Abstract
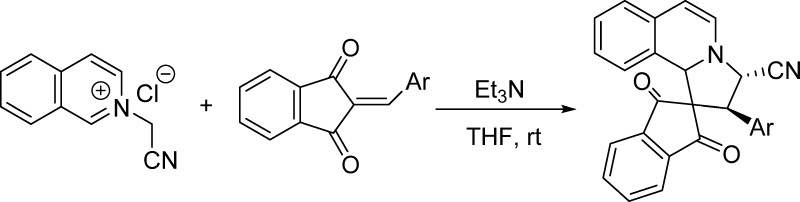
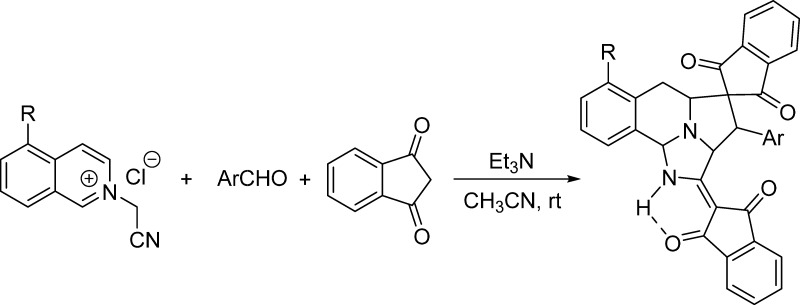
The base-promoted cycloaddition reaction of N-cyanomethylisoquinolinium chloride with 2-arylidene-1,3-indanediones in dry tetrahydrofuran resulted in the expected spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline] derivatives. However, the triethylamine-promoted three-component reaction of N-cyanomethylisoquinolinium chloride, aromatic aldehydes, and two molecules of 1,3-indanediones in acetonitrile afforded unique spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene] derivatives in satisfactory yields through tandem double [3 + 2] cycloaddition reactions.
Introduction
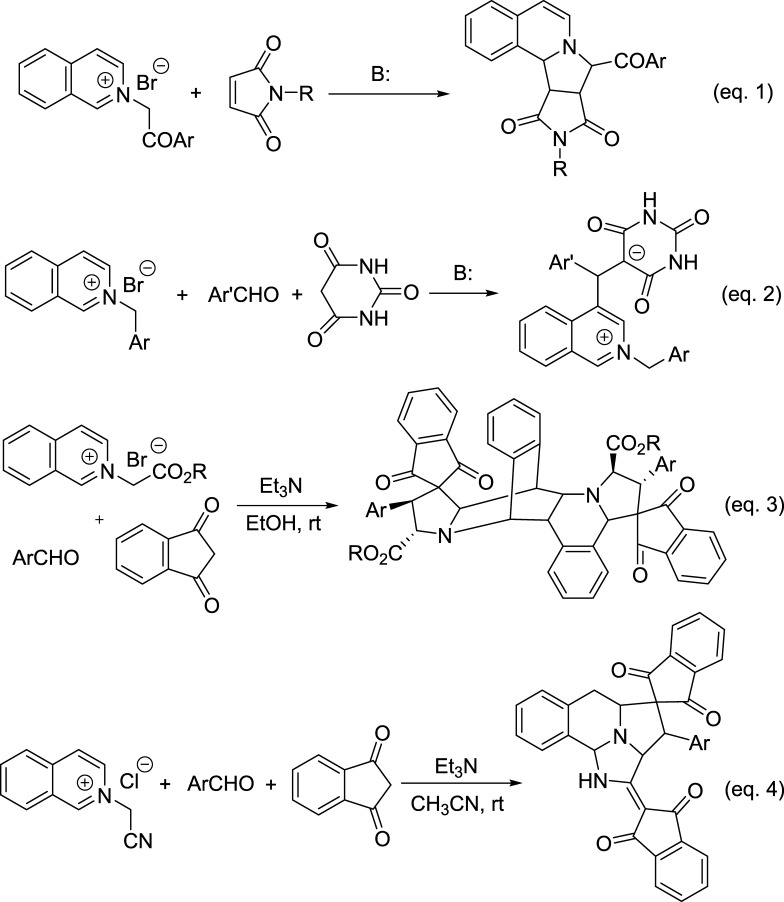
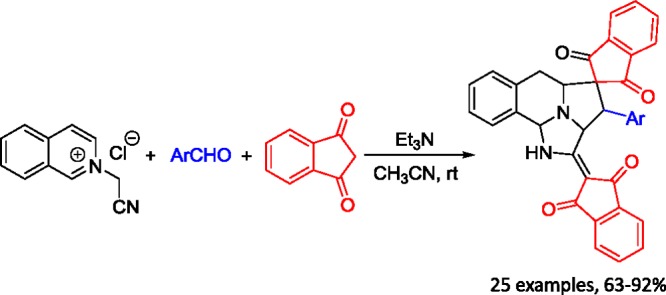
As one of the reactive heteroaromatic N-ylides, isoquinolinium ylides have been attracting continuous attention due to their easy preparation and versatile reactivity and become one of the most valuable synthons for the construction of nitrogen-containing heterocyclic systems.1,2 Isoquinolinium ylides can be conveniently generated in situ from deprotonation of the corresponding N-substituted methylisoquinolinium salts in basic media.3 The most common reaction of isoquinolinium ylide is the [3 + 2] cycloaddition reaction with various 1,3-dipolarophiles to give pyrrolo[2,1-a]isoquinoline derivatives (eq 1 in Scheme 1).4 By employing various substrates and adopting different reaction conditions, these kinds of reactions have resulted in many pyrrolo[2,1-a]isoquinoline derivatives and related spiro or polycyclic systems with valuable molecular diversity and regulated diastereoselectivity.5 In recent years, new synthetic reaction patterns of isoquinolinium ylides have been successfully revealed, which indicated that there are many unknown characters in the chemistry of isoquinolinium ylides.6 For examples, the base-promoted reaction of N-phenacylisoquinolinium ylides with arylidene Meldrum acid resulted in stable zwitterionic salts.7a More notably, the isoquinolinium zwitterionic salts with an unusual C-4 substitution pattern were also obtained from the reaction of N-benzylisoquinolinium ylides with aromatic aldehydes and cyclic 1,3-dicarbonyl compounds (eq 2 in Scheme 1).7b The base-mediated three-component reaction of N-alkoxycarbonylmethylisoquinolinium salts, aromatic aldehydes, and 1,3-indanedione afforded the unprecedented complex polycyclic compounds, in which the generated isoquinilonium ylide not only behaved as a reactive 1,3-dipole but also acted as a useful diene to accomplish sequential 1,3-dipolar cycloaddition and the Diels–Alder reaction (eq 3 in Scheme 1).8 In continuation of our aim to exploit the potential synthetic applications of the heteroaromatic N-ylides,8 herein, we wish to reveal the unprecedented reactions of N-cyanomethylisoquinolinium chloride with 2-arylidene-1,3-indanediones under different reaction conditions. The reaction not only gave the normal spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline] derivatives through the normal 1,3-dipolar cycloaddition reaction but also afforded unique spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene] derivatives by the domino cyclization process (eq 4 in Scheme 1).
Scheme 1. Illustration of Typical Reaction Patterns of Isoquinolinium Ylides.
Results and Discussion
Initially, the conditions for the reaction of N-cyanomethylisoquinolinium chloride with 2-benzylidene-1,3-indanedione were briefly examined on the basis of our previously established reaction conditions for the N-phenacyl and N-ethoxycarbonylmethylisoquinolinium salts.8 When the reaction was carried out in the solvent of MeOH, EtOH, CH3CN and dry tetrahydrofuran (THF) in the presence of triethylamine as base for about 10 h, the expected spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline], 1a, was obtained in 47, 51, 56, and 81% yields. When 1,4-diazabicyclo[2.2.2]octane (DABCO), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and piperidine were used as base, the reaction in dry THF gave 45, 59, and 57% yields. When the triethylamine-promoted reaction was carried out in THF at about 50 °C for 5 h, the yield of product 1a greatly decreased to 31%. Thus, in the presence of slight excess triethylamine, the reactions of N-cyanomethylisoquinolinium chloride with various 2-arylidene-1,3-indanediones in dry THF at room temperature (rt) for about 10 h afforded spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1a–f in good yields. The structures of spiro compounds were successfully characterized by IR, high-resolution mass spectrometry (HRMS), 1H NMR, and 13C NMR spectra. It should be pointed out that spiro compounds 1a–f are not very stable in solution, especially in the protonic solvent or hydrous solvent. It must be quick to separate samples and record their 1H and 13C NMR spectra. The formation of spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1a–f obviously took place from the normal [3 + 2] cycloaddition of the in situ-generated isoquinolinium ylide with 2-arylidene-1,3-indanediones. The 1H NMR and 13C NMR spectra clearly indicated that only one diastereoisomer exists in the obtained samples of the products. On the basis of NMR spectra and previously obtained results, we tentatively assigned the spiro compounds 1a–f having trans-configuration.
Because 2-arylidene-1,3-indanediones were initially prepared from the base-catalyzed condensation of aromatic aldehydes with 1,3-indanedione, it is natural to test the possibility of converting the above reaction to a one-pot three-component reaction. When equal amounts of N-cyanomethylisoquinolinium chloride, benzaldehyde, and 1,3-indanedione were stirred in ethanol in the presence of triethylamine, instead of the formation of above spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline], a unique spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene] 2a was obtained in 31% yield, in which two scaffolds of 1,3-indanedione were eventually included in the molecule. When excess 1,3-indanedione was employed and triethylamine was used as the base, the reaction in MeOH, EtOH, and CH3CN gave new product 2a in 51, 67, and 83% yields, respectively. When other bases such as DABCO, DBU, and Na2CO3 were used as the base, the yield of the product did not increase. Prolonging reaction time or carrying out the reaction at an elevated temperature also caused a decrease in yield. Thus, the best conditions for this reaction are carrying out the reaction in acetonitrile at room temperature in the presence of triethylamine as the base promoter. Under these convenient reaction conditions, the reaction scope was investigated, and the obtained results are summarized in Tables 1 and 2. We were pleased to find that various aromatic aldehydes with different substituents reacted smoothly to give spiro polycyclic compounds 2a–o in high yields. Some heterocylic substituted aldehydes, such as 2-furfural, 2-thiophenecarbaldehyde, picolinaldehyde, nicotinaldehyde, and isonicotinaldehyde, also gave desired products 2p–t in good yields. n-Heptaldehyde was also successfully employed in the reaction to give spiro products 2x and 2y in good yields. Phenylglyoxal also afforded benzoyl-substituted product 2y in 85% yield. On the other hand, the reaction with substituted isoquinolines such as 5-bromoisoquinoline also resulted in spiro products 2v and 2w in satisfactory yields. These results indicated that this reaction has a wide variety of substrates.
Table 1. Synthesis of Spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1a–fa.
| entry | compd | Ar | yield (%)b |
|---|---|---|---|
| 1 | 1a | C6H5 | 81 |
| 2 | 1b | o-CH3OC6H4 | 84 |
| 3 | 1c | p-CH3C6H4 | 79 |
| 4 | 1d | p-BrC6H4 | 83 |
| 5 | 1e | p-ClC6H4 | 81 |
| 6 | 1f | p-(t-Bu)C6H4 | 61 |
Reaction conditions: isoquinolinium salt (0.5 mmol), 2-arylidene-1,3-indanedione (0.5 mmol), Et3N (0.6 mmol), THF (15.0 mL), rt, 10 h.
Isolated yields.
Table 2. Synthesis of Polycyclic Spiro Compounds 2a–ua.
| entry | compd | R | Ar | yield (%)b |
|---|---|---|---|---|
| 1 | 2a | H | C6H5 | 83 |
| 2 | 2b | H | o-HOC6H4 | 78 |
| 3 | 2c | H | o-CH3OC6H4 | 87 |
| 4 | 2d | H | o-ClC6H4 | 89 |
| 5 | 2e | H | o-BrC6H4 | 85 |
| 6 | 2f | H | m-CH3C6H4 | 86 |
| 7 | 2g | H | m-FC6H4 | 81 |
| 8 | 2h | H | m-NO2C6H4 | 82 |
| 9 | 2i | H | p-CH3C6H4 | 92 |
| 10 | 2j | H | p-(CH3)2NC6H4 | 73 |
| 11 | 2k | H | p-BrC6H4 | 87 |
| 12 | 2l | H | p-ClC6H4 | 91 |
| 13 | 2m | H | p-t-C(CH3)3C6H4 | 81 |
| 14 | 2n | H | p-NO2C6H4 | 79 |
| 15 | 2o | H | 2-HO-4-ClC6H3 | 72 |
| 16 | 2p | H | 2-furan | 69 |
| 17 | 2q | H | 2-thiophen | 78 |
| 18 | 2r | H | 2-Py | 83 |
| 19 | 2s | H | 3-Py | 76 |
| 20 | 2t | H | 4-Py | 74 |
| 21 | 2u | H | benzoyl | 85 |
| 22 | 2v | Br | p-ClC6H4 | 81 |
| 23 | 2w | Br | p-NO2C6H4 | 83 |
| 24 | 2x | H | n-Hex | 70 |
| 25 | 2y | Br | n-Hex | 63 |
Reaction conditions: isoquinolinium salt (0.5 mmol), aldehyde (0.5 mmol), 1,3-indanedione (1.1 mmol), Et3N (1.2 mmol), CH3CN (15.0 mL), rt, 8 h.
Isolated yields.
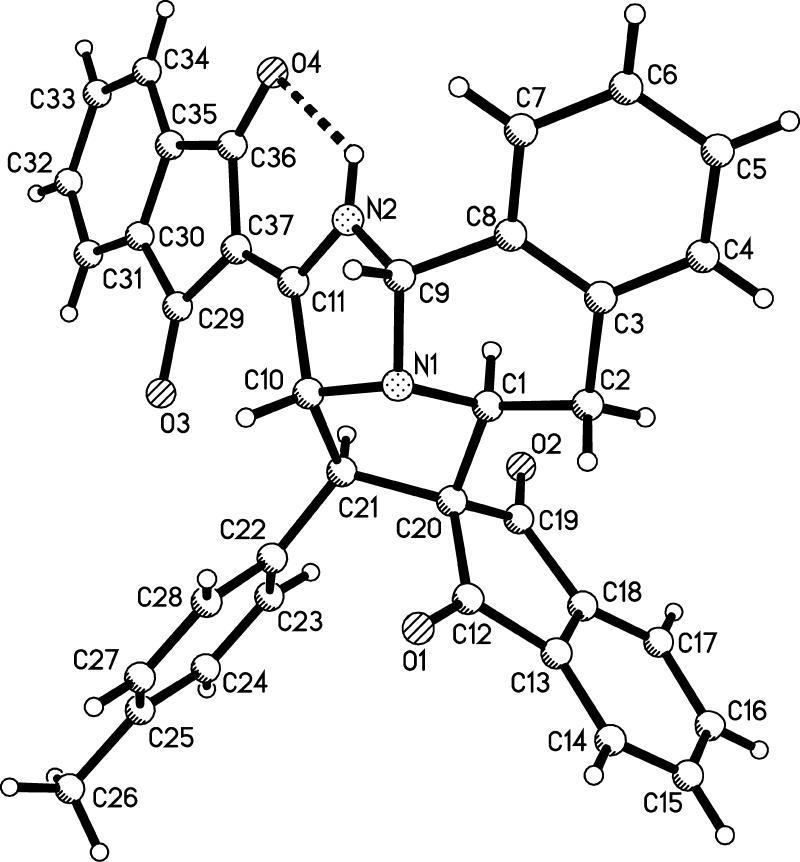
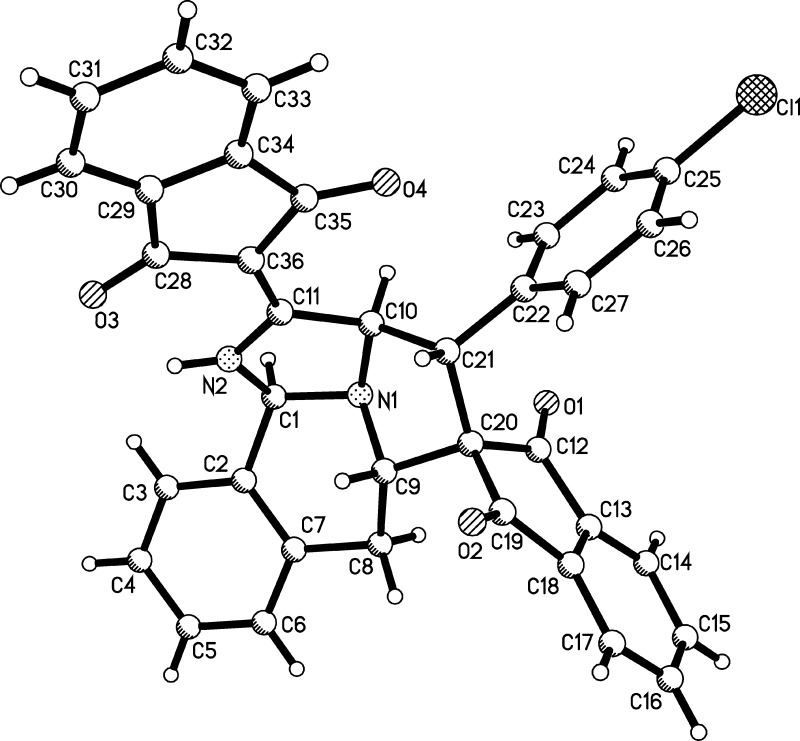
The structures of spiro compounds 2a–y were successfully determined by various spectroscopy techniques. The single-crystal structures of two representative compounds 2i and 2l were successfully determined by the X-ray diffraction method (Figure 1). From Figures 1 and 2, it can be seen that both C-1 and C-3 atoms of isoquinolinium ylide took part in the reaction. Although the pyridinium salts sometimes undergo the double [3 + 2] cycloaddition reaction at both C-1 and C-6 atoms to give novel pyrrolo[2,1,5-cd]indolizine derivatives,10 it is rare for isoquinolinium salts to undergo such double [3 + 2] cycloaddition reactions at both C-1 and C-3 atoms of the isoquinoline ring. The only example is the early report about the formation of benzocyclo[3.2.2]azines in lower yield from the double [3 + 2] cycloaddition reaction of isoquinolinium ylide with N-methylmaleimide.11a Another related example is the NHC-catalyzed enantioselective dearomatizing double Mannich reaction at both C-1 and C-3 atoms of isoquinolinium salts to give 8-azabicyclo[3.2.1]octane derivatives.11b On the other hand, there are several reports about the cyano group to take part in the sequential cyclization process in the reactions of N-cyanomethylpyridinium salts and N-cyanomethylisoquinolinium salts.12 Here, the normal [3 + 2] cycloaddition at 1,2-positions of isoquinolinium ylide was replaced by the abnormal [3 + 2] cycloaddition at 2,3-positions of isoquinolinium ylide and internal cyclization at the C-1 position. This kind of reaction pattern might be observed for the first time in the reactions of isoquinolinium ylides. It is also noticed that this reaction successfully provided a convenient dearomatizing method for the isoquinoline ring.
Figure 1.
Single-crystal structure of compounds 2i.
Figure 2.
Single-crystal structure of compounds 2l.
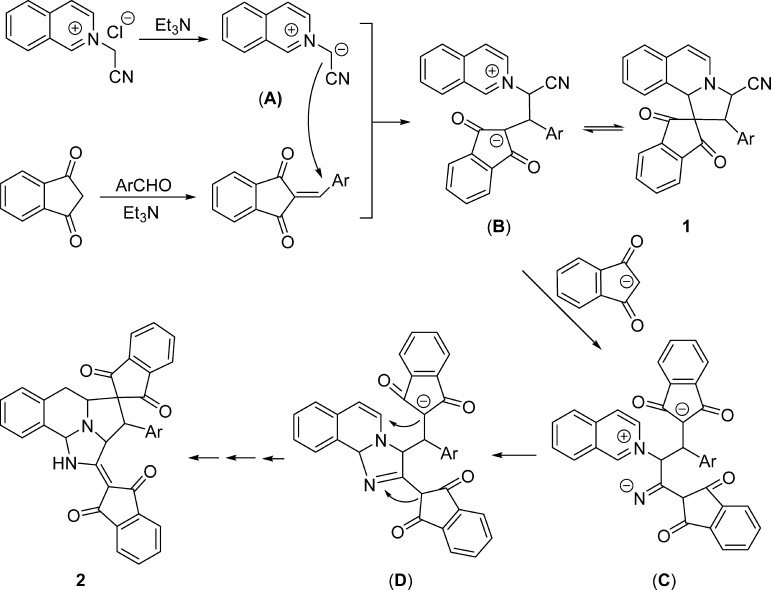
Although the exact mechanism is not completely understood at the present time, a plausible reaction mechanism is proposed on the basis of the above results and the previously reported reactions (Scheme 2).9−12 First, an isoquinolinium ylide (A) was generated from deprotonation of N-cyanomethylisoquinolinium salt. In the meantime, 2-arylidene-1,3-indanedione was also formed from the Knoevenagel condensation of the respective aromatic aldehyde with 1,3-indanedione in the presence of triethylamine. Second, a Michael addition of a isoquinolinium ylide (A) to 2-arylidene-1,3-indanedione afforded a zwitterionic intermediate (B). Third, the intermolecular cyclization resulted in spiro compound 1 in dry THF. Spiro compound 1 is not very stable and is in equilibrium with the zwitterionic intermediate (B) in acetonitrile. Then, the nucleophilic addition of the carbanion of 1,3-indanedione to the cyano group generated an imine intermediate (C), which in turn added to the cyclic iminium ion to give a cyclic intermediate (D). Finally, the intermolecular nucleophilic addition of carbanion to enamine resulted in polycyclic product 2 with the corresponding protonation and proton immigration process. Here, the unique dual reactivities of the cyano group and 1,3-indanedione are the crucial factors for completing this reaction sequence.
Scheme 2. Proposed Reaction Mechanism.
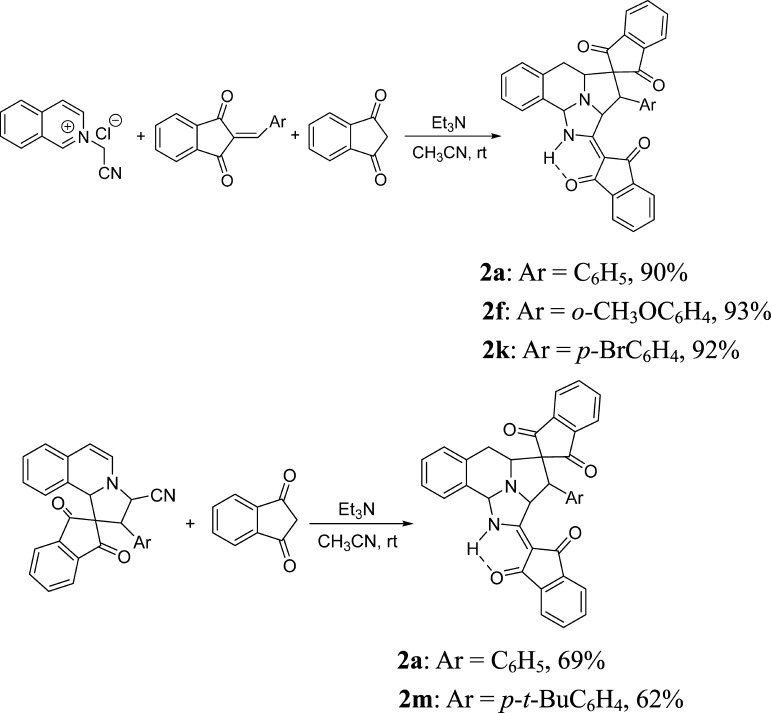
To probe the credibility of our proposed mechanistic scheme and shed more light on the formation of polycyclic spiro compounds, further control experiments were carried out (Scheme 3). First, an equal combination of 1,3-indanedione and initially prepared 2-arylidene-1,3-indanedione was used in the three-component reaction, and corresponding spiro compounds 2a, 2f, and 2k were obtained in very high yields, which clearly indicated that 2-arylidene-1,3-indanedione was the intermediate in the reaction. Second, the reaction of initially prepared spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1 with 1,3-indandione in acetonitrile in the presence of triethylamine for overnight resulted in polycyclic spiro compounds 2a and 2m in moderate yields, which clearly showed that spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1 decomposed to the zwitterionic intermediate (B) in the solution, which is the key intermediate in the above proposed mechanism.
Scheme 3. Control Experiments.
Conclusions
In summary, we have successfully investigated the base-promoted cycloaddition reaction of N-cyanomethylisoquinolinium chloride with 2-arylidene-1,3-indanediones under different reaction conditions. The reaction not only gave the normal spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline] derivatives through the normal 1,3-dipolar cycloaddition reaction but also afforded unique spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene] derivatives via tandem double [3 + 2] cycloaddition reactions. The reaction originated from the versatile reactivity of the well-known heteroaromatic N-ylides and successfully revealed a new reaction pattern for isoquinolinium ylides in heterocyclic synthesis. The potential applications of this convenient synthetic protocol in organic and medicinal chemistry might be significant.
Experimental Section
General Procedure for the Preparation of Spiro[indene-2,1′-pyrrolo[2,1-a]isoquinolines] 1a–f
A mixture of N-cyanomethylisoquinolinium chloride (0.5 mmol), 2-arylidene-1,3-indanedione (0.5 mmol), and triethylamine (0.6 mmol) in dry tetrahydrofuran (15.0 mL) was stirred at room temperature for 10 h. The solvent was removed at reduced pressure by rotatory evaporation. The residue was titrated with a mixture of light petroleum and methylene dichloride to give the pure solid.
1,3-Dioxo-2′-phenyl-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1a)
Yellow solid, 81%, mp 161–163 °C 1H NMR (400 MHz, DMSO-d6) δ: 7.78–7.76 (m, 4H, Ar H), 7.20–7.16 (m, 5H, Ar H), 6.94 (t, J = 7.2 Hz, 1H, Ar H), 6.85 (d, J = 7.6 Hz, 1H, Ar H), 6.59–6.35 (m, 2H, Ar H), 6.23 (d, J = 7.6 Hz, 1H, CH), 5.84 (s, 1H, CH), 5.68 (d, J = 10.0 Hz, 1H, CH), 5.36 (d, J = 7.6 Hz, 1H, CH), 4.29 (d, J = 10.0 Hz, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 198.1, 142.6, 137.0, 134.0, 132.5, 132.1, 129.3, 129.2, 129.0, 128.5, 125.7, 125.2, 125.1, 125.0, 123.2, 119.3, 100.3, 71.1, 71.0, 57.1, 53.9; IR (KBr) υ: 3061, 2924, 2860, 2238, 1810, 1696, 1592, 1491, 1420, 1244, 1033, 854, 760 cm–1; HRMS (ESI) calcd for C27H19N2O2([M + H]+): 403.1441, found: 403.1452.
2′-(2-Methoxyphenyl)-1,3-dioxo-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1b)
Yellow solid, 84%, mp 162–164 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.79–7.71 (m, 4H, Ar H), 7.41 (d, J = 7.2 Hz, 1H, Ar H), 7.15 (t, J = 7.6 Hz, 1H, Ar H), 6.98–6.91 (m, 3H, Ar H), 6.65 (d, J = 7.6 Hz, 1H, CH), 6.61–6.56 (m, 2H, Ar H), 6.19 (d, J = 7.6 Hz, 1H, Ar H), 5.66 (d, J = 7.6 Hz, 1H, CH), 5.47–5.45 (m 2H, CH), 4.23–4.22 (m, 1H, CH), 3.12 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) δ: 198.1, 156.7, 142.2, 136.5, 132.6, 129.7, 128.9, 127.9, 125.6, 125.2, 124.9, 122.9, 120.8, 110.7, 69.8, 68.2, 56.0, 54.6, 49.4; IR (KBr) υ: 3031, 2928, 2269, 1738, 1704, 1593, 1548, 1540, 1359, 1262, 836, 813, 769, 675 cm–1; HRMS (ESI) calcd for C28H21N2O3([M + H]+): 433.1547, found: 433.1560.
1,3-Dioxo-2′-(p-tolyl)-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1c)
Yellow solid, 79%, mp 181–183 °C; 1H NMR (400 MHz, CDCl3) δ: 7.91–7.90 (m, 1H, Ar H), 7.73–7.69 (m, 1H, Ar H), 7.63–7.62 (m, 2H, Ar H), 7.06 (d, J = 8.0 Hz, 2H, Ar H), 6.97–6.92 (m, 3H, Ar H), 6.83 (d, J = 7.2 Hz, 1H, Ar H), 6.54 (d, J = 7.6 Hz, 1H, Ar H), 6.33 (d, J = 7.2 Hz, 1H, CH), 6.25 (d, J = 7.6 Hz, 1H, Ar H), 5.84 (s, 1H, CH), 5.39 (d, J = 7.2 Hz, 1H, CH), 5.16 (d, J = 10.4 Hz, 1H, CH), 4.29 (d, J = 10.8 Hz, 1H, CH), 2.18 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 198.2, 142.7, 138.4, 137.1, 134.1, 132.4, 129.7, 129.1, 128.9, 128.4, 125.6, 125.2, 125.1, 125.0, 123.2, 119.3, 100.1, 70.9, 69.4, 57.2, 53.7, 20.9; IR (KBr) υ: 3059, 2916, 2275, 1740, 1704, 1591, 1562, 1540, 1353, 878, 803, 772, 680 cm–1; HRMS (ESI) calcd for C28H21N2O2([M + H]+): 417.1598, found: 417.1611.
2′-(4-Bromophenyl)-1,3-dioxo-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1d)
Yellow solid, 83%, mp 177–179 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.78–7.75 (m, 4H, Ar H), 7.41 (d, J = 8.0 Hz, 2H, Ar H), 7.16 (d, J = 8.0 Hz, 2H, Ar H), 6.98 (d, J = 7.2 Hz, 1H, Ar H), 76.89 (d, J = 7.6 Hz, 1H, Ar H), 6.64–6.60 (m, 2H, Ar H), 6.29 (d, J = 7.6 Hz, 1H, CH), 5.91 (s, 1H, CH), 5.73 (d, J = 10.0 Hz, 1H, CH), 5.44 (d, J = 7.6 Hz, 1H, CH), 4.31 (d, J = 10.4 Hz, 1H, CH),; 13C NMR (100 MHz, DMSO-d6) δ: 197.7, 142.5, 137.0, 133.9, 132.5, 132.1, 131.9, 130.8, 129.5, 125.9, 125.3, 125.3, 125.0, 123.1, 122.3, 119.0, 101.0, 71.7, 71.7, 57.2, 52.9; IR (KBr) υ: 3060, 2911, 2231, 1736, 1702, 1593, 1489, 1418, 1248, 766, 718 cm–1; HRMS (ESI) calcd for C27H18BrN2O2([M + H]+): 781.0546, found: 481.0542.
2′-(4-Chlorophenyl)-1,3-dioxo-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1e)
Yellow solid, 81%, mp 180–183 °C; 1H NMR (600 MHz, CDCl3) δ: 7.91–7.90 (m, 1H, Ar H), 7.74–7.73 (m, 1H, Ar H), 7.67–7.62 (m, 2H, Ar H), 7.17–7.13 (m, 4H, Ar H), 6.93 (t, J = 7.2 Hz, 1H, Ar H), 6.84 (d, J = 7.2 Hz, 1H, Ar H), 6.54 (d, J = 7.2 Hz, 1H, Ar H), 6.32 (d, J = 6.6 Hz, 1H, CH), 6.24 (d, J = 7.8 Hz, 1H, Ar H), 5.81 (s, 1H, CH), 5.41 (d, J = 6.0 Hz, 1H, CH), 5.14 (d, J = 10.2 Hz, 1H, CH), 4.28 (d, J = 10.2 Hz, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.1, 142.9, 136.0, 136.0, 134.8, 132.3, 131.8, 129.7, 129.6, 129.1, 128.7, 125.4, 125.3, 124.8, 124.8, 123.1, 109.9, 101.1, 70.1, 68.6, 57.7, 53.6; IR (KBr) υ: 3064, 2914, 2240, 1739, 1704, 1590, 1489, 1456, 1276, 1095, 849, 737 cm–1; HRMS (ESI) calcd for C27H18ClN2O2([M + H]+): 437.1051, found: 437.1045.
2′-(4-(tert-Butyl)phenyl)-1,3-dioxo-1,2′,3,3′-tetrahydro-10b′H-spiro[indene-2,1′-pyrrolo[2,1-a]isoquinoline]-3′-carbonitrile (1f)
Yellow solid, 61%, mp 176–178 °C; 1H NMR (400 MHz, CDCl3) δ: 7.91–7.90 (m, 1H, Ar H), 7.72–7.69 (m, 1H, Ar H), 7.63–7.62 (m, 2H, Ar H), 7.16 (d, J = 8.4 Hz, 2H, Ar H), 7.09 (d, J = 8.4 Hz, 2H, Ar H), 6.93 (t, J = 7.6 Hz, 1H, Ar H), 6.83 (d, J = 7.2 Hz, 1H, Ar H), 6.54 (t, J = 7.6 Hz, 1H, Ar H), 6.33 (d, J = 7.2 Hz, 1H, CH), 6.25 (d, J = 7.6 Hz, 1H, Ar H), 5.83 (s, 1H, CH), 5.39 (d, J = 6.8 Hz, 1H, CH), 5.16 (d, J = 10.4 Hz, 1H, CH), 4.30 (d, J = 10.8 Hz, 1H, CH), 1.16 (s, 9H, 3CH3); 13C NMR (100 MHz, DMSO-d6) δ: 198.1, 151.4, 142.7, 137.0, 134.2, 132.4, 129.2, 129.1, 128.3, 126.0, 125.6, 125.2, 125.1, 125.0, 123.2, 119.4, 100.2, 100.1, 71.1, 70.0, 57.4, 53.4, 34.6, 31.2; IR (KBr) υ: 3057, 2961, 2867, 2257, 1739, 1700, 1595, 1500, 1267, 1084, 951, 805, 762, 721 cm–1; HRMS (ESI) calcd for C31H27N2O2([M + H]+): 459.2067, found: 459.2081.
General Procedure for the Preparation of Spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indenes] 2a–y
A mixture of N-cyanomethylisoquinolinium chloride (0.5 mmol), aromatic aldehyde (0.5 mmol), 1,3-indanedione (1.1 mmol), and triethylamine (1.2 mmol) in acetonitrile (15.0 mL) was stirred at room temperature for 8 h. The resulting precipitates were collected by filtration and washed with cold ethanol to give pure products 2a–v for analysis. In the cases of reactions with n-heptanal, the crude products were subjected to column chromatography with a mixture of light petroleum and ethyl acetate (v/v = 2:1) as an eluent to give pure products 2x and 2y for analysis.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-phenyl-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2a)
White solid, 83%, mp 249–251 °C; 1H NMR (400 MHz, CDCl3) δ: 10.29 (s, 1H, NH), 7.39 (d, J = 7.6 Hz, 1H, Ar H), 7.76 (t, J = 7.6 Hz, 1H, Ar H), 7.72–7.65 (m, 3H, Ar H), 7.61 (d, J = 6.8 Hz, 1H, Ar H), 7.57–7.51 (m, 2H, Ar H), 7.44 (d, J = 7.2 Hz, 1H, Ar H), 7.33 (t, J = 7.6 Hz, 1H, Ar H), 7.27 (t, J = 7.6 Hz, 1H, Ar H), 7.19–7.11 (m, 5H, Ar H), 7.01 (d, J = 7.2 Hz, 1H, Ar H), 5.99 (s, 1H, CH), 5.44 (d, J = 6.8 Hz, 1H, CH), 4.66–4.65 (m, 1H, CH), 3.54 (d, J = 10.4 Hz, 1H, CH), 2.93 (t, J = 12.4 Hz, 1H, CH), 2.41–2.36 (m, 1H, CH); 13C NMR (100 MHz, CDCl3) δ: 199.7, 197.5, 194.0, 188.7, 165.7, 142.9, 141.5, 139.9, 139.7, 137.6, 136.1, 135.5, 134.8, 133.2, 132.8, 129.7, 129.1, 129.0, 128.0, 127.9, 127.3, 127.1, 123.1, 123.0, 122.0, 121.2, 100.9, 77.3, 74.5, 67.8, 62.4, 56.3, 30.1; IR (KBr) υ: 3276, 3026, 2916, 1738, 1703, 1570, 1457, 1273, 1087, 859, 739 cm–1; HRMS (ESI) calcd for C36H25N2O4([M + H]+): 549.1809, found: 549.1818.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(2-hydroxyphenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2b)
White solid, 78%, mp 247–249 °C; 1H NMR (400 MHz, CDCl3) δ: 10.43 (s, 1H, NH), 9.00 (s, 1H, OH), 7.91–7.88 (m, 3H, Ar H), 7.83–7.80 (m, 1H, Ar H), 7.66–7.60 (m, 4H, Ar H), 7.50–7.45 (m, 2H, Ar H), 7.34 (t, J = 7.6 Hz, 1H, Ar H), 7.29–7.26 (m, 1H, Ar H), 7.08 (d, J = 7.6 Hz, 1H, Ar H), 6.90–6.86 (m, 1H, Ar H), 6.74 (t, J = 7.2 Hz, 1H, Ar H), 6.38 (d, J = 8.0 Hz, 1H, Ar H), 5.95 (s, 1H, CH), 5.51–5.50 (m, 1H, CH), 4.64 (d, J = 3.6 Hz, 1H, CH), 3.21 (dd, J1 = 11.6 Hz, J2 = 2.4 Hz, 1H, CH), 2.62–2.55 (m, 1H, CH), 2.35 (dd, J1 = 15.2 Hz, J2 = 2.4 Hz, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.7, 197.4, 191.9, 188.2, 167.3, 154.3, 142.3, 141.4, 139.7, 139.5, 136.4, 136.2, 135.4, 133.8, 133.6, 130.4, 129.7, 129.3, 129.1, 128.5, 127.5, 127.2, 125.8, 123.2, 122.9, 121.6, 121.2, 118.7, 114.4, 100.0, 77.7, 73.7, 65.9, 63.4, 48.7, 29.9; IR (KBr) υ: 3265, 3069, 2910, 1745, 1707, 1566, 1457, 1206, 1139, 997, 862, 741, 694, 655 cm–1; HRMS (ESI) calcd for C36H25N2O5([M + H]+): 565.1758, found: 565.1757.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(2-methoxyphenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2c)
White solid, 87%, mp 266–268 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.45 (s, 1H, NH), 8.02 (d, J = 7.2 Hz, 1H, Ar H), 7.94–7.92 (m, 2H, Ar H), 7.85 (t, J = 7.2 Hz, 1H, Ar H), 7.68–7.60 (m, 5H, Ar H), 7.52 (d, J = 6.8 Hz, 1H, Ar H), 7.37 (t, J = 7.6 Hz, 1H, Ar H), 7.31 (t, J = 7.6 Hz, 1H, Ar H), 7.12–7.10 (m, 2H, Ar H), 6.97 (t, J = 7.6 Hz, 1H, Ar H), 6.58 (d, J = 8.0 Hz, 1H, Ar H), 6.00 (s, 1H, CH), 5.63 (s, 1H, CH), 4.67–4.66 (m, 1H, CH), 3.30–3.28 (m, 1H, CH), 2.95 (s, 3H, CH3), 2.64 (t, J = 7.2 Hz, 1H, CH), 2.43 (d, J = 10.8 Hz, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.4, 197.2, 191.9, 188.4, 167.1, 155.5, 142.1, 141.1, 139.7, 139.5, 136.5, 136.3, 135.4, 133.8, 133.7, 130.4, 129.7, 129.3, 129.1, 128.1, 128.0, 127.4, 127.2, 123.1, 122.9, 121.6, 121.2, 120.5, 110.2, 100.0, 77.8, 73.0, 65.6, 63.1, 54.3, 49.1, 29.8; IR (KBr) υ: 3252, 3072, 2833, 1746, 1708, 1649, 1567, 1492, 1462, 1360, 1275, 1206, 1029, 933, 853, 697 cm–1; HRMS (ESI) calcd for C37H27N2O5([M + H]+): 579.1914, found: 579.1920.
3-(2-Chlorophenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2d)
White solid, 89%, mp 264–266 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.46 (s, 1H, NH), 7.98–7.92 (m, 3H, Ar H), 7.88 (t, J = 7.2 Hz, 1H, Ar H), 7.74 (d, J = 7.6 Hz, 1H, Ar H), 7.70–7.65 (m, 4H, Ar H), 7.56–7.55 (m, 1H, Ar H), 7.38–7.32 (m, 3H, Ar H), 7.17–7.12 (m, 3H, Ar H), 6.02 (s, 1H, CH), 5.46 (s, 1H, CH), 4.84–4.83 (m, 1H, CH), 3.37–3.34 (m, 1H, CH), 2.74 (t, J = 12.4 Hz, 1H, CH), 2.52–2.51 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.9, 196.9, 191.6, 188.5, 166.0, 141.9, 141.8, 139.7, 139.5, 137.3, 136.8, 136.7, 135.3, 133.9, 133.7, 133.4, 131.0, 130.3, 129.9, 129.3, 129.2, 129.0, 128.7, 127.2, 127.0, 123.6, 123.1, 121.6, 121.3, 99.9, 78.0, 76.1, 65.8, 63.4, 52.1, 29.7; IR (KBr) υ: 3279, 3069, 2907, 2836, 1746, 1709, 1659, 1572, 1461, 1436, 1205, 1036, 891, 850, 792, 685 cm–1; HRMS (ESI) calcd for C36H24ClN2O4([M + H]+): 583.1419, found: 583.1421.
3-(2-Bromophenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2e)
White solid, 85%, mp 270–272 °C; 1H NMR (600 MHz, CDCl3) δ: 10.21 (s, 1H, NH), 8.42–8.37 (m, 2H, Ar H), 7.97 (d, J = 7.2 Hz, 1H, Ar H), 7.83–7.73 (m, 5H, Ar H), 7.63–7.55 (m, 3H, Ar H), 7.46 (d, J = 7.8 Hz, 1H, Ar H), 7.36 (t, J = 7.8 Hz, 1H, Ar H), 7.30 (t, J = 7.2 Hz, 1H, Ar H), 7.26–7.24 (m, 1H, Ar H), 7.03 (d, J = 7.2 Hz, 1H, Ar H), 6.00 (s, 1H, CH), 5.39–5.38 (m, 1H, CH), 4.65–4.64 (m, 1H, CH), 3.56 (d, J = 12.0 Hz, 1H, CH), 2.91 (t, J = 13.2 Hz, 1H, CH), 2.40 (d, J = 15.0 Hz, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.0, 196.9, 191.5, 188.5, 165.9, 142.1, 141.9, 139.8, 139.6, 138.5, 137.2, 136.8, 135.4, 133.9, 133.7, 132.3, 131.4, 130.3, 129.9, 129.3, 129.2, 128.9, 127.5, 127.2, 124.9, 123.7, 123.1, 121.6, 121.3, 100.0, 78.1, 76.8, 65.8, 63.3, 54.7, 29.7; IR (KBr) υ: 3278, 3066, 2914, 2836, 1741, 1703, 1584, 1464, 1431, 1265, 1132, 852, 746 cm–1; HRMS (ESI) calcd for C36H24BrN2O4([M + H]+): 627.0914, found: 627.0900.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(m-tolyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2f)
White solid, 86%, mp 233–235 °C; 1H NMR (400 MHz, CDCl3) δ: 10.23 (s, 1H, NH), 7.93 (d, J = 7.6 Hz, 1H, Ar H), 7.76 (t, J = 7.2 Hz, 1H, Ar H), 7.72–7.69 (m, 2H, Ar H), 7.66 (d, J = 8.0 Hz, 1H, Ar H), 7.62 (d, J = 6.8 Hz, 1H, Ar H), 7.57–7.50 (m, 1H, Ar H), 7.44 (d, J = 7.6 Hz, 1H, Ar H), 7.32 (t, J = 7.6 Hz, 1H, Ar H), 7.28–7.25 (m, 1H, Ar H), 7.04–7.00 (m, 3H, Ar H), 6.92–6.89 (m, 2H, Ar H), 6.00 (s, 1H, CH), 5.45 (d, J = 3.6 Hz, 1H, CH), 4.63 (d, J = 4.0 Hz, 1H, CH), 3.55–3.52 (m, 1H, CH), 2.99–2.92 (m, 1H, CH), 2.39 (dd, J1 = 15.2 Hz, J2 = 2.0 Hz, 1H, CH), 2.20 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 199.8, 197.5, 193.9, 188.7, 165.7, 143.0, 141.6, 139.9, 139.7, 137.5, 137.4, 136.0, 135.4, 134.9, 133.2, 132.8, 129.7, 129.1, 129.0, 128.5, 127.9, 127.8, 127.2, 125.1, 123.1, 123.0, 122.0, 121.2, 100.9, 77.3, 77.0, 76.7, 74.4, 67.8, 62.3, 56.3, 30.1, 21.4; IR (KBr) υ: 3273, 3099, 3018, 2918, 1741, 1706, 1570, 1489, 1461, 1365, 1278, 1137, 1062, 934, 868, 730, 700, 653 cm–1; HRMS (ESI) calcd for C37H26KN2O4([M + K]+): 601.1524, found: 601.1527.
2-(1,3-Bioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(3-fluorophenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2g)
White solid, 81%, mp 259–261 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.24 (s, 1H, NH), 7.95 (d, J = 7.6 Hz, 1H, Ar H), 7.82–7.70 (m, 4H, Ar H), 7.63–7.61 (m, 1H, Ar H), 7.58–7.54 (m, 2H, Ar H), 7.44 (d, J = 7.6 Hz, 1H, Ar H), 7.33 (t, J = 7.6 Hz, 1H, Ar H), 7.29–7.25 (m, 1H, Ar H), 7.16–7.12 (m, 1H, Ar H), 7.02–6.93 (m, 3H, Ar H), 6.85–6.80 (m, 1H, Ar H), 5.98 (s, 1H, CH), 5.39 (d, J = 3.6 Hz, 1H, CH), 4.64 (d, J = 4.4 Hz, 1H, CH), 3.51 (dd, J1 = 11.6 Hz, J2 = 2.4 Hz, 1H, CH), 2.94–2.87 (m, 1H, CH), 2.37 (dd, J1 = 15.2 Hz, J2 = 2.8 Hz, 1H, CH); 13C NMR (100 MHz, CDCl3) δ: 199.4, 197.3, 193.8, 188.8, 165.3, 162.4 (d, J = 244.5 Hz), 142.8, 141.4, 140.4, 140.3, 139.9, 139.7, 134.6, 133.3, 133.0, 129.5, 129.5, 129.2, 129.1, 128.0, 127.3, 123.7, 123.3, 123.2, 121.9, 121.2, 115.2 (d, J = 22.6 Hz), 114.1 (d, J = 20.9 Hz), 109.9, 100.8, 77.2, 74.3, 67.6, 62.7, 55.6, 30.1; IR (KBr) υ: 3280, 3067, 3020, 2916, 2838, 1742, 1709, 1659, 1589, 1488, 1202, 1090, 959, 828, 739 cm–1; HRMS (ESI) calcd for C36H24FN2O4([M + H]+): 567.1715, found: 567.1709.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(3-nitrophenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2h)
White solid, 82%, mp 252–254 °C; 1H NMR (400 MHz, CDCl3) δ: 10.18 (s, 1H, NH), 8.16–8.15 (m, 1H, Ar H), 8.04–8.01 (m, 1H, Ar H), 7.97 (d, J = 7.6 Hz, 1H, Ar H), 7.84–7.80 (m, 1H, Ar H), 7.78–7.74 (m, 1H, Ar H), 7.72–7.71 (m, 1H, Ar H), 7.60–7.53 (m, 4H, Ar H), 7.45 (d, J = 7.2 Hz, 1H, Ar H), 7.41–7.33 (m, 2H, Ar H), 7.30–7.26 (m, 1H, Ar H), 7.01 (d, J = 7.6 Hz, 1H, Ar H), 5.99 (s, 1H, CH), 5.44 (d, J = 3.6 Hz, 1H, CH), 4.73 (d, J = 4.0 Hz, 1H, CH), 3.53 (dd, J1 = 8.0 Hz, J2 = 2.4 Hz, 1H, CH), 2.91–4.84 (m, 1H, CH), 2.37 (dd, J1 = 14.8 Hz, J2 = 2.4 Hz, 1H, CH); 13C NMR (100 MHz, CDCl3) δ: 198.8, 197.2, 193.7, 189.0, 164.8, 147.8, 142.5, 141.3, 140.2, 139.8, 139.6, 136.6, 136.1, 134.4, 134.3, 133.4, 133.1, 129.4, 129.2, 129.0, 128.0, 127.4, 123.4, 123.3, 123.2, 122.2, 121.9, 121.3, 100.8, 77.2, 74.3, 67.6, 63.2, 55.0, 30.1; IR (KBr) υ: 3282, 3068, 2911, 2835, 1743, 1708, 1660, 1569, 1529, 1462, 1203, 994, 831, 737 cm–1; HRMS (ESI) calcd for C36H24N3O6([M + H]+): 594.1660, found: 594.1651.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(p-tolyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2i)
White solid, 92%, mp 233–235 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.48 (s, 1H, NH), 7.97–7.95 (m, 1H, Ar H), 7.92–7.86 (m, 3H, Ar H), 7.68–7.62 (m, 4H, Ar H), 7.50 (d, J = 6.4 Hz, 1H, Ar H), 7.34 (t, J = 7.2 Hz, 1H, Ar H), 7.27 (t, J = 6.4 Hz, 1H, Ar H), 7.07 (d, J = 7.2 Hz, 1H, Ar H), 6.92–6.90 (m, 3H, Ar H), 5.96–5.95 (m, 1H, CH), 5.47–5.45 (m, 1H, CH), 4.37 (d, J = 4.0 Hz, 1H, CH), 3.18 (dd, J1 = 12.0 Hz, J2 = 2.4 Hz, 1H, CH), 2.64–2.57 (m, 1H, CH), 2.45–2.42 (m, 1H, CH), 2.16 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 200.5, 196.9, 192.0, 188.3, 166.7, 142.7, 141.3, 139.6, 139.5, 137.5, 136.7, 135.9, 135.7, 135.1, 133.9, 133.7, 130.3, 129.6, 129.4, 129.2, 128.7, 128.0, 127.3, 123.7, 123.3, 121.7, 121.2, 100.0, 77.3, 74.6, 67.6, 63.0, 55.4, 29.8, 21.0; IR (KBr) υ: 3275, 3018, 2918, 1738, 1704, 1569, 1459, 1357, 1274, 1135, 861, 740 cm–1; HRMS (ESI) calcd for C37H27N2O4([M + H]+): 563.1965, found: 563.1979.
3-(4-(Bimethylamino)phenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2j)
White solid, 73%, mp 262–264 °C; 1H NMR (400 MHz, CDCl3) δ: 10.35 (s, 1H, NH), 7.92–7.91 (m, 1H, Ar H), 7.74–7.69 (m, 4H, Ar H), 7.62–7.61 (m, 1H, Ar H), 7.53–7.52 (m, 2H, Ar H), 7.43–7.41 (m, 1H, Ar H), 7.32–7.30 (m, 2H, Ar H), 7.04–7.01 (m, 3H, Ar H), 6.54–6.51 (m, 2H, Ar H), 5.98 (s, 1H, CH), 5.34 (s, 1H, CH), 4.60 (s, 1H, CH), 3.51 (d, J = 12.4 Hz, 1H, CH), 2.96 (t, J = 10.4 Hz, 1H, CH), 2.84 (s, 6H, 2CH3), 2.37 (d, J = 13.2 Hz, 1H, CH); 13C NMR (100 MHz, CDCl3) δ: 200.1, 197.9, 194.1, 188.6, 166.0, 149.2, 143.1, 141.7, 139.9, 139.7, 135.9, 135.2, 135.0, 133.1, 132.7, 129.8, 129.1, 129.0, 128.8, 127.9, 127.2, 125.3, 123.2, 122.9, 122.0, 121.1, 112.1, 101.0, 77.1, 75.2, 68.0, 62.1, 56.0, 40.4, 30.2; IR (KBr) υ: 3270, 3071, 2903, 2832, 1744, 1708, 1665, 1568, 1520, 1459, 1362, 1340, 1246, 1058, 858, 743, 693 cm–1; HRMS (ESI) calcd for C38H29N3NaO4([M + Na]+): 614.2050, found: 614.2041.
3-(4-Bromophenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2k)
White solid, 81%, mp 278–281 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.46 (s, 1H, NH), 7.98–7.89 (m, 4H, Ar H), 7.73–7.71 (m, 1H, Ar H), 7.62–7.57 (m, 4H, Ar H), 7.36–7.30 (m, 3H, Ar H), 7.28 (t, J = 7.6 Hz, 1H, Ar H), 7.08–6.95 (m, 3H, Ar H), 5.93 (s, 1H, CH), 5.48 (s, 1H, CH), 4.36–4.35 (m, 1H, CH), 3.19 (d, J = 10.4 Hz, 1H, CH), 2.58 (t, J = 12.4 Hz, 1H, CH), 2.45–2.43 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.0, 196.9, 191.9, 191.9, 191.9, 188.5, 188.4, 188.4, 166.3, 142.6, 141.3, 138.4, 137.6, 136.9, 135.0, 133.9, 133.9, 133.8, 133.8, 133.8, 131.0, 130.4, 130.3, 129.7, 129.4, 129.2, 127.3, 123.8, 123.3, 120.0, 100.0, 77.4, 74.3, 67.5, 63.2, 54.9, 40.4, 40.2, 39.9, 29.9; IR (KBr) υ: 3279, 3066, 2902, 1738, 1704, 1569, 1488, 1279, 1137, 1003, 934, 862, 744 cm–1; HRMS (ESI) calcd for C36H23BrKN2O4([M + K]+): 665.0473, found: 665.0460.
3-(4-Chlorophenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2l)
White solid, 91%, mp 269–272 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.50 (s, 1H, NH), 8.02–8.00 (m, 2H, Ar H), 7.97–7.94 (m, 2H, Ar H), 7.75 (d, J = 7.2 Hz, 1H, Ar H), 7.70–7.68 (m, 2H, Ar H), 7.54 (d, J = 6.8 Hz, 1H, Ar H), 7.42–7.40 (m, 1H, Ar H), 7.33–7.29 (m, 2H, Ar H), 7.24–7.22 (m, 3H, Ar H), 7.12–7.08 (m, 2H, Ar H), 5.98 (s, 1H, CH), 5.54–5.50 (m, 1H, CH), 4.42–4.41 (m, 1H, CH), 3.24 (d, J = 10.8 Hz, 1H, CH), 2.64 (t, J = 12.4 Hz, 1H, CH), 2.49–2.47 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.0, 196.9, 191.9, 188.4, 166.3, 142.6, 141.3, 139.7, 139.5, 138.0, 137.6, 136.9, 135.0, 133.9, 133.7, 131.4, 130.3, 130.1, 129.7, 129.3, 129.2, 128.1, 127.3, 123.8, 123.3, 121.7, 121.3, 100.0, 77.4, 74.4, 67.6, 63.2, 54.9, 29.9; IR (KBr) υ: 3287, 3042, 2938, 1739, 1704, 1661, 1568, 1487, 1283, 1141, 1020, 936, 897, 732 cm–1; HRMS (ESI) calcd for C36H24ClN2O4([M + H]+): 583.1419, found: 583.1421.
3-(4-(tert-Butyl)phenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2m)
White solid, 81%, mp 255–257 °C; 1H NMR (400 MHz, CDCl3) δ: 10.35 (s, 1H, NH), 7.92 (d, J = 7.6 Hz, 1H, Ar H), 7.76–7.73 (m, 1H, Ar H), 7.71–7.65 (m, 2H, Ar H), 7.63–7.61 (m, 1H, Ar H), 7.58–7.56 (m, 1H, Ar H), 7.55–7.50 (m, 2H, Ar H), 7.44 (d, J = 7.2 Hz, 1H, Ar H), 7.33 (t, J = 7.2 Hz, 1H, Ar H), 7.29–7.25 (m, 1H, Ar H), 7.15–7.06 (m, 4H, Ar H), 7.03 (d, J = 7.6 Hz, 1H, Ar H), 6.01 (s, 1H, CH), 5.40–5.38 (m, 1H, CH), 4.63 (d, J = 4.0 Hz, 1H, CH), 3.55 (dd, J1 = 12.0 Hz, J2 = 2.4 Hz, 1H, CH), 3.03–2.96 (m, 1H, CH), 2.40 (dd, J1 = 15.6 Hz, J2 = 2.4 Hz, 1H, CH), 1.20 (s, 9H, 3CH3); 13C NMR (100 MHz, CDCl3) δ: 199.7, 197.6, 197.6, 194.0, 188.8, 165.7, 149.5, 143.1, 141.6, 139.9, 139.6, 135.8, 135.3, 135.0, 134.4, 133.1, 132.8, 129.7, 129.2, 129.0, 127.9, 127.7, 127.2, 124.9, 122.9, 122.0, 121.2, 100.9, 77.3, 74.7, 68.0, 61.8, 56.4, 34.3, 31.2, 30.1; IR (KBr) υ: 3280, 3063, 3019, 2960, 2872, 1741, 1703, 1569, 1461, 1356, 1274, 1136, 931, 824, 742 cm–1; HRMS (ESI) calcd for C40H33N2O4([M + H]+): 605.2435, found: 605.2432.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(4-nitrophenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2n)
White solid, 79%, mp 265–267 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.48 (s, 1H, NH), 8.12–8.00 (m, 4H, Ar H), 7.98–7.92 (m, 3H, Ar H), 7.74 (d, J = 7.6 Hz, 1H, Ar H), 7.70–7.65 (m, 3H, Ar H), 7.52 (d, J = 6.8 Hz, 1H, Ar H), 7.44–7.43 (m, 1H, Ar H), 7.38 (t, J = 7.2 Hz, 1H, Ar H), 7.32 (t, J = 7.6 Hz, 1H, Ar H), 7.11 (d, J = 7.6 Hz, 1H, Ar H), 6.00 (s, 1H, CH), 5.66 (s, 1H, CH), 4.56 (d, J = 3.6 Hz, 1H, CH), 3.26–3.25 (m, 1H, CH), 2.62 (t, J = 12.0 Hz, 1H, CH), 2.52–2.51 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.4, 196.7, 191.8, 188.6, 166.0, 147.0, 146.5, 142.4, 141.2, 139.6, 139.5, 137.8, 137.1, 134.9, 133.9, 133.8, 130.2, 129.7, 129.5, 129.4, 129.2, 127.3, 124.0, 123.4, 123.3, 121.7, 121.3, 99.9, 77.4, 74.0, 67.7, 63.6, 54.7, 29.9; IR (KBr) υ: 3245, 3017, 2900, 2832, 1741, 1704, 1571, 1571, 1460, 1206, 1050, 896, 789, 698 cm–1; HRMS (ESI) calcd for C36H23KN3O4([M + K]+): 632.1218, found: 632.1216.
3-(4-Chloro-2-hydroxyphenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2o)
White solid, 72%, mp 240–242 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.45 (s, 1H, NH), 9.44 (s, 1H, OH), 7.98–7.92 (m, 3H, Ar H), 7.88 (t, J = 7.2 Hz, 1H, Ar H), 7.75–7.73 (m, 1H, Ar H), 7.69–7.66 (m, 3H, Ar H), 7.55–7.54 (m, 2H, Ar H), 7.37 (t, J = 7.6 Hz, 1H, Ar H), 7.31 (t, J = 7.6 Hz, 1H, Ar H), 7.11 (d, J = 7.6 Hz, 1H, Ar H), 7.00–6.97 (m, 1H, Ar H), 6.45 (d, J = 8.8 Hz, 1H, Ar H), 5.98 (s, 1H, CH), 5.59 (s, 1H, CH), 4.61 (d, J = 3.6 Hz, 1H, CH), 3.24–3.21 (m, 1H, CH), 2.63–2.56 (m, 1H, CH), 2.40–2.37 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.4, 197.5, 191.8, 188.5, 167.0, 153.3, 142.2, 141.3, 139.7, 136.6, 136.5, 135.2, 133.7, 130.4, 129.8, 129.3, 129.2, 128.3, 127.3, 127.2, 123.3, 123.1, 122.6, 121.6, 115.9, 100.0, 77.8, 73.4, 65.7, 63.7, 48.3, 29.9; IR (KBr) υ: 3351, 3304, 3058, 2926, 1741, 1705, 1667, 1570, 1490, 1462, 1203, 1033, 928, 869, 787, 740, 700, 659 cm–1; HRMS (ESI) calcd for C36H24ClN2O5([M + H]+): 599.1368, found: 599.1364.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(furan-2-yl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2p)
White solid, 69%, mp 221–223 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.54 (s, 1H, NH), 8.08–8.04 (m, 3H, Ar H), 7.96–7.92 (m, 2H, Ar H), 7.75–7.71 (m, 3H, Ar H), 7.62–7.60 (m, 1H, Ar H), 7.39–7.34 (m, 2H, Ar H), 7.26–7.25 (m, 1H, Ar H), 7.12–7.11 (m, 1H, Ar H), 6.38–6.34 (m, 2H, Ar H), 5.97 (s, 1H, CH), 5.56 (s, 1H, CH), 4.50–4.48 (m, 1H, CH), 3.16–3.10 (m, 1H, CH), 2.60–2.58 (m, 1H, CH), 2.50–2.46 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.3, 195.7, 191.5, 188.2, 165.4, 152.3, 142.0, 141.2, 140.6, 139.2, 139.0, 137.2, 136.4, 134.3, 133.6, 133.4, 129.8, 129.2, 129.0, 128.8, 126.9, 123.5, 122.9, 121.3, 120.9, 110.5, 106.4, 99.6, 76.6, 72.5, 65.6, 62.7, 47.7, 39.5, 29.2; IR (KBr) υ: 3278, 3069, 2913, 2831, 1744, 1707, 1680, 1593, 1564, 1462, 1244, 1135, 888, 697 cm–1; HRMS (ESI) calcd for C34H23N2O5([M + H]+): 539.1601, found: 539.1620.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(thiophen-2-yl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2q)
White solid, 78%, mp 233–235 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.55 (s, 1H, NH), 8.06–8.04 (m, 1H, Ar H), 7.99–7.97 (m, 2H, Ar H), 7.94 (d, J = 8.0 Hz, 1H, Ar H), 7.82 (d, J = 8.0 Hz, 1H, Ar H), 7.69–7.67 (m, 3H, Ar H), 7.59–7.57 (m, 3H, Ar H), 7.37 (t, J = 8.0 Hz, 1H, Ar H), 7.32 (t, J = 7.6 Hz, 1H, Ar H), 7.21 (d, J = 4.4 Hz, 1H, Ar H), 7.11 (d, J = 7.6 Hz, 1H, Ar H), 6.87–6.86 (m, 2H, Ar H), 5.99 (s, 1H, CH), 5.53 (s, 1H, CH), 4.67 (d, J = 4.0 Hz, 1H, CH), 3.21–3.18 (m, 1H, CH), 2.62–2.59 (m, 1H, CH), 2.50–2.48 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.0, 196.2, 192.0, 188.3, 165.8, 142.8, 142.0, 141.4, 139.6, 139.5, 137.6, 136.8, 134.8, 134.0, 133.8, 130.2, 129.6, 129.4, 129.2, 127.3, 126.9, 125.5, 124.1, 123.8, 123.3, 121.7, 121.3, 100.1, 77.1, 75.6, 67.6, 63.0, 50.3, 29.8; IR (KBr) υ: 3277, 3068, 2915, 2836, 1742, 1705, 1568, 1462, 1245, 1203, 1060, 892, 738, 692 cm–1; HRMS (ESI) calcd for C34H23N3O2S ([M + H]+): 555.1373, found: 555.1383.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(pyridin-2-yl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2r)
White solid, 83%, mp 270–272 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.55 (s, 1H, NH), 8.07 (d, J = 7.6 Hz, 1H, Ar H), 8.01–7.94 (m, 4H, Ar H), 7.89 (d, J = 6.8 Hz, 1H, Ar H), 7.75 (d, J = 7.6 Hz, 1H, Ar H), 7.70–7.64 (m, 4H, Ar H), 7.55 (d, J = 6.8 Hz, 1H, Ar H), 7.37 (t, J = 7.6 Hz, 1H, Ar H), 7.30 (t, J = 7.6 Hz, 1H, Ar H), 7.13–7.09 (m, 2H, Ar H), 5.97 (s, 1H, CH), 5.91 (s, 1H, CH), 4.73 (d, J = 4.0 Hz, 1H, CH), 3.15–3.11 (m, 1H, CH), 2.48–2.45 (m, 1H, CH), 2.42–2.38 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.7, 197.7, 192.0, 188.7, 167.0, 158.6, 147.9, 147.9, 142.8, 141.4, 139.6, 139.5, 137.0, 136.5, 136.4, 134.7, 134.0, 133.8, 130.3, 129.6, 129.4, 129.2, 127.3, 123.9, 122.9, 122.4, 121.9, 121.7, 121.3, 100.0, 77.0, 77.0, 71.4, 67.5, 63.1, 55.8, 55.7, 29.8, 29.8; IR (KBr) υ: 3271, 3062, 2925, 1745, 1710, 1648, 1568, 1466, 1338, 1212, 935, 682 cm–1; HRMS (ESI) calcd for C35H24N3O4 ([M + H]+): 550.1761, found: 550.1769.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(pyridin-3-yl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2s)
White solid, 76%, mp 255–257 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.50 (s, 1H, NH), 8.33–8.32 (m, 1H, Ar H), 8.25–8.24 (m, 1H, Ar H), 8.02 (d, J = 7.2 Hz, 1H, Ar H), 7.99–7.92 (m, 3H, Ar H), 7.74–7.68 (m, 5H, Ar H), 7.55–7.53 (m, 1H, Ar H), 7.38 (t, J = 7.6 Hz, 1H, Ar H), 7.32 (t, J = 7.2 Hz, 1H, Ar H), 7.26–7.24 (m, 1H, Ar H), 7.12 (d, J = 7.6 Hz, 1H, Ar H), 5.99 (s, 1H, CH), 5.58 (s, 1H, CH), 4.43 (d, J = 3.2 Hz, 1H, CH), 3.28 (d, J = 12.0 Hz, 1H, CH), 2.66 (t, J = 12.0 Hz, 1H, CH), 2.54–2.51 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.7, 197.0, 191.8, 188.5, 166.1, 149.4, 148.1, 142.5, 141.3, 139.7, 139.5, 137.7, 137.1, 135.8, 135.0, 134.6, 133.9, 133.8, 130.2, 129.7, 129.4, 129.2, 127.3, 123.9, 123.3, 123.2, 121.7, 121.3, 99.9, 77.4, 74.2, 67.5, 63.3, 53.1, 29.8; IR (KBr) υ: 3284, 3070, 2969, 2916, 1741, 1704, 1571, 1484, 1202, 1089, 932, 713, 682, 618 cm–1; HRMS (ESI) calcd for C35H24N3O4([M + H]+): 550.1761, found: 550.1783.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(pyridin-4-yl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2t)
White solid, 74%, mp 265–267 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.50 (s, 1H, NH), 8.37–8.36 (m, 2H, Ar H), 8.05 (d, J = 6.8 Hz, 1H, Ar H), 8.01–7.95 (m, 3H, Ar H), 7.75 (t, J = 7.2 Hz, 1H, Ar H), 7.69–7.66 (m, 3H, Ar H), 7.55–7.53 (m, 1H, Ar H), 7.38 (t, J = 7.2 Hz, 1H, Ar H), 7.32 (t, J = 7.2 Hz, 1H, Ar H), 7.12–7.10 (m, 3H, Ar H), 5.98 (s, 1H, CH), 5.66 (s, 1H, CH), 4.41 (d, J = 3.2 Hz, 1H, CH), 3.24 (d, J = 11.2 Hz, 1H, CH), 2.59 (t, J = 11.6 Hz, 1H, CH), 2.49–2.48 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.5, 196.6, 191.8, 188.6, 166.1, 149.6, 147.8, 142.5, 141.2, 139.6, 137.8, 137.1, 134.9, 133.9, 133.8, 130.2, 129.7, 129.4, 129.2, 127.3, 124.0, 123.4, 123.2, 121.7, 121.3, 99.9, 77.3, 73.0, 67.4, 63.4, 54.2, 29.8; IR (KBr) υ: 3293, 3071, 3038, 2901, 2834, 1742, 1705, 1668, 1567, 1461, 1203, 1140, 1054, 892, 815, 740, 680 cm–1; HRMS (ESI) calcd for C35H24N3O4 ([M + H]+): 550.1761, found: 550.1783.
3-Benzoyl-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2u)
White solid, 85%, mp 284–286 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.68 (s, 1H, NH), 8.18 (d, J = 7.6 Hz, 1H, Ar H), 8.10–8.05 (m, 3H, Ar H), 7.99–7.91 (m, 4H, Ar H), 7.82 (d, J = 6.4 Hz, 1H, Ar H), 7.63–7.58 (m, 6H, Ar H), 7.52 (t, J = 7.2 Hz, 2H, Ar H), 7.41 (t, J = 7.2 Hz, 2H, Ar H), 7.30 (d, J = 7.6 Hz, 1H, Ar H), 6.26 (s, 1H, CH), 6.10 (s, 1H, CH), 5.27 (d, J = 3.6 Hz, 1H, CH), 3.40–3.37 (m, 1H, CH), 2.68–2.65 (m, 1H, CH), 2.61–2.57 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.5, 196.7, 193.8, 191.8, 189.4, 166.4, 141.5, 140.8, 139.8, 139.4, 137.5, 136.9, 136.5, 134.6, 134.0, 133.9, 133.3, 130.2, 129.8, 129.4, 129.1, 128.8, 128.2, 127.3, 123.6, 123.2, 121.8, 121.3, 99.7, 77.6, 69.7, 64.9, 64.7, 55.8, 29.6; IR (KBr) υ: 3239, 3065, 2913, 2828, 1739, 1708, 1653, 1580, 1461, 1266, 1144, 792, 704, 637 cm–1; HRMS (ESI) calcd for C37H25N2O5 ([M + H]+): 577.1758, found: 577.1765.
6-Bromo-3-(4-chlorophenyl)-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2v)
White solid, 81%, mp 266–268 °C; 1H NMR (600 MHz, DMSO-d6) δ: 10.56 (s, 1H, NH), 8.04 (d, J = 7.8 Hz, 1H, Ar H), 8.01 (d, J = 7.8 Hz, 1H, Ar H), 7.97 (d, J = 7.8 Hz, 1H, Ar H), 7.93 (d, J = 7.2 Hz, 1H, Ar H), 7.73 (d, J = 7.8 Hz, 1H, Ar H), 7.71–7.67 (m, 4H, Ar H), 7.56 (d, J = 7.2 Hz, 1H, Ar H), 7.38 (t, J = 7.8 Hz, 1H, Ar H), 7.24–7.23 (m, 2H, Ar H), 7.12–7.11 (m, 1H, Ar H), 6.00 (s, 1H, CH), 5.51 (s, 1H, CH), 4.40 (d, J = 3.6 Hz, 1H, CH), 3.29 (dd, J1 = 11.4 Hz, J2 = 2.4 Hz, 1H, CH), 2.61–2.58 (m, 1H, CH), 2.53–2.52 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.5, 196.8, 191.6, 188.4, 165.9, 134.6, 132.8, 131.4, 130.1, 129.6, 128.9, 127.9, 124.3, 123.8, 123.2, 100.0, 77.0, 74.5, 67.1, 62.2, 55.4, 30.9; IR (KBr) υ: 3288, 3066, 2927, 1738, 1702, 1652, 1571, 1492, 1452, 1280, 1136, 1012, 892, 819, 779, 698 cm–1; HRMS (ESI) calcd for C36H22BrClN2NaO4 ([M + Na]+): 683.0344, found: 683.0351.
6-Bromo-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-(4-nitrophenyl)-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2w)
White solid, 83%, mp 275–277 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.59 (s, 1H, NH), 8.08–8.03 (m, 4H, Ar H), 7.99 (t, J = 7.2 Hz, 1H, Ar H), 7.93 (t, J = 7.2 Hz, 1H, Ar H), 7.74 (d, J = 7.6 Hz, 1H, Ar H), 7.67–7.65 (m, 3H, Ar H), 7.61–7.60 (m, 2H, Ar H), 7.40–7.35 (m, 3H, Ar H), 5.99 (s, 1H, CH), 5.62 (s, 1H, CH), 4.53 (d, J = 3.6 Hz, 1H, CH), 3.31 (dd, J1 = 11.2 Hz, J2 = 2.4 Hz, 1H, CH), 2.61–2.58 (m, 1H, CH), 2.48–2.44 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 199.2, 196.8, 165.8, 147.0, 146.5, 142.4, 141.2, 139.7, 137.8, 137.2, 134.6, 133.7, 133.0, 129.7, 129.7, 129.1, 124.4, 124.1, 123.4, 123.3, 121.3, 100.1, 78.0, 77.9, 77.9, 74.5, 67.5, 62.7, 55.4, 31.1; IR (KBr) υ: 3288, 3074, 2912, 1744, 1704, 1658, 1578, 1518, 1421, 1214, 1011, 942, 839, 706, 690 cm–1; HRMS (ESI) calcd for C36H22BrKN3O6 ([M + K]+): 710.0324, found: 710.0319.
2-(1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-hexyl-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2x)
White solid, 70%, mp 275–277 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.52 (s, 1H, NH), 8.10–8.03 (m, 4H, Ar H), 7.86 (t, J = 8.0 Hz, 1H, Ar H), 7.71–7.70 (m, 4H, Ar H), 7.35 (t, J = 7.6 Hz, 1H, Ar H), 7.28 (d, J = 7.2 Hz, 1H, Ar H), 7.08 (d, J = 7.2 Hz, 1H, Ar H), 5.86(s, 1H, CH), 4.70 (s, 1H, CH), 3.19 (d, J = 12.0 Hz, 1H, CH), 2.97 (d, J = 11.6 Hz, 1H, CH), 2.70–2.69 (m, 1H, CH), 2.49–2.46 (m, 1H, CH), 2.37 (d, J = 10.8 Hz, 1H, CH), 1.99–1.91 (m, 1H, CH), 1.10–1.05 (m, 2H, CH), 0.96–0.94 (m, 4H, CH), 0.79–0.78 (m, 1H, CH), 0.69 (t, J = 5.6 Hz, 3H, CH3), 0.51–0.50 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.8, 198.3, 192.3, 188.9, 167.1, 142.3, 140.3, 139.7, 139.3, 137.6, 137.1, 134.7, 134.0, 133.8, 130.2, 129.4, 129.1, 127.3, 123.9, 123.3, 121.7, 121.3, 100.2, 76.9, 76.7, 65.0, 64.3, 49.3, 31.1, 31.0, 29.6, 28.4, 28.1, 21.8, 14.1; IR (KBr) υ: 3237, 3071, 2952, 2849, 1741, 1703, 1647, 1593, 1493, 1359, 1334, 1273, 1203, 1002, 897, 785, 737, 685 cm–1; HRMS (ESI) calcd for C36H32N2NaO4([M + Na]+): 579.2254, found: 579.2254.
6-Bromo-2-(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)-3-hexyl-1,2a,3,4a,5,9b-hexahydro-2H-spiro[benzo[f]imidazo[5,1,2-cd]indolizine-4,2′-indene]-1′,3′-dione (2y)
White solid, 63%, mp 251–253 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.54 (s, 1H, NH), 8.12–8.04 (m, 4H, Ar H), 7.95 (t, J = 7.2 Hz, 1H, Ar H), 7.72–7.70 (m, 4H, Ar H), 7.62 (d, J = 7.6 Hz, 1H, Ar H), 7.33 (t, J = 7.2 Hz, 1H, Ar H), 5.86(s, 1H, CH), 4.71 (s, 1H, CH), 3.21 (d, J = 11.6 Hz, 1H, CH), 3.01 (d, J = 11.2 Hz, 1H, CH), 2.68–2.66 (m, 1H, CH), 2.41 (d, J = 15.0 Hz, 1H, CH), 2.33 (d, J = 12.4 Hz, 1H, CH), 1.98–1.95 (m, 1H, CH), 1.09–1.06 (m, 2H, CH), 0.96–0.92 (m, 4H, CH), 0.80–0.78 (m, 1H, CH), 0.70 (t, J = 5.6 Hz, 3H, CH3), 0.52–0.50 (m, 1H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 200.6, 198.2, 192.2, 188.9, 166.9, 142.2, 140.4, 139.7, 139.3, 137.7, 137.2, 134.2, 134.0, 133.8, 133.0, 132.9, 129.4, 129.1, 124.5, 124.0, 123.3, 121.7, 121.3, 100.4, 76.7, 64.8, 63.8, 49.7, 31.1, 31.0, 30.8, 28.4, 28.1, 21.8, 14.1; IR (KBr) υ: 3247, 3071, 2925, 2853, 1742, 1707, 1669, 1569, 1451, 1359, 1276, 1215, 1090, 953, 833, 777, 696, 652 cm–1; HRMS (ESI) calcd for C36H32BrN2NaO4([M + H]+): 635.1540, found: 635.1544.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (No. 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support (No. BK2013016). The Analytical Center of Yangzhou University is acknowledged for analytical assistance.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01391.
The authors declare no competing financial interest.
Supplementary Material
References
- a Sliwa W. Cycloaddition reactions of pyridinium ylides and oxidopyridiniums. Heterocycles 1996, 43, 2005–2029. 10.3987/REV-96-478. [DOI] [Google Scholar]; b Jacobs J.; Van Hende E.; Claessens S.; De Kimpe N. Pyridinium ylides in heterocyclic synthesis. Curr. Org. Chem. 2011, 15, 1340–1362. 10.2174/138527211795378209. [DOI] [Google Scholar]; c Kakehi A. Reactions of pyridinium N-ylides and their related pyridinium salts. Heterocycles 2012, 85, 1529–1577. 10.3987/REV-12-735. [DOI] [Google Scholar]; d Bull J. A.; Mousseau J. J.; Pelletier G.; Charette A. B. Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines. Chem. Rev. 2012, 112, 2642–2713. 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- a Katritzky A. R.; Grzeskowiak N. E.; Alvarez-Builla J. Preparation of tetrahydroindolizines from pyridinium and isoquinolinium ylides. J. Chem. Soc., Perkin Trans. 1 1981, 1180–1185. 10.1039/p19810001180. [DOI] [Google Scholar]; b Tsuge O.; Kanemasa S.; Takenaka S. Stereochemical study on 1,3-dipolar cycloaddition reactions of heteroaromatic N-ylides with symmetrically substituted cis and trans olefins. Bull. Chem. Soc. Jpn. 1985, 58, 3137–3157. 10.1246/bcsj.58.3137. [DOI] [Google Scholar]; c Tsuge O.; Kanemasa S.; Takenaka S. Stereochemical study on 1,3-dipolar cycloaddition reactions of heteroaromatic N-ylides with unsymmetrically substituted olefinic dipolarophiles. Bull. Chem. Soc. Jpn. 1985, 58, 3320–3336. 10.1246/bcsj.58.3320. [DOI] [Google Scholar]; d Ruano J. L.; Fraile A.; Martin M. R.; Gonzalez G.; Fajardo C.; Martin-Castro A. M. Asymmetric synthesis of pyrrolo[2,1-a]isoquinoline derivatives by 1,3-dipolar cycloadditions of stabilized isoquinolinium N-ylides with sulfinyl dipolarophiles. J. Org. Chem. 2011, 76, 3296–3305. 10.1021/jo200191c. [DOI] [PubMed] [Google Scholar]; e Allgäuer D. S.; Mayr H. Electrophilicities of 1,2-Disubstituted Ethylenes. Eur. J. Org. Chem. 2014, 2014, 2956–2963. 10.1002/ejoc.201301779. [DOI] [Google Scholar]; f Allgäuer D. S.; Mayer P.; Mayr H. Nucleophilicity Parameters of Pyridinium Ylides and Their Use in Mechanistic Analyses. J. Am. Chem. Soc. 2013, 135, 15216–15224. 10.1021/ja407885h. [DOI] [PubMed] [Google Scholar]; g Allgäuer D. S.; Mayr H. One-Pot Two-Step Synthesis of 1-(Ethoxycarbonyl)indolizines via Pyridinium Ylides. Eur. J. Org. Chem. 2013, 2013, 6379–6388. 10.1002/ejoc.201300784. [DOI] [Google Scholar]
- a Zhang X.-c.; Huang W.-y. A one-step approach to 1-(fluoroalkyl)indolizine derivatives. Synthesis 1999, 1999, 51–54. 10.1055/s-1999-3675. [DOI] [Google Scholar]; b Zhu S. Z.; Qin C. Y.; Wang Y. L.; Chu Q. L. Preparation of 1-(trifluoroacetyl)indolizines and their derivatives via cycloaddition of pyridinium N-ylides with 4-ethoxy-1,1,1-trifluorobut-3-en-2-one. J. Fluorine Chem. 1999, 99, 183–187. 10.1016/S0022-1139(99)00145-1. [DOI] [Google Scholar]; c Peng W.; Zhu S. Reactions of N-benzyl-pyridinium or -isoquinolinium ylides with ethyl 3-fluoro-3-(fluoroalkyl)acrylates to give fluoroalkyl-substituted indolizine and pyrrolo[2,1-a]isoquinoline derivatives. J. Chem. Soc., Perkin Trans. 1 2001, 3204–3210. 10.1039/b103586j. [DOI] [Google Scholar]; d Wu K.; Chen Q.-Y. Synthesis of fluorinated indolizines and 4H-pyrrolo[1,2-a]benzimidazoles via 1,3-dipolar cycloaddition of fluoroalkenes to N-ylides. Synthesis 2003, 0035–0040. 10.1055/s-2003-36246. [DOI] [Google Scholar]; e Liu Y.; Sun J. W. Copper(II)-Catalyzed Synthesis of Benzo[f]pyrido[1,2-a]indole-6,11-dione Derivatives via Naphthoquinone Difunctionalization Reaction. J. Org. Chem. 2012, 77, 1191–1197. 10.1021/jo2023312. [DOI] [PubMed] [Google Scholar]; f Wang F.; Shen Y.; Hu H.; Wang X.; Wu H.; Liu Y. Copper(II)-Catalyzed Indolizines Formation Followed by Dehydrogenative Functionalization Cascade to Synthesize 1-Bromoindolizines. J. Org. Chem. 2014, 79, 9556–9566. 10.1021/jo501626b. [DOI] [PubMed] [Google Scholar]; g Brioche J.; Meyer C.; Cossy J. Synthesis of 2-Aminoindolizines by 1,3-Dipolar Cycloaddition of Pyridinium Ylides with Electron-Deficient Ynamides. Org. Lett. 2015, 17, 2800–2803. 10.1021/acs.orglett.5b01205. [DOI] [PubMed] [Google Scholar]
- a Gupta R. B.; Franck R. W. Cycloadditions of isoquinolinium salts: evidence for a two-step mechanism in a stereocontrolled synthesis of substituted tetralins. J. Am. Chem. Soc. 1987, 109, 5393–5403. 10.1021/ja00252a015. [DOI] [Google Scholar]; b Fernández N.; Carrillo L.; Vicario J. L.; Badia D.; Reyes E. Organocatalytic enantioselective (3+2) cycloaddition using stable azomethine ylides. Chem. Commun. 2011, 47, 12313–12315. 10.1039/c1cc15671c. [DOI] [PubMed] [Google Scholar]; c Ruano J. L. G.; Fraile A.; Martin M. R.; Gonzalez G.; Fajardo C.; Martin-Castro A. M. Asymmetric synthesis of pyrrolo[2,1-a]isoquinoline derivatives by 1,3-dipolar cycloadditions of stabilized isoquinolinium N-ylides with sulfinyl dipolarophiles. J. Org. Chem. 2011, 76, 3296–3305. 10.1021/jo200191c. [DOI] [PubMed] [Google Scholar]; d Bakshi D.; Singh A. Transition-Metal-Free Synthesis of Nitrogen Containing Heterocycles With Fully Substituted N-fused Pyrrole Rings. Asian J. Org. Chem. 2016, 5, 70–73. 10.1002/ajoc.201500324. [DOI] [Google Scholar]; e Yang W. J.; Yuan C. H.; Liu Y.; Mao B. M.; Sun Z. H.; Guo H. C. [4+3] Cycloaddition of Phthalazinium Dicyanomethanides with Azoalkenes Formed in Situ: Synthesis of Triazepine Derivatives. J. Org. Chem. 2016, 81, 7597–7603. 10.1021/acs.joc.6b01296. [DOI] [PubMed] [Google Scholar]
- a Pilli R. A.; Rodrigues J. A. R.; Kascheres A. Reaction of N-(p-tolylsulfonyl)diphenylcyclopropenimine with pyridinium and isoquinolinium ylides. J. Org. Chem. 1983, 48, 1084–1091. 10.1021/jo00155a031. [DOI] [Google Scholar]; b Fang X.; Wu Y. M.; Deng J.; Wang S. W. Synthesis of monofluorinated indolizines and their derivatives by the 1,3-dipolar reaction of N-ylides with fluorinated vinyl tosylates. Tetrahedron 2004, 60, 5487–5493. 10.1016/j.tet.2004.04.012. [DOI] [Google Scholar]; c Liu Y.; Hu H. Y.; Liu Q. J.; Hu H. W.; Xu J. H. Synthesis of polycyclic indolizine derivatives via one-pot tandem reactions of N-ylides with dichloro substituted α,β-unsaturated carbonyl compounds. Tetrahedron 2007, 63, 2024–2033. 10.1016/j.tet.2006.12.050. [DOI] [Google Scholar]; d An J.; Yang Q. Q.; Wang Q.; Xiao W. J. Direct synthesis of pyrrolo[2,1-a]isoquinolines by 1,3-dipolar cycloaddition of stabilized isoquinolinium N-ylides with vinyl sulfonium salts. Tetrahedron Lett. 2013, 54, 3834–3837. 10.1016/j.tetlet.2013.05.053. [DOI] [Google Scholar]; e Xu X.; Zavalij P. Y.; Doyle M. P. Catalytic Asymmetric Syntheses of Quinolizidines by Dirhodium-Catalyzed Dearomatization of Isoquinolinium/Pyridinium Methylides-The Role of Catalyst and Carbene Source. J. Am. Chem. Soc. 2013, 135, 12439–12447. 10.1021/ja406482q. [DOI] [PubMed] [Google Scholar]; f Li F.; Chen J. F.; Hou Y. D.; Li Y. J.; Wu X. Y.; Tong X. F. 1,3-Dipolar Cycloadditions of 4-Acetoxy Allenoates: Access to 2,3-Dihydropyrazoles, 2,3-Dihydroisoxazoles, and Indolizines. Org. Lett. 2015, 17, 5376–5379. 10.1021/acs.orglett.5b02724. [DOI] [PubMed] [Google Scholar]
- a Yamaguchi R.; Nakayasu T.; Hatano B.; Nagura T.; Kozima S.; Fujita K. Facile addition reactions of allylsilanes to quinolines and isoquinolines activated by chloroformate ester and a catalytic amount of triflate ion. Tetrahedron 2001, 57, 109–118. 10.1016/S0040-4020(00)00993-5. [DOI] [Google Scholar]; b Lin C. H.; Jhang J. F.; Yang D. Y. One-Pot Synthesis of Coumarin-Based Oxazabicyclic and Oxazatricyclic Compounds and Their Fluorescence Redox Switching Properties. Org. Lett. 2009, 11, 4064–4067. 10.1021/ol901505e. [DOI] [PubMed] [Google Scholar]; c Lin C. H.; Chen J. R.; Yang D. Y. Syntheses of Quaternary Carbon-Containing Oxazatricycle and Spiropyran Libraries via Multicomponent Reactions and Their Molecular Switching Properties. J. Comb. Chem. 2010, 12, 119–124. 10.1021/cc900127g. [DOI] [PubMed] [Google Scholar]; d Cabrera-Pardo J. R.; Chai D. I.; Kozmina S. A. Silver-Promoted Benzannulations of Siloxyalkynes with Pyridinium and Isoquinolinium Salts. Adv. Synth. Catal. 2013, 355, 2495–2498. 10.1002/adsc.201300443. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu L. J.; Yan C. G. Efficient Synthesis of Complex Oxazatricycles via Three-Component Reaction of Isoquinolinium Salts, Acetone and Cyclic Diketones. J. Heterocycl. Chem. 2015, 52, 1513–1517. 10.1002/jhet.2201. [DOI] [Google Scholar]
- a Wang Q. F.; Hui L.; Hou H.; Yan C. G. Synthesis of Zwitterionic Salts of Pyridinium-Meldrum Acid and Barbiturate through Unique Four-component Reactions. J. Comb. Chem. 2010, 12, 260–265. 10.1021/cc900161z. [DOI] [PubMed] [Google Scholar]; b Hou H.; Zhang Y.; Yan C. G. One-pot synthesis of 4-substituted isoquinolinium zwitterionic salts by metal-free C-H bond activation. Chem. Commun. 2012, 48, 4492–4494. 10.1039/c2cc30708a. [DOI] [PubMed] [Google Scholar]
- Liu R.; Shi R. G.; Sun J.; Yan C. G. A [3+2]-[4+2]-[3+2] cycloaddition sequence of isoquinolinium ylide. Org. Chem. Front. 2017, 4, 354–357. 10.1039/C6QO00615A. [DOI] [Google Scholar]
- a Fang J.; Yan C. G. Synthesis of 6a,6b,13,13a-tetrahydro-6H-5-oxa-12a-azadibenzo[a,g]fluorene derivatives via cycloaddition reactions of isoquinolinium salts with 3-nitrochromenes. Mol. Diversity 2014, 18, 91–99. 10.1007/s11030-013-9489-z. [DOI] [PubMed] [Google Scholar]; b Wu L.; Sun J.; Yan C. G. Facile synthesis of spiro[indoline-3,3′-pyrrolo[1,2-a]quinolines] and spiro[indoline-3,1′-pyrrolo[2,1-a]isoquinolines] via 1,3-dipolar cycloaddition reactions of heteroaromatic ammonium salts with 3-phenacylideneoxindoles. Org. Biomol. Chem. 2012, 10, 9452–9436. 10.1039/c2ob26849c. [DOI] [PubMed] [Google Scholar]; c Wang X. H.; Yan C. G. Facile synthesis of spiro[indane-2,1′-pyrrolo[2,1-a]isoquinolines] via three-component reaction of isoquinolinium salts, indane-1,3-dione, and isatins. Synthesis 2014, 46, 1059–1066. 10.1055/s-0033-1340815. [DOI] [Google Scholar]; d Sun J.; Shen G. L.; Huang Y.; Yan C. G. Formation of diverse polycyclic spirooxindoles via three-component reaction of isoquinolinium salts, isatins and malononitrile. Sci. Rep. 2017, 7, 41024 10.1038/srep41024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Belei D.; Abuhaie C.; Bicu E.; Jones P. G.; Hopf H.; Birsa L. M. A direct synthesis of octahydropyrrolo[2,1,5-cd]indolizin-6-one derivatives. Synlett 2012, 23, 545–548. 10.1055/s-0031-1290337. [DOI] [Google Scholar]; b Hopf H.; Jones P. G.; Nicolescu A.; Bicu E.; Birsa L. M.; Belei D. A Facile Synthesis of Pechmann Dyes. Chem. – Eur. J. 2014, 20, 5565–5568. 10.1002/chem.201400329. [DOI] [PubMed] [Google Scholar]; c Liu R. Z.; Wang X. Y.; Sun J.; Yan C. G. A facile synthesis of tricyclic skeleton of alkaloid 261C by double [3+2] cycloaddition of pyridinium ylide. Tetrahedron Lett. 2015, 56, 6711–6714. 10.1016/j.tetlet.2015.10.049. [DOI] [Google Scholar]
- a Kanemasa S.; Takenaka S.; Watanabe H.; Tsuge O. Tandem 1,3-dipolar cycloadditions of pyridinium or isoquinolinium methylides with olefinic dipolarophiles leading to cycl[3.2.2]azines. Enamine route as a new generation method of azomethine ylides. J. Org. Chem. 1989, 54, 420–424. 10.1021/jo00263a030. [DOI] [Google Scholar]; b Xu J. H.; Zheng S. C.; Zhang J. W.; Liu X. Y.; Tan B. Construction of Tropane Derivatives by the Organocatalytic Asymmetric Dearomatization of Isoquinolines. Angew. Chem., Int. Ed. 2016, 55, 11834–11839. 10.1002/anie.201605736. [DOI] [PubMed] [Google Scholar]
- a Voskressensky L. G.; Kulikova L. N.; Listratova A. V.; Borisov R. S.; Kukaniev M. A.; Varlamov A. V. A novel cascade Kroehnke condensation - an intramolecular nucleophilic cyclization approach toward annulated chromenes. Tetrahedron Lett. 2010, 51, 2269–2270. 10.1016/j.tetlet.2010.02.102. [DOI] [Google Scholar]; b Voskressensky L. G.; Festa A. A.; Sokolova E. A.; Varlamov A. V. Synthesis of chromeno[2′,3′:4,5]imidazo[2,1-a]isoquinolines via a novel domino reaction of isoquinoline-derived immonium salts. Scope and limitations. Tetrahedron 2012, 68, 5498–5504. 10.1016/j.tet.2012.04.087. [DOI] [Google Scholar]; c Voskressensky L. G.; Sokolova E. A.; Festa A. A.; Varlamov A. V. A novel domino condensation-intramolecular nucleophilic cyclization approach towards annulated thiochromenes. Tetrahedron Lett. 2013, 54, 5172–5173. 10.1016/j.tetlet.2013.07.040. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.