Abstract
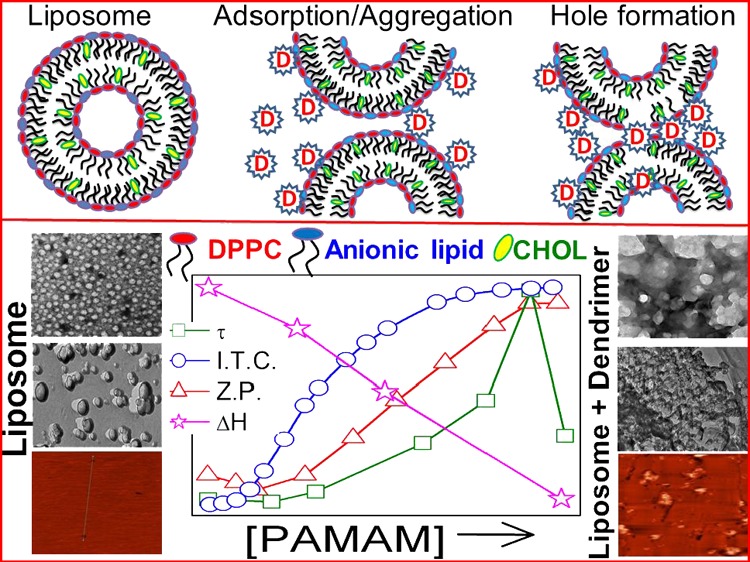
Interaction between negatively charged liposomes and cationic polyamidoamine dendrimers of different generations was investigated through size, zeta potential, turbidity, electron microscopy, atomic force microscopy, fluorescence spectroscopy, and calorimetric studies. Liposomes with the binary combination of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) + dihexadecyl phosphate, DPPC + 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol, DPPC + 1,2-dipalmitoyl-sn-glycero-3-phosphate, and DPPC + 1,2-dipalmitoyl-sn-glycero-3-phosphoethanol were stable up to 60 days. The electrostatic nature of dendrimer–lipid bilayer interaction was evidenced through charge neutralization and subsequent reversal upon added dendrimer to liposome. Dendrimer–liposome interaction depended on its generation (5 > 4 > 3) in addition to the charge, head groups, and hydrocarbon chain length of lipids. Fluorescence anisotropy and differential scanning calorimetry studies suggest the fluidization of the bilayer, although the surface rigidity was enhanced by the added dendrimers. Thermodynamic parameters of the interaction processes were evaluated by isothermal titration and differential scanning calorimetric studies. The binding processes were exothermic in nature. The enthalpy of transition of the chain melting of lipids decreased systematically with increasing dendrimer concentration and generation. Dendrimer–liposome aggregates were nontoxic to healthy human blood cell, suggesting the potential of such aggregates as drug delivery systems.
1. Introduction
All living cells have membrane bilayers; besides the subcellular components such as mitochondria and Golgi bodies also possess the lipid bilayer structures.1 Because of their easy manifestation and ready flexibility in nature, liposomes have received substantial considerations because of the simplified version of cell membrane. Physicochemistry of liposomes depends on its constituents (head group charge, head group moiety, and hydrocarbon chain length), intra-/intermolecular interactions, and the environmental conditions.2,3 Thus, biophysical correlates on the composition, function, and structure of membrane bilayer structure in understanding the function of cell membranes are considered to be relevant. Reports on the interaction studies between dendrimers and liposomes are fragmentary in nature; no comprehensive and systematic studies have been done previously to assess the impact of dendrimer generation, concentration, and the variation of the liposome type.
Dendrimers are synthetically prepared hyperbranched macromolecules with huge number of active termini that describe their properties and functions.4,5 Because of perfect branching, dendrimers have the maximum number of terminal functionalities among any polymeric material at a given molecular weight and are perfectly monodispersed. Dendrimers, compared to the corresponding linear polymers, possess architectural advantages in terms of drug delivery: (i) reproducible pharmacokinetic behavior (having monodispersity);10 (ii) globular shape (provides superior biological and rheological properties);6,7 and (iii) controlled multivalency that can attach several molecules such as drugs, imaging agents, cell-penetrating peptides, targeting groups, and solubilizing moieties.4,8−10
Natural cell membranes carry the overall negative charge with a bland of lipids. However, the preference of a mixture of lipids over the individual components (where lipids mixtures exhibit superior performance than single components) by nature is still not completely understood. It is, therefore, worthwhile to investigate the physicochemistry of lipid mixtures whereby the anionic components could be varied. 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) is a naturally occurring zwitterionic phospholipid found in pulmonary surfactant. It can achieve high surface pressure upon compression at the air–liquid interface; however, being solid in nature, it is unable to get fluidized by its own upon expansion. To maintain parity in terms of charge, 30 mol % phosphatidyl glycerol is present in the pulmonary surfactant. Such a combination of lipid mixtures is also capable of forming liposomes with a net negative charge. With the intention to mimic these combinations, we have formulated a variety of liposomes, whereby 30 mol % anionic lipids were separately used in combination with DPPC. The anionic lipids used herein include dihexadecyl phosphate (DHP), 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG), 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPP), and 1,2-dipalmitoyl-sn-glycero-3-phospho ethanol (DPPEth). DHP lipid is different from other phospholipids even though it can form stable liposome.11,12 Phosphatidyl alcohols are highly potent promoters of membrane curvature, and their transbilayer movement is three times higher than any naturally occurring phospholipid at physiological pH.2,13 Although the aforementioned anionic lipids (except DMPG) are not directly relevant to biological cell membranes, however, it is expected that such a combination of lipids in the form of liposome can be explored as antimicrobial drug delivery systems (DDSs),14 if they are used in combination with dendrimers.11,15,16 Cholesterol, another component of cell membrane, modulates its fluidity/rigidity. Cholesterol (30 mol %) was used for each combination along with the other lipids in the present set of studies. It is known that the most active compounds (drug molecule) cannot attain therapeutical efficacy because of their inability to reach the target side by crossing the cell membrane barrier. To overcome this constraint, the interactions between dendrimers with liposomes are carried out. The present sets of liposomes with negative charge are expected to be biocompatible. Here, the liposomes are expected to act as a drug carrier or, more generally, as a platform for theranostics.11,15,16
Disruption of membrane bilayer with added linear or coiled polymer peptides or detergents occurs through interfacial adsorption on the membrane.17−19 Because of the differences in the type and nature of interaction, studies involving dendrimer–lipid bilayers are in the ascendance. Oppositely charged dendrimers can create holes in the lipid bilayer or can be incorporated into lipid aggregates.20,21 Charge and size mainly govern the strength of dendrimer function.22 Positively charged dendrimers interact more effectively with cell membranes or other model bilayer carrying net negative charge because of obvious reasons. Since the first successful production of poly(amidoamine) (PAMAM) dendrimer in the mid-1980s,23,24 dendrimers have received significant attention. In particular, biomedical applications of dendrimers in various medical and pharmaceutical fields include drug delivery, gene transfection, nanoscale drug, and diagnostic tools. Such systems have been regarded as highly promising and have drawn eminent interest and studies. The possibility to introduce several functionalities has opened the door for their applications in theranostics. Dendrimers usually cross cell membrane barriers by endocytosis; thus, they are entrapped in endosomes.1,25 The translocation mediated by PAMAM dendrimers in combination with liposomes are considered as promising drug delivery systems (DDSs). Although the mechanism of spontaneous translocation of dendrimers through the bilayers are yet not properly understood; such studies can motivate changes in the bilayer structure, which need to be taken into account in designing the DDSs.23,24 The phenomenon of dendrimer-mediated cell membrane crossing needs the understanding of its interactions with lipid bilayers. Liposomes are the ideal model systems because of their simple and membrane-like arrangement, easy preparation, biocompatibility, biodegradability, and satisfactory stability above time.4,26 Some dendrimers can interact with lipids through hydrophobic interactions that exist between the lipid acyl chains and the hydrophobic dendrimer interior. Strength of the interaction also depends on the size and head group of the lipid molecule.11,12,27
Adsorption of dendrimer on liposome surface and the formation of dendrimer–liposome aggregates are some of the most common aspects of dendrimer–liposome interaction. Dendrimers can act as “glue” for oppositely charged liposomes.28 Most of the studies have focused on the interactions between positively charged dendrimers and cell membranes because greater interaction potency is expected in comparison with other neutral or negatively charged dendrimers. Physicochemical properties of dendrimers influencing the dendrimer–lipid bilayer interaction include the dendrimer type, generation, and surface charge as well as the composition of the lipid bilayer. Prevalence of electrostatic interaction can be evaluated through the zeta potential (Z.P.) measurements. Calorimetric studies on liposome and dendrimers can lead to the evaluation of thermodynamic parameters of the interaction process such as the chain melting temperature (Tm), width of the chain melting peak (T1/2), enthalpy change of the chain melting process (ΔH), heat capacity change (ΔCP), and binding constant (K). Such studies can shed light on the effect of the PAMAM dendrimer–liposome aggregates. Size, turbidity, electron microscopy [transmission electron microscopy (TEM)/freeze-fracture TEM (FF-TEM)/cryogenic TEM], atomic force microscopy (AFM), differential scanning calorimetry (DSC), fluorescence spectroscopy, and isothermal titration calorimetry (ITC) measurements are the effective tools to elucidate the dendrimer–liposome interactions to elaborate the design of new DDSs that consist of dendrimers incorporating bioactive molecules.
Different modes of interactions between dendrimers and liposomes are reported in the literature. The talent of PAMAM dendrimers in forming aggregates with liposomes carrying a net negative charge is expected to enlighten how dendrimers act as drug delivery vehicles across the cell membrane. Dendrimers can either pass through the lipid bilayer or form dendrimer–lipid aggregates. Some dendrimers can interact with lipids by hydrophobic interactions between the lipid acyl chains and the hydrophobic dendrimer interior. The strength of the interaction mainly depends on the size and charge of the molecule. Ultimately, the formation of dendrimer–liposome aggregates or complex will be used as new DDSs.
2. Results and Discussion
2.1. DLS and Turbidity Studies
2.1.1. Characterization of Liposomes
Hydrodynamic diameter (dh), polydispersity index (PDI), and Z.P. of four different liposomes (DHP + DPPC), (DMPG + DPPC), (DPP + DPPC), and (DPPEth + DPPC) were measured at pH 7.4 as a function of time by dynamic light scattering. DHP, DMPG, DPP, and DPPEth are all negatively charged phospholipids, whereas DPPC is zwitterionic with the phosphocholine head group. The formation of stable liposome depends on the hydrophobicity of the chain and the hydrophilic nature of the lipid head group. In the case of DPPC, the quaternary ammonium group showed a hydrophilic nature, and palmitoyl group is hydrophobic in nature for the bilayer formation. Data for DPPEth + DPPC are shown in Figure 1 whereas some other results are shown in Figure S1 (Supporting Information section). Initial constriction in size, followed by its increase with increasing time (up to 5–18 days), were due to the structural reorganization of the lipidic components.37 The size remained almost constant up to 60 days for all of the systems, indicating their substantial stability.
Figure 1.
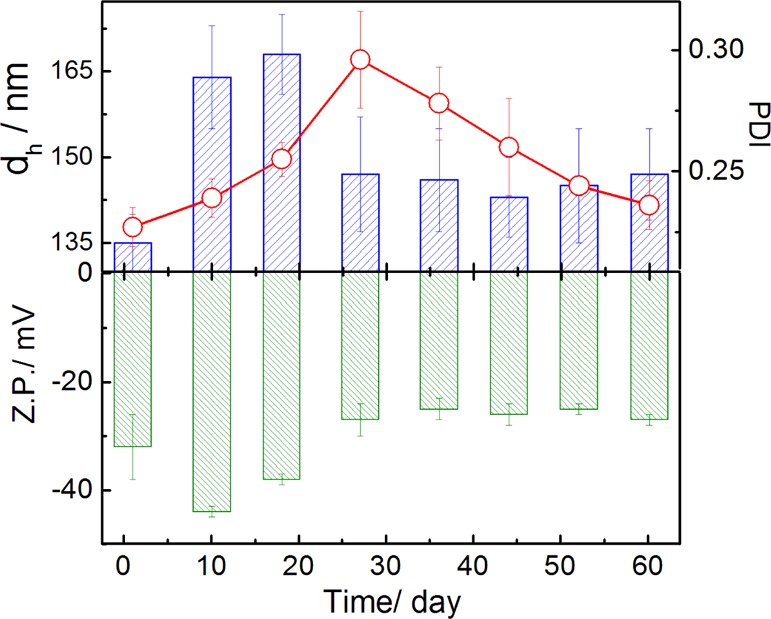
Variation in the hydrodynamic diameter (dh), PDI (line graphs), and Z.P. of DPPETh + DPPC liposomes with time. Cholesterol (30 mol %) was used in each case. DPPEth/DPPC lipid ratio is 3:7 (M/M). Line graph indicates PDI values. Temperature: 25 °C. Phospholipid concentration: 0.1 mM.
Lower PDI values support monodispersity of the formulations.30,38 The size of the liposome depends on the lateral packing of lipid molecules within the membrane bilayer and is mainly governed by the van der Waals interactions between the hydrocarbon chains.4,12dh values for DHP + DPPC liposome, higher than other liposomes (DMPG + DPPC, DPP + DPPC, and DPPEth + DPPC), are due to the fact that the fraction of the head group region of higher density decreases with increasing hydrocarbon-chain length.39,40 DHP has two hexadecyl groups which are directly connected with the phosphate group. Thus, the hydrophobicity of DHP lipid is higher than the other lipids and showed a maximum size in DHP + DPPC liposome. DMPG differs from other lipids in their head group and tail group. In case of DMPG, it contains lesser number of (14, myristoyl) methylene hydrocarbon chains than other phospholipids (16, palmitoyl and hexadecyl groups). Thus, the hydrophobicity of the tail group is lower than other lipids. On the other hand, because of presence of glycerol moiety in the head group, it forms stronger hydrogen bonding with neighbor molecules in aqueous solution. Thus, the size of DMPG + DPPC liposome is lower than other systems. This may be explained as a consequence of the orientation of polar head groups to compensate the close packing imposed by the lateral stronger van der Waals interactions of the acyl hydrocarbon chains. The PDI values follow the sequence DPPEth + DPPC > DMPG + DPPC > DPP + DPPC > DHP + DPPC.
The phosphate group in DHP is directly connected to the long hydrocarbon chains, which strongly pushes the electron toward the phosphate group and enhances the electron density. Hence, the Z.P. of DHP + DPPC liposome is higher than other systems. In case of DPP and DPPEth lipids, with the same kind of dipalmitoyl groups, unlike DPPEth (phosphatidyl alcohol), where the phosphate group is directly connected to −CH2CH3 group, it generates greater positive inductive effect. Hence, the Z.P. of DPPEth + DPPC liposome is higher than that of DPP + DPPC liposome but not higher than DHP. On the other hand, DMPG has two myristoyl groups and also the phosphate group directly connected with a glycerol moiety.39,41 At the same time, the hydroxyl group of glycerol moiety forms hydrogen bond with the phosphate group of DPPC or neighboring DMPG moiety in aqueous medium. Effectively, the enhancement of electron density on the phosphate group in DMPG is less pronounced than the other systems; hence, the magnitude of the Z.P. is lower in the case of DMPG + DPPC liposome. The Z.P. for different liposome follows the sequence DHP + DPPC > DPPEth + DPPC > DPP + DPPC > DMPG + DPPC (Figures 1 and S1).
2.2. Impact of Dendrimer on Liposome
Interaction between dendrimers and liposomes can be assessed by the turbidity measurements. Figure 2 (panel A) shows the concentration effect of different-generation PAMAM dendrimers on the turbidity (τ), size (dh), and Z.P. of (DPPEth + DPPC) liposome as a representative. Turbidity values of the dendrimer–liposome complexes pass through maxima, likewise the size after a threshold dendrimer concentration.32 Initial size or turbidity increment and the attainment of maxima are due to the aggregation of liposomes assisted by dendrimers.32 Dendrimers, being oppositely charged, adsorb on to the liposome surface.27 The decrease in size and turbidity upon further addition of dendrimers was due to the formation of water-soluble dendrimer–liposome aggregates.28,32 The ability in imparting turbidity or size enhancement depends on the dendrimer generation. PAMAM dendrimers follow the order 5G > 4G > 3G while considering the size and turbidity variation. There are 128, 64, and 32 end groups in the 5G, 4G, and 3G PAMAM dendrimers, respectively. With increasing dendrimer generation, the number of end groups increases and hence lesser number of dendrimers are required for an effective interaction. It is, therefore, reasonable to consider the dendrimer activity in terms of the end groups’ concentration, as shown in Figure S2 (Supporting Information section). The activity of dendrimer was independent of dendrimer generation while the end groups were taken into account. Earlier reports reveal that the activities follow the opposite trend Glower generation > Ghigher generation.32 The lower the generation, the higher number of end groups become accessible for effective interactions. With increasing dendrimer generation, the end groups tend to back fold.32 Thus, for higher generation dendrimers, lesser number of end groups could effectively participate in the interaction process.
Figure 2.
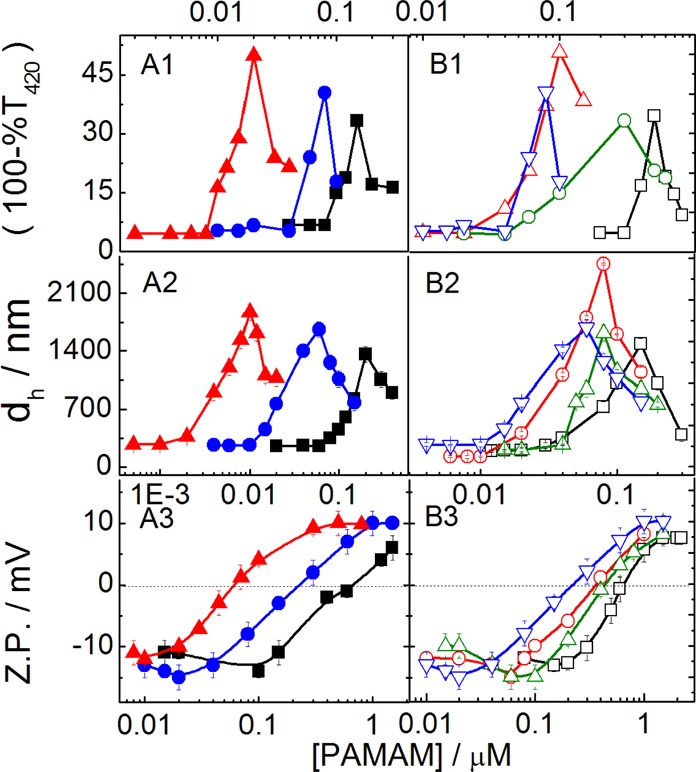
Effect of PAMAM dendrimer generation: (■, 3G; ●, 4G and ▲, 5G) on the turbidity (A1), size (dh, A2), and Z.P. (A3) of (DPPEth + DPPC) liposome and the effect of 4G dendrimer on the turbidity (B1), size (B2), and Z.P. (B3) of different liposomes (□, DHP + DPPC; ○, DMPG + DPPC; △, DPP + DPPC; and ▽, DPPEth + DPPC). Cholesterol (30 mol %) was used in each case. Phospholipid concentration: 0.1 mM.
In addition to the dendrimer generation, the extent of interaction also depended on the lipid composition. Dendrimer–liposome complex is stable because of strong electrostatic interactions and hydrogen bonding between amine end groups of dendrimer and the phosphate moiety of the anionic lipids.3,42 As DHP shows higher negative Z.P., it requires higher amount of dendrimer in achieving the threshold concentration. Thus, the interaction between dendrimer and DHP + DPPC liposome was less sensitive than other systems. Although considering the head groups’ bulkier nature with respect to palmitoyl group for each lipids (except DHP, dihexadecyl group), the size of the group follows the order DMPG > DPPEth > DPP [DMPG, −CH2CH(OH)CH2OH; DPP, −H and DPPEth, −CH2CH3]. Although initially DMPG + DPPC liposome was smaller than the other systems, it’s size increased with increasing the dendrimer concentration significantly, higher than the other liposomes because of stronger hydrogen bonding of a glycerol group with positively charged PAMAM dendrimers and glycerol moiety or phosphate group of DMPG.2,39,43 Thus, the DMPG + DPPC liposome displays stronger interactions with dendrimers than other liposomes. On the other hand, DPPEth comprising liposomes was more sensitive toward dendrimer than other liposomes because of the presence of −CH2CH3 group, which is directly connected with the phosphate group.2 Interactions between the negatively charged liposome surface and positively charged dendrimers were further explored by Z.P. measurements. Representative results are summarized in panel A3 and B3 of Figure 2. Magnitude of the negative Z.P. decreased with the dendrimer concentration, and there occurred charge reversal upon further addition of dendrimers.32,44 Further increase in Z.P. toward the positive direction suggests the formation of nonstoichiometric aggregates, indicating the saturation point. The post-stoichiometric aggregation is governed by hydrogen bonding and/or hydrophobic interaction induced by amine groups. It was observed that the other liposome combinations also interacted strongly with the dendrimers, ascertained by the higher slopes of Z.P.–dendrimer concentration profile that required lesser amount of dendrimers.11,12 However, when the dendrimer activities were expressed in terms of total end group concentrations, the pattern became opposite (Figure S2).
Results suggest that all of the end groups of dendrimers could not effectively take part in the interaction process. End groups of higher generation dendrimer can backfold. DPPEth + DPPC liposome was more sensitive toward dendrimers than the other liposomal formulations, which could better be explained by further experiments, as described later.
2.3. Morphological Analyses (TEM, FF-TEM, and AFM)
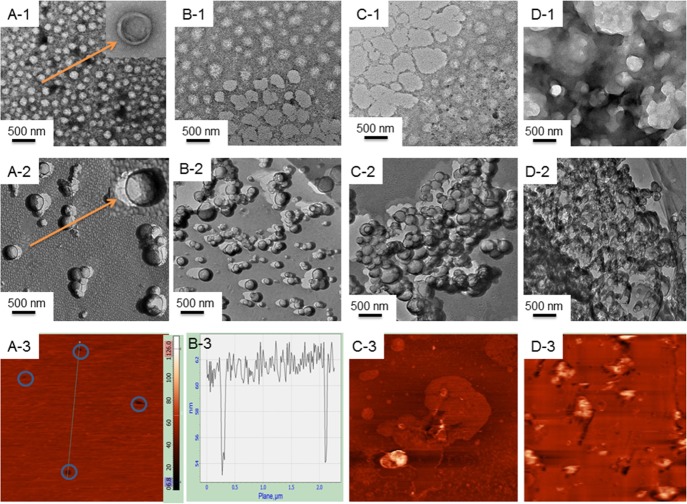
Spherical morphology with smooth surfaces was observed for all of the liposomes; sizes were comparable to the DLS data, as shown in Figure 3 (panel A1). Conventional TEM analysis is associated with the drying of samples which may alter the size and morphology. To double check the morphological information, FF-TEM studies were performed. While considering the impact of dendrimers, it was observed that with increasing concentration of PAMAM, liposome surfaces lost its homogeneity with the ultimate formation of aggregated heterogeneous entities. PDI values, as determined by DLS, also support this proposition; PDI values of liposomes increased nonsystematically with increasing dendrimer concentration (Figure S3). It is known that the oppositely charged dendrimer can act as glue to liposomes.28 The effect of dendrimer on the structure of solid-supported (mica substrate) lipid bilayer was further investigated by AFM studies. Results are shown in the bottom panels of Figure 3. Height of the solid supported bilayer was in the range 5–6 nm, as also reported by others.12,30,40 The existence of holes in the solid-supported lipid bilayer (Figure 3, panel A3) is a natural phenomenon, which probably is responsible in the transport processes. At lower dendrimer concentration, white patches with higher height profiles were noticed (panel C3 of Figure 3), which were due to the preferential adsorption of positively charged PAMAM dendrimers on the mica and/or the edge of the bilayer holes. At lower dendrimer concentrations, homogeneity of the membrane bilayer was significantly perturbed (panel D3); holes were expanded because of the disruption of bilayer through the formation of water-soluble dendrimer–lipid aggregates. These results further support the proposition of the formation of dendrimer–liposome complexes that are discussed in the DLS studies.
Figure 3.
Effect of 4G PAMAM on the (DPPC + DPPEth) bilayer. (A) No dendrimer; (B) 10 nmol dm–3 4G PAMAM; (C) 100 nmol dm–3 4G PAMAM, and (D) 500 nmol dm–3 4G PAMAM [images: (1) TEM; (2) FF-TEM, and (3) AFM]. Panel (B3) height analysis for bilayer thickness. Cholesterol (30 mol %) was used in each case. Phospholipid concentration: 0.1 mM.
2.4. Steady-State Fluorescence Anisotropy and Lifetime Analyses
State of polarity of a liposome surface and packing of bilayer are other two important parameters while considering the dendrimer–liposome interaction. Solvatochromic dye 7-hydroxycoumarin (7-HC) was used as the molecular probe to understand the state of polarity as well as the rigidity/fluidity of the palisade layer of the liposome and the effect of dendrimers. Additionally, the hydrophobic probe 6-diphenyl-1,3,5-hexatriene (DPH) was used to understand the bilayer packing of dendrimer–liposome complexes. Fluorescence anisotropy value of 7-HC-loaded liposomes was lower (DHP + DPPC, 0.048; DMPG + DPPC, 0.125; DPP + DPPC, 0.145; and DPPEth + DPPC, 0.093) than DPH-loaded liposomes (DHP + DPPC, 0.078; DMPG + DPPC, 0.155; DPP + DPPC, 0.175; and DPPEth + DPPC, 0.123). DPH, being completely hydrophobic, resides inside the bilayer unlike 7-HC that resides on the palisade layer. Increase in fluorescence anisotropy of 7-HC with increasing dendrimer concentration was recorded (Figure 4, panel A and B). Dendrimers led to significant changes in the fluorescence anisotropy of 7-HC. Its increase was due to the adsorption of dendrimer liposome surface; further, addition of dendrimers led to mild downshift probably because of the formation of holes in the bilayer, reflecting the fact that some dendrimers probably crossed through the liposome bilayer,20,21 which was further reported, by using DPH as the molecular probe. DPH, being completely hydrophobic, will preferentially reside within the lipid acyl chain with a parallel orientation that results in the increase in the anisotropy value. Results, as summarized in Figure 4 (panel C and D), suggest that the membrane fluidity decreases with increasing concentration of dendrimer. These fluorescent probes monitor interactions between the external and internal regions of the membrane with dendrimers. The change in fluorescence anisotropy with increasing dendrimer concentration indicates alterations in membrane fluidity.45 Significant changes in fluorescence anisotropy were also observed with the variation of the dendrimer generation shown (Figure 4, panel A and C). It was observed that the extent of interaction between the dendrimers of 3G and 4G with liposome was less than 5G because of the formation of rigid bilayer.20,41 Higher generation of dendrimers having larger number of end groups could effectively interact with greater magnitude with the liposome surface.
Figure 4.
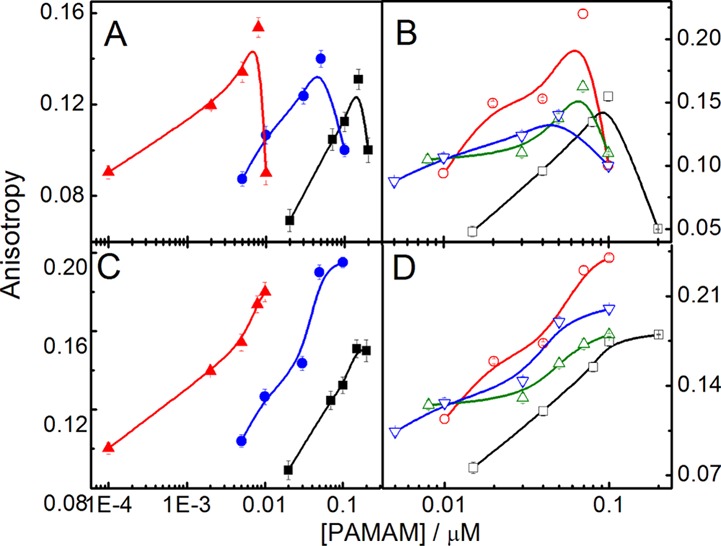
Effect of PAMAM dendrimer generation (3G, ■; 4G, ● and 5G, ▲) on the fluorescence anisotropy of 7-HC (panel A) and DPH (panel C) in DPPEth + DPPC liposome. Effect of 4G PAMAM dendrimer on the anisotropy values of 7-HC (panel B)- and DPH (panel D)-loaded liposomes (DHP + DPPC, □; DMPG + DPPC, ○; DPP + DPPC, △; and DPPEth + DPPC, ▽). Cholesterol (30 mol %) was used in each case. Phospholipid concentration: 0.1 mM.
Time-resolved fluorescence decay studies of 7-HC were carried out to further understand the emission decay parameters.46 Fluorescence lifetime measures the duration of the excited state of a probe. It can also study the interaction phenomena as it can provide information on the change in the binding environment of the fluorophores. Figure S4 in panel C (Supporting Information section) explains the variation in the excited state lifetime (τ) in liposomes with dendrimer concentrations. Fluorescence lifetime of 7-HC was 5.01, 5.65, 5.20, and 5.38 ns for DHP + DPPC, DMPG + DPPC, DPP + DPPC, and DPPEth + DPPC, respectively. Initially, with the progressive addition of dendrimer, fluorescence life time increased, which finally attained constancy. Rotational diffusion of 7-HC decreased with increasing dendrimer concentration because of the formation of stable dendrimer–liposome complexes that led to an overall increase in the viscosity of the medium, as also revealed from the anisotropy studies. The lifetime did not change significantly with the variation of dendrimer generation.
2.5. DSC Studies
DSC studies were carried out to understand the thermal behavior of dendrimer–liposome interaction process.45 DPPC, DPP, and DPPEth contain two palmitoyl chains, whereas DMPG has two myristoyl chains and DHP contains two hexadecyl hydrocarbon side chains.41,47 Lipids with lower hydrocarbon chain length are expected to have lower pretransition and main transition temperature (Tm).41,48 DMPG shows the main phase-transition temperature at 23.9 °C, whereas for DPPC, it was 41.4 °C. For the DMPG + DPPC liposome, the chain melting temperature was 25.5 °C, as shown in Figure S5 (Supporting Information section). DPP + DPPC and DPPEth + DPPC liposomes show the chain melting temperature at 44.7 and 42.0 °C, respectively, about the same as DPPC at 41.4 °C (as all of them have similar chain lengths). The chain melting temperature of DHP + DPPC liposome was 57.8 °C.49 However, the change in enthalpy of DMPG + DPPC liposome was found to be higher because of intra-/intermolecular hydrogen bonding (Table 1). Phospholipids are known to exist in two different mesomorphic phases; highly ordered gel phase and more disordered liquid crystalline fluid phase. Transition from the gel phase to the liquid-crystalline phase can be reached upon heating with increasing temperature; the intermolecular motion around C–C bonds, lateral and rotational diffusion among the lipid molecules also increase. The thermally induced transition of liposome can be affected by dendrimers (DSC peaks are shifted toward lower enthalpies) and this perturbation was found to be concentration-dependent. A downshift in the Tm values was recorded with increasing dendrimer concentration. Pretransition temperature is an approachable parameter for discovering the interactions between dendrimer and phospholipid bilayers. Even at low concentrations of dendrimer, the pretransition temperature was significantly affected. With increasing dendrimer concentration, the pretransition temperature of lipids declined and the main transition peak became lower and wider. Figure 5 shows that a higher concentration of dendrimer led to the abolition of DPPEth + DPPC lipid bilayer transition peak indicating its perturbation. A decrease in the pretransition temperature of the DPPEth + DPPC liposome (Figure 5) suggests the interaction between dendrimer and liposome, whereas alteration of the main transition peak suggests that dendrimers can generate holes. The bilayer can lose its homogeneity that depends on the dendrimer concentration.41 Interaction between dendrimers and bilayer occur mainly in the palisade region.21 With increasing dendrimer concentration, change in the enthalpy (ΔH) decreased (32.51–17.16 kcal·mol–1, for DPPEth + DPPC liposome), leading to the abolition of the peak indicating the bilayer disruption.50 Results on the DSC studies are summarized in Table 1 along with the other data. In the case of DMPG + DPPC, the carbonyl groups and glycerol backbone favor the hydrogen bonding between dendrimer and liposome that showed higher enthalpy values than other systems. In aqueous medium, the hydration of the head group increases its effective volume and decreases the orderedness of hydrocarbon chains. The increase in head group volume, induced by the dendrimers (through the formation of dendrimer lipid aggregates), creates energetically unfavorable voids in the hydrocarbon region of non-interdigitated membranes. Decrease in Tm and broadening of the transition peaks indicate the increased size of the DMPG + DPPC liposomes as a consequence of their interaction with PAMAM dendrimers (similar observation was found from the size measurement by DLS).41,50 Results further suggest that at higher dendrimer concentration, the lipid bilayer loses its homogeneity in a concentration-dependent manner (similar observation was found by PDI measurement as shown in Figure S3). With increasing dendrimer concentration, the membrane fluidity increases and the endothermic heat change decreases that lead to the lowering of the enthalpy changes of the chain-melting processes.
Table 1. Calorimetric Data for the Interaction of Dendrimer and Liposomea.
| DSC | |||||
|---|---|---|---|---|---|
| liposome | liposome–4G dendrimer | Tm/°C | ΔT1/2/°C | ΔH/kcal·mol–1 | ΔCP/kcal·mol–1 C–1 |
| DHP + DPPC + CHOL | only liposome | 58.10 | 6.60 | 32.11 | 8.12 |
| 1:0.0001 | 57.37 | 9.51 | 26.45 | 4.64 | |
| 1:0.001 | 56.65 | 13.20 | 20.11 | 2.54 | |
| 1:0.005 | 55.90 | 15.77 | 10.12 | 1.10 | |
| DMPG + DPPC + CHOL | only liposome | 25.55 | 5.97 | 50.22 | 14.76 |
| 1:0.0001 | 24.91 | 6.66 | 45.11 | 11.88 | |
| 1:0.001 | 24.29 | 7.60 | 40.21 | 9.28 | |
| 1:0.005 | 23.10 | 7.35 | 30.21 | 7.21 | |
| DPP + DPPC + CHOL | only liposome | 44.72 | 11.10 | 29.67 | 4.68 |
| 1:0.0001 | 43.80 | 12.97 | 27.24 | 3.67 | |
| 1:0.001 | 42.84 | 13.30 | 19.11 | 2.51 | |
| 1:0.005 | 42.20 | 14.90 | 7.48 | 0.87 | |
| DPPEth + DPPC + CHOL | only liposome | 42.56 | 2.54 | 32.51 | 22.61 |
| 1:0.0001 | 42.00 | 3.96 | 29.53 | 13.14 | |
| 1:0.001 | 40.78 | 4.91 | 24.96 | 8.97 | |
| 1:0.005 | 40.17 | 5.70 | 17.16 | 5.30 | |
| ITC | ||||
|---|---|---|---|---|
| dendrimer | K/mol–1 × 10–6 | 10–4 × ΔH/kcal·mol–1 | ΔS/kcal·mol–1·C–1 | |
| DHP + DPPC + CHOL | 3G | 9.6 | –111 | –11.40 |
| 4G | 8.1 | –197 | –34.50 | |
| 5G | 7.6 | –219 | –41.90 | |
| DMPG + DPPC + CHOL | 3G | 15.0 | –300 | –71.80 |
| 4G | 8.0 | –417 | –111.00 | |
| 5G | 6.6 | –513 | –141.00 | |
| DPP + DPPC + CHOL | 3G | 12.0 | –221 | –41.00 |
| 4G | 4.2 | –181 | –26.00 | |
| 5G | 1.5 | –300 | –67.90 | |
| DPPEth + DPPC + CHOL | 3G | 12.0 | –225 | –42.90 |
| 4G | 4.2 | –290 | –67.20 | |
| 5G | 1.1 | –454 | –120.00 | |
Tm is chain-melting temperature; ΔT1/2 is the peak width; ΔH is the enthalpy change; ΔCP is the heat capacity change; K is binding constant; and ΔS is the entropy change.
Figure 5.
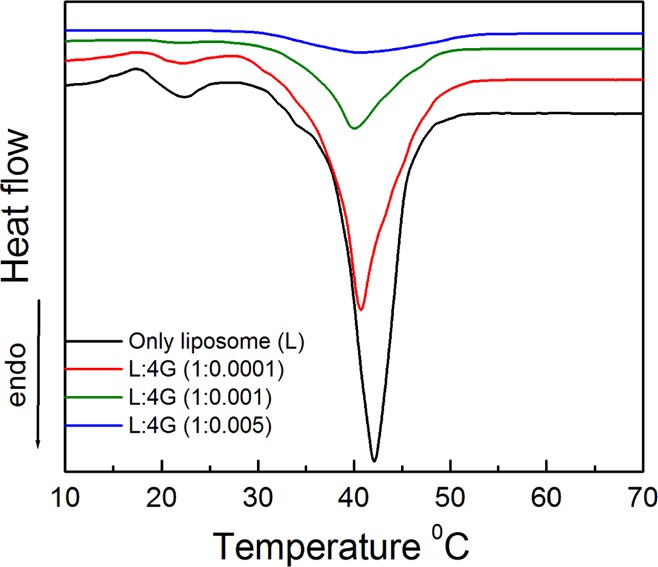
DSC thermogram of DPPEth + DPPC liposome at different concentrations of 4G PAMAM dendrimer. Liposome–4G PAMAM ratio (M/M) are mentioned inside the figure. Cholesterol (30 mol %) was used in each case.
2.6. Determination of Interaction Constant by Absorption Spectroscopy
Binding constant is another important parameter to quantify the extent of the interaction processes between the dendrimer and liposome. Concentration of dendrimer was varied in a liposome of fixed concentration, whereby the concentration of the free and bound (to liposome) dendrimer was estimated colorimetrically. Results are summarized in Table S1 (Supporting Information section). The binding tendency of dendrimer on liposomes follows the order DMPG + DPPC > DPP + DPPC ≈ DPPEth + DPPC > DHP + DPPC. Because of the formation of hydrogen bonding between dendrimer and hydroxyl group of DMPG, DMPG + DPPC liposome shows highest binding constant value than that of other systems. With increasing dendrimer generation, less binding affinity were found. Because, for higher the generation of dendrimer having higher number of end groups, it requires lesser amount of effective interaction. The binding constant study by this technique is less sensitive than other process because of lower concentration of dendrimer. To be acquainted with the details about binding phenomena, isothermal titration calorimetric titrations were done.
2.7. Isothermal Titration Calorimetric Studies
Binding properties of the dendrimer with liposome in phosphate buffer solution were explored by employing the ITC. Negatively charged liposomal dispersions were titrated by the dendrimers of different generations. The exothermic enthalpy changes related to the interaction reached a saturation plateau quite fast, as shown in Figure 6. The observed exothermic enthalpy changes include contributions from the binding of dendrimers by the lipid phosphate groups. The effect is clearly demonstrated by the titration experiments.11,12 The control experiments for the interaction of dendrimers with liposomes have also been carried out by diluting the dendrimers into phosphate-buffered saline (PBS) without having the liposomes. Upon subtraction of the calorimetric contribution from the control experiment, a single-site binding model was applied for the interaction between amino groups of the dendrimers with liposome phosphate groups, leading to ΔH. In this case, a significantly less binding enthalpy change was recorded during the titration (Figure 6), suggesting the weak binding of the dendrimers by the phosphate groups of the lipids.51 The much weaker interaction emerges because of the competition between the liposomal and the buffer phosphate groups.51 The apparent binding constants (K) are listed in Table 1. Because of the presence of the glycerol moiety of DMPG phospholipid liposome, the binding constant is higher than for other liposomes (reasons already mentioned earlier). Dendrimers get strongly absorbed onto the liposome surface through the combined electrostatic interactions and hydrogen bonding between amino groups of the dendrimers and hydroxyl groups of DMPG.51 With increasing dendrimer generation, the binding constant values decrease. Because of the presence of larger number of polymeric amino groups, higher generation of dendrimers interact effortlessly than that of lower generation of the dendrimer.
Figure 6.
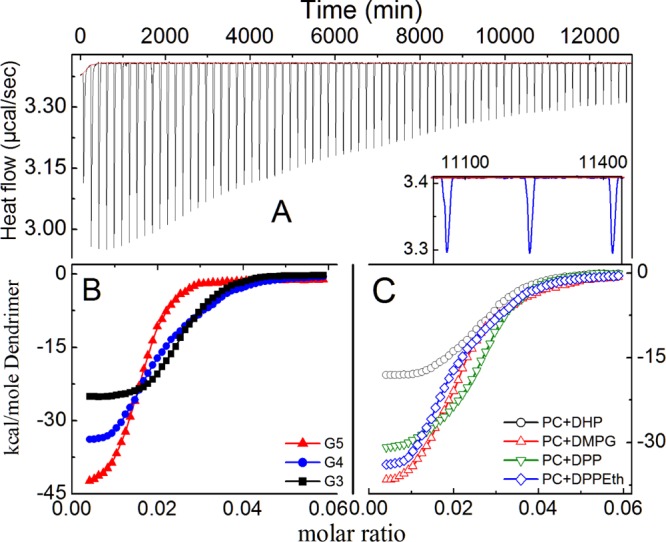
Isothermal titration calorimetric profile of dendrimer–liposome interaction process. Panel (A): raw ITC data of DPPEth + DPPC liposome–PAMAM 4G dendrimer; panel (B): effect of dendrimer generation on DPPEth + DPPC liposomes; and panel (C): effect of lipid variation on 4G PAMAM dendrimers. Cholesterol (30 mol %) was used in each case. Phospholipid concentration: 0.1 mM.
2.8. Cytotoxicity Studies
Understanding the mechanism of dendrimer–liposome interaction is important as the cytotoxicity issue is vital for acceptance and development of dendrimer–liposome aggregates as pharmaceutical agents. Cytotoxicity results obtained from 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay clearly demonstrate that all of the liposome formulations and dendrimer–liposome aggregates were completely nontoxic toward normal human blood lymphocyte. The nontoxicity of a bioactive compound is the most important requirement for its therapeutic application. The obtained results showed that the liposome and dendrimer–liposome aggregates were almost nontoxic and possessed no effect on cell viability, as shown in Figure 7. The nontoxic nature of dendrimer–liposome complexes was also found even with the change of dendrimer concentrations (Figure S6, Supporting Information section). Results indicate that the dendrimer–liposome aggregates could be considered as a good DDS. In PBS buffer solution, the components did not affect the ionic strength of the solution. Thus, the formulations could be considered safe in terms of drug delivery. However, further in vivo studies are warranted to make final conclusions on this issue. Moreover, the hemolysis results showed that all of the formulations of a liposome (0.1 mM) and dendrimer–liposome aggregates (liposome–dendrimer, M/M; 1:0.0001, 1:0.001, and 1:0.005) were nontoxic toward human RBCs with only <1.1% hemolysis, as shown in Figure S7 (Supporting Information section).52 It has been reported earlier that the materials with <5% hemolysis were regarded as hemocompatible.53 Thus, the dosage of dendrimer–liposome complexes (liposome–dendrimer, M/M; 1:0.0001, 1:0.001, and 1:0.005) were found to be hemocompatible. Hence, the formation of dendrimer–liposome aggregates enfolding by positively charged entity can act as a mimic to the biologically simulated systems.
Figure 7.
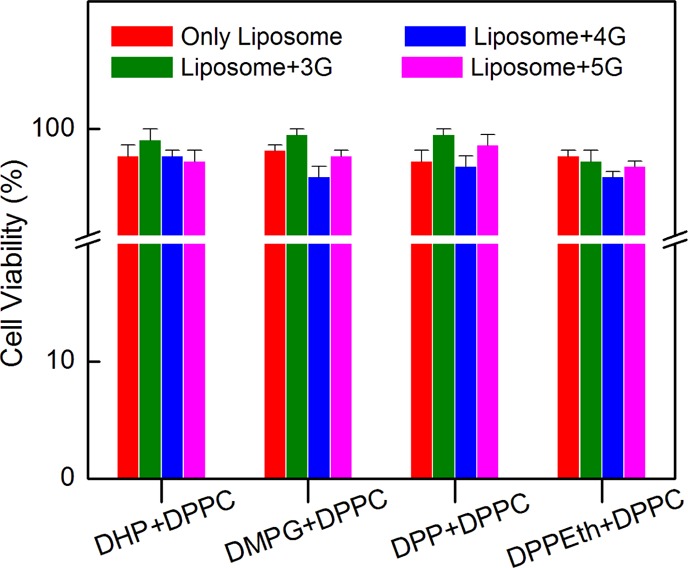
Dose response on various liposomes and dendrimer–liposome complexes on human blood cell lymphocyte. Liposome–dendrimer (1:0.001, M/M). Cholesterol (30 mol %) was used in each case. Phospholipid concentration: 0.1 mM.
3. Conclusions
The manuscript describes the interaction between negatively charged liposomes with cationic PAMAM dendrimer through assessing different biophysical properties of dendrimer–liposome aggregates. The type and strength of the interaction are dependent on the charge and size of the liposomes as well as the dendrimer generation. Larger size of DHP + DPPC, DPP + DPPC, and DPPEth + DPPC in the gel state than for DMPG + DPPC in the same state is rationalized through the lateral packing of lipid molecules within the membrane because of the stronger van der Waals interactions between the hydrocarbon chains. Z.P. of the liposomes depends on the charge and electron density of the phospholipid head group with an exception for DHP, and it is higher for DHP + DPPC liposome. All of the liposomes carry net negative charges, promoting the cationic dendrimers to interact with it electrostatically. Higher generation (5G) dendrimer causes greater perturbation in the lipid bilayer and thus could interact more effectively with liposomes. Formation of dendrimer–liposome aggregates at higher dendrimer concentration (liposome–dendrimer, 1:0.005, M/M) was also visualized through the TEM, FF-TEM, and AFM studies. Increase in the fluorescence anisotropy shows that the liposomal membranes attain rigidity at a particular dendrimer concentration, reflecting the fact that dendrimers could move across the liposome bilayer. DSC and fluorescence anisotropy studies showed that the dendrimers interact not only with the hydrophilic part of the membranes but also with the hydrocarbon chain. In the case of DMPG + DPPC, the carbonyl groups and glycerol backbone favor the hydrogen bonding interactions with dendrimers, and it shows higher enthalpy changes than other systems. Extent of binding during the formation of dendrimer–liposome complexes depends on the head group moiety of the lipids and the generation of the dendrimers. Cytotoxicity and hemolysis studies led to conclude that both liposomes and dendrimer–liposome complexes are nontoxic. In conclusion, it is clear that the exploration of the dendrimer–liposome aggregates (on a particular range of dendrimer–liposome ratio) as a potential drug carrier has significant prospects.
4. Experimental Section
4.1. Materials
DPPC, DHP, DMPG, DPP, DPPEth, 7-HC, DPH, and PAMAM dendrimer of different generations [generation 3 (3G), generation 4 (4G), and generation 5 (5G) as methanolic solutions] were the products from Sigma-Aldrich Chemicals Pvt. Ltd. (USA). AR grade disodium hydrogen phosphate (Na2HPO4·2H2O), sodium dihydrogen phosphate (NaH2PO4·2H2O), sodium chloride (NaCl), HPLC grade chloroform, and methanol were the products of Merck Specialties Pvt. Ltd, India. Double distilled water with a specific conductance of 2–4 μS (at 25 ○C) was used in preparing the solutions. All of the chemicals were stated to be ≥99.5% pure and were used as received.
4.2. Methods
4.2.1. Preparation of Liposome
Liposomes were prepared by the well-known thin-film hydration technique.29,30 Quantitative amount of lipids were dissolved in chloroform and methanol (C/M, 3:1, v/v) in a round-bottom flask. The solvent was removed by using a rotary evaporator at 40 °C. The trace amount of solvent was removed by using vacuum desiccator for 6 h. Then, 10 mM PBS was mixed and then hydrated for 1 h at 70 °C (temperature above the chain-melting temperature). Salinity was maintained at 100 mM using sodium chloride. Then, the systems were frozen at −20 °C and thawed followed by sonication at 45 °C. This procedure (freeze–thaw sonication) was continued up to four cycles to get small unilamellar liposomes. Liposomes (0.1 mM) were prepared separately using DHP + DPPC, DMPG + DPPC, DPP + DPPC, and DPPEth + DPPC along with 30 mol % cholesterol at pH 7.4 (anionic lipid/DPPC, 3:7 M/M). In case of dye (7-HC and DPH)-loaded liposomes, dyes were mixed along with the lipids in C/M before thin-film generation. Dispersions were filtered through 0.45 μm cellulose nitrate membrane filter before size and Z.P. measurements.
4.3. Instrumental Analyses
4.3.1. Turbidity, Size, and Z.P. Measurements
Dendrimer–liposome interaction was studied by measuring the % transmittance (% T) at 420 nm.31,32 At this wavelength, the turbidity (τ) of a solution is assumed to be proportional to (100 – % T). Measurements were done using a Cary 1E UV–visible spectrophotometer (UVD-2950, Labomed Inc., USA). Liposome solution without dendrimer was used as blank. Size and Z.P. of liposomes in combination with dendrimers were measured using a Zeta Sizer Nano (Malvern Instruments, U.K.). He–Ne laser emitting light at 632.8 nm was used. The size measurements were done using a quartz cell of 1.0 cm path length, whereas for Z.P., a different special kind of plastic cell was used.
4.3.2. Electron Microscopic Studies
A drop of dilute (10–4 M) liposome dispersion was placed on Formver carbon-coated 200 mesh copper grid and dried under air. It was then analyzed to obtain TEM images using Hitachi H-600 transmission electron microscope (Japan) operating at 80 kV. In the case of FF-TEM studies, a drop of the sample was placed onto the sample holders and frozen in liquid propane. FR-7000A (Hitachi High Technologies Ltd., Japan) was used at −150 °C for freeze fracturing. Samples were then replicated by evaporation using platinum carbon. The replica was placed on a 300 mesh copper grid, dried, and examined in a transmission electron microscope (H-7650, Hitachi High Technologies Ltd., Japan) with an accelerating voltage of 120 kV.
4.3.3. Atomic Force Microscopy
AFM images on a solid-supported bilayer were obtained using a Multi-mode Nanoscope III (Digital Instruments, Santa Barbara, CA, USA). Liposomal solution (100 μL) was placed on a 1 cm2 freshly cleaved mica incubated for an hour at 37 °C. Overload liposome was then smoothly washed off with 1 mmol·dm–3 NaCl solution. AFM images were taken in tapping mode by the use of a liquid cell. Silicon nitride cantilever with a spring constant of 0.06 N m–1 operating at a driving frequency of 7–9 kHz was used. Bilayer images were taken at altered resolution. Then, dendrimer solutions of preferred concentration were used to rinse the bilayer. After 30 min, yet again scanned to visualize the effect of dendrimers. Addition of dendrimer was done slowly and carefully so that the bilayer does not get disturbed.
4.3.4. Fluorescence Spectroscopy
Fluorescence anisotropy and lifetime measurements were carried out by a bench-top spectrofluorimeter (QuantaMaster-40, Photon Technology International Inc., NJ, USA). 7-HC and DPH, used as the probes, were excited at 321 and 350 nm, respectively. Data were recorded at the emission wavelength 451 nm (for 7-HC) and 422 nm (for DPH). Fluorescence anisotropy (r) was determined according to the equation33
| 1 |
where, IVV is the parallel polarized and IVH is the perpendicularly polarized fluorescence intensity, G = IHV/IHH is the monochromator grating correction factor. Felix Gx software was used to calculate the anisotropy value.
Fluorescence lifetime of 7-HC loaded in liposomes and dendrimer–liposome aggregates was determined with a HORIBA Jobin Yvon FluoroMax (HORIBA JobinYvon, UK) using the time-correlated single-photon counting technique. Scattering was measured by using a Ludox (colloidal dispersion of silica) solution; excitation was performed at 288 nm with a delta diode-C1 diode controller.
The experimental results of time-resolved fluorescence decay profiles, R(t), were estimated by inbuilt Horiba EZ time software unit according to the following expression equation.34
| 2 |
Here, n = number of distinct decay components and τi and αi are the excited-state fluorescence lifetimes and the pre-exponential factors related to the ith component, respectively.
4.3.5. Differential Scanning Calorimetry
DSC studies were carried out to understand the changes in the thermal properties of a lipid bilayer upon interaction with dendrimers, using a differential scanning calorimeter (DSC 1, STARe system, Mettler Toledo, Switzerland) with a scan rate of 2 °C/min. Quantitative amount of phospholipid mixture (7:3) with cholesterol was dissolved in chloroform–methanol (3:1) in a 40 μL Al pan and after that, the solvent was evaporated under a stream of nitrogen. It was then placed under vacuum to remove traces of solvent. Dendrimer in 10 mM phosphate buffer was added to the dry lipid film, and it was then hydrated at 70 °C. Samples were subsequently scanned by DSC in the temperature at 10–70 °C. A pan filled with dendrimer in buffer solution was used as a reference. The enthalpies and characteristic temperatures were calculated using Mettler-Toledo STARe software.
4.3.6. Isothermal Titration Calorimetry
ITC experiments were studied by using VP-ITC titration calorimeter (MicroCal, Northampton, MA). At first, the samples were degassed carefully. The liposomes were kept in a sample cell, and the dendrimers (ligand) were put in a syringe of volume of 300 μL. Total 75 injections were performed by 15 s each at a 3 min time interval. Per injection, 3 μL aliquots (ligand solution) were added sequentially in the sample cell. This order of sequence was maintained to ensure complete occupancy of the binding sites and titrating with the same ligand without removing the samples from the cell until the titration signal was essentially constant. The corresponding heat of dilution was deducted from the binding experiments prior to curve fitting. The titration experiments were repeated three times.
4.3.7. Binding Constant
Binding constant of the dendrimer–liposome aggregate was also determined colorimetrically. Different amounts of dendrimer solution were separately added to a fixed amount of liposome solution. After homogenization, the solutions were kept for 2 h in attaining the equilibrium. It was then centrifuged at 20 000 rpm for 1 h, whereby the dendrimer–liposome aggregate got sedimented. The supernatant, which contained the free dendrimer, was estimated colorimetrically using a UV–vis spectrophotometer (JASCO V-30, USA) at 282 nm. The corresponding liposome without dendrimer was used as a reference. Binding constant, K, is associated with the binding and unbinding reaction of the receptor (L) and ligand (D) molecules, which is formalized as
| 3 |
The reaction is characterized by the forward constant k1 and the backward rate constant k–1. Under equilibrium, the forward binding transition D + L → DL should be balanced by the backward unbinding transition DL → D + L. That is, k1[L][D] = k–1[LD], where [L], [D], and [LD] represent the concentration of unbound free receptor (liposome), the concentration of unbound free ligand (dendrimer), and the concentration of ligand–receptor complexes. The binding constant K is defined by
| 4 |
4.3.8. Cytotoxicity Analyses
The blood sample was collected from the healthy human subjects (n = 3) for the separation of lymphocytes, as described previously.35 Human blood (5.0 mL) was diluted with PBS (1:1) and layered onto Histopaque 1077, as described earlier.33 After the treatment with liposome and dendrimer–liposome aggregates, the peripheral blood mononuclear cell (PBMCs) (2 × 105 cells in each set) was washed with PBS (1×) for three times using centrifugation (2200 rpm for 3 min per wash) and was subjected to quantitative estimation for cytotoxicity by a nonradioactive, colorimetric assay systems using tetrazolium salt, MTT. The percentage of proliferation was calculated as described previously.33
4.3.9. Hemolysis Assay
The hemocompatibility of liposomes and dendrimer–liposome aggregates were determined in terms of the percent hemolysis36
| 5 |
where AS is the sample absorbance, AN is the absorbance of the negative control, and AP is the absorbance of the positive control.
All of the experiments, except the DSC studies, were carried out at ambient but controlled temperature.
Acknowledgments
The research fund provided by the Council of Scientific & Industrial Research (project no. 01(2533)/11/EMR-II), Government of India, New Delhi, is sincerely acknowledged. B.R. acknowledges the University Grants Commission (UGC BSR), Government of India, New Delhi, for research fellowship and the Department of Chemistry, University of North Bengal, Darjeeling, for providing laboratory facilities. A.K.P. sincerely acknowledges the support from the Indo-Russian Collaborative research project, funded by the Department of Science and Technology, Government of India (Project INT/RUS/RFBR/P-220), and the Russian Foundation of Basic Research (RFBR no. 15-53-45043_IND_a).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01187.
Results on the binding constant measured colorimetrically, dynamic light scattering studies, fluorescence spectroscopic studies, DSC studies, cytotoxicity study, and hemolysis assay techniques (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kitchens K. M.; Kolhatkar R. B.; Swaan P. W.; Ghandehari H. Endocytosis Inhibitors Prevent Poly(amidoamine) Dendrimer Internalization and Permeability across Caco-2 Cells. Mol. Pharm. 2008, 5, 364–369. 10.1021/mp700089s. [DOI] [PubMed] [Google Scholar]
- Jaikishan S.; Björkbom A.; Slotte J. P. Phosphatidyl alcohols: effect of head group size on domain forming properties and interactions with sterols. Biochim. Biophys. Acta 2010, 1798, 1615–1622. 10.1016/j.bbamem.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Morini M. A.; Sierra M. B.; Pedroni V. I.; Alarcon L. M.; Appignanesi G. A.; Disalvo E. A. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloids Surf., B 2015, 131, 54–58. 10.1016/j.colsurfb.2015.03.054. [DOI] [PubMed] [Google Scholar]
- Falanga A.; Tarallo R.; Carberry T.; Galdiero M.; Weck M.; Galdiero S. Elucidation of the interaction mechanism with liposomes of gH625-peptide functionalized dendrimers. PLoS ONE 2014, 9, 112128. 10.1371/journal.pone.0112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechet J. Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Sci. Inf. 1994, 263, 1710–1715. 10.1126/science.8134834. [DOI] [PubMed] [Google Scholar]
- Klajnert B.; Bryszewska M. Dendrimers: properties and applications. Acta Biochim. Pol. 2001, 48, 199–208. [PubMed] [Google Scholar]
- Nunez C. M.; Chiou B.-S.; Andrady A. L.; Khan S. A. Solution rheology of hyperbranched polyesters and their blends with linear polymers. Macromolecules 2000, 33, 1720–1726. 10.1021/ma991044z. [DOI] [Google Scholar]
- Lee C. C.; MacKay J. A.; Fréchet J. M. J.; Szoka F. C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- Liu M.; Fréchet J. M. J. Designing dendrimers for drug delivery. Pharm. Sci. Technol. 1999, 2, 393–401. 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- Qiu L. Y.; Bae Y. H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30. 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- Pantos A.; Tsiourvas D.; Nounesis G.; Paleos C. M. Interaction of Functional Dendrimers with Multilamellar Liposomes: Design of a Model System for Studying Drug Delivery. Langmuir 2005, 21, 7483–7490. 10.1021/la0510331. [DOI] [PubMed] [Google Scholar]
- Tsogas I.; Tsiourvas D.; Nounesis G.; Paleos C. M. Interaction of poly-L-arginine with dihexadecyl phosphate/phosphatidylcholine liposomes. Langmuir 2005, 21, 5997–6001. 10.1021/la050475+. [DOI] [PubMed] [Google Scholar]
- Lee Y. C.; Taraschi T. F.; Janes N. Support for the shape concept of lipid structure based on a headgroup volume approach. Biophys. J. 1993, 65, 1429–1432. 10.1016/s0006-3495(93)81206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukavina Z.; Vanić Z. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18. 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. O.; Laszlo J. A.; Compton D. L. Carboxyl-terminated PAMAM dendrimer interaction with 1-palmitoyl-2-oleoyl phosphocholine bilayers. Biochim. Biophys. Acta 2014, 1838, 445–455. 10.1016/j.bbamem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Papagiannaros A.; Dimas K.; Papaioannou G. T.; Demetzos C. Doxorubicin-PAMAM dendrimer complex attached to liposomes: cytotoxic studies against human cancer cell lines. Int. J. Pharm. 2005, 302, 29–38. 10.1016/j.ijpharm.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U.; le Maire M.; Møller J. V. The Mechanism of Detergent Solubilization of Liposomes and Protein-Containing Membranes. Biophys. J. 1998, 75, 2932–2946. 10.1016/s0006-3495(98)77735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg D.; Ahyayauch H.; Goñi F. M. The mechanism of detergent solubilization of lipid bilayers. Biophys. J. 2013, 105, 289–299. 10.1016/j.bpj.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H.; Feix J. B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1245. 10.1016/j.bbamem.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Wrobel D.; Kłys A.; Ionov M.; Vitovic P.; Waczulikowa I.; Hianik T.; Gomez-Ramirez R.; de la Mata J.; Klajnert B.; Bryszewska M. Cationic carbosilane dendrimers-lipid membrane interactions. Chem. Phys. Lipids 2012, 165, 401–407. 10.1016/j.chemphyslip.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Ionov M.; Gardikis K.; Wróbel D.; Hatziantoniou S.; Mourelatou H.; Majoral J.-P.; Klajnert B.; Bryszewska M.; Demetzos C. Interaction of cationic phosphorus dendrimers (CPD) with charged and neutral lipid membranes. Colloids Surf., B 2011, 82, 8–12. 10.1016/j.colsurfb.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Yu S.; Larson R. G. Monte-Carlo simulations of PAMAM dendrimer-DNA interactions. Soft Matter 2014, 10, 5325–5336. 10.1039/c4sm00452c. [DOI] [PubMed] [Google Scholar]
- Abbasi E.; Aval S.; Akbarzadeh A.; Milani M.; Nasrabadi H.; Joo S.; Hanifehpour Y.; Nejati-Koshki K.; Pashaei-Asl R. Dendrimers: synthesis, applications, and properties. Nano. Res. Lett. 2014, 9, 247. 10.1186/1556-276x-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D. P.; de Villiers M. M. Poly(amidoamine) Dendrimers as a Pharmaceutical Excipient. Are We There yet?. J. Pharm. Sci. 2018, 107, 75–83. 10.1016/j.xphs.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Najlah M.; Demanuele A. Crossing cellular barriers using dendrimer nanotechnologies. Curr. Opin. Pharmacol. 2006, 6, 522–527. 10.1016/j.coph.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Simons K.; Vaz W. L. C. Model systems, lipid rafts, and cell membranes. Anals. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Yanez Arteta M.; Ainalem M.-L.; Porcar L.; Martel A.; Coker H.; Lundberg D.; Chang D. P.; Soltwedel O.; Barker R.; Nylander T. Interactions of PAMAM dendrimers with negatively charged model biomembranes. J. Phys. Chem. B 2014, 118, 12892–12906. 10.1021/jp506510s. [DOI] [PubMed] [Google Scholar]
- Sideratou Z.; Foundis J.; Tsiourvas D.; Nezis I. P.; Papadimas G.; Paleos C. M. A Novel Dendrimeric ″Glue″ for Adhesion of Phosphatidyl Choline-Based Liposomes. Langmuir 2002, 18, 5036–5039. 10.1021/la020150i. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh S.; Valizadeh H.; Zakeri-Milani P. Application of response surface methodology in development of sirolimus liposomes prepared by thin film hydration technique. BioImpacts 2013, 3, 75–81. 10.5681/bi.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B.; Guha P.; Bhattarai R.; Nahak P.; Karmakar G.; Chettri P.; Panda A. K. Influence of Lipid Composition, pH, and Temperature on Physicochemical Properties of Liposomes with Curcumin as Model Drug. J. Oleo Sci. 2016, 65, 399–411. 10.5650/jos.ess15229. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H. K.; Buschmann C. Extraction of Phtosynthetic Tissues:Chlorophylls and Carotenoids. Curr. Protoc. Food. Analyt. Chem. 2001, 1, F4.3.1–F4.3.8. 10.1002/0471142913.faf0403s01. [DOI] [Google Scholar]
- Roy B.; Panda A. K.; Parimi S.; Ametov I.; Barnes T.; Prestidge C. A. Physico-chemical studies on the interaction of dendrimers with lipid bilayers. 1. Effect of dendrimer generation and liposome surface charge. J. Oleo Sci. 2014, 63, 1185–1193. 10.5650/jos.ess14081. [DOI] [PubMed] [Google Scholar]
- Koirala S.; Roy B.; Guha P.; Bhattarai R.; Sapkota M.; Nahak P.; Karmakar G.; Mandal A. K.; Kumar A.; Panda A. K. Effect of double tailed cationic surfactants on the physicochemical behavior of hybrid vesicles. RSC Adv. 2016, 6, 13786–13796. 10.1039/c5ra17774j. [DOI] [Google Scholar]
- Liu S.; Lv P.; Li D.; Guo X.; Zhang B.; Yu M.; Li D.; Xiong Y.; Zhang L.; Tian C. K+ preference at the NaK channel entrance revealed by fluorescence lifetime and anisotropy analysis of site-specifically incorporated (7-hydroxycoumarin-4-yl)ethylglycine. Chem. Commun. 2015, 51, 15971–15974. 10.1039/c5cc06124e. [DOI] [PubMed] [Google Scholar]
- Mandal A. K.; Sen I. K.; Maity P.; Chattopadhyay S.; Chakraborty R.; Roy S.; Islam S. S. Structural elucidation and biological studies of a novel exopolysaccaride from Klebsiella pneumoniae PB12. Int. J. Biol. Macromol. 2015, 79, 413–422. 10.1016/j.ijbiomac.2015.04.077. [DOI] [PubMed] [Google Scholar]
- Jana S. K.; Mandal A. K.; Kumar A.; Puschmann H.; Hossain M.; Dalai S. Sensing of tryptophan by a non-toxic cobalt(ii) complex. RSC Adv. 2016, 6, 95888–95896. 10.1039/c6ra16086g. [DOI] [Google Scholar]
- Guha P.; Roy B.; Karmakar G.; Nahak P.; Koirala S.; Sapkota M.; Misono T.; Torigoe K.; Panda A. K. Ion-Pair Amphiphile: A Neoteric Substitute That Modulates the Physicochemical Properties of Biomimetic Membranes. J. Phys. Chem. B 2015, 119, 4251–4262. 10.1021/jp512212u. [DOI] [PubMed] [Google Scholar]
- Giustini M.; Bellinazzo C.; Galantini L.; Mallardi A.; Palazzo G.; Sennato S.; Bordi F.; Rissanen K. Incorporation of the bacterial reaction centre into dendrimersomes. Colloids Surf., A 2012, 413, 38–43. 10.1016/j.colsurfa.2012.01.040. [DOI] [Google Scholar]
- Winther L. R.The Phase Transition of DMPG and its Dependence on pH. Bachelor Thesis, Niels Bohr Institute, Copenhagen University, 2008. [Google Scholar]
- Wrobel D.; Appelhans D.; Signorelli M.; Wiesner B.; Fessas D.; Scheler U.; Voit B.; Maly J. Interaction study between maltose-modified PPI dendrimers and lipidic model membranes. Biochim. Biophys. Acta 2015, 1848, 1490. 10.1016/j.bbamem.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Wrobel D.; Ionov M.; Gardikis K.; Demetzos C.; Majoral J.-P.; Palecz B.; Klajnert B.; Bryszewska M. Interactions of phosphorus-containing dendrimers with liposomes. Biochim. Biophys. Acta 2011, 1811, 221–226. 10.1016/j.bbalip.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Singler R. E.; Sennett M. S.; Willingham R. A.. Phosphazene polymers: synthesis, structure, and properties. Inorganic and Organometallic Polymers; ACS Publications, 1988; Vol. 360, pp 268–276. [Google Scholar]
- Lozano N.; Pinazo A.; La Mesa C.; Perez L.; Andreozzi P.; Pons R. Catanionic Vesicles Formed with Arginine-Based Surfactants and 1,2-Dipalmitoyl-sn-glycero-3-phosphate Monosodium Salt. J. Phys. Chem. B 2009, 113, 6321–6327. 10.1021/jp810671p. [DOI] [PubMed] [Google Scholar]
- Tsogas I.; Tsiourvas D.; Nounesis G.; Paleos C. M. Modeling Cell Membrane Transport: Interaction of Guanidinylated Poly(propylene imine) Dendrimers with a Liposomal Membrane Consisting of Phosphate-Based Lipids. Langmuir 2006, 22, 11322–11328. 10.1021/la0620861. [DOI] [PubMed] [Google Scholar]
- Wrobel D.; Appelhans D.; Signorelli M.; Wiesner B.; Fessas D.; Scheler U.; Voit B.; Maly J. Interaction study between maltose-modified PPI dendrimers and lipidic model membranes. Biochim. Biophys. Acta 2015, 1848, 1490–1501. 10.1016/j.bbamem.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva L.; Simkovitch R.; Huppert D.; Esteves da Silva J. C. G. Combined experimental and theoretical study of the photochemistry of 4- and 3-hydroxycoumarin. J. Photochem. Photobiol., A 2017, 338, 23–36. 10.1016/j.jphotochem.2017.01.032. [DOI] [Google Scholar]
- Smith E. A.; Dea P. K.. Differential Scanning Calorimetry Studies of Phospholipid Membranes: the Interdigitated Gel Phase; INTECH, 2013. [Google Scholar]
- Feitosa E.; Jansson J.; Lindman B. The effect of chain length on the melting temperature and size of dialkyldimethylammonium bromide vesicles. Chem. Phys. Lipids 2006, 142, 128–132. 10.1016/j.chemphyslip.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ringsdorf H.; Schlarb B.; Tyminski P. N.; O’Brien D. F. Permeability characteristics of liposomes in a net-membranes of dihexadecyl phosphate with polymerizable gegenions. Macromolecules 1988, 21, 671–677. 10.1021/ma00181a023. [DOI] [Google Scholar]
- Ionov M.; Wróbel D.; Gardikis K.; Hatziantoniou S.; Demetzos C.; Majoral J.-P.; Klajnert B.; Bryszewska M. Effect of phosphorus dendrimers on DMPC lipid membranes. Chem. Phys. Lipids 2012, 165, 408–413. 10.1016/j.chemphyslip.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Pantos A.; Tsiourvas D.; Nounesis G.; Paleos C. M. Interaction of functional dendrimers with multilamellar liposomes: design of a model system for studying drug delivery. Langmuir 2005, 21, 7483–7490. 10.1021/la0510331. [DOI] [PubMed] [Google Scholar]
- Lovelock J. E. The haemolysis of human red blood-cells by freezing and thawing. Biochim. Biophys. Acta 1953, 10, 414–426. 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- Lin J.-J.; Lin W.-C.; Dong R.-X.; Hsu S.-h. The cellular responses and antibacterial activities of silver nanoparticles stabilized by different polymers. Nanotechnology 2012, 23, 065102. 10.1088/0957-4484/23/6/065102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.