold font indicates significant difference from 0 mg/70 kg; for psilocybin doses, values not sharing a common letter are significantly different (Fisher’s LSD p < 0.05)
indicates significant difference from 400 mg/70 kg dextromethorphan
Items commonly associated with mystical experiences
Table 4.
Volunteer ratings on Mystical Experience, Psychological Insight, and Challenging Experience Questionnaires completed 7 hours after drug administration
| Questionnaire and Subscale Description | Placebo | Psilocybin dose (mg/ 70 kg) | Dextromethorphan dose (mg/ 70 kg) | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 400 | |
| Mystical Experience Questionnaire (MEQ30) # | |||||
| Mystical | 6.53 (2.08) | 34.80 (4.42)a | 48.47 (6.28)*,b | 61.27 (4.81)*,b | 29.67 (4.82) |
| Deeply felt positive mood† | 15.83 (2.63) | 49.33 (4.03)a | 60.50 (5.44)*,a,b | 66.33 (4.58)*,b | 46.33 (4.95) |
| Transcendence of time and space† | 6.33 (219) | 35.17 (3.63)*,a | 51.50 (4.86)b | 59.83 (4.00)b | 49.00 (4.74) |
| Ineffability† | 4.67 (2.06) | 45.67 (4.33)*,a | 66.33 (5.22)b | 72.00 (4.21)*,b | 59.00 (4.20) |
| Total† | 8.17 (1.96) | 38.87 (3.67)a | 53.27 (5.09)*,b | 63.07 (3.98)*,b | 39.80 (3.88) |
| Mysticism Scale | |||||
| Interpretation (max score=108) † | 41.15 (4.64) | 75.20 (4.15)a | 83.45 (4.63)*,a,b | 90.45 (3.91)*,b | 69.45 (5.09) |
| Introvertive (max score=108) † | 35.30 (4.13) | 69.60 (4.83)a | 82.80 (3.44)b | 87.25 (3.07)b | 78.40 (3.60) |
| Extrovertive (max score=72) † | 22.50 (3.00) | 43.05 (3.83)a | 50.45 (3.44)a,b | 57.05 (3.41)*,b | 42.10 (3.70) |
| Total (max score=288) † | 98.95 (11.27) | 187.85 (12.12)a | 216.70 (10.45)a,b | 234.75 (9.84)*,b | 189.95 (11.79) |
| Psychological Insight Questionnaire | |||||
| Total# | 9.82 (2.82) | 39.18 (4.52)*,a | 55.32 (4.77)*,b | 53.45 (3.84)*,b | 22.13 (4.78) |
| Challenging Experience Questionnaire (CEQ) # | |||||
| Physical Distress | 3.00 (1.42) | 20.25 (4.18)a | 26.00 (4.50)a | 25.00 (4.65)a | 29.50 (3.91)a |
| Grief | 4.63 (2.62) | 17.00 (4.53)a | 34.42 (5.65)*,b | 27.42 (5.23)*,a,b | 9.21 (2.45) |
| Fear | 0.97 (0.54) | 7.80 (2.10)a | 16.84 (3.70)b | 19.79 (4.06)b | 13.25 (2.92) |
| Insanity | 0.05 (0.05) | 6.28 (2.29)a | 16.48 (4.38)b | 23.38 (4.85)*,b | 10.20 (3.69) |
| Isolation | 1.07 (0.66) | 8.57 (2.36)a | 19.00 (4.75)b | 12.50 (3.34)a,b | 14.43 (3.69) |
| Death | 0.00 (0.00) | 4.25 (2.62)a | 16.38 (6.88)b | 18.88 (4.71)b | 14.25 (3.77) |
| Paranoia | 0.00 (0.00) | 5.00 (2.56) | 4.50 (1.98) | 10.50 (3.87) | 3.00 (2.19) |
| Total | 1.96 (0.92) | 11.74 (2.63)a | 21.88 (3.43)b | 21.34 (3.59)b | 14.52 (2.04) |
Data are mean scores with 1 SEM shown in parentheses (N= 20); within a row, bold font indicates significant difference from 0 mg/70 kg; for psilocybin doses, values not sharing a common letter are significantly different (Fisher’s LSD p<0.05)
Indicates significant difference from 400 mg/70 kg dextromethorphan
Maximum score for each subscale of this questionnaire was 100
Items commonly associated with mystical experiences
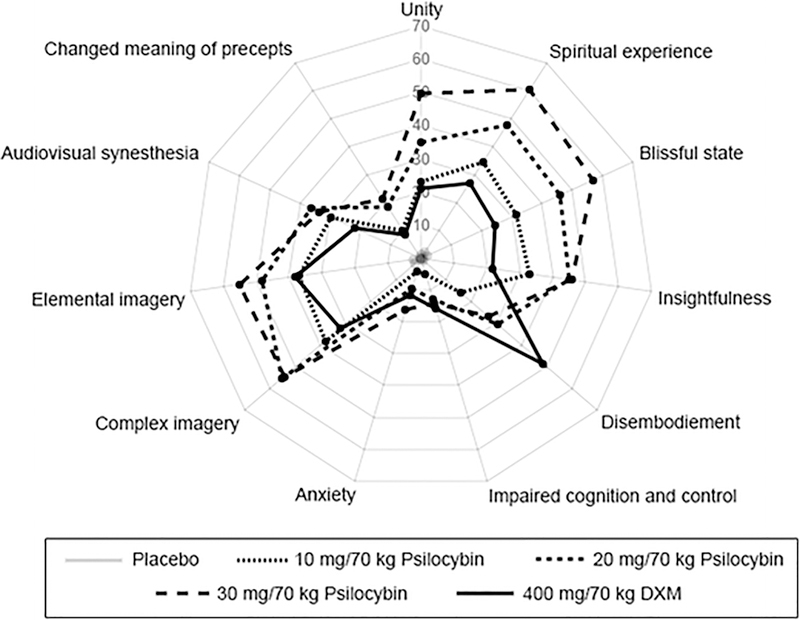
However, the 30 mg/70 kg dose of psilocybin (and often 20 mg/70 kg dose) produced significantly greater effects than DXM on 6 of 11 subscales of the 5D-ASC, 4 of 6 subscales of the HRS (Table 3), the total score and most of the subscale scores on the Mystical Experience Questionnaire, total score and all subscales of the Mysticism Scale, total score on the Psychological Insight Questionnaires (Table 4), and 11 of 15 visual effects and music significance items (Table 5). Notably, DXM produced significantly greater increases on the Disembodiment Scale of the 5D-ASC than each of the psilocybin doses. The orderly psilocybin dose effects and differences between psilocybin and DXM on the subscales of the 5D-ASC are presented in Fig. 2.
Fig 2.
Effects of placebo, psilocybin (10, 20, and 30 mg/70 kg), and dextromethorphan (DXM, 400 mg/70 kg) on the 11 subscale scores of the Altered States of Consciousness scale (5 D-ASC). Data points show means (N= 20); maximum score = 100.
The proportion of volunteers who met a priori criteria for having had a “complete” mystical experience on the MEQ30 was: 0%, 0%, 20%, 40%, and 0% after placebo, 10, 20, and 30 mg/70 kg psilocybin, and DXM, respectively. The incidence of complete mystical experience after the 30 mg/70 kg psilocybin dose is significantly greater than after placebo, 10 mg/kg/70 kg psilocybin, and DXM.
On the Pharmacological Class Questionnaire, when participants were required to choose the single drug class best representing the drug effect they experienced that session, most participants (70% 14 of 20) chose Placebo after receiving placebo, with 25% participants selecting Sedative-hypnotic/Muscle relaxant/Anti-anxiety medication, and 5% selecting MDMA. Most volunteers chose Classic hallucinogen (e.g., like LSD, psilocybin, DMT, and mescaline) after receiving psilocybin, with 85%, 80%, and 90% choosing this drug class at 10, 20, and 30 mg/70 kg psilocybin, respectively. Sixty percent of participants chose Dissociative anesthetic hallucinogen after receiving DXM, with 10% selecting Classic hallucinogen, 10% selecting MDMA, 5% selecting Sedative-hypnotic/Muscle relaxant/Anti-anxiety, and 15% indicating that they did not know. Participant ratings of the degree of similarity of drug session experiences to the 14 possible drugs or drug classes are presented in Online Supplementary Table 1. Psilocybin doses were rated as 75%, 83% and 90% similar to the Classic hallucinogen class at 10, 20 and 30 mg/70 kg, respectively. Ratings of these doses as similar to the Dissociative anesthetic hallucinogen were 11%, 21%, and 15%, respectively. DXM, in contrast was rated as 65% similar to the Dissociative class, 28% similar to the Classic hallucinogen class, and 27% similar to an unidentified “Other” class.
Phenotyping of DXM metabolism
Of the 20 participants assessed for CYP2D6 status, one was a poor metabolizer. Inspection of results across a range of measure suggested no obvious difference in data from this participant.
Discussion
The present study provided the first within-subject comparison of psilocybin and dextromethorphan, and under conditions designed to minimize participant and staff expectancy effects. High doses of both drugs produced similar increases in participant ratings of peak overall drug effect strength, with similar times to maximal effect and time-course (Figure 1, Tables 2 and 3). Likewise peak and time-course of monitor ratings of overall drug effects of DXM and the high dose of psilocybin were similar (Figure 1, Table 1). These results suggest that both the perceived and observed intensity of overall drug effects and time-course of DXM and the high dose of psilocybin were similar.
In the present study, psilocybin produced orderly dose-related increases on almost all participant-rated subjective measures during sessions and 7 hours after drug administration (Tables 2, 3, 4 and 5). The orderliness of these data across doses, in combination with the consistency of these observations with a prior study that demonstrated similar psilocybin dose effects in volunteers without histories of hallucinogen use who were informed that they would receive a range of psilocybin doses (Griffiths et al. 2011), suggests that the robustness of the methodology and replicability of the psilocybin dose-effect findings. Most participant-and monitor-rated qualitative measures of psilocybin evaluated in both the present study and the previous study (Griffiths et al. 2011) were similar, including increases on participant completed questionnaires assessing typical hallucinogen subjective drug effects, mystical experience, and visual effects. Three differences from previous studies were that the hallucinogen-naive participants (Griffiths et al. 2011) showed significant increases in monitor ratings of psilocybin-induced anxiety or fearfulness, paranoid thinking, and unresponsive to questions. In contrast, the hallucinogen experienced participants in the present study showed no such increases. Whether this difference reflects an acquired tolerance to these possibly unpleasant effects of psilocybin or a selection bias in enrolling hallucinogen experienced participants is unknown.
With regard to physiological and behavioral effects, the present study showed that psilocybin produced significant dose-related increases in systolic blood pressure and heart rate, and pupil diameter, along with decreases in Circular Lights and Balance Task performance. The absence of a significant increase in diastolic blood pressure contrasts with the previous psilocybin dose-effect study (Griffiths et al. 2011).
In the present study, the effects of a high dose DXM (400 mg/70 kg) were similar to those demonstrated in a previous study of a high dose of DXM in experienced hallucinogen users (Reissig et al. 2012; penultimate and maximum DXM doses). In both studies, DXM increased participant-rated somatic effects of numbness/tingling, light-headed/dizzy, queasy/sick to stomach, and hot/flushed; monitor ratings of, restless/fidgety, peace, joy, and nausea/vomiting; post-session participant-rated questionnaire measures of hallucinogen drug effects (HRS) and mystical experience; and the incidence of emesis. Also similar to the previous study, DXM increased systolic blood pressure and heart rate, and decreased circular lights and balance task performance. Like psilocybin, an absence of a significant increase in diastolic blood pressure after DXM contrasts with the previous DXM study (Reissig et al. 2012; penultimate DXM dose).
Comparing psilocybin and DXM
Given that participants reported similar overall drug effect intensity after DXM and the high dose of psilocybin, examination of similarities and differences between DXM and psilocybin is of particular interest. Furthermore, because both psilocybin and DXM are considered to be hallucinogens and hallucinogens are defined by alterations of subjective experience, the comparisons of most interest are measures of subjective states reported by participants during and retrospectively after drug administration (11 peak subjective measures in Table 2; 51 subjective effect measures in Tables 3, 4 and 5). Consistent with the classification of these compounds as hallucinogens, both the high dose of psilocybin and DXM significantly increased all of these 62 measures, with the only exceptions being that DXM did not significantly increase scores on the Psychological Insight Questionnaire or visual effect ratings of room overlaid with visual patterns, kaleidoscopic images, and visual synesthesia.
Differences in visual effects
Although both drugs increased a wide range of diverse visual effects, psilocybin produced greater effects than DXM. Of the 18 measures of visual effects (1, 3, and 14 items in Tables 2, 3 and 5 respectively), the high dose of psilocybin produced significantly greater effects than DXM on 12 measures (75%), indicating greater movement and more frequent, brighter, distinctive, and complex (including textured and kaleidoscopic) images and visions. Furthermore, with eyes open, psilocybin also produced greater visual effects than DXM. These differences between psilocybin and DXM are consistent with vivid, complex, patterned, colorful imagery commonly reported after psilocybin and other tryptamine hallucinogens such as DMT relative to more dreamlike less vivid visual imagery associated with ketamine (Shulgin and Shulgin 1997; Jansen 2004; Gouzoulis-Mayfrank et al. 2005; Studerus et al. 2010). These results are also consistent with a double-blind study showing significantly greater scores on a measure of visionary restructuralization after intravenous DMT than ketamine (Gouzoulis-Mayfrank et al. 2005), and several studies suggesting greater visual effects after oral or intravenous psilocybin than intravenous ketamine (Studerus et al. 2012; Schmidt et al. 2013; Schartner et al. 2017). Visions of white light, which have been reported at higher doses of DXM (White 2002) did not differ between DXM and psilocybin.
Differences in mystical experience-type effects
Of the 17 subjective dimensions commonly associated with mystical experience (5, 3, and 9 items in Table 2, 3 and 4, respectively), the high dose of psilocybin produced significantly greater effects than DXM on 10 measures (59%). Furthermore, the proportion of participants of meeting criteria for having had a complete mystical experience was significantly higher after the high dose of psilocybin than DXM (40% vs. 0%). It is interesting to note that none of the 5 mystical experience items assessed during the session (Table 2) statistically differentiated the high dose of psilocybin from DXM, which may reflect greater memory impairing effects of DXM than psilocybin (unpublished data from this study). Of the 12 mystical experience measures assessed at the end of the session, only two (one subscale each from the MEQ30 and Mysticism Scale) did not distinguish between the high dose of psilocybin and DXM. The endorsement of transcendence of time and space is common to both these measures. That DXM produced such effects would seem to be consistent with the high Disembodiment scores after DXM in the current study and with anecdotal reports suggesting that out-of-body experiences and near death experiences, are not uncommon effects of NMDA antagonist dissociative anesthetics such as DXM and ketamine (White 2002; Jansen 2004).
Differences in psychological insight
DXM and all three doses of psilocybin significantly increased the two measures assessing the attribution of insight to the drug experiences. Both measures (100%) were significantly higher after psilocybin than DXM (Tables 3 and 4). The more detailed of these measures is the 37-item Psychological Insight Questionnaire, which probes psychological insights into emotions, beliefs, memories, and relationships. Scores on that measure were significantly higher after each of the three psilocybin doses than after DXM. Although the “Insightfulness” subscale of the 5D-ASC also showed significant differences between DXM and the highest two doses of psilocybin, a study comparing oral psilocybin to intravenous ketamine did not show a difference on this subscale (Schmidt et al., 2012). Notably, this subscale is comprised of only three items, two of which may not reflect psychological insight per se (“I felt very profound” and “I had very original thoughts”). A valuable future research direction with psychedelics will be establishing the factor structure, reliability, and internal validity of the Psychological Insight Questionnaire.
Differences in the experience of music
Although DXM and all three doses of psilocybin significantly increased the two measures assessing the absorption or significance of music, the high dose of psilocybin produced significantly greater effects than DXM on both measures (100%). Music is often used in the context of therapeutic sessions with psilocybin and other psychedelics (Bonny and Pahnke 1972; Johnson et al. 2008; Barrett et al. 2017) and a recent controlled trial showed that LSD enhanced the positive emotional responses to music (Kaelen et al. 2015). Although the present study suggests differences between psilocybin and DXM in regard to engagement with music, only two questionnaire items were assessed. Future research comparing hallucinogens should use validated measures assessing a broader range music-elicited experiences (Zentner et al. 2008).
Differences in the experience of disembodiment
Although both DXM and psilocybin significantly increased the disembodiment subscale of the 5D-ASC, DXM produced significantly greater increases than all three doses of psilocybin. Higher scores after DXM on this 3-item subscale (which probes feelings of being out of the body, not having a body, and floating) are consistent with reports of out-of-body experiences after DXM and ketamine (White 2002; Jansen 2004). The greater scores after DXM than psilocybin on the disembodiment subscale of the 5D-ASC are also consistent with several studies suggesting greater disembodiment scores after oral psilocybin than intravenous ketamine (Studerus et al. 2012; Schmidt et al. 2013).
Differences in somatic symptoms and emesis
Although both DXM and psilocybin increased various somatic effects, DXM produced significantly greater increases in participant- and monitor-rated nausea or sick to stomach, participant-rated lightheaded/dizzy, and the incidence of emesis. All three doses of psilocybin produced significantly higher monitor-ratings of yawning than DXM.
The Pharmacological Class Questionnaire
This questionnaire provided data suggesting both similarities and differences between psilocybin and DXM. The high dose of psilocybin was often accurately classified as a classic hallucinogen (90% of participants; 90% of maximum on analogue ratings of similarity). In contrast, only somewhat more than half of participants (60%) accurately classified DXM as a dissociative hallucinogen, with analogue ratings of similarity to a dissociative anesthetic of 65% and similarity to a classic hallucinogen of 28%. These results contrast results from a previous study that compared DXM and triazolam under similar instruction and blinding conditions and also in participants with histories of hallucinogen use (Reissig et al. 2012). In that study, 400 mg/70 kg DXM was classified as a classic hallucinogen by 92% of participants, with an analogue similarity rating of 93%. The difference between these studies suggests the possibility that the context in which drugs are evaluated for subjective effects (e.g. in this case, what comparator drugs are being evaluated) may be an important determinant of subjective perceptions of drugs. The finding underscores the importance of controlling for context and expectancy effects in comparative studies of different hallucinogens. The finding also raises questions about the validity of commonly reported differences in the phenomenology between different hallucinogens (e.g. the allegedly relatively high rates of serpent imagery after ayahuasca (Narby 1999) or autonomous entity encounter experiences after DMT (Strassman 2001)).
Conclusions
This study compared subjective experiences of two mechanistically different hallucinogens psilocybin and dextromethorphan in participants with histories of hallucinogen use. A unique feature of the study design was that instructions to participants and staff did not provide information about which specific hallucinogens would be administered. High doses of both drugs produced similar increases in participant and monitor ratings of peak overall drug effect strength, indicating that the perceived and observed intensity of overall drug effects were similar. Consistent with their classification as hallucinogens, both drugs increased a wide range of measures indicating typical hallucinogen subjective drug. However, at high doses that produced comparable ratings of overall drug effect intensity, psilocybin produced relatively greater visual, mystical-type, and insightful experiences, and greater absorption in music. DXM, in contrast, produced greater feelings of disembodiment and nausea, and a greater incidence of emesis.
Supplementary Material
Acknowledgements
We thank Mary Cosimano, M.S.W, Taylor Marcus, Darrick May, M.D. and 5 other staff members for their roles as session monitors, Dr. Annie Umbricht for medical management, Frederick Barrett, Ph.D. for contributing to the study design, Lisa Shade for technical assistance, Linda Felch for statistical assistance, and the pharmacy and medical staff. We also thank David Nichols, Ph.D. for synthesizing the psilocybin and Michelle Rudek, Pharm.D., Ph.D. and Nichole Anders of the Analytical Pharmacology Core for analyzing dextromethorphan metabolism. The study was conducted in compliance with United States laws.
Conduct of this research was supported by NIH R01DA03889 and T32 DA07209. Support for dextromethorphan metabolic analysis was supported NIH grants P30CA006973 and UL1TR001079, and the Shared Instrument Grant S10OD020091 to the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center.
Footnotes
Compliance with ethical standards
The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. Participants gave their written informed consent before beginning the study procedures and were paid for their participation.
Disclosures: Dr. Carbonaro is an employee of the U.S. Food and Drug Administration (FDA), however, the views presented in this article do not necessarily reflect those of the FDA and no official support or endorsement of this article by the FDA is intended or should be inferred. Roland Griffiths is on the Board of Directors of the Heffter Research Institute.
Supplementary material: Supplementary material is available for this article and is accessible for authorized users.
Contributor Information
Theresa M. Carbonaro, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Matthew W. Johnson, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA
Ethan Hurwitz, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA.
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA Department of Neuroscience, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224-6823, USA.
References
- Aghajanian GK Marek GJ (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21(2 Suppl):16S–23S [DOI] [PubMed] [Google Scholar]
- Banken JA, Foster H (2008) Dextromethorphan. Ann N Y Acad Sci, 1139:402–411 [DOI] [PubMed] [Google Scholar]
- Barrett FS, Workman CI, Sair HI, Savonenko AV, Kraut MA, Sodums DJ, Joo JJ, Nassery N, Marano CM, Munro CA, Brandt J, Zhou Y, Wong DF, Smith GS (2017) Association between serotonin denervation and resting-state functional connectivity in mild cognitive impairment. Hum Brain Mapp. Epub before print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR (2015) Validation of the revised mystical experience questionnaire in experimental sessions with psilocybin. J Psychopharmacol 29(11):1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos JS, Johnson MW, Griffiths RR (2016) The challenging experience questionnaire: Characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol 30(12):1279–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Robbins H, Smooke D, Brown JL, Griffiths RR (2017). Qualitative and quantitative features of music reported to support peak mystical experiences during psychedelic therapy sessions. Frontiers in Psychology, Auditory Cognitive Neuroscience Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny HL Pahnke WN (1972) The use of music in psychedelic (LSD) psychotherapy. J Music Therapy Vol IX:64–87 [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, Gassman MC, Cooper KM, Muller D, Thomas CD, Hetzel SJ, Henriquez KM, Ribaudo AS, Hutson PR (2017) Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults, Clin Pharmacokinet [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology 232(1):275–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR (2006) Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology 31(11):2537–2551 [DOI] [PubMed] [Google Scholar]
- Carter LP, Reissig CJ, Johnson MW, Klinedinst MA, Griffiths RR, Mintzer MZ (2013) Acute cognitive effects of high doses of dextromethorphan relative to triazolam in humans. Drug Alcohol Depend 128(3):206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A (1998) The standardized psychometric assessment of altered states of conciousness (ACSs) in humans. Pharmacopsychiatry 31 Suppl 2:80–4 [DOI] [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M (2012) Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 62:2184–2191 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ (2008) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75(1):17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–98 [DOI] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adnewy SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD Jr, Brezina V, Sealfron SC, Filizola M, Gonzalez-Maeso J, Logothetis DE (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147(5): 1011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptormediated signaling pathways to affect behavior. Neuron 53:439–452 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar K-A (2005) Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): A double blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38:301–11 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187(3):268–83; discussion 284–92 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R (2011) Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 218(4) :649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX (2004) Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled doseeffect study. Psychopharmacology 172(2):145–156 [DOI] [PubMed] [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, Bar T, Vollenweider FX (1997) Determinaton of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv 72(3):175–84 [DOI] [PubMed] [Google Scholar]
- Hood RW, Hill PC, Bernard S (2009) The psychology of religion: An empirical approach. New York: Guilford Press [Google Scholar]
- Jansen KLR (2004) Ketamine: Dreams and Realities. Sarasota, FL: Multidisciplinary Association for Psychedelic Studies (MAPS) [Google Scholar]
- Johnson M, Richards WA, Griffiths RR (2008) Human hallucinogen research: Guidelines for safety. J Psychopharmacol 22(6):603–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Barrett FS, Roseman L, Lorenz R, Family N, Bolstridge M, Carhart-Harris RL (2015) LSD enhances the emotional response to music. Psychopharmacology 232(19): 3607–3614 [DOI] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR (2015) Hallucinogens and club drugs In: Galanter M, Kleber HD, Bradu L, editors. The American Psychiatric Press Textbook of Substance Abuse Treatment. Fifth ed. Arlington, VA.: The American Psychiatric Press; p. 209–222 [Google Scholar]
- MacLean KA, Leoutsakos JM, Johnson MW, Griffiths RR (2012) Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig 51(4):721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL,Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493:76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H Wallach J (2014) From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal 7(5):358–67 [DOI] [PubMed] [Google Scholar]
- Metzner R, Litwin G, Weil G (1965) The relation of expectation and mood to psilocybin reactions: A questionnaire study. Psychedelic Review 5:3–39 [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR (1995) Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther 272(2):570–80 [PubMed] [Google Scholar]
- Narby J (1999) The cosmic serpent DNA and the origins of knowledge. Jeremy P. Tarcher/Putnam, New York, NY [Google Scholar]
- Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR (2016) Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther 159:1–22 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016) Psychedelics. Pharmacol Rev 68:264–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM (2016) The pharmacology of psilocybin. Addict Biol 7(4):357–364 [DOI] [PubMed] [Google Scholar]
- Preller KH, Vollenweider FX (2016) Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR (2012) High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology 223(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, Liechti ME (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26: 1327–37. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2016) 2015 national survey on drug use and health: Detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Rockville, MD [Google Scholar]
- SAMHSA (2015) 2014 national survey on drug use and health: Detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Rockville, MD [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD (2017) Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Scientific Reports 7:46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Bircher J, Preisig R, Kupfer A (1985) Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther 38(6):618–24 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F (2013) The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacol 225:227–239 [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A (1997) TiHKAL. Transform Press, Berkeley, CA [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R (1994) Dose response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108 [DOI] [PubMed] [Google Scholar]
- Strassman R (2001) DMT: The spirit molecule. Park Street Press, Rochester, VA [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5(8):e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR (2016) Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (nuedexta(R)) clinical use. Pharmacol Ther 164:170–182 [DOI] [PubMed] [Google Scholar]
- Tyls F, Palenicek T, Horacek J (2014) Psilocybin--summary of knowledge and new perspectives. Eur Neuropsychopharmacol 24(3):342–356 [DOI] [PubMed] [Google Scholar]
- Vengurlekar SS, Heitkamp J, McCush F, Velagaleti PR, Brisson JH, Bramer SL (2002) J Pharm Biomed Anal 30(1):113–24 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11(9):642–51 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 9(17):3897–3902 [DOI] [PubMed] [Google Scholar]
- White W The DXM experience. https://www.erowid.org/chemicals/dxm_experience.shtml#toc.5. Accessed 15 July 2017.
- Winter JC, Eckler JR, Rabin RA (2004) Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology 172:233–240 [DOI] [PubMed] [Google Scholar]
- Winter JC (2009) Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology 203:251–263 [DOI] [PubMed] [Google Scholar]
- Zentner M, Grandjean D, Scherer KR (2008) Emotions evoked by the sound of music: characterization, classification, and measurement. Emotion 8:494–521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.