Abstract

Careful design of the structures of interfaces between nanofillers and polymer matrices can significantly improve the mechanical and thermal properties of the overall nanocomposites. Here, we investigate how the grafting density on the surface of nanocelluloses influences the properties of nanocellulose/cellulose triacetate (CTA) composites. The surface of nanocellulose, which was prepared by 2,2,6,6-tetramethylpiperidine-1-oxyl oxidation, was modified with long poly(ethylene glycol) (PEG) chains at different grafting densities. The PEG-grafted nanocelluloses were homogeneously embedded in CTA matrices. The mechanical and thermal properties of the nanocomposites were characterized. Increasing the grafting density caused the soft PEG chains to form denser and thicker layers around the rigid nanocelluloses. The PEG layers were not completely miscible with the CTA matrix. This structure considerably enhanced the energy dissipation by allowing sliding at the interface, which increased the toughness of the nanocomposites. The thermal and mechanical properties of the composites could be tailored by controlling the grafting density. These findings provide a deeper understanding about interfacial design for nanocellulose-based composite materials.
Introduction
In polymer composite materials, interfacial properties between the soft polymer matrix and rigid fillers are an important factor governing their mechanical properties.1−5 Under strain, load transfer occurs at the interfaces. Well-designed interfaces can therefore improve the strength, stiffness, and toughness of composite materials. This design becomes more important in the case of “nanofiller”-reinforced polymer composites, because the interfacial layer occupies a large volume fraction in the composite, due to the large specific surface area of the nanofillers. Controlling the chemical and morphological features at the interface is of great importance in nanocomposites.
Surface modification of nanofillers is usually carried out to control the properties of the interface. The surface energy, balance between hydrophobicity and hydrophilicity, and dispersibility of nanofillers have all been modified to achieve good compatibility at the interface.6−8 Polymer grafting is one of the most effective methods.6,7,9−11 This approach allows the surface chemistry of the fillers and thickness of the grafted layer to be controlled, by varying the molecular weight (Mw) and grafting density of the grafted polymer. Polymer-grafted nanofillers can form versatile interfacial structures with polymer matrices.
In our previous study, we modified the surface of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose nanofibrils (TOCNs) with an amine-terminated poly(ethylene glycol) (PEG-NH2) at the same grafting density. The reinforcing properties of the PEG-grafted TOCNs (PEG-TOCNs) were investigated using cellulose triacetate (CTA) as a polymer matrix. The addition of PEG-TOCN to the CTA matrix resulted in increases in not only the modulus and tensile strength but also the ductility and toughness,12 which is unusual for other TOCN/polymer composites. Subsequently, we prepared PEG-TOCNs using PEG-NH2 samples with different Mw values on TOCN at the same grafting density.13 The properties of the PEG-TOCN/CTA composite films were studied to obtain the effect of chain lengths of the grafted PEG molecules on the interfacial interactions in the CTA matrix.13 These results showed that the thermal and mechanical properties of the PEG-TOCN/CTA composite films could be tuned by the interfacial layer thickness formed by the grafted PEGs and CTA. However, there was no information about the effect of the grafting density of PEG chains on TOCN surfaces on the properties of PEG-TOCN/CTA composite films, despite the PEG density directly affecting the chain conformation on the TOCN surfaces.
Herein, the contribution of the grafting density to the mechanical, thermal, and dynamic mechanical thermal properties of nanocellulose-reinforced polymer composites was investigated. PEG chains having the same Mw were grafted onto TOCN surfaces at different grafting densities via ionic bonds (see Figure 1). The PEG-TOCN/CTA composite films were prepared by solvent casting using N,N-dimethylacetamide (DMAc). The relationship between the grafting density and thermal and mechanical properties of the composite films is discussed.
Figure 1.

Structures of TOCNs and digital photographs of TOCNs/DMAc dispersions.
Results and Discussion
The protonated TOCN (TOCN-COOH) and a series of PEG-TOCNs containing different grafting ratios (27, 44, 60, and 72% mol/mol to the surface COOH groups) were prepared (see Figure S1 and Table S1). These TOCNs were abbreviated to P0-T, P27-T, P44-T, P60-T, and P72-T, respectively, with the numeral indicating the grafting ratio. The structures of the TOCNs and digital photographs of the TOCNs/DMAc dispersions are shown in Figure 1. The TOCN-COOH and PEG-TOCN elements were individually dispersed in DMAc without aggregation, regardless of the grafting density. TOCN-COOH disperses in DMAc due to the osmotic pressure between the electric double layers on the TOCN surface.13 The PEG-TOCNs disperse due to the osmotic pressure between the grafted PEG layers.14−16 According to the calculation for polymer conformation in a good solvent,17−19 the grafted PEG chains had a polymer brush conformation in the vicinity of the TOCN surface, irrespective of the grafting density (see Supporting Information). From these homogeneous dispersions, TOCN/CTA nanocomposite films with different PEG grafting densities were prepared by the solvent casting method.
Transmission Electron Microscopy (TEM) Observations
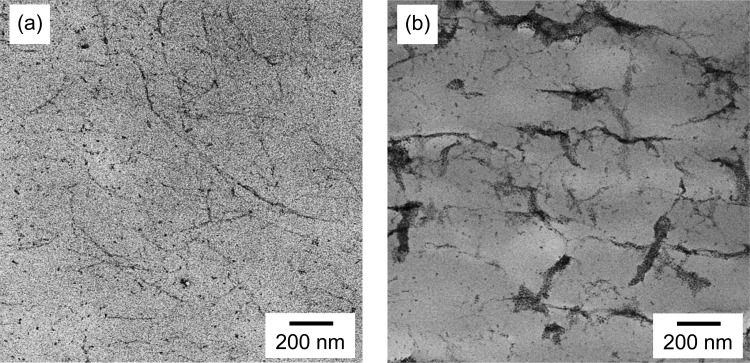
Figure 2 shows TEM images of cross-sections of the nanocomposite films. TOCN elements were well dispersed in the CTA matrix with and without PEG grafting. Thin fibrillar elements with clear grain boundaries were observed in the TEM image of the P0-T/CTA composite film. Thick PEG-TOCN elements with indistinct boundaries were observed in the TEM image of the P72-T/CTA composite film. It was likely that both TOCNs and grafted PEG layers were stained and thus observed as indistinct elements in the P72-T/CTA composite film. These results show that the nanocomposites with different grafting densities had similar homogeneous distributions of TOCNs in the CTA matrix. This suggests that PEG grafting did not affect the dispersibility of the TOCNs. Therefore, changes in the other properties observed in the present study can be related solely to the interfacial PEG density.
Figure 2.
TEM images of cross-section of (a) P0-T/CTA and (b) P72-T/CTA composite films.
Mechanical Properties
Figure 3 shows representative stress–strain curves of the TOCN/CTA nanocomposites. The nanocomposites exhibited improved mechanical properties compared with neat CTA, with the properties depending on the surface grafting density of the TOCNs (Figure 4 and Table 1). The density of the nanocomposite and crystallinity of the CTA matrix were almost the same (approximately 1.15 and 25–30%, respectively), irrespective of the grafting density (Table S2 in Supporting Information). The different nanocomposites had identical TOCN compositions (Table S3 in Supporting Information). Therefore, the differences in mechanical properties of the nanocomposites were attributed to the different grafting densities on the TOCN surfaces.
Figure 3.
Representative stress–strain curves of the nanocomposite films.
Figure 4.
Mechanical properties of the nanocomposite films: (a) Young’s modulus, (b) ultimate strength, (c) toughness, and (d) elongation at break.
Table 1. Mechanical Properties of Nanocomposite Films.
| sample | Young’s modulus (GPa) | ultimate strength (MPa) | yield strength (MPa) | elongation at break (%) | toughness (MJ/m3) |
|---|---|---|---|---|---|
| neat CTA | 1.03 ± 0.07 | 40.8 ± 2.2 | 40.8 ± 2.3 | 11.6 ± 4.1 | 3.6 ± 1.7 |
| P0-T/CTA | 1.33 ± 0.11 | 44.1 ± 2.8 | 39.1 ± 2.4 | 12.7 ± 6.4 | 4.0 ± 2.9 |
| P27-T/CTA | 1.27 ± 0.09 | 47.1 ± 4.0 | 45.9 ± 3.1 | 16.6 ± 9.4 | 6.4 ± 3.9 |
| P44-T/CTA | 1.25 ± 0.09 | 42.2 ± 3.5 | 40.0 ± 3.9 | 25.3 ± 12.2 | 9.1 ± 6.0 |
| P60-T/CTA | 1.12 ± 0.08 | 37.7 ± 3.9 | 35.4 ± 3.8 | 37.3 ± 13.0 | 14.0 ± 5.5 |
| P72-T/CTA | 1.03 ± 0.12 | 35.0 ± 3.2 | 32.7 ± 3.3 | 45.7 ± 11.8 | 14.8 ± 6.2 |
Figure 4a shows the Young’s modulus of the nanocomposites. The modulus increased upon the addition of P0-T (Figure 4a). Increasing the grafting density of TOCNs caused the nanocomposites to become softer, which was most likely due to sliding at the PEG/CTA interfaces. In general, soft interfacial layers surrounding rigid fillers have a low capability to transfer stress between the filler and the polymer matrix.20−23 Thus, the soft PEG layer in this study increased the sliding at the TOCN/CTA interface, resulting in the lower stiffness with increasing grafting density. The TOCN content in the present study was also sufficiently high to form a percolation network.24 The rigid percolation network of nanocellulose25,26 may have been loosened by PEG grafting, which could have influenced the changes in Young’s modulus. The PEG-TOCN/CTA composites showed lower Young’s modulus values than the P0-T/CTA composite. However, the modulus was still as high as that of neat CTA with the addition of P72-T. The ultimate strength of the PEG-TOCN/CTA composites showed a trend similar to that of Young’s modulus (Figure 4b). This was most likely due to changes in stress-transfer behavior by the surface PEG layers as discussed above.
The elongation at break and toughness significantly increased with increasing grafting density (Figure 4c,d). The toughness, which was calculated from the area under the stress–strain curve, significantly increased with increasing grafting density. As previously reported, soft interlayers between fillers and polymer matrices support the plastic deformation of the polymer matrices, and enhance energy dissipation during deformation.27−31 Therefore, the dense and soft PEG layers of the nanocomposites enhanced the deformation and energy dissipation of the nanocomposites after the yielding point. Accordingly, PEG grafting resulted in the toughness of CTA increasing by up to 4 times. Nanocellulose/polymer nanocomposites generally exhibit a stiff yet brittle behavior, as was the case for the current P0-T/CTA composite. In contrast, the PEG-TOCN/CTA composites exhibited an unusual reinforcing effect, which greatly improved the toughness without sacrificing the stiffness of the CTA matrix. This toughening effect was not significantly caused by the plasticizing effect of the PEG chains, which is discussed in the next section.
Dynamic Mechanical Thermal Analysis
Storage modulus (E′) and loss tangent (tan δ; loss modulus/storage modulus) curves of the nanocomposites are shown in Figure 5, and characteristic values calculated from the data are given in Table 2. The E′ of the nanocomposites was 2.6–3.3 GPa at 23 °C, and showed a trend similar to that of Young’s modulus in the tensile tests (Figure 4a). At −80 °C (<grass transition temperature of PEG32), the difference in the E′ values of the nanocomposites was less significant because the grafted PEG chains were in the solid state.
Figure 5.
Representative curves of (a) storage modulus and (b) tan δ of the nanocomposite films.
Table 2. Dynamic Mechanical Thermal Properties of Nanocomposite Films.
| sample | E′ at –80 °C (GPa) | E′ at 23 °C (GPa) | Tg (°C) |
|---|---|---|---|
| neat CTA | 4.38 ± 0.04 | 2.94 ± 0.04 | 180.3 ± 2.7 |
| P0-T/CTA | 4.72 ± 0.30 | 3.27 ± 0.22 | 183.2 ± 2.8 |
| P27-T/CTA | 4.73 ± 0.56 | 3.23 ± 0.42 | 185.2 ± 1.5 |
| P44-T/CTA | 4.53 ± 0.40 | 2.79 ± 0.25 | 185.0 ± 0.9 |
| P60-T/CTA | 4.39± 0.28 | 2.75 ± 0.08 | 186.2 ± 0.5 |
| P72-T/CTA | 4.36 ± 0.40 | 2.60 ± 0.37 | 182.8 ± 1.7 |
The glass transition temperatures (Tg) of the nanocomposites were calculated from the position of the α-transition peak in the tan δ curves (Figure 5b). The Tg of the CTA matrix slightly increased upon adding TOCN and PEG-TOCNs (Table 2). The Tg of polymer matrices can reportedly be increased by adding nanofillers that have good interactions with the polymer matrix or that form a percolated network that confines the molecular mobility of the polymer matrix.4,33−36 In the present study, TOCNs or PEG-TOCNs likely formed a network and confined the chain mobility of the CTA matrix. When only PEG-NH2 was incorporated, the Tg of the CTA matrix decreased due to the plasticizing effect (Figure S2 and Table S5 in Supporting Information). This suggested that PEG and CTA were not completely miscible in the nanocomposite system, possibly due to crowding of the dense grafted PEG chains.
Thermomechanical Properties
Figure 6 shows coefficient of thermal expansion (CTE) values for the nanocomposites. The CTE of the P0-T/CTA composites was 13% lower than that of neat CTA. This was because P0-T formed a rigid and thermally stable percolating network, and restrained the thermal expansion of the CTA matrix. The CTE of the PEG-TOCN/CTA nanocomposites increased with increasing grafting density. This was because the PEG layers surrounded the TOCN surfaces and loosened the TOCN network. The CTE values of the PEG-NH2/CTA films were higher than that of neat CTA because of the increased free volume caused by the PEG chains (see Figure S3 in Supporting Information). The present PEG-TOCN/CTA nanocomposites showed almost the same CTE values as that of neat CTA, until the grafting density reached 60%. This resulted from both restraint by the TOCN network, and the enhanced thermal expansion caused by the PEG chains.12
Figure 6.
(a) Thermal expansion behavior and (b) coefficient of thermal expansion of the nanocomposite films.
Conclusions
We systematically investigated the contribution of the grafting density to the properties of nanocellulose-reinforced polymer composites, where TOCNs with different grafting densities were well dispersed in CTA matrices. The present study is one of the case studies for nanocellulose/polymer composites to understand the influence of interfacial layers on the composite properties. In the nanocomposites, the surface PEG layer and CTA matrix were not completely miscible, which caused a reinforcing effect. Specifically, an increase in grafting density caused the grafted PEG to form a denser layer around the TOCN surfaces. This enhanced sliding between the PEG and CTA, which significantly increased the toughness of the material. Importantly, the Young’s modulus of CTA was not decreased by the addition of PEG-TOCNs. This strategy allows the thermal and mechanical properties to be tuned by simply changing the grafting density. These findings provide insights into the interfacial design of CNF-based composite materials.
Materials and Methods
Materials
Never-dried softwood bleached kraft pulp (SBKP) produced by Nippon Paper Industries Co., Ltd., Japan, was used as wood cellulose. CTA was produced by Daicel Corp., Japan.12,13 PEG-NH2 (SUNBRIGHT MEPA-20H, Mw = 2182) was produced by NOF Corp., Japan. Uranyl acetate, lead citrate, and ruthenium tetroxide were purchased from Mitsuwa Chemicals Co., Ltd., Japan, TAAB Laboratories Equipment Ltd., England, and Nacalai Tesque, Inc., Japan, respectively. All other reagents and solvents were purchased from Sigma-Aldrich, Japan, or Fuji Film Wako Pure Chemical Industries, Ltd., Japan.
TEMPO/NaBr/NaClO Oxidation
TEMPO/NaBr/NaClO oxidation was performed according to a previous study.12,13,37 TEMPO and NaBr (0.016 and 0.1 g, respectively) were added to distilled water (100 mL), and this mixture was stirred until a homogeneous TEMPO/NaBr/water solution was obtained. SBKP (1.0 g) was added to the solution, and the SBKP/TEMPO/NaBr slurry was stirred with a magnetic stir bar for 30 min. Then, 2 M sodium hypochlorite (NaClO, 10 mmol/g-SBKP, 5 mL) was added to the slurry to initiate oxidation. The oxidation continued in the slurry at pH 10 and room temperature for 6 h. A pH stat (AUT-501, DKK-TOA Corp., Japan) was used to maintain the pH 10 during oxidation. After oxidation, ethanol was added to the slurry to consume excess NaClO and to finish the oxidation. The TEMPO-oxidized pulp (TOP) was washed with deionized water until pH 7 by filtration using a glass filter. The TOP (1.0 g) was postoxidized with sodium chlorite (NaClO2, 1.1 g) in a 0.1 M acetate buffer (pH 4.8, 100 mL) at room temperature for 48 h. After the NaClO2 oxidation, the TOP was washed with deionized water until pH 7 by filtration using a glass filter. The TOP was freeze-dried to determine the carboxylate content by conductometric titration. The carboxylate content of the TOP was 1.78 mmol/g.
Preparation of TOCN-COOH/DMAc and PEG-TOCN/DMAc Dispersion
The TOCN-COOH gel particle was prepared according to a previous method.38 The TOCN-COOH gel was suspended in DMAc (0.1% w/v). Then, PEG-NH2 was dissolved in DMAc and added to the TOCN-COOH/DMAc suspension to ionically graft PEG-NH2 chains on the TOCN surfaces.13 The suspension was mechanically agitated for 24 h with a Rotamax 120.13 The amount of PEG-NH2 added was controlled to prepare PEG-TOCNs with different grafting densities. The PEG-TOCN/DMAc and TOCN-COOH gel/DMAc suspensions were sonicated and centrifuged to prepare PEG-TOCN/DMAc and TOCN-COOH/DMAc dispersions, respectively, according to the previous study.13
Preparation of TOCN-COOH/CTA and PEG-TOCN/CTA Composite Films
A 2% w/v CTA/DMAc solution and either TOCN-COOH/DMAc or PEG-TOCN/DMAc dispersion were mixed, and this mixture was stirred for 30 min. The mixture was then cast-dried in vacuo at 40 °C for 1 day and subsequently at 70 °C for 1 week. The TOCN content in the nanocomposites was calculated according to the following equation and adjusted to 2.5% w/w
The PEG-NH2/CTA films were prepared as a reference using the same procedure. The amount of PEG-NH2 in the composites was adjusted to be identical to that in the corresponding PEG-TOCN/CTA composites.
Analyses
Fourier-transform infrared (FT-IR) spectra of cast-dried TOCN-COOH or PEG-TOCN films were measured with a spectrometer (JASCO FT/IR-6100, transmission mode from 400 to 4000 cm–1, 4 cm–1 resolution). The grafting ratios of the PEG chains onto the surface COOH groups were calculated from the intensity of the absorption peak at 1720 cm–1.12,13 TEM observations were conducted using a Hitachi HT 7700 Exalens TEM apparatus, at an accelerating voltage of 100 kV. For TEM observations, the nanocomposites were embedded in epoxy resin, which was then sectioned in the direction parallel to the film surface using a diamond knife. The 80 nm-thick sections were stained with 5% uranyl acetate and 2% lead citrate or with ruthenium tetroxide on a collodion film to provide clear contrast between the stained TOCN elements and CTA matrix. Tensile tests were performed according to our previous study.13 Twenty specimens were tested for each sample. Dynamic mechanical thermal analysis (DMTA) was performed using a dynamic mechanical analyzer (DMA Q800, TA instruments, Inc., DE) at temperatures from −100 to 200 °C with a heating rate of 3 °C/min and a frequency of 1 Hz. The specimens for DMTA were rectangular shaped (40 mm × 5 mm). Three specimens were tested for each sample. Thermal analysis was performed using the same procedure used in the previous study.13
Acknowledgments
This research was supported by Grants-in-Aid for Scientific Research (Grant number 16J02980) from the Japan Society for the Promotion of Science (JSPS), the JSPS Overseas Challenge Program for Young Researchers (Grant number 201780257), in part by the Core Research for Evolutional Science and Technology (CREST, Grant number JPMJCR13B2) of the Japan Science and Technology Agency (JST), and in part by the JST-Mirai Program (Grant number JPMJMI17ED). The authors thank Aidan G. Young, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01616.
FT-IR spectra of PEG-TOCN films, calculation for grafting density of TOCN surfaces, fundamental properties of PEG-TOCN/CTA composites, and mechanical, dynamic mechanical thermal, and thermal properties of PEG-NH2/CTA composites (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Vaia R. A.; Emmanuel P. Polymer Nanocomposites: Status and Oppotunites. MRS Bull. 2001, 26, 394–401. 10.1557/mrs2001.93. [DOI] [Google Scholar]
- Wagner H. D.; Vaia R. A. Nanocomposites: Issues at the Interface. Mater. Today 2004, 7, 38–42. 10.1016/S1369-7021(04)00507-3. [DOI] [Google Scholar]
- Thostenson E. T.; Li C.; Chou T. W. Nanocomposites in Context. Compos. Sci. Technol. 2005, 65, 491–516. 10.1016/j.compscitech.2004.11.003. [DOI] [Google Scholar]
- Crosby A. J.; Lee J. Y. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. 10.1080/15583720701271278. [DOI] [Google Scholar]
- Vaia R. A.; Wagner H. D. Framework for Nanocomposites. Mater. Today 2004, 7, 32–37. 10.1016/S1369-7021(04)00506-1. [DOI] [Google Scholar]
- Kango S.; Kalia S.; Celli A.; Njuguna J.; Habibi Y.; Kumar R. Surface Modification of Inorganic Nanoparticles for Development of Organic-Inorganic Nanocomposites - A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. 10.1016/j.progpolymsci.2013.02.003. [DOI] [Google Scholar]
- Missoum K.; Belgacem M. N.; Bras J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. 10.3390/ma6051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia S.; Boufi S.; Celli A.; Kango S. Nanofibrillated Cellulose: Surface Modification and Potential Applications. Colloid Polym. Sci. 2014, 292, 5–31. 10.1007/s00396-013-3112-9. [DOI] [Google Scholar]
- Xie L.; Xu F.; Qiu F.; Lu H.; Yang Y. Single-Walled Carbon Nanotubes Functionalized with High Bonding Density of Polymer Layers and Enhanced Mechanical Properties of Composites. Macromolecules 2007, 40, 3296–3305. 10.1021/ma062103t. [DOI] [Google Scholar]
- Spitalsky Z.; Tasis D.; Papagelis K.; Galiotis C. Carbon Nanotube-Polymer Composites: Chemistry, Processing, Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. 10.1016/j.progpolymsci.2009.09.003. [DOI] [Google Scholar]
- Chadwick R. C.; Khan U.; Coleman J. N.; Adronov A. Polymer Grafting to Single-Walled Carbon Nanotubes: Effect of Chain Length on Solubility, Graft Density and Mechanical Properties of Macroscopic Structures. Small 2013, 9, 552–560. 10.1002/smll.201201683. [DOI] [PubMed] [Google Scholar]
- Soeta H.; Fujisawa S.; Saito T.; Berglund L.; Isogai A. Low-Birefringent and Highly Tough Nanocellulose-Reinforced Cellulose Triacetate. ACS Appl. Mater. Interfaces 2015, 7, 11041–11046. 10.1021/acsami.5b02863. [DOI] [PubMed] [Google Scholar]
- Soeta H.; Fujisawa S.; Saito T.; Isogai A. Interfacial Layer Thickness Design for Exploiting the Reinforcement Potential of Nanocellulose in Cellulose Triacetate Matrix. Compos. Sci. Technol. 2017, 147, 100–106. 10.1016/j.compscitech.2017.05.010. [DOI] [Google Scholar]
- Milner S. T. Polymer Brushes. Science 1991, 905–914. 10.1126/science.251.4996.905. [DOI] [PubMed] [Google Scholar]
- Currie E. P. K.; Norde W.; Cohen Stuart M. A. C. Tethered Polymer Chains: Surface Chemistry and Their Impact on Colloidal and Surface Properties. Adv. Colloid Interface Sci. 2003, 100–102, 205–265. 10.1016/S0001-8686(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Fujisawa S.; Saito T.; Kimura S.; Iwata T.; Isogai A. Surface Engineering of Ultrafine Cellulose Nanofibrils toward Polymer Nanocomposite Materials. Biomacromolecules 2013, 14, 1541–1546. 10.1021/bm400178m. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N.Intermolecular and Surface Forces; Academic press, 2011. [Google Scholar]
- de Gennes P. G. Conformations of Polymers Attached to an Interface. Macromolecules 1980, 13, 1069–1075. 10.1021/ma60077a009. [DOI] [Google Scholar]
- Zhao B.; Brittain W. J. Polymer Brushes: Surface-Immobilized Macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. 10.1016/S0079-6700(00)00012-5. [DOI] [Google Scholar]
- Wan H.; Delale F.; Shen L. Effect of CNT Length and CNT-Matrix Interphase in Carbon Nanotube (CNT) Reinforced Composites. Mech. Res. Commun. 2005, 32, 481–489. 10.1016/j.mechrescom.2004.10.011. [DOI] [Google Scholar]
- Al Masud M. A.; Masud A. K. M. Effect of Interphase Characteristic and Property on Axial Modulus of Carbon Nanotube Based Composites. J. Mech. Eng. 2010, 41, 15–24. 10.3329/jme.v41i1.5358. [DOI] [Google Scholar]
- Joshi P.; Upadhyay S. H. Effect of Interphase on Elastic Behavior of Multiwalled Carbon Nanotube Reinforced Composite. Comput. Mater. Sci. 2014, 87, 267–273. 10.1016/j.commatsci.2014.02.029. [DOI] [Google Scholar]
- Guru K.; Sharma T.; Shukla K. K.; Mishra S. B. Effect of Interface on the Elastic Modulus of CNT Nanocomposites. J. Nanomech. Micromech. 2015, 6, 04016004 10.1061/(asce)nm.2153-5477.0000109. [DOI] [Google Scholar]
- Fujisawa S.; Ikeuchi T.; Takeuchi M.; Saito T.; Isogai A. Superior Reinforcement Effect of TEMPO-Oxidized Cellulose Nanofibrils in Polystyrene Matrix: Optical, Thermal, and Mechanical Studies. Biomacromolecules 2012, 13, 2188–2194. 10.1021/bm300609c. [DOI] [PubMed] [Google Scholar]
- Eichhorn S. J.; Dufresne A.; Aranguren M.; Marcovich N. E.; Capadona J. R.; Rowan S. J.; Weder C.; Thielemans W.; Roman M.; Renneckar S.; Gindl W.; Veigel S.; Keckes J.; Yano H.; Abe K.; Nogi M.; Nakagaito A. N.; Mangalam A.; Simonsen J.; Benight A. S.; Bismarck A.; Berglund L. A.; Peijs T. Review: Current International Research into Cellulose Nanofibres and Nanocomposites. J. Mater. Sci. 2010, 45, 1–33. 10.1007/s10853-009-3874-0. [DOI] [Google Scholar]
- Svagan A. J.; Azizi Samir M. A. S.; Berglund L. A. Biomimetic Polysaccharide Nanocomposites of High Cellulose Content and High Toughness. Biomacromolecules 2007, 8, 2556–2563. 10.1021/bm0703160. [DOI] [PubMed] [Google Scholar]
- Rong M. Z.; Zhang M. Q.; Pan S. L.; Friedrich K. Interfacial Effects in Polypropylene – Silica Nanocomposites. J. Appl. Polym. Sci. 2004, 92, 1771–1781. 10.1002/app.20139. [DOI] [Google Scholar]
- Wu C. L.; Zhang M. Q.; Rong M. Z.; Friedrich K. Silica Nanoparticles Filled Polypropylene: Effects of Particle Surface Treatment, Matrix Ductility and Particle Species on Mechanical Performance of the Composites. Compos. Sci. Technol. 2005, 65, 635–645. 10.1016/j.compscitech.2004.09.004. [DOI] [Google Scholar]
- Fang M.; Zhang Z.; Li J.; Zhang H.; Lu H.; Yang Y. Constructing Hierarchically Structured Interphases for Strong and Tough Epoxy Nanocomposites by Amine-Rich Graphene Surfaces. J. Mater. Chem. 2010, 20, 9635. 10.1039/c0jm01620a. [DOI] [Google Scholar]
- Zappalorto M.; Salviato M.; Quaresimin M. A Multiscale Model to Describe Nanocomposite Fracture Toughness Enhancement by the Plastic Yielding of Nanovoids. Compos. Sci. Technol. 2012, 72, 1683–1691. 10.1016/j.compscitech.2012.07.010. [DOI] [Google Scholar]
- Guan L.-Z.; Wan Y.-J.; Gong L.-X.; Yan D.; Tang L.-C.; Wu L.-B.; Jiang J.-X.; Lai G.-Q. Toward Effective and Tunable Interphases in Graphene Oxide/Epoxy Composites by Grafting Different Chain Lengths of Polyetheramine onto Graphene Oxide. J. Mater. Chem. A 2014, 2, 15058. 10.1039/C4TA02429J. [DOI] [Google Scholar]
- Craig D. Q. M. Polyethyelene Glycols and Drug Release. Drug Dev. Ind. Pharm. 1990, 16, 2501–2526. 10.3109/03639049009058544. [DOI] [Google Scholar]
- Rittigstein P.; Torkelson J. M. Polymer–Nanoparticle Interfacial Interactions in Polymer Nanocomposites: Confinement Effects on Glass Transition Temperature and Suppression of Physical Aging. J. Polym. Sci., Part B: Polym. Phys. 2006, 44, 2935–2943. 10.1002/polb.20925. [DOI] [Google Scholar]
- Zou H.; Wu S.; Shen J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. 10.1021/cr068035q. [DOI] [PubMed] [Google Scholar]
- Sengupta R.; Chakraborty S.; Bandyopadhyay S.; Dasgupta S.; Mukhopadhyay R.; Auddy K.; Deuri A. S. A Short Review on Rubber / Clay Nanocomposites With Emphasis on Mechanical Properties. Polym. Eng. Sci. 2007, 47, 1956–1974. 10.1002/pen.20921. [DOI] [Google Scholar]
- Rahmat M.; Hubert P. Carbon Nanotube-Polymer Interactions in Nanocomposites: A Review. Compos. Sci. Technol. 2011, 72, 72–84. 10.1016/j.compscitech.2011.10.002. [DOI] [Google Scholar]
- Saito T.; Nishiyama Y.; Putaux J. L.; Vignon M.; Isogai A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. 10.1021/bm060154s. [DOI] [PubMed] [Google Scholar]
- Okita Y.; Fujisawa S.; Saito T.; Isogai A. TEMPO-Oxidized Cellulose Nanofibrils Dispersed in Organic Solvents. Biomacromolecules 2011, 12, 518–522. 10.1021/bm101255x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.