Abstract
Setting:
A resource-limited urban setting in Zimbabwe with a high burden of tuberculosis (TB) and human immunodeficiency virus (HIV).
Objectives:
To determine the feasibility and yield of diabetes mellitus (DM) screening among TB patients in primary health care facilities.
Design:
A descriptive study.
Results:
Of the 1617 TB patients registered at 10 pilot facilities, close to two thirds (60%) were male and 798 (49%) were bacteriologically confirmed. The median age was 37 years; two thirds (67%) were co-infected with HIV. A total of 1305 (89%) were screened for DM, and 111 (8.5%, 95% CI 7.0–10.2) were newly diagnosed with DM. Low TB notifying sites were more likely than high TB notifying sites to screen patients using random blood glucose (RBG) (83% vs. 79%; P < 0.04). Screening increased gradually per quarter over the study period. There were, however, notable losses along the screening cascade, the reasons for which will need to be explored in future studies.
Conclusion:
The study findings indicate the feasibility of DM screening among TB patients, with considerable yield of persons newly diagnosed with DM. Scaling up of this intervention will need to address the observed losses along the screening cascade.
Keywords: unidirectional, comorbidity, Southern Africa
Abstract
Contexte :
Un contexte très affecté par la tuberculose (TB) et le virus de l'immunodéficience humaine (VIH) dans une zone urbaine à ressources limitées du Zimbabwe.
Objectif:
Déterminer la faisabilité et le rendement du dépistage du diabète (DM) parmi les patients TB des structures de santé primaires.
Schéma:
Une étude descriptive.
Résultats:
Sur les 1617 patients TB enregistrés dans 10 structures pilotes, près de deux tiers (60%) ont été des hommes et 798 (49%) ont été confirmés par bactériologie. L'âge médian a été de 37 ans et deux tiers (67%) ont été co-infectés par le VIH. Un total de 1305 (89%) patients ont eu un dépistage du DM et 111 (8,5% ; IC 95% 7,0–10,2) ont été des diagnostics nouveaux de DM. Les sites notifiant peu de TB ont été plus susceptibles de dépister davantage de patients (test de glycémie aléatoire) plutôt que les sites notifiant de nombreuses TB (83% contre 79% ; P < 0,04). Le dépistage a progressivement augmenté à chaque trimestre de la période d'étude. Il y a cependant eu des pertes notables tout au long de la cascade de dépistage, pour des raisons qui devraient être explorées dans de futures études.
Conclusion:
Les résultats de l'étude sont en faveur de la faisabilité du dépistage du DM parmi les patients TB avec un rendement considérable de nouveaux diagnostics de DM. L'expansion de cette intervention devra prendre soin d'examiner le problème des pertes observées au long de la cascade de dépistage.
Abstract
Marco de Referencia:
Un entorno con alta carga de morbilidad por tuberculosis (TB) e infección por el virus de la inmunodeficiencia humana (VIH) en Zimbabwe.
Objetivos:
Determinar la factibilidad y el rendimiento de la detección sistemática de la diabetes (DM) en los pacientes con TB en los establecimientos de atención primaria de salud.
Método:
Fue este un estudio descriptivo.
Resultados:
De los 1617 pacientes con TB registrados en 10 establecimientos piloto, cerca de dos tercios (60%) eran de sexo masculino y en 798 casos se obtuvo la confirmación bacteriológica del diagnóstico (49%). La mediana de la edad fue 37 años y dos tercios de los pacientes presentaban coinfección por el VIH (67%). Se realizó el tamizaje de la DM en 1305 pacientes (89%) y en 111 se estableció un diagnóstico nuevo de DM (8,5%; IC 95% 7,0–10,2). La probabilidad de practicar una prueba de glucemia sin ayuno fue mayor en los centros con baja notificación de TB que en los centros con notificación alta (83% contra 79%; P < 0,04). El tamizaje aumentó gradualmente cada trimestre durante el período del estudio. Sin embargo, se observaron pérdidas considerables a lo largo de la secuencia de detección, cuyas causas se deben investigar en estudios futuros.
Conclusión:
Los resultados del estudio indican que es factible practicar la detección sistemática de la DM en los pacientes con TB, con un rendimiento diagnóstico notable de casos nuevos de DM. Durante la ampliación de escala de esta intervención será necesario abordar las pérdidas observadas a lo largo de la secuencia de la detección sistemática.
Despite significant progress in tuberculosis (TB) care and prevention in recent decades, TB remains a major public health problem. In 2017, 10.0 million people worldwide developed TB and 1.6 million people died from the disease.1 Zimbabwe is a high TB burden country, with an estimated incidence of 208 per 100 000 population.1 In 2017, 26 401 people were diagnosed with the disease and approximately two thirds were co-infected with human immunodeficiency virus (HIV).1
Along with socio-economic development, rapid urbanisation, high caloric diet coupled with physical inactivity, the global burden of diabetes mellitus (DM) is increasing, particularly in low- and middle-income countries.2,3 One in every 11 people (425 million) worldwide was estimated to have DM in 2017, and this is expected to rise to 629 million by 2045.2 Africa is anticipated to have the highest increase in DM, from 20 million in 2013 to almost 42 million by 2035.4,5
Zimbabwe is one of the countries that is experiencing a growing burden of non-communicable diseases (NCDs), including DM.6 In 2017, the prevalence of DM among adults in Zimbabwe was reported to be 1.3%, translating to 99 400 persons living with DM, with 76.3% estimated to be undiagnosed. DM prevalence is predicted to increase to 1.6%, with 265 000 estimated to have DM by 2045, a more than 2.5-fold increase.3
People with DM have a two to three-fold risk of developing TB compared with those without DM.7 Between 10–15% of all TB incidence in some sub-Saharan African countries, such as neighbouring South Africa and the Democratic Republic of Congo, has been attributed to DM.8 TB patients with DM have more severe clinical presentation, with higher likelihood of bacteriologically confirmed disease and cavities on chest radiographs, than patients without DM,9 and their TB treatment outcomes are frequently worse than of patients without DM, with delayed sputum smear conversion, and higher risk of treatment failure and relapse after successful completion of TB treatment.7–10
In 2011, the WHO and the International Union Against Tuberculosis and Lung Disease (The Union) launched a collaborative framework for the care and control of persons with DM and TB to guide policy makers and implementers in combatting the dual epidemic, with emphasis on the need for operational research to build an evidence base for public health action. Over the past decade, various countries (mainly in Asia) have applied this framework, reporting a high yield from screening.13,14 We do not know if the same approach can be implemented in sub-Saharan African countries with limited resources and high HIV burden.
We implemented a project of screening TB patients for DM supported by the World Diabetes Foundation (WDF; Bagsværd, Denmark) and conducted an operational study using the project data to assess the burden of DM among TB patients and the feasibility of screening DM among TB patients as measured by 1) the yield of DM among TB patients, 2) the number of patients screened, and 3) trends in screening over time. Findings are likely to contribute to the pool of emerging evidence, and most importantly, inform possible scale-up in resource-limited, high TB-HIV burden settings.
METHODS
Study design
This was a descriptive study using routine programme data.
Study site
This study was carried out in the capital city of Harare, Zimbabwe, with an estimated population of 2 231 433.13 The City Health Department is responsible for providing primary health care (PHC) services to Harare residents. The city has two infectious disease hospitals and a network of 25 clinics across the city. Comprehensive opportunistic infection/antiretroviral treatment (OI/ART) services have been decentralised to 90% of the clinics from the two hospitals, and treatment and prevention of priority public health conditions such as TB and HIV, have been standardised using national treatment guidelines.14 In 2017, a total of 3310 TB cases were notified in the city, translating to a notification rate of 148/100000.15
Ten clinics were selected to pilot test the feasibility of implementing routine DM screening among TB patients due to their relatively high out-patient department (OPD) attendance. These were Warren Park, Kuwadzana, Rutsanana, Budiriro, Rujeko, Mabvuku, Mbare, Highfield, Mufakose and Glenview Clinics. These facilities are located in poor, densely populated, urban suburbs and covered a population of 1 025 507. All pilot sites were included in the study.
TB and DM services were integrated with other PHC services at these clinics. HIV diagnosis and ART services were also offered in opportunistic infection clinics situated at these facilities. Provider-initiated routine TB screening and investigations were available to persons living with HIV (PLHIV) in care at every clinic visit. TB services were predominantly nurse-led, but complicated cases were referred to a doctor during a scheduled weekly visit to each clinic. The visiting doctor managed patients diagnosed with DM, with diabetic emergencies being referred to central hospitals. For the purposes of this study, facilities were defined as high-volume if they had an average daily OPD attendance of ⩾200 individuals, and low-volume if <200. Clinics with TB notification rates > 140/100 000 in 2017 were defined as high TB notifying sites, and low notifying if ⩽140/100 000. Nursing coverage per population served was defined as high if ⩾25/100 000, and low if <25/100000.
Screening for diabetes mellitus among tuberculosis patients
During the pilot period from April 2016 to October 2017, TB patients notified at the selected facilities and not known to have DM were screened initially with the random blood glucose (RBG) test using capillary blood from a finger prick. Persons with a RBG of ⩾6.1 mmol/l were requested to have a fasting blood glucose (FBG) test on a subsequent visit after a fasting period of at least 10 h. Diagnosis of DM was made based on an FBG result of ⩾7.0 mmol/l. Patients newly diagnosed with DM were initiated on treatment by a physician either at the clinic or were referred to a central hospital.
Study population
Newly registered TB patients aged ⩾15 years at any of the above-mentioned clinics from April 2016 to October 2017 were included in the study.
Data variables, data sources and data collection
A structured questionnaire was used to collect the following variables from the clinics' TB registers: age, sex, type of TB, HIV status, ART status, weight, RBG result, FBG result, DM screening status and the number diagnosed with DM. A desk review of the Harare City Health Department's 2017 annual report was used to collect facility data on the catchment population, nursing coverage, average daily OPD attendance and the number of registered TB patients in 2017.16
Statistical analysis
Data from the questionnaire were double-entered and analysed using EpiData v3.1 (EpiData Association, Odense, Denmark). A logic check was used to identify errors made during data entry, and appropriate corrections made. Categorical data were analysed using frequencies and proportions, and a Z-test to determine differences in proportions, with the significance level set at 5%. The burden of DM among TB patients was measured by the percentage yield of DM among TB patients screened; the feasibility of screening was determined by the number of patients screened and trend of screening over time.
Ethical approval
Ethical approval was obtained from the institutional review board of Harare City Health Department, Harare, and the Medical Research Council, Harare, Zimbabwe (document number MRCZ/A/2208).
RESULTS
Characteristics of the health facilities
Characteristics of the 10 facilities are shown in Table 1. The TB notification rates per 100 000 ranged from 100 in Highfields to 276 in Mabvuku, while the average daily OPD attendance ranged from 142 in Mufakose to 247 in Mbare. The nursing coverage by facility ranged from 15/100 000 served in Budiriro and Mabvuku, to 52/100 000 in Kuwadzana.
TABLE 1.
Characteristics of participating health facilities, Harare, Zimbabwe, 2017
| Facility | Catchment population n | Nurses in post as of 31 December 2017 | Nursing coverage/100000 population | Average daily OPD attendance n | Registered TB patients n | Notification rate/100000 population |
|---|---|---|---|---|---|---|
| Budiriro | 138416 | 21 | 15 | 238 | 142 | 102 |
| Glenview | 129551 | 23 | 18 | 207 | 167 | 128 |
| Highfield | 108958 | 17 | 16 | 170 | 110 | 100 |
| Kuwadzana | 181304 | 28 | 15 | 228 | 246 | 135 |
| Mabvuku | 50335 | 26 | 52 | 193 | 139 | 276 |
| Mbare | 91882 | 29 | 32 | 247 | 158 | 171 |
| Mufakose | 88542 | 19 | 21 | 142 | 126 | 142 |
| Rujeko | 77850 | 22 | 28 | 176 | 144 | 184 |
| Rutsanana | 83256 | 24 | 29 | 190 | 120 | 144 |
| Warren Park | 75413 | 19 | 25 | 202 | 96 | 127 |
| All facilities | 1025 507 | 228 | 22 | 199 | 1448 | 141 |
Source: 16
OPD = out-patients department; TB = tuberculosis.
Characteristics of the study participants
A total of 1617 TB patients were notified by the pilot facilities; 966 (60%) were males. The median age was 37 years (interquartile range 30–44). Two thirds of the patients (1091/1617) were HIV-positive, 607 (38%) of whom had been on ART for <2 years (Table 2). Of the 1 617 patients, 798 (49%) were bacteriologically confirmed.
TABLE 2.
Characteristics of TB patients screened for DM in participating health facilities in Harare, Zimbabwe, April 2016–October 2017
| Characteristic | Study participants (n = 1617) n (%) |
|---|---|
| Sex | |
| Male | 966 (60) |
| Female | 651 (40) |
| Age, years, median (Q1–Q3) | 37 [30–44] |
| Type of TB | |
| Bacteriologically confirmed | 798 (49) |
| Clinically diagnosed | 818 (51) |
| Unknown | 1 (0.1) |
| HIV status | |
| Negative | 501 (31) |
| Positive | 1091 (67) |
| Unknown | 25 (2) |
| Duration on ART, years | |
| <2 | 607 (38) |
| ⩾2 | 123 (8) |
| Unknown | 361 (22) |
| Not applicable* | 526 (33) |
| Screened for DM | |
| Yes | 1305 (81) |
| No | 312 (19) |
| DM diagnosed | |
| No | 1194 (91) |
| Yes | 111 (9) |
* HIV-negative or of unknown HIV status.
TB = tuberculosis; DM = diabetes mellitus; Q = quarter; HIV = human immunodeficiency virus; ART = antiretroviral therapy.
Screening cascade and yield for DM
A total of 1305 (81%) were screened using RBG, 617 (47%) of whom had an RBG of ⩾6.1 mmol/l and were therefore eligible for an FBG test. Of those eligible, 427 (69%) underwent FBG testing, and 111 (26%) were diagnosed with DM (Figure 1). This translates to an overall DM yield of 9% (111/1305) among TB patients screened using RBG. The DM yield among participants with RBG ⩾ 6.1 mmol/l ranged from 11% (Kuwadzana) to 38% (Mbare), with an overall yield of respectively 3% and 16% among all TB patients screened. The Highfield and Kuwadzana clinics that had the lowest TB notification rates (100–135/100 000) screened the highest proportions of these patients with RBG, respectively 99% and 98% (Table 3).
FIGURE 1.
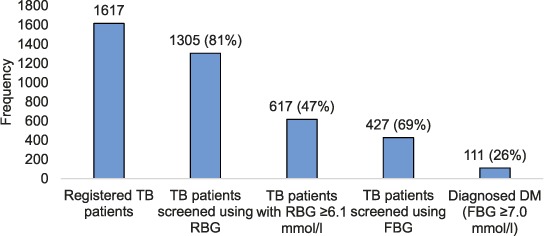
Screening cascade and yield for DM in TB patients in participating health facilities in Harare, Zimbabwe, April 2016–October 2017. TB = tuberculosis; RBG = random blood glucose; FBG = fasting blood glucose; DM = diabetes mellitus.
TABLE 3.
DM screening in TB patients per health facility, Harare, Zimbabwe, April 2016–October 2017
| Facility | Registered in TB clinic n | Screened using RBG n (%) | RBG ⩾ 6.1 mmol/l n (%) | Screened using FBG n (%) | Newly diagnosed DM with FBG ⩾ 7.0 mmol/l n (%) |
|---|---|---|---|---|---|
| Budiriro | 132 | 115 (87) | 57 (50) | 37 (65) | 9 (24) |
| Glenview | 216 | 159 (74) | 69 (43) | 45 (65) | 13 (29) |
| Highfield | 133 | 132 (99) | 77 (58) | 63 (81) | 19 (30) |
| Kuwadzana | 92 | 90 (98) | 39 (43) | 28 (72) | 3 (11) |
| Mabvuku | 190 | 127 (67) | 47 (37) | 33 (70) | 8 (24) |
| Mbare | 154 | 132 (86) | 71 (54) | 55 (77) | 21 (38) |
| Mufakose | 183 | 119 (65) | 57 (48) | 31 (54) | 4 (13) |
| Rujeko | 216 | 186 (86) | 79 (42) | 41 (52) | 7 (17) |
| Rutsanana | 156 | 145 (93) | 70 (48) | 55 (79) | 14 (25) |
| Warren Park | 145 | 100 (69) | 51 (51) | 39 (76) | 13 (33) |
| All facilities | 1617 | 1 305 (81) | 617 (47) | 427 (69) | 111 (26) |
DM = diabetes mellitus; TB = tuberculosis; RBG = random blood glucose; FBG = fasting blood glucose.
Low TB notifying sites were likely to screen more patients with RBG than high TB notifying sites (83% vs. 79%; P < 0.04). There was, however, no significant differences in screening by out-patient volume (high-compared to low-volume facilities) or nursing coverage (high-compared to low-coverage facilities) (Table 4).
TABLE 4.
Diabetes mellitus screening in TB patients by out-patient volume, TB burden and nursing coverage, Harare, Zimbabwe, April 2016–October 2017
| Variable | Registered in TB clinic n | Screened for RBG n (%) | P value* | RBG 3 6.1 mmol/l n | Screened for FBG n (%) | P value* |
|---|---|---|---|---|---|---|
| By patient volume | ||||||
| High-volume sites† | 739 | 596 (81) | 287 | 204 (71) | ||
| Low-volume sites‡ | 878 | 709 (81) | 1 | 303 | 223 (68) | 0.42 |
| By TB burden | ||||||
| High TB notifying§ | 899 | 709 (79) | 324 | 215 (66) | ||
| Low TB notifying¶ | 718 | 596 (83) | <0.04 | 293 | 212 (72) | 0.11 |
| By nursing coverage | ||||||
| High coverage# | 861 | 690 (80) | 318 | 223 (70) | ||
| Low coverage** | 756 | 615 (81) | 0.61 | 299 | 204 (68) | 0.59 |
* Z-test for difference in proportions.
† Average daily OPD attendance ⩾200 persons.
‡ Average daily OPD attendance <200 persons.
§ TB notification rate ⩾ 140/100 000.
¶ TB notification rate <140/100 000.
#Nursing coverage ⩾25/100 000.
**Nursing coverage < 25/100000.
TB = tuberculosis; RBG = random blood glucose; FBG = fasting blood glucose; OPD = out-patient department.
Trend of DM screening by quarter
The proportion of TB patients screened with RBG rose from 36% in the April–June 2016 quarter to a peak of 89% in the April–June 2017 quarter. The proportion of patients eligible and who returned for FBG screening was consistently lower than the proportion screened with RBG in all quarters, ranging from 27% in the April–June 2016 quarter to 61% in the October–December 2016 quarter (Figure 2).
FIGURE 2.
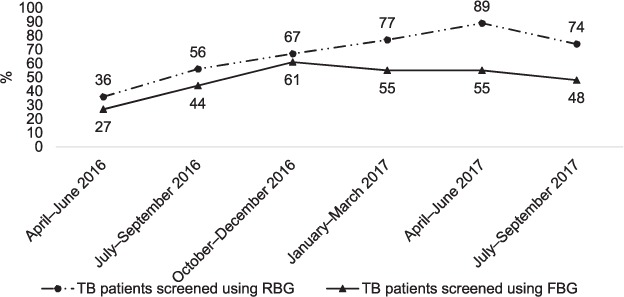
Trends in diabetes mellitus screening in TB patients at participating health facilities in Harare, Zimbabwe by quarter, April 2016–September 2017. TB = tuberculosis; RBG = random blood glucose; FBG = fasting blood glucose.
DISCUSSION
This is the first study in Zimbabwe to explore the feasibility and yield of routine DM screening among TB patients. The study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines on the conduct of observational studies.17 It was carried out in routine programme settings at urban primary health care facilities with limited resources, and therefore reflects the actual situation in the field.
The prevalence of DM among TB patients in our study was 8.5% (95% CI 7.1–10.1), similar to that reported in Ethiopia (8.3%).18 This was, however, lower than the prevalence reported in Tanzania (16.7%) and in Nigeria (12.3%).19,20 The prevalence of DM among TB patients in our study was more than four times higher than what has been reported in the general adult population of Zimbabwe.3 Our findings echo the findings from a systematic review where DM patients had a 3.6-fold increased risk of active TB compared to non-DM patients in four prospective studies.21
Over 80% of registered TB patients were screened with RBG, the trend increasing over time, which could reflect an incremental increase in adoption of the intervention by health workers over time. Ongoing mentorship and supervision at the sites might have also contributed towards the improved output. Among patients with RBG ⩾ 6.1 mmol/l, a consistently lower proportion of patients returned for FBG every quarter. Speculative reasons for the drop off could be patient-related factors such as patients unable to return for a second visit due to transport cost-related barriers, or health service factors such as possible lapses in inter-personal communication between health care worker and patient on the need for a return visit. These possible reasons require further exploration in future studies. Given these losses along the screening cascade, it is likely that the observed yield of DM in this study was an under-estimate of the actual burden of DM among TB patients in Harare. In better resourced, middle-income settings, such as China and India, over 95% of TB patients were successfully screened for DM.11,12,22,23 Furthermore, any intention to scale up this intervention beyond these pilot sites should pay attention to ‘closing the leaking tap’ along the screening cascade.
In this study, health facilities with a lower TB case load (low TB notifying sites) were likely to screen more patients for DM. In contrast, there was no difference in screening uptake when comparing sites with different workloads, as measured by daily OPD attendance or nursing staffing coverage. This is contrary to findings for other public health programmes, where quantity and maldistribution of staff have been documented as important barriers to care.24
The strengths of this study included the fact that it was carried out in a programme setting using routinely collected data. There were also certain limitations. First, capillary blood samples were analysed using a glucometer with no quality assurance, instead of testing venous blood samples using a biochemical analyser. This may have caused some testing bias.25 Second, DM was diagnosed using FBG alone, without oral glucose tolerance or glycosylated haemoglobin testing. This may have led to an underestimation of DM prevalence in TB patients.26 Third, blood glucose was measured only once immediately after TB registration, without a confirmation test at the end of TB treatment. We were therefore not able to ascertain whether or not there were any cases of hyperglycaemia induced by transient infections, as reported in the literature. This should be explored in future studies.27
CONCLUSION
Screening TB patients for DM in a PHC setting in Zimbabwe was found to be feasible. The intervention was high-yielding and should be considered for phased national scale-up. It is, however, critical that regular supportive, data-driven supervision and provision of essential consumables such as gluco strips be provided to ensure a high quality of DM-TB integrated care with a minimum number of patients dropping out of the care cascade.
Acknowledgments
This research was conducted in partnership with the Harare City Health Department, Harare, the Zimbabwean National Tuberculosis Control Programme, Harare, Zimbabwe, and the International Union Against Tuberculosis and Lung Diseases, Paris, France. The study, including open access publishing costs, was funded by the World Diabetes Foundation, Bagsværd, Denmark. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.World Health Organization Global tuberculosis control, 2018. Geneva, Switzerland: WHO; 2018. WHO/CDS/TB/2018.20. www.who.int/tb/publications/global_report/tb18_ExecSum_web_4Oct18.pdf Accessed May 2019. [Google Scholar]
- 2.Ojuka E O, Goyaram V. Increasing prevalence of type 2 diabetes in sub-Saharan Africa: not only a case of inadequate physical activity. Med Sport Sci. 2014;60:27–35. doi: 10.1159/000357333. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation The IDF Diabetes Atlas. 8th ed. Brussels, Belgium: IDF; 2017. www.diabetesatlas.org Accessed May 2019. [Google Scholar]
- 4.Peer N, Kengne A P, Motala A A. Diabetes in the Africa Region: an update. Diabetes Res Clin Pract. 2014;103:197–205. doi: 10.1016/j.diabres.2013.11.006. et.al. [DOI] [PubMed] [Google Scholar]
- 5.Khazrai Y M, Defeudis G, Pozzilli P. Effect of diet on type 2 diabetes mellitus: a review. Diabetes Metab Res Rev. 2014;30(Suppl 1):24–33. doi: 10.1002/dmrr.2515. [DOI] [PubMed] [Google Scholar]
- 6.Young F, Critchley J A, Johnstone L K et al. A review of co-morbidity between infectious and chronic disease in sub-Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome and the impact of globalization. Global Health. 2009;5:9. doi: 10.1186/1744-8603-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker M A, Harries A D, Jeon C Y et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries A D, Kumar A M V, Satyanarayana S et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis. 2015;19:879–886. doi: 10.5588/ijtld.15.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley K E, Chaisson R E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher-Hoch S P, Whitney E, McCormick J B et al. Type 2 diabetes and multi-drug-resistant tuberculosis. Scand J Infect Dis. 2008;40:888–893. doi: 10.1080/00365540802342372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Li L, Mi F et al. Screening patients with diabetes mellitus for tuberculosis in China. Trop Med Int Health. 2012;17:1302–1308. doi: 10.1111/j.1365-3156.2012.03069.x. [DOI] [PubMed] [Google Scholar]
- 12.Dave P, Shah A, Chauhan M et al. Screening patients with tuberculosis for diabetes mellitus in Gujarat, India. Public Health Action. 2013;3(Suppl):S29–S33. doi: 10.5588/pha.13.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimbabwe National Statistics Agency Zimbabwe Population Census. Harare, Zimbabwe: ZIMSTAT; 2012. [Google Scholar]
- 14.Health Department, Zimbabwe City of Harare Progress Report on HIV/PMTCT indicators. Harare, Zimbabwe: Health Department; 2014. [Google Scholar]
- 15.Ministry of Health and Child Care National Tuberculosis Control Programme 2017 Annual Report. Harare, Zimbabwe: Ministry of Health and Child Care; 2018. [Google Scholar]
- 16.City of Harare 2017 Annual Report. Harare, Zimbabwe: City of Harare Health Department; 2018. [Google Scholar]
- 17.von Elm E, Altman D G, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS Med. 2007;4 doi: 10.1371/journal.pmed.0040296. e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workneh M H, Bjune G A, Yimer S A. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0147621. e0147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faurholt-Jepsen D, Range N, PrayGod G et al. Diabetes is a risk factor for pulmonary tuberculosis: a case-control study from Mwanza, Tanzania. PLOS ONE. 2011;6 doi: 10.1371/journal.pone.0024215. e24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogbera A O, Kapur A, Chinenye S et al. Undiagnosed diabetes mellitus in tuberculosis: a Lagos report. Indian J Endocrinol Metab. 2014;18:475. doi: 10.4103/2230-8210.137488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Rifai R H, Pearson F, Critchley J A et al. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLOS ONE. 2017;12 doi: 10.1371/journal.pone.0187967. e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Jain DC, Gupta D et al. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18:636–645. doi: 10.1111/tmi.12084. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Lin Y, Mi F et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health. 2012;17:1294–1301. doi: 10.1111/j.1365-3156.2012.03068.x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization & Stop TB Partnership The Global Plan to Stop TB 2011–2015. Geneva, Switzerland: WHO; 2010. WHO/HTM/STB/2010.2. [Google Scholar]
- 25.World Health Organization & International Diabetes Federation Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Geneva, Switzerland: WHO & IDF; 2006. www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ Accessed May 2019. [Google Scholar]
- 26.World Health Organization Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Geneva, Switzerland: WHO; 2011. WHO/NMH/CHP/CPM/11.1. http://www.who.int/cardiovascular_diseases/report-hba1c_2011_edited.pdf Accessed May 2019. [PubMed] [Google Scholar]
- 27.Boillat-Blanco N, Ramaiya K L, Mganga M et al. Transient hyperglycemia in patients with tuberculosis in Tanzania: implications for diabetes screening algorithms. J Infect Dis. 2016;213:1163–1172. doi: 10.1093/infdis/jiv568. [DOI] [PubMed] [Google Scholar]


