Abstract
2-Chloro-4-nitrobenzoic acid (2c4n) is an antiviral agent used for the treatment of HIV infection and to boost the immune response in immune deficiency diseases. In the present study, a series of eight molecular salts of 2c4n with pyridyl and benzoic acid derivatives have been synthesized by a crystal engineering approach and were characterized structurally by various spectroscopic, thermal, and X-ray diffraction techniques. Crystal structures of all synthesized molecular salts were determined by single-crystal X-ray diffraction techniques. In all synthesized molecular salts, the charge-assisted acid···pyridine/amine heterosynthon was found to be the primary supramolecular synthon. The synthesized salts, namely, 2c4n.g and 2c4n.h salts were found to be isostructural. Further, in the current work, the occurrence of weak halogen bonds in the presence of strong hydrogen bonds in the synthesized and in the reported molecular salts/cocrystals of 2c4n has been investigated. A detailed inspection of the crystal structures of salts/cocrystals of 2c4n was carried out to demonstrate the importance of halogen bonds in these crystal structures. It was found that 4 out of 8 synthesized molecular salts and 12 out of 24 reported molecular adducts of 2c4n were found to exhibit halogen bonds in their crystal structures. A similar kind of conformational change was observed for molecular salts exhibiting halogen bonds in their crystal structures; however, the conformations were found to be slightly different in other molecular salts. It was observed that two-point primary supramolecular synthon and stronger intramolecular Cl···O halogen bonds in the molecular adducts of 2c4n are found to be more susceptible to exhibit halogen bonds in their crystal structures. Halogen bond interactions played a vital role in the crystal stabilization of these molecular adducts.
Introduction
Crystal engineering has played a major role in understanding the intermolecular interactions in the self-assembly of molecules and invoked it to generate new supramolecular assemblies without forming or breaking covalent bonds.1−3 In the rational design of the supramolecular structure, various noncovalent interactions such as hydrogen bonds,4−13 halogen bonds,14−24 van der Waals interactions, and π–π interactions25,26 play a vital role. In particular, these noncovalent interactions have been extensively used in solid-state chemistry to assemble supramolecular building blocks into well-defined crystal structures. Hydrogen bonds are the unique tool in molecular recognition and play a master key role in the packing of crystal structures.27−31 Halogen bonds32−34 have attracted significant attention from the scientific community in recent years because of their vital role in crystal packing. In the last decade, Resnati and Metrangolo et al. have used halogen bonds extensively for studying the crystal packing of iodoperflurocarbon molecules with aliphatic amines.35−39 Jennifer and Muthiah designed and synthesized the cocrystals/salts of nitrogenous bases and thiophene carboxylic acid through a combination of hydrogen and halogen bonds.40 Saha et al. studied the halogen bond interactions in iodopyridine derivatives.41 Priimagi et al. reported the importance of halogen bonds in designing the functional materials in the fields of biomimetics, optics/photonics, functional surfaces, and photoswitchable supramolecules.42 Anion-sensing property of receptors composed of halogen bond donor groups has been studied by Chudzinski et al.43 Further, applications in the fields of liquid crystals, nanomaterials, polymer chemistry, inorganic chemistry, medicinal chemistry, and biochemistry have been achieved using halogen bond interactions.44
Halogen bonds are not well-defined as hydrogen bonds, although they have strength, directionality, and specificity similar to those of hydrogen bonds.45,46 In general, halogen bonds are defined as a noncovalent interaction between the halogen atom and electron donors such as N, O, and S, similar to hydrogen bonds. The theoretical and experimental studies on halogen bonds revealed that there is an anisotropic distribution of electron clouds along the covalently bonded halogen atom.47−50 As a result, a localized positive potential region is formed at the surface of the halogen atom, known as a σ hole.51 The atoms having a nucleophilic character approach the halogen atom toward the σ hole and facilitate the formation of halogen bonds. Therefore, halogen bonds can be considered as the attractive interaction between the positive electrostatic potential region of a halogen atom and a negative site (halogen or electronegative atoms). This was well-explained by Politzer et al. in 2014 based on the computational methods.52 However, in late 1980s, Desiraju and Parthasarathy demonstrated that because of the anisotropically segregated region of complementary electrostatic potential, halogen–halogen interaction is possible in the halogen atom which exhibits both electrophilic and nucleophilic characters.53 As a consequence of the nonspherical atomic charge distribution among halogen atoms, the interhalogen bond (especially Cl···Cl bond) occurs, which is systematically explained by Price et al.54 Weak Cl···O interaction was coined in organic chemistry in the year 1950, and in recent years, it has been used extensively for building supramolecular assemblies in crystal engineering.55
The importance of halogen bond interactions in the pharmaceutical industry is that we can exploit these interactions in the design of new pharmaceutical drug products with definable structural and biological properties. A significant number of new drug molecules in the clinical development are halogenated compounds.56 The incorporation of the halogen atoms in the new drug molecules could lead to an increase in the permeability of the molecules, thereby increasing their oral bioavailability.57−59 A few cocrystals/salts are designed and synthesized based on the halogen bond interactions.60−62 The first example of a pharmaceutical cocrystal involving halogen bonds as a major interaction was reported by Baldrighi et al. in the year 2013, wherein an antimicrobial drug 3-iodo-2-propynyl-N-butylcarbamate (IPBC) has been chosen as the key molecule.63 Better physicochemical properties such as powder flowability and thermal stability were obtained for the cocrystal compared to those of the parent IPBC.
2-Chloro-4-nitrobenzoic acid (2c4n) is a derivative of benzoic acid which contains one electrophilic substituent and one labile group. This compound and most of its isomers (namely, 5-chloro-4-nitrobenzoic acid) are used as antiviral agents64 in the pharmaceutical field. They are useful in the treatment of HIV infection and boost the immune response in immune deficiency diseases.65 In the last two decades, numerous cocrystals/salts of 2c4n have been reported. Ishida et al. synthesized a series of cocrystals and salts of 2c4n with N-containing heterocycles. These include (2c4n)2·(pyrazine),66 (2c4n)·(2,4,6-trimethyl pyridine),67 (2c4n)·(3-cyanopyridine),68 (2c4n)·(1,2,3-benzotriazole),69 (2c4n)·(4-benzoylpyridine),70 and (2c4n)·(quinoline).71 In all reported molecular assemblies, acid···pyridine heterosynthon was found to be the most robust synthon. 4-Amino pyridine,72 imidazole,73 isopropylideneisonicotinohydrazide,74 and 3,5-lutidine75 were known to form salts with 2c4n using charge-assisted acid···pyridine hydrogen bonds. Lemmerer et al. synthesized a series of nine cocrystals/salts of 2c4n with various pyridine derivatives such as 2-bromopyridine, 3-amino-2-bromopyridine, 3-amino-2-chloropyridine, 2-amino-5-nitropyridine, 2-amino-3-bromopyridine, 2-chloro-3-hydroxypyridine, 2-amino-5-bromopyridine, 2,6-dimethylpyridine, and 2-amino-5-chloropyridine.76 Further, solvates and polymorphic phase transformation of 2c4n were reported by Aitipamula et al. in the year 2011.77
The interaction of carboxylic acid with pyridine nitrogen includes conventional and nonconventional hydrogen bonds and has been extensively studied in the design of various supramolecular nanostructures such as rosettes, rods, layers, tapes, sheets, ribbons, and sphere aggregates.78−80 Although numerous reports on cocrystals/salts of 2c4n are reported, the presence of weak secondary interactions, especially the presence of halogen bonds, and their importance in crystal packing were not illustrated much in the literature. Further, reports on the salt formation in the presence of other competing functionalities (carboxylic acid) are fewer in the literature. Hence, it is interesting to explore synthon formation when diverse functional groups are present that compete for synthon formation. Studies pertaining to the product formation in the presence of competitive functional groups such as amine (−NH2) are relatively sparse. Hence, heterocyclic amines have been chosen as well for studying the salt formation.
In this regard, an attempt was made to synthesize the molecular salts of 2c4n with various benzoic acid and pyridyl derivatives based on the pKa values of 2c4n and the salt formers. The salt formers used are 2-aminobenzoic acid, 3-aminobenzoic acid, 4-aminobenzoic acid, 2-aminothiazole, 2-aminobenzimidazole, 2-aminopyrimidine, 2-aminopyridine, and 3-aminopyridine (Scheme 1). Further, the present work aims to study and understand the nature of weak secondary interactions involved in the molecular salts, especially halogen bonds, and compare these nonbonded interactions with the reported salts/cocrystals of 2c4n to infer the presence of halogen bonds in these supramolecular architectures.
Scheme 1. Chemical Diagrams of 2c4n, 2-Aminobenzoic Acid (a), 3-Aminobenzoic Acid (b), 4-Aminobenzoic Acid (c), 2-Aminothiazole (d), 2-Aminobenzimidazole (e), 2-Aminopyrimidine (f), 2-Aminopyridine (g), and 3-Aminopyridine (h).
Results and Discussion
Crystal Structure of Molecular Salts
2c4n.a
The asymmetric unit in the molecular salt contains one molecule each of 2c4n and 2-aminobenzoic acid which was crystallized in a triclinic crystal system with the space group P1̅. The proton transfer from a carboxylic acid of 2c4n to 2-aminobenzoic acid was observed with a N–H···O bond distance of 2.734 Å (∠164.40°) (Figure 1a). Two neighboring supramolecular units are interconnected through a N–H···O hydrogen bond (2.756 Å) between the amine group and the carboxylate of 2c4n, resulting in a cyclic tetrameric unit with an R42 (8) graph set (Figure 1b). This tetrameric unit further self-assembled through a complementary bifurcated N–H···O hydrogen bond (2.987 Å) and an −O–H···O hydrogen bond (2.521 Å) between the acid group of 2-aminobenzoic acid and the carboxylate of 2c4n, resulting in a linear one-dimensional (1D) tape (Figure 2). The 1D structure is further converted to three-dimensional (3D) by utilizing a weak C–H···O hydrogen bond (3.239 Å) between aromatic C–H of 2c4n and O of the carboxylate of 2-aminobenzoic acid (Figure 3). The crystallographic data and the hydrogen bond parameters are displayed in Tables 1 and 2.
Figure 1.

Asymmetric unit (a) and the tetrameric unit (b) of salt 2c4n.a.
Figure 2.

One-dimensional representation of the 2c4n.a salt.
Figure 3.

Three-dimensional representation of the 2c4n.a salt stabilized by a C–H···O hydrogen bond.
Table 1. Crystallographic Data and the Refinement Parameters of the 2c4n Salts.
| structure details | 2c4n.a | 2c4n.b | 2c4n.c | 2c4n.d | 2c4n.e | 2c4n.f | 2c4n.g | 2c4n.h |
| CCDC number | 961164 | 961165 | 961166 | 961167 | 961168 | 1023271 | 961169 | 992132 |
| formula | C14H12ClN2O6 | C28H24Cl2N4O13 | C14H12ClN2O6 | C10H8ClN3O4S | C14H11ClN4O4 | C11H9ClN4O4 | C12H10ClN3O4 | C12H10ClN3O4 |
| Mr | 338.70 | 695.42 | 338.70 | 301.71 | 334.72 | 296.67 | 295.68 | 295.68 |
| crystal system | triclinic | triclinic | orthorhombic | triclinic | monoclinic | triclinic | triclinic | triclinic |
| space group | P1̅ | P1̅ | Pbca | P1̅ | P21/c | P1̅ | P1̅ | P1̅ |
| a/Å | 8.1717(14) | 10.856(3) | 7.2428(3) | 7.0003(11) | 11.7902(5) | 6.7950(5) | 5.7224(4) | 5.9445(3) |
| b/Å | 8.2522(14) | 11.154(3) | 13.0520(6) | 8.2219(19) | 11.3110(5) | 7.4766(5) | 10.5903(7) | 10.7390(5) |
| c/Å | 10.856(18) | 14.213(3) | 29.5375(13) | 10.551(17) | 12.3892(6) | 13.7306(9) | 11.2992(7) | 10.9139(5) |
| α/deg | 79.563(2) | 106.325(3) | 90.00 | 85.243(3) | 90.00 | 77.211(4) | 95.938(10) | 98.910(2) |
| β/deg | 89.113(2) | 104.731(3) | 90.00 | 78.471(2) | 109.790(10) | 88.200(4) | 103.751(10) | 95.465(2) |
| γ/deg | 83.600(2) | 106.209(3) | 90.00 | 79.703(2) | 90.00 | 69.323(4) | 94.7420(10) | 105.836(2) |
| volume/Å3 | 715.5(2) | 1479.1(6) | 2792.3(2) | 584.77(19) | 1554.63(12) | 635.6(8) | 657.47(8) | 655.22(5) |
| Z | 2 | 2 | 8 | 2 | 4 | 2 | 2 | 2 |
| density [g/cm3] | 1.577 | 1.562 | 1.611 | 1.714 | 1.430 | 1.5 | 1.494 | 1.499 |
| μ (Mo Kα) [mm–1] | 0.302 | 0.297 | 0.309 | 0.519 | 0.271 | 0.320 | 0.308 | 0.309 |
| T/K | 296(2) | 296(2) | 293(2) | 296(2) | 296(2) | 296(2) | 296 (2) | 296 (2) |
| reflections collected | 2773 | 5795 | 3236 | 2349 | 3181 | 2396 | 2567 | 2591 |
| unique reflections | 2227 | 4932 | 2824 | 2164 | 2440 | 2117 | 2184 | 2328 |
| parameter refined | 252 | 520 | 208 | 176 | 224 | 213 | 193 | 193 |
| R1 (I > 2σ) | 0.0468 | 0.0438 | 0.0449 | 0.0265 | 0.0495 | 0.0726 | 0.0319 | 0.0326 |
| wR2 (I < 2σ) | 0.1341 | 0.1510 | 0.1616 | 0.0659 | 0.1360 | 0.2368 | 0.0878 | 0.1155 |
| GOF | 0.922 | 1.149 | 1.354 | 1.263 | 1.525 | 1.447 | 0.625 | 0.951 |
Table 2. Geometrical Parameters of the Intermolecular Interactions in the Synthesized Molecular Salts of 2c4n.
| salt | D–H···Aa | H···A (Å) | D···A (Å) | ∠D–H···A (deg) | symmetry code |
|---|---|---|---|---|---|
| 2c4n.a | O6–H12···O1 | 1.59 | 2.521 | 167 | x – 1, y, z |
| N1–H10···O2 | 1.85 | 2.756 | 156 | –x + 1, –y + 1, –z + 1 | |
| N1–H8···O3 | 2.61 | 3.062 | 115 | –x, –y + 2, –z + 1 | |
| N1–H9···O2 | 1.81 | 2.734 | 164 | x, y, z | |
| N1–H8···O5 | 1.92 | 2.659 | 144 | x, y, z | |
| 2c4n.b | O13–H24···O9 | 2.26 | 2.973 | 151 | x, y, z |
| O12–H21···O1 | 1.71 | 2.581 | 175 | x + 1, y, z – 1 | |
| N4–H19···O2 | 1.74 | 2.658 | 158 | –x + 1, –y + 2, –z + 2 | |
| N4–H22···O7 | 2.60 | 3.029 | 110 | –x + 1, –y + 2, –z + 1 | |
| N4–H20···O6 | 1.89 | 2.787 | 167 | –x + 1, –y + 2, –z + 1 | |
| O5–H1···O8 | 1.78 | 2.612 | 163 | x, y, z | |
| N2–H13···O13 | 1.93 | 2.775 | 177 | x – 1, y, z + 1 | |
| N2–H12···Cl1 | 2.87 | 3.338 | 111 | –x, –y + 1, –z + 1 | |
| N2–H12···O8 | 1.74 | 2.677 | 167 | –x, –y + 1, –z + 1 | |
| 2c4n.c | N2–H10···O6 | 1.92 | 2.828 | 171.0 | x, y, z |
| N2–H9···O5 | 2.62 | 3.229 | 124.7 | x – 1/2, –y + 1/2, −z | |
| N2–H9···O6 | 1.91 | 2.825 | 178.8 | x – 1/2, –y + 1/2, −z | |
| N2–H8···O5 | 1.83 | 2.788 | 171.0 | –x + 5/2, y – 1/2, z | |
| O1–H11···O2 | 1.95 | 2.723 | 160.7 | x – 1/2, y, –z + 1/2 | |
| 2c4n.d | N2–H6···O2 | 2.014 | 2.846 | 162.5 | x – 1, y + 1, z – 1 |
| N3–H8···O1 | 1.69 | 2.634 | 174 | x – 1, y + 1, z – 1 | |
| N2–H7···O2 | 2.174 | 2.892 | 140.91 | –x + 1, −y, –z + 1 | |
| N2–H7···O3 | 2.543 | 3.143 | 127.68 | x – 1, y, z | |
| 2c4n.e | N4–H11···O2 | 1.92 | 2.827 | 170 | –x + 2, −y, –z + 1 |
| N4–H10···O1 | 2.09 | 2.859 | 156 | –x + 2, y + 1/2, –z + 1/2 | |
| N2–H8···O1 | 2.60 | 3.298 | 130 | –x + 2, y + 1/2, –z + 1/2 | |
| N2–H8···O2 | 1.80 | 2.759 | 173 | –x + 2, y + 1/2, –z + 1/2 | |
| N3–H9···O1 | 1.89 | 2.70 | 163 | x, y, z | |
| 2c4n.f | N4–H7···N2 | 2.13 | 3.036 | 174 | –x + 1, –y + 1, –z + 1 |
| N3–H9···O2 | 1.69 | 2.602 | 166 | x, y, z – 1 | |
| N4–H8···O1 | 1.98 | 2.823 | 166 | x, y, z – 1 | |
| 2c4n.g | C12–H7···O4 | 2.314 | 3.229 | 167.98 | –x, –y + 1, −z |
| N3–H6···O4 | 1.862 | 2.743 | 169.81 | x – 1, y, z | |
| N2–H4···O3 | 1.924 | 2.804 | 178.01 | x – 1, y, z | |
| N2–H5···O3 | 2.101 | 2.823 | 141.77 | –x + 1, –y + 1, –z + 1 | |
| 2c4n.h | C12–H4···O2 | 2.439 | 3.310 | 156.02 | –x, –y + 1, −z |
| C9–H2···O4 | 2.492 | 3.239 | 137.47 | –x + 2, –y + 1, –z + 1 | |
| N1–H5···O2 | 1.725 | 2.615 | 171.18 | x, y, z | |
| N2–H6···O1 | 2.174 | 2.935 | 163.38 | –x, –y + 1, −z | |
| N2–H7···O1 | 2.127 | 2.873 | 152.08 | x + 1, y + 1, z |
D: donor and A: acceptor.
2c4n.b
Salt 2c4n.b is crystallized in a triclinic crystal system with the P1̅ space group, and the asymmetric unit contains two ion pairs of 2c4n and 3-aminobenzoic acid along with one molecule of water (Figure 4). In the first instance, one molecule of 3-aminobenzoic acid interacted with one molecule each of 2c4n and 3-aminobenzoic acid through a −N–H···O hydrogen bond (3.029 and 2.787 Å, respectively) and a C–H···O hydrogen bond (3.165 Å); second, a 3-aminobenzoic acid molecule involved in a hydrogen bond with a water molecule with a −N–H···O bond distance of 2.776 Å. The water molecule further forms a hydrogen bond with the oxygen of 4-nitro group of 2c4n with an O–H···O bond distance of 2.973 Å. These molecular units further self-assembled through strong N–H···O and weak C–H···Cl and C–H···O hydrogen bonds, resulting in a two-dimensional (2D) network of the 2c4n.b salt (Figure 5). The unit repeats to form a 3D network which is stabilized by the π–π interaction between 2c4n and 3-aminobenzoic acid with a centroid–centroid distance of 3.636 Å (Figure 6).
Figure 4.

Asymmetric unit in the molecular salt of 2c4n.b.
Figure 5.

Two-dimensional network-like structure of the 2c4n.b salt.
Figure 6.

Three-dimensional representation of the 2c4n.b salt stabilized by the π–π interaction.
2c4n.c
This molecular salt is crystallized in an orthorhombic crystal system with the space group Pbca, and the asymmetric unit contains one molecule each of 2c4n and 4-aminobenzoic acid (Figure 7a). The primary synthon was found to be N+–H···O– heterosynthon with a distance of 2.828 Å. These supramolecular units are connected to the neighboring units through three different complementary N–H···O hydrogen bonds (2.788, 2.825, and 3.229 Å), resulting in the cyclic tetrameric unit (Figure 7b). These units further repeat to form a 1D sheet (Figure 8) via a C–H···O hydrogen bond (between two 2c4n molecules) and an O–H···O hydrogen bond (between two 4-aminobenzoic acid molecules). It is further converted to a 3D ladder kind of arrangement that is stabilized by the π–π interaction between 2c4n and 4-aminobenzoic acid with a centroid–centroid distance of 3.615 Å (Figure 9).
Figure 7.

Asymmetric unit (a) and tetrameric unit (b) of the 2c4n.c salt.
Figure 8.
One-dimensional representation of the 2c4n.c salt.
Figure 9.

Three-dimensional representation of the 2c4n.c salt.
2c4n.d
The product is crystallized in a triclinic crystal system with the space group P1̅ with one molecule each of 2c4n and 2-aminothiazole in the asymmetric unit. The primary interaction is the N+–H (azole)···O– (carboxylate) hydrogen bond (2.634 Å, ∠173.9°) and N–H···O hydrogen bond (2.846 Å, ∠162.45°) between 2c4n and 2-aminothiazole (Figure 10a). Two neighboring supramolecular adducts are interconnected through a strong complementary N–H···O hydrogen bond (2.892 Å) and a weak Cl···S halogen bond (3.405 Å), resulting in a cyclic tetrameric unit with R42 (8) ring motifs (Figure 10b). The tetramers are interconnected to form a 1D and then a 2D chain along the b axis via Cl···S and Cl···O halogen bonds (3.405 and 3.102 Å, respectively) and a S···O chalcogen bond (3.016 Å) (Figures 11 and 12). These further recur to form a 3D network that is stabilized by a C–H (azole)···O (carboxylate) hydrogen bond (3.339 Å).
Figure 10.

Asymmetric unit (a) and tetrameric unit (b) of the 2c4n.d salt.
Figure 11.
Linear 1D chain of the 2c4n.d salt.
Figure 12.

Two-dimensional chain of the 2c4n.d salt stabilized via a Cl···O halogen bond.
2c4n.e
2c4n.e is crystallized in a monoclinic crystal system with the space group P21/c. The asymmetric unit contains one molecule each of 2c4n and the 2-aminobenzimidazolium ion (Figure 13). The proton transfer was observed from 2c4n to the imidazole nitrogen of 2-aminobenzimidazole. Each of the carboxylate oxygen of 2c4n is involved in a hydrogen bond with the imidazole via N+–H (imidazole)···O– (2.759 Å, ∠172.99°) and N–H (amine)···O– hydrogen bonds (2.859 Å, ∠156.37°). The dimers are connected to each other through a N–H (imidazole)···O– hydrogen bond (2.703 Å) and a Cl···O halogen bond (3.205 Å) on the other side that recurs to form a V-shaped network (Figure 14). The overall crystal structure features weak secondary interactions such as a C–H···O hydrogen bond (3.532 Å) between two 2c4n molecules and a C–H···O hydrogen bond (3.410 Å) between aromatic C–H of 2-aminobenzimidazole and the nitro group of 2c4n (Figure 15).
Figure 13.

Asymmetric unit of the 2c4n.e salt.
Figure 14.

V-shaped network-like structure of the 2c4n.e salt stabilized by a Cl···O halogen bond.
Figure 15.

Three-dimensional representation of the 2c4n.e salt.
2c4n.f
The product is crystallized in a triclinic crystal system with the space group P1̅, and the asymmetric unit comprised one molecule each of 2c4n anion and 2-amino pyrimidinium cation. The primary synthon in the crystal structure comprised N+–H (pyrimidine)···O– (carboxylate of 2c4n) and N–H (amine of 2-aminopyrimidine)···O– (carboxylate) hydrogen bonds with bond distances of 2.602 and 2.823 Å, respectively (Figure 16). It is further interconnected to the neighboring unit via a complementary N–H···N hydrogen bond (3.036 Å) to form a heterodimer. These units repeat to form a 1D linear chain mediated by a C–H···O hydrogen bond between 2c4n molecules (3.355 Å) (Figure 17). It is further converted to a 3D structure by utilizing a weak C–H···Cl hydrogen bond (3.695 Å) and a C–H···O hydrogen bond between the oxygen of the nitro group of 2c4n and C–H of the 2-aminopyrimidine molecule. The overall crystal structure also features the π–π interaction between 2-aminopyridine molecules with a centroid-to-centroid bond distance of 3.409 Å (Figure 18).
Figure 16.

Asymmetric unit of the 2c4n.f salt.
Figure 17.
One-dimensional representation of the 2c4n.f salt mediated by a C–H···O hydrogen bond.
Figure 18.
Three-dimensional representation of the 2c4n.f salt.
2c4n.g
A 1:1 molecular salt of 2c4n and 2-aminopyridine was obtained and is crystallized in the triclinic space group P1̅. In the crystal structure, the asymmetric unit contains two crystallographically independent molecules, each of 2c4n and 2-aminopyridine. The primary synthon is a two-point supramolecular synthon which is assembled through a charge-assisted complementary hydrogen bond between carboxylic acid and aminopyridine moiety, resulting in a dimeric unit (2.743 Å) (Figure 19). Adjacent dimers are further linked through a self-complementary N–H···O (2.823 Å) hydrogen bond to form cyclic heterotetramers. These heterotetramers are mediated by a secondary C–H···O (3.229 Å) interaction to form a supramolecular 1D column (Figure 20). The 1D column is interconnected to the neighboring column through a secondary C–H···O interaction involving C–H of the pyridine ring and the oxygen of the nitro group of 2c4n to form a 2D supramolecular sheetlike structure (Figure 21). The dimensionality of the supramolecular structure of 2c4n.g increased by a weak Cl···Cl interaction. The supramolecular sheets are interconnected to the neighboring ones in a vertical plane through a Cl···Cl halogen bond (3.481 Å) (Figure 22).
Figure 19.
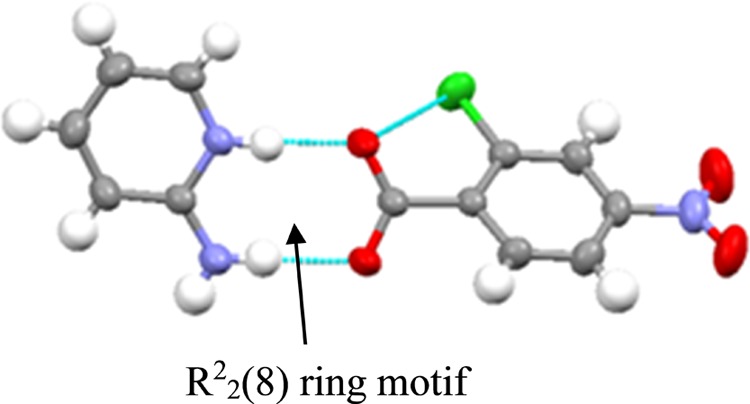
Asymmetric unit in salt 2c4n.g.
Figure 20.
One-dimensional column stabilized by a C–H···O interaction in salt 2c4n.g.
Figure 21.
Two-dimensional supramolecular sheet stabilized by a C–H···O interaction in salt 2c4n.g.
Figure 22.
Cl···Cl interaction in the crystal structure of salt 2c4n.g.
2c4n.h
2c4n and 3-aminopyridine afforded a crystalline salt in a 1:1 ratio which is crystallized in a triclinic crystal system with the space group P1̅. The asymmetric unit of the salt contains one 3-aminopyridine cation and one 2c4n anion. The primary synthon is a single-point supramolecular synthon in which a proton from the carboxylic group is transferred to the pyridine nitrogen atom with a N–H···O bond distance of 2.615 Å (Figure 23). The proton transfer from the acid to the N atom of pyridine is reflected by an increase in the C–N–C bond angle (123.44°) compared to that of the neutral molecule (117.7°–118.5°). The two neighboring ion pairs are linked through complementary N–H···O (2.935 Å) hydrogen bonds to form a cyclic heterotetramer. These heterotetramers are further combined through N–H···O hydrogen bonds (2.873 Å) to form a 1D column (Figure 24). These supramolecular 1D columns are interconnected to the neighboring column through a secondary C–H···O hydrogen bond (3.239 Å) which results in a 2D supramolecular sheet (Figure 25). Similar to salt 2c4n.g, the dimensionality of the crystal structure of 2c4n.h is increased by a weak Cl···Cl interaction. The supramolecular columns are interconnected to the neighboring columns through a chlorine–chlorine interaction with a Cl···Cl distance of 3.305 Å (Figure 26).
Figure 23.

Asymmetric unit in salt 2c4n.h.
Figure 24.
One-dimensional column held by a N–H···O hydrogen bond in salt 2c4n.h.
Figure 25.
Two-dimensional supramolecular sheet stabilized by a C–H···O interaction in salt 2c4n.h.
Figure 26.
Cl···Cl interaction in the crystal structure of salt 2c4n.h.
Role of Halogen Bonds in the Present Work and the Reported Salts/Cocrystals of 2c4n in Building a Supramolecular Network
Among the 24 reported molecular adducts and 8 synthesized molecular salts of 2c4n, only 12 and 4 molecular salts/cocrystals exhibited halogen bonds in their crystal structures. Molecular structures of the reported salts/coformers of 2c4n are shown in Scheme 2. Conformational changes in the carboxylate group were observed in the synthesized molecular salts and in the reported molecular adducts of 2c4n as well. Synthesized molecular salts such as 2c4n.d, 2c4n.e, 2c4n.g, and 2c4n.h exhibited halogen bonds in their crystal structures, which along with hydrogen bonds are responsible for the overall crystal stability of the molecular salts. However, the crystal structures of the remaining salts were stabilized via C–H···Cl and C–H···O hydrogen bonds except for the 2c4n.a salt, where chlorine was not involved in the intermolecular bond formation. It is worth noticing that the synthesized as well as the reported molecular adducts showed a similar kind of conformational change for the adducts involving halogen bonds in their crystal structures, whereas the conformation was found to be slightly twisted in the rest of the salts to attain the stable forms (Figure 27). Torsion angles of the synthesized molecular salts of 2c4n are listed in the Supporting Information (Table S1).
Scheme 2. Molecular Structures of the Salt Formers/Coformers Reported with 2c4n.
Figure 27.

Conformational plot of the crystal structure of 2c4n derived from the synthesized molecular salts (red: 2c4n.a, blue: 2c4n.b, orange: 2c4n.c, green: 2c4n.d, purple: 2c4n.e, magenta: 2c4n.f, gray: 2c4n.g, and yellow: 2c4n.h).
Attempts have been made to systematically study the presence of halogen bonds in the 24 reported molecular adducts and 8 synthesized salts. Efforts were made to correlate the presence of halogen bonds in these crystal structures with the arrangement of molecules in the crystal lattice. Further, the effect of substituent groups in the counterion molecule was studied. It was observed that the molecular components interacted through a two-point primary supramolecular synthon rather than a single-point supramolecular synthon in the crystal structure (Figure 28) were more likely to exhibit halogen bonds. However, there were exceptions where the molecular salt/cocrystal was found to be stabilized via a C–H···Cl hydrogen bond rather than a halogen bond. It was further understood that stronger intramolecular halogen bonds in 2c4n are more susceptible to form intermolecular halogen bonds (Table 3). Lommerse et al.81 defined the parameter R to bring interacting halogen bond distances into a standardized scale, which is given by
where d is the X–D distance and rx and rd are the standard van der Waals radii of the participating atoms. The parameter R for the molecular adducts that exhibited halogen bonds is listed in Table 4.
Figure 28.

Two-point and single-point heterosynthon representation.
Table 3. Intramolecular Halogen Bond Distances and the Observed Primary Synthon in the 2c4n Salts.
| molecular salts of 2c4n | intramolecular Cl···O halogen bond distance (Å) | presence of halogen bonds | observed primary synthon |
|---|---|---|---|
| 2c4n.a | 3.210 | × | single point |
| 2c4n.b | 3.070 & 3.003 | × | single point |
| 2c4n.c | 3.200 | × | single point |
| 2c4n.d | 2.992 | √ | two point |
| 2c4n.e | 3.038 | √ | two point |
| 2c4n.f | 3.161 | × | two point |
| 2c4n.g | 3.055 | √ | two point |
| 2c4n.h | 3.141 | √ | single point |
| benzotriazole | 2.917 | √ | single point |
| 2-amino-3-bromopyridine | × | √ | two point |
| 2-amino-5-bromopyridine | × | √ | two point |
| 2-amino-5-chloropyridine | × | √ | two point |
| 2-amino-5-nitropyridine | 3.023 | √ | single point |
| 2-bromopyridine | × | √ | single point |
| bipyridine82 | 3.011 | √ | single point |
| 2-chloro-3-hydroxypyridine | × | √ | single point |
| 4-aminopyridine | 2.889 | × | single point |
| lutidine83 | 2.898 | √ | single point |
| morpholine84 | × | × | single point |
| nicotinamide85 | 2.750 | √ | single point |
| quinoline | 3.031 | √ | single point |
| pyrazine | 3.004 | × | single point |
| 2,6-dimethylpyridine | × | × | single point |
| 2-bromo-3-aminopyridine | × | × | single point |
| 3-aminophenol86 | × | × | single point |
| 3-cyanopyridine | 3.063 | × | single point |
| 2-chloro-3-aminopyridine | × | × | single point |
| benzimidazole87 | × | × | single point |
| imidazole | 3.250 | × | single point |
| isoniazid88 | 3.079 | × | single point |
| isopropylideneisonicotinohydrazide | 2.910, 2.892 | √ | single point |
| 4-benzoylpyridine | × | × | single point |
Table 4. Relevant Halogen Bonds and Standardized Parameter R for the Molecular Saltsa.
| molecular adducts of 2c4n | d(Cl···Cl) Å, d(Br···Br)* Å | d(Cl···O) Å, d(Br···O)* Å | d(Cl···S) Å, d(Cl···Br)* Å, d(Cl···N)§ Å | R |
|---|---|---|---|---|
| 2c4n.d | 3.102 | 3.405 | 0.95 & 0.96 | |
| 2c4n.e | 3.205 | 0.98 | ||
| 2c4n.g | 3.481 | 0.99 | ||
| 2c4n.h | 3.305 | 0.94 | ||
| benzotriazole | 3.164 | 0.97 | ||
| 2-amino-3-bromopyridine* | 3.527 | 0.98 | ||
| 2-amino-5-bromopyridine* | 3.145 | 0.93 | ||
| 2-amino-5-chloropyridine | 3.143 | 0.96 | ||
| 2-amino-5-nitropyridine | 3.108 | 0.95 | ||
| 2-bromopyridne* | 3.630 | 0.98 | ||
| bipyridine | 3.117 | 0.95 | ||
| 2-chloro-3-hydroxypyridine | 3.388 | 0.97 | ||
| lutidine | 3.120 | 0.95 | ||
| nicotinamide§ | 3.077 | 0.93 | ||
| quinoline | 3.115 | 0.95 | ||
| isopropylideneisonicotinohydrazide | 3.127, 3.180 | 0.96, 0.97 |
* and § symbols were given to show the exact interaction distances (like Cl···Br and Cl···N).
From Table 4, it is clear that quite strong Cl···O, Br···O, and Cl···N halogen bonds with R values of 0.94, 0.93, and 0.93 were formed in the molecular adducts of 2c4n with 3-aminopyridne, 2-amino-5-bromopyridine, and nicotinamide, respectively. A moderately strong halogen bond was observed in the cases of 2c4n.d, 2c4n.h, 2-amino-5-nitropyridne, bipyridine, lutidine, and quinoline adducts of 2c4n. In the remaining molecular adducts, only weaker halogen bond interactions were observed which is attributed to a higher R value.
Significance of the pKa Value as a Tool to Predict Cocrystals or Salts
The prediction of formation of salts or cocrystals is one of the major challenges before crystal engineering.89 Earlier literature suggested that the proton transfer from the acid to a nitrogen atom of the N-heterocyclic compound occurs based on the difference in the pKa values of acid and the conjugate acid of the N-heterocyclic base. Proton transfer will occur when ΔpKa > 3, whereas cocrystal will form for ΔpKa < 3. There is an ambiguity in the region 0 < ΔpKa < 3, where the probability of formation of salts increases as the ΔpKa increases.90−94 However, in reality, the pKa value of acids and bases is bound to vary based on the chosen solvent and other components associated with the reaction. As a result, it is difficult to predict the nature of the resultant compounds.
The ΔpKa values for various reported salt formers used with 2c4n are shown in Table 5. In general, it is expected that cocrystal formation is predominant when ΔpKa < 0 and salt formation is predominant when ΔpKa is greater than 3. A similar trend is observed in the reported molecular adducts of 2c4n. In most of the cases, salt formation is observed when the ΔpKa lies above 3 units. However, in few cases, cocrystal formation is observed if the ΔpKa value is close to 3 units, as shown in Table 5. Cocrystal formation is found to be predominant when the ΔpKa lies below 0 units. Therefore, the region in between 0 and 3 (ΔpKa) is considered as a salt–cocrystal continuum.90−94
Table 5. Comparison of ΔpKa of Salts/Cocrystals of 2c4n with Different Coformersa.
| active pharmaceutical ingredient/salt former | pKa of the base | ΔpKa | salt/cocrystal | ΔDC–O |
|---|---|---|---|---|
| 2c4n (pKa: 1.96) | ||||
| 2-aminobenzoic acid | 4.95 | 2.99 | salt | 0.006 |
| 3-aminobenzoic acid | 5.10 | 3.14 | salt | 0.034, 0.041 |
| 4-aminobenzoic acid | 4.87 | 2.91 | salt | 0.027 |
| 2-aminothiazole | 5.5 | 3.54 | salt | 0.013 |
| 2-aminobenzimidazole | 7.51 | 5.55 | salt | 0.003 |
| 2-aminopyrimidine | 3.54 | 1.58 | salt | 0.038 |
| 2-aminopyridne | 6.82 | 4.86 | salt | 0.003 |
| 3-aminopyridne | 5.98 | 4.02 | salt | 0.014 |
| benzotriazole | <0 | –1.96 | cocrystal | 0.108 |
| 2-amino-3-bromopyridine | 4.14 | 2.18 | salt | 0.005 |
| 2-amino-5-bromopyridine | 4.65 | 2.69 | salt | 0.017 |
| 2-amino-5-chloropyridine | 4.67 | 2.71 | salt | 0.017 |
| 2-amino-5-nitro pyridine | 2.78 | 0.82 | cocrystal | 0.095 |
| 2-bromopyridine | 0.79 | –1.17 | cocrystal | 0.133 |
| bipyridine | 3.27 | 1.31 | cocrystal | 0.086 |
| 2-chloro-3-hydroxypyridine | 4.32 | 2.36 | cocrystal | 0.101 |
| 4-amino pyridine | 9.11 | 7.15 | salt | 0.019 |
| lutidine | 6.14 | 4.18 | salt | 0.117 |
| morpholine | 8.36 | 6.4 | salt | 0.044 |
| nicotinamide | 3.33 | 1.37 | cocrystal | 0.073 |
| quinoline | 4.85 | 2.89 | cocrystal | 0.101 |
| pyrazine | 0.65 | –1.31 | cocrystal | 0.127 |
| 2,6-dimethylpyridine | 6.60 | 4.64 | salt | 0.036 |
| 2-bromo-3-aminopyridine | 1.72 | –0.24 | cocrystal | 0.103 |
| 3-aminophenol | 9.87 | 7.91 | salt | 0.001 |
| 3-cyanopyridine | 1.36 | –0.6 | cocrystal | 0.102 |
| 2-chloro-3-aminopyridine | 2.42 | 0.46 | cocrystal | 0.115 |
| benzimidazole | 5.4 | 3.44 | salt | 0.013 |
| imidazole | 6.95 | 4.99 | salt | 0.004 |
| isoniazid | 3.35 | 1.39 | cocrystal | 0.105 |
| 4-benzoylpyridine | 4.2 | 2.24 | cocrystal | 0.100 |
ΔpKa = pKa1 (conjugate acid of the base) – pKa2 (acid); ΔDC–O = d(carboxylate group) – d(carboxyl group).
A plot of all cocrystals and salts including some of the compounds reported in the literature is shown in Figure 29. The x-axis corresponds to ΔpKa [pKa (conjugate acid of the base) – pKa (acid)] while the y-axis corresponds to the ΔD (the difference between the lengths of the two C–O bonds in a carboxyl group) bond length. The ΔD value for a cocrystal is found to be high; therefore, the top left corner of the scatter plot corresponds to cocrystal formation and the bottom right corner corresponds to salt formation. A similar kind of scattergram plot is reported in the literature as well.95
Figure 29.
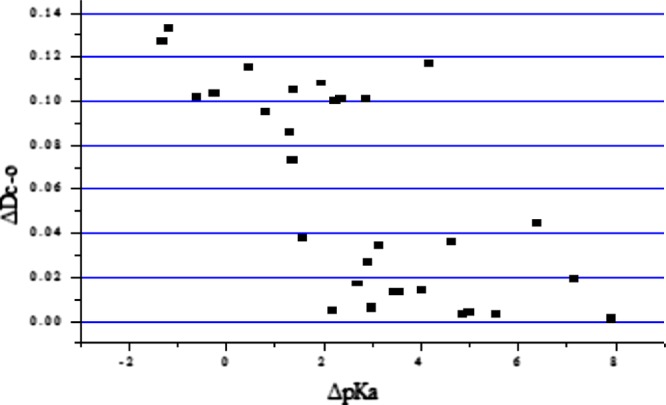
Plot of ΔpKa vs ΔDC–O for 2c4n with different coformers.
Thermal Study
Differential scanning calorimetry (DSC) analysis was performed to study the thermal stability of the synthesized molecular salts. Salts with benzoic acid derivatives (2c4n.a, 2c4n.b, and 2c4n.c) melt in between/nearly equal to the melting temperatures of 2c4n and salt formers. No correlation of the melting point of these salts with the crystal density is observed. In 2c4n.b and 2c4n.c, phase transformation was observed which is possibly due to the existence of polymorphism in these salts. A higher melting temperature was observed for the pyridine derivative salt of 2c4n when compared to its respective starting materials. The melting points of 2c4n and the salts are shown in Table 6. Because of the isostructurality of 2c4n.g and 2c4n.h, their densities and melting temperatures were found to be approximately similar. It is surprising that the melting temperature of 2c4n.e was found to be higher when compared to those of all other synthesized salts even though the crystal density was low, which indicates the high thermal stability of salt 2c4n.e. It is further observed that a higher melting temperature was observed for those salts that exhibited halogen bonds in their crystal structures. DSC thermogram is displayed in the Supporting Information Figure S1.
Table 6. Melting Points of 2c4n and the Molecular Salts.
| sl. no. | active pharmaceutical ingredient/salt | melting point (°C) | melting point of the salt (°C) | molecular adduct |
|---|---|---|---|---|
| 1 | 2c4n | 145–148 | ||
| 2 | 2-aminobenzoic acid (a) | 146–148 | 150.1 | salt |
| 3 | 3-aminobenzoic acid (b) | 176–180 | 155.93, 174.18 | salt |
| 4 | 4-aminobenzoic acid (c) | 187–189 | 160.1, 165.9 | salt |
| 5 | 2-aminothiazole (d) | 86–89 | 167.23 | salt |
| 6 | 2-aminobenzimidazole (e) | 226–230 | 248.3 | salt |
| 7 | 2-aminopyrimidine (f) | 59–60 | 161.4 | salt |
| 8 | 2-aminopyridine (g) | 54–58 | 165.21 | salt |
| 9 | 3-aminopyridine (h) | 60–63 | 162.91 | salt |
Fourier Transform Infrared Spectroscopy
Generally, IR spectroscopy is used to identify the functional group present in the molecule. It also gives information about the hydrogen bonds present in the molecule. Salt formation can be confirmed by observing changes in the carbonyl stretching frequency as the ionization causes a reduction in the carbonyl stretching frequency. On the basis of the changes in these stretching frequencies, we ascertained the formation of new solid forms. −C=O stretching frequency for 2c4n was observed at 1704 cm–1. In all molecular salts, ionization caused a large shift in the carbonyl stretching frequency for pyridyl derivative salts; however, in the case of benzoic acid derivative salts, a considerable shift in the stretching frequency was observed. The Fourier transform infrared (FT-IR) spectra and the stretching values are shown in the Supporting Information (Figures S10–S17 and Table S2).
Powder X-ray Diffraction Analysis
Powder X-ray diffraction (PXRD) analysis is the prerequisite for the confirmation of formation of new molecular components. In the present study, PXRD analysis is carried out to confirm the bulk purity of the synthesized molecular salts. All synthesized molecular salts were found to be pure in the bulk. The PXRD pattern is shown in the Supporting Information (Figures S2–S9).
1H NMR Spectroscopy
All synthesized molecular salts exhibited a 1:1 molecular stoichiometry in their asymmetric unit. The 1H NMR spectra and the spectral details are given in the Supporting Information (Figures S18–S25).
Conclusions
In summary, a series of eight molecular salts of 2c4n have been synthesized in view of studying the presence of halogen bonds in the molecular salts/cocrystals of 2c4n. Four synthesized molecular salts and 12 out of 24 reported molecular adducts of 2c4n were found to exhibit halogen bonds in their crystal structures. The synthesized molecular salts were characterized by various spectroscopic, thermal, and X-ray diffraction techniques. In all synthesized salts, proton transfer from the carboxylic acid group to the pyridyl nitrogen/amino group was observed. All synthesized salts were found to be anhydrous except for 2c4n.b, which is monohydrate. Conformational analysis of the synthesized and the reported molecular salts revealed that 2c4n exhibited two different conformations in the molecular adducts; a similar kind of conformation was observed for the salts exhibiting halogen bonds in their crystal structures. Two of the synthesized molecular salts were found to be isostructural as well (2c4n.g and 2c4n.h). In the synthesized and the reported molecular adducts of 2c4n, the acid···pyridine heterosynthon was found to be the robust. Detailed inspection of the reported and the synthesized molecular adducts of 2c4n revealed that two-point primary supramolecular synthon and stronger intramolecular Cl···O halogen bonds in the crystal structures are more prone to exhibit halogen bonds in their crystal structures. From the single-crystal X-ray diffraction (SC-XRD) study, it is strongly suggested that two-point heterosynthon is more likely to exhibit halogen bonds though the conformational change of the carboxylate group of 2c4n occurs. Halogen bonds played a major role in the crystal packing of these molecular adducts, based on the SC-XRD study.
Experimental Section
Materials and Methods
All chemicals were purchased from Sigma-Aldrich and used as such without any further purification. All solvents used were of analytical or chromatographic grade.
Synthesis of Molecular Salts
2c4n.a
A mixture of 2c4n (200 mg, 0.99 mmol) and 2-aminobenzoic acid (136 mg, 0.99 mmol) was taken in 5 mL of methanol, sonicated for complete dissolution, and then kept for crystallization at ambient temperature. Cream-colored columnar crystals were obtained in 2 days.
2c4n.b
A mixture of 2c4n (200 mg, 0.99 mmol) and 3-aminobenzoic acid (136 mg, 0.99 mmol) was taken in a 1:1 mixture of acetonitrile and methanol (4 mL) and sonicated for complete dissolution. It was then allowed for crystallization at room temperature. Block-shaped brown-colored crystals were obtained in 2 days.
2c4n.c
A mixture of 2c4n (200 mg, 0.99 mmol) and 4-aminobenzoic acid (136 mg, 0.99 mmol) was dissolved in a 5:2 mixture of acetonitrile and methanol (7 mL) by sonication. It was then allowed for slow evaporation at room temperature. Plate-shaped pink-colored crystals were obtained in 2 days.
2c4n.d
A mixture of 2c4n (200 mg, 0.99 mmol) and 2-aminothiazole (99.1 mg, 0.99 mmol) was dissolved in a 5:1 mixture of acetonitrile and dimethyl sulfoxide (DMSO) (6 mL) by sonication and then allowed for slow evaporation. Block-shaped brown-colored crystals were obtained in 4 days.
2c4n.e
A mixture of 2c4n (200 mg, 0.99 mmol) and 2-aminobenzimidazole (132.11 mg, 0.99 mmol) was dissolved in a 5:2 mixture of methanol and dimethylformamide (7 mL) by sonication and then allowed for slow evaporation. Transparent plate-shaped crystals were obtained in 3 days.
2c4n.f
A mixture of 2c4n (200 mg, 0.99 mmol) and 2-aminopyrimidine (94.37 mg, 0.99 mmol) was dissolved in a 7:1 mixture of methanol and 1,4-dioxane (8 mL) by sonication and then allowed for slow evaporation. Plate-shaped cream-colored crystals were obtained in 5 days.
2c4n.g
A 1:1 stoichiometric amount of 2c4n (200 mg, 0.99 mmol) and 2-aminopyridine (93.38 mg, 0.99 mmol) was mixed together, dissolved in 5 mL of methanol solvent by sonication, and then allowed for slow evaporation at room temperature. Plate-shaped pale yellow-colored crystals were obtained after 4 days.
2c4n.h
A 1:1 stoichiometric amount of 2c4n (200 mg, 0.99 mmol) and 3-aminopyridine (93.38 mg, 0.99 mmol) was mixed together, then dissolved in 6 mL of methanol solvent by sonication, and allowed for slow evaporation at room temperature. Block-shaped pale yellow-colored crystals were obtained after 4 days.
X-ray Crystallography
X-ray crystal data were collected for the synthesized molecular salts at room temperature using a Bruker Smart Apex Duo single-crystal X-ray diffractometer with a dual system charge-coupled device detector. Monochromatic Mo Kα radiation (λ = 0.7103 Å) was used for the measurements. The data integration and reduction were carried out using SAINT-Plus software. An empirical absorption correction was applied to the collected reflections with SADABS. All structures were solved by direct methods using SHELXS97 and SHELXL2007/2014, and refinement was carried out by the full-matrix least-squares technique. Anisotropic displacement parameters were calculated for all nonhydrogen atoms. H atoms attached to the N atoms were located in a difference Fourier density map and refined isotropically. The program PLATON96 was used to generate the hydrogen bond table, whereas MERCURY was used for all graphical representations of the results.
1H NMR Spectroscopy
1H NMR spectrum is recorded on a Bruker BioSpin 500 MHz/400 MHz spectrometer (Bruker, Germany). 1H NMR provides information related to the molecular structure and the stoichiometry in the crystal lattice. 1H NMR was recorded in a DMSO-d6 solvent with tetramethylsilane as the internal reference standard (δ = 0). For the analysis, approximately 5–10 mg of the samples was dissolved in a DMSO-d6 solvent and the spectra were recorded with 16 numbers of scans. MestReNova 7.0.0.8331 software was used to process the raw NMR data.
Powder X-ray Diffraction
PXRD was recorded on a JEOL JDX-8 PXRD instrument in the scan range of 2θ = 5°–50°. For a typical experiment, samples were placed on the standard sample holder and then scanned continuously at a scan rate of 2° min–1.
Infrared Spectroscopy
FT-IR spectra were recorded on a Nicolet Avatar 330 instrument in the scan range of 400–4000 cm–1. In a typical experiment, approximately 10–15 mg of the samples was mixed with KBr, ground well using a mortar, and pressed with a steel die into a pellet. The FT-IR spectra were collected for 16 scans at a resolution of 4 cm–1.
Differential Scanning Calorimetry
DSC analysis was performed on a DSC 60 differential scanning calorimeter (Shimadzu, Japan) which was calibrated for temperature and enthalpy using a Tin standard material. For the analysis, 2–5 mg of the samples was placed into an aluminum pan, and it was covered with a crimped lid. This weighed, crimped aluminum pan was kept in the sample reference cell and scanned from 30 to 350 °C at a heating rate of 10 °C/min under a continuously purged dry nitrogen atmosphere.
Acknowledgments
D.R.T., S.K.N., M.O., and P.A.K. acknowledge the Director and the HOD (Department of Chemistry), NITK, Surathkal, for providing research infrastructure and facilities. The authors are also thankful to the MIT Manipal and the College of Pharmaceutical Sciences, Manipal, for providing analytical support. They thank the DST (Department of Science and Technology, Government of India, New Delhi, India) for the SC-XRD facility (procured under the FIST programme). D.R.T., S.K.N., and M.O. are indebted to the CSMCRI for extending the NMR facility. S.K.N. and M.O. are grateful to NITK, Surathkal, for research fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00878.
DSC plot and PXRD comparison plots; comparison of FT-IR spectra of the molecular salts with those of their starting materials, 1H NMR spectra of the molecular salts; isostructurality comparison; torsion angles; list of FT-IR stretching frequencies; and 1H NMR chemical shifts (PDF)
Crystallographic data of the molecular salts of 2c4n (CIF)
Author Contributions
M.O. and S.K.N. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Vishweshwar P.; McMahon J. A.; Bis J. A.; Zaworotko M. J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. 10.1002/jps.20578. [DOI] [PubMed] [Google Scholar]
- Lemmerer A.; Esterhuysen C.; Bernstein J. Synthesis characterization and molecular modeling of a pharmaceutical co-crystal: (2-chloro-4-nitrobenzoic acid):(nicotinamide). J. Pharm. Sci. 2010, 99, 4054–4071. 10.1002/jps.22211. [DOI] [PubMed] [Google Scholar]
- Santra R.; Ghosh N.; Biradha K. Crystal engineering with acid and pyridine heteromeric synthon: neutral and ionic co-crystals. New J. Chem. 2008, 32, 1673–1676. 10.1039/b803280g. [DOI] [Google Scholar]
- Aakeröy C. B.; Salmon D. J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. 10.1039/b505883j. [DOI] [Google Scholar]
- MacGillivray L. On substituents, steering, and stacking to control properties of the organic solid state. CrystEngComm 2004, 6, 77–78. 10.1039/b403015j. [DOI] [Google Scholar]
- Lehn J.-M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. 10.1126/science.1071063. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R. Hydrogen bridges in crystal engineering: interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. 10.1021/ar010054t. [DOI] [PubMed] [Google Scholar]
- Moulton B.; Zaworotko M. J. From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. 10.1021/cr9900432. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R. Supramolecular synthons in crystal engineering—a new organic synthesis. Angew. Chem., Int. Ed. Engl. 1995, 34, 2311–2327. 10.1002/anie.199523111. [DOI] [Google Scholar]
- Wenger M.; Bernstein J. Designing a Cocrystal of γ-Amino Butyric Acid. Angew. Chem., Int. Ed. 2006, 45, 7966–7969. 10.1002/anie.200603241. [DOI] [PubMed] [Google Scholar]
- Childs S. L.; Hardcastle K. I. Cocrystals of piroxicam with carboxylic acids. Cryst. Growth Des. 2007, 7, 1291–1304. 10.1021/cg060742p. [DOI] [Google Scholar]
- Bosch E. Chain-link hydrogen-bonded capsules. CrystEngComm 2007, 9, 191–198. 10.1039/b615899d. [DOI] [Google Scholar]
- Pedireddi V. R.; Chatterjee S.; Ranganathan A.; Rao C. N. R. Noncovalent synthesis of layered and channel structures involving sulfur-mediated hydrogen bonds. J. Am. Chem. Soc. 1997, 119, 10867–10868. 10.1021/ja972289z. [DOI] [Google Scholar]
- Metrangolo P.; Neukirch H.; Pilati T.; Resnati G. Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc. Chem. Res. 2005, 38, 386–395. 10.1021/ar0400995. [DOI] [PubMed] [Google Scholar]
- Metrangolo P.; Meyer F.; Pilati T.; Proserpio D. M.; Resnati G. Highly Interpenetrated Supramolecular Networks Supported by N···I Halogen Bonding. Chem.—Eur. J. 2007, 13, 5765–5772. 10.1002/chem.200601653. [DOI] [PubMed] [Google Scholar]
- De Santis A.; Forni A.; Liantonio R.; Metrangolo P.; Pilati T.; Resnati G. N···Br Halogen Bonding: One-Dimensional Infinite Chains through the Self-Assembly of Dibromotetrafluorobenzenes with Dipyridyl Derivatives. Chem.—Eur. J. 2003, 9, 3974–3983. 10.1002/chem.200204655. [DOI] [PubMed] [Google Scholar]
- Walsh R. B.; Padgett C. W.; Metrangolo P.; Resnati G.; Hanks T. W.; Pennington W. T. Crystal engineering through halogen bonding: complexes of nitrogen heterocycles with organic iodides. Cryst. Growth Des. 2001, 1, 165–175. 10.1021/cg005540m. [DOI] [Google Scholar]
- Cinčić D.; Friščić T.; Jones W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. 10.1021/ja801164v. [DOI] [PubMed] [Google Scholar]
- Cinčić D.; Friščić T.; Jones W. Isostructural Materials Achieved by Using Structurally Equivalent Donors and Acceptors in Halogen-Bonded Cocrystals. Chem.—Eur. J. 2008, 14, 747–753. 10.1002/chem.200701184. [DOI] [PubMed] [Google Scholar]
- Shirman T.; Freeman D.; Posner Y. D.; Feldman I.; Facchetti A.; van der Boom M. E. Assembly of crystalline halogen-bonded materials by physical vapor deposition. J. Am. Chem. Soc. 2008, 130, 8162–8163. 10.1021/ja8029784. [DOI] [PubMed] [Google Scholar]
- Nguyen H. L.; Horton P. N.; Hursthouse M. B.; Legon A. C.; Bruce D. W. Halogen bonding: a new interaction for liquid crystal formation. J. Am. Chem. Soc. 2004, 126, 16–17. 10.1021/ja036994l. [DOI] [PubMed] [Google Scholar]
- Triguero S.; Llusar R.; Polo V.; Fourmigué M. Halogen bonding interactions of sym-triiodotrifluorobenzene with halide anions: a combined structural and theoretical study. Cryst. Growth Des. 2008, 8, 2241–2247. 10.1021/cg7008489. [DOI] [Google Scholar]
- Meyer E. A.; Castellano R. K.; Diederich F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem., Int. Ed. 2003, 42, 1210–1250. 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]
- Espallargas G. M.; Brammer L.; Sherwood P. Designing Intermolecular Interactions between Halogenated Peripheries of Inorganic and Organic Molecules: Electrostatically Directed M-X···X′-C Halogen Bonds. Angew. Chem., Int. Ed. 2006, 45, 435–440. 10.1002/anie.200502586. [DOI] [PubMed] [Google Scholar]
- Barooah N.; Sarma R. J.; Baruah J. B. Solid-state hydrogen bonded assembly of N,N′-bis(glycinyl)-pyromellitic diimide with aromatic guests. CrystEngComm 2006, 8, 608–615. 10.1039/b607323a. [DOI] [Google Scholar]
- Nishio M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. 10.1039/b313104a. [DOI] [Google Scholar]
- Aakeröy C. B.; Beatty A. M. Crystal engineering of hydrogen-bonded assemblies-a progress report. Aust. J. Chem. 2001, 54, 409–421. 10.1071/ch01133. [DOI] [Google Scholar]
- Steiner T. The hydrogen bond in the solid state. Angew. Chem., Int. Ed. 2002, 41, 48–76. . [DOI] [PubMed] [Google Scholar]
- Bernstein J.; Davis R. E.; Shimoni L.; Chang N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem., Int. Ed. Engl. 1995, 34, 1555–1573. 10.1002/anie.199515551. [DOI] [Google Scholar]
- Kollman P. A. Theory of hydrogen bond directionality. J. Am. Chem. Soc. 1972, 94, 1837–1842. 10.1021/ja00761a008. [DOI] [Google Scholar]
- Aakeröy C. B.; Seddon K. R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. 10.1039/cs9932200397. [DOI] [Google Scholar]
- Cavallo G.; Metrangolo P.; Milani R.; Pilati T.; Priimagi A.; Resnati G.; Terraneo G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. 10.1021/acs.chemrev.5b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aakeröy C. B.; Chopade P. D.; Desper J. Establishing a Hierarchy of Halogen Bonding by Engineering Crystals without Disorder. Cryst. Growth Des. 2013, 13, 4145–4150. 10.1021/cg400988m. [DOI] [Google Scholar]
- Karanam M.; Choudhury A. R. Study of halogen-mediated weak interactions in a series of halogen-substituted azobenzenes. Cryst. Growth Des. 2013, 13, 4803–4814. 10.1021/cg400967k. [DOI] [Google Scholar]
- Metrangolo P.; Resnati G. Halogen versus hydrogen. Science 2008, 321, 918–919. 10.1126/science.1162215. [DOI] [PubMed] [Google Scholar]
- Amico V.; Meille S. V.; Corradi E.; Messina M. T.; Resnati G. Perfluorocarbon–Hydrocarbon Self-Assembling. 1D Infinite Chain Formation Driven by Nitrogen···Iodine Interactions. J. Am. Chem. Soc. 1998, 120, 8261–8262. 10.1021/ja9810686. [DOI] [Google Scholar]
- Cardillo P.; Corradi E.; Lunghi A.; Meille S. V.; Messina M. T.; Metrangolo P.; Resnati G. The N···I intermolecular interaction as a general protocol for the formation of perfluorocarbon–hydrocarbon supramolecular architectures. Tetrahedron 2000, 56, 5535–5550. 10.1016/s0040-4020(00)00476-2. [DOI] [Google Scholar]
- Corradi E.; Meille S. V.; Messina M. T.; Metrangolo P.; Resnati G. Halogen bonding versus hydrogen bonding in driving self-assembly processes. Angew. Chem. 2000, 112, 1852–1856. . [DOI] [PubMed] [Google Scholar]
- Corradi E.; Meille S. V.; Messina M. T.; Metrangolo P.; Resnati G. Perfluorocarbon-hydrocarbon self-assembly. Part 6: 1 α,ω-Diiodoperfluoroalkanes as pseudohalogens in supramolecular synthesis. Tetrahedron Lett. 1999, 40, 7519–7523. 10.1016/s0040-4039(99)01479-3. [DOI] [Google Scholar]
- Jennifer S.; Muthiah P. Design of co-crystals/salts of some Nitrogenous bases and some derivatives of thiophene carboxylic acids through a combination of hydrogen and halogen bonds. Chem. Cent. J. 2014, 8, 20. 10.1186/1752-153x-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B. K.; Nangia A.; Jaskólski M. Crystal engineering with hydrogen bonds and halogen bonds. CrystEngComm 2005, 7, 355–358. 10.1039/b501693b. [DOI] [Google Scholar]
- Priimagi A.; Cavallo G.; Metrangolo P.; Resnati G. The halogen bond in the design of functional supramolecular materials: recent advances. Acc. Chem. Res. 2013, 46, 2686–2695. 10.1021/ar400103r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudzinski M. G.; McClary C. A.; Taylor M. S. Anion receptors composed of hydrogen- and halogen-bond donor groups: modulating selectivity with combinations of distinct noncovalent interactions. J. Am. Chem. Soc. 2011, 133, 10559–10567. 10.1021/ja202096f. [DOI] [PubMed] [Google Scholar]
- Legon A. C. The halogen bond: an interim perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. 10.1039/c002129f. [DOI] [PubMed] [Google Scholar]
- Metrangolo P.; Neukirch H.; Pilati T.; Resnati G. Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc. Chem. Res. 2005, 38, 386–395. 10.1021/ar0400995. [DOI] [PubMed] [Google Scholar]
- Rissanen K. Halogen bonded supramolecular complexes and networks. CrystEngComm 2008, 10, 1107–1113. 10.1039/b803329n. [DOI] [Google Scholar]
- Politzer P.; Murray J. S.; Clark T. Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. 10.1039/c004189k. [DOI] [PubMed] [Google Scholar]
- Lommerse J. P. M.; Stone A. J.; Taylor R.; Allen F. H. The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J. Am. Chem. Soc. 1996, 118, 3108–3116. 10.1021/ja953281x. [DOI] [Google Scholar]
- Bilewicz E.; Rybarczyk-Pirek A. J.; Dubis A. T.; Grabowski S. J. Halogen bonding in crystal structure of 1-methylpyrrol-2-yl trichloromethyl ketone. J. Mol. Struct. 2007, 829, 208–211. 10.1016/j.molstruc.2006.06.032. [DOI] [Google Scholar]
- Nyburg S. C.; Wong-Ng W. Anisotropic atom-atom forces and the space group of solid chlorine. Proc. R. Soc. London, Ser. A 1979, 367, 29–45. 10.1098/rspa.1979.0074. [DOI] [Google Scholar]
- Clark T.; Hennemann M.; Murray J. S.; Politzer P. Halogen bonding: the σ-hole. J. Mol. Model. 2007, 13, 291–296. 10.1007/s00894-006-0130-2. [DOI] [PubMed] [Google Scholar]
- Politzer P.; Murray J. S.; Clark T.. σ-Hole bonding: a physical interpretation. Halogen Bonding I; Topics in Current Chemistry; Springer, 2014; pp 19–42. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R.; Parthasarathy R. The nature of halogen. cntdot.. cntdot.. cntdot. halogen interactions: are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms?. J. Am. Chem. Soc. 1989, 111, 8725–8726. 10.1021/ja00205a027. [DOI] [Google Scholar]
- Price S. L.; Stone A. J.; Lucas J.; Rowland R. S.; Thornley A. E. The Nature of-Cl. cntdot.. cntdot.. cntdot. Cl-Intermolecular Interactions. J. Am. Chem. Soc. 1994, 116, 4910–4918. 10.1021/ja00090a041. [DOI] [Google Scholar]
- Auffinger P.; Hays F. A.; Westhof E.; Ho P. S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 16789–16794. 10.1073/pnas.0407607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes M.; Cavalcanti S. M.; Moreira D. R.; de Azevedo W.; Leite A. C. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets 2010, 11, 303–314. 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]
- Geronikaki A. A.; Lagunin A. A.; Hadjipavlou-Litina D. I.; Eleftheriou P. T.; Filimonov D. A.; Poroikov V. V.; Alam I.; Saxena A. K. Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J. Med. Chem. 2008, 51, 1601–1609. 10.1021/jm701496h. [DOI] [PubMed] [Google Scholar]
- Greenbaum D. C.; Mackey Z.; Hansell E.; Doyle P.; Gut J.; Caffrey C. R.; Lehrman J.; Rosenthal P. J.; McKerrow J. H.; Chibale K. Synthesis and structure–activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004, 47, 3212–3219. 10.1021/jm030549j. [DOI] [PubMed] [Google Scholar]
- Motta C. L.; Sartini S.; Mugnaini L.; Salerno S.; Simorini F.; Taliani S.; Antonioli L. Exploiting the pyrazolo[3,4-d] pyrimidin-4-one ring system as a useful template to obtain potent adenosine deaminase inhibitors. J. Med. Chem. 2009, 52, 1681–1692. 10.1021/jm801427r. [DOI] [PubMed] [Google Scholar]
- Cinčić D.; Friščić T.; Jones W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. 10.1021/ja801164v. [DOI] [PubMed] [Google Scholar]
- Präsang C.; Whitwood A. C.; Bruce D. W. Halogen-bonded cocrystals of 4-(N,N-dimethylamino)pyridine with fluorinated iodobenzenes. Cryst. Growth Des. 2009, 9, 5319–5326. 10.1021/cg900823d. [DOI] [Google Scholar]
- Cinčić D.; Friščić T.; Jones W. Isostructural Materials Achieved by Using Structurally Equivalent Donors and Acceptors in Halogen-Bonded Cocrystals. Chem.—Eur. J. 2008, 14, 747–753. 10.1002/chem.200701184. [DOI] [PubMed] [Google Scholar]
- Baldrighi M.; Cavallo G.; Chierotti M. R.; Gobetto R.; Metrangolo P.; Pilati T.; Resnati G.; Terraneo G. Halogen bonding and pharmaceutical cocrystals: the case of a widely used preservative. Mol. Pharmaceutics 2013, 10, 1760–1772. 10.1021/mp300574j. [DOI] [PubMed] [Google Scholar]
- Lemmerer A.; Esterhuysen C.; Bernstein J. Synthesis characterization molecular modeling of a pharmaceutical co-crystal: (2-chloro-4-nitrobenzoic acid):(nicotinamide). J. Pharm. Sci. 2010, 99, 4054–4071. 10.1002/jps.22211. [DOI] [PubMed] [Google Scholar]
- Kinchington D.; Ng T.; Mathews N.; Tisdale M.; Devine D.; Ayuko W. O. T cell costimulation by derivatives of benzoic acid. Antiviral Chem. Chemother. 1997, 8, 121–130. 10.1177/095632029700800206. [DOI] [Google Scholar]
- Ishida H.; Rahman B.; Kashino S. 2:1 complexes of 2-chloro-4-nitrobenzoic acid and 2-chloro-5-nitrobenzoic acid with pyrazine. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2001, 57, 876–879. 10.1107/s0108270101007016. [DOI] [PubMed] [Google Scholar]
- Tolstoy P. M.; Smirnov S. N.; Shenderovich I. G.; Golubev N. S.; Denisov G. S.; Limbach H.-H. NMR studies of solid state—solvent and H/D isotope effects on hydrogen bond geometries of 1:1 complexes of collidine with carboxylic acids. J. Mol. Struct. 2004, 700, 19–27. 10.1016/j.molstruc.2004.02.023. [DOI] [Google Scholar]
- Ishida H.; Fukunaga T. 3-Cyanopyridine–2-chloro-4-nitrobenzoic acid (1/1). Acta Crystallogr., Sect. E: Struct. Rep. Online 2004, 60, o1664–o1665. 10.1107/s1600536804021087. [DOI] [Google Scholar]
- Ishida H.; Fukunaga T.; Kashino S. The 1:1 complex of 2-chloro-4-nitrobenzoic acid and 1, 2, 3-benzotriazole. Acta Crystallogr., Sect. E: Struct. Rep. Online 2002, 58, o1083–o1084. 10.1107/s1600536802015969. [DOI] [Google Scholar]
- Sugiyama T.; Meng J.; Matsuura T. Intermolecular interactions in the formation of two-component molecular crystals composed of chloronitrobenzoic acids and 4-benzoylpyridine. J. Mol. Struct. 2002, 611, 53–64. 10.1016/s0022-2860(02)00037-6. [DOI] [Google Scholar]
- Gotoh K.; Ishida H. 2-Chloro-4-nitrobenzoic acid–quinoline (1/1). Acta Crystallogr., Sect. E: Struct. Rep. Online 2011, 67, o2883. 10.1107/s160053681104075x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T.; Meng J.; Matsuura T. Two-component molecular crystals composed of chloronitrobenzoic acids and 4-aminopyridine. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2002, 58, o242–o246. 10.1107/s0108270102003657. [DOI] [PubMed] [Google Scholar]
- Ishida H.; Rahman B.; Kashino S. Imidazolium 2-chloro-4-nitrobenzoate. Acta Crystallogr., Sect. E: Struct. Rep. Online 2001, 57, o744–o745. 10.1107/s1600536801011977. [DOI] [PubMed] [Google Scholar]
- Lemmerer A. Covalent assistance to supramolecular synthesis: modifying the drug functionality of the antituberculosis API isoniazid in situ during co-crystallization with GRAS and API compounds. CrystEngComm 2012, 14, 2465–2478. 10.1039/c1ce06310c. [DOI] [Google Scholar]
- Ishida H.; Rahman B.; Kashino S. 3,5-Lutidinium–2-chloro-4-nitrobenzoate–2-chloro-4-nitrobenzoic acid (1/1/1). Acta Crystallogr., Sect. E: Struct. Rep. Online 2004, 60, o1661–o1663. 10.1107/s1600536804021075. [DOI] [Google Scholar]
- Lemmerer A.; Govindraju S.; Johnston M.; Motloung X.; Savig K. L. Co-crystals and molecular salts of carboxylic acid/pyridine complexes: can calculated pKa’s predict proton transfer? A case study of nine complexes. CrystEngComm 2015, 17, 3591–3595. 10.1039/c5ce00102a. [DOI] [Google Scholar]
- Aitipamula S.; Chow P. S.; Tan R. B. Solvates and polymorphic phase transformations of 2-chloro-4-nitrobenzoic acid. CrystEngComm 2011, 13, 1037–1045. 10.1039/c0ce00361a. [DOI] [Google Scholar]
- Vishweshwar P.; Nangia A.; Lynch V. M. Recurrence of Carboxylic Acid–Pyridine Supramolecular Synthon in the Crystal Structures of Some Pyrazinecarboxylic Acids. J. Org. Chem. 2002, 67, 556–565. 10.1021/jo0162484. [DOI] [PubMed] [Google Scholar]
- Aitipamula S.; Thallapally P. K.; Thaimattam R.; Jaskólski M.; Desiraju G. R. Topological equivalences between organic and coordination polymer crystal structures: An organic ladder formed with three-connected molecular and supramolecular synthons. Org. Lett. 2002, 4, 921–924. 10.1021/ol017284s. [DOI] [PubMed] [Google Scholar]
- Desiraju G. R.; Steiner T.. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, 1999. [Google Scholar]
- Lommerse J. P. M.; Stone A. J.; Taylor R.; Allen F. H. The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J. Am. Chem. Soc. 1996, 118, 3108–3116. 10.1021/ja953281x. [DOI] [Google Scholar]
- Gotoh K.; Ishida H. 4,4′-Bipyridyl–2-chloro-4-nitrobenzoic acid (1/2). Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, o4500. 10.1107/s1600536807052865. [DOI] [Google Scholar]
- Ishida H.; Rahman B.; Kashino S. 3,5-Lutidinium–2-chloro-4-nitrobenzoate–2-chloro-4-nitrobenzoic acid (1/1/1). Acta Crystallogr., Sect. E: Struct. Rep. Online 2004, 60, o1661–o1663. 10.1107/s1600536804021075. [DOI] [Google Scholar]
- Ishida H.; Rahman B.; Kashino S. Morpholinium 2-chloro-4-nitrobenzoate, 2-chloro-5-nitrobenzoate and 4-chloro-3-nitrobenzoate. Acta Crystallogr., Sect. E: Struct. Rep. Online 2001, 57, 1450–1453. 10.1107/s0108270101016389. [DOI] [PubMed] [Google Scholar]
- Lemmerer A.; Esterhuysen C.; Bernstein J. Synthesis characterization molecular modeling of a pharmaceutical co-crystal: (2-chloro-4-nitrobenzoic acid):(nicotinamide). J. Pharm. Sci. 2010, 99, 4054–4071. 10.1002/jps.22211. [DOI] [PubMed] [Google Scholar]
- Mani R.; Rietveld I. B.; Nicolaï B.; Varadharajan K.; Louhi-Kultanen M.; Narasimhan S. Fluorescence and physical properties of the organic salt 2-chloro-4-nitrobenzoate–3-ammonium-phenol. Chem. Phys. 2015, 458, 52–61. 10.1016/j.chemphys.2015.07.004. [DOI] [Google Scholar]
- Ishida H.; Fukunaga T.; Kashino S. Benzimidazolium 2-chloro-4-nitrobenzoate. Acta Crystallogr., Sect. E: Struct. Rep. Online 2002, 58, o1085–o1087. 10.1107/s1600536802015970. [DOI] [Google Scholar]
- Lemmerer A. Covalent assistance to supramolecular synthesis: modifying the drug functionality of the antituberculosis API isoniazid in situ during co-crystallization with GRAS and API compounds. CrystEngComm 2012, 14, 2465–2478. 10.1039/c1ce06310c. [DOI] [Google Scholar]
- Sarma B.; Nath N. K.; Bhogala B. R.; Nangia A. Synthon competition and cooperation in molecular salts of hydroxybenzoic acids and aminopyridines. Cryst. Growth Des. 2009, 9, 1546–1557. 10.1021/cg801145c. [DOI] [Google Scholar]
- Bhogala B. R.; Basavoju S.; Nangia A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. 10.1039/b509162d. [DOI] [Google Scholar]
- Stahl P. H.; Wermuth C. G.. Handbook of Pharmaceutical Salts: Properties, Selection, and Use; Wiley-VCH, 2002. [Google Scholar]
- Cruz-Cabeza A. J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. 10.1039/c2ce26055g. [DOI] [Google Scholar]
- Childs S. L.; Stahly G. P.; Park A. The salt–cocrystal continuum: the influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. 10.1021/mp0601345. [DOI] [PubMed] [Google Scholar]
- Stilinović V.; Kaitner B. Salts and co-crystals of gentisic acid with pyridine derivatives: the effect of proton transfer on the crystal packing (and vice versa). Cryst. Growth Des. 2012, 12, 5763–5772. 10.1021/cg301267h. [DOI] [Google Scholar]
- Aakeröy C. B.; Desper J.; Urbina J. F. Supramolecular reagents: versatile tools for non-covalent synthesis. Chem. Commun. 2005, 2820–2822. 10.1039/b503718b. [DOI] [PubMed] [Google Scholar]
- Spek A. L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr., Sect. A: Found. Crystallogr. 1990, 46, c34. 10.1107/S0108767390099780. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.