Abstract
Background
Knowledge of risk factors for drug-related hospitalizations (DRHs) is limited.
Aim
To examine the prevalence of DRHs and the relationships between DRHs and various variables in multimorbid patients admitted to an internal medicine ward.
Methods
Multimorbid patients ≥ 18 years, using minimum of four regular drugs from minimum two therapeutic classes, were included from the Internal Medicine ward, Oslo University Hospital, Norway, from August 2014 to March 2016. Clinical pharmacists prospectively conducted medicines reconciliations and reviews to reveal drug-related problems (DRPs). Blinded for identified DRPs, an interdisciplinary group retrospectively made comprehensive, clinical assessments of each patient case to classify hospitalizations as drug-related (DRH) or non-drug-related (non-DRH). Age, sex distribution, Charlson Comorbidity Index (CCI), renal function, aberrant genotype frequencies, body-mass index, number of drugs, proportion of patients which received assistance for drug administration from the home care service, and/or through multidose-dispensed drugs, and occurrence of specific DRP subgroups, were compared separately between patients with DRHs versus non-DRHs, followed by multiple logistic regression analysis.
Results
Hospitalizations were classified as drug-related in 155 of the 404 included patients (38%). Factors significantly associated with DRHs were occurrence of adverse effect DRPs (adjusted odds ratio (OR) 3.3, 95% confidence interval (CI) 1.4–8.0), adherence issues (OR 2.9, 1.1–7.2), home care (OR 1.9, 1.1–3.5), drug monitoring DRPs (OR 1.9, 1.2–3.0), and CCI score ≥6 (OR 0.33, 0.14–0.77). Frequencies of aberrant genotypes did not differ between the patient groups, but in 41 patients with DRHs (26.5%), gene-drug interactions influenced the assessments of DRHs.
Conclusion
DRHs are prevalent in multimorbid patients with adverse effect DRPs and adherence issues as the most important risk factors.
Introduction
Many previous studies have investigated the occurrence of drug-related hospitalizations (DRHs) in different patient populations [1–5]. A systematic review representing various patient groups reported that around 10% of all hospitalizations are drug-related [6]. In older adults, the reported prevalence of DRHs is higher, comprising around 30% of all hospitalizations [7, 8].
Preventing hospitalizations is important both for the benefit of the patients and society. A high proportion of DRHs are preventable [1–3, 6, 7, 9, 10], but effective prevention requires knowledge of important risk factors. The causality of DRHs may be complex and involve many predisposing factors, such as patient characteristics, disease state, living situation, and drug-related problems (DRPs) [1–3, 6, 7, 11–15]. A DRP is defined as an event or circumstance that actually or potentially interferes with desired health outcomes [16], which can manifest as drug-related morbidity or death if no action is taken [17]. Typical DRPs comprise suspected adverse effects, unnecessary drugs, adherence issues and drug-drug interactions (DDIs). Moreover, pharmacogenetic variability is a potential source of DRPs [18], which has not previously been investigated in relation to risk of DRHs.
Multimorbid patients represent a group with a high risk of DRPs [17]. This is a heterogeneous patient group, but a common feature is increased health care utilization, including frequent hospitalizations, and multiple drug treatments [19–23]. The population of multimorbid patients is growing due to steadily improving health care and increasing life expectancy [24, 25]. These patients are often admitted to internal medicine wards due to the complex nature of their disease state. They, therefore, comprise a resource-demanding patient group, where prevention of hospitalizations is crucial to reduce social costs and improve patient health.
Several studies have previously investigated the prevalence and risk factors for DRHs in various patient populations, e.g. cancer patients, geriatric patients and older patients with dementia [4, 8, 26]. Advanced age and polypharmacy are the main risk factors for DRHs identified in most studies [6, 27, 28]. However, to the best of our knowledge, no studies have investigated multimorbid patients with respect to prevalence and risk factors for DRHs. The aim of the present study was therefore to examine the prevalence of DRHs and the relationships between DRHs and various variables in multimorbid patients admitted to an internal medicine ward.
Materials and methods
Study design and setting
This observational study, approved by the Regional Committee for Medical and Health Research Ethics (2014/704/REK south-eastern D) and the Privacy Ombudsman, was conducted at an internal medicine ward of Oslo University hospital (Ullevaal location), Norway, from August 2014 to March 2016. The current study is based on observational data collected during the inclusion of patients to a randomized controlled trial (RCT) studying the effect of a pharmacist intervention on readmissions, ClinicalTrials.gov Identifier: NCT02336113. Baseline data at inclusion was used to assess drug-related hospitalizations. Patients were considered for inclusion by clinical pharmacists Monday to Friday during regular daytime working hours until a target number of 400 patients, based on the power calculation of the RCT, were enrolled. Eligible patients were prospectively invited and enrolled in the study following written informed consent.
Fig 1 illustrates the outline of the study with the various steps and processes performed after patient inclusion. During the inclusion period, six experienced clinical pharmacists, all with a master degree in clinical pharmacy and had undergone a thorough, standardized course in IMM, prospectively performed medicine reviews and identified DRPs at the time of admission, based on the reconciled drug list as described below. As the pharmacists worked daytime shifts, more than one pharmacist was often involved in an individual participant`s medicines reconciliations and/or reviews. Information about sex, age, body-mass index (BMI) and living situation. If the patient received assistance with drug administration from home care, in the following text referred to as ‘home care’, and/or through multidose-dispensed drugs, this was also registered. Multidose-dispensed drugs mean that the individual patient’s drugs (tablets or capsules) were prepacked by a dispensing pharmacy in small, labeled plastic bags; each bag contains all drugs prescribed to be administered at the same time.
Fig 1. Illustration of the outline of the study.
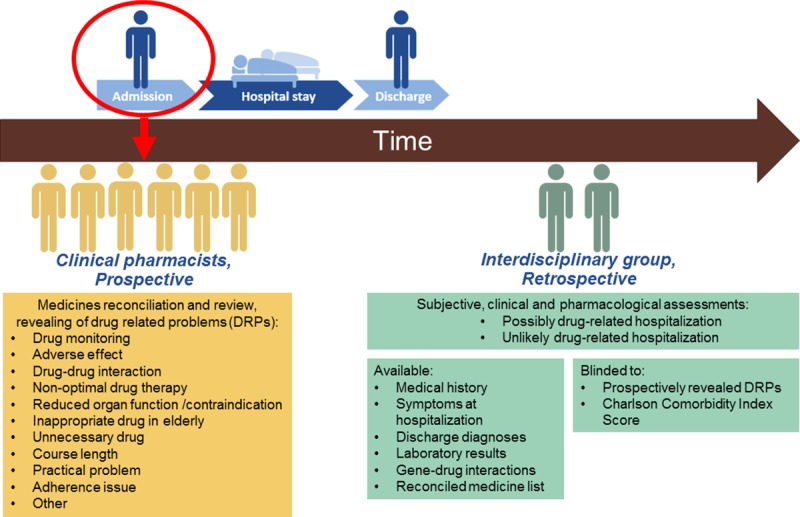
In addition, blood samples were collected for biochemical measurements and pharmacogenetic analyses of drug-metabolizing cytochrome P450 (CYP) enzymes and the transporter mediating uptake of statins from the blood into the liver (OAPTP1B1). Glomerular filtration rate (GFR) was calculated using the Cockcroft-Gault formula [29], except for obese patients (BMI > 30) where the Salazar-Corcoran formula was used [30]. An experienced senior physician retrospectively collected information of diagnoses from the medical records to calculate the Charlson Comorbidity Index (CCI) score of each patient [31].
Inclusion and exclusion criteria
Inclusion criteria were: acute admission, age ≥ 18 years and use of at least four regular drugs from at least two Anatomical Therapeutic Chemical (ATC) groups [32] at 1st level, at admission. The latter was a surrogate for multimorbidity, defined as the presence of minimum two conditions, a commonly used definition [33]. Exclusion criteria were i) terminally ill, ii) isolated due to severe infections, or iii) unable to communicate in Norwegian or English in absence of a translator. Patients readmitted during the study period were not invited for ‘a second’ inclusion.
Prospective medicine reconciliation and review
A Norwegian translation of the Integrated Medicines Management (IMM) model [34], adapted to the Norwegian setting, was used as the method for the systematic medicines reconciliations and reviews. Medicines reconciliation was performed by clinical pharmacists according to a structured IMM interview form, which included questions to assess patient adherence. Based on the reconciled medicine list, the clinical pharmacists performed systematic medicines reviews and identified DRPs at the time of admission. These medicines reviews were defined as advanced reviews, according to the PCNE classification [35]. The medicine reviews only included drugs used prior to admission and not drugs initiated during transport to or following hospital admission. The pharmacists had access to the patient’s medical history and laboratory results (up to and including admission time) when performing medicine reviews.
A DRP was defined according to the Pharmaceutical Care Network Europe (PCNE) as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’ [16]. As IMM was used by the clinical pharmacists to conduct medicine reviews, the identified DRPs were classified in the following subgroups according to the IMM procedure; ‘drug monitoring’, ‘adverse effect’, ‘drug-drug-interaction’, ‘non-optimal drug therapy’, ‘reduced organ function / contraindication’, ‘inappropriate drug in elderly’, ‘unnecessary drug’, ‘course length’, ‘practical problem’, ‘adherence issue’ and ‘other’. Each DRP subgroup is described in detail in S1 Table. When information obtained during medicines reconciliation led to the identification of a DRP, this was also recorded.
Pharmacogenetic analyses
Blood samples drawn from the included patients were used for pharmacogenetic analyses at Diakonhjemmet Hospital, Oslo, Norway. The analyses were conducted retrospectively due to time constraints and therefore not included as a basis for the medicine reviews. For all included patients, variant allele analyses of CYP2D6, CYP2C19, CYP2C9, CYP3A5 and SLCO1B1 (encoding OATP1B1; uptake transporter of statins into the liver) were performed. For warfarin-treated patients, VKORC1 genotyping was also conducted. An overview of the variant alleles (mutations) of the various pharmacogenes included in the genotyping panels, and the respective genotype-predicted aberrant phenotypes, is provided in S2 Table. Briefly, for CYP2D6 and CYP2C19, the most relevant polymorphic enzymes involved in drug metabolism, homozygous carriers of non-coding (null) alleles were defined as ‘poor metabolizers’ (PMs) of the respective enzymes, while heterozygous carriers of null alleles and homozygous carriers of reduced-function alleles were defined as ‘intermediate metabolizers’ (IMs). Patients carrying three or more functional CYP2D6 gene copies were classified as CYP2D6 ‘ultrarapid metabolizers’ (UMs), while patients carrying CYP2C19*17 were classified as CYP2C19 UMs.
Identification of gene-drug interactions (GDIs)
Retrospectively, the authors EM and ML identified gene-drug interactions (GDIs) by assessing the reconciled drug list against the respective patients`genotype results to identify drugs being substrates of the respective polymorphic enzymes or transporters. Assessments were restricted to aberrant phenotypes, as defined in S2 Table. The concept of defining a gene-drug interaction (GDI) was that a patient’s variant genotype likely determined an at least 50% change in the plasma concentration/exposure of the affected drug. As there is currently no gene-drug interaction databases available, each of the cases was assessed by manual reviews with author EM as the responsible person, who is head of research at the Center for Psychopharmacology, where genotyping is performed as a clinical service on a routine basis. In the assessments, a conservative approach was applied concerning i) the variant genotype’s effect on function/phenotype of the enzyme or transporter (expected reduction or increased in activity score by a factor of 2 or more), and ii) the estimated relative involvement of the enzyme or transporter in the overall clearance of the drug (expected fraction of clearance ≥1/3). The presumed clinical relevance of the identified GDIs was included in the assessments of drug-related hospitalizations.
Assessment of drug-related hospitalization (DRH)
After patient inclusion, a senior geriatrician/internal medicine physician (author MM) and a pharmacologist (author EM) made comprehensive, clinical and pharmacological assessments for each individual patient as to whether the hospitalization was possibly drug-related (classified as DRH) or unlikely to be drug-related (classified as non-DRH). This method for hospitalization assessment with only two categories, i.e. ‘possibly’ or ‘unlikely’ drug-related, was applied due to the limitations of using scoring tools to grade the probability of DRHs in this population, where patients are characterized by complex symptoms and drug treatments.
Prior to the assessments, the authors MM and EM received case report forms (CRFs) prefilled by the clinical pharmacist who organized the study (author ML). The following information was included in the CRFs: sex, age, brief medical history, symptoms at hospitalization, laboratory results, reconciled drug list at hospitalization, discharge diagnoses, and results from the pharmacogenetic analyses. MM and EM were blinded to the CCI score and the DRPs prospectively identified by the study pharmacists, and they had no training or knowledge of identification of DRPs according to IMM. The main principle during the DRH assessments was whether the diversity of symptoms, laboratory values and/or current admission reasons mentioned in the medical record were possibly or unlikely explained by the patient`s drug use or lack of drug use. The assessments were made in physical meetings, where EM and MM together discussed each case thoroughly (15–20 minutes per patient) until agreement was reached, to classify hospitalizations as possibly or unlikely drug-related. Drugs involved in possible DRHs, and GDIs that influenced the DRH assessments, were systematically registered.
Statistics
All registered patient variables, i.e. age, sex, number of prescribed drugs, living situation, use of home care, multidose-dispensed drugs, BMI, GFR, CCI score, genotype frequencies and occurrence of specific DRP subgroups, were initially compared between patients with DRHs versus non-DRHs using chi-square test for proportions and Mann-Whitney test for continuous variables. Variables with p values < 0.2 in the simple comparisons were included in the subsequent multiple logistic regression analysis. Backward elimination of non-significant variables was performed, and the final model was restricted to include explanatory variables with p values < 0.05. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Excluded variables were introduced one by one to the final model to verify that they did not significantly affect the effect of the variables that remained in the model.
IBM SPSS Software version 25.0 (IBM Corp. NY), was used for all statistical analyses. P values < 0.05 were defined as statistically significant.
Results
During the study period, 2174 patients were admitted to the internal medicine ward and 1769 patients (81%) were considered for inclusion. Of these, 913 patients did not meet the predefined inclusion criteria and 258 patients were not asked to participate for practical reasons. Among the remaining 598 patients, 175 (29%) declined the invitation to participate in the study (permission to register reasons for declining not obtained). After excluding patients due to i) medicines reconciliation revealed use of less than four regular drugs (n = 3), ii) erroneous reinclusion (n = 1), iii) withdrawal of consent (n = 8), and iv) acute events preventing data collection (in-hospital death; n = 3, transfer to other wards; n = 3, rapid discharge; n = 1), a total of 404 participants were enrolled in the study.
The median age of the included patients was 79.4 years (range 23.1–96.4) and 216 patients (54%) were females. Most patients (n = 373, 92%) were living at home prior to hospitalization, and the majority (n = 283, 70%) administered the drugs themselves. The median number of regular and as needed drugs in the study population was 8 (range 4–19) and 2 (range 0–11) and the median number of diagnoses was 7 (range 2–17), emphasizing the multimorbidity of the study participants. The median CCI score was 3 (range 0–12). The five most frequent reasons for hospitalization described in the medical admission record were infections (n = 72), breathlessness (n = 44), heart failure exacerbation (n = 37), chest pain (n = 35) and general functional impairment/failure (n = 27). During the hospital stay, 11 of the 404 included participants died. We did not have permission to register the causes of death or to assess these as drug-related or not.
In the medication reviews, the study pharmacists identified a total number of 5527 DRPs, whereof 950 (17%) were identified as a result of completing medicine lists during medicines reconciliation. The median number of DRPs per patient was 13 (range 3–42), and 315 patients (78%) had a minimum of one DRP identified based on information revealed during the medicines reconciliation.
After comprehensive clinical and pharmacological assessments using information about patient characteristics, disease status and history, laboratory results, reconciled medicine lists, discharge diagnoses and pharmacogenetic profiles of all included patients, authors MM and EM retrospectively classified hospitalizations as possibly drug-related in 155 patients (38%). S3 Table shows the drugs most frequently involved in the underlying causes of hospitalization in the cases assessed as DRHs. Metoprolol was the drug most frequently suspected to be related to DRHs, i.e. in as many as 39 of the cases (25%). The second most frequent drug suspected to be related to hospitalization was bumetanide (21 cases), followed by zopiclone (15 cases) and insulin or insulin analogues (13 cases).
In 287 of the patients (71%), at least one gene-drug interaction (GDI) was identified (total number of GDIs 538). One or more GDIs were found to be of relevance for the DRH assessments in 41 of the patients (26.5%). In five patients, GDIs were decisive for classifying hospitalizations as possibly drug-related. S4 Table shows frequencies of the various GDIs identified in the patient population, as well as those relevant for DRH assessments. The most frequently involved agents in GDIs were metoprolol, comprising 125 of the 538 GDIs (23%), and warfarin, comprising 60 of the 538 GDIs (11%). Proton pump inhibitors, statins, and opioids were also commonly involved in GDIs, with 84, 81 and 74 identified cases.
For 9 patients the relationship between drug treatment and hospitalization was not assessed due to insufficient information in the medical record. These patients were excluded in the subsequent comparative analyses of patients with possibly DRHs versus unlikely DRHs, in the following text described as ‘DRHs’ (155 patients) versus ‘non-DRHs’ (240 patients).
In Table 1, patients with DRHs and non-DRHs are compared with respect to age, sex, number of prescribed drugs, living situation, home care, multidose-dispensed drugs, BMI, GFR, CCI score and frequencies of variant genotypes encoding reduced/absent enzyme or transporter activities. The proportion receiving multidose-dispensed drugs was the only of these variables being significantly different between the cases of DRHs versus non-DRHs in the unadjusted analysis, i.e. 30% versus 20% (p = 0.035). In addition, home care, CCI score, BMI and number of prescribed drugs had p values < 0.2 for comparisons between DRHs and non-DRHs, and were included as candidate variables in the subsequent multiple logistic regression analysis.
Table 1. Comparisons of characteristics in patients with drug-related hospitalizations (DRHs) versus non-drug-related hospitalizations (non-DRHs) in the study population (n = 395a).
| Characteristic | DRHs (n = 155) | Non-DRHs (n = 240) | p value |
|---|---|---|---|
| Female/male | 79/76 | 135/105 | 0.304 |
| Age, median (range) | 78.4 (32.9–94.9) | 80.3 (23.1–96.4) | 0.666 |
| Number of prescribed drugs, median (range) | |||
| • Regular | 9 (4–19) | 8 (4–19) | 0.107 |
| • As needed | 2 (0–10) | 2 (0–11) | 0.174 |
| Living at home before admittance, n (%) | 146 (94) | 218 (91) | 0.225 |
| Assistance with drug administration: | |||
| • Nursing home, n (%) | 9 (6) | 22 (9) | 0.225 |
| • Multidose, n (%) | 46 (30) | 49 (20) | 0.035 |
| • Home care, n (%) | 30 (19) | 31 (13) | 0.084 |
| Charlson Comorbidity Index, median score (range) | 3 (0–11) | 3 (0–11) | 0.189 |
| mean score (SD) | 2.77 (1.97) | 3.12 (2.16) | |
| Body-mass index b, median (range) | 23.8 (14.4–48.4) | 25.0 (13.1–43.0) | 0.145 |
| Glomerular filtration rate (ml/min), median (range) | 49.0 (9–182) | 52.5 (5–235) | 0.268 |
| CYP2D6 poor metabolizers c, n (%) | 8 (5) | 20 (9) | 0.214 |
| CYP2C19 poor metabolizers c, n (%) | 4 (3) | 11 (5) | 0.289 |
| CYP2C9 *3 carriers c, n (%) | 18 (12) | 23 (10) | 0.569 |
| SLCO1B1 *5 carriers c, n (%) | 38 (25) | 66 (29) | 0.472 |
a Nine of the included patients were excluded from the comparison since defining hospitalizations as drug-related or not was impossible.
b Body-mass index was registered for 121/155 patients with DRHs and 175/240 patients with non-DRHs.
c Blood samples for genotyping were available for 150 patients (SLCO1B1) and 151 patients (cytochrome P450 (CYP)-enzymes) of 155 patients with DRHs and 230/240 patients with non-DRH
Table 2 shows comparisons between patients with DRHs and non-DRHs regarding the occurrence of DRPs. Patients with DRHs had significantly more DRPs in total, and significantly more of the DRP subgroups ‘drug monitoring’, ‘adverse effect’, ‘other’, ‘non-optimal drug therapy’, ‘reduced organ function / contraindication’ and ‘drug-drug interaction’. ‘Adherence issues’ were also observed more frequently in patients with DRHs versus non-DRHs (p = 0.050), and included in the multiple logistic regression analysis as a DRP subgroup.
Table 2. Overview of drug-related problems (DRPs) at hospitalization, identified by clinical pharmacists, in patients with drug-related hospitalization (DRH) versus non-drug-related hospitalizations (non-DRHs) in the study population (n = 395a).
| DRPs | DRHs (155 patients) |
Non-DRHs (240 patients) |
p value |
|---|---|---|---|
| Total | |||
| • Number of patients (%) | 155 (100) | 240 (100) | |
| • Number per patient, median (range) | 15 (4–42) | 12 (3–30) | <0.001 |
| Subgroups | |||
| Drug monitoring | |||
| • Number of patients (%) | 63 (41) | 61 (25) | 0.001 |
| • Number per patient, median (range) | 0 (0–4) | 0 (0–2) | 0.001 |
| Adverse effect | |||
| • Number of patients (%) | 147 (95) | 203 (85) | 0.002 |
| • Number per patient, median (range) | 3 (0–10) | 2 (0–8) | <0.001 |
| Non-optimal drug therapy | |||
| • Number of patients (%) | 152 (98) | 236 (98) | 1.000 |
| • Number per patient, median (range) | 4 (0–10) | 4 (0–11) | 0.006 |
| Reduced organ function / contraindication | |||
| • Number of patients (%) | 87 (56) | 114 (48) | 0.094 |
| • Number per patient, median (range) | 1 (0–8) | 0 (0–7) | 0.031 |
| Adherence issue | |||
| • Number of patients (%) | 13 (8) | 9 (4) | 0.050 |
| • Number per patient, median (range) | 0 (0–2) | 0 (0–2) | 0.051 |
| Drug-drug-interaction | |||
| • Number of patients (%) | 117 (76) | 180 (75) | 0.913 |
| • Number per patient, median (range) | 2 (0–10) | 1 (0–10) | 0.047 |
| Inappropriate drug in elderly | |||
| • Number of patients (%) | 90 (58) | 136 (57) | 0.784 |
| • Number per patient, median (range) | 1 (0–6) | 1 (0–6) | 0.446 |
| Unnecessary drug | |||
| • Number of patients (%) | 114 (74) | 181 (75) | 0.677 |
| • Number per patient, median (range) | 1 (0–7) | 1 (0–7) | 0.627 |
| Course length | |||
| • Number of patients (%) | 57 (37) | 93 (39) | 0.693 |
| • Number per patient, median (range) | 0 (0–5) | 0 (0–7) | 0.921 |
| Practical problem | |||
| • Number of patients (%) | 22 (14) | 45 (19) | 0.239 |
| • Number per patient, median (range) | 0 (0–2) | 0 (0–4) | 0.240 |
| Other | |||
| • Number of patients (%) | 57 (37) | 64 (27) | 0.033 |
| • Number per patient, median (range) | 0 (0–4) | 0 (0–5) | 0.021 |
a Nine of the included patients were excluded from the comparison since defining hospitalizations as drug-related or not was impossible.
Drug monitoring; Need for therapeutic drug monitoring. Adverse effect; Presence of symptoms or changes in laboratory values possibly caused by drug(s). Non-optimal drug therapy; Lack of drug treatment or non-optimal drug treatment of a symptom/disease. Course length; Consideration of appropriate duration of course length. Other; DRPs not applicable in other subgroups, e.g. prescription errors, documentation errors. The rest of the DRP subgroups are described in S1 Table.
In addition to the comparisons presented in Tables 1 and 2, it was observed that patients with multidose-dispensed drugs had significantly more DRPs in total than other patients (median number 15 (range 4–34) versus 14 (range 3–44); p = 0.038).
Table 3 shows adjusted OR with 95% CIs for the variables statistically significantly associated with DRHs, comprising occurrence of adverse effect DRPs (adjusted odds ratio (OR) 3.3, 95% confidence interval (CI) 1.4–8.0), adherence issues (OR 2.9, 1.1–7.2), home care (OR 1.9, 1.1–3.5), drug monitoring DRPs (OR 1.9, 1.2–3.0), and CCI score ≥6 (OR 0.33, 0.14–0.77).
Table 3. Adjusted odds ratios (OR) with 95% confidence intervals (CI) for the characteristics related to drug-related hospitalizations estimated in multiple logistic regression analysis.
| Characteristic | Adjusted OR (95% CI) | p value |
|---|---|---|
| Adverse effect DRPsa | 3.29 (1.36–7.99) | 0.008 |
| Adherence issue | 2.86 (1.14–7.17) | 0.025 |
| Home care | 1.93 (1.07–3.50) | 0.030 |
| Drug monitoring DRPsa | 1.91 (1.21–3.00) | 0.005 |
| Charlson Comorbidity Index Score | ||
| Score 0–1 (ref) | 1 | 0.033 |
| Score 2 | 0.70 (0.38–1.29) | 0.249 |
| Score 3 | 1.17 (0.63–2.16) | 0.621 |
| Score 4 | 0.55 (0.27–1.13) | 0.102 |
| Score 5 | 1.21 (0.50–2.91) | 0.670 |
| Score ≥6 | 0.33 (0.14–0.77) | 0.011 |
a drug-related problem
Discussion
In this study on multimorbid internal medicine patients, almost 40% of hospitalizations were assessed as possibly being drug-related. To the best of our knowledge, the study is the first to characterize drug-related hospitalizations in multimorbid patients. The frequency of drug-related hospitalizations was very high and substantially higher than in many other patient populations. Drug groups associated with DRHs in previous studies [7, 11, 13], e.g. beta-blockers and diuretics, were similarly frequently involved in DRHs in the present study. Important considerations are that morbidity increases the vulnerability towards drug-related problems [36–38] and that the included patients used a minimum of four regular drugs, which generally increases the risk of DRPs [17, 39]. These results clearly indicate the necessity of managing DRPs in multimorbid patients.
Presence of three specific DRP subgroups was associated with significantly higher odds for DRHs among the included patients. Patients with suspected adverse effects and adherence issues had around a threefold increased odds of DRHs, and patients with drug monitoring DRPs had nearly a twofold increased odds. Similar findings have been reported in previous studies investigating the type of DRPs most frequently associated with DRHs in various patient populations [1–3, 6, 7]. However, the present study is the first to include specific DRP subgroups together with non-drug-related factors in a multiple logistic regression analysis to investigate the impact on risks of DRHs. By adjusting for different non-drug-related factors, adherence issues and adverse effects DRPs were identified as major risk factors for DRHs in this population of multimorbid internal medicine patients.
Patients with the highest CCI score had significantly reduced odds for DRHs compared to the reference group with the lowest CCI scores. This is an interesting finding as several other studies on other patient populations have found the opposite, i.e. that high comorbidity is associated with increased risk of being hospitalized due to drug-related issues [11, 40, 41]. The result from our study probably reflects that complex multimorbid patients are frequently hospitalized due to disease progression and that a high CCI score in the current study population indicates the presence of such an extensive disease/symptom complexity, which more often resulted in classifying a hospitalization as disease-related rather than possibly drug-related. The finding suggests that focus should be on optimizing drug treatment in the healthiest of the multimorbid patients, which is in accordance with the results of a previous Swedish study, where a pharmacist intervention was more effective in preventing emergency department visits in patients using fewer drugs [42]. However, additional studies are required to evaluate which patients will benefit the most from medication reviews to prevent DRHs.
Home care was associated with increased risk of DRHs in the study population. Immediately this appears as unexpected and provoking findings, but it is important to specify that patients requiring home care assistance to drug administration usually have increased frailty and impaired cognition. Thus, despite that the statistical analysis accounted for differences in CCI scores and identified DRPs, it might be that the statistically significant associations of home care to DRHs are confounded by factors related to the patient vulnerability that not were measured and included in the multivariate model, e.g. impaired cognition. On the other hand, it could not be excluded that drug management by the home care service is of such low quality that it increases the risk for DRHs. Actually, the latter is to some extent supported by reported findings in previous studies [43–45], which possibly may reflect insufficient cooperation between different health care providers [46, 47].
In contrast to several other studies [7, 11, 27], we did not find that the overall number of regularly used drugs was associated with an increased risk of DRHs. This might have been different if the study had investigated patient readmissions shortly after hospital discharge, as the 30-day readmission rate is in previous studies closely related to the number of drugs [48–50]. However, in the context of multimorbid patients, it appears that reducing the number of drugs per se is not a sufficient action to prevent DRHs and that closer follow-up of specific DRP subgroups, i.e. suspected adverse effects, adherence issues and drug monitoring, may potentially be rational.
A unique feature of the present study was that we investigated pharmacogenetic factors related to hospitalizations. While the frequencies of genotype-predicted poor metabolizers (PMs) did not differ between patients with and without DRHs, GDIs of potential clinical relevance were identified in the majority of the patients and considered to influence about 25% of the hospitalizations assessed as possibly drug-related. A drug often involved in the identified GDIs was metoprolol, which is mainly metabolized by the enzyme CYP2D6. In white Europeans, 5–10% are CYP2D6 PMs. These patients obtain a five-fold higher dose exposure and are therefore at substantially increased risk of excessive beta-blockade and accompanying consequences like hypotension, dizziness, and falls. This is the main reason why metoprolol was frequently linked to DRHs, but an important point is also that the use of metoprolol likely prevents many hospitalizations as well [51, 52]. Nevertheless, the frequent occurrence of clinically relevant GDIs in the study population suggests that pharmacogenetics should be included as an aspect in future studies investigating risk factors for DRHs.
We did not consider the preventability of the DRHs in the study, which represents an important limitation. However, the significantly higher frequencies of several DRP subgroups, which generally are regarded as preventable (i.e. adverse effects, adherence issues and drug monitoring), in patients with DRHs versus non-DRHs indicates that a major proportion of the cases assessed as DRHs could have been avoided.
In multimorbid patients, symptoms and disease states at hospital admission are complex, which limits the suitability of objective scoring tools as a basis for assessing relationships between drug use and hospitalizations. Thus, we decided to use a method for individual and comprehensive assessments using complete sets of clinical and pharmacological data to classify hospitalization as ‘possibly’ or ‘unlikely’ drug-related. This is a time-consuming method but enables detailed assessments of clinical characteristics related to the admissions. On the other hand, the validity of the methodological approach is difficult to evaluate. The occurrence of adherence issues, adverse effect, and drug monitoring DRPs were found associated with increased risk of DRHs as in other studies involving older patients, which supports the validity of the DRH assessments in the present study.
It is likely that the findings of our study will reflect other multimorbid patient populations using multiple drugs from different therapeutic classes. However, as for other studies investigating DRHs, the generalisability of the findings are not necessarily transferable to other hospitals or clinical setting. Thus, further studies on the same patient group, preferably also including additional factors of potential importance for the risk of hospitalization, e.g. social factors, should be conducted to compare and evaluate the generalisability of our findings.
Important limitations of the study include i) the lack of measuring inter-rater reliability between the six clinical pharmacists involved in the identification and classification of DRPs, ii) the lack of using of an internationally agreed classification system for DRPs (IMM classification used instead), iii) the lack of assessing preventability of DRHs and iv) that the DRH assessments were performed by only two researchers/experts without applying a validated scale. However, regarding the first mentioned limitation, a point is that the involvement of several clinical pharmacists also is a study strength, as it also may reduce the probability of operator-biased findings. The thorough, comprehensive DRH assessments covering all aspects related to the hospitalizations is another strength of the study.
Conclusions
This study indicates that DRHs are prevalent in multimorbid internal medicine patients using at least four regular drugs from different therapeutic classes. Several factors were associated with risk of DRHs in these patients, with adverse effect DRPs and adherence issues being most important. The results clearly indicate the necessity of managing DRPs in multimorbid patients.
Supporting information
(PDF)
(PDF)
(PDF)
GDIs of potential relevance for assessments of drug-related hospitalizations (DRHs) were determined based on expected consequences of GDIs and information available in the medical records, including causes of hospitalization.
(PDF)
Acknowledgments
The authors thank the study pharmacists Anne Schwinghammer, Anette Engnes, Elin Trapnes, Hanne Steen, and Petra Foynland for their valuable contribution in patient inclusion and medicines reconciliation and review, senior physician Jo Fuglestved for summarizing the CCI scores, project group members Anne Mette Njaastad, Kristin Hestad Solheim and Kristin Thomassen for valuable input on the study design, employees at the internal medicine ward for positive attitude to the study, and finally the laboratory technicians at Diakonhjemmet Hospital for performing pharmacogenetic analyses.
Data Availability
The data that supports the findings of this study are available upon request from Oslo University Hospital due to ethical restrictions involving patient-sensitive information imposed by the Regional Committee for Medical and Health Research Ethics in Norway (REC) and the Privacy Ombudsman at Oslo University Hospital. Data are available from the authors and REC upon reasonable request and with permission of Oslo University Hospital. Interested researchers can contact REC via email at rek-sorost@medisin.uio.no or the corresponding author.
Funding Statement
The study was funded by South-Eastern Norway Regional Health Authority (PhD grant number 12/00718 to author ML, https://www.helse-sorost.no/south-eastern-norway-regional-health-authority), Hospital Pharmacies Enterprise, South Eastern Norway (https://sykehusapotekene.no/), Oslo University Hospital (https://oslo-universitetssykehus.no/oslo-university-hospital) and Diakonhjemmet Hospital (http://diakonhjemmetsykehus.no/#!/diakon/forside/om-sykehuset/brief-information-in-english). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pattanaik S, Dhamija P, Malhotra S, Sharma N, Pandhi P. Evaluation of cost of treatment of drug-related events in a tertiary care public sector hospital in Northern India: a prospective study. British journal of clinical pharmacology. 2009;67(3):363–9. Epub 2009/06/16. 10.1111/j.1365-2125.2008.03346.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcum ZA, Pugh MJ, Amuan ME, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of potentially preventable unplanned hospitalizations caused by therapeutic failures and adverse drug withdrawal events among older veterans. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67(8):867–74. Epub 2012/03/06. 10.1093/gerona/gls001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samoy LJ, Zed PJ, Wilbur K, Balen RM, Abu-Laban RB, Roberts M. Drug-related hospitalizations in a tertiary care internal medicine service of a Canadian hospital: a prospective study. Pharmacotherapy. 2006;26(11):1578–86. Epub 2006/10/27. 10.1592/phco.26.11.1578 . [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson M, Sjolander M, Pfister B, Jonsson J, Schneede J, Lovheim H. Drug-related hospital admissions among old people with dementia. European journal of clinical pharmacology. 2016;72(9):1143–53. Epub 2016/07/06. 10.1007/s00228-016-2084-3 . [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M, et al. Adverse drug reactions as a cause of hospital admissions: A 6-month experience in a single center in Greece. European journal of internal medicine. 2008;19(7):505–10. 10.1016/j.ejim.2007.06.030 [DOI] [PubMed] [Google Scholar]
- 6.Al Hamid A, Ghaleb M, Aljadhey H, Aslanpour Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. British journal of clinical pharmacology. 2014;78(2):202–17. Epub 2013/11/29. 10.1111/bcp.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Internal medicine journal. 2001;31(4):199–205. Epub 2001/07/18. . [DOI] [PubMed] [Google Scholar]
- 8.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. European journal of clinical pharmacology. 2017;73(6):759–70. Epub 2017/03/03. 10.1007/s00228-017-2225-3 . [DOI] [PubMed] [Google Scholar]
- 9.Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. The Annals of pharmacotherapy. 2002;36(7–8):1238–48. Epub 2002/06/28. 10.1345/aph.1A225 . [DOI] [PubMed] [Google Scholar]
- 10.Easton KL, Chapman CB, Brien JA. Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. British journal of clinical pharmacology. 2004;57(5):611–5. Epub 2004/04/20. 10.1111/j.1365-2125.2004.02052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Archives of internal medicine. 2008;168(17):1890–6. Epub 2008/09/24. 10.1001/archinternmed.2008.3 . [DOI] [PubMed] [Google Scholar]
- 12.Varga S, Alcusky M, Keith SW, Hegarty SE, Del Canale S, Lombardi M, et al. Hospitalization rates during potentially inappropriate medication use in a large population-based cohort of older adults. British journal of clinical pharmacology. 2017;83(11):2572–80. Epub 2017/07/02. 10.1111/bcp.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. British journal of clinical pharmacology. 2007;63(2):136–47. Epub 2006/06/29. 10.1111/j.1365-2125.2006.02698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claydon-Platt K, Manias E, Dunning T. Medication-related problems occurring in people with diabetes during an admission to an adult teaching hospital: a retrospective cohort study. Diabetes research and clinical practice. 2012;97(2):223–30. Epub 2012/05/01. 10.1016/j.diabres.2012.03.003 . [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. European journal of clinical pharmacology. 2002;58(4):285–91. Epub 2002/07/24. 10.1007/s00228-002-0467-0 . [DOI] [PubMed] [Google Scholar]
- 16.Parmaceutical Care Network Europe (PCNE). Classification for Drug related problems V 6.2. [cited 2018 April 3]. Available from: http://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf.
- 17.Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P, et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. European journal of clinical pharmacology. 2004;60(9):651–8. Epub 2004/11/30. 10.1007/s00228-004-0830-4 . [DOI] [PubMed] [Google Scholar]
- 18.Elliott LS, Henderson JC, Neradilek MB, Moyer NA, Ashcraft KC, Thirumaran RK. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: A prospective pilot randomized controlled trial. PloS one. 2017;12(2):e0170905 Epub 2017/02/06. 10.1371/journal.pone.0170905 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassell A, Edwards D, Harshfield A, Rhodes K, Brimicombe J, Payne R, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. The British journal of general practice: the journal of the Royal College of General Practitioners. 2018. Epub 2018/03/14. 10.3399/bjgp18X695465 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glynn LG, Valderas JM, Healy P, Burke E, Newell J, Gillespie P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Family Practice. 2011;28(5):516–23. 10.1093/fampra/cmr013 [DOI] [PubMed] [Google Scholar]
- 21.Muth C, Blom JW, Smith SM, Johnell K, Gonzalez-Gonzalez AI, Nguyen TS, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. Journal of internal medicine. 2018. [Epub ahead of print]. Epub 2018/10/26. 10.1111/joim.12842 . [DOI] [PubMed] [Google Scholar]
- 22.Aubert CE, Streit S, Da Costa BR, Collet TH, Cornuz J, Gaspoz JM, et al. Polypharmacy and specific comorbidities in university primary care settings. European journal of internal medicine. 2016;35:35–42. Epub 2016/06/13. 10.1016/j.ejim.2016.05.022 . [DOI] [PubMed] [Google Scholar]
- 23.Navickas R, Visockiene Z, Puronaite R, Rukseniene M, Kasiulevicius V, Jureviciene E. Prevalence and structure of multiple chronic conditions in Lithuanian population and the distribution of the associated healthcare resources. European journal of internal medicine. 2015;26(3):160–8. Epub 2015/03/03. 10.1016/j.ejim.2015.02.015 . [DOI] [PubMed] [Google Scholar]
- 24.Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. The European journal of general practice. 2008;14 Suppl 1:28–32. Epub 2008/10/31. 10.1080/13814780802436093 . [DOI] [PubMed] [Google Scholar]
- 25.Jureviciene E, Onder G, Visockiene Z, Puronaite R, Petrikonyte D, Gargalskaite U, et al. Does multimorbidity still remain a matter of the elderly: Lithuanian national data analysis. Health policy (Amsterdam, Netherlands). 2018. Epub 2018/04/01. 10.1016/j.healthpol.2018.03.003 . [DOI] [PubMed] [Google Scholar]
- 26.Chan A, Soh D, Ko Y, Huang YC, Chiang J. Characteristics of unplanned hospital admissions due to drug-related problems in cancer patients. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(7):1875–81. Epub 2014/02/22. 10.1007/s00520-014-2160-0 . [DOI] [PubMed] [Google Scholar]
- 27.Peyriere H, Cassan S, Floutard E, Riviere S, Blayac JP, Hillaire-Buys D, et al. Adverse drug events associated with hospital admission. The Annals of pharmacotherapy. 2003;37(1):5–11. Epub 2002/12/31. 10.1345/aph.1C126 . [DOI] [PubMed] [Google Scholar]
- 28.Pedros C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. European journal of clinical pharmacology. 2014;70(3):361–7. Epub 2013/12/24. 10.1007/s00228-013-1630-5 . [DOI] [PubMed] [Google Scholar]
- 29.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. Epub 1976/01/01. 10.1159/000180580 . [DOI] [PubMed] [Google Scholar]
- 30.Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. The American journal of medicine. 1988;84(6):1053–60. Epub 1988/06/01. 10.1016/0002-9343(88)90310-5 . [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Collaborating Centre for Drug Statistics Methodology. ATC index with DDDs [cited 2018 September 20]. Available from: https://www.whocc.no/atc_ddd_index/. [Google Scholar]
- 33.Mercer S, Salisbury C, Fortin M. ABC of Multimorbidity. First ed: John Wiley & Sons; 2014. [Google Scholar]
- 34.Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. Journal of evaluation in clinical practice. 2007;13(5):781–8. Epub 2007/09/11. 10.1111/j.1365-2753.2006.00753.x . [DOI] [PubMed] [Google Scholar]
- 35.Griese-Mammen N, Hersberger KE, Messerli M, Leikola S, Horvat N, van Mil JWF, et al. PCNE definition of medication review: reaching agreement. International journal of clinical pharmacy. 2018;40(5):1199–208. Epub 2018/08/04. 10.1007/s11096-018-0696-7 . [DOI] [PubMed] [Google Scholar]
- 36.Cheng PY, Morgan ET. Hepatic cytochrome P450 regulation in disease states. Current drug metabolism. 2001;2(2):165–83. Epub 2001/07/27. . [DOI] [PubMed] [Google Scholar]
- 37.Philips BJ, Lane K, Dixon J, Macphee I. The effects of acute renal failure on drug metabolism. Expert opinion on drug metabolism & toxicology. 2014;10(1):11–23. Epub 2013/10/01. 10.1517/17425255.2013.835802 . [DOI] [PubMed] [Google Scholar]
- 38.Hubbard RE, O'Mahony MS, Calver BL, Woodhouse KW. Plasma esterases and inflammation in ageing and frailty. European journal of clinical pharmacology. 2008;64(9):895–900. Epub 2008/05/29. 10.1007/s00228-008-0499-1 . [DOI] [PubMed] [Google Scholar]
- 39.Nivya K, Sri Sai Kiran V, Ragoo N, Jayaprakash B, Sonal Sekhar M. Systemic review on drug related hospital admissions—A pubmed based search. Saudi pharmaceutical journal: SPJ: the official publication of the Saudi Pharmaceutical Society. 2015;23(1):1–8. Epub 2015/02/17. 10.1016/j.jsps.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). Journal of the American Geriatrics Society. 2002;50(12):1962–8. Epub 2002/12/11. 10.1046/j.1532-5415.2002.50607.x . [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Holman CD, Price SD, Sanfilippo FM, Preen DB, Bulsara MK. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ (Clinical research ed). 2009;338:a2752 Epub 2009/01/09. 10.1136/bmj.a2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alassaad A, Bertilsson M, Gillespie U, Sundstrom J, Hammarlund-Udenaes M, Melhus H. The effects of pharmacist intervention on emergency department visits in patients 80 years and older: subgroup analyses by number of prescribed drugs and appropriate prescribing. PloS one. 2014;9(11):e111797 Epub 2014/11/05. 10.1371/journal.pone.0111797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohman MC, Cotton BP, Zagaria AB, Bao Y, Greenberg RL, Fortuna KL, et al. Hospitalization Risk and Potentially Inappropriate Medications among Medicare Home Health Nursing Patients. Journal of general internal medicine. 2017;32(12):1301–8. Epub 2017/08/30. 10.1007/s11606-017-4157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao Y, Shao H, Bishop TF, Schackman BR, Bruce ML. Inappropriate medication in a national sample of US elderly patients receiving home health care. Journal of general internal medicine. 2012;27(3):304–10. Epub 2011/10/07. 10.1007/s11606-011-1905-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brody AA, Gibson B, Tresner-Kirsch D, Kramer H, Thraen I, Coarr ME, et al. High Prevalence of Medication Discrepancies Between Home Health Referrals and Centers for Medicare and Medicaid Services Home Health Certification and Plan of Care and Their Potential to Affect Safety of Vulnerable Elderly Adults. Journal of the American Geriatrics Society. 2016;64(11):e166–e70. Epub 2016/09/28. 10.1111/jgs.14457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, O'Kelly S, O'Dowd T. GPs' and pharmacists' experiences of managing multimorbidity: a 'Pandora's box'. The British journal of general practice: the journal of the Royal College of General Practitioners. 2010;60(576):285–94. Epub 2010/07/03. 10.3399/bjgp10X514756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sondergaard E, Willadsen TG, Guassora AD, Vestergaard M, Tomasdottir MO, Borgquist L, et al. Problems and challenges in relation to the treatment of patients with multimorbidity: General practitioners' views and attitudes. Scandinavian journal of primary health care. 2015;33(2):121–6. Epub 2015/07/15. 10.3109/02813432.2015.1041828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwab C, Korb-Savoldelli V, Escudie JB, Fernandez C, Durieux P, Saint-Jean O, et al. Iatrogenic risk factors associated with hospital readmission of elderly patients: A matched case-control study using a clinical data warehouse. Journal of clinical pharmacy and therapeutics. 2018;43(3):393–400. Epub 2018/02/16. 10.1111/jcpt.12670 . [DOI] [PubMed] [Google Scholar]
- 49.Sehgal V, Bajwa SJS, Sehgal R, Bajaj A, Khaira U, Kresse V. Polypharmacy and potentially inappropriate medication use as the precipitating factor in readmissions to the hospital. J Family Med Prim Care. 2013;2(2):194–9. 10.4103/2249-4863.117423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picker D, Heard K, Bailey TC, Martin NR, LaRossa GN, Kollef MH. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC health services research. 2015;15:282–. 10.1186/s12913-015-0950-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franciosa JA. Beta-adrenergic blocking agents: past, present, and future perspectives. Coronary artery disease. 1999;10(6):369–76. Epub 1999/09/04. . [DOI] [PubMed] [Google Scholar]
- 52.Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC medicine. 2015;13:78 Epub 2015/04/17. 10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
GDIs of potential relevance for assessments of drug-related hospitalizations (DRHs) were determined based on expected consequences of GDIs and information available in the medical records, including causes of hospitalization.
(PDF)
Data Availability Statement
The data that supports the findings of this study are available upon request from Oslo University Hospital due to ethical restrictions involving patient-sensitive information imposed by the Regional Committee for Medical and Health Research Ethics in Norway (REC) and the Privacy Ombudsman at Oslo University Hospital. Data are available from the authors and REC upon reasonable request and with permission of Oslo University Hospital. Interested researchers can contact REC via email at rek-sorost@medisin.uio.no or the corresponding author.


