Abstract
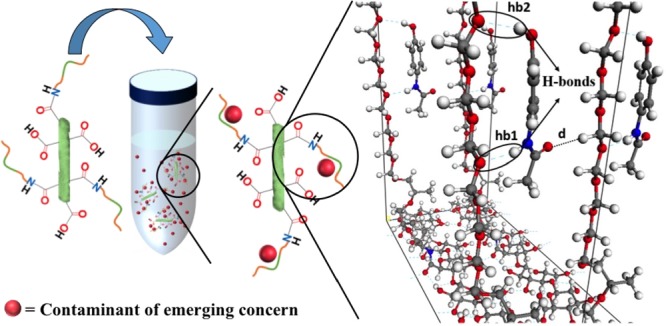
The occurrence of contaminants of emerging concern (CECs) in water is an environmental issue that must be addressed to avoid damage to ecosystems and human health. Inspired by this current issue, in this work, we fabricated nanocellulose (NC) particles grafted with the block copolymer Jeffamine ED 600 (NC–Jeffamine) capable of adsorbing acetaminophen, sulfamethoxazole, and N,N-diethyl-meta-toluamide (DEET) from aqueous solution by electrostatic interactions. NC–Jeffamine composites were prepared by carboxylation of the NC surface via 2,2,6,6-tetramethyl-1-piperidinyloxy oxidation followed by the covalent attachment of Jeffamine using the N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide/N-hydroxysulfosuccinimide sodium salt reaction. The reaction was followed and confirmed by Fourier transform infrared and conductometric titration. The physical characterization was performed by thermogravimetric analysis, Brunauer–Emmett–Teller analysis, scanning electron microscopy, dynamic light scattering, and Z-potential analysis. This material was used to study the adsorption profile of three CECs in deionized water, namely, acetaminophen, sulfamethoxazole, and DEET. The adsorption isotherms were obtained at pH 3, 7, and 9, where the best adsorption results corresponded to pH 9 because of the uniform dispersion of the adsorbate in solution. A computational study based on the density functional theory determined that the possible interactions of the CECs with the adsorbent material were related to hydrogen bonds and/or van der Waals forces. The calculated binding energies were used as a descriptor to characterize the optimum adsorption site of CECs onto NC–Jeffamine.
1. Introduction
Recent investigations have focused on the study and removal of contaminants of emerging concern (CECs) to meet the current quality criteria of potable water.1 Pharmaceutical compounds and personal care products represent the majority of these contaminants, which once entered in water streams are difficult to remove because of their low concentration (ng−μg/L).2 Moreover, water treatment plants lack cost-effective strategies to address the analytical removal of these CECs.3 Indeed, the common water treatment methods such as ultraviolet, chlorination, and ozonation are somewhat effective in degrading most of these contaminants. Nonetheless, these processes do not account as actual removal methods, and the byproducts that are created can be even more toxic than the initial compounds.4 Presently, the adsorption of CECs with activated carbon is a preferred method because of the high porosity surface area of the material, which allows for an effective adsorption of the contaminants.5 However, production of activated carbon demands considerable amounts of energy. In addition, activated carbon pores can easily undergo fouling, drastically reducing its adsorption efficiency.6
These drawbacks have motivated research in the development of environmentally friendly adsorption materials from natural sources such as nanocellulose (NC). NC is primarily obtained from naturally occurring cellulose sources and are getting considerable attention because they are inexpensive, biodegradable, and practically renewable, making them sustainable and nontoxic materials for several applications.7 NC is produced by the acid treatment of cellulose to remove the naturally occurring amorphous domains, thereby obtaining a crystal structure with high amounts of primary hydroxyl groups on the surface. This characteristic makes NC a competitive material for the adsorption of contaminants via electrostatic interactions onto its surface.8 However, the hydrophobic nature of most of CECs makes the electrostatic interactions of NC ineffective to remove this type of contaminants. Therefore, it is appropriate to incorporate new functionalities by chemical modification of the NC primary hydroxyl groups to improve the adsorption capabilities toward CECs. Typically, polymers are preferred for this type of modification because of the versatility and variability of their structure.9 In terms of the removal of CECs from water, an environmentally friendly and biodegradable polymer such as polyethylene glycol (PEG) represents a feasible option.10 PEG is a nontoxic polymer widely used as an excipient in pharmaceutical products with high hydrophilicity that allows an easier metabolization of prescribed drugs within the human body.11 The latter characteristic makes PEG-based polymers an ideal alternative for the removal of CECs because low water-soluble pharmaceuticals represent a considerable percentage of CECs. Other researchers have explored the chemical modification of cellulose with PEG-based polymers,12 but there are no relevant studies regarding the use of PEG–NC to remove CECs from water.
To contribute to this area of research, herein, we present the synthesis of a PEG–modified NC composite. This composite is generated by the formation of an amide bond between a polyether diamine, with an approximate molecular weight of 600 g/mol (Jeffamine ED 600), and the previously carboxylated surface of NC. Besides the synthesis of the proposed adsorbent, extensive chemical and physical characterization is presented. Moreover, as a proof-of-concept, adsorption isotherms for different contaminants are executed, and the results are confirmed using computational modeling.
2. Materials and Methods
2.1. Materials
Cellulose nanocrystals (NC) 11.8 wt % aqueous slurry was purchased from The Process Development Center of the University of Maine. To oxidize the surface of NC, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), sodium hypochlorite, and sodium bromide were employed. Surface grafting of O,O′-bis(2-aminopropyl) polypropylene glycol-block-polyethylene glycol-block-polypropylene glycol (Jeffamine ED 600, 600 MW, 30 mequiv NH2/g) onto NC was achieved using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and N-hydroxysulfosuccinimide sodium salt (sulfo-NHS). Analytical standards, acetaminophen, sulfamethoxazole, and N,N-diethyl-m-toluamide (DEET), served as the CECs. All above reagents were purchased from Sigma-Aldrich, and the experiments were conducted using deionized water (18.23 MΩ/cm).
2.2. Methods
2.2.1. TEMPO-Mediated Oxidation of NC
Carboxylation of the NC surface via TEMPO-mediated oxidation is a well-known method reported elsewhere.13 First, we suspended NC (1.02 g, 6.3 mmol anhydroglucose units, AGU), NaBr (324 mg, 3.15 mmol), and TEMPO (29.5 mg, 0.19 mmol) in 50 mL of deionized water under continuous magnetic stirring. The reaction was initiated by adding 0.94 mL of 12 wt % NaOCl dropwise to the suspension for a molar ratio of 0.3 NaOCl/AGU. pH was maintained at 10 using 0.5 M NaOH until no fluctuations were observed. At this point, 10 mL of methanol was added to halt the reaction, and pH was lowered to 7 using 0.5 M HCl. The resulting suspension was dialyzed in a 3.5 kD membrane against deionized water at 4 °C for 48 h to eliminate excess reagents.
2.2.2. Polymer Coupling onto the NC Surface by EDC/sulfo-NHS
The surface of carboxylated polysaccharides can be easily modified with amine-terminated compounds via the formation of peptide bonds mediated by an EDC/sulfo-NHS coupling reaction.14 EDC (110 mg, 5.77 mmol) and sulfo-NHS (121 mg, 5.57 mmol) were dissolved in a 100 mL suspension of dialyzed oxidized NC. Amine-terminal Jeffamine ED 600 (184 mg, 5.52 mequiv of NH2) was added to the suspension with respect to the carboxylic content of oxidized NC (determined by conductometric titration), pH was set to 8, and the reaction was kept undisturbed for 24 h at room temperature. Afterward, pH was lowered to acidity, and the suspension was dialyzed for 72 h in deionized water. To obtain solid samples of the modified NC (NC–Jeffamine), suspensions were freeze-dried until complete dehydration.
2.2.3. Characterization of NC Grafted with a Block Copolymer
Fourier transform infrared (FTIR) spectra were recorded on a Bruker Tensor 27 spectrometer using the attenuated total reflectance mode. The spectral width ranged from 400 to 4000 cm–1, with a resolution of 4 cm–1 and an accumulation of 32 scans. A PerkinElmer STA 6000 simultaneous analyzer was used for the thermal analysis. All samples were heated from 30 to 750 °C with a ramp of 20 °C/min in an air atmosphere. A Malvern Zetasizer Nano series with a 4 mW 632.8 nm laser was used to determine the average diameter and surface charge of the samples in aqueous suspension (∼0.1 wt %) at pH 7. A JEOL 6480LV scanning electron microscope and a Bruker Dimension Edge AFM was used to record the micrographs. Samples for the scanning electron microscopy (SEM) analysis were suspended in deionized water, and then a drop was placed onto a carbon-coated copper grid under an air atmosphere until dryness was achieved. In the case of atomic force microscopy (AFM), a diluted solution of the sample was spin-coated at 3000 rpm for 20 s in a silicon substrate (1–10 Ω cm) from the International Wafer Service, Inc. A TriStar II 3020 surface area analyzer was used to determine the specific surface area of the samples by applying the Brunauer–Emmett–Teller (BET) method. The degassing temperature was set at 120 °C for 8 h under a N2 flow.
2.2.4. Determination of the Block Copolymer Content on the NC Surface
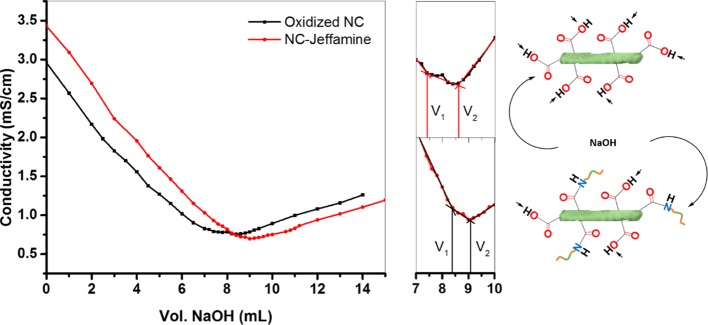
Conductometric titration was used to determine the content of carboxylic acid groups on the surface of NC after the oxidation and coupling reactions. An Accumet Basic AB30 conductivity meter was utilized to monitor the titration of NC samples with an excess of 0.01 M HCl, using 0.01 M NaOH as the titrant. The resulting plots showed the volume of NaOH necessary to determine the degree of oxidation (DO) using the following equation15
where C is the concentration of NaOH (mol/L), V2 and V1 are the volumes of NaOH (L), and w is the sample weight (g). Because the coupling reaction modifies the carboxylic acid into an amide functional group, we can indirectly determine the block copolymer content on the surface of NC by subtracting the DO before and after the EDC/sulfo-NHS reaction.
2.2.5. Batch Adsorption Experiments of Contaminants
Batch adsorption isotherm experiments in the presence of the CECs, sulfamethoxazole, acetaminophen, and DEET, were carried out at room temperature under different pH conditions using 50 mL tubes containing 20 mL of the contaminant solution (10–50 mg/L) and modified NC (0.1 g) as adsorbents. An orbital shaker was employed to create turbulence, and it was set at 250 rpm to maintain uniformity until equilibrium (24 h). Then, the solutions were centrifuged at 40 000 rpm to remove the adsorbent. By determining the concentration of the contaminants before and after the adsorption experiments, the removal efficiency of the adsorbents was calculated using this equation
where qe is the equilibrium adsorption amount (mg/g), Co is the initial concentration of the contaminant solution (mg/L), Ce is the equilibrium concentration of the contaminant (mg/L) after adsorption, V is the volume of the contaminant solution (L), and w is the mass of modified NC. Emerging contaminant concentrations were measured by the high-pressure liquid chromatography analysis using an Agilent 1100 Series VWD equipped with a Hypersil BDS C18 column (150 × 4.6 mm, 5 μm) at 25 °C. The flow rate was set at 1 mL/min, and the injection volume was 30 μL.
2.2.6. Computational Methodological Details
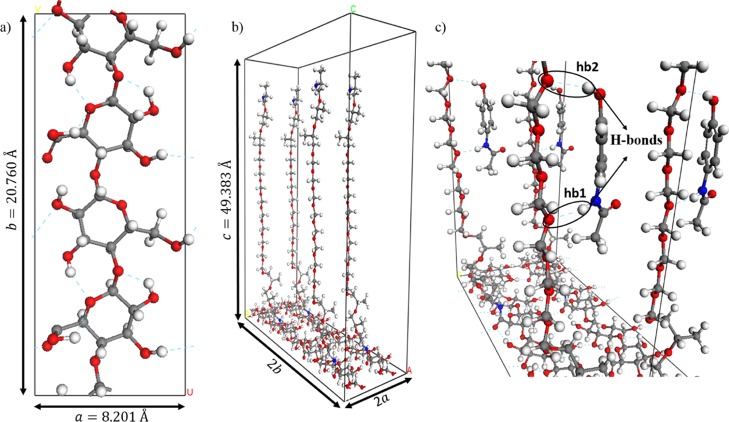
The calculations were carried out based on the state-of-the-art density functional theory (DFT) in a plane-wave pseudopotential method, as implemented in the Vienna Ab initio Simulation Package.16 The Perdew–Burke–Ernzerhof exchange-correlation functional with an energy cutoff of 400 eV for the plane wave expansion and 4 × 2 × 1 Monkhorst–Pack grid for k-point sampling were used in the calculations. The long-range interactions [van der Waals (vdW)] were considered by adding a semiempirical correction to the conventional Kohn–Sham DFT energy, that is, dispersion-corrected DFT-D3 method of Grimme.17 The projector augmented-wave pseudopotentials16,18 were used for all elements except hydrogen atoms. To better mimic the experimental conditions related with the block copolymer content on NC, an orthorhombic supercell (a = 8.201, b = 20.760, and c = 49.383 Å, see Figure 8) was built based on the experimental lattice parameters of bulk nanocellulose (NC).19 All atoms were allowed to move during the gradient-minimization procedure without any internal-coordinates constraint while the cell parameters were fixed. The binding energy (BE) was calculated by using the following formula
where ENC/Pol:CEC, ENC/Pol, and ECEC denote the total (DFT + vdW) energy of the equilibrium structures for the adsorbent material + CEC, the adsorbent material (Jeffamine polymer attach to functionalized NC), and CEC, respectively. The level of accuracy of the present computational methodology is indicated by comparison with high-level many-body theory computations of BEs for hydrogen-bonded dimers available in the literature.20 Specifically, the absolute differences of the computed BEs for formamide and N-methylacetamide dimers are within ∼1 kcal/mol.
Figure 8.
Atomic structures models for (a) 1 × 2-surface of oxidized NC, (b) 2 × 2 × 1 supercell of NC–Jeffamine, and (c) a 2 × 2 × 1 supercell representation of minimum-energy structure for acetaminophen adsorbed on NC–Jeffamine through two hydrogen bonds.
3. Results and Discussion
3.1. TEMPO-Mediated Oxidation of NC
We performed the TEMPO oxidation of NC by varying the molar ratio of NaOCl/AGU to assess the optimal reaction conditions. After the reaction, FTIR was conducted, and the intensity of the carbonyl band corresponding to the oxidation process was tracked (see Figure S1A). From the intensity ratios of the carbonyl bands from the FTIR spectra, it was possible to determine the optimal concentration of NaOCl to successfully complete the reaction. These results were plotted against the different molar ratios of NaOCl/AGU ranging from 0.05 to 1.0 (Figure S1B). From these results, it was observed that molar ratios higher than 0.5 NaOCl/AGU did not significantly increase the DO. This finding suggests that the limit of possible modifiable hydroxyl groups was reached given the size of the NC particles.13c The information obtained from the FTIR spectra was compared to the TGA analysis at different stages of the reaction (Figure S2), and it was shown that this molar ratio was enough to significantly decrease the thermostability of NC. Because NaOCl was the only variable in the different oxidation reactions, it can be argued that NaOCl plays a major contribution in the structural integrity of NC. Therefore, to maintain the physical integrity of NC, we opted for lowering the molar ratio to 0.3 NaOCl/AGU to perform further experiments.
3.2. Block Copolymer Grafting onto Oxidized NC
Jeffamine ED 600 is a commercially available block copolymer with a polyether frame consisting of PEG and polypropylene glycol with terminal amino functional groups (Figure 1). These types of block copolymers are used to prepare thermo-responsive hydrogels, given their hydrophilicity at room temperature and formation of sol–gels at higher temperatures.21 Here, Jeffamine ED 600 was grafted onto oxidized NC to obtain an enhanced hydrophilic composite with increased adsorption capacities.
Figure 1.
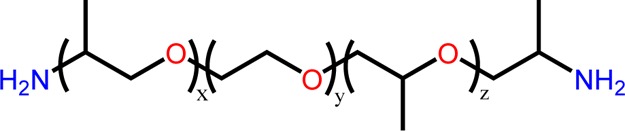
Molecular structure of Jeffamine ED 600.
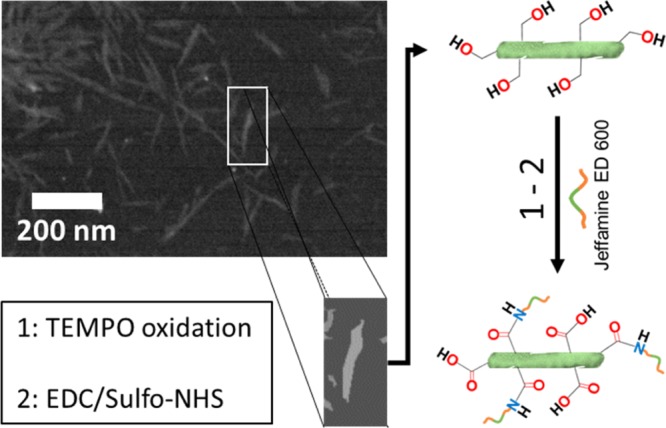
EDC/sulfo-NHS allowed the coupling reaction between the carboxylic groups on the surface of oxidized NC and the primary amines of Jeffamine to obtain a covalent amide bond. The ratios of EDC, sulfo-NHS, and Jeffamine (equivalents of NH2) were set to 4 times in relation to the oxidized NC.12aScheme 1 shows the details regarding the chemical changes on the surface before and after grafting Jeffamine ED 600 onto the surface of NC.
Scheme 1. Steps for Grafting of NC with Block Copolymer Jeffamine ED 600.
FTIR was utilized to measure the amide functional groups that are expected to form after the coupling reaction. Figure 2 shows the FTIR spectra of NC (Figure 2a), NC after oxidation (Figure 2b), and NC–Jeffamine (Figure 2c). The pictograms at the right side of each spectrum in the same figure represent the physical structure of NC, with the major functional groups on its surface. It can be clearly observed that after the TEMPO-mediated reaction, an intense band around 1600 cm–1 corresponding to the free salt of carboxyl groups was identified,22 providing strong evidence that corroborates the oxidation of NC. Moreover, after polymer grafting, a broad band centered at 1650 cm–1 can be observed, which was assigned to the new amide functional group.
Figure 2.
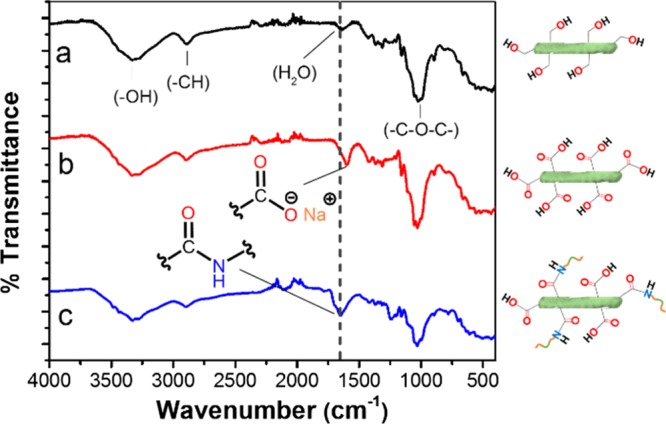
FTIR spectra and structural pictograms of (a) NC, (b) oxidized NC, and (c) NC–Jeffamine.
Because the band around 1650 cm–1 in Figure 2c appears to be composed of several bands, a Gaussian deconvolution was employed (Figure 3) to better determine the species that were present after the coupling reaction. This deconvolution revealed that this broad band is composed of at least four different bands located at ca. 1700, 1650, 1600, and 1550 cm–1. The band around 1550 cm–1 is characteristic of the bending of N–H, also known as amide II. This band is a confirmation of the formation of the covalent bond between the block copolymer and NC.23 Additionally, a band at 1600 cm–1 corresponding to unreactive carboxylic groups is present in the final sample. This appreciation is reasonable if we take into consideration that this band appears at the same position as that of the FTIR spectrum of oxidized NC. In addition, unreacted Jeffamine molecules might have remained after the reaction because of steric impediment with bulky Jeffamine already attached to the surface of NC. These molecules were further removed after dialysis. Lastly, a band around 1700 cm–1 was tentatively interpreted as impurities from the coupling agents sulfo-NHS and EDC, as seen in Figure S3.
Figure 3.
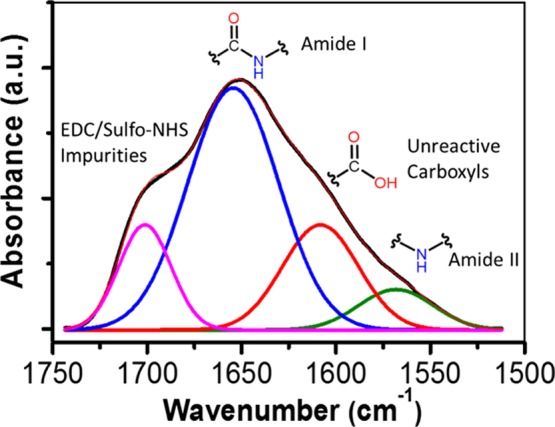
Gaussian deconvolution of the broad band around 1650 cm–1 of the FTIR spectrum of NC–Jeffamine.
After confirmation of the successful grafting, we indirectly determined the amount of block copolymer on the surface of NC using conductometric titration. Figure 4 shows the titration plots of oxidized NC and NC–Jeffamine. The initial steep slope in both curves corresponds to the titration of excess of HCl, followed by stabilization of the conductivity because of the titration of the available carboxylic acids on the surface of NC. The titration finishes with an increase in the conductivity because of excess of NaOH. After TEMPO oxidation, DO was 0.075, whereas this value lowered to 0.041 when the block copolymer was grafted. The degree of substitution of the block copolymer was determined from these results, and it was found that the difference between the values before and after block copolymer grafting is 0.033, which indicates that ca. 45% of the carboxylic groups on the NC surface were converted or modified to amide groups. This value is in good agreement with those found in the literature.12a
Figure 4.
Conductometric titration curves of oxidized NC and NC–Jeffamine and pictograms showing their reacting groups.
To study the thermal stability of NC and modified NC, TGA was conducted and is shown in Figure 5. Initially, there is a loss of 5% in mass around 100 °C, followed by a sharp loss in mass around 300 °C that slowly decreases until almost no material remains. This sharp decrease in the mass corresponds to the rapid depolymerization of the outer cellulose chains, whereas the last step of the process is characteristic of a hierarchical depolymerization and total carbonization caused by the crystallinity of the NC.7b It is worth to mention that NC–Jeffamine appears to be less thermally stable than the other species because a significant decrease in the mass starts at around 250 °C. Other minor variations in the thermogram trends suggest that the core structure of NC remains intact, suggesting that the oxidation and grafting conditions were adequate for the modification of the NC surface.
Figure 5.
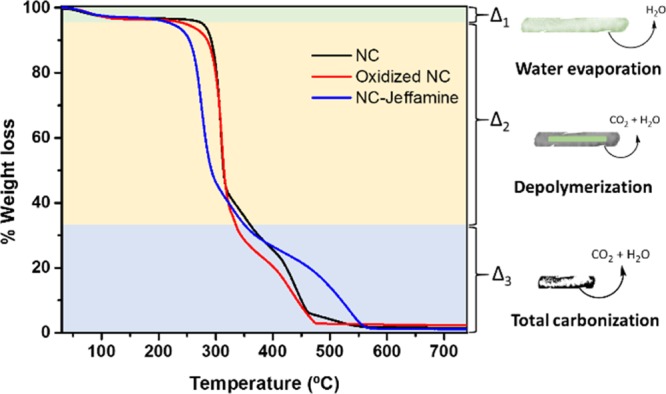
Thermograms of NC, oxidized NC, and NC–Jeffamine. The pictograms at the right show the major process during the heating process.
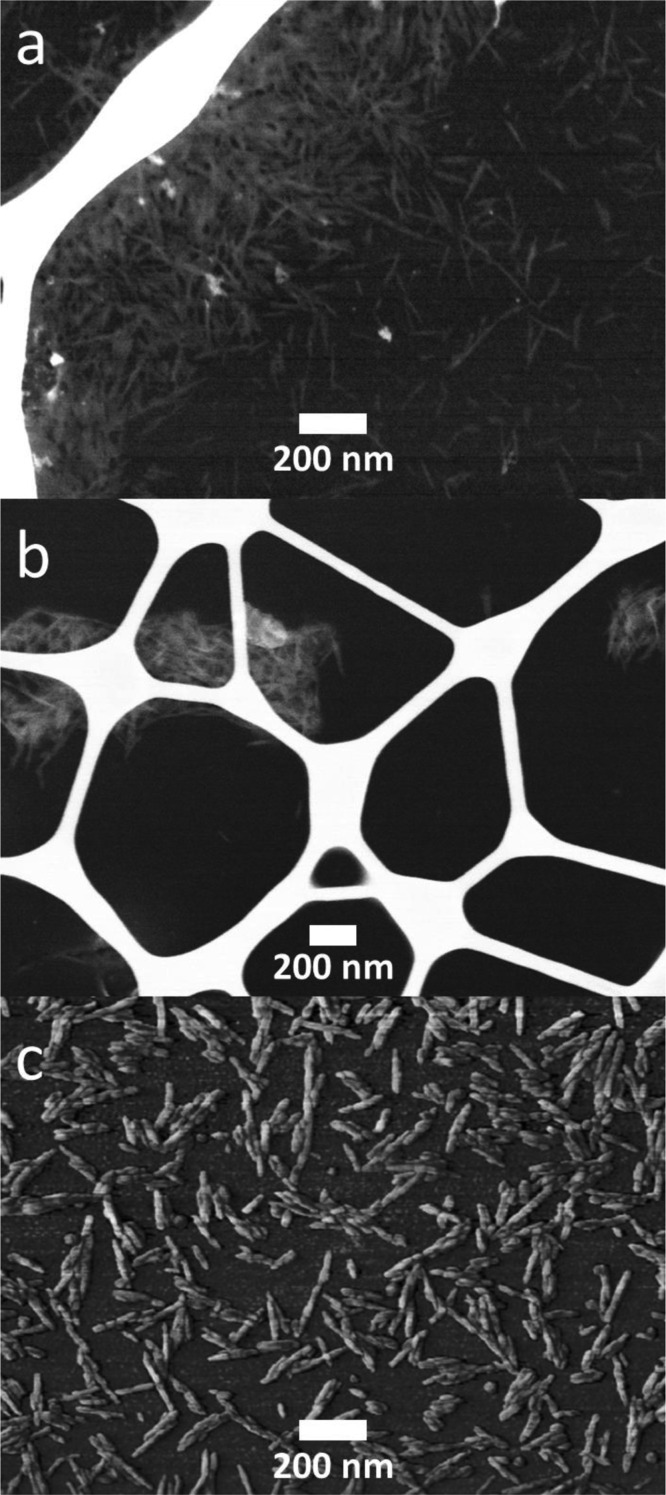
After chemical characterization, we were intrigued to determine the surface area of the material before and after modification. The specific surface areas of NC, oxidized NC, and NC–Jeffamine were measured using the N2 adsorption–desorption method at −196 °C. The BET surface area was 2.4 m2/g for NC, 8.4 m2/g for the oxidized NC, and 15.1 m2/g for NC–Jeffamine. These results represent an increase in the surface area after oxidation and grafting by almost 4 and 5 times, respectively, compared to that of NC. These resulting values of the BET surface area studies of NC are consistent with tightly packed and low porous structures due to strong hydrogen bonding.7a,24 The increase in the surface area after oxidation and grafting can be attributed to the new functionalities (carboxylic groups and block copolymer). It is worth mentioning that NaOCl could have promoted surface roughness because of slight degradation of the glycosidic bonds in NC during the oxidation; however, because NaOCl was not used in the grafting reaction, the further increase of the surface area can only be related to the presence of the block copolymer in the NC surface. SEM images were acquired to account for morphological changes in the sample that would provide more insights on the modification process. From the SEM images of NC (Figures 6a and S4a), oxidized NC (Figures 6b and S4b), and an AFM image of NC–Jeffamine (Figures 6c and S4b), it can be observed that the structure of NC has no significant differences after oxidation and grafting. This result suggests that only the surface of NC underwent modification. Moreover, this corroborates the findings from the surface analysis and can explain why only a minor increase in the surface area was obtained after the modifications.
Figure 6.
SEM images of (a) NC, (b) oxidized NC, and (c) AFM deflection image of NC–Jeffamine.
To characterize NC and modified NC in aqueous solutions, analysis with dynamic light scattering (DLS) and Z-potential was performed to determine the average size and surface charge, respectively. Figure 7 show the DLS histograms, and Table 1 summarizes the findings for this analysis.
Figure 7.
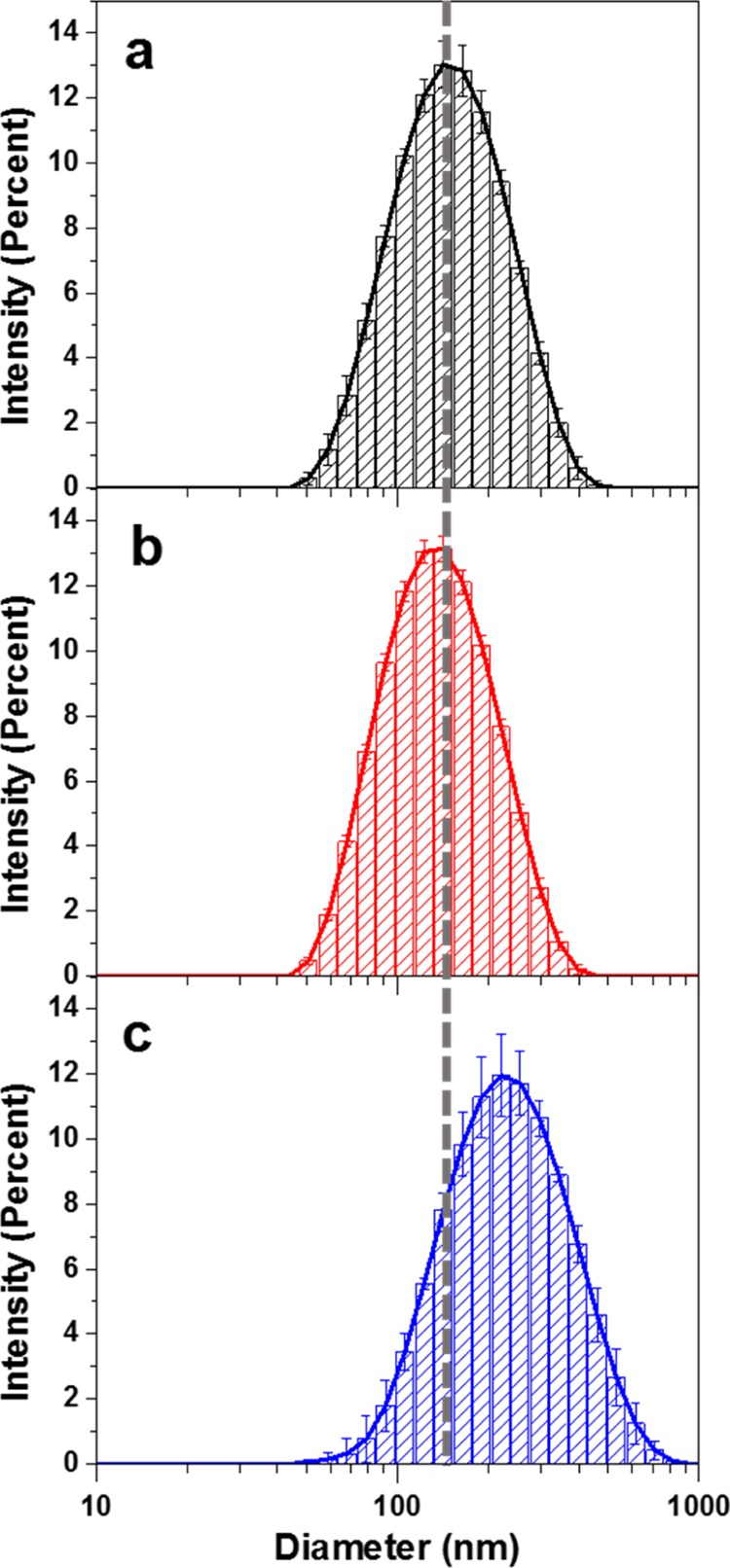
DLS histograms of (a) NC, (b) oxidized NC, and (c) NC–Jeffamine.
Table 1. Z-Average Size and Z-Potential of NC and Modified NC.
| sample | Z-average (nm) | polydispersive index | Z-potential (mV) |
|---|---|---|---|
| NC | 130 ± 2 | 0.189 ± 0.008 | –54 ± 2 |
| oxidized NC | 121.0 ± 0.7 | 0.18 ± 0.01 | –58 ± 1 |
| NC–Jeffamine | 190 ± 2 | 0.243 ± 0.05 | –33 ± 1 |
DLS measures the hydrodynamic diameter of the particles assuming a spherical shape and taking into consideration their translational diffusion coefficient. NC has a rodlike shape; because the overall translational diffusion coefficients of rods with a defined length are equivalent to the sphere counterparts, the hydrodynamic diameter strongly correlates with the particle length.25 The measured sizes of NC and oxidized NC are similar, with a slight decrease in the case of oxidized NC. On the other hand, NC–Jeffamine shows a significant increase in the diameter, from 130 to 190 nm. The resulting size distributions in DLS are in good agreement with those observed in SEM except for NC–Jeffamine, which is significantly bigger when it was measured by DLS. NC–Jeffamine in aqueous solution has a greater hydrodynamic diameter, given the contribution of the extended hydrophilic block copolymer toward the aqueous medium. This last statement reinforces the idea of successful attachment of the block copolymer Jeffamine ED 600 to the surface of NC.
In the case of the Z-potential results, it is clear that the addition of Jeffamine decreases the surface charge of NC. In this case, the surface charge measured by the Z-potential was useful to determine the presence of Jeffamine on the surface of NC. Aqueous dispersion of NC has a surface charge of −54 mV, which is related to the presence of negatively charged sulfate groups on its surface that maintain the particles in suspension and avoid aggregation by electrostatic repulsion.7b After oxidation, these sulfate groups are replaced by negatively charged carboxyl groups that result in a slight increase of the negative charge on the surface. On the other hand, the introduction of the neutral Jeffamine polymer substitutes almost 50% of the carboxylates on the surface for amide groups. This explains why the net charge is less negative than the other species.
3.3. Sorption Isotherms of CECs
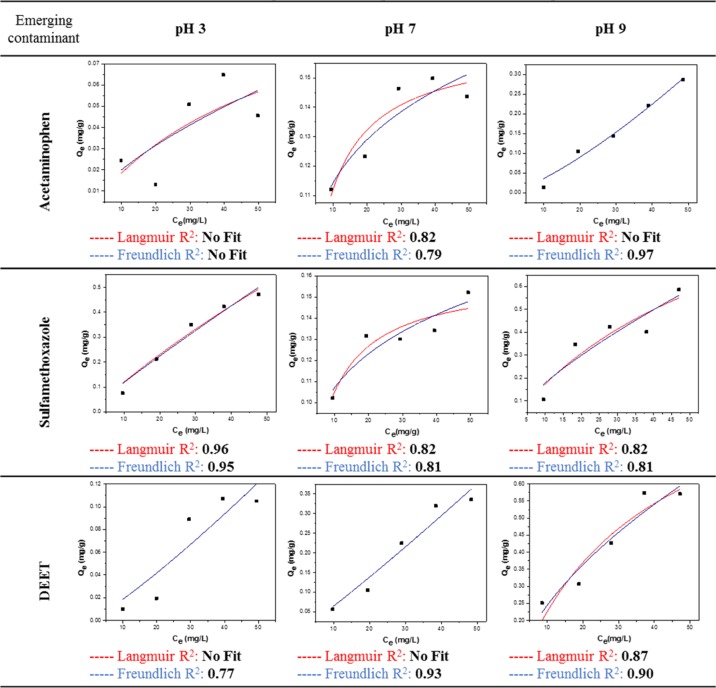
To measure the adsorption capacity of NC–Jeffamine toward acetaminophen, sulfamethoxazole, and DEET, batch experiments were performed at pH 3.0, 7.0, and 9.0, and the results were fit to the mathematical nonlinear models of Langmuir and Freundlich.26 The results are presented in Table 2. For acetaminophen, there was no significant adsorption at acidic pH, whereas at neutral and basic pH values, adsorption was evident, it being higher under basic conditions. However, for sulfamethoxazole, higher adsorption was observed at both acidic and basic pH values, with adsorption at pH 9.0 being slightly higher. On the other hand, DEET adsorption increased as a function of pH. In terms of the mathematical models, acetaminophen had a better fitting with the Freundlich model at basic pH, meanwhile, sulfamethoxazole had a better fitting at acidic pH with both Langmuir and Freundlich models. DEET was consistent along the pH range measured, with the Freundlich models presenting a better fit. Batch adsorption experiments using NC or oxidized NC were not successful because no significant adsorption was obtained (Table S1).
Table 2. Isotherms Plot for the Adsorption of CECs at Different pH values.
To analyze the adsorption isotherms of the different CECs, it is important to take into consideration the structural changes that both NC–Jeffamine and CECs undergo at different pH values. Because of the presence of unreactive carboxylic groups on the surface of NC–Jeffamine, the dispersion of the particles in aqueous solution is affected by pH. As seen in Figure S5, at acidic pH, the particles are in an aggregated state forming a viscous slurry. This phenomenon occurs due to protonation of the carboxylates to form neutral carboxylic acids. Under this condition, hydrogen bonds overcome the electrostatic repulsions of the particles. As pH increases, the formation of negatively charged carboxylates restores the electrostatic interactions of the particles to obtain well-defined aqueous suspensions. The adsorption of all of the CECs increases at pH 9, as seen in Table 2. It can be suggested that the better dispersity of NC–Jeffamine in the solution favors the interaction with the contaminants and enhances the adsorption. In the case of CECs, pKa plays an important role in the solubility and the net charge of each one. Acetaminophen has a pKa of 9.5,27 which means that at basic pH, it has a net negative charge. That behavior can explain why there is almost no adsorption at pH 3; it is possible that acetaminophen remained insoluble in the aqueous solution during the adsorption experiment, which decreased the possibility of effective interactions with NC–Jeffamine. Sulfamethoxazole and DEET have acid pKa values of 5.6 and 2.00, respectively.28 Besides the aggregation of NC at acid pH, the adsorption of sulfamethoxazole is considerable. The adsorption is possibly driven by the electrostatic interactions of positively charged sulfamethoxazole and the surface of NC–Jeffamine. This phenomenon can also help to understand the relatively good fitting of both Langmuir and Freundlich models in terms of the possibility that ions of sulfamethoxazole can form successive layers on the surface of aggregated NC–Jeffamine.29 In the case of DEET, the adsorption is more consistent with the increase of the solution pH. Because pKa is considerably low, there is no chance to obtain a charged molecule under the studied conditions. The formation of nonspecific interactions between DEET and NC–Jeffamine is more probable, given the poor correlation with the two mathematical models.
3.4. Computed BEs
On the basis of the finding that the experimental adsorption of CECs onto NC and oxidized NC was negligible in this study, the computational effort was focused on NC–Jeffamine. Figure 8 shows the atomistic models for the oxidized NC surface (composed of four chemical formulas of oxidized cellulose), a supercell containing four unit cells of NC–Jeffamine, and the equilibrium structure for the adsorption site of acetaminophen onto NC–Jeffamine. The elucidation of the minimum-energy atomistic structures and BEs computationally obtained helps to understand how the adsorption of CECs onto NC–Jeffamine takes place and the nature of interaction between them. A variety of intermolecular interactions, such as moderate hydrogen bonds and vdW forces, are the reason for adsorption of CECs onto NC–Jeffamine. In the case of acetaminophen, two hydrogen bonds (hb1 and hb2) are established (see Figure 8); the bond lengths are 2.02 Å for hb1 and 2.29 Å for hb2, and the contribution of vdW interactions to its BE is 14.549 kcal/mol of 21.17 kcal/mol. For sulfamethoxazole, one hydrogen bond is made with a bond length of 2.28 Å (see Figure S6a), and the vdW contribution to the BE is 7.21 kcal/mol of 14.32 kcal/mol. Finally, for DEET, the contribution to the BE is almost because of the vdW interactions, which account for 5.70 kcal/mol of the 8.37 kcal/mol total BE (see Figure S6b). In all cases, the adsorption of CECs onto NC–Jeffamine does not affect the atomistic structure of overall NC–Jeffamine drastically; this can be observed by direct comparison of Figures 8c and S6.
4. Conclusions
In this research, we demonstrate the feasibility of the adsorption of CECs using the environmental friendly NC–Jeffamine that we synthetized/characterized by experimental (adsorption isotherms) and computational methods (atomistic structures and BEs energies). We successfully attached Jeffamine ED 600 to oxidized NC to obtain nanoparticles with an aspect ratio similar to that of the precursor, as suggested from the SEM imaging and DLS analysis, but with a higher surface area (BET analysis) and hydrophilicity conferred by the polymer chains. These new physical properties allowed the adsorption of acetaminophen, sulfamethoxazole, and DEET at pH 3, 7, and 9 by means of electrostatic interactions with the polymer arrangements on the surface of the nanoparticle. The computational calculations resulted in BEs of 21.17, 14.32, and 8.37 kcal/mol for acetaminophen, sulfamethoxazole, and DEET, respectively, related to hydrogen bonds and/or vdW interactions with the polymer chain. These computational results clearly indicate the adsorption of CECs onto NC–Jeffamine on the molecular level and its agreement with the adsorption trends that were experimentally obtained for all those CECs at neutral pH. This investigation has placed a fundamental basis that encourages further research on the production of eco-friendly materials based on the polymer grafted onto a biomaterial surface and their use for the remediation of contaminants from aqueous media.
Acknowledgments
This work was supported by the NASA Experimental Program to Stimulate Competitive Research (EPSCoR) under grant #NNX14AN18A. The authors acknowledge the UPR Materials Characterization Center (MCC) for the support provided during the attainment of this work. We would also like to thank the Institute for Functional Nanomaterials Nanoscopy Facility.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01053.
FTIR spectra, TGA results, and computational results (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. J.H.-M. is the main author of this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Fawell J.; Ong C. N. Emerging Contaminants and the Implications for Drinking Water. Int. J. Water Resour. D. 2012, 28, 247–263. 10.1080/07900627.2012.672394. [DOI] [Google Scholar]; b Petrović M.; Gonzalez S.; Barceló D. Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC, Trends Anal. Chem. 2003, 22, 685–696. 10.1016/S0165-9936(03)01105-1. [DOI] [Google Scholar]; c Rivera-Utrilla J.; Sánchez-Polo M.; Ferro-García M. Á.; Prados-Joya G.; Ocampo-Pérez R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. 10.1016/j.chemosphere.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Pal A.; Gin K. Y.-H.; Lin A. Y.-C.; Reinhard M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. 10.1016/j.scitotenv.2010.09.026. [DOI] [PubMed] [Google Scholar]
- a Stackelberg P. E.; Furlong E. T.; Meyer M. T.; Zaugg S. D.; Henderson A. K.; Reissman D. B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. 10.1016/j.scitotenv.2004.03.015. [DOI] [PubMed] [Google Scholar]; b Richardson S. D. Environmental mass spectrometry: emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778. 10.1021/ac202903d. [DOI] [PubMed] [Google Scholar]
- a Sichel C.; Garcia C.; Andre K. Feasibility studies: UV/chlorine advanced oxidation treatment for the removal of emerging contaminants. Water Res. 2011, 45, 6371–6380. 10.1016/j.watres.2011.09.025. [DOI] [PubMed] [Google Scholar]; b Ikehata K.; El-Din M. G.; Snyder S. A. Ozonation and Advanced Oxidation Treatment of Emerging Organic Pollutants in Water and Wastewater. Ozone: Sci. Eng. 2008, 30, 21–26. 10.1080/01919510701728970. [DOI] [Google Scholar]; c Papageorgiou A.; Voutsa D.; Papadakis N. Occurrence and fate of ozonation by-products at a full-scale drinking water treatment plant. Sci. Total Environ. 2014, 481, 392–400. 10.1016/j.scitotenv.2014.02.069. [DOI] [PubMed] [Google Scholar]; d Richardson S. D. Disinfection by-products and other emerging contaminants in drinking water. TrAC, Trends Anal. Chem. 2003, 22, 666–684. 10.1016/s0165-9936(03)01003-3. [DOI] [Google Scholar]; e Richardson S. D.; Plewa M. J.; Wagner E. D.; Schoeny R.; DeMarini D. M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res., Rev. Mutat. Res. 2007, 636, 178–242. 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- a Rossner A.; Snyder S. A.; Knappe D. R. U. Removal of emerging contaminants of concern by alternative adsorbents. Water Res. 2009, 43, 3787–3796. 10.1016/j.watres.2009.06.009. [DOI] [PubMed] [Google Scholar]; b Yu Z.; Peldszus S.; Huck P. M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound—Naproxen, carbamazepine and nonylphenol—on activated carbon. Water Res. 2008, 42, 2873–2882. 10.1016/j.watres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- a Ahmadpour A.; Do D. D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. 10.1016/0008-6223(95)00204-9. [DOI] [Google Scholar]; b Matsui Y.; Fukuda Y.; Inoue T.; Matsushita T. Effect of natural organic matter on powdered activated carbon adsorption of trace contaminants: characteristics and mechanism of competitive adsorption. Water Res. 2003, 37, 4413–4424. 10.1016/s0043-1354(03)00423-8. [DOI] [PubMed] [Google Scholar]
- a Lu P.; Hsieh Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. 10.1016/j.carbpol.2011.08.022. [DOI] [PubMed] [Google Scholar]; b Lin N.; Dufresne A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. 10.1039/c3nr06761k. [DOI] [PubMed] [Google Scholar]; c Dufresne A. Nanocellulose: a new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. 10.1016/j.mattod.2013.06.004. [DOI] [Google Scholar]
- a Kardam A.; Raj K. R.; Srivastava S.; Srivastava M. M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Envir. 2013, 16, 385–393. 10.1007/s10098-013-0634-2. [DOI] [Google Scholar]; b Yu X.; Tong S.; Ge M.; Wu L.; Zuo J.; Cao C.; Song W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. 10.1016/s1001-0742(12)60145-4. [DOI] [PubMed] [Google Scholar]
- Roy D.; Semsarilar M.; Guthrie J. T.; Perrier S. Cellulose modification by polymer grafting: a review. Chem. Soc. Rev. 2009, 38, 2046–2064. 10.1039/b808639g. [DOI] [PubMed] [Google Scholar]
- a Glastrup J. Degradation of polyethylene glycol. A study of the reaction mechanism in a model molecule: Tetraethylene glycol. Polym. Degrad. Stab. 1996, 52, 217–222. 10.1016/0141-3910(95)00225-1. [DOI] [Google Scholar]; b Ohta T.; Tani A.; Kimbara K.; Kawai F. A novel nicotinoprotein aldehyde dehydrogenase involved in polyethylene glycol degradation. Appl. Microbiol. Biotechnol. 2005, 68, 639–646. 10.1007/s00253-005-1936-z. [DOI] [PubMed] [Google Scholar]
- a Basit A. W.; Newton J. M.; Short M. D.; Waddington W. A.; Ell P. J.; Lacey L. F. The Effect of Polyethylene Glycol 400 on Gastrointestinal Transit: Implications for the Formulation of Poorly-Water Soluble Drugs. Pharm. Res. 2001, 18, 1146–1150. 10.1023/a:1010927026837. [DOI] [PubMed] [Google Scholar]; b Strickley R. G. Solubilizing Excipients in Oral and Injectable Formulations. Pharm. Res. 2004, 21, 201–230. 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- a Azzam F.; Heux L.; Putaux J.-L.; Jean B. Preparation By Grafting Onto, Characterization, and Properties of Thermally Responsive Polymer-Decorated Cellulose Nanocrystals. Biomacromolecules 2010, 11, 3652–3659. 10.1021/bm101106c. [DOI] [PubMed] [Google Scholar]; b Wu W.; Huang F.; Pan S.; Mu W.; Meng X.; Yang H.; Xu Z.; Ragauskas A. J.; Deng Y. Thermo-responsive and fluorescent cellulose nanocrystals grafted with polymer brushes. J. Mater. Chem. A 2015, 3, 1995–2005. 10.1039/c4ta04761c. [DOI] [Google Scholar]
- a Isogai A.; Saito T.; Fukuzumi H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. 10.1039/c0nr00583e. [DOI] [PubMed] [Google Scholar]; b Eyley S.; Thielemans W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. 10.1039/c4nr01756k. [DOI] [PubMed] [Google Scholar]; c Montanari S.; Roumani M.; Heux L.; Vignon M. R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. 10.1021/ma048396c. [DOI] [Google Scholar]
- a Wu J.-l.; Liu C. g.; Wang X. l.; Huang Z. h. Preparation and characterization of nanoparticles based on histidine–hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci.: Mater. Med. 2012, 23, 1921–1929. 10.1007/s10856-012-4665-8. [DOI] [PubMed] [Google Scholar]; b Bulpitt P.; Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. . [DOI] [PubMed] [Google Scholar]
- da Silva Perez D.; Montanari S.; Vignon M. R. TEMPO-Mediated Oxidation of Cellulose III. Biomacromolecules 2003, 4, 1417–1425. 10.1021/bm034144s. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. 10.1103/physrevb.47.558. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y.; Langan P.; Chanzy H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. 10.1021/ja0257319. [DOI] [PubMed] [Google Scholar]
- Ireta J.; Neugebauer J.; Scheffler M. On the Accuracy of DFT for Describing Hydrogen Bonds: Dependence on the Bond Directionality. J. Phys. Chem. A 2004, 108, 5692–5698. 10.1021/jp0377073. [DOI] [Google Scholar]
- a Dulong V.; Mocanu G.; Picton L.; Le Cerf D. Amphiphilic and thermosensitive copolymers based on pullulan and Jeffamine: Synthesis, characterization and physicochemical properties. Carbohydr. Polym. 2012, 87, 1522–1531. 10.1016/j.carbpol.2011.09.049. [DOI] [Google Scholar]; b Belbekhouche S.; Ali G.; Dulong V.; Picton L.; Le Cerf D. Synthesis and characterization of thermosensitive and pH-sensitive block copolymers based on polyetheramine and pullulan with different length. Carbohydr. Polym. 2011, 86, 304–312. 10.1016/j.carbpol.2011.04.053. [DOI] [Google Scholar]
- Habibi Y.; Chanzy H.; Vignon M. R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. 10.1007/s10570-006-9075-y. [DOI] [Google Scholar]
- Araki J.; Wada M.; Kuga S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. 10.1021/la001070m. [DOI] [Google Scholar]
- Lu P.; Hsieh Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. 10.1016/j.carbpol.2010.04.073. [DOI] [Google Scholar]
- Boluk Y.; Danumah C. Analysis of cellulose nanocrystal rod lengths by dynamic light scattering and electron microscopy. J. Nanopart. Res. 2013, 16, 2174. 10.1007/s11051-013-2174-4. [DOI] [Google Scholar]
- Wang P.; Du M.; Zhu H.; Bao S.; Yang T.; Zou M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. 2015, 286, 533–544. 10.1016/j.jhazmat.2014.12.034. [DOI] [PubMed] [Google Scholar]
- de Martino M.; Chiarugi A. Recent Advances in Pediatric Use of Oral Paracetamol in Fever and Pain Management. Pain Ther. 2015, 4, 149–168. 10.1007/s40122-015-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland K. C.; Dickenson E. R. V.; Drewes J. E.; Higgins C. P. Sorption of ionized and neutral emerging trace organic compounds onto activated sludge from different wastewater treatment configurations. Water Res. 2012, 46, 1958–1968. 10.1016/j.watres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Aloulou F.; Boufi S.; Belgacem N.; Gandini A. Adsorption of cationic surfactants and subsequent adsolubilization of organic compounds onto cellulose fibers. Colloid Polym. Sci. 2004, 283, 344–350. 10.1007/s00396-004-1143-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.