Abstract

In this work, we synthesized gold nanoclusters within a Zirconium-based metal–organic framework (AuNCs@UiO-66) that may create new prospects for the development of novel sensing materials for biosensor applications. The resulting AuNCs@UiO-66 nanocomposite exhibits red fluorescence with a high quantum yield (11%), and the AuNCs are homogeneously distributed along UiO-66. Analytical and morphological characterizations of the resulting material were carried out by UV–visible spectroscopy, photoluminescence spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, and high-resolution transmission electron microscopy. The synthesized AuNCs@UiO-66 nanocomposite was used for the effective detection of Hg2+ ions with a detection limit as low as 77 pM. Moreover, the fabricated sensors also successfully detected Hg2+ in real water samples. This sensor is stable and highly fluorescent, developed using a simple fabrication method, and would be constructive for the detection of other metal ions and in biological applications.
Introduction
Routine sensing of mercury ions (Hg2+) is a major part of environmental monitoring in water, food sources, soil, and other environmental systems because of their harmful outcome for the environment and human health.1 Water and soil can be contaminated by the long atmospheric residence time of Hg0 vapors that can be oxidized to inorganic Hg.2 High levels of exposure to Hg can have harmful effects on the brain, kidney, lungs, heart, and immune system.3 Therefore, there is a great need to fabricate a highly sensitive and low-cost Hg sensor that can illustrate the accurate determination of Hg level in the food, water, and the environment. Several analytical techniques such as electrochemical sensing, enzymatic techniques, chemiluminescence, capillary electrophoresis, and spectrofluorometry are commonly used in this field.4 Nevertheless, these methods possess several disadvantages such as high cost, hazardous solvents, labor intensive operation, and the generation of waste products.5 To overcome these issues, analytical methods using fluorescent probes have attracted more attention in the sensor field due to their selectivity, speed, and sensitivity. Fluorescence intensity quenching of the analyte provides biochemical information in a simple way.6 Various fluorescent materials, such as carbon quantum dots, organic dyes, organic polymers, metal nanoparticles, and metal–organic frameworks (MOFs), have been studied.7−9
Metal–organic frameworks (MOFs), based on the coordination of metal cations with organic linkers, form a class of hybrid materials.10 In this class, UiO-66 has been developed with different pore and cavity diameters and a variety of dimensionally connected UiO-66 materials have been reported. The well-developed pores in UiO-66 provide a platform for small molecules and ion pathways in transformation processes, allowing for the formation of porous nanostructures.11 UiO-66 has received considerable attention due to its porosity, surface area, structure, and chemical stability.12−14 UiO-66 can easily be developed as a possible template under appropriate conditions.15 These advanced and unique properties make UiO-66 attractive for various applications such as chemical sensors, adsorption, drug delivery, gas storage, and catalysis.16 Recently, nanomaterials have been fabricated by embedding or encapsulating nanoparticles within UiO-66; these nanomaterials have received increasing interest for many applications. The fabricated UiO-66 nanomaterials have been proven to enhance sensing, catalytic, and electric activity. Noble metals like copper, silver, and gold and carbon dots have attracted considerable attention for fabricating nanostructures with UiO-66. Gold nanomaterials in particular have unique properties, which enhance their functionality in all applications.11
Gold nanoparticles and nanoclusters (AuNCs) have received a lot of attention in the fields of chemistry, biology, and materials.2,17,18 In particular, Au clusters are subnanometer in size, have fluorescent properties, are biocompatible, and exhibit an energy transition between the highest occupied molecular orbital and lowest unoccupied molecular orbital (LUMO) levels.19 The strong fluorescence of the AuNCs can sense the target molecules with single-particle sensitivity.20 Proteins can play a major role in the synthesis of subnanometer-sized AuNCs under mild conditions, as thiol, amine, and carboxyl groups present in the protein can be used as reducing and stabilizing agents.21 On the basis of this unique property, the fluorescence quenching effects of gold have been used to detect a limited set of analytes, including Hg2+, Cu2+, and CN–.22 Several procedures are available to synthesize AuNCs using DNA, polymers, ligands, and dendrimers as a template.23 In the latter, bovine serum albumin (BSA) has been found to stabilize the metal ions at subnanometer size. BSA reacts with Au3+ ions and reduces them into Au+ ions with the help of biomolecules present in the protein.24 Recent studies suggest that forces between closed-shell atoms are physically powerful; this can particularly affect the interactions between Hg2+ and Au+.25
The aim of this research is to develop highly fluorescent, ecofriendly, and small-sized gold nanoclusters incorporated into a UiO-66 framework and illustrate highly fluorescent (red) emission at 650 nm. The synthesized nanocomposite has a lot of advantages such as high sensitivity, stability, and cost-effectiveness. We demonstrate that the synthesized nanocomposite can detect Hg2+ metal ions in both river and tap water samples via fluorescence intensity quenching.
Experimental Section
Materials
Zirconium chloride (Aldrich, 99.8%), 1,4-benzenedicarboxylic acid (H2BDC, Aldrich, 99%), HCl (Aldrich, 37%), N,N-dimethylformamide (DMF, Aldrich), gold(III) chloride trihydrate (HAuCl4·3H2O), sodium hydroxide (NaOH), hydrochloride human serum albumin, mercury nitrate monohydrate (Hg(NO3)2·H2O), and other metal ions were purchased from Sigma-Aldrich.
Instrumentation
Synthesized AuNCs@UiO-66 samples were diluted in deionized water for taking dark field images using a Photon Technology International (PTI) UV illuminator at 365 nm. The prepared materials were diluted well, and their UV spectra were measured using a Varian Cary 100 UV–vis spectrophotometer. High-resolution transmission electron microscopy (HRTEM) images were taken using Tecnai G2 F30 Series, the final nanoanalysis system that was set with a 300 kV Schottky FEG, with a high maximum beam current, patented S-TWIN objective lens, 0.102 nm of line resolution, 0.17 nm of high-resolution scanning TEM resolution, and 0.20 nm of point resolution, which was purchased from Hillsboro, OR. Photoluminescence (PL) fluorescence spectra were analyzed at an excitation wavelength of 450 nm using a fluorescence spectrometer (QuantaMaster, Photon Technology International, NJ) equipped with a xenon lamp (Arc Lamp Housing, A-1010B), monochromator, and power supply (Brytexbox). X-ray diffraction (XRD) patterns of the nanocomposites were acquired using a Rigaku Rint 2200 Series X-ray automatic diffractometer (Cu Kα radiation at a wavelength of 1.5406 Å) from Rigaku, TX. A Bruker vortex high-resolution 70 Fourier transform infrared (FTIR) spectrometer, equipped with BRUKER FTIR Vertex 70 with microplate extension high throughput screening extension and attenuated total reflection units, from Billerica, MA, was used to analyze FTIR spectra of the synthesized nanocomposite. The overall fluorescence lifetime decay curves were obtained using a Photon Technology International (PTI) EL series of nanosecond pulsed light-emitting diodes designed for the EasyLife II excitation wavelength ranging from 260 to 650 nm. For the background signal, we have used silica suspension in water, and the instrument response function was recorded using a 1% LUDOX LS. The curve fitting data were obtained using EasyLife II via a serial RS232 and USB connection under the control of the EasyLife II program.
Synthesis of UiO-66
UiO-66 was synthesized in accordance with previously reported methods with some modification.26,27 A mixture of zirconium chloride (Aldrich, 99.8%), 1,4-benzenedicarboxylic acid (H2BDC, Aldrich, 99%), and HCl (Aldrich, 37%) in N,N-dimethylformamide (DMF, Aldrich) was prepared by stirring. After that, the mixture was transferred to a Teflon-lined autoclave and kept at 393 K for 24 h in a conventional oven.
Synthesis of AuNCs@UiO-66
The AuNCs@UiO-66 samples were synthesized by mixing 5 mL of UiO-66 (50 mg/mL in ethanol) with 5 mL of HAucl4 and adding the mixture to BSA solution (50 mg/mL, 5 mL) and then stirred at 800 rpm. After 30 min, sodium hydroxide (1 M, 500 μL) was added and the solution was kept at 50 °C and vigorously stirred for 3–4 h. The color started to change from pale yellow to light brown. This color change indicates that AuNCs@UiO-66 was synthesized. Powder samples were collected by freeze-drying and used for further experiments.
Results and Discussion
Characterization of AuNCs@UiO-66
Figure 1a presents the UV–vis spectroscopy analysis of UiO-66 and the AuNCS@UiO-66 composite. The absorption band of UiO-66 is located at 320 nm; it could be caused by ligand-to-metal charge transfer. After the introduction of AuNCs, a small peak is present at the same position and another peak observed at 340 nm indicates the presence of AuNCs in UiO-66. In Figure 1b, we display the fluorescence spectra of the synthesized AuNCs@UiO-66 in PBS buffer. Deep red fluorescence was observed at 650 nm (emission) from an excitation wavelength of 450 nm. The inset image (i) shows the AuNCs@UiO-66 nanocomposite under visible light. Inset image (ii) shows the strong red fluorescence of AuNCs@UiO-66 under UV illumination. The synthesized AuNCs@UiO-66 nanocomposite exhibits a better quantum yield of 11%. In Figure 2a, we show the fluorescence intensity of AuNCs@UiO-66 under different excitation wavelengths and its pH stability. When the excitation wavelength was gradually changed by 20 nm from 340 to 600 nm, the PL peak red-shifted and the intensity also changed rapidly. The excitation-dependent PL characteristics of the different emission spectra are the result of the localization of electron hole pairs due to the isolated sp2 clusters within the sp3 matrix.28 However, we have analyzed the optimum pH for this sensing experiment (Figure 2b). A pH of 7.2 gives higher intensity than other pH values, and moreover, the synthesized AuNCs@UiO-66 nanocomposite was constant from 5 to 11 pH. Therefore, the whole experiment was done at pH 7.2.
Figure 1.
Analysis of UV–visible spectra (a) and PL spectrum (b) of synthesized materials. The inset images in figure (b): (i) is AuNCs@UiO-66 in visible light and (ii) AuNCs@UiO-66 under a UV lamp.
Figure 2.
Different excitation and pH response of AuNCs@UiO-66.
Figure 3a shows the powder XRD patterns for the AuNCs@UiO-66 nanocomposite compared to those for UiO-66. These results ensure that the crystallinity of UiO-66 is maintained after the formation of the composite with AuNCs is formed; distinct peaks were observed for AuNCs@UiO-66. This result suggests that the parent UiO-66 was well preserved during the in situ synthesis of AuNCs. The distinct 2θ peaks for UiO-66 were confirmed at around 7 and 8°, belonging to the (111) and (002) lattice planes. The same peaks were observed after the incorporation of AuNCs into UiO-66. The peak at around 46° for the (111) plane corresponds to the BSA–AuNCs. Moreover, further evidence that UiO-66 is well conjugated with the AuNCs was obtained from the FTIR spectrum in Figure 3b. The peak at around 1600 cm–1 represents the organic aromatic linkers in UiO-66.29 The peak at 1190–1240 cm–1 represents the −SO3H group, and the same peak is present in the composite with AuNCs. The −C–H, −OH, and −N–H stretching frequencies in the range of 2955–3300 cm–1 originate from the conformation of the AuNCs.
Figure 3.
Study of XRD spectrum (a) and FTIR spectrum (b) of AuNCs@UiO-66.
Morphology and elemental analyses of UiO-66 and AuNCs@UiO-66 were carried out by HRTEM (Figure 4). However, it is very difficult to acquire high-quality images of small-size (1–3 nm) gold nanoclusters due to the size of the electron beam. Figure 4a displays a low-magnification image of AuNCs@UiO-66, and the AuNCs are well dispersed on the surface of UiO-66.30,31 Moreover, the high-resolution TEM (Figure 4b) clearly shows that some of the AuNCs enter into the UiO-66 and some of them are present on the surface of UiO-66; the yellow circle and lines highlight the AuNCs present in UiO-66. An energy-dispersive X-ray spectroscopy (EDX) chemical map taken from the AuNCs@UiO-66 is shown in Figure 4c. From this image, it can be seen that the Au is completely conjugated with Zr in UiO-66. The overall TEM image clearly shows that AuNCs were fully functionalized with UiO-66.
Figure 4.
TEM morphological analysis of AuNCs@UiO-66. (a) Low-resolution image, (b) high-resolution image, and (c) EDX study of the composite. Here, we point the AuNCs in UiO-66 as yellow circles and arrows.
Detection of Mercury
We explored the feasibility of using the AuNCs@UiO-66 nanocomposite for the detection of Hg2+. AuNCs@UiO-66 exhibits a strong fluorescence at 650 nm in the absence of Hg2+. The AuNCs@UiO-66 fluorescence intensity was constantly quenched when Hg2+ concentration was increased from 800 nM to 10 μM (Figure 5a), and explicit PL quenching by 64% was observed after the addition of Hg2+ to the AuNCs@UiO-66. The PL quenching ratio (F0 – F/F0) at 450 nm was fitted to a linear function of Hg2+ ion concentration from 800 nM to 10 μM, with a high linear coefficient (R2 = 0.996), which coincidences with a detection limit of 77 pM (Figure 5b). A comparison of detection performance of various sensors toward Hg2+ is illustrated in Table 1. This observation indicates that Hg2+ are able to suppress the fluorescence of AuNCs@UiO-66, presumably via electron or energy transfer. The fluorescence quenching is illustrated by the Stern–Volmer equation.32
| 1 |
Here, F0 and F are the fluorescence quenching intensities in the absence and presence of metal ion, Kq represents the affinity between the fluorophore and the quenching ion by means of the Stern–Volmer constant, and [M] is the concentration of Hg2+. The sensitivity of Hg2+ was evaluated depending on the detection limit value. The detection limit was calculated by the following equation
| 2 |
Here, σ is the standard deviation of the least concentration and m is the slope of the concentration-dependent response.33
Figure 5.
PL emission spectra of AuNCs@UiO-66 with Hg2+ and different metal ions. (a) Hg2+ detection with various concentrations from 800 nM to 10 μM. (b) Linear relation between the F0 – F/F0 value and the various concentrations of Hg2+. (c) Comparison between the selectivity of as-synthesized AuNCs@UiO-66 for Hg2+ and for other metal ions.
Table 1. Detection Performance of Different Sensors for Detection of Hg2+.
Selectivity of AuNCs@UiO-66 toward Other Metal Ions
To estimate the selectivity of the fluorescence quenching sensor system, we measured the difference in the PL intensity of AuNCs@UiO-66 with various metal ions such as As3+, Co2+, Cd2+, Hg2+, Cr2+, Ni2+, Fe3+, Pb2+, Mg2+, and Mn2+ under the same conditions (Figure 5c). The developed sensor should exhibit not only high sensitivity but also augmented specificity. The prepared metal ions were incubated for 10 min, and PL analysis was carried out at 450 nm excitation wavelength. The PL intensity of AuNCs@UiO-66 was quenched (∼91%) with 10 μM Hg2+. The PL of AuNCs@UiO-66 showed much lesser intensity in the presence of Hg2+ compared to that in the presence of the rest of the metal ions. Zirconium oxide could be considered as an active metal node of UiO-66. The mechanism behind the detection of Hg2+ by AuNCs@UiO-66 is mainly the easy binding of Hg2+ ions to the chemically active sites on the UiO-66 framework.34 Moreover, the physical properties of the AuNCs@UiO-66 sensor, such as the organic nature of UiO-66, its high surface area, its morphology, and size of the particle, are significant for the detection of the target ion and its binding to the sensor.
Detection Performance of AuNCs@UiO-66 toward Hg2+ in Real Water Samples
For the realistic application of the synthesized AuNCs@UiO-66-based sensor, various concentrations of Hg2+ ranging from 1 to 4 μM were spiked into river and tap water samples. The environmental aqua samples were collected from river (Hangang) and tap water (Gachon University) (Seoul, South Korea) sources. The river water samples were filtered with 5 mm qualitative filter paper to remove impurities and centrifuged at 10 000 rpm for 30 min before the experiments. The quenching efficiency (F0/F) showed a better linear correlation coefficient (R2 = 0.9907) with respect to the different concentrations of Hg2+, as shown in Figure 6a. The limit of detection was achieved at 193 pM by AuNCs@UiO-66 in river water (Figure 6b). Furthermore, recovery values of the spiked river water sample are obtained ranging from 101.6 to 98.5% (Table 2).
Figure 6.
(a) PL emission spectra of AuNCs@UiO-66 in Hg2+-spiked river water and (b) linear fit of fluorescence quenching as a function of Hg2+ concentration.
Table 2. Hg2+ Detection in the Spiked River Water Sample.
| sample | spiked | found ± SD | % recovery | RSD (%) |
|---|---|---|---|---|
| river water | 1 | 1.016 ± 0.066 | 101.6 | 6.30 |
| 2 | 2.11 ± 0.090 | 105.5 | 4.27 | |
| 3 | 2.82 ± 0.047 | 94 | 1.67 | |
| 4 | 3.94 ± 0.080 | 98.5 | 2.04 |
The tap water samples were spiked with Hg2+ in concentrations ranging from 1 to 4 μM (Figure 7a). A plot of F0 – F/F0 against the spiked Hg2+ concentration shows a linear squared correlation of 0.983 (Figure 7b), and the limit of detection was achieved at 223 pM. The calculated recovery values of Hg2+ in the spiked tap water samples are shown in Table 3.
Figure 7.
(a) Fluorescence emission spectra of AuNCs@UiO-66 in Hg2+-spiked tap water and (b) linear fit of fluorescence quenching as a function of Hg2+ concentration.
Table 3. Detection of Hg2+ in the Spiked Tap Water Sample.
| sample | spiked | found ± SD | % recovery | RSD (%) |
|---|---|---|---|---|
| tap water | 1 | 1.009 ± 0.080 | 100.9 | 7.92 |
| 2 | 2.14 ± 0.100 | 107 | 4.70 | |
| 3 | 3.02 ± 0.085 | 100.6 | 2.82 | |
| 4 | 3.74 ± 0.065 | 93.5 | 1.74 |
Fluorescence Quenching Mechanism of AuNCs@ UiO-66 for Hg2+
The fluorescence quenching mechanism of synthesized AuNCs@UiO-66 in the presence of Hg2+ metal ions is shown in Figure 8. The fluorescence decay curves can be built in a double exponential function using the following equation
| 3 |
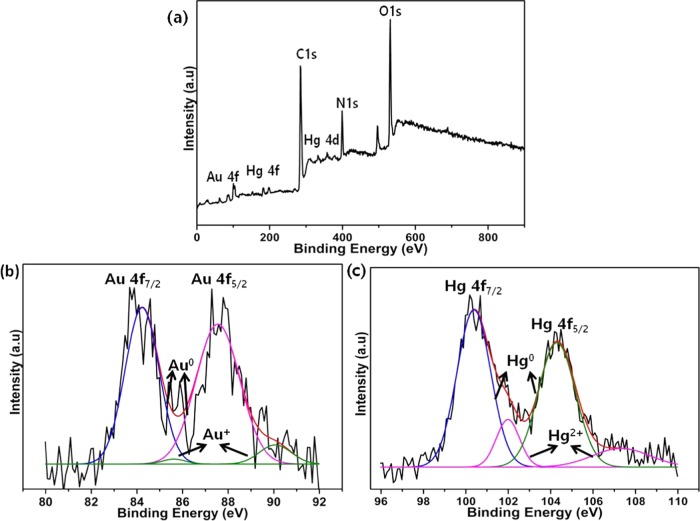
where D is the PL decay, τ is the lifetime, τi is the PL lifetime of different fluorescent types, and ai is the equivalent pre-exponential factor.35 Bare AuNCs@UiO-66 shows lifetimes of τ1 = 12.01 and τ2 = 7.07 ns in the absence of Hg2+ metal ions. After the addition of Hg2+, a drastic decrease in the lifetimes of 5.30 and 0.58 ns was observed for τ1 and τ2, respectively, due to electron transfer. This decrease in lifetime clearly indicates that electrons transferred to the LUMO energy level from the conduction band due to the Hg2+ metal ion.36 Moreover, we have investigated the interaction between AuNCs and Hg2+ by X-ray photoelectron spectroscopy (XPS) (Figure 9). The survey scan clearly shows the presence of C, Au, N, Hg, and O (Figure 9a). The peaks located at around 84.13 and 87.5 eV, corresponding to Au 4f, were allocated to metallic gold (Au0), and the peaks at around 85.7 and 90.01 were assigned to ionic gold (Au+) (Figure 9b).37,38 In Figure 9c, the peaks at 100.9 and 104.2 eV for Hg 4f spectrum corresponded to metallic mercury (Hg0).39,40 These results indicate that the Hg2+ ions were reduced by AuNCs and formed a Au–Hg amalgam.41 Therefore, it is predictable here that the amino group containing BSA performs the fluorescence quenching after interaction of Hg2+ ions with BSA and Hg2+ ions, binding them tightly to each other.
Figure 8.

Time-resolved fluorescence decay of AuNCs@UiO-66 in the presence and absence of Hg2+.
Figure 9.
XPS analysis of AuNCs@UiO-66 interacting with Hg2+. (a) Survey scan, (b) Au after treatment with Hg2+, and (c) Hg 4f spectrum.
Conclusions
We have developed high red fluorescence AuNCs incorporated in UiO-66 frameworks with a high QY (11%). The AuNCs were well dispersed, spherical in shape, and 2 nm in size on average. Due to the porosity and surface area of UiO-66, the AuNCs were homogeneously distributed along the UiO-66 framework. The AuNCs@UiO-66 nanocomposite shows high Hg2+ detection sensitivity (77 pM) and also high sensitivity to the real water samples, including river and tap water, with the limit of detection being 193 and 223 pM, respectively, and high levels of recovery. The developed sensor may prove useful because of the simple procedure, high fluorescence, and selective sensitive methods to detect the Hg2+ ions and also be advantageous for applications in nanotechnology and other biosensor fields.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (No. 2017R1A2B4004700) and by the Research program of Ministry of Trade, Industry and Energy (Grant no.: 20000580).
The authors declare no competing financial interest.
References
- Wei H.; Wang Z.; Yang L.; Tian S.; Hou C.; Lu Y. Lysozyme-stabilized gold fluorescent cluster: synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. 10.1039/c0an00046a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi Y.; Takasugi Y.; Sato S.; Yao H.; Kimura K.; Tsukuda T. Ix-s Structures, Stabilities and Physicochemical Properties of Organometallic Hybrid Clusters. J. Am. Chem. Soc. 2004, 126, 6518–6519. 10.1021/ja0483589. [DOI] [PubMed] [Google Scholar]
- Darbha G. K.; Ray A.; Ray P. C. Gold nanoparticle-based miniaturized nanomaterial surface energy transfer probe for rapid and ultrasensitive detection of mercury in soil, water, and fish. ACS Nano 2007, 1, 208–214. 10.1021/nn7001954. [DOI] [PubMed] [Google Scholar]
- Liu J. M.; Cui M. L.; Jiang S. L.; Wang X. X.; Lin L. P.; Jiao L.; Zhang L. H.; Zheng Z. Y. BSA-protected gold nanoclusters as fluorescent sensor for selective and sensitive detection of pyrophosphate. Anal. Methods 2013, 5, 3942–3947. 10.1039/c3ay00054k. [DOI] [Google Scholar]
- Venkateswarlu S.; Viswanath B.; Reddy A. S.; Yoon M. Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Sens. Actuators, B 2017, 258, 172–183. 10.1016/j.snb.2017.11.044. [DOI] [Google Scholar]
- Ma Y.; Yang C.; Li N.; Yang X. A sensitive method for the detection of catecholamine based on fluorescence quenching of CdSe nanocrystals. Talanta 2005, 67, 979–983. 10.1016/j.talanta.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Tatay S.; Gaviña P.; Coronado E.; Palomares E. Optical mercury sensing using a benzothiazolium hemicyanine dye. Org. Lett. 2006, 8, 3857–3860. 10.1021/ol0615580. [DOI] [PubMed] [Google Scholar]
- Li M.; Wang Q.; Shi X.; Hornak L. A.; Wu N. Detection of mercury by quantum dot/DNA/gold nanoparticle ensemble based nanosensor via nanometal surface energy transfer. Anal. Chem. 2011, 83, 7061–7065. 10.1021/ac2019014. [DOI] [PubMed] [Google Scholar]
- Li W. X.; Li H. X.; Li H. Y.; Chen M. M.; Shi Y. X.; Lang J. P. 1,4-Bis(2-(pyridin-4-yl) vinyl) naphthalene and its Zinc Coordination Polymers: Synthesis, Structural Characterization and Selective Luminescent Sensing of Mercury ion. Cryst. Growth Des. 2017, 3948–3959. 10.1021/acs.cgd.7b00575. [DOI] [Google Scholar]
- Lü Y.; Zhan W.; He Y.; Wang Y.; Kong X.; Kuang Q.; Xie Z.; Zheng L. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. 10.1021/am405858v. [DOI] [PubMed] [Google Scholar]
- Xia J.; Diao K.; Zheng Z.; Cui X. Porous Au/ZnO nanoparticles synthesised through a metal organic framework (MOF) route for enhanced acetone gas-sensing. RSC Adv. 2017, 7, 38444–38451. 10.1039/C7RA06690B. [DOI] [Google Scholar]
- Yaghi O. M.; O’keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Férey G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. 10.1039/B618320B. [DOI] [PubMed] [Google Scholar]
- Horike S.; Shimomura S.; Kitagawa S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. 10.1038/nchem.444. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Lü Y.; Zhan W.; Xie Z.; Kuang Q.; Zheng L. Synthesis of porous Cu2O/CuO cages using Cu-based metal–organic frameworks as templates and their gas-sensing properties. J. Mater. Chem. A 2015, 3, 12796–12803. 10.1039/C5TA01108F. [DOI] [Google Scholar]
- Wu R.; Qian X.; Zhou K.; Liu H.; Yadian B.; Wei J.; Zhu H.; Huang Y. Highly dispersed Au nanoparticles immobilized on Zr-based metal–organic frameworks as heterostructured catalyst for CO oxidation. J. Mater. Chem. A 2013, 1, 14294–14299. 10.1039/c3ta13114a. [DOI] [Google Scholar]
- Wu Z.; Jin R. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010, 10, 2568–2573. 10.1021/nl101225f. [DOI] [PubMed] [Google Scholar]
- Lin C.-A. J.; Yang T. Y.; Lee C. H.; et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- Chevrier D. M.; Chatt A.; Zhang P. Properties and applications of protein-stabilized fluorescent gold nanoclusters: short review. J. Nanophotonics 2012, 6, 064501 10.1117/1.JNP.6.064504. [DOI] [Google Scholar]
- Cho E. S.; Kim J.; Tejerina B.; et al. Ultrasensitive detection of toxic cations through changes in the tunnelling current across films of striped nanoparticles. Nat. Mater. 2012, 11, 978–985. 10.1038/nmat3406. [DOI] [PubMed] [Google Scholar]
- Guo C.; Irudayaraj J. Fluorescent Ag clusters via a protein-directed approach as a Hg [2] ion sensor. Anal. Chem. 2011, 83, 2883–2889. 10.1021/ac1032403. [DOI] [PubMed] [Google Scholar]
- Wen F.; Dong Y.; Feng L.; Wang S.; Zhang S.; Zhang X. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing. Anal. Chem. 2011, 83, 1193–1196. 10.1021/ac1031447. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Han M. S.; Mirkin C. A. Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew. Chem., Int. Ed. 2007, 46, 4093–4096. 10.1002/anie.200700269. [DOI] [PubMed] [Google Scholar]
- Govindaraju S.; Ankireddy S. R.; Viswanath B.; Kim J.; Yun K. Fluorescent gold nanoclusters for selective detection of dopamine in cerebrospinal fluid. Sci. Rep. 2017, 7, 40298 10.1038/srep40298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.; Zheng Y.; Ying J. Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem. Commun. 2010, 46, 961–963. 10.1039/B920748A. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Zlotea C.; Phanon D.; Mazaj M.; et al. Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs. Dalton Trans. 2011, 40, 4879–4881. 10.1039/c1dt10115c. [DOI] [PubMed] [Google Scholar]
- Lin T. N.; Chih K.; Yuan C.; Shen J.; Lin C.; Liu W. Laser-ablation production of graphene oxide nanostructures: from ribbons to quantum dots. Nanoscale 2015, 7, 2708–2715. 10.1039/C4NR05737F. [DOI] [PubMed] [Google Scholar]
- Kandiah M.; Usseglio S.; Svelle S.; Olsbye U.; Lillerud K. P.; Tilset M. Post-synthetic modification of the metal–organic framework compound UiO-66. J. Mater. Chem. 2010, 20, 9848–9851. 10.1039/c0jm02416c. [DOI] [Google Scholar]
- Pourkhosravani M.; Dehghanpour S.; Farzaneh F. Palladium Nanoparticles Supported on Zirconium Metal Organic Framework as an Efficient Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction. Catal. Lett. 2016, 146, 499–508. 10.1007/s10562-015-1674-5. [DOI] [Google Scholar]
- Bakuru V. R.; Velaga B.; Peela N. R.; Kalidindi S. B. Hybridization of Pd Nanoparticles with UiO-66 (Hf) Metal–Organic Framework and the Effect of Nanostructure on the Catalytic Properties. Chem. - Eur. J. 2018, 10.1002/chem.201803200. [DOI] [PubMed] [Google Scholar]
- Liu S.; Tian J.; Wang L.; Zhang Y.; Qin X.; Luo Y.; Asiri A. M.; Al-Youbi A. O.; Sun X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu[2] Ions. Adv. Mater. 2012, 24, 2037–2041. 10.1002/adma.201200164. [DOI] [PubMed] [Google Scholar]
- Li N.; Than A.; Wang X.; Xu S.; Sun L.; Duan H.; Xu C.; Chen P. Ultrasensitive profiling of metabolites using tyramine-functionalized graphene quantum dots. ACS Nano 2016, 10, 3622–3629. 10.1021/acsnano.5b08103. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Shen B.; Zhu S.; Xu H.; Tian L. UiO-66 and its Br-modified derivates for elemental mercury removal. J. Hazard. Mater. 2016, 320, 556–563. 10.1016/j.jhazmat.2016.08.039. [DOI] [PubMed] [Google Scholar]
- Raut S.; Rich R.; Fudala R.; Butler S.; Kokate R.; Gryczynski Z.; Luchowski R.; Gryczynski I. Resonance energy transfer between fluorescent BSA protected Au nanoclusters and organic fluorophores. Nanoscale 2014, 6, 385–391. 10.1039/C3NR03886F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Xu C.; Tian Z.; Lin Y.; Shi Z. Facilely synthesized N-doped carbon quantum dots with high fluorescent yield for sensing Fe3+. New J. Chem. 2016, 40, 2083–2088. 10.1039/C5NJ03252K. [DOI] [Google Scholar]
- Das S. K.; Dickinson C.; Lafir F.; Brougham D. F.; Marsili E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. 10.1039/c2gc16676c. [DOI] [Google Scholar]
- Ballestero D.; Juan R.; Gómez-Giménez C.; García-Díez E.; Ruiz C.; Rubio B.; Izquierdo M. T. Novel methodology for gold nanoparticles deposition on carbon monolith supports. Colloids Surf., A 2014, 441, 91–100. 10.1016/j.colsurfa.2013.08.055. [DOI] [Google Scholar]
- Singh A.; Pasricha R.; Sastry M. Ultra-low level optical detection of mercuric ions using biogenic gold nanotriangles. Analyst 2012, 137, 3083–3090. 10.1039/c2an35162e. [DOI] [PubMed] [Google Scholar]
- Ren W.; Zhu C.; Wang E. Enhanced sensitivity of a direct SERS technique for Hg2+ detection based on the investigation of the interaction between silver nanoparticles and mercury ions. Nanoscale 2012, 4, 5902–5909. 10.1039/c2nr31410j. [DOI] [PubMed] [Google Scholar]
- Senthamizhan A.; Celebioglu A.; Uyar T. Real-time selective visual monitoring of Hg2+ detection at ppt level: an approach to lighting electrospun nanofibers using gold nanoclusters. Sci. Rep. 2015, 5, 10403 10.1038/srep10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C.; Yang Z.; Lee K. H.; Chang H. T. Synthesis of highly fluorescent gold nanoparticles for sensing mercury (II). Angew. Chem., Int. Ed. 2007, 46, 6824–6828. 10.1002/anie.200700803. [DOI] [PubMed] [Google Scholar]
- Wei H.; Zidong W.; Limin Y.; Shiliang T.; Changjun H.; Yi L. Lysozyme-stabilized gold fluorescent cluster: synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406–1410. 10.1039/c0an00046a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L.; Yang L.; Stockmar F.; Popescu R.; Trouillet V.; Bruns M.; Gerthsen D.; Nienhaus G. U. Microwave-assisted rapid synthesis of luminescent gold nanoclusters for sensing Hg2+ in living cells using fluorescence imaging. Nanoscale 2012, 4, 4155–4160. 10.1039/c2nr30219e. [DOI] [PubMed] [Google Scholar]
- Chen G. H.; Chen W. Y.; Yen Y. C.; Wang C. W.; Chang H. T.; Chen C. F. Detection of mercury (II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. 10.1021/ac5008688. [DOI] [PubMed] [Google Scholar]