Abstract
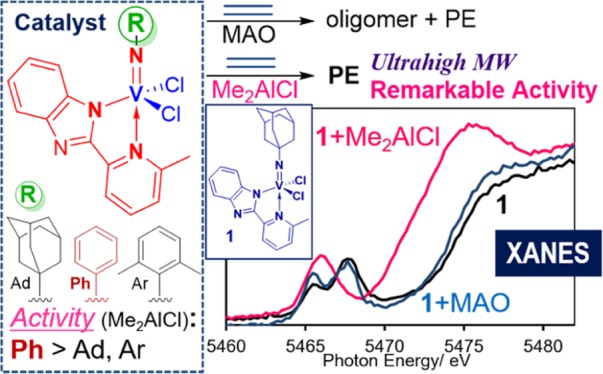
(Imido)vanadium(V) dichloride complexes containing 2-(2′-benzimidazolyl)-6-methylpyridine ligand (L) of type V(NR)Cl2(L) [R = 1-adamantyl (Ad, 1), C6H5 (2), and 2,6-Me2C6H3 (3)] have been prepared, and their structures were determined by X-ray crystallography as distorted trigonal bipyramidal structures around vanadium. Reactions with ethylene using 1–3 in the presence of methylaluminoxane (MAO) afforded a mixture of oligomer and polymers, and the compositions were affected by the imido ligand employed. By contrast, 1–3 exhibited remarkable catalytic activities for ethylene polymerization in the presence of Me2AlCl; the phenylimido complex (2) exhibited the highest activity [80 100 kg-PE/mol-V·h turn over frequency (TOF, 2 850 000 h–1, 792 s–1)]. The ethylene copolymerizations with norbornene afforded ultrahigh-molecular-weight copolymers with uniform molecular weight distributions and compositions [e.g., Mn = 1.71–2.66 × 106, Mw/Mn = 2.27–2.53]. On the basis of V nuclear magnetic resonance (51V NMR), electron spin resonance, and V K-edge X-ray absorption near-edge structure (XANES) spectra of the catalyst solution, the observed difference in the catalyst performance in the presence of (between) MAO and Me2AlCl cocatalyst should be due to the formation of different catalytically active species with different oxidation states. Apparent changes in the oxidation state were observed in the (especially in the NMR and XANES) spectra upon addition of Me2AlCl, whereas no significant changes in the spectra were observed in presence of MAO.
1. Introduction
Metal-catalyzed olefin polymerization/oligomerization is one of the key reactions in the chemical industry, and design of efficient molecular catalysts has been considered as an important subject.1−33 Because of the attractive characteristics (notable reactivity toward olefins) displayed by the classical Ziegler-type vanadium catalyst systems [V(acac)3, VOCl3 and Et2AlCl, EtAlCl2, nBuLi, etc.; employed as catalysts for production of EPDM (synthetic rubber) etc.],24,34−45 development of efficient vanadium complex catalysts is considered to be an attractive and important subject.24,42−45 It has been reported that (imido)vanadium(V) complexes containing the anionic ancillary donor ligand (aryloxo,46−49 imidazolidin-2-iminato,50 etc.)24,42−50 and the chelate anionic donor ligand,51−58 as shown in Chart 1, exhibit promising catalyst behaviors in the presence of the Al cocatalyst.59−64 In the reaction with ethylene using the chelate (2-anilidomethyl)pyridine analogues,52,54−58 V(NR)Cl2[2-ArNCH2(C5H4N)] [R = 1-adamantyl (Ad), C6H5, Ar(2,6-Me2C6H3), etc.], a steric bulk in the imido ligand affects the reactivity (dimerization vs polymerization) in the presence of methylaluminoxane (MAO) cocatalyst.52,54,56 For instance, adamantylimido/phenylimido complexes exhibited both high catalytic activity and selectivity in ethylene dimerization,54 whereas the 2,6-Me2C6H3 analogue afforded polyethylene under the same conditions.52 Moreover, on the basis of recent results in the reaction chemistry (of the dimethyl and the cationic methyl, etc.), nuclear magnetic resonance (NMR) and electron spin resonance (ESR) spectra, and X-ray absorption spectroscopy (XAS) analysis of the catalyst solution, it has been postulated that the catalytically active species in this catalysis should be the cationic vanadium(V) alkyl species preserving the basic ligand frameworks [imido, (2-anilidomethyl)pyridine].58
Chart 1. Selected Examples for Effective (Imido)vanadium(V) Dichloride Complexes for Ethylene Polymerization (Left) and Dimerization (Right)46−50,52,54−58.
As described above, it has been suggested that a steric environment around vanadium plays a role for the reaction pathway (dimerization vs polymerization). Therefore, it could be assumed that use of the 2-(2′-benzimidazolyl)pyridine [or 2-(2′-pyridyl)benzimidazole] ligand65,66 would provide more open space around vanadium compared to the reported 2-(anilidomethyl)pyridine ligand (Chart 2, 1–3) because the ligand would construct a plane consisting of vanadium, three nitrogen (imido, pyridine, and benzimidazolyl ligands; marked with red), and the aromatic ring, which could be perpendicular to a plane consisting of two chloride and vanadium (and nitrogen) in the trigonal bipyramidal structure, as also demonstrated later in the text by their structural analyses. This should be a unique contrast to that in the 2-(anilidomethyl)pyridine ligand (4),52,54−58 in which the phenyl group connected to the nitrogen forms a plane (marked with blue, Chart 2) parallel to the plane consisting of two Cl atoms and nitrogen on the anilide ligand. It might be thus assumed that the structure would facilitate the β-hydrogen elimination after olefin insertion.52,54,56 In this paper, we thus prepared a series of (imido)vanadium(V) dichloride complexes containing 2-(2′-benzimidazolyl)-6-methylpyridine ligands and explored their catalyst performances in the reaction of ethylene in the presence of the Al cocatalyst. As we observed a unique effect of Al cocatalysts (MAO vs Me2AlCl) toward the activity and product distribution, we further explored more details in these catalyses by NMR and ESR spectra and XAS analysis.
Chart 2. List of (Imido)vanadium(V) Dichloride Complexes (1–3) Employed in This Study.
2. Results and Discussion
2.1. Synthesis and Structural Analysis of (Imido)vanadium(V) Dichloride Complexes Containing 2-(2′-Benzimidazolyl)-6-methylpyridine Ligand
Three (imido)vanadium(V) dichloride complexes containing the 2-(2′-benzimidazolyl)-6-methylpyridine ligand (L) could be prepared from the corresponding trichloride complexes, V(NR)Cl3 [R = 1-adamantyl (Ad),67 C6H5,54 2,6-Me2C6H3 (Ar),68 in toluene by treating the ligand potassium salts (LKs), which were prepared in advance by treatment of 2-(2′-benzimidazole)-6-methylpyridine (LH)69 with KH in tetrahydrofuran (THF). This is a somewhat analogous procedure for the synthesis of titanium(IV) dichloride complexes containing the same ligands,65,66 although the syntheses were conducted in THF using the sodium salts. Resultant complexes were purified by recrystallization from the chilled dichloromethane solution layered by n-hexane. The isolated complexes were identified as V(NR)Cl2(L) [R = Ad (1), phenyl (2), and Ar (3); L = 2-(2′-benzimidazolyl)-6-methylpyridine] by NMR spectra and elemental analysis (Scheme 1).
Scheme 1. Synthesis of (Imido)vanadium(V) Dichloride Complexes Containing 2-(2′-Benzimidazolyl)-6-methylpyridine Ligand (1–3).
However, attempted reactions of V(NAr)Cl3 with 2-(2′-benzimidazolyl)pyridine70 conducted under the same conditions failed, affording a mixture of several complexes, which seemed difficult to separate on the basis of 51V NMR spectra of the reaction mixture [Figure S1, monitored the time course; additional experiments for the synthesis of 2-(2′-benzimidazolyl)-pyridine and attempted reactions with V(N-2,6-Me2C6H3)Cl3 are shown in the Supporting Information]. Therefore, we could not isolate the desired (methyl-free) complexes at this moment.
Figure 1 shows the Oak Ridge thermal ellipsoid plot (ORTEP) drawings for complexes 1–3, and the selected bond distances and angles are summarized in Table 1 (structure reports and xyz files for complexes 1–3 are shown in the Supporting Information). Both the adamantylimido complex (1) and the phenylimido complex (2) fold a distorted trigonal bipyramidal geometry around vanadium, with the nitrogen on pyridyl of the bidentate ligand (L) and the nitrogen atom in the imido ligand lying on the axis, and an equatorial plane consisted of two chloride ligands and the N atom in L. The axial N(1)–V–N(2) bond angles in 1 and 2 [178.37(8) and 179.1(2)°, respectively] are larger than that in the reported 2-(anilidomethyl)pyridine complex, V(NAd)Cl2[2-ArNCH2(C5H4N)] (4) [174.90(4)°],54 and the total bond angles of the equatorial Cl(1)–V–N(3) and Cl(2)–V–N(3) and Cl(1)–V–Cl(2) are 355.55 and 356.21° for 1 and 2, respectively. These results clearly suggest that the nitrogen atoms in the pyridine ligand locate at the trans position of the imido ligand. The vanadium–imido bond distances in 1 and 2 [V–N(1): 1.647(2) and 1.651(4) Å, respectively] are apparently shorter than the V–N bond distances in L [V–N(3): 1.918(2) and 1.896(5) Å, respectively] and those in the pyridine ligand [V–N(2): 2.279(2) and 2.264(5) Å, respectively]. These results also indicate that three nitrogen atoms coordinate with vanadium in different fashions. Moreover, the V–N bond distances in the pyridine ligand [1 and 2: V–N(2): 2.279(2) and 2.264(5) Å, respectively] are slightly longer than that in 4 [2.2241(11) Å]54 but apparently shorter that those in the (1-adamantylimido)vanadium(V) dichloride complexes with 2- or 8-(anilidomethyl)quinoline ligands [2.2911(14) and 2.3338(18) Å, respectively].56
Figure 1.
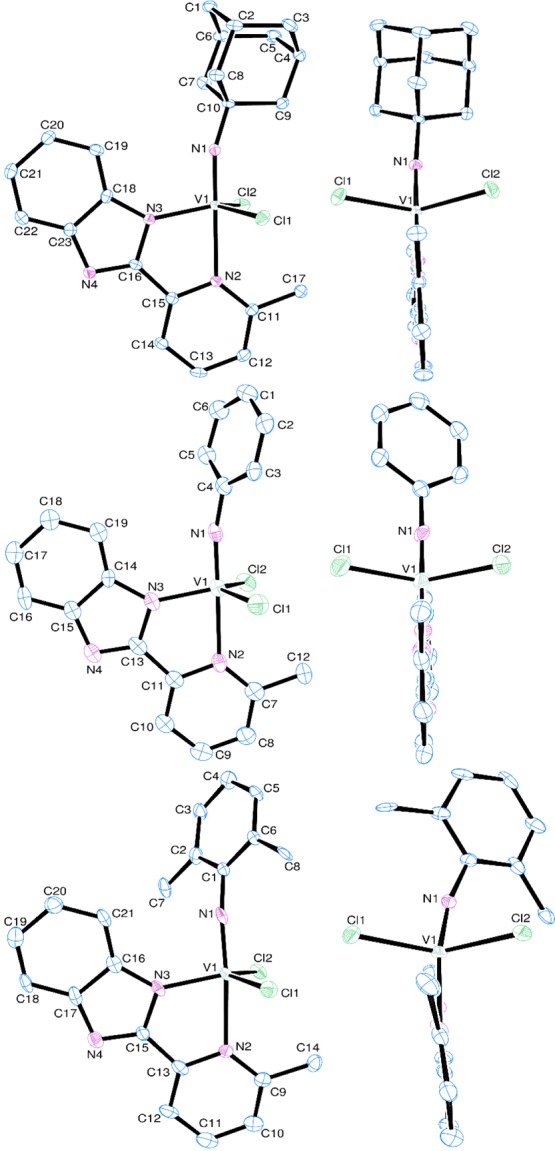
ORTEP drawings for (top) V(NAd)Cl2(L) (1), (middle) V(NC6H5)Cl2(L) (2), and (bottom) V(NAr)Cl2(L) (3) [Ad = 1-adamantyl, Ar = 2,6-Me2C6H3; L = 2-(2′-benzimidazolyl)-6-methylpyridine]. Thermal ellipsoids are drawn at the 50% probability level, and H atoms are omitted for clarity.
Table 1. Selected Bond Distances and Angles for V(NR)Cl2(L) [R = Ad (1), C6H5 (2), and 2,6-Me2C6H3 (3); L = 2-(2′-Benzimidazolyl)-6-methylpyridine]a.
| complex (R) | 1 (Ad) | 2 (C6H5) | 3 (2,6-Me2C6H3) |
|---|---|---|---|
| Bond Distances (Å) | |||
| V–N(1) | 1.647(2) | 1.651(4) | 1.679(6) |
| V–N(2) | 2.279(2) | 2.264(5) | 2.263(6) |
| V–N(3) | 1.918(2) | 1.896(5) | 1.909(5) |
| V–Cl(1) | 2.2376(7) | 2.248(2) | 2.240(2) |
| V–Cl(2) | 2.2532(7) | 2.2418(18) | 2.266(2) |
| Bond Angles (deg) | |||
| V–N(1)–C(10) | 162.03(18) | 168.3(4), V–N(1)–C(4) | 164.7(5), V–N(1)–C(1) |
| Cl(1)–V–Cl(2) | 124.04(3) | 128.38(7) | 125.01(8) |
| N(1)–V–N(2) | 178.37(8) | 179.1(2) | 171.1(3) |
| N(1)–V–N(3) | 101.27(10) | 99.7(2) | 98.2(3) |
| N(2)–V–N(3) | 78.99(8) | 79.33(19) | 78.3(2) |
| Cl(1)–V–N(1) | 96.12(7) | 94.9(2) | 101.6(2) |
| Cl(1)–V–N(2) | 85.20(5) | 85.38(15) | 87.35(16) |
| Cl(1)–V–N(3) | 114.99(6) | 113.88(17) | 112.02(19) |
| Cl(2)–V–N(1) | 94.06(7) | 95.16(17) | 91.1(2) |
| Cl(2)–V–N(2) | 84.40(5) | 85.31(13) | 83.59(16) |
| Cl(2)–V–N(3) | 116.52(6) | 113.95(17) | 118.78(19) |
Structure reports and xyz files for complexes 1–3 are shown in the Supporting Information.
The V–N(1)–C (in the imido ligand) bond angles in 1 and 2 [162.03(18) and 168.3(4)°] are apparently smaller than that in 4 [170.94(10)°],54 and the Cl(1)–V–N(1) and Cl(2)–V–N(1) bond angles [1: 96.12(7)° and 94.06(7)°; 2: 94.9(2)° and 95.16(17)°] are also slightly smaller than those in 4 [98.90(3)° and 96.02(4)°].54 The imido substituents are slightly bent toward the chloride ligands, probably due to a steric influence of the phenyl group in the benzimidazolyl ligand. These Cl(1)–V–N(1) and Cl(2)–V–N(1) bond angles are larger than the Cl(1)–V–N(2) and Cl(2)–V–N(2) angles [1: 85.20(5)° and 84.40(5)°; 2: 85.38(15)° and 85.31(13)°], which are similar to those in 4 [85.49(3)° and 83.93(3)°].54
It turned out that the mean deviations in the plane consisting of vanadium, imido nitrogen, and three nitrogens in the 2-(2′-benzimidazolyl)-6-methylpyridine ligand including aromatic rings in 1–3 are 0.0315, 0.0234, and 0.00965 Å, respectively. The results thus clearly indicate that these ligand frames possessed a plane perpendicular to a plane consisting of two chlorine and vanadium atoms [dihedral angles in 1: 90.053° and 2: 89.298°], as observed previously in the related titanium complexes,65,66 although the dihedral angle in 3 is somewhat low [86.682°] due to a steric bulk, as described below. The results also indicate that these complexes provide more open space for the catalytic reaction compared to the reported 2-(anilidomethyl)pyridine complex (4).54
The 2,6-dimethylphenylimido analogue (3) also folds a distorted trigonal bipyramidal geometry around vanadium, as observed in 1 and 2. Probably because of a steric bulk in the two methyl groups in the imido ligand (Figure 1), the N(1)–V–N(2) bond angle [171.1(3)°] is apparently smaller than those in the others [178.37(8) and 179.1(2)°, for 1 and 2, respectively], and the V–N(1) bond distance [1.679(6) Å] is longer than those in others [1.647(2) and 1.651(4) Å, for 1 and 2, respectively]. Moreover, the Cl(1)–V–N(1) and Cl(1)–V–N(2) bond angles [101.6(2) and 87.35(16)°, respectively] are apparently larger than the Cl(2)–V–N(1) and Cl(2)–V–N(2) bond angles [91.1(2)° and 83.59(16), respectively]. Similarly, the Cl(1)–V–N(3) bond angle [112.02(19)°] is smaller than the Cl(2)–V–N(3) bond angle [118.78(19)°], and the V–Cl(2) distance [2.266(2) Å] is apparently longer than that in V–Cl(1) [2.240(2) Å]. These would be due to a steric bulk (the phenyl substituent in the imido ligand is bent).
2.2. Reaction with Ethylene in the Presence of Al Cocatalysts
Reactions with ethylene using V(NR)Cl2(L) [R = Ad (1), C6H5 (2), and 2,6-Me2C6H3 (3)] were conducted in toluene at 25 °C in the presence of the Al cocatalyst [Me2AlCl or the methylaluminoxane white solid prepared by removing toluene and AlMe3 from the commercially available sample, TMAO, 9.5 wt % (Al) toluene solution, Tosoh Finechem Co.].57 The results are summarized in Tables 2 and 3.
Table 2. Reaction with Ethylene Using V(NR)Cl2(L) [R = Ad (1), C6H5 (2), and Ar (3, Ar = 2,6-Me2C6H3); L = 2-(2′-Benzimidazolyl)-6-methylpyridine] in the Presence of Al Cocatalystsa.
| PEc |
oligomer (C4–C22)f |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| run | V(NR)Cl2(L), R (μmol) | Al cocat. | Al/Vb | wt % | yield/mg | activityd | TOFe/h–1 | activityd | TOFe/h–1 | C4–C22g total | C4g/wt % | C6g/wt % | C8g/wt % | otherh/wt % |
| 1 | Ad (0.5) | MAO | 500 | 16.7 | 9.6 | 115 | 4100 | 574 | 20 500 | 68.1 | 30.5 | 15.8 | 11.8 | 15.1 |
| 2 | Ad (0.5) | MAO | 1000 | 23.7 | 13.8 | 165 | 5890 | 532 | 19 000 | 66.4 | 26.6 | 15.2 | 9.8 | 10.0 |
| 3 | Ad (0.5) | MAO | 2000 | 22.0 | 11.2 | 134 | 4780 | 476 | 17 000 | 64.5 | 26.0 | 14.8 | 9.5 | 13.3 |
| 4 | Ad (0.2) | MAO | 500 | 29.5 | 7.7 | 231 | 8220 | 434 | 15 500 | 55.6 | 20.1 | 15.5 | 9.2 | 14.9 |
| 5 | Ad (0.2) | MAO | 1000 | 28.4 | 8.7 | 261 | 9290 | 534 | 19 000 | 58.3 | 20.5 | 15.9 | 10.6 | 13.2 |
| 6 | Ad (0.2) | Me2AlCl | 1250 | >99 | 311 | 9320 | 332 000 | |||||||
| 7 | Ad (0.2) | Me2AlCl | 2500 | >99 | 327 | 9790 | 349 000 | |||||||
| 8 | C6H5 (0.2) | MAO | 1000 | 49.5 | 7.6 | 228 | 8110 | 232 | 8290 | 30.7 | 12.5 | 7.9 | 6.7 | 19.8 |
| 9 | Ar (0.2) | MAO | 500 | 96.1 | 102 | 3050 | 109 000 | 124 | 4420 | 1.9 | 1.0 | 0.2 | 0.6 | 2.0 |
| 10 | Ar (0.2) | MAO | 1000 | 93.1 | 109 | 3270 | 117 000 | 244 | 8700 | 3.9 | 2.1 | 1.2 | 0.5 | 3.1 |
Conditions: ethylene 8 atm, toluene 30 mL, 25 °C, 10 min.
Al/V molar ratio.
Isolated as MeOH/HCl insoluble portion; details are shown in the Experimental Section.
Activity in kg-ethylene reacted/mol-V·h [also kg-PE/mol-V·h].
TOF = (molar amount of ethylene reacted)/(mol-V·h).
Oligomer (analyzed by GC and CHCl3 extracted portion); details are shown in the Experimental Section.
Oligomer (C4–C22 fraction) analyzed by GC vs internal standards.
Wax fraction (isolated as CHCl3 soluble fraction after pouring the mixture into MeOH/HCl).
Table 3. Ethylene Polymerization Using V(NR)Cl2(L) [R = Ad (1), C6H5 (2), and Ar (3, Ar = 2,6-Me2C6H3); L = 2-(2′-Benzimidazolyl)-6-methylpyridine] in the Presence of Al Alkyl Cocatalystsa,f.
| run | V(NR)Cl2(L), R (μmol) | Al cocat. | Al/Vb | ETA/Vc | time/min | yield/mg | activityd | TOFe/h–1 (s–1) |
|---|---|---|---|---|---|---|---|---|
| 6 | Ad (0.2) | Me2AlCl | 1250 | 0 | 10 | 311 | 9320 | 332 000 (92) |
| 7 | Ad (0.2) | Me2AlCl | 2500 | 0 | 10 | 327 | 9790 | 349 000 (97) |
| 11 | Ad (0.1) | Me2AlCl | 2500 | 0 | 10 | 221 | 13 300 | 472 000 (131) |
| 12 | Ad (0.1) | Me2AlCl | 2500 | 0 | 6 | 143 | 14 300 | 508 000 (141) |
| 13 | Ad (0.1) | Me2AlCl | 5000 | 0 | 6 | 265 | 26 500 | 946 000 (263) |
| 14 | Ad (0.05) | Me2AlCl | 5000 | 0 | 6 | 108 | 21 700 | 773 000 (215) |
| 15 | Ad (0.05) | Me2AlCl | 10 000 | 0 | 6 | 278e | 55 700 | 1 990 000 (553) |
| 16 | Ad (0.05) | Me2AlCl | 10 000 | 10 | 6 | 155 | 31 000 | 1 110 000 (308) |
| 17 | Ad (0.05) | Me2AlCl | 10 000 | 50 | 6 | 234 | 46 700 | 1 670 000 (464) |
| 18 | Ad (0.05) | Me2AlCl | 10 000 | 100 | 6 | 410 | 82 000 | 2 930 000 (814) |
| 19 | Ad (0.05) | Me2AlCl | 10 000 | 1000 | 6 | 269 | 53 700 | 1 920 000 (533) |
| 20 | C6H5 (0.05) | Me2AlCl | 5000 | 0 | 6 | 400 | 80 100 | 2 850 000 (792) |
| 21 | C6H5 (0.05) | Me2AlCl | 10 000 | 0 | 6 | 332 | 66 400 | 2 370 000 (658) |
| 22 | C6H5 (0.05) | Et2AlCl | 10 000 | 0 | 6 | 7.3 | 1460 | 52 000 (14.5) |
| 23 | C6H5 (0.5) | Et2AlCl | 100 | 0 | 6 | 127 | 2540 | 90 700 (25.2) |
| 24 | C6H5 (0.5) | Et2AlCl | 1000 | 0 | 6 | 73.7 | 1470 | 52 400 (14.6) |
| 25 | C6H5 (0.05) | AliBu3 | 5000 | 0 | 6 | trace | ||
| 26 | C6H5 (0.5) | AliBu3 | 100 | 0 | 6 | trace | ||
| 27 | C6H5 (0.05) | AlEt3 | 5000 | 0 | 6 | trace | ||
| 28 | C6H5 (0.5) | AlEt3 | 100 | 0 | 6 | trace | ||
| 29 | Ar (0.1) | Me2AlCl | 2500 | 0 | 6 | 299 | 29 900 | 1 070 000 (297) |
| 30 | Ar (0.1) | Me2AlCl | 5000 | 0 | 6 | 186 | 18 600 | 662 000 (184) |
| 31 | Ar (0.05) | Me2AlCl | 5000 | 0 | 6 | 106 | 21 100 | 753 000 (209) |
| 32 | Ar (0.05) | Me2AlCl | 2000 | 0 | 6 | 178 | 35 700 | 151 000 (42) |
| 33 | Ar (0.05) | Me2AlCl | 1000 | 0 | 6 | 117 | 23 400 | 834 000 (232) |
Conditions: ethylene 8 atm, toluene 30 mL, 25 °C, 10 min.
Al/V molar ratio.
ETA = Cl3CCO2Et (molar ratio).
Activity in kg-PE/mol-V·h.
TOF = (molar amount of ethylene reacted)/(mol-V·h).
Melting temperature at 136 °C by DSC thermogram (shown in the Supporting Information).
It turned out that the reactions by the 1–MAO catalyst system afforded a mixture of oligomer [C4–C22 fractions, mostly 1-butene and 1-hexene analyzed by gas chromatography (GC) of the reaction mixture] in addition to higher oligomers [CHCl3 extracted portion (that could not be measured by GC) after pouring the reaction solution into a mixture consisting of MeOH and HCl aqueous solutions and removal of volatiles] and polyethylene. Although the activities [and turn over frequency (TOF) values] by the 1–MAO catalyst system were affected by the Al/V molar ratio employed (runs 1–5), significant differences in the compositions by varying the ratio were not observed. The resultant polymers isolated (MeOH insoluble fraction) were insoluble in ordinary high-temperature gel permeation chromatography (GPC) analysis (conducted in o-dichlorobenzene at 140 °C), which suggest a possibility of obtainment of polymers with an ultrahigh molecular weight.47−50,55,56 These results thus assume that at least two catalytically active species play roles (yielding oligomers and ultrahigh-molecular-weight polymer) in the reaction mixture.
It also turned out that the reactions by the V(NAr)Cl2(L) (3)–MAO catalyst system afforded polymers that were insoluble for ordinary GPC analysis in addition to small amount of oligomers (runs 9 and 10); this is the same observation in the reaction using the 2-(anilidomethyl)pyridine analogue, V(NAr)Cl2[2-ArNCH2(C5H4N)].52 The phenylimido analogue (2) exhibited the lowest activity affording a mixture of the polymer and oligomer (run 8), whereas another phenylimido analogue, V(NC6H5)Cl2[2-ArNCH2(C5H4N)], showed higher activity than the 2,6-arylimido analogue in the reaction with ethylene under similar conditions.52,54 The ratio of oligomer/polymer (composition) was thus affected by the imido ligand employed in these catalyses.
It should be noted that the reaction by the adamantylimido analogue (1) exhibited a remarkably high catalytic activity for ethylene polymerization in the presence of the Me2AlCl cocatalyst (runs 6 and 7, Table 2), and the amount of oligomers (should be detected by GC or extracted with CHCl3 from the reaction solution) formed were negligible. Noteworthy, the activities by 1 in the presence of Me2AlCl were much higher than those in the presence of MAO. Moreover, the activities by 1 (9320 and 9790 kg-PE/mol-V·h, runs 6 and 7) were higher than that by V(NAd)Cl2[2-ArNCH2(C5H4N)] (4, e.g., 704 kg-PE/mol-V·h)55 conducted under similar conditions. Although it has often been observed in ethylene polymerization and ethylene/propylene copolymerization using (especially, classical Ziegler-type) vanadium catalysts that the use of halogenated Al alkyls is more effective than MAO,24,42−50 the present catalyst systems exhibited remarkably high catalytic activities compared to the reported systems (shown in Table 3).
Table 3 summarizes the results of ethylene polymerization by 1–3 in the presence of the Me2AlCl cocatalyst. These reactions afforded polymers exclusively, which were insoluble for the ordinary GPC analysis (in o-dichlorobenzene at 140 °C), suggesting a possibility of formation of ultrahigh-molecular-weight polymers (as also suggested from the copolymerization results with norbornene (NBE), shown below in Table 4).47−50,55,56 Differential scanning calorimetry (DSC) thermogram in the resultant polymer possesses a melting temperature at 136 °C (sample run 15, shown in the Supporting Information), strongly suggesting that the resultant polymers are linear polyethylene. Attempts for reactions with ethylene by 1 in the presence of AliBu3 (1, 0.05 μmol, Al/V = 1000, molar ratio, in toluene, ethylene 8 atm for 10 min) afforded negligible amounts of polymers/oligomers. Moreover, attempts for reactions with ethylene by 2 in the presence of AlEt3 or AliBu3 afforded a negligible amount of polymers/oligomers (runs 25–28), and the activities by 2 in the presence of Et2AlCl (in place of Me2AlCl) were rather low (runs 22–24).
Table 4. Copolymerization of Ethylene with Cyclic Olefin Using the V(NC6H5)Cl2(L) [2; L = 2-(2′-Benzimidazolyl)-6-methylpyridine]–Me2AlCl Catalyst Systema.
| run | 2/μmol | comonomer (M)b | Al/Vc | ETA/Vd | time/min | yield/mg | activitye | Mnf × 10–6 | Mw/Mnf | Tm (Tg)g/°C |
|---|---|---|---|---|---|---|---|---|---|---|
| 34 | 0.05 | NBE (0.25) | 20 000 | 0 | 10 | 58.2 | 6970 | 1.74 | 2.27 | 71 |
| 35 | 0.05 | NBE (0.25) | 10 000 | 0 | 10 | 67.6 | 8100 | 1.78 | 2.41 | 71 |
| 36 | 0.05 | NBE (0.25) | 10 000 | 50 | 10 | 118 | 14 200 | 2.49 | 2.53 | 66 |
| 37 | 0.05 | NBE (0.25) | 10 000 | 100 | 10 | 272 | 32 600 | 2.66 | 2.36 | 67 |
| 38 | 0.05 | NBE (0.25) | 10 000 | 0 | 15 | 297 | 23 700 | 1.71 | 2.30 | |
| 39 | 0.05 | NBE (0.25) | 5000 | 0 | 15 | 78.8 | 5660 | |||
| 40 | 0.05 | NBE (0.5) | 20 000 | 0 | 10 | 47.1 | 5640 | |||
| 41 | 0.05 | NBE (0.5) | 10 000 | 0 | 10 | 78.8 | 9440 | |||
| 42 | 0.05 | NBE (0.5) | 10 000 | 0 | 15 | 93.6 | 7490 | |||
| 43 | 0.05 | NBE (0.5) | 5000 | 0 | 10 | 141 | 16 900 | 3.37h | 2.73h | 42 (−10) |
| 44 | 0.05 | NBE (0.5) | 5000 | 0 | 15 | 198 | 15 900 | 3.44h | 2.75h | 45 (−8.5) |
| 45 | 0.50 | TCD (0.5) | 1000 | 0 | 10 | trace | ||||
| 46 | 0.50 | TCD (0.5) | 5000 | 0 | 10 | trace | ||||
| 47I | 0.50 | CPE (5.0) | 2000 | 0 | 10 | trace | ||||
| 48I | 0.05 | CHE (5.0) | 20 000 | 0 | 10 | trace | ||||
| 49I | 0.50 | CHE (5.0) | 2000 | 10 | 11.3 | 136 | 4.00 | 2.15 | 129 |
Conditions: ethylene 8 atm, comonomer + toluene total 30 mL, 25 °C.
Initial cyclic olefin concentration in mmol/mL.
Al/V molar ratio.
ETA = Cl3CCO2Et (molar ratio).
Activity in kg-polymer/mol-V·h.
GPC data in o-dichlorobenzene at 140 °C vs polystyrene standard.
By DSC thermograms (shown in the Supporting Information).
Measured under low polymer concentration (data with hot o-dichlorobenzene soluble portion).
Cyclic olefin (CPE, CHE) + toluene total 10.0 mL, ethylene 2 atm. .
It turned out that the activities by V(NR)Cl2(L)–Me2AlCl catalysts were affected by the Al/V molar ratio employed, and the activity under the optimized conditions increased in the order 3 (R = 2,6-Me2C6H3, 35 700 kg-PE/mol-V·h, run 32) < 1 (R = Ad, 55 700, run 15) < 2 (R = C6H5, 80 100, run 20). The phenylimido analogue (2) exhibited the highest activities, and the activity [80 100 kg-PE/mol-V·h (TOF 2 850 000 h–1, 792 s–1)] is higher than those by V(N-2,6-Cl2C6H3)Cl2(OAr)–Et2AlCl (55 800 kg-PE/mol-V·h)49 and V(NAr)Cl2(OAr)–iBu2AlCl catalyst systems (64 800 kg-PE/mol-V·h),48 which are known to exhibit the highest activities in toluene among the reported aryloxo-modified (imido)vanadium dichloride complex catalysts. It would be interesting to note that the activity by the adamantylimido analogue (1) increased upon the addition of Cl3CCO2Et (ETA) that has been known as an effective promoter (reoxidants, for the reactivation of the assumed catalytically active species),24,42−44,71 whereas a similar effect was not observed in ethylene polymerization using the V(NAr)Cl2(OAr)–Et2AlCl catalyst system.48
Copolymerizations of ethylene with various cyclic olefins [NBE, tetracyclododecene (TCD), cyclopentene (CPE), and cyclohexene (CHE)] were conducted in the presence of the V(NC6H5)Cl2(L) (2)–Me2AlCl catalyst system in toluene at 25 °C. This is because the aryloxo-modified (imido)vanadium complexes, exemplified as V(NAr)Cl2(OAr), exhibited both high catalytic activities and efficient NBE incorporation in the ethylene/NBE copolymerization,47−49 and the resultant cyclic olefin copolymers are promising materials with a high thermal resistance [glass transition temperature (Tg)], transparency with low water absorption, and so forth.72−76 The results are summarized in Table 4. Selected 13C NMR spectrum (Figure S3) and DSC thermograms (Figure S4) in the resultant polymers are shown in the Supporting Information.
It was revealed that the copolymerizations with NBE afforded ultrahigh-molecular-weight polymers with unimodal molecular weight distributions (e.g., Mn = 1.71–2.66 × 106, Mw/Mn = 2.27–2.53, runs 34–38). The activity was affected by the Al/V molar ratio and the NBE concentration employed, and the activity seemed to decrease upon increasing the NBE concentration. This is consistent with the fact that the activity in the copolymerization became low compared with that in the ethylene homopolymerization [e.g., activity: 23 700 kg-polymer/mol-V·h (run 38) vs 80 100 kg-PE/mol-V·h (run 20)]. The Mn values in the copolymer increased upon increasing the NBE contents (runs 34–38 vs 43 and 44), and the Mn values were not affected by the Al/V molar ratio. As observed in the ethylene polymerization,24,42−44,71 the activities increased upon addition of ETA, affording ultrahigh-molecular-weight copolymers (runs 35–37). The resultant polymers possessed uniform molecular weight distributions (as described above, measured by GPC in o-dichlorobenzene at 140 °C) and compositions confirmed by DSC thermograms (single melting temperature, glass transition temperature, Figure S4). These results clearly suggest that the polymerization proceeded with uniform catalytically active species.
Figure S3 shows the selected 13C NMR spectrum in the resultant poly(ethylene-co-NBE) (in 1,1,2,2-tetrachloroethane-d2 at 110 °C). All resonances could be assigned according to the previous reports,73,74,77,78 and the resultant polymer possessed microstructures corresponding to the isolated NBE incorporations in addition to the alternating NBE incorporations to a small extent. The attempted measurement of samples prepared under a high NBE concentration failed (because of poor solubility for measurement of the NMR spectrum because of ultrahigh-molecular-weight). The NBE content (8.5 mol %) estimated by the NMR spectrum is close to that conducted by the aryloxo analogue under similar conditions,47−49 demonstrating that the present catalyst (2) exhibited a similar copolymerization ability. Moreover, both the melting temperature and glass transition temperature observed by DSC thermograms (especially, samples in runs 43 and 44, shown in the Supporting Information, Figure S4) well-corresponded to those in the samples with the same NBE content prepared by the reported catalysts.73,74,77,78 The results thus strongly suggest that the resultant polymers are copolymers with random NBE incorporations.
Although the 2–Me2AlCl catalyst system gave poly(ethylene-co-NBE)s, attempted ethylene copolymerizations with TCD afforded a negligible amount of polymers (runs 45 and 46). Attempted copolymerizations with CPE and CHE conducted under a low ethylene pressure (2 atm) with a high comonomer concentration (5.0 M) afforded trace amount of polymers (runs 47 and 48) or a small amount of polyethylene (confirmed by the DSC thermogram, run 49, Figure S4).
2.3. Analysis of the Catalyst Solution Consisting of V(NAd)Cl2(L) (1) and Al Cocatalysts in Toluene by NMR, ESR, and Solution-Phase XAS
51V NMR spectra of toluene-d8 solution containing V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] and MAO or Me2AlCl (10 equiv) were measured at 25 °C, and the results are shown in Figure S2 (Supporting Information). It was revealed that a resonance ascribed to 1 at −87.0 ppm disappeared upon addition of Me2AlCl (Figure S2c), whereas no significant changes in the spectrum was observed upon addition of MAO (Figure S2b). As the disappearance of resonance upon addition of Me2AlCl suggests a possibility of formation of certain paramagnetic species, ESR spectra of similar solutions consisting of 1 and MAO or Me2AlCl were measured (Figure 2).55,79−83
Figure 2.
ESR spectra (in toluene at 25 °C, vanadium 2.5 μmol/mL) for (a–c) V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] upon addition of Al cocatalyst (10 equiv) and (d) V(NAd)Cl2[N(H)Me2]2.
It was revealed that no significant differences (no resonances ascribed to the formation of paramagnetic species) in the spectrum were observed when a toluene solution of 1 was added to MAO (10.0 equiv, Figure 2b). By contrast, resonances ascribed to the formation of a paramagnetic species were observed upon addition of Me2AlCl (10.0 equiv, Figure 2c). However, the intensity was low compared to that of the (imido)vanadium(IV) analogue, V(NAd)Cl2[N(H)Me2]280 (Figure 2d), under the same conditions. This would suggest that the percentage of ESR-observed species, probably vanadium(IV), might be low. Because disappearance of the signal in the 51V NMR spectrum was observed when 1 was added to Me2AlCl, a detailed analysis is required to explore the oxidation state of the probable catalytically active species.
We thus focus on the synchrotron XAS because the method (V–K edge analysis, 5.46 keV, through synchrotron radiation at SPring-8, BL01B1 beamline) provides information concerning the oxidation state [by V–K pre-edge and edge peaks in the X-ray absorption near-edge structure (XANES) analysis] and coordination atoms around the vanadium [by FT extended X-ray absorption fine structure (FT-EXAFS) analysis]. Figure 3 shows the XANES spectra of toluene solution containing V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] and the solution in the presence of MAO or Me2AlCl (10.0 equiv). The spectra of V(NAd)Cl2[2-ArCH2(C5H4)] (4),58 V(NAd)Cl3,67 and the (imido)vanadium(IV) complex, V(NAd)Cl2[N(H)Me2]2,80 measured under the same conditions are also shown for comparison (Figure 3a).
Figure 3.

Solution-phase V K-edge XANES spectra (in toluene at 25 °C) for (a) V(NR)Cl2(L) [R = Ad (1), C6H5 (2), L = 2-(2′-benzimidazolyl)-6-methylpyridine]. Spectra for V(NAd)Cl2[2-ArCH2(C5H4)],58 V(NAd)Cl3,58 and V(NAd)Cl2[N(H)Me2] measured under the same conditions are also placed for comparison. (b) V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] in the presence of Al cocatalyst [MAO, Me2AlCl, Me2AlCl, and ETA10.0 equiv].
As shown in Figure 3a, V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] shows two pre-edge peaks at 5465.5 and 5467.7 eV in addition to a small shoulder-edge peak at 5477.0 eV. Similarly, V(NPh)Cl2(L) (2) shows pre-edge peak(s) at 5465.7 and 5467.5 eV in addition to a small shoulder-edge peak at 5478.1 eV. These are similar to the observed facts that two pre-edge peaks were observed in V(NAd)Cl2[2-ArCH2(C5H4)] (4, 5465.2 and 5467.3 eV) and V(NAd)Cl3 (5465.6 and 5467.1 eV), and these complexes also showed a shoulder-edge peak (5477.8 and 5477.6 eV, respectively) that would be ascribed to the V–Cl bond.58 Two pre-edge peaks are probably due to a transition from 1s to 3d + 4p,84,85 although examples of solution V K-edge XANES spectra by synchrotron radiation still have been limited.86,87 By contrast, the related (imido)vanadium(IV) dichloride, V(NAd)Cl2[N(H)Me2]2, showed a broad pre-edge peak at 5466.4 eV and a shoulder-edge peak at 5477.5 eV. It is clear from the spectra that the edge peak of the vanadium(IV) dichloride complex low-shifted compared to the vanadium(V) dichloride complexes. These results also suggest that the observed shoulder-edge peak would be ascribed to the V–Cl bond.58
It was revealed that no significant differences in the XANES spectrum (pre-edge peaks and edge) from that in 1 was observed upon addition of MAO [5465.5 and 5467.7 eV (pre-edge), 5476.8 eV (shoulder-edge), Figure 3b]. These results thus suggest that the oxidation state of 1 was preserved upon addition of MAO, and the results would be in good agreement with those in the NMR and ESR spectra. By contrast, it should be noted that remarkable changes in the XANES (pre-edge and edge regions) spectrum were observed when 1 [5465.5 and 5467.7 eV (pre-edge), 5477.0 eV (shoulder-edge)] was treated with Me2AlCl [5466.0 eV (pre-edge), 5475.8 eV (shoulder-edge), Figure 4]. Apparently, the low-energy shift in the edge peak (in addition to change from two to one pre-edge peak) strongly suggests that complex 1 was reduced by reaction with Me2AlCl, and the result is consistent with that observed especially in the 51V NMR spectrum (disappearance of signal because of the generation of paramagnetic species). The remarkable changes in the XANES (pre-edge and edge regions) spectrum suggest the structural changes upon addition of Me2AlCl, especially by reduction. Moreover, the intensity in the shoulder-edge (5475.7 eV) increased upon addition of ETA on decreasing the intensity of the pre-edge peak (5465.5 eV), and this corresponds to the fact that the activity increased upon addition of ETA. The results thus suggest that the addition of ETA would be effective for the generation (increased percentage) of catalytically active species by certain structural changes with the reduction of 1.
Figure 4.

(a) Solution-phase (in toluene at 25 °C) V K-edge EXAFS oscillations and the simulated spectrum for V(NAd)Cl2(L) (1). In this simulation (on the basis of X-ray crystallographic data), the contributions from the neighbor atoms within 3.34 Å distance from the V atom were considered, and 0.0036 Å2 of the Debye–Waller factor was applied. (b) Solution-phase V K-edge FT-EXAFS spectra (in toluene at 25 °C) for V(NAd)Cl2(L) (1) upon addition of Me2AlCl (10 equiv). Additional analysis data are shown in the Supporting Information.
Figure 4a shows the EXAFS oscillations and the simulated spectrum of 1 in toluene.82 The observed spectrum shows a good fitting with that estimated from the X-ray crystallographic data (Figure 4a). Three nitrogen atoms are coordinated to vanadium [coordination number (CN) 1.7(2), V–N = 1.683 Å; CN 1.2(8), V–N = 2.290(42) Å], which probably corresponds to three vanadium–nitrogen bonds [V–N(1): 1.647(2), V–N(2): 2.279(2), and V–N(3): 1.918(2) Å, Table 1]. The spectrum also suggests the presence of two V–Cl bonds [CN 1.6(2), V–Cl = 2.293(3) Å], which also correspond well to the two vanadium–chloride bonds [V–Cl(1): 2.2376(7) and V–Cl(2): 2.2532(7) Å]. The results clearly indicate that complex 1 preserves the basic trigonal bipyramidal structure (determined by X-ray crystallography) in solution. It was revealed that the CN of the vanadium–nitrogen bond decreased upon addition of Me2AlCl [CN 0.9(3), V–N: 1.64(2) Å, additional analysis data are shown in the Supporting Information], suggesting that two nitrogen bonds (maybe in L) would probably be dissociated upon addition of Me2AlCl (Figure 4b). We are unsure about the reason for the presence of two/three V–Cl bonds [CN 2.6(1), V–Cl = 2.455(7) Å], which became apparently weak compared to those in the original (complex 1, V–Cl = 2.293(3) Å). Taking into account the result in Figure 4, we can at least say that reduction occurred from 1 (probably by dissociation of two vanadium–nitrogen bonds) upon addition of Me2AlCl, although the detailed structure in the real catalytically active species is still unclear at this moment.
Through 51V NMR, ESR, and XAS analyses, it was revealed that (i) no resonances in the 51V NMR spectrum were observed when V(NAd)Cl2(L) [1, L = 2-(2′-benzimidazolyl)-6-methylpyridine] was treated with 10.0 equiv of Me2AlCl in toluene-d8 at 25 °C, (ii) formation of paramagnetic species (but weak intensity) was observed in the toluene solution of 1 upon addition of Me2AlCl (10 equiv) in the ESR spectrum, whereas no resonances were observed upon addition of MAO in place of Me2AlCl, and (iii) remarkable changes in the XANES (pre-edge and edge regions) spectrum was observed when 1 was added with Me2AlCl in toluene, whereas no significant differences were observed upon addition of MAO. These results strongly suggest the observed difference as the effect of the Al cocatalyst (MAO vs Me2AlCl) in the reaction with ethylene using 1 because of the formation of different catalytically active species with different oxidation states. Because the observed XANES spectra upon addition of Me2AlCl are different (especially, the edge region) from those in (adamantylimido)vanadium(IV) dichloride, V(NAd)Cl2[N(H)Me2]2, and the intensity of resonances observed in the ESR spectrum (Figure 3c) was weak, it thus seems likely and may be assumed that certain vanadium(III) species by reduction was generated from 1 upon addition of Me2AlCl, although we could not come to any detailed conclusion (structure, oxidation state) at this stage from the EXAFS spectrum.
3. Concluding Remarks
Three (imido)vanadium(V) dichloride complexes containing 2-(2′-benzimidazolyl)-6-methylpyridine ligand (L) of type, V(NR)Cl2(L) [R = 1-adamantyl (Ad, 1), C6H5 (2), 2,6-Me2C6H3 (3)], were prepared, and their structures could be determined by X-ray crystallographic analysis. These complexes fold distorted trigonal bipyramidal structures around vanadium and a plane consisting of three nitrogen atoms in the 2-(2′-benzimidazolyl)pyridine ligand and the imido ligand positioned perpendicular to a plane consisting of two chloride and vanadium. These complexes (1–3) exhibited catalytic activities for the reaction with ethylene in the presence of MAO, affording a mixture of oligomer and polymers, and the compositions were affected by the imido ligand employed. By contrast, the complexes (1–3) exhibited remarkable catalytic activities for ethylene polymerization in the presence of Me2AlCl. The phenylimido complex (2) exhibited an exceptionally high activity [80 100 kg-PE/mol-V·h (TOF 2 850 000 h–1, 792 s–1)], which was higher than those reported previously by the aryloxo-modified (imido)vanadium dichloride complexes, demonstrating its promising capability as the efficient ethylene polymerization catalyst. Complex 2 was also effective for ethylene copolymerization with NBE to afford ultrahigh-molecular-weight copolymers with uniform molecular weight distributions and compositions, suggesting that the polymerization proceeds with uniform catalytically active species.
On the basis of the studies by NMR and ESR spectra and solution V K-edge XAS (XANES and EXAFS) analysis, the observed difference in the catalyst performances in the presence of (between) MAO and Me2AlCl cocatalyst should be due to the formation of different catalytically active species with different oxidation states. In particular, significant changes in the oxidation state were observed upon addition of Me2AlCl in the XANES spectrum (in addition to NMR and ESR spectra), and the intensity in the shoulder-edge increased upon addition of ETA with decreasing intensity of the preedge peak, whereas no significant changes in the spectra were observed upon the presence of MAO. Although the detailed structural analysis of the catalyst solution consisting of 1 and Me2AlCl could not be done at this moment, the observed fact should provide important information for a better understanding of the catalysis mechanism and catalyst design. Moreover, we believe that a combination of all these analyses (NMR and ESR spectra and XAS analysis) should be helpful in providing more clear information for better understanding and explanation. We are now exploring more details, including further analyses of the catalyst solutions (and complexes) through solution XAS (XANES, EXAFS) analysis, including assignments of resonances; these would be introduced in the future.
4. Experimental Section
4.1. General Procedure
All experiments were conducted in a Vacuum Atmospheres drybox under a nitrogen atmosphere. Toluene, n-hexane, and dichloromethane of anhydrous grade (Kanto Kagaku Co., Ltd.) were stored in a bottle containing molecular sieves (a mixture of 3A 1/16, 4A 1/8, and 13X 1/16) in the drybox; solvents were further purified by passing through an alumina short column under a nitrogen stream prior to use. V(NAd)Cl3 (Ad = 1-adamantyl),67 V(NC6H5)Cl3,54 V(N-2,6-Me2C6H3)Cl3,68 and V(NAd)Cl2[N(H)Me2]280 were prepared according to a published method. Me2AlCl (1.0 M in n-hexane, Kanto Kagaku Co., Ltd.) and ethylene for polymerization (purity >99.9%, Sumitomo Seika Co. Ltd.) were used as received. Toluene and AlMe3 in methylaluminoxane [TMAO, 9.5 wt % (Al) toluene solution, Tosoh Finechem] were removed in vaccuo (at ca. 50 °C for removal of toluene and AlMe3, and then heated at >100 °C for 1 h for completion) in the drybox to afford white solids.57
Elemental analyses were performed by using a EAI CE-440 CHN/O/S elemental analyzer (Exeter Analytical, Inc.). All 1H, 13C, and 51V NMR spectra were recorded on a Bruker AV500 spectrometer (500.13 MHz for 1H, 125.77 MHz for 13C, and 131.55 MHz for 51V). All spectra were obtained in the solvent indicated at 25 °C, unless otherwise noted. Chemical shifts are given in ppm and are referenced to SiMe4 (δ 0.00 ppm, 1H, 13C) and VOCl3 (δ 0.00 ppm, 51V). Coupling constants and half-width values, Δν1/2, are given in Hz. 13C NMR spectra for the resultant polymers were recorded with proton decoupling, the pulse interval was 5.2 s, the acquisition time was 0.8 s, the pulse angle was 90°, and the number of transients accumulated was about 6000. The copolymer samples for analysis were prepared by dissolving the polymers in 1,1,2,2-tetrachloroethane-d2 solution, and the spectra was measured at 110 °C. GC analysis was performed with a SHIMADZU GC-2025AF gas chromatograph (Shimadzu Co. Ltd.) equipped with a flame ionization detector.
Molecular weights and molecular weight distributions of the prepared polymers were measured by GPC (Tosoh HLC-8121GPC/HT) using an RI-8022 detector (for high temperature; Tosoh Co.) with a polystyrene gel column (TSK gel GMHHR-H HT32, 30 cm, 37.8 mm i.d.), ranging from <102 to <2.8 × 108 MW) at 140 °C using o-dichlorobenzene containing 0.05 w/v % 2,6-di-tert-butyl-p-cresol as the solvent. DSC data for the polymer were recorded by means of a Hitachi DSC-7020 instrument under a nitrogen atmosphere [preheating from 30 to 300 °C (20 °C/min), cooling to −100 °C under N2, and measurement from −100 to 300 °C (20 °C/min) under N2]. This heating and cooling was repeated two times. Tg values were determined from the middle point of the phase transition of the second heating scan.
4.1.1. Synthesis of V(NAd)Cl2(L) [1, L = 2-(2′-Benzimidazolyl)-6-methylpyridine]
(i) Synthesis of LK. Into the THF solution (15 mL) containing 2-(2′-6′-methylpyridiyl)-benzimidazole (836 mg, 4.0 mmol),69 KH powder (160 mg, 4.0 mmol) was added at −30 °C in the drybox. The reaction mixture was warmed slowly to room temperature and stirred for 4 h. The resultant white solid (insoluble in THF) was then collected and dried in vacuo (935 mg, 94%). (ii) Synthesis of complex 1. To a toluene solution (20 mL) containing V(NAd)Cl3 (568 mg, 1.82 mmol), LK (450 mg, 1.82 mmol) was added at −30 °C. The reaction mixture was warmed slowly to room temperature and stirred overnight (ca. 16 h). The resultant red brown solution was filtered and extracted with toluene. The extracts were filtered through a Celite pad, and the filtercake was washed with toluene. The toluene extract (combined filtrate and the wash) was then placed in vacuo to remove volatiles, giving deep red solids. The resultant solids were then dissolved in a minimum amount of dichloromethane layered by n-hexane. The chilled solution placed in the freezer (−30 °C) yielded deep red microcrystals, which were dried in vacuo. Yield 403 mg (46%). 1H NMR (CDCl3): δ 8.32 (d, J = 7.55 Hz, 1H), 8.16 (s, 1H), 7.85 (s, 1H), 7.66 (s, 1H), 7.35–7.41 (m, 3H), 2.94 (s, 3H), 2.61 (br s, 6H), 2.28 (br s, 1H), 1.82 (br s, 2H), 1.79 (br s, 2H), 1.74 (br s, 2H), 1.71 (br s, 1H). 13C NMR (CDCl3): δ 159.5, 147.4, 142.9, 139.5, 128.4, 126.2, 125.4, 118.9, 116.0, 42.8, 35.8, 29.4, 23.9. 51V NMR (CDCl3): δ −78.33 (Δν1/2 = 1950 Hz). Anal. Calcd for C23H25Cl2N4V: C, 57.63; H, 5.26; N, 11.69%. Found: C, 57.81; H, 5.46; N, 11.80%.
4.1.2. Synthesis of V(NC6H5)Cl2(L) (2)
The procedure for the synthesis of 2 was similar to that for 1, except that V(NC6H5)Cl3 (485 mg, 1.95 mmol) in place of V(NAd)Cl3 and LK (483 mg, 1.95 mmol) were used. After the reactions and extraction with toluene, the resultant solids after the removal of volatiles were dissolved with a minimum amount of dichloromethane layered by n-hexane. The chilled solution placed in the freezer (−30 °C) yielded deep red microcrystals, which were dried in vacuo. Yield 621 mg (75%). 1H NMR (CDCl3): δ 8.10 (d, J = 8.05 Hz, 1H), 7.93 (d, J = 7.70 Hz, 1H), 7.75 (d, J = 7.75 Hz, 2H), 7.71 (d, J = 7.85 Hz, 1H), 6.99 (t, J = 7.58 Hz, 1H), 6.84 (td, J = 7.71 Hz, 0.98, 1H), 6.73–6.78 (m, 3H), 6.65 (t, J = 7.53 Hz, 1H), 6.38 (d, J = 7.65 Hz, 1H), 2.87 (s, 3H, CH3). 13C NMR (CDCl3): δ 24.0, 117.5, 118.9, 125.9, 126.2, 128.7, 129.2, 129.7, 131.4, 139.9, 142.8, 145.34, 147.4, 158.2, 159.7, 162.0. 51V NMR (CDCl3): δ 41.70 (Δν1/2 = 1470 Hz). Anal. Calcd for C19H15Cl2N4V: C, 54.18; H, 3.59; N, 13.30%. Found: C, 53.89; H, 3.57; N, 13.22%.
4.1.3. Synthesis of V(N-2,6-Me2C6H3)Cl2(L) (3)
The procedure for synthesis of 3 was similar to that for 1, except that V(N-2,6-Me2C6H3)Cl3 (206 mg, 0.744 mmol) in place of V(NAd)Cl3 and LK (184 mg, 0.744 mmol) were used. After the reactions and extraction with toluene, the resultant solids after the removal of volatiles were dissolved with a minimum amount of dichloromethane layered by n-hexane. The chilled solution placed in the freezer (−30 °C) yielded deep red microcrystals, which were dried in vacuo. Yield 184 mg (55%). 1H NMR (CDCl3): δ 8.18 (br s, 1H), 7.88 (br s, 1H), 7.74 (d, 1H, J = 7.8 Hz), 7.64 (br s, 1H), 7.42 (br s, 1H), 7.07 (br s, 4H), 3.05 (s, 3H), 2.92 (s, 6H). 13C NMR (CDCl3): δ 20.1, 24.2, 117.1, 118.8, 125.9, 126.0, 128.5, 128.7, 130.8, 139.7, 143.0, 144.9, 146.4, 147.3, 158.6, 159.8, 162.8. 51V NMR (CDCl3): δ 131.19 (Δν1/2 = 1950 Hz). Anal. Calcd for C21H19Cl2N4V·0.5 toluene: C, 59.41; H, 4.68; N, 11.31%. Found: C, 58.91; H, 4.61; N, 11.55%.
4.2. Reaction with Ethylene
Reactions with ethylene were conducted in a 100 mL scale stainless steel autoclave, and the typical reaction procedure is as follows. Toluene (29.0 mL) and the prescribed amount of MAO or Me2AlCl (1.0 M n-hexane) were added into the autoclave in the drybox. The reaction apparatus was then filled with ethylene (1 atm), and the prescribed amount of complex, V(NR)Cl2(L) [L = 2-(2′-benzimidazolyl)-6-methylpyridine], in toluene (1.0 mL) was added into the autoclave. The reaction apparatus was then immediately pressurized to 7 atm (total 8 atm, kept constant during the reaction), and the mixture was magnetically stirred for 10 or 15 min. After the reaction, the remaining ethylene was purged at −30 °C, and 0.5 g of n-heptane (internal standard) was added. Both the activity and product distribution were then analyzed by GC. After the above oligomerization procedure, the mixture remaining in the reactor was poured into MeOH containing HCl; the resultant white precipitate was collected on a filter paper by filtration, and the powder was adequately washed with MeOH. The resultant polymer was then dried in vacuo at 60 °C for 2 h. The MeOH soluble portion was extracted with CHCl3, washed with water, and dried through Na2SO4. Removal of volatiles afforded a high-molecular-weight oligomer (or a low-molecular-weight PE).
4.3. Copolymerization of Ethylene with Cyclic Olefins by V(NC6H5)Cl2(L) (2)–Me2AlCl Catalyst System
The polymerization procedures conducted were similar to those conducted in ethylene polymerization/oligomerization. Toluene (29.0 or 9.0 mL) and the prescribed amount of cyclic olefin (NBE etc.) and Me2AlCl (1.0 M n-hexane) were added into the autoclave in the drybox. The reaction apparatus was then filled with ethylene (1 atm), and the prescribed amount of the complex, V(NC6H5)Cl2(L) (2), in toluene (1.0 mL) was added into the autoclave. The reaction apparatus was then immediately pressurized to 1 or 7 atm (total 2 or 8 atm), and the mixture was stirred for 6 or 10 min. After the reaction, the mixture in the reactor was poured into MeOH containing HCl, and the resultant polymer was collected on a filter paper by filtration, which was adequately washed with MeOH. The resultant polymer was then dried in vacuo at 60 °C for 2 h. Selected 13C NMR spectra (Figure S3) and DSC thermograms (Figure S4) in the resultant polymers are shown in the Supporting Information.
4.4. Crystallographic Analysis
All measurements were made on a Rigaku XtaLAB P200 diffractometer using multilayer mirror monochromated Mo Kα radiation. The crystal collection parameters are listed in Table 5. The data were collected and processed using CrystalClear (Rigaku)88 or CrysAlisPro (Rigaku Oxford Diffraction),89 and the structure was solved by direct methods90 and expanded using Fourier techniques. The nonhydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. All calculations were performed using the CrystalStructure91 crystallographic software package, except for refinement, which was performed using SHELXL version 2014/7.92,93 Structure reports and xyz files for V(NR)Cl2(L) [R = 1-adamantyl (Ad, 1), C6H5 (2), 2,6-Me2C6H3 (3); L = 2-(2′-benzimidazolyl)-6-methylpyridine] are shown in the Supporting Information. CCDC reference numbers of 1–3 are CCDC 1544623–1544625, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Table 5. Crystal Data and Collection Parameters of [V(NR)Cl2(L)] [R = Ad (1), C6H5 (2), 2,6-Me2C6H3 (3); L = 2-(2′-Benzimidazolyl)-6-methylpyridyl]a.
| 1b | 2 | 3 | |
|---|---|---|---|
| formula | C24H27Cl4N4V | C19H15Cl2N4V | C42H38Cl4N8V2 |
| formula weight | 564.26 | 421.20 | 898.51 |
| crystal color, habit | red, block | red, plate | red, block |
| crystal size (mm) | 0.120 × 0.110 × 0.060 | 0.100 × 0.080 × 0.060 | 0.100 × 0.060 × 0.020 |
| crystal system | monoclinic | triclinic | orthorhombic |
| space group | P21 (#4) | P1̅ (#2) | P212121 (#19) |
| a (Å) | 8.3276(11) | 7.4884(7) | 7.2388(3) |
| b (Å) | 11.0967(15) | 9.3222(9) | 15.3494(8) |
| c (Å) | 13.592(2) | 13.6821(15) | 36.6025(16) |
| α (deg) | 70.369(10) | ||
| β (deg) | 99.934(4) | 87.341(8) | |
| γ (deg) | 88.592(8) | ||
| V (Å3) | 1237.2(3) | 898.60(17) | 4066.9(3) |
| Z value | 2 | 2 | 4 |
| Dcalcd (g/cm3) | 1.515 | 1.557 | 1.467 |
| F000 | 580.00 | 428.00 | 1840.00 |
| temp (K) | 93(2) | 93(2) | 93(2) |
| μ (Mo Kα) (cm–1) | 8.533 | 8.590 | 7.642 |
| no. of reflections measured (Rint) | total: 10 045, unique: 4251 (0.0327) | total: 7640, unique: 3837 (0.0756) | total: 31 734, unique: 8255 (0.1129) |
| 2θmax (deg) | 55.0 | 55.0 | 55.0 |
| no. of observations [I > 2.00σ(I)] | 4251 | 3837 | 8255 |
| no. of variables | 298 | 235 | 505 |
| R1 [I > 2.00σ(I)] | 0.0214 | 0.0730 | 0.0657 |
| wR2 [I > 2.00σ(I)] | 0.0538 | 0.1938 | 0.1595 |
| goodness of fit | 1.019 | 0.999 | 1.007 |
Structure reports and xyz files for complexes 1–3 are shown in the Supporting Information. CCDC reference numbers for 1–3 are 1544623–1544625, respectively.
Structure for 1 contains CH2Cl2.
4.5. 51V NMR Experiments in the Reaction of V(NAd)Cl2(L) [1, L = 2-(2′-Benzimidazolyl)-6-methylpyridine] with Al Cocatalysts in Toluene-d8
The typical procedure is as follows. Into a toluene-d8 solution (ca. 0.6 mL) containing 1 (25 μmol, ca. 42 μmol/mL) placed in the freezer (−30 °C), Al cocatalyst (Me2AlCl or MAO; 250 μmol, 10.0 equiv) was added. The mixture was then measured by 51V NMR spectrum at 25 °C within 10 min after the preparation.
4.6. ESR Measurements
ESR measurement was performed with a Bruker ER073 spectrometer. A toluene solution containing V(NAd)Cl2(L) (1) and a toluene solution containing MAO (10.0 equiv) or Me2AlCl (10.0 equiv) were mixed with their concentrations being kept at 2.50 μmol/mL for 1 and 25.0 μmol/mL for MAO or Me2AlCl. The tube was then placed into the instrument preset (ESR measurements were started in less than 10 min after the preparation). The experimental parameters were as follows: 9.4 GHz frequency, 0.10 mT modulation amplitude, and power 1.0 mW for 1 + Me2AlCl and 1 + MAO [9.85 GHz frequency for 1 and V(NAd)Cl2{N(H)Me2}2].
4.7. Analysis of the Catalyst Solution by Solution-Phase XAS
V K-edge XANES and XAFS measurements were carried out at the BL01B1 beam line at the SPring-8 facility of the Japan Synchrotron Radiation Research Institute (proposal nos. 2016A1455 and 2016B1509). The measurements were conducted at room temperature. A Si(111) two-crystal monochromator was used for the incident beam. V K-edge XAFS spectra of V complex samples (prepared as toluene solution, 50 μmol/mL) were recorded in the fluorescence mode using an ionization chamber as the I0 detector and 19 solid-state detectors as the I detector. The X-ray energy was calibrated using V2O5, and the data analysis was performed with REX2000 ver. 2.5.9 software package (Rigaku Co.). The XANES data were analyzed by removing the atomic absorption background using a cubic spline from the χ spectra and normalization of them to the edge height.
Acknowledgments
This project was partly supported by grant-in-aid for Scientific Research on Innovative Areas (“3D Active-Site Science”, no. 26105003) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and grant-in-aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS, no. 15H03812). The synchrotron XAFS analysis was performed at SPring-8 beam lines of BL01B1 with the approval of JASRI (2016A1455, 2016B1509, and 2017A1512). The authors also express their heartfelt thanks to Prof. Z. Maeno, Prof. K. Jitsukawa, and Prof. K. Kaneda (Osaka University) for their big support for collaboration of XAFS analysis at SPring-8. K.N. expresses his thanks to Takumi Yamada, Takuya Omiya, and Dr. Shunsuke Sueki (Tokyo Metropolitan Univ., TMU) for helping with the measurement of synchrotron XAS analysis at SPring 8, to Profs. S. Komiya and A. Inagaki (TMU) for discussions, and to Tosoh Finechem Co. for donating MAO. K.N. expresses his thanks to the Chinese Academy of Sciences, President’s International Fellowship Initiative (PIFI) for the support to conduct international collaboration research.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01225.
Additional experimental results in the attempted reaction of V(NAd)Cl3 with the potassium salt of 2-(2′-benzimidazolyl)pyridine (51V NMR data), selected 13C NMR spectrum and DSC thermograms in the resultant polymers in the (co)polymerization using the V(NR)Cl2(L) [R = Ad (1), C6H5 (2), L = 2-(2′-benzimidazolyl)-6-methylpyridine]–Me2AlCl catalyst system, additional EXAFS spectra and curve fittings for V(NAd)Cl2(L) (1) by calculations on the basis of X-ray crystallographic structure (PDF)
Structure reports for V(NR)Cl2(L) [R = 1-adamantyl (Ad, 1), C6H5 (2), 2,6-Me2C6H3 (3); L = 2-(2′-benzimidazolyl)-6-methylpyridine] (XYZ)
The authors declare no competing financial interest.
Supplementary Material
References
- Selected early reviews/accounts (polymerization, refs (1)–9):Jordan R. F. Chemistry of cationic dicyclopentadienyl group 4 metal-alkyl I complexes. Adv. Organomet. Chem. 1991, 32, 325–387. 10.1016/S0065-3055(08)60482-7. [DOI] [Google Scholar]
- Marks T. J. Surface-bound metal hydrocarbyls. Organometallic connections between heterogeneous and homogeneous catalysis. Acc. Chem. Res. 1992, 25, 57–65. 10.1021/ar00014a001. [DOI] [Google Scholar]
- Brintzinger H. H.; Fischer D.; Mülhaupt R.; Rieger B.; Waymouth R. M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem., Int. Ed. Engl. 1995, 34, 1143–1170. 10.1002/anie.199511431. [DOI] [Google Scholar]
- Kaminsky W.; Arndt M. Metallocenes for polymer catalysis. Adv. Polym. Sci. 1997, 127, 143–187. 10.1007/bfb0103631. [DOI] [Google Scholar]
- McKnight A. L.; Waymouth R. M. Group 4ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. 10.1021/cr940442r. [DOI] [PubMed] [Google Scholar]
- Suhm J.; Heinemann J.; Wörner C.; Müller P.; Stricker F.; Kressler J.; Okuda J.; Mülhaupt R. Novel polyolefin materials via catalysis and reactive processing. Macromol. Symp. 1998, 129, 1–28. 10.1002/masy.19981290103. [DOI] [Google Scholar]
- Coates G. W.; Hustad P. D.; Reinartz S. Catalysts for the living insertion polymerization of alkenes: access to new polyolefin architectures using Ziegler–Natta chemistry. Angew. Chem., Int. Ed. 2002, 41, 2236–2257. . [DOI] [PubMed] [Google Scholar]
- Britovsek G. J. P.; Gibson V. C.; Wass D. F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem., Int. Ed. 1999, 38, 428–447. . [DOI] [PubMed] [Google Scholar]
- Gibson V. C.; Spitzmesser S. K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. 10.1021/cr980461r. [DOI] [PubMed] [Google Scholar]
- Recent selected reviews (oligomerization, refs (10)–15):Pillai S. M.; Ravindranathan M.; Sivaram S. Dimerization of ethylene and propylene catalyzed by transition-metal complexes. Chem. Rev. 1986, 86, 353–399. 10.1021/cr00072a004. [DOI] [Google Scholar]
- Skupinska J. Oligomerization of α-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. 10.1021/cr00004a007. [DOI] [Google Scholar]
- McGuinness D. S. Olefin oligomerization via metallacycles: Dimerization, trimerization, tetramerization, and beyond. Chem. Rev. 2011, 111, 2321–2341. 10.1021/cr100217q. [DOI] [PubMed] [Google Scholar]
- Agapie T. Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 2011, 255, 861–880. 10.1016/j.ccr.2010.11.035. [DOI] [Google Scholar]
- van Leeuwen P. W. N. M.; Clément N. D.; Tschan M. J.-L. New processes for the selective production of 1-octene. Coord. Chem. Rev. 2011, 255, 1499–1517. 10.1016/j.ccr.2010.10.009. [DOI] [Google Scholar]
- Small B. L. Discovery and development of pyridine-bis(imine) and related catalysts for olefin polymerization and oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. 10.1021/acs.accounts.5b00252. [DOI] [PubMed] [Google Scholar]
- For selected pioneering examples (oligomerization, refs (16)–22):Killian C. M.; Johnson L. K.; Brookhart M. Preparation of linear α-olefins using cationic nickel(II) α-diimine catalysts. Organometallics 1997, 16, 2005–2007. 10.1021/om961057q. [DOI] [Google Scholar]
- Svejda S. A.; Brookhart M. Ethylene oligomerization and propylene dimerization using cationic (α-diimine)nickel(II) catalysts. Organometallics 1999, 18, 65–74. 10.1021/om980736t. [DOI] [Google Scholar]
- Komon Z. J. A.; Bu X.; Bazan G. C. Synthesis, characterization, and ethylene oligomerization action of [(C6H5)2PC6H4C(O-B(C6F5)3)O-κ2P,O]Ni(η3-CH2C6H5). J. Am. Chem. Soc. 2000, 122, 12379–12380. 10.1021/ja002042t. [DOI] [Google Scholar]
- Speiser F.; Braunstein P.; Saussine L. Catalytic ethylene dimerization and oligomerization: Recent developments with nickel complexes containing P,N-chelating ligands. Acc. Chem. Res. 2005, 38, 784–793. 10.1021/ar050040d. [DOI] [PubMed] [Google Scholar]
- Small B. L.; Brookhart M. Iron-based catalysts with exceptionally high activities and selectivities for oligomerization of ethylene to linear α-olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. 10.1021/ja981317q. [DOI] [Google Scholar]
- Britovsek G. J. P.; Mastroianni S.; Solan G. A.; Baugh S. P. D.; Redshaw C.; Gibson V. C.; White A. J. P.; Williams D. J.; Elsegood M. R. J. Oligomerisation of ethylene by bis(imino)pyridyliron and -cobalt complexes. Chem.—Eur. J. 2000, 6, 2221–2231. . [DOI] [PubMed] [Google Scholar]
- Reagan W. K. (Phillips Petroleum Company) EP 0417477, 1991.
- Selected recent reviews/accounts (polymerization, refs (23)–33):; Organometallic Reactions and Polymerization; Osakada K., Ed.; The Lecture Notes in Chemistry 85; Springer-Verlag: Berlin, 2014. [Google Scholar]
- Nomura K.; Zhang S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. 10.1021/cr100207h. [DOI] [PubMed] [Google Scholar]; . References (concerning vanadium complex catalyzed olefin polymerization) are cited therein
- Makio H.; Terao H.; Iwashita A.; Fujita T. FI Catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. 10.1021/cr100294r. [DOI] [PubMed] [Google Scholar]
- Delferro M.; Marks T. J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. 10.1021/cr1003634. [DOI] [PubMed] [Google Scholar]
- McInnis J. P.; Delferro M.; Marks T. J. Multinuclear group 4 catalysis: Olefin polymerization pathways modified by strong metal–metal cooperative effects. Acc. Chem. Res. 2014, 47, 2545–2557. 10.1021/ar5001633. [DOI] [PubMed] [Google Scholar]
- Valente A.; Mortreux A.; Visseaux M.; Zinck P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. 10.1021/cr300289z. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Liu J.; Padmanabhan S.; Kitiyanan B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A: Chem. 2007, 267, 1–29. 10.1016/j.molcata.2006.11.006. [DOI] [Google Scholar]
- Nomura K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerisation. Dalton Trans. 2009, 0, 8811–8823. 10.1039/b910407k. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Liu J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. 10.1039/c1dt10086f. [DOI] [PubMed] [Google Scholar]
- Redshaw C.; Tang Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. 10.1039/c2cs35028a. [DOI] [PubMed] [Google Scholar]
- Miyake G. M.; Chen E. Y.-X. Synthesis of highly syndiotactic polymers by discrete catalysts or initiators. Polym. Chem. 2011, 2, 2462–2480. 10.1039/c1py00245g. [DOI] [Google Scholar]
- Carrick W. L. Mechanism of ethylene polymerization with vanadium catalysts. J. Am. Chem. Soc. 1958, 80, 6455–6456. 10.1021/ja01556a073. [DOI] [Google Scholar]
- Carrick W. L.; Kluiber R. W.; Bonner E. F.; Wartman L. H.; Rugg F. M.; Smith J. J. Transition metal catalysts. I. Ethylene polymerization with a soluble catalyst formed from an aluminum halide, tetraphenyltin and a vanadium Halide. J. Am. Chem. Soc. 1960, 82, 3883–3887. 10.1021/ja01500a025. [DOI] [Google Scholar]
- Phillips G. W.; Carrick W. L. Transition metal catalysts. VIII. The role of oxygen in ethylene polymerizations with the AlBr3-VXn-Sn(C6H5)4 catalyst. J. Polym. Sci. 1962, 59, 401–412. 10.1002/pol.1962.1205916816. [DOI] [Google Scholar]
- Junghanns V. E.; Gumboldt A.; Bier G. Polymerisation von äthylen und propylen zu amorphen copolymerisaten mit katalysatoren aus vanadiumoxychlorid und aluminiumhalogenalkylen. Makromol. Chem. 1962, 58, 18–42. 10.1002/macp.1962.020580102. [DOI] [Google Scholar]
- Natta G.; Mazzanti G.; Valvassori A.; Sartori G.; Fiumani D. Ethylene–propylene copolymerization in the presence of catalysts prepared from vanadium triacetylacetonate. J. Polym. Sci. 1961, 51, 411–427. 10.1002/pol.1961.1205115603. [DOI] [Google Scholar]
- Christman D. L.; Keim G. I. Reactivities of nonconjugated dienes used in preparation of terpolymers in homogeneous systems. Macromolecules 1968, 1, 358–363. 10.1021/ma60004a017. [DOI] [Google Scholar]
- Doi Y.; Ueki S.; Keii T. “Living” coordination polymerization of propene initiated by the soluble V(acac)3-Al(C2H5)2Cl system. Macromolecules 1979, 12, 814–819. 10.1021/ma60071a004. [DOI] [Google Scholar]
- Doi Y.; Koyama T.; Soga K. Synthesis of a propene—methyl methacrylate diblock copolymer via “living” coordination polymerization. Makromol. Chem. 1985, 186, 11–15. 10.1002/macp.1985.021860102. [DOI] [Google Scholar]
- For selected reviews (vanadium catalysts for olefin polymerization, refs (24)(42)–45):Hagen H.; Boersma J.; van Koten G. Homogeneous vanadium-based catalysts for the Ziegler–Natta polymerization of α-olefins. Chem. Soc. Rev. 2002, 31, 357–364. 10.1039/b205238e. [DOI] [PubMed] [Google Scholar]
- Gambarotta S. Vanadium-based Ziegler–Natta: challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. 10.1016/s0010-8545(02)00298-9. [DOI] [Google Scholar]
- Redshaw C. Vanadium procatalysts bearing chelating aryloxides: structure–activity trends in ethylene polymerisation. Dalton Trans. 2010, 39, 5595–5604. 10.1039/b924088h. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Zhang W.. Organometallic Reactions and Polymerization; Osakada K., Ed.; The Lecture Notes in Chemistry 85; Springer-Verlag: Berlin, 2014; pp 89–118. [Google Scholar]
- Nomura K.; Sagara A.; Imanishi Y. Olefin polymerization and ring-opening metathesis polymerization of norbornene by (arylimido)(aryloxo)vanadium(V) complexes of the type VX2(NAr)(OAr‘). Remarkable effect of aluminum cocatalyst for the coordination and insertion and ring-opening metathesis polymerization. Macromolecules 2002, 35, 1583–1590. 10.1021/ma0117413. [DOI] [Google Scholar]
- Wang W.; Nomura K. Remarkable effects of aluminum cocatalyst and comonomer in ethylene copolymerizations catalyzed by (arylimido)(aryloxo)vanadium complexes: Efficient synthesis of high molecular weight ethylene/norbornene copolymer. Macromolecules 2005, 38, 5905–5913. 10.1021/ma050629s. [DOI] [Google Scholar]
- Wang W.; Nomura K. Notable effects of aluminum alkyls and solvents for highly efficient ethylene (co)polymerizations catalyzed by (arylimido)- (aryloxo)vanadium complexes. Adv. Synth. Catal. 2006, 348, 743–750. 10.1002/adsc.200505446. [DOI] [Google Scholar]
- Diteepeng N.; Tang X.; Hou X.; Li Y.-S.; Phomphrai K.; Nomura K. Ethylene polymerisation and ethylene/norbornene copolymerisation by using aryloxo-modified vanadium(V) complexes containing 2,6-difluoro-, dichloro-phenylimido complexes. Dalton Trans. 2015, 44, 12273–12281. 10.1039/c4dt04026k. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Bahuleyan B. K.; Zhang S.; Sharma P. M. V.; Katao S.; Igarashi A.; Inagaki A.; Tamm M. Synthesis and structural analysis of (imido)vanadium(V) dichloride complexes containing imidazolin-2-iminato- and imidazolidin-2-iminato ligands, and their use as catalyst precursors for ethylene (co)polymerization. Inorg. Chem. 2014, 53, 607–623. 10.1021/ic402747d. [DOI] [PubMed] [Google Scholar]
- Onishi Y.; Katao S.; Fujiki M.; Nomura K. Synthesis and structural analysis of (arylimido)vanadium(V) complexes containing phenoxyimine ligands: New, efficient catalyst precursors for ethylene polymerization. Organometallics 2008, 27, 2590–2596. 10.1021/om800177g. [DOI] [Google Scholar]
- Zhang S.; Katao S.; Sun W.-H.; Nomura K. Synthesis of (arylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands and their use as the catalyst precursors for olefin polymerization. Organometallics 2009, 28, 5925–5933. 10.1021/om900633u. [DOI] [Google Scholar]
- Igarashi A.; Kolychev E. L.; Tamm M.; Nomura K. Synthesis of (imido)vanadium(V) dichloride complexes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety: New MAO-free ethylene polymerization catalysts. Organometallics 2016, 35, 1778–1784. 10.1021/acs.organomet.6b00200. [DOI] [Google Scholar]
- Zhang S.; Nomura K. Highly efficient dimerization of ethylene by (imido)vanadium complexes containing (2-anilidomethyl)pyridine ligands: Notable ligand effect toward activity and selectivity. J. Am. Chem. Soc. 2010, 132, 4960–4965. 10.1021/ja100573d. [DOI] [PubMed] [Google Scholar]
- Igarashi A.; Zhang S.; Nomura K. Ethylene dimerization/polymerization catalyzed by (adamantylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands: Factors affecting the ethylene reactivity. Organometallics 2012, 31, 3575–3581. 10.1021/om3000532. [DOI] [Google Scholar]
- Nomura K.; Igarashi A.; Katao S.; Zhang W.; Sun W.-H. Synthesis and structural analysis of (imido)vanadium(V) complexes containing chelate (anilido)methyl-imine ligands: Ligand effect in ethylene dimerization. Inorg. Chem. 2013, 52, 2607–2614. 10.1021/ic302633y. [DOI] [PubMed] [Google Scholar]
- Tang X.-Y.; Igarashi A.; Sun W.-H.; Inagaki A.; Liu J.; Zhang W.; Li Y.-S.; Nomura K. Synthesis of (imido)vanadium(V) complexes containing 8-(2,6-dimethylanilide)-5,6,7-trihydroquinoline ligands: Highly active catalyst precursors for ethylene dimerization. Organometallics 2014, 33, 1053–1060. 10.1021/om401119y. [DOI] [Google Scholar]
- Nomura K.; Mitsudome T.; Igarashi A.; Nagai G.; Tsutsumi K.; Ina T.; Omiya T.; Takaya H.; Yamazoe S. Synthesis of (adamantylimido)vanadium(V) dimethyl complex containing (2-anilidomethyl)pyridine ligand and selected reactions: Exploring the oxidation state of the catalytically active species in ethylene dimerization. Organometallics 2017, 36, 530–542. 10.1021/acs.organomet.6b00727. [DOI] [Google Scholar]; . Observed XANES and EXAFS spectra of V(NAd)Cl2[2-ArCH2(C5H4)] are good fitting of those estimated by both X-ray crystallographic analysis and the DFT calculation; good curve-fitting between the XANES spectrum of V(NAd)Cl3 and that by the DFT calculation was observed
- The other reports concerning olefin polymrization with vanadium complex catalysts containing calix arene or bis- or tetra-(phenolate) ligands (refs (59)–64):Gibson V. C.; Redshaw C.; Elsegood M. R. J. Calix[6] and [8]arene complexes of vanadium. J. Chem. Soc., Dalton Trans. 2001, 767–769. 10.1039/b010248m. [DOI] [Google Scholar]
- Redshaw C.; Rowan M. A.; Warford L.; Homden D. M.; Arbaoui A.; Elsegood M. R. J.; Dale S. H.; Yamato T.; Casas C. P.; Matsui S.; Matsuura S. Oxo- and imidovanadium complexes incorporating methylene- and dimethyleneoxa-bridged calix[3]- and -[4]arenes: Synthesis, structures and ethylene polymerisation catalysis. Chem.—Eur. J. 2007, 13, 1090–1107. 10.1002/chem.200600679. [DOI] [PubMed] [Google Scholar]
- Arbaoui A.; Redshaw C.; Homden D. M.; Wright J. A.; Elsegood M. R. J. Vanadium-based imido-alkoxide pro-catalysts bearing bisphenolate ligands for ethylene and ε-caprolactone polymerisation. Dalton Trans. 2009, 8911–8922. 10.1039/b902402f. [DOI] [PubMed] [Google Scholar]
- Clowes L.; Redshaw C.; Hughes D. L. Vanadium-based pro-catalysts bearing depleted 1,3-calix[4]arenes for ethylene or ε-caprolactone polymerization. Inorg. Chem. 2011, 50, 7838–7845. 10.1021/ic2010198. [DOI] [PubMed] [Google Scholar]
- Redshaw C.; Walton M. J.; Lee D. S.; Jiang C.; Elsegood M. R. J.; Michiue K. Vanadium(V) oxo and imido calix[8]arene complexes: Synthesis, structural studies, and ethylene homo/copolymerisation capability. Chem.—Eur. J. 2015, 21, 5199–5210. 10.1002/chem.201406084. [DOI] [PubMed] [Google Scholar]
- Redshaw C.; Walton M. J.; Elsegood M. R. J.; Prior T. J.; Michiue K. Vanadium(V) tetra-phenolate complexes: synthesis, structural studies and ethylene homo-(co-)polymerization capability. RSC Adv. 2015, 5, 89783–89796. 10.1039/c5ra20177b. [DOI] [PubMed] [Google Scholar]
- Zuo W.; Zhang S.; Liu S.; Liu X.; Sun W.-H. Half-titanocene complexes bearing dianionic 6-benzimidazolylpyridyl-2-carboximidate ligands: Synthesis, characterization, and their ethylene polymerization. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 3396–3410. 10.1002/pola.22693. [DOI] [Google Scholar]
- Liu S.; Zuo W.; Zhang S.; Hao P.; Wang D.; Sun W.-H. Bis(2-(6-methylpyridin-2-yl)-benzimidazolyl)titanium dichloride and titanium bis(6-benzimidazolylpyridine-2-carboxylimidate): Synthesis, characterization, and their catalytic behaviors for ethylene polymerization. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 3411–3423. 10.1002/pola.22694. [DOI] [Google Scholar]
- Zhang W.; Nomura K. Synthesis of (1-adamantylimido)vanadium(V) complexes containing aryloxo, ketimide ligands: Effect of ligand substituents in olefin insertion/metathesis polymerization. Inorg. Chem. 2008, 47, 6482–6492. 10.1021/ic800347n. [DOI] [PubMed] [Google Scholar]
- Buijink J.-K. F.; Teuben J. H.; Kooijman H.; Spek A. L. Synthesis, molecular structure, and reactivity of a half-sandwich vanadium(III) imido complex: The first vanadium(V) alkylidene. Organometallics 1994, 13, 2922–2924. 10.1021/om00020a003. [DOI] [Google Scholar]
- Sun W.-H.; Hao P.; Zhang S.; Shi Q.; Zuo W.; Tang X.; Lu X. Iron(II) and cobalt(II) 2-(benzimidazolyl)-6-(1-(arylimino)ethyl)pyridyl complexes as catalysts for ethylene oligomerization and polymerization. Organometallics 2007, 26, 2720–2734. 10.1021/om0700819. [DOI] [Google Scholar]
- For example,Liu Q.-D.; Jia W.-L.; Wang S. Blue luminescent 2-(2’-pyridyl)benzimidazole derivative ligands and their orange luminescent mononuclear and polynuclear organoplatinum(II) complexes. Inorg. Chem. 2005, 44, 1332–1343. 10.1021/ic0487530. [DOI] [PubMed] [Google Scholar]
- Christman D. L. Preparation of polyethylene in solution. J. Polym. Sci., Part A-1: Polym. Chem. 1972, 10, 471. 10.1002/pol.1972.150100213. [DOI] [Google Scholar]
- Reviews (refs (72).–76):Kaminsky W. Olefinpolymerisation mittels metallocenekatalysatoren. Angew. Makromol. Chem. 1994, 223, 101–120. 10.1002/apmc.1994.052230108. [DOI] [Google Scholar]
- Tritto I.; Boggioni L.; Ferro D. R. Metallocene catalyzed ethene- and propene co-norbornene polymerization: Mechanisms from a detailed microstructural analysis. Coord. Chem. Rev. 2006, 250, 212–241. 10.1016/j.ccr.2005.06.019. [DOI] [Google Scholar]
- Nomura K. Nonbridged half-titanocenes containing anionic ancillary donor ligands: Promising new catalysts for precise synthesis of cyclic olefin copolymers (COCs). Chin. J. Polym. Sci. 2008, 26, 513–523. 10.1142/s0256767908003217. [DOI] [Google Scholar]
- Li X.; Hou Z. Organometallic catalysts for copolymerization of cyclic olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. 10.1016/j.ccr.2007.11.027. [DOI] [Google Scholar]
- Zhao W.; Nomura K. Design of efficient molecular catalysts for synthesis of cyclic olefin copolymers (COC) by copolymerization of ethylene and α-olefins with norbornene or tetracyclododecene. Catalysts 2016, 6, 175. 10.3390/catal6110175. [DOI] [Google Scholar]
- Tritto I.; Marestin C.; Boggioni L.; Zetta L.; Provasoli A.; Ferro D. R. Ethylene–norbornene copolymer microstructure. assessment and advances based on assignments of 13C NMR spectra. Macromolecules 2000, 33, 8931–8944. 10.1021/ma000795u. [DOI] [Google Scholar]
- Nomura K.; Tsubota M.; Fujiki M. Efficient ethylene/norbornene copolymerization by (aryloxo)(indenyl)titanium(IV) complexes–MAO catalyst system. Macromolecules 2003, 36, 3797–3799. 10.1021/ma034215f. [DOI] [Google Scholar]; . Detailed analysis data were shown in the Supporting Information
- Pioneering ESR study for monitoring the catalytically active species (VCl4 – Et2AlCl catalyst system),Lehr M. H.; Carman C. J. Electron spin resonance evidence of inactive V(III) precursor to catalytically active V(III) in vanadium tetrachloride Ziegler catalysts. Macromolecules 1969, 2, 217–219. 10.1021/ma60008a025. [DOI] [Google Scholar]
- Bigmore H. R.; Zuideveld M. A.; Kowalczyk R. M.; Cowley A. R.; Kranenburg M.; McInnes E. J. L.; Mountford P. Synthesis, structures, and olefin polymerization capability of vanadium(4+) imido compounds with fac-N3 donor ligands. Inorg. Chem. 2006, 45, 6411–6423. 10.1021/ic060454i. [DOI] [PubMed] [Google Scholar]
- Soshnikov I. E.; Semikolenova N. V.; Shubin A. A.; Bryliakov K. P.; Zakharov V. A.; Redshaw C.; Talsi E. P. EPR Monitoring of vanadium(IV) species formed upon activation of vanadium(V) polyphenolate precatalysts with AlR2Cl and AlR2Cl/ethyltrichloroacetate (R = Me, Et). Organometallics 2009, 28, 6714–6720. 10.1021/om900515h. [DOI] [Google Scholar]
- Soshnikov I. E.; Semikolenova N. V.; Bryliakov K. P.; Zakharov V. A.; Redshaw C.; Talsi E. P. An EPR study of the vanadium species formed upon interaction of vanadyl N and C-capped tris(phenolate) complexes with AlEt3 and AlEt2Cl. J. Mol. Catal. A: Chem. 2009, 303, 23–29. 10.1016/j.molcata.2008.12.013. [DOI] [Google Scholar]
- Soshnikov I. E.; Semikolenova N. V.; Bryliakov K. P.; Shubin A. A.; Zakharov V. A.; Redshaw C.; Talsi E. P. An EPR study of the V(IV) species formed upon activation of a vanadyl phenoxyimine polymerization catalyst with AlR3 and AlR2Cl (R = Me, Et). Macromol. Chem. Phys. 2009, 210, 542–548. 10.1002/macp.200800556. [DOI] [Google Scholar]
- For example (account),Yamamoto T. Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole?. X-Ray Spectrom. 2008, 37, 572–584. 10.1002/xrs.1103. [DOI] [Google Scholar]; The transition of a 1s electron to 3d orbital gives weak pre-edge peaks due to the electric quadrupole transition for any symmetries. An intense pre-edge peak is assigned to an electric dipole transition to p-character in the d-p hybridized orbital, and the mixing of a metal 4p orbital with the 3d orbital strongly depends on the coordination symmetry.
- Srivastava U. C.; Higam H. L. X-ray absorption edge spectrometry (xaes) as applied to coordination chemistry. Coord. Chem. Rev. 1973, 9, 275–310. 10.1016/s0010-8545(00)82080-9. [DOI] [Google Scholar]; . References cited therein
- Wong J.; Lytle F. W.; Messmer R. P.; Maylotte D. H. K-edge absorption spectra of selected vanadium compounds. Phys. Rev. B: Condens. Matter Mater. Phys. 1984, 30, 5596–5610. 10.1103/physrevb.30.5596. [DOI] [Google Scholar]; . Reports for solid structural analysis of vanadium compounds by XAS spectra
- Asakura H.; Shishido T.; Yamazoe S.; Teramura K.; Tanaka T. Structural analysis of group V, VI, and VII metal compounds by XAFS. J. Phys. Chem. C 2011, 115, 23653–23663. 10.1021/jp2034104. [DOI] [Google Scholar]
- CrystalClear: Data Collection and Processing Software; Rigaku Corporation. Tokyo 196-8666: Japan,1998–2015.
- CrysAlisPro: Data Collection and Processing Software; Rigaku Corporation. Tokyo 196-8666: Japan, 2015.
- Sheldrick G. SHELXT: Integrating space group determination and structure solution. Acta Crystallogr., Sect. A: Found. Adv. 2014, 70, C1437. 10.1107/s2053273314085623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CrystalStructure 4.2: Crystal Structure Analysis Package; Rigaku Corporation. Tokyo 196-8666: Japan, 2000–2015.
- SHELXL Version 2014/7:Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Adv. 2008, 64, 112–122. 10.1107/s0108767307043930. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






