Abstract
Behavioral experience has long been known to influence functional outcome after brain injury, but only recently has its pervasive role in the reorganization of the adult brain after damage become appreciated. We briefly review findings from animal models on the role of experience in shaping neuronal events after stroke-like injury. Experience-dependent neural plasticity can be enhanced or impaired by brain damage, depending upon injury parameters and timing. The neuronal growth response to some experiences is heightened due to interactions with denervation-induced plasticity. This includes compensatory behavioral strategies developed in response to functional impairments. Early behavioral experiences can constrain later experience-dependent plasticity, leading to suboptimal functional outcome. Time dependencies and facets of neural growth patterns are reminiscent of experience-expectant processes that shape brain development. As with sensitive periods in brain development, this process may establish behavioral patterns early after brain injuries that are relatively resistant to later change.
Keywords: stroke, learned nonuse, behavioral compensation, dendritic pruning, reactive synaptogenesis, rehabilitative training, motor skill learning, motor cortex
INTRODUCTION
“… genes need only roughly outline the pattern of neural connectivity…leaving the more specific details to be determined through the organism’s interactions with its environment.” (Greenough, Black & Wallace, 1987, p. 543)
In the development and maturation of the nervous system, experience is ubiquitously involved in shaping neural and non-neural structure and function, from the level of gene expression to activity patterns across systems. This is an understanding propelled by the findings and conceptual insights of Greenough and his colleagues (e.g., Greenough, et al., 1987; 2001; Weiler, Wang, & Greenough, 1994). Brain injury in adulthood results in widespread degenerative effects that initiate a prolonged cascade of reorganization of synaptic connections and activity patterns of surviving neurons. Though the adult brain seems reticent to grow axons long distances (Cheatwood, Emerick, & Kartje, 2008), injury sometimes reveals an impressive capacity for reactive collateral sprouting (Nudo, 2007), dendritic remodeling (Brown and Murphy, 2008), cell proliferation (Carmichael, 2006; Lichtenwalner & Parent, 2006), and glial and vascular plasticity (Carmichael, 2006). Many studies link aspects of the regenerative responses to functional improvement (reviewed in Allred & Jones, 2008a; Cheatwood et al., 2008; Darian-Smith, 2009; Johansson, 2004; Nudo, 2007).
The similarities between the developing brain and the dynamic milieu of the adult injured brain have been noted by many (e.g., Carmichael, 2006; Cramer & Chopp, 2000; Finger & Almli, 1985; Murphy & Corbett, 2009). When examined in detail, the analogy to brain development is stretched, e.g., by the maturity of remaining circuits, injury-induced pathological events and differences in microenvironments (Carmichael, 2006; Finger and Almli 1985; Galván, 2010; Kolb & Teskey, 2010). We nevertheless believe that the comparison with brain development is useful when considering the central role of behavioral experience in re-shaping the brain after injury. Some post-injury neural events seem especially reminiscent of the brain developmental process that Greenough and colleagues refer to as “experience-expectant” (Greenough et al., 1987; 2001). This is the process in which experiences available during early sensitive periods sculpt neural circuits by selective stabilization of some synapses at the expense of insufficiently activated ones. We review findings indicating that this process of synaptic competition is at play in reorganizing the adult injured brain.
There is a growing understanding of the cellular and molecular changes that follow injury to the adult nervous system (Carmichael, 2006; Keyvani & Schallert, 2002). However, outside of our animal models, we still cannot precisely predict neural events and their behavioral consequences after any given CNS injury. The problem seems daunting since the effects of injury vary with injury locus, size (Dancause et al., 2006) and type (Carmichael & Chesselet, 2002), as well as the age, sex and various other characteristics of the individual (see Dobkin, 2007). However, such breadth can also be said to exist within the fields of development and learning, in which model systems have been used to divide and conquer the problems (see Churchill et al., 2002). This strategy has yielded many general principles of experience-dependent plasticity (Kleim & Jones, 2008). It has also made it clear that, without considering the role of experience, we are unlikely to tackle the problem of how the brain reorganizes when it is damaged. This is the inspiration and context for our model systems approach for investigating experience-injury interactions after stroke.
Using Rodents to Model Upper Extremity Impairment after Stroke
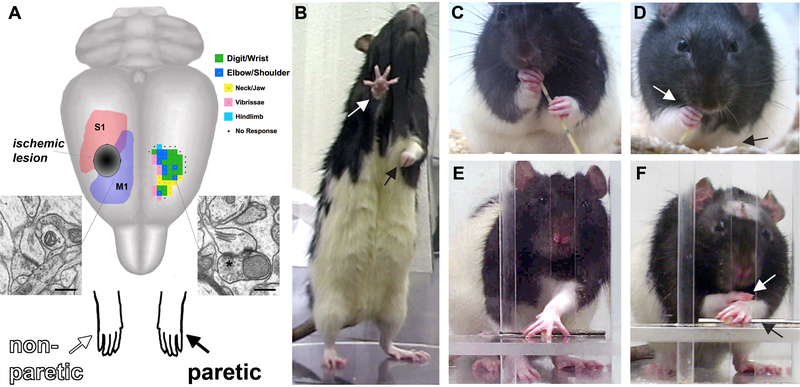
Upper extremity (hand and arm) impairments are a leading long-term disability in stroke survivors. Rats use their forepaws in dexterous ways that bear some homologies to human hand use (Whishaw & Coles, 1996). There is no reliable population bias for limb dominance in rats, but they show specializations in the way they use either paw and a strong limb preference for lateralized tasks such as reaching to grasp small food pieces (Allred et al., 2008; Peterson, 1934; Whishaw & Coles, 1996). Several behavioral tasks can sensitively characterize and manipulate forelimb behavior in rats (Fig. 1). Mice are also extremely dexterous in forelimb use (Farr & Whishaw, 2002; Tennant & Jones, 2009; Tennant, Asay, Allred, Ozburn & Jones, 2010; Xu et al., 2009). Their intrinsic manual dexterity, and certain homologies with primates in motor systems organization, including the large territory devoted to forelimb movements in motor cortex (Fig. 1A; Donoghue & Wise, 1983; Tennant, Adkins, Donlan et al., 2010) have made mice and rats increasingly popular for studies of motor skill learning and recovery.
Figure 1.
(A) Ischemic lesions of the sensorimotor cortex result in impairments in the contralesional, paretic, forelimb (black arrows) and compensation with the nonparetic limb (white arrows). Synaptic structural plasticity in the contralesional motor cortex occurs due to denervation of transcallosal projections and increased reliance on the nonparetic limb. The residual cortex of the injured hemisphere can be driven to undergo greater neuronal plasticity with focused training (“rehabilitative training) of the paretic limb. A representative map of forelimb movement representations (green and blue, an average of n = 7 maps) is shown in the intact hemisphere. M1, primary motor cortex, S1, primary somatosensory cortex, *perforated synapse, scale bars = 400nm. (B) Rats rely more on the nonparetic limb for postural support. (C) In handling food pieces (in this example, a piece of uncooked vermicelli pasta), normal rats use dexterous forepaw movements. (D) After unilateral SMC lesions, rats use the paretic forelimb less and in less coordinated ways. (E) In skilled reaching, normal rats retrieve food pellets using a coordinated sequence of proximal and distal forelimb movements. (F) After unilateral SMC lesions, rats have movement abnormalities and frequently compensate with the nonparetic paw.
The motor cortex undergoes neural plasticity during motor skill learning (Monfils, Plautz, & Kleim, 2005). Greenough, Larson and Withers (1985) were the first to report that learning a skilled forelimb reaching task leads to neuronal structural plasticity (growth of dendrites) in the adult rat cortex (see also Withers & Greenough, 1989). Since then, many studies in humans and animal models have investigated the relationships between motor skill acquisition and motor cortical plasticity (e.g., Kleim, Lussnig, Schwarz, Comery, & Greenough, 1996; Luft, Buitrago, Ringer, Dichgans, & Schulz, 2004; Monfils & Teskey, 2004; Monfils et al., 2005; Nudo, Milliken, Jenkins, & Merzenich, 1996; Pavlides, Miyashita, & Asanuma, 1993; Rioult-Pedotti, Friedman, & Donoghue, 2000; Withers & Greenough, 1989; Xu et al., 2009). Studies of rodent motor cortex have largely followed the precedent of Greenough et al. (1985) by focusing on how they learn to skillfully perform reach-to-grasp tasks. Skill acquisition in adult animals involves activity-dependent plasticity that is reflected in both neuronal structural changes and in reorganization of movement representations within the primary motor cortex (Monfils et al., 2005). Cortical synaptic structural and functional changes are initiated during early stages of practice (Kleim et al., 1996; Monfils & Teskey, 2004; Xu et al., 2009) and stabilized by repetition and time (Kleim et al., 2004; Tennant, Adkins, Scalco et al., 2010). Skill acquisition is impaired when plasticity of the motor cortex is disrupted (Conner, Culberson, Packowski, Chiba, & Tuszynski, 2003; Luft et al., 2004).
In rats and mice, the primary motor and somatosensory regions of cortex partially overlap in the forelimb representation area (Fig. 1A); hence, this region is termed “sensorimotor” cortex (SMC). After unilateral SMC lesions, including stroke-like ischemic infarcts, rats have sensory and motor deficits in the contralateral-to-the-lesion (contralesional) forelimb (Jones & Schallert, 1992; Whishaw, 2000). We refer to this as the “paretic” limb to use terminology consistent with the clinical literature in reference to the weakness and partial loss of movement resulting from unilateral cerebral injury. Although some of these impairments recover over time, using sufficiently sensitive tasks, such as the skilled reaching task (Fig. 1), profound and permanent behavioral impairments can occur. The rats begin to disuse the paretic limb, as is evident in home cage observations of postural support and in measures of dexterous food handling (Allred et al., 2008; Jones & Schallert, 1992). They also begin to rely on the ipsilesional, “nonparetic”, forelimb (Fig. 1D & F) despite subtle deficits in this limb (Hsu & Jones, 2006). This disuse of the paretic forelimb and compensation with the nonparetic limb in rats resembles the phenomenon of learned nonuse in human stroke survivors with upper extremity impairments (Taub, Uswatte, Mark, & Morris, 2006).
Converging Experience- and Injury-Induced Plasticity
“In the harsher world of nature, any incapacitating or debilitating injury affects the probability of survival… Thus, characteristics which enhance rate of recovery, such as the ability to benefit from experience, may be naturally selected.” (Greenough, Fass, & DeVoogd, 1976, p. 12)
It can be expected that injury- and learning-related plasticity interact when they converge upon the same neuronal circuits. They are both activity-dependent (Brus-Ramer, Carmel, Chakrabarty, & Martin, 2007; Di Filippo et al., 2008) and they have overlapping cellular mechanisms for the formation and strengthening of synapses, the restructuring of dendrites and coordination of glial and vascular plasticity (Grossman, Churchill, Bates, Kleim, & Greenough, 2002; Jones & Greenough, 2002; Nudo, 2007). The neural mechanisms of learning are proposed to have an evolutionary basis in primitive cellular responses to injury (Sung & Ambron, 2004; Walters & Ambron, 1995). (This idea stems from findings of strong overlap in the molecular pathways activated in Aplysia neurons during learning and after injury.) Furthermore, their convergence is likely widespread in the injured brain. After surviving brain damage, animals undergo dramatic behavioral change because they are coping with, and learning to compensate for, functional deficits resulting from the injury (Jones & Schallert, 1994; Taub et al., 2006; Whishaw, 2000). The resulting behavioral changes can have a profound impact on the underlying neuronal populations that are simultaneously responding to the degenerative effects of the initial injury.
Unilateral SMC lesions result in a loss of its transcallosal projections. The degeneration of these projections alone triggers glial reactivity, neuronal plasticity and other changes in the contralesional cortex, as can be demonstrated by corpus callosum transections (Adkins, Bury, & Jones, 2002; Bury, Eichhorn, Kotzer, & Jones, 2000; Gomide & Chadi, 1999). However, after unilateral SMC lesions, this occurs at the same time that animals are learning to rely on the nonparetic limb, which asserts behavioral pressures on the same region (Jones & Schallert, 1992). Thus, there is a convergence in both time and location of behavioral and degenerative pressures. This convergence results in neuronal structural plasticity in the contralesional cortex that cannot be reproduced as a result of either transcallosal denervation or behavioral manipulations alone (Adkins et al., 2002; Bury, Adkins et al., 2000; Jones & Schallert, 1994), or if the denervation and behavioral change are uncoupled temporally (Jones & Allred, 2004).
The neuronal structural response to this experience-injury interaction resembles the sculpting associated with “experience-expectant” development of cortex (Greenough et al., 1987), in that the dendrites in the contralesional cortex overgrow and then are partially eliminated (Jones & Schallert, 1992). The pruning phase corresponds with the appearance of greater numbers of mature, efficacious synapses (e.g., perforated synapses; Jones, 1999). On the behavioral level, animals show a paradoxical enhancement in the ability to learn new motor skills with the nonparetic limb (Bury & Jones, 2002, reviewed in Allred & Jones, 2008a). Rats have subtle impairments in using the nonparetic limb in motor skills established before the injury; however, they can more quickly acquire a novel motor skill (skilled reaching) with this limb compared to intact animals (Hsu & Jones, 2006). This is also seen if animals are trained on a novel motor skill soon after transecting the corpus callosum (Bury & Jones, 2004). Thus, the convergence of learning (training in a novel task) and anatomical denervation is sufficient for the effect.
These results indicate that neural responses to degeneration can sometimes enhance experience-dependent plasticity. In this case, it enhances plasticity in the same circuit being relied upon for the development of behavioral compensation with the nonparetic body side. In the context of the evolutionary selection pressures discussed by Greenough et al. (1976), this might enable an animal to rapidly benefit from behavioral experiences in a way that optimizes its chances of survival after neural injury. However, this more rapid solution is probably suboptimal for the return of more normal function in the paretic limb. In human stroke survivors, debility of the paretic arm leads to its disuse, which limits functional improvement, a phenomenon termed “learned non-use” (Taub et al., 2006). This could be exacerbated by the facilitation of neural plasticity underlying learning with the nonparetic limb. Consistent with this, when rats are trained with the nonparetic limb on a skilled reaching task, it impairs performance of this task with the paretic limb and also exaggerates its disuse in other behavioral measures (Allred, Maldonado, Hsu & Jones, 2005; Allred & Jones, 2008a).
Skill acquisition with the nonparetic limb can also negatively impact the way the cortex of the injured hemisphere responds to experience with the paretic limb. During repetitive activity, the transcription factor ΔFosB accumulates gradually in neurons and persists over time spans of at least days (McClung et al., 2004), making it amenable for assaying neuronal activation resulting from gradual, practice-dependent, behavioral change. Neuronal immunolabeling for FosB/ΔFosB in the perilesion cortex after training with the paretic limb is reduced in rats that had previously been trained with the nonparetic limb (Allred & Jones, 2008b). The reason for this apparent constraint of neuronal activation in the injured hemisphere by the nonparetic limb is not yet clear, but it may reflect experience-dependent alterations in interhemispheric activity. Cutting the callosal connections between the injured and intact hemisphere mitigates the maladaptive effects of learning with the nonparetic limb (Allred, Cappellini, & Jones, 2010). In human stroke survivors and in animal models, recovery of the paretic limb has been linked to more normal lateralization of hemispheric activity (Cramer & Riley, 2008) and plasticity in the residual cortex of the injured hemisphere (discussed below). Behavioral experience with the nonparetic limb may exacerbate abnormal interhemispheric activity and restrict adaptive plasticity in the injured hemisphere.
Here we focus on findings from our own animal model, but many other studies have found that aspects of the brain’s response to injury can be altered, often bi-directionally, by behavioral manipulations, as reviewed elsewhere (Johansson, 2004; Kleim, Jones, & Schallert, 2003; Nudo, 2007; Vaynman & Gomez-Pinilla, 2005). The ease of finding these effects suggests that the convergence of behavior and injury on the same neural systems is a very common occurrence after stroke and other types of CNS injury. Furthermore, experience has the capacity to influence degenerative and regenerative responses in ways that can both enhance and impair function, sometimes simultaneously, as in our example of training the nonparetic limb following stroke. It is therefore important to understand these interactions in order to avoid worsening function, in addition to finding new ways to improve it.
Using Behavioral Manipulations to Improve Brain Reorganization after Injury
“…whatever occurs during these early critical periods, whether or not appropriate, appears to “set” the brain, such that in later functioning its range of response is limited by those experiences.” (Greenough et al, 1976, p. 13)
We have many tools for manipulating neural activity and connectivity, but there is currently no better way of doing this in precisely the manner needed to improve function than to use the neural mechanisms of learning. The concept of using experience-dependent neural plasticity as a tool to remodel the damaged brain has been embraced in the stroke rehabilitation research community (e.g., Gonzalez Rothi, Musson, Rosenbek, & Sapienza, 2008; Krakauer, 2006; Taub et al., 2006), but in current clinical practice this is primarily a conceptual awareness. A better understanding of experience effects on the reorganization of the damaged brain may yield more concrete neural targets for behavioral interventions.
Many studies have now found that “rehabilitative” training (that which improves function, typically with task practice) can directly influence the structural and functional reorganization of residual tissue after cerebral damage (Johansson, 2004; Jones & Adkins, 2010; Nudo, 2007). Instrumental to this concept was a pivotal study by Nudo, Wise, SiFuentes, & Milliken (1996), in which adult squirrel monkeys were given ischemic lesions of the distal forelimb representation of the primary motor cortex. Following several weeks of spontaneous recovery, in the absence of training, the remaining distal forelimb representation was reduced in the perilesion cortex. Post-lesion rehabilitative training in skilled reaching with the paretic limb prevented the loss of hand representation territory in perilesion cortex and improved hand function. Similarly, in rats following unilateral cortical lesions, extensive skilled reach training with the paretic forelimb improves motor function and restructuring of motor maps within the remaining cortex of the injured hemisphere (Ramanathan, Conner, & Tuszynski, 2006; Winship & Murphy, 2009). It also enhances the survival of newly generated cells in this region (Maldonado & Jones, 2009).
These findings indicate that behavioral experience in the form of rehabilitative training can modulate cortical plasticity and motor recovery following cerebral injury. However, unlike the example of heightened sensitivity to experience in the contralesional cortex discussed above, the injured cortex can have impaired experience-dependent plasticity (Carmichael, 2006; Di Filippo et al., 2008; Jablonka, Witte, & Kossut, 2007). There is a major increase in many growth promoting signals after injury (Carmichael, 2006; Cramer & Chopp, 2000; Keyvani & Schallert, 2002; Murphy & Corbett, 2009). However, surviving neurons being called upon to grow new connections may have to overcome time-dependent elevations in growth inhibitory molecules (Benowitz & Carmichael., 2010; Carmichael, 2006; Cheatwood et al., 2008). They may also have to surmount dysfunction and metabolic limitations resulting from partial denervation, disturbed glial support (Anderson et al., 2003), impaired neurovascular coupling (del Zoppo, 2010), and altered cortical and interhemispheric patterns of excitatory and inhibitory activity (Neumann-Haefelin & Witte, 2000; Ward & Cohen, 2004). An enduring elevation in tonic inhibition in peri-infarct cortex (Clarkson et al., 2010) also probably works against activity-dependent synapse formation. Other types of brain injury (ablation and traumatic brain injury) may result in an even more unfavorable environment for experience-dependent neuronal plasticity (Carmichael & Chesselet, 2002; Phillips & Reeves, 2001). Dendrites and axons do grow after stroke, sometimes spectacularly (e.g., Brown, Boyd & Murphy, 2010; Carmichael & Chesselet, 2002; Dancause et al., 2005; Winship & Murphy, 2009). However, the functional improvements that can be driven by rehabilitative training alone are laborious to achieve and often far from sufficient to restore normal performance levels. Thus, an increasing focus of current research is to combine behavioral manipulations with other treatments intended to facilitate experience-dependent plasticity after brain injury (e.g., Huang & Krakauer, 2009; Page, Szaflarski, Eliassen, Pan, & Cramer, 2009; Plow, Carey, Nudo, & Pascual-Leone, 2009).
Other behavioral manipulations, including complex environment housing and exercise, can influence post-injury neural and non-neural events. For example, exercise can enhance recovery on cognitive tasks in traumatic brain injury models (Vaynman & Gomez-Pinilla, 2005). This is linked with increased expression of synaptic-plasticity related molecules in the hippocampus. Complex environment housing has diverse effects on neural events after brain injury, and is typically found to be beneficial to behavioral outcome, as reviewed in detailed elsewhere (Johansson, 2004; Will et al., 2004).
The time of onset of behavioral training following focal brain injury is an important variable in its effects. This is consistent with the interaction of training with injury-induced plasticity, which evolves over time after the initial damage (Carmichael, 2006). In monkeys, a delay in the onset of rehabilitative reach training until one month after motor cortex damage resulted in diminished neural reorganization compared with earlier training (Barbay et al., 2006). In rats receiving focal ischemic brain injury, early (5 days post-injury) initiation of rehabilitative training, enhanced dendritic growth and skilled forelimb function as compared to delayed training (Biernaskie, Chernenko, & Corbett, 2004). Here again it is tempting to draw parallels with brain development. If experience interacts with time sensitive injury-induced changes, then it might shape brain reorganization in a manner that is long lasting and that could not be accomplished at more chronic post-injury time points.
There is also the capacity to exacerbate degeneration and worsen behavioral function, especially with early behavioral manipulations. This has been found with both intense forced forelimb use procedures early after cortical lesions (Schallert, Fleming, & Woodlee, 2003) and with voluntary exercise early after traumatic brain injury, an effect that interacts with injury severity (Griesbach, Gomez-Pinilla, & Hovda, 2007; see also Ploughman et al., 2007). Thus, a major unresolved issue in the field is how to safely capitalize upon early sensitive periods after brain injury, when the neuronal growth-permissive environment is at its peak, without worsening function by exaggerating degeneration or promoting maladaptive neural plasticity.
CONCLUSIONS
Injury places the brain in a malleable state in which it is unusually responsive to some behavioral experiences. Experiences that have the capacity to shape neural reorganization after CNS damage include not just overt manipulations, but also the changes in behavioral strategies that animals develop on their own to compensate for impairments. The sensitivity to experience is reflected in exuberant neuronal growth responses in the cortex that resemble aspects of the experience-expectant processes involved in refinement of cortical circuits during development. However, the heightened sensitivity can have maladaptive functional consequences. Furthermore, some brain regions show impaired experience-dependent plasticity, including areas proximal to an injury that may be particularly important for functional outcome. Degenerative processes in these same areas can also be exacerbated by too early and too intense behavioral activity.
At present, we lack a sufficient understanding of experience-injury interactions to predict their contribution after CNS injury and to manipulate them to optimize functional outcome. Outside of our animal models, it is unclear how to capitalize on sensitive time windows after injury without risking an exacerbation of degenerative effects and functional impairments. It is also unclear how to take advantage of neuronal growth permissive environments that facilitate learning-related plasticity without promoting neural changes that reinforce suboptimal, behavioral strategies. As noted above, this topic seems daunting in its breadth and complexity (see Dobkin, 2007). We take inspiration in the remarkable success achieved by Greenough and his colleagues in uncovering and conceptualizing principles of experience-dependent plasticity and, as a result, dramatically advancing our understanding of normal and abnormal brain development and adult brain function.
Acknowledgments
The work reviewed here was supported by NS056839. We thank Cole Husbands for editing of the text.
REFERENCES
- Adkins DL, Bury SD, & Jones TA (2002). Laminar-dependent dendritic spine alterations in the motor cortex of adult rats following callosal transection and forced forelimb use. Neurobiology of Learning and Memory, 78, 35–52. [DOI] [PubMed] [Google Scholar]
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, & Jones TA (2008). The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. Journal of Neuroscience Methods, 170, 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RA, Cappellini C and Jones TA (2010). The “good” limb makes the “bad” limb worse: Experience-dependent interhemispheric disruption of functional outcome after cortical stroke in rats. Behavioral Neuroscience, 124, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, & Jones TA (2008a). Experience--a double edged sword for restorative neural plasticity after brain damage. Future Neurology, 3, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, & Jones TA (2008b). Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Experimental Neurology, 210, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, Maldonado MA, Hsu And JE, & Jones TA (2005). Training the “less-affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurology and Neuroscience, 23, 297–302. [PubMed] [Google Scholar]
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, & Nilsson M (2003). Astrocytes and stroke: networking for survival?, Neurochemical Research, 28, 293–305. [DOI] [PubMed] [Google Scholar]
- Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, et al. (2006). Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Experimental Brain Research, 169, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI & Carmichael ST (2010). Promoting axonal rewiring to improve outcome after stroke. Neurobiology of Disease, 37, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Chernenko G, & Corbett D (2004). Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. Journal of Neuroscience, 24, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Boyd JD, Murphy TH (2010). Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. Journal of Cerebral Blood Flow and Metabolism, 30, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, & Martin JH (2007). Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. Journal of Neuroscience, 27, 13793–13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SD, Adkins DL, Ishida JT, Kotzer CM, Eichhorn AC, & Jones TA (2000). Denervation facilitates neuronal growth in the motor cortex of rats in the presence of behavioral demand. Neuroscience Letters, 287, 85–88. [DOI] [PubMed] [Google Scholar]
- Bury SD, Eichhorn AC, Kotzer CM, & Jones TA (2000). Reactive astrocytic responses to denervation in the motor cortex of adult rats are sensitive to manipulations of behavioral experience. Neuropharmacology, 39, 743–755. [DOI] [PubMed] [Google Scholar]
- Bury SD, & Jones TA (2002). Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. Journal of Neuroscience, 22, 8597–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury SD, & Jones TA (2004). Facilitation of motor skill learning by callosal denervation or forced forelimb use in adult rats. Behavioural Brain Research, 150, 43–53. [DOI] [PubMed] [Google Scholar]
- Carmichael ST (2006). Cellular and molecular mechanisms of neural repair after stroke: making waves. Annals of Neurology, 59, 735–742. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, & Chesselet MF (2002). Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. Journal of Neuroscience, 22, 6062–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatwood JL, Emerick AJ, & Kartje GL (2008). Neuronal plasticity and functional recovery after ischemic stroke. Top Stroke Rehabil, 15(1), 42–50. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Grossman AW, Irwin SA, Galvez R, Klintsova AY, Weiler IJ, & Greenough WT (2002). A converging-methods approach to fragile X syndrome. Developmental Psychobiology, 40, 323–338. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, & Carmichael ST (2010). Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature, 468, 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, & Tuszynski MH (2003). Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron, 38, 819–829. [DOI] [PubMed] [Google Scholar]
- Cramer SC, & Chopp M (2000). Recovery recapitulates ontogeny. Trends in Neurosciences, 23, 265–271. [DOI] [PubMed] [Google Scholar]
- Cramer SC, & Riley JD (2008). Neuroplasticity and brain repair after stroke. Current Opinion in Neurology, 21, 76–82. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, & Nudo RJ (2005). Extensive cortical rewiring after brain injury. Journal of Neuroscience, 25, 10167–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, & Nudo RJ. (2006). Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. Journal of Neurophysiology, 96, 3506–3511. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C (2009). Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist, 15, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ (2010). The neurovascular unit in the setting of stroke. Journal of Internal Medicine, 267, 156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Tozzi A, Costa C, Belcastro V, Tantucci M, Picconi B, et al. (2008). Plasticity and repair in the post-ischemic brain. Neuropharmacology, 55, 353–362. [DOI] [PubMed] [Google Scholar]
- Dobkin BH (2007). Curiosity and cure: translational research strategies for neural repair-mediated rehabilitation. Developmental Neurobiology, 67, 1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP & Wise SP (1982). The motor cortex of the rat: cytoarchitecture and microstimulation mapping. Journal of Comparative Neurology, 212, 76–88. [DOI] [PubMed] [Google Scholar]
- Farr TD, & Whishaw IQ (2002). Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke, 33, 1869–1875. [DOI] [PubMed] [Google Scholar]
- Finger S, & Almli CR (1985). Brain damage and neuroplasticity: mechanisms of recovery or development? Brain Research, 357, 177–186. [DOI] [PubMed] [Google Scholar]
- Galván A (2010). Neural plasticity of development and learning. Human Brain Mapping, 31, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomide VC, & Chadi G (1999). The trophic factors S-100beta and basic fibroblast growth factor are increased in the forebrain reactive astrocytes of adult callosotomized rat. Brain Research, 835, 162–174. [DOI] [PubMed] [Google Scholar]
- Gonzalez Rothi LJ, Musson N, Rosenbek JC, & Sapienza CM (2008). Neuroplasticity and rehabilitation research for speech, language, and swallowing disorders. Journal of Speech, Language, and Hearing Research, 51, S222–224. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 58, 539–559. [PubMed] [Google Scholar]
- Greenough WT, Fass B, & DeVoogd TJ (1976). The influence of experience on recovery following brain damage in rodents: Hypotheses based on development research In Walsh RN & Greenough WT (Eds.), Environments as therapy for brain dysfunction (pp. 10–50). New York, Plenum Press. [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, & Weiler IJ (2001). Synaptic regulation of protein synthesis and the fragile X protein. Proceedings of the National Academy of Sciences of the United States of America, 98, 7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, & Withers GS (1985). Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behavioral and Neural Biology, 44, 301–314. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, & Hovda DA (2007). Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. Journal of Neurotrauma, 24, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, Bates KE, Kleim JA, & Greenough WT (2002). A brain adaptation view of plasticity: is synaptic plasticity an overly limited concept? Progress in Brain Research, 138, 91–108. [DOI] [PubMed] [Google Scholar]
- Hsu JE, & Jones TA (2006). Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Experimental Neurology, 201, 479–494. [DOI] [PubMed] [Google Scholar]
- Huang VS, & Krakauer JW (2009). Robotic neurorehabilitation: a computational motor learning perspective. Journal of Neuroengineering and Rehabilitation, 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka JA, Witte OW, & Kossut M (2007). Photothrombotic infarct impairs experience-dependent plasticity in neighboring cortex. NeuroReport, 18, 165–169. [DOI] [PubMed] [Google Scholar]
- Johansson BB (2004). Functional and cellular effects of environmental enrichment after experimental brain infarcts. Restorative Neurology and Neuroscience, 22, 163–174. [PubMed] [Google Scholar]
- Jones TA (1999). Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. Journal of Comparative Neurology, 414, 57–66. [PubMed] [Google Scholar]
- Jones TA, & Adkins DL (2010). Behavioral influences on neuronal events after stroke, Section I, Chapter 3 In Cramer SC & Nudo RJ (Eds.), Brain Repair after Stroke (pp. 23–33). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Jones TA, & Allred RP (2004). Dendritic growth in the cortex opposite unilateral sensorimotor cortex lesions in rats is inhibited by prior transection of the corpus callosum. Society for Neuroscience Abstracts, 30, 681617. [Google Scholar]
- Jones TA, & Greenough WT (2002). Chapter 19, Behavioral experience-dependent plasticity of glial-neuronal interactions In Volterra A, Magistretti P & Haydon PG (Eds.), Glia in Synaptic Transmission (pp. 248–265). Oxford University Press. [Google Scholar]
- Jones TA, & Schallert T (1992). Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Research, 581, 156–160. [DOI] [PubMed] [Google Scholar]
- Jones TA, & Schallert T (1994). Use-dependent growth of pyramidal neurons after neocortical damage. Journal of Neuroscience, 14, 2140–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyvani K, & Schallert T (2002). Plasticity-associated molecular and structural events in the injured brain. Journal of Neuropathology and Experimental Neurology, 61, 831–840. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, & Remple M (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. Journal of Neuroscience, 24, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, & Jones TA (2008). Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech Language and Hearing Research, 51, S225–239. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA, & Schallert T (2003). Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical Research, 28, 1757–1769. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, & Greenough WT (1996). Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience, 16, 4529–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, & Teskey GC (2010). ge, experience, injury, and the changing brain. Developmental Psychobiology, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Krakauer JW (2006). Motor learning: its relevance to stroke recovery and neurorehabilitation. Current Opinion in Neurology, 19, 84–90. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, & Parent JM (2006). Adult neurogenesis and the ischemic forebrain. Journal of Cerebral Blood Flow and Metabolism, 26, 1–20. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, & Schulz JB (2004). Motor skill learning depends on protein synthesis in motor cortex after training. Journal of Neuroscience, 24, 6515–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MA, & Jones TA (2009). Rehabilitative motor training promotes the survival of newly generated cells in perilesion motor cortex after unilateral ischemic lesions in rats. Society for Neuroscience Abstracts, program no. 333.22. Neuroscience Meeting Planner, Online. [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, & Nestler EJ (2004). DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Research Molecular Brain Research, 132, 146–154. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, & Kleim JA (2005). In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist, 11, 471–483. [DOI] [PubMed] [Google Scholar]
- Monfils MH, & Teskey GC (2004). Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience, 125, 329–336. [DOI] [PubMed] [Google Scholar]
- Murphy TH, & Corbett D (2009). Plasticity during stroke recovery: from synapse to behaviour. Nature Reviews Neuroscience, 10, 861–872. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, & Witte OW (2000). Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. Journal of Cerebral Blood Flow and Metabolism, 20, 45–52. [DOI] [PubMed] [Google Scholar]
- Nudo RJ (2007). Postinfarct cortical plasticity and behavioral recovery. Stroke, 38(2 Suppl), 840–845. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, & Merzenich MM (1996). Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience, 16, 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, & Milliken GW (1996). Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science, 272, 1791–1794. [DOI] [PubMed] [Google Scholar]
- Page SJ, Szaflarski JP, Eliassen JC, Pan H, & Cramer SC (2009). Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabilitation and Neural Repair, 23, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, & Asanuma H (1993). Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. Journal of Neurophysiology, 70, 733–741. [DOI] [PubMed] [Google Scholar]
- Peterson GM (1934). Mechanism of handedness in the rat. Comparative Psychology Monographs, 9, 1–67. [Google Scholar]
- Phillips LL, & Reeves TM (2001). Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restorative Neurology and Neuroscience, 19, 213–235. [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, & Corbett D (2007). Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Research, 1150, 207–216. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, & Pascual-Leone A (2009). Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke, 40, 1926–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, & Tuszynski MH (2006). A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proceedings of the National Academy of Sciences of the United States of America, 103, 11370–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, & Donoghue JP (2000). Learning-induced LTP in neocortex. Science, 290, 533–536. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, & Woodlee MT (2003). Should the injured and intact hemispheres be treated differently during the early phases of physical restorative therapy in experimental stroke or parkinsonism? Physical Medicine and Rehabilitation Clinics of North America, 14, S27–46. [DOI] [PubMed] [Google Scholar]
- Sung YJ, & Ambron RT (2004). PolyADP-ribose polymerase-1 (PARP-1) and the evolution of learning and memory. Bioessays, 26, 1268–1271. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, & Morris DM (2006). The learned nonuse phenomenon: implications for rehabilitation. Europa Medicophysica, 42, 241–256. [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA and Jones TA (2010). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral Cortex, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, Kleim JA and Jones TA (2010). The effects of duration and intensity of motor skill training on plasticity of the forelimb representation in the motor cortex of C57BL/6 mice. Society for Neuroscience Abstracts, program no. 493.7 Neuroscience Meeting Planner, Online. [Google Scholar]
- Tennant KA, Asay AL, Allred RP, Ozburn AR and Jones TA (2010). The Vermicelli and Capellini Handling Tests: Simple quantitative measures of dexterous forepaw function in rats and mice. Journal of Visualized Experiments, 41, pii: 2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, & Jones TA (2009). Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. Journal of Neuroscience Methods, 181, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, & Gomez-Pinilla F (2005). License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and Neural Repair, 19, 283–295. [DOI] [PubMed] [Google Scholar]
- Walters ET, & Ambron RT (1995). Long-term alterations induced by injury and by 5-HT in Aplysia sensory neurons: convergent pathways and common signals? Trends in Neurosciences, 18, 137–142. [DOI] [PubMed] [Google Scholar]
- Ward NS, & Cohen LG (2004). Mechanisms underlying recovery of motor function after stroke. Archives of Neurology, 61, 1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Wang X, & Greenough WT (1994). Synapse-activated protein synthesis as a possible mechanism of plastic neural change. Progress in Brain Research, 100, 189–194. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ (2000). Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology, 39, 788–805. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, & Coles BL (1996). Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behavioural Brain Research, 77, 135–148. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, & Rosenzweig MR (2004). Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002). Progress in Neurobiology, 72, 167–182. [DOI] [PubMed] [Google Scholar]
- Winship IR, & Murphy TH (2009). Remapping the somatosensory cortex after stroke: insight from imaging the synapse to network. Neuroscientist, 15, 507–524. [DOI] [PubMed] [Google Scholar]
- Withers GS, & Greenough WT (1989). Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia, 27, 61–69. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant KA, et al. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 462, 915–919 [DOI] [PMC free article] [PubMed] [Google Scholar]