Abstract
Exosomes might have an unimproved potential to serve as effective delivery vehicles. However, when exosomes are developed for therapeutic applications, a method to enhance their delivery is important. This study aimed to evaluate wheather calcium chloride (CaCl2) or other chloride compounds could enhance exosome delivery to various cells without causing toxicity. Exosomes were purified from human serum by using the ExoQuick exosome precipitation kit. Isolated exosomes were mixed with CaCl2 at concentrations ranging from 100 μM to 1 mM, and then washed using Amicon filter for treating the cells. The delivery efficiency of exosomes and the viability of the cells [HEK 293 (human kidney cells) and H9C2 (rat cardiomyocytes)] were evaluated. Cellular uptake of exosomes was observed using a confocal microscope based on PKH26 labeling of exosomes. CaCl2 increased the delivery of exosomes in a dose- and treatment time-dependent manner. In HEK 293 cells, a CaCl2 concentration of 400 μM and exposure time of 12 h increased the delivery of exosomes by >20 times compared with controls. In H9C2 cells, a CaCl2 concentration of 400 μM and exposure time of >24 h increased the delivery of exosomes by >400 times compared with controls. The viability of both cell lines was maintained up to a CaCl2 concentration of 1 mM. However, cobalt chloride, cupric chloride, and magnesium chloride did not change the delivery of exosomes in both cell lines. These results suggest that the use of CaCl2 treatment might be a useful method for enhancing the delivery of exosomes.
Introduction
Exosomes are 40–200nm vesicles secreted by many types of cells [1]. They exist in cell culture medium and in numerous body fluids including plasma and serum [2]. The biogenesis of exosomes involves the fusion of multivesicular bodies enclosing pools of endosomal vesicles with the cell plasma membrane and the subsequent release of the vesicles into intercellular space [3]. These vesicles are known to carry a variety of signaling molecules, including nucleic acids, predominantly mRNA and microRNA, functional proteins, and lipids [4]. Owing to their outstanding cell-to-cell communication characteristic, numerous studies have investigated the role of exosomes in physiological and pathophysiological processes, including immune modulation [5,6], tumor metastasis [7], and neurodegenerative diseases [8,9].
Many studies have suggested that exosomes can be ideal candidates for use as carriers for drug delivery [10,11]. Exosomes have been used as delivery vehicles of small nucleic acids such as micro-RNAs and small interfering RNAs or low-molecular-weight medicines [12,13]. Exosome-liposome hybrid nanoparticles can deliver the CRISPR-Cas9 system in mesenchymal stem cells, and thus can be promising tools in in vivo gene manipulation [14]. The organ-specific delivery of exosomes was improved by expressing target peptides with Lamp2 on the surface of exosomes [12,15–17]. Pseudotyping exosomes have been suggested as vehicles for the enhanced delivery of protein reporters and protein therapeutics to target cells [18]. However, a simpler method for enhancing the delivery efficacy of exosomes is needed [19].
To date, calcium chloride (CaCl2)-associated transfection methods have been widely used to introduce DNA into mammalian cells, with relatively low cost and low toxicity [20]. However, whether CaCl2 can increase the delivery efficiency of exosomes to the target cells has not been evaluated. In a recent study, CaCl2 and subsequent heat shock-mediated miR-15a-loaded exosomes showed higher delivery of miR-15a to target cells than miR-15a-electroporated exosome [21]. Taken together, we hypothesized that CaCl2 or other chloride compounds can affect the delivery efficacy of exosomes into target cells.
Thus, we aimed to investigate whether exosomes mixed with CaCl2 can show improved delivery to cells compared with normal exosomes, thus providing an effective method for drug delivery in the future.
Materials and methods
Exosome purification and labeling
Human peripheral blood samples from non-atrial fibrillation patients were obtained at Yonsei University Health System (Seoul, Korea). The study protocol conformed to the principles outlined in Declaration of Helsinki, and was approved by the local ethics committee (YUMC 4-2011-0872). Informed consent was obtained from all patients. The name of ethics committee is as follows: Severance Hospital.
Exosomes were purified using the ExoQuick exosome precipitation kit (SBI System Bioscience, Mountain View, CA, USA), according to manufacturer’s instructions. A 250 μL volume of human serum was mixed with 63 μL ExoQuick solution and incubated overnight at 4°C. After centrifugation (1500g for 30 min), the supernatant was discarded and tubes were centrifuged again (1500g for 5 min). All traces of fluid were aspirated, then pellets were resuspended in 200 μL phosphate-buffered saline (PBS) [22].
After exosome treatment to cells, the uptake of exosomes by cultured HEK 293 cells (human kidney cells) and H9C2 cells (rat cardiomyocytes) was assessed using a confocal microscope (LSM710; Carl Zeiss GmbH, Jena, Germany). For this evaluation, purified exosomes were labeled using PKH26 dye (Sigma, Germany) according to the manufacturer’s instructions, as described previously [23]. Briefly, exosomes were suspended in 1 mL diluent C containing 5 μM PKH26 and incubated for 5 min. The labeling action was stopped by incubating for 1 min with an equal volume of 1% bovine serum albumin (Bovogen, Melbourne, Australia). The exosomes were washed twice with Amicon ultrafilter (10 KDa cut-off, Millipore, MA, USA) with cold PBS. Thereafter, the exosomes were resuspended in 200 μL PBS.
Mixture of exosomes and CaCl2
To deliver exosomes efficiently to cells, a modified method of CaCl2 transfection was developed. PKH26-labeled exosomes (50 μg in PBS) were mixed with CaCl2 solution (0.2 M stock). The final volume was adjusted to 150 μL using sterile PBS. The mixture was incubated at 37°C in a shaker for 10 min at 25 rpm, and then the tube was immediately placed on ice. The ExoQuick reagent (30 μL) was added, and the mixture was placed on ice for 30 min. The sample was centrifuged for 3 min at 13,000–14,000 rpm in a microfuge. The supernatant was removed, and the mixture pellet was resuspended in 1 mL PBS. The pellet was washed using Amicon Ultra tubes with cold PBS. Thereafter, the exosomes were resuspended in 200 μL PBS.
Western blot analysis
Western blot analysis was performed as we described previously [16]. Briefly, ultracentrifuged exosomal pellets were lysed with radioimmunoprecipitation buffer (ATTO, NY, USA) containing a protease inhibitor cocktail (ATTO). The total amount of protein was determined using a 660-nm protein assay (Pierce, MA, USA), and equal amounts (20 μg) of exosomal proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The blots were probed overnight at 4°C with anti-Lamp2 (SC-18822; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-CD81 (SC-166029, Santa Cruz Biotechnology), or anti-Alix (SC-99010, Santa Cruz Biotechnology), as indicated. The membranes were then exposed to horseradish peroxidase-conjugated mouse or rabbit anti-mouse secondary antibodies (Santa Cruz Biotechnology), and the results were visualized using chemiluminescence (Advansta, Menlo Park, CA, USA).
Transmission electron microscopy
A Formvar-carbon-coated electron microscope grid was placed with the formvar side down on top of an exosome drop for approximately 1 min. The grid was removed, blotted with filter paper, and placed onto a drop of 2% uranyl acetate for 15 s. The excess uranyl acetate was removed, and the electron microscope grid was examined and photographed for transmission electron microscopy (TEM). All thin sections were observed under a transmission electron microscope (JEM-1011; JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV. Images were captured with a side-mounted Camera-Megaview III (Soft Imaging System, Münster, Germany) [24].
Nanoparticle tracking analysis
The number of nanoparticles in serum-derived exosomes was assessed using the Nanosight LM10-HS nanoparticle characterization system (Nanosight Ltd., Amesbury, UK). Three recordings were performed for each sample. The Nanosight Tracking Analysis 3.2 software was then used to analyze the video, and to determine the particle concentration and the size distribution of the particles. Three videos of 10-s duration were recorded for each sample.
Cell culture
HEK 293 cells and H9C2 cells were cultivated as we described previously [16]. Briefly, HEK 293 cells (Korean Cell Line Bank, Seoul, Korea) and H9C2 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s medium (Welgene, Daegu, Korea) containing 10% fetal bovine serum (Young In Frontier, Seoul, Korea) and 1% penicillin-streptomycin (Gibco, NY, USA). Cells were cultured in a humidified incubator at 37°C with 5% CO2.
Immunocytochemistry and confocal microscopy
Immunocytochemistry and confocal microscopy were executed as we described previously [16]. After treating HEK 293 and H9C2 cells with or without exosomes for 24 h, the cells were fixed with 4% paraformaldehyde for 60 min at room temperature and washed with PBS. Cell nuclei were stained with 4′6‐diamidino‐2‐phenylindole (Santa Cruz Biotechnology). Fluorescence images were obtained using a Zeiss LSM710 confocal microscope with 2 excitation filters (405 and 543 nm). The data were recorded as serial optical sections, each consisting of 1024 × 1024 pixels, overlaid to distinguish between the separate emission channels, and saved as TIFF (tagged image file format) files. Quantification was performed using Image J program. PKH26 expression and DAPI expression were measured respectively using the RGB function of histogram. The same slide was averaged by taking three different parts, and the number of samples was three.
Cell proliferation assay
The cytotoxic potential of exosomes was assessed using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (Promega, Madison, WI, USA). HEK 293 cells were treated with exosomes in triplicate in a 96-well plate. Cell survival was determined using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Menlo Park, CA, USA).
Mouse and NIRF imaging
Male C57BL/6 mice (25 g) were purchased from Orient Bio (Seoul, Korea). All animal experiments were approved by the Institutional Animal Care and Use Committee of Yonsei University College of Medicine (Seoul, Korea), and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. We injected 150 μg of PKH26 labeled exosomes per animal. After 24 h, the heart, liver, and spleen of each mouse were harvested and IVIS Spectrum imaging system (PerkinElmer, Waltham, MA, USA) was employed to capture Near-infrared fluorescence (NIRF) images. PKH26-related fluorescence signals were discriminated from the auto-fluorescence signals using Living Image software (PerkinElmer).
Statistical analysis
Data analyses were performed with Student's t-test between two groups. A p-value of <0.05 was considered statistically significant. All statistical analyses were conducted with SPSS version 23.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Isolation and characterization of serum exosomes
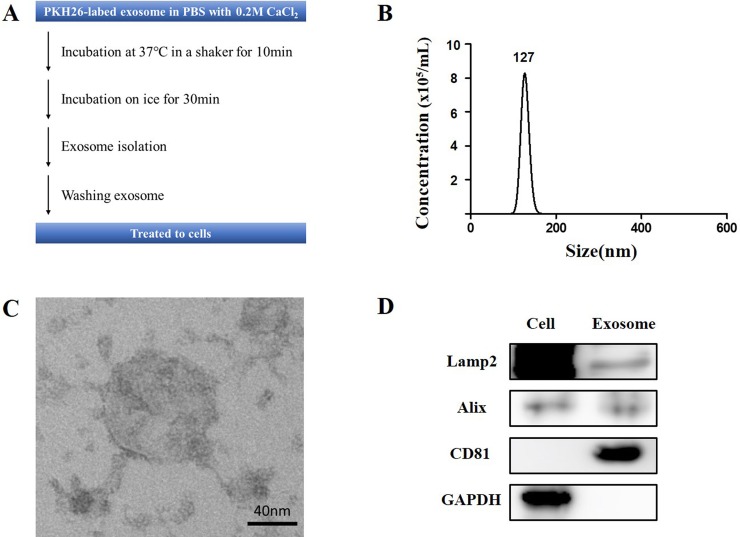
The protocol of preparing the mixture of exosomes and CaCl2 is shown in Fig 1A. CaCl2-associated transfection methods are widely used for introducing DNA into mammalian cells [20]. We used a modified protocol in which incubation at 37°C in a shaker was performed to increase exosome delivery to cells. Exosomes were isolated from peripheral blood by using the ExoQuick reagent, as described in Materials and Methods. The profiles of serum-derived exosomes were characterized using nanoparticle tracking analysis, TEM, and western blotting. According to the Nanosight instrument, the mean particle diameter was 136.6 nm, and mean concentration was 8.59×107 particles/ml (Fig 1B). And structurally intact exosomes were detected by TEM analysis (Fig 1C). There was no significant difference in the size and concentration of exosomes before and after addition of CaCl2 when TEM and NTA were compared (S1 Fig). When cell lysate and exosome were examined by western blot analysis, the exosomes were positive for exosomal markers including Alix, CD81, and Lamp2 (Fig 1D). Taken together, the exosomes were successfully purified.
Fig 1. Characterization of exosomes.
(A) Study protocol. (B) Nanoparticle tracking analysis of exosomes showing the number and size distribution of particles. (C) Representative electron microscopic image of the exosomes (scale bar, 40 nm). (D) Western blot analysis of isolated exosomes and cell lysate.
CaCl2 dose-dependent delivery of exosomes to HEK 293 and H9C2 cells
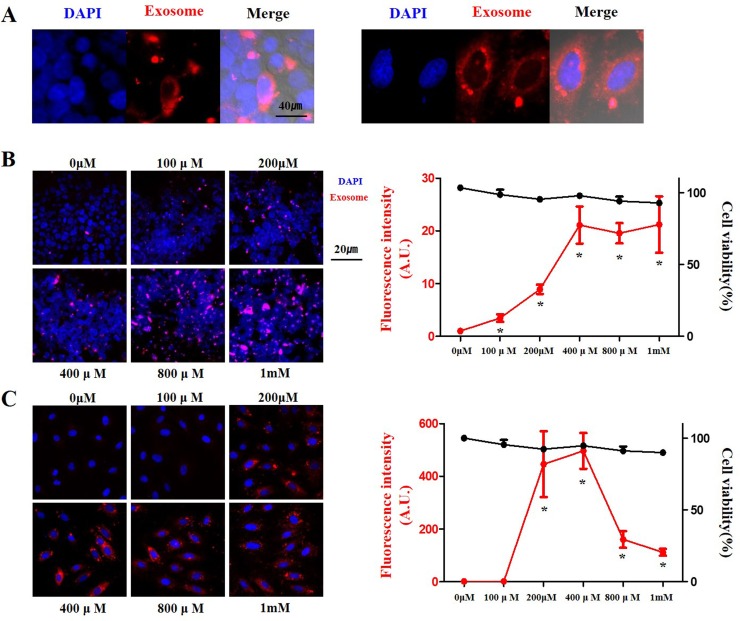
To identify whether CaCl2 treatment improves delivery of exosomes, we added several concentrations of CaCl2 to exosomes and these mixtures were incubated at 37°C in a shaker. The mixture of exosomes and CaCl2 was used to treat the HEK 293 and H9C2 cells, which were then incubated for 24 h. The most commonly used method for monitoring exosome uptake is to stain exosomal membranes with fluorescent lipid membrane dyes such as PKH26 [25], PKH67 [26], and DiI [27]. In this study, PKH26 was used to label the exosomes and to monitor the uptake of labeled exosomes by cells. The uptake of labeled exosomes by cells was confirmed using a confocal microscope (Fig 2A).
Fig 2. Delivery efficiency of exosomes with CaCl2 in a dose-dependent manner.
(A) Typical example of delivery of exosomes to HEK 293 cells (left panels) and H9C2 cells (right panels). (B) and (C) Delivery efficiency and cell viability of exosomes labeled with PKH26 (red) at different concentrations of CaCl2 in HEK 293 (B) and H9C2 (C) cells. Fluorescent microscopic images (left panels), and fluorescence intensity and cell viability (right panels). The data are presented as the mean ± s.e.m. *P < 0.01.
In HEK 293 cells, the delivery of exosomes was dose-dependently increased. At a CaCl2 concentration of 400 μM (P < 0.001), the delivery of exosomes increased the most, and then reached a plateau. In H9C2 cells, the delivery of exosomes was significantly increased over 400 times (P = 0.003) at a CaCl2 concentration of 200 μM, After peaking at 400 μM, it decreased at higher concentrations (P < 0.001).
In order for exosomes to be used as therapeutic application, there should be no damage to the cells. Thus, we checked cell viability after exosomes incubation. Cell viability was maintained up to a CaCl2 concentration of 1 mM in both cells (Fig 2B and 2C). As a result, we found that the CaCl2 treatment improved the delivery of exosomes to cells without toxicity.
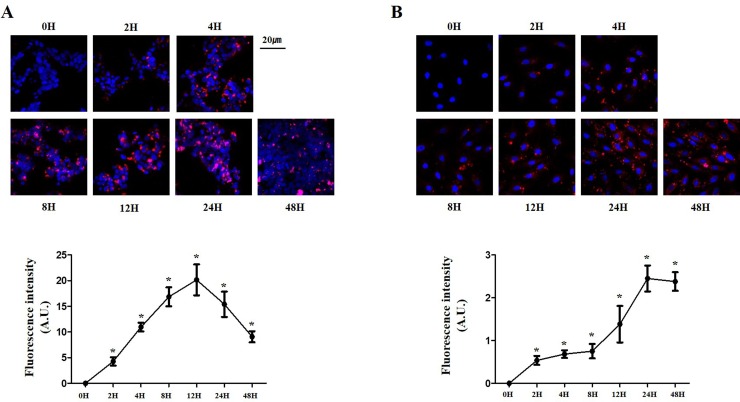
Optimal time for exosome uptake by cells
Because 400 μM was the CaCl2 concentration with the maximum delivery of exosomes, we evaluated the cellular uptake of exosomes at different durations of exposure to these CaCl2-treated exosomes. In HEK 293 cells, the cellular uptake of exosomes increased as the incubation time increased, with saturation at around 12 h and a decrease after 24 h (Fig 3A, P < 0.0001). In H9C2 cells, the uptake increased as the incubation time increased, with saturation at around 24 h and a decrease after 48 h (Fig 3B, P < 0.0001). Taken together, we found the most efficient concentration of CaCl2 and the appropriate treatment time.
Fig 3. Delivery efficiency of exosomes with CaCl2 in a time-dependent manner.
(A) and (B) Delivery efficiency of exosomes labeled with PKH26 (red) at different time exposures to CaCl2-treated exosomes in HEK 293 (A) and H9C2 (B) cells. Fluorescent microscopic images (upper panels) and fluorescence intensity (lower panels). *P < 0.01.
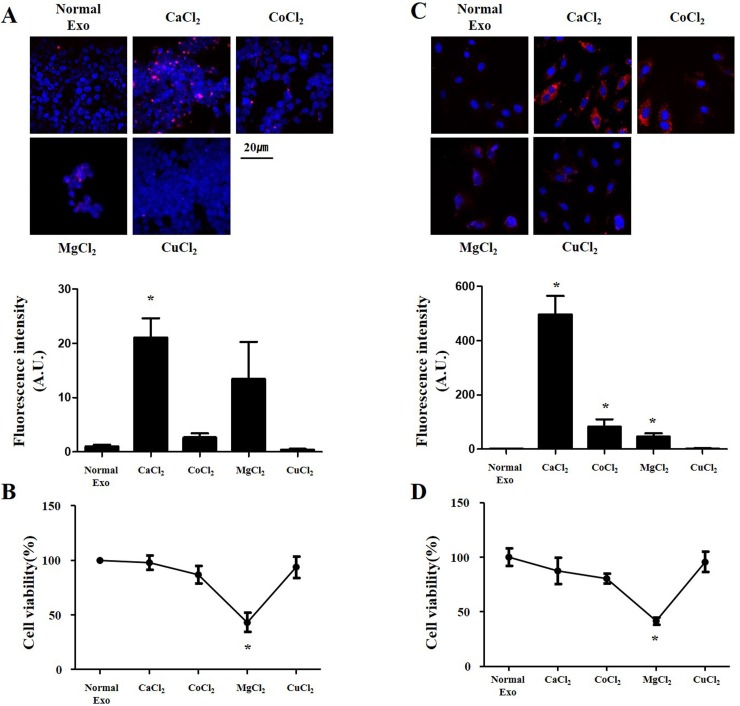
CaCl2 is more effective than other chloride compounds
Next, we assessed whether the cellular uptake of exosomes will be increased by other chloride compounds. Cobalt chloride (CoCl2), magnesium chloride (MgCl2) and cupric chloride (CuCl2) were treated exosome at a concentration of 400μM for 24 hours. Exosome delivery and cell viability were measured by the above method. In HEK 293 cells, the cellular delivery of exosomes was not significantly improved by CoCl2 or MgCl2, but was rather decreased by CuCl2 (Fig 4A). In H9C2 cells, the cellular delivery of exosomes was improved by CoCl2 (P = 0.017) and MgCl2 (P = 0.004), but not by CuCl2 (Fig 4B). Moreover, the cell viability was significantly decreased by MgCl2 in both cell lines. These results show that CaCl2 significantly enhances intracellular delivery of exosomes compared to other chloride compounds.
Fig 4. Delivery efficiency of exosomes with chloride compounds.
(A, C) Delivery efficiency and (B, D) cell viability of exosomes labeled with PKH26 (red) added with different chloride compounds in HEK 293 (A,B) and H9C2 (C,D) cells. Fluorescent microscopic images (upper panels), and fluorescence intensity and cell viability (lower panels). *P < 0.01.
In vivo delivery of CaCl2-treated exosomes
We also performed in vivo experiments to support the claim that CaCl2 enhances the delivery of exosomes. Exosomes were injected via tail vein in mice and sacrificed 24 hours later. The level of PKH26 was measured using In Vivo Imaging System (IVIS) of organs. We confirmed that CaCl2-treated exosomes significantly increased the delivery in heart, lung, kidney, and spleen (S3 Fig). Based on these results, we suggest that CaCl2-treated exosomes allow higher delivery than normal exosomes in vivo.
Discussion
In this study, we attempted to enhance the delivery efficiency of exosomes by using CaCl2, which is commonly used for transfection of DNA into mammalian cells. Exosomes incubated with CaCl2 increased delivery efficiency in a dose-dependent manner in HEK 293 and H9C2 cells. The optimum duration of treatment with exosomes added with CaCl2 was 12 h for HEK 293 cells and 24 h for H9C2 cells. Finally, it was confirmed that CaCl2 significantly increased the intracellular uptake of exosomes when compared with other chloride compounds at the same concentration. On the basis of these results, we suggest that adding CaCl2 to exosomes for treating the cells is an efficient method of increasing the delivery of exosomes.
Exosomes are vesicles of endocytic origin released by many cells [28]. They are crucial in distant cell-cell communication because they can enter the circulatory system when they are secreted and can pass through additional biological barriers [29]. R. Liu et al. recent accumulated evidence suggests that these nano-sized vesicles can deliver various RNAs into cells from the natural pathway, to deliver genetic material in organisms [30]. As exosomes are promising for use as vectors in clinical applications owing to their strong biocompatibility, enhancement of exosome delivery is an important research topic [31]. One of the limitations of exosomes is the requirement for high capacity production for clinical use [19,32]. Our findings provide a solution to this limitation.
In the standard method for transforming of Escherichia coli with external DNA, cells are known to be suitable for DNA uptake by incubating in ice-cold 100 mM CaCl2 [33]. CaCl2 assists the interactions between DNA molecules and the cell surface and helps endocytosis of the DNA molecules [34]. We modified this protocol to increase the uptake of exosomes into cells. Also, calcium influx induces endocytosis and exocytosis, and is known to trigger vesicle fusion [35–37]. Therefore, we anticipate that the addition of CaCl2 increases the efficiency of exosome delivery. However, more research is needed to confirm these mechanisms. In our study, up to 1 mM CaCl2 was used, but no cytotoxicity was observed. Moreover, CaCl2 is a common compound used in various research institutes. In this experiment, a low CaCl2 concentration was used, thus providing the advantages of low cost and high accessibility. We also compared CaCl2 with other chloride compounds. Our results showed that CaCl2 was the only common enhancer of exosome delivery between HEK 293 and H9C2 cells. Our findings provide useful technological insights for the development of exosome-mediated drug delivery.
Supporting information
(A) Representative electron microscopic image of CaCl2-Exo (scale bar, 40 nm). (B) Size distribution of CaCl2-Exo measured from TEM images. (C) Nanoparticle tracking analysis of CaCl2-Exo showing the concentration of particles.
(TIF)
(TIF)
(A) Representative NIRF images (overlaid with photograph) of mice organs which received administration of PBS, PKH26-labeled exosomes, or CaCl2-Exo. (B) Quantitation of fluorescence intensity in the lesion region. *P<0.05.
(TIF)
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by research grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2017R1A2B3003303), and from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare (HI16C0058). The funders had no role in study design data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9: 581–593. 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft BA, de Sonneville J, Yuana Y, Osanto S, Bertina R, Kuil ME, et al. Determination of the size distribution of blood microparticles directly in plasma using atomic force microscopy and microfluidics. Biomed Microdevices. 2012;14: 641–649. 10.1007/s10544-012-9642-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29: 116–125. 10.1016/j.ceb.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7: 10872 10.1038/ncomms10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caradec J, Kharmate G, Hosseini-Beheshti E, Adomat H, Gleave M, Guns E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem. 2014;47: 1286–1292. 10.1016/j.clinbiochem.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9: 17 10.1038/s41467-017-02406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, et al. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun. 2017;8: 1319 10.1038/s41467-017-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6: 37043–37053. 10.18632/oncotarget.6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287: 3842–3849. 10.1074/jbc.M111.277061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219: 396–405. 10.1016/j.jconrel.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellavia D, Raimondi L, Costa V, De Luca A, Carina V, Maglio M, et al. Engineered exosomes: A new promise for the management of musculoskeletal diseases. Biochim Biophys Acta Gen Subj. 2018;1862: 1893–1901. 10.1016/j.bbagen.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29: 341–345. 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35: 2383–2390. 10.1016/j.biomaterials.2013.11.083 [DOI] [PubMed] [Google Scholar]
- 14.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, et al. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv Sci (Weinh). 2018;5: 1700611 10.1002/advs.201700611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7: 1525–1541. 10.2147/IJN.S29661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Yun N, Mun D, Kang JY, Lee SH, Park H, et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun. 2018;499: 803–808. 10.1016/j.bbrc.2018.03.227 [DOI] [PubMed] [Google Scholar]
- 17.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7: 1333–1345. 10.7150/thno.17092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer C, Losacco J, Stickney Z, Li L, Marriott G, Lu B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int J Nanomedicine. 2017;12: 3153–3170. 10.2147/IJN.S133430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita T, Takahashi Y, Takakura Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol Pharm Bull. 2018;41: 835–842. 10.1248/bpb.b18-00133 [DOI] [PubMed] [Google Scholar]
- 20.Kingston RE, Chen CA, Rose JK. Calcium phosphate transfection. Curr Protoc Mol Biol. 2003;Chapter 9: Unit 9.1. 10.1002/0471142727.mb0901s63 [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312: L110–l121. 10.1152/ajplung.00423.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, et al. Paclitaxelresistant gastric cancer MGC803 cells promote epithelialtomesenchymal transition and chemoresistance in paclitaxelsensitive cells via exosomal delivery of miR1555p. Int J Oncol. 2019;54: 326–338. 10.3892/ijo.2018.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed-Zidane M, Linden L, Salilew-Wondim D, Held E, Neuhoff C, Tholen E, et al. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS One. 2017;12: e0187569 10.1371/journal.pone.0187569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Kim HJ, Kim HJ, Kim DH, Han JH, Byeon HK, et al. HSPA5 negatively regulates lysosomal activity through ubiquitination of MUL1 in head and neck cancer. Autophagy. 2018;14: 385–403. 10.1080/15548627.2017.1414126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller L, Simms P, Hong CS, Nishimura MI, Jackson EK, Watkins SC, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6: e1261243 10.1080/2162402X.2016.1261243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017;16: 132 10.1186/s12943-017-0694-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5 10.1161/jaha.115.002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9: 654–659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 29.Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521: 167–175. 10.1016/j.ijpharm.2017.02.038 [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Liu J, Ji X, Liu Y. Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases. Metab Brain Dis. 2013;28: 551–562. 10.1007/s11011-013-9434-y [DOI] [PubMed] [Google Scholar]
- 31.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38: 754–763. 10.1038/aps.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195: 72–85. 10.1016/j.jconrel.2014.07.049 [DOI] [PubMed] [Google Scholar]
- 33.Aich P, Patra M, Chatterjee AK, Roy SS, Basu T. Calcium chloride made E. coli competent for uptake of extraneous DNA through overproduction of OmpC protein. Protein J. 2012;31: 366–373. 10.1007/s10930-012-9411-z [DOI] [PubMed] [Google Scholar]
- 34.Wigler M, Silverstein S, Lee LS, Pellicer A, Cheng Y, Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11: 223–232 10.1016/0092-8674(77)90333-6 [DOI] [PubMed] [Google Scholar]
- 35.Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, et al. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12: 1003–1010. 10.1038/nn.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, et al. Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J Gen Physiol. 2011;137: 111–132. 10.1085/jgp.201010468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyoung M, Zhang Y, Diao J, Chu S, Brunger AT. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nat Protoc. 2013;8: 1–16. 10.1038/nprot.2012.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative electron microscopic image of CaCl2-Exo (scale bar, 40 nm). (B) Size distribution of CaCl2-Exo measured from TEM images. (C) Nanoparticle tracking analysis of CaCl2-Exo showing the concentration of particles.
(TIF)
(TIF)
(A) Representative NIRF images (overlaid with photograph) of mice organs which received administration of PBS, PKH26-labeled exosomes, or CaCl2-Exo. (B) Quantitation of fluorescence intensity in the lesion region. *P<0.05.
(TIF)
Data Availability Statement
All relevant data are within the paper.