Abstract
Increasing seed oil content is one of the most important breeding goals for soybean due to a high global demand for edible vegetable oil. However, genetic improvement of seed oil content has been difficult in soybean because of the complexity of oil metabolism. Determining the major variants and molecular mechanisms conferring oil accumulation is critical for substantial oil enhancement in soybean and other oilseed crops. In this study, we evaluated the seed oil contents of 219 diverse soybean accessions across six different environments and dissected the underlying mechanism using a high-resolution genome-wide association study (GWAS). An environmentally stable quantitative trait locus (QTL), GqOil20, significantly associated with oil content was identified, accounting for 23.70% of the total phenotypic variance of seed oil across multiple environments. Haplotype and expression analyses indicate that an oleosin protein-encoding gene (GmOLEO1), colocated with a leading single nucleotide polymorphism (SNP) from the GWAS, was significantly correlated with seed oil content. GmOLEO1 is predominantly expressed during seed maturation, and GmOLEO1 is localized to accumulated oil bodies (OBs) in maturing seeds. Overexpression of GmOLEO1 significantly enriched smaller OBs and increased seed oil content by 10.6% compared with those of control seeds. A time-course transcriptomics analysis between transgenic and control soybeans indicated that GmOLEO1 positively enhanced oil accumulation by affecting triacylglycerol metabolism. Our results also showed that strong artificial selection had occurred in the promoter region of GmOLEO1, which resulted in its high expression in cultivated soybean relative to wild soybean, leading to increased seed oil accumulation. The GmOLEO1 locus may serve as a direct target for both genetic engineering and selection for soybean oil improvement.
Author summary
Soybean seed oil is an important quality trait targeted during domestication and breeding. However, the molecular mechanism of soybean oil regulation is largely unknown due to its complex genetic architecture and environmental sensitivity. In this paper, we integrated GWAS across multiple environments, haplotype analysis, genetic transformation, and diversity analysis to study the genetic architecture of oil content and the underlying mechanism in soybean. This combined analysis enabled us to identify an environmentally stable QTL (GqOil20) and functionally verified that GmOLEO1 positively regulates total seed oil accumulation in soybean seeds. In addition, we found that GmOLEO1 showed a higher level of expression in cultivated soybean seeds than in wild soybean seeds, possibly as the result of the positive selection of the promoter, resulting in seed oil accumulation. Moreover, we identified an elite GmOLEO1 haplotype that correlated strongly with high oil content in soybean, holding great potential for assisting oil improvement in soybean breeding. Our study provided a new genetic resource for oil content improvement in soybean and other oilseed crops.
Introduction
Soybean (Glycine max (L.) Merr.) is an important food and oil crop. Soybean seeds accumulate large amounts of oil and protein and have been intensively targeted for human consumption during long-term domestication and cultivation. Given the high percentage of oil in soybean seeds, the demand for soybean oil production has increased dramatically due to the increasing demand for vegetable oils and expanded use of biodiesel, and the seed composition improvement is of particular interest in terms of increasing awareness of health issues around dietary fats [1]. However, oil accumulation in the seed is a complex metabolic process that is environmentally sensitive; thus, stably expressed oil-enhancing key genes that can be applied to soybean molecular breeding have rarely been reported, and the mechanism of the variance of oil content in soybean remains largely unknown.
In plants, accumulated oil in seeds is generally stored as triacylglycerols (TAGs). TAG synthesis is initiated from glucose in the cytosol, and the resulting products from glycolysis are transported into the plastid for fatty acid (FA) synthesis. The FAs are processed by a series of key enzymes to produce C16:0 and C18:0 acyl chains and desaturated products, such as C18:1. FA products are then exported to the endoplasmic reticulum (ER) to form TAGs via the acyl-CoA-dependent and acyl-CoA-independent pathways [2]. The resulting TAGs are present in subcellular spherical lipid droplets in various plant tissues; the lipid droplets stored in seeds are usually called oil bodies (OBs) and have been extensively investigated previously in studies of, for instance, the structure and composition of an OB and the essential role of OB-related proteins, such as oleosins, in OB formation, mobilization, and oil accumulation [3–10]. Previous studies have indicated that oleosins play conserved roles in OB formation in seeds in several oilseed plants [7–8]. Suppression of a soybean oleosin produces micro-OBs [10], while the effects of OBs on seed oil accumulation have rarely been reported in soybean. The soybean genome contains 13 putative oleosin-encoding genes [11], and if any of them are involved in oil accumulation remain unexploited.
By linkage and linkage disequilibrium mapping, over 300 quantitative trait loci (QTLs) associated with seed oil content have been identified across all 20 chromosomes in the soybean genome over the past decades (SoyBase, https://soybase.org). These studies have revealed the polygenic nature of oil regulation, and the majority of loci were found to have varying additive, epistatic or QTL×environment effects [12–15], implying that traditional breeding based on genetic crossing and phenotypic selection may be inadequate for oil improvement. Recent studies have shown that increased oil in soybean could be achieved by genetic engineering of transcription factors involved in oil accumulation [16–18] or a QTL gene controlling seed coat bloom [19]. However, QTLs directly related to seed oil accumulation in soybean have not been cloned; thus, the underlying mechanism has not been thoroughly elucidated to date. Therefore, identifying an environmentally stable major QTL regulating seed oil content is urgently needed to substantially enhance seed oil content and understand the underlying regulatory mechanism in soybean.
To reveal the genetic basis of seed oil content and elucidate how oil accumulation is regulated, we investigated the oil content variation in 219 diverse soybean genotypes across six different environments and conducted a high-density genome-wide association study (GWAS) using 201,994 genome-wide single nucleotide polymorphisms (SNP). In total, three QTLs were identified to be significantly associated with soybean oil content across at least two environments, with GqOil20 on chromosome 20 stably expressing across all six environments. We also found that an oleosin-encoding gene, GmOLEO1, in the GqOil20 linkage disequilibrium (LD) block was exclusively expressed in developing seeds and that its expression level was significantly correlated with oil content within selected genotypes. We subsequently verified that GmOLEO1 contributed to oil accumulation in soybean seeds by conducting a series of molecular assays. Our results reveal an environmentally stable QTL/gene controlling oil accumulation in soybean seeds, provide new insight into oil accumulation in soybean and offer new directions for breeding soybean varieties with enhanced seed oil content.
Results
GWAS identified a stably expressed QTL associated with oil content
To identify the genetic variation in seed oil content, we measured the oil contents of 219 soybean genotypes with diverse genetic backgrounds across six different environments. Seed oil content exhibited large amounts of natural variation within the association panel in each environment and showed relative consistency across the six environments (S1A and S1B Fig, S1 Table). The mean oil content for the 219 accessions ranged from 18.10% to 18.97% across the six environments, and the observed maximum oil content reached 27.69% in Environment 1 (E1), which was approximately three times higher than the minimum value (9.64%) observed in E6 (S1A Fig, S1 Table). The distribution of oil content for the association panel in each environment was approximately normal (S1C Fig). Analysis of variance (ANOVA) indicated a significant difference (p < 0.001) in oil content among the genotypes, the oil content was significantly affected by environments (p < 0.001) (S1 Table), and the heritability was 0.64.
Because of the wide variation in seed oil content in the panel across the environments, we performed GWAS for the oil content in six environments (E1 to E6) and the best linear unbiased prediction (BLUP) using 201,994 genome-wide SNPs with a minor allele frequency (MAF) ≥ 0.05 in an effort to identify the genetic loci associated with soybean oil content. In total, 110 SNPs on three chromosomes (8, 12, and 20) were identified as significantly associated with oil content across at least two environments (S2 Fig, S2 Table). For the sake of simplicity, we empirically classified closely adjacent SNPs located within 5 Mb into one locus, as previously described [20]. The 110 SNPs were classified into three genomic loci, which were subsequently designated GqOil8, GqOil12, and GqOil20 (S2 Fig, S2 Table). Of these QTLs, the most significantly associated SNPs were identified in GqOil20, which was in physical proximity to oil-related QTLs identified in previous studies (S2 Table) [21–24]. Importantly, GqOil20 was consistently identified across all the environments and BLUP except E4 (Fig 1A), and it explained 13.4–24.4% of oil variation, representing the most stably expressed QTL for oil content in soybean.
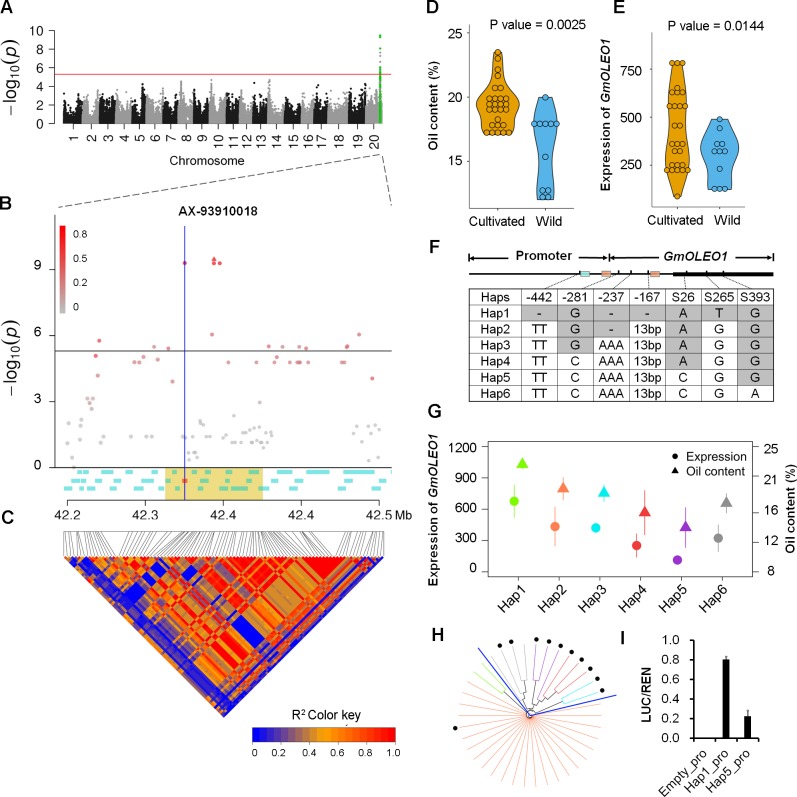
Fig 1. GWAS for oil content in soybean seeds and candidate gene selection analyses.
A, A Manhattan plot for the BLUP of soybean oil content across six environments by association mapping. A red horizontal line depicts the Bonferroni-adjusted significance threshold (P<4.95×10−6). The x-axis shows the 20 soybean chromosomes, and the y-axis shows the significance expressed as the -log10P value. B, A zoomed-in Manhattan plot of the 0.2-Mb genomic region on either side of the most significant SNP at the QTL GqOil20 on chromosome 20. The red solid triangle represents the leading SNP (AX-93910018). The color intensity of other SNPs is shown according to their LDs (r2 value) with the leading SNP. Gene models within the region are indicated with blue rectangles, and the red rectangle represents the candidate gene Glyma.20G196600. The 50-kb genomic regions on both sides of the leading SNP are highlighted in light yellow. C, The extent of linkage disequilibrium (LD) in the 0.2-Mb genomic region on either side of the leading SNP based on pairwise r2 values. The r2 values are indicated using the color intensity index. D, Comparisons of seed oil content (%) between cultivated and wild soybeans. E, Comparison of GmOLEO1 expression between cultivated and wild soybeans. F, haplotypes of GmOLEO1 among 38 soybean genotypes. The orange and cyan rectangles on the promoter region indicate the cis-acting regulatory elements involved in ABA response and seed-specific regulation, respectively. G, Comparative analyses of the GmOLEO1 expression and oil content between the six different haplotypes. H, A neighbor-joining tree of the 38 accessions using variants from GmOLEO1. The edge color pattern was the same as that indicated by the six different haplotypes in (G). Black solid dots represent G. soja accessions. I, Ratios of LUC and REN activity in Arabidopsis protoplasts transformed with recombinant plasmids containing the GmOLEO1 promoters from two different haplotypes (Hap1_pro, Hap5_pro) and the control vector. Significance analysis was performed using Fisher’s protected least significant difference (LSD) test. ** indicates a significant difference at the 0.01 level.
GmOLEO1 is a candidate gene for GqOil20 and encodes oleosin
It is known that oil content is a key domestication trait undergoing artificial selection [25], and the regulatory genes involved were likely selected during domestication. Thus, a comparison of the genetic diversity at the three loci between cultivated soybean (G. max) and wild soybean (G. soja), the progenitor of G. max, could be helpful in determining the most likely regions containing the oil-controlling gene(s). To this end, we calculated genetic differentiation (Fst) within the 140 kb regions upstream and downstream of each leading SNP per locus within a group containing this association panel (272 G. max accessions) and a panel of 122 G. soja accessions genotyped with the same microarray, as previously described [26]. After the comparison, we found that Fst showed variation, and the Fst across the entire group (G. max and G. soja) was lower than the average Fst in the association panel (G. max only) in two QTLs (GqOil8 and GqOil12). In contrast, most of the Fst values for GqOil20 were significantly higher in the G. max-G. soja group than in the association panel, suggesting that artificial selection might have occurred in this genomic region in relation to oil accumulation (S3 Fig), consistent with the fact that soybean oil content is a domestication trait [25]. In this regard, GqOil20 likely harbors a gene or genes that have important functions in the regulation of soybean oil accumulation. Thus, we next focused on GqOil20 to identify oil-related genes.
To identify the candidate gene, we analyzed the LD region harboring the leading SNPs using BLUP as a phenotype. GqOil20 contained a total of 33 significant SNPs located within a strong LD with an average r2 = 0.66 (Fig 1C). Of these genes within the LD according to the G. max Wm82.a2v1 reference genome (https://phytozome.jgi.doe.gov) (S2 Table), we found that a gene, Glyma.20G196600, encoding a putative oleosin protein, colocated with the significant SNP AX-93661332 (P = 4.98 × 10−10) (Fig 1A and 1B, S3 Table). Glyma.20G196600 is an ortholog of Arabidopsis AtOLE1 (AT4G25140), an oleosin-encoding gene with demonstrated roles in oil body formation [9], while other gene models in this block are annotated to be involved in defense responses (S3 Table). Thus, Glyma.20G196600 might be the candidate gene underlying GqOil20, and we designated it GmOLEO1 for further study.
Genetic variation and expression of GmOLEO1 correlated with seed oil content
To investigate whether GmOLEO1 underlies the domestication region GqOil20, we examined the expression patterns and sequence variations of GmOLEO1 alleles in 38 soybean accessions comprising 27 cultivated and 11 wild genotypes with significant differences in oil content between two subgroups (Fig 1D). Consistent with the observed high oil content in G. max relative to G. soja, GmOLEO1 showed significantly higher expression in cultivated soybeans than in wild soybeans (Fig 1D and 1E), indicating a correlation between the transcript abundance of GmOLEO1 and oil content.
Next, a 2.3-kb genomic region extending from -1,500 bp upstream of the start codon (ATG) to the 3’-untranslated region (UTR) of GmOLEO1 was sequenced and analyzed. Sequence analyses identified 12 nucleotide variants that divided the 38 germplasm into six haplotypes (Hap), which were clearly classified into two subgroups (cultivated and wild) by a phylogenetic tree (Fig 1H). Moreover, the six haplotypes represented six levels of seed oil content (Fig 1F and 1G), with Hap1 seeds containing the highest oil content. Of the 12 nucleotide variants, seven variants were found to be significantly associated with soybean oil content (Fig 1F), with four located at -442 (A/C/-, P = 5.22×10−4), -281 (G/C, P = 2.83×10−4), -237 (AAA/—, P = 1.26×10−3), and -167 (13-bp insertion/deletion, P = 3.13×10−3) being detected in the promoter region and three (PS26 = 5.36×10−3, PS265 = 0.02, PS393 = 0.04) occurring in the exon. Of these variants, those in the promoter region represent the most significant variation associated with the variation in seed oil, suggesting that resulting differences in the expression of GmOLEO1 among the haplotypes might account for the oil content variation.
To further determine whether variation in the promoter affected gene expression, we compared the transcriptional activity of the promoters of Hap1 and Hap5 (Hap1_pro and Hap5_pro) using a dual luciferase reporter gene assay. As shown in Fig 1I, Hap1_pro exhibited 3.58-fold higher activity than Hap5_pro, consistent with the observed higher expression of Hap1 than Hap5 (Fig 1I). These results suggest that GmOLEO1 is a strong candidate for GqOil20 and that expression level instead of exon variation is an important factor affecting seed oil content.
GmOLEO1 is localized to OBs and exclusively expressed in maturing seeds
Given that GmOLEO1 was a strong candidate associated with seed oil content, we characterized its protein structure, phylogeny, and expression pattern. BLASTp showed that GmOLEO1 is an ortholog of Arabidopsis AtOLE1 (AT4G25140), an oleosin-like protein with demonstrated roles in OB formation [9] (Fig 2A and 2B). Similar to previously described OLE orthologs in other species, GmOLEO1 contains three conserved structural domains (Fig 2A) [5]. Two amphipathic domains are located at the N- and C-termini, respectively, and a hydrophobic domain is located at the center. In the central hydrophobic domain, GmOLEO1 also contains the conserved “proline knot” sequence (PX5SPX3P), which can form a loop including a hydrophobic hairpin that penetrates into the TAG matrix and two arms located on both sides of the knot (Fig 2A) [5]. This domain organization allows oleosins to be anchored on the surface of an OB, as illustrated in a previous study [3]. Phylogenetic analysis revealed that OLEO-like proteins from the Faboideae, Brassicaceae, and grass clades clustered separately, suggesting functional conservation within the clade and possible functional diversity between clades (Fig 2B). In addition, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses showed that GmOLEO1 has a low molecular mass (~16 KD) (Fig 2C), consistent with previous findings [9, 27]. These results indicated that the uncharacterized gene GmOLEO1 encodes a putative OB protein that might play roles associated with OB formation or oil accumulation in soybean.
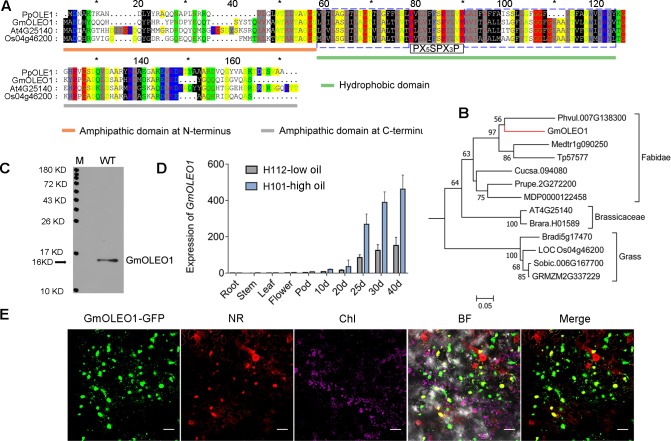
Fig 2. Sequence and expression analysis of GmOLEO1.
A, Domain structure and full-length amino acid alignment of representative OLE protein orthologs, including the structurally well-studied PpOLE1 (Physcomitrella patens), GmOLEO1, At4G25140 (Arabidopsis thaliana), and Os04g46200 (Oryza sativa). The amphipathic domains at the N-terminus and C-terminus are highlighted by blue and gray solid boxes, respectively. The conserved hydrophobic domain between two amphipathic proteins is shown by a red solid box. Dotted boxes indicate the two hydrophobic arms that can form a hairpin (blue in D); (PX5SPX3P) indicate the conserved sequence that forms a loop (black in D). B, Phylogenetic relationship between GmOLEO1 and its orthologs. All amino acid sequences were retrieved from Phytozome (https://phytozome.jgi.doe.gov). C, Gel image showing the molecular mass of GmOLEO1 (15.76 KD) using Western blot. M, protein marker, WT, proteins extracted from developing seeds of Williams 82 at 40 DAF. D, The relative transcript level of GmOLEO1 in different soybean tissues and developing seeds at 10, 20, 25, 30, and 40 days after flowering between the high-oil soybean cultivar H101 and low-oil cultivar H112; ** P<0.01 t-test. E, Subcellular localization analysis of GmOLEO1. GmOLEO1-GFP signal (GFP) colocalizes with oil bodies stained with Nile Red (NR). Chl represents chloroplast, BF represents bright field, and Merge shows both GFP and NR. Scale bar, 20 μm. White arrows indicate oil bodies.
To determine the temporal and spatial expression pattern of GmOLEO1, the expression levels of GmOLEO1 were examined in ten different tissues and two soybean varieties with different seed oil contents (H101, a high-oil variety; H112, a low-oil variety) (Fig 2D). Quantitative real-time PCR (qPCR) results showed that GmOLEO1 transcripts were undetectable in nonseed tissues, including the roots, stems, leaves, and flowers of both varieties, but its transcripts could be detected in developing seeds beginning at the seed-filling stage (Fig 2D). The abundance of GmOLEO1 transcripts in seeds increased with the number of days after flowering (DAF), with the highest expression level observed in developing seeds at 40 DAF, which was immediately before that seeds had completely matured (Fig 2D). Overall, the expression level of GmOLEO1 in the developing seeds of H101 was significantly greater than that in H112 seeds at all tested stages. These results indicated that GmOLEO1 functions specifically during seed maturation and that transcript abundance positively correlated with oil content (Fig 1G).
We next investigated whether GmOLEO1 was spatially related to OBs. We expressed a 35S::GmOLEO1-GFP (green fluorescent protein) construct in tobacco (Nicotiana benthamiana) leaf epidermal cells by agro-infiltration followed by staining with Nile Red, a lipophilic dye used to visualize OBs [4]. Confocal microscopy analysis revealed that GmOLEO1-linked GFP fluorescence and Nile Red fluorescence signal from OBs were colocalized in seed cells (Fig 2E), indicating that GmOLEO1 was localized to accumulated OBs.
Taken together, the results of haplotype analysis, diversity analysis, phylogenetic analysis, expression analysis, and subcellular localization supported the GWAS results and collectively indicated that GmOLEO1 was a strong candidate gene underlying GqOil20 associated with oil accumulation in soybean seeds.
Overexpression of GmOLEO1 increased seed oil content with pleiotropic effects on seed-related traits
To further demonstrate whether GmOLEO1 is functionally involved in oil accumulation in soybean seeds, we overexpressed GmOLEO1 in soybean using an improved cot-node transformation protocol [28]. Successful transformation was determined by detecting both the expression of the selective bar gene using the strip test and the presence of 35S::GmOLEO1 (Fig 3A) using polymerase chain reaction (PCR) analysis in T0 plant leaves (S4 Fig). Transgenic soybean lines were self-pollinated through three generations to obtain homozygous lines harboring 35S::GmOLEO1. Three independent homozygous transgenic lines (OE-9, OE-16, and OE-18) were selected and used for further analysis.
Fig 3. Expression analyses of GmOLEO1 in transgenic plants.
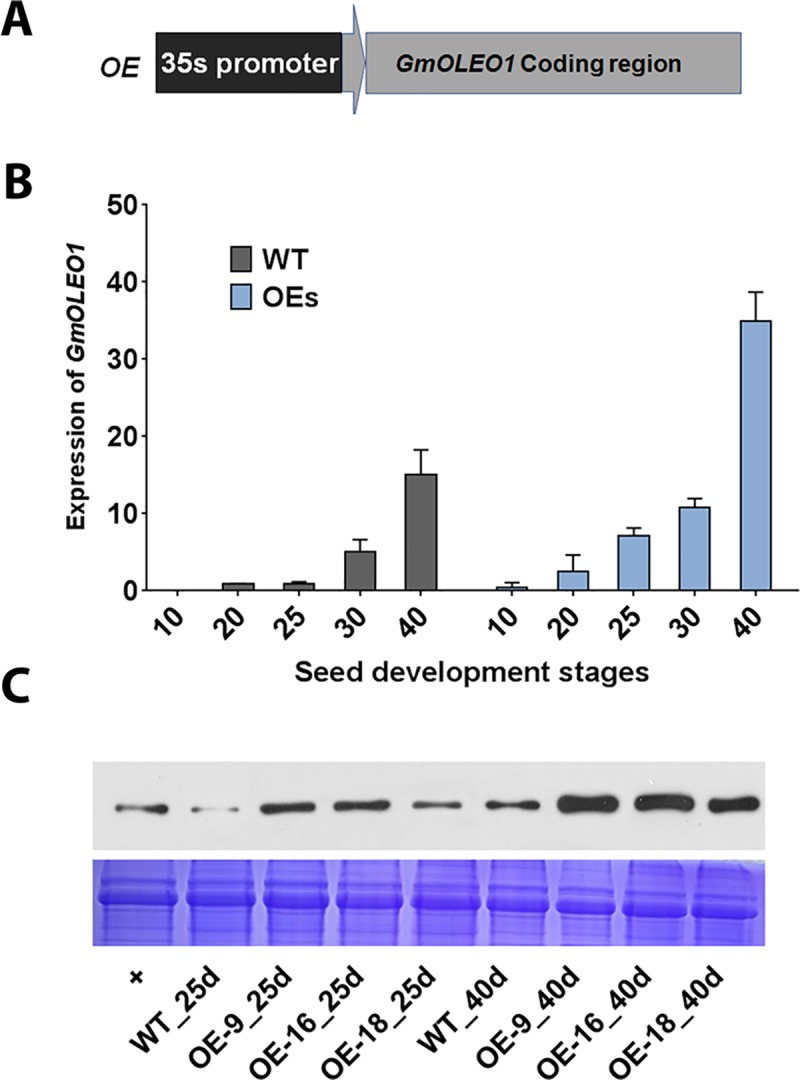
A, Diagram of the plasmid construct (35S::GmOLEO1) that was used for soybean transformation. B, Expression analysis of GmOLEO1 in developing seeds (10, 20, 25, 30, 40 DAF) of T3 homozygous OE lines and the wild type (WT). C, Western blot image showing the expression pattern of GmOLEO1 protein between WT and OE seeds at 25 and 40 DAF. Protein bands were detected by Coomassie blue staining (CBB, bottom) or Western blot (WB, top) probed with antibodies to GmOLEO1.
We first quantified the expression of GmOLEO1 in developing seeds (10, 20, 25, 30, and 40 DAF). As shown in Fig 3B, GmOLEO1 expression increased during seed development in both the OE lines and wild type (WT), while its expression in the OE seeds was significantly higher in OE than in WT at each stage of seed development. GmOLEO1 exhibited a sharp increase in its expression in the OE lines at 25 DAF, which was five days earlier than the increase in expression observed (30 DAF) in WT seeds. In agreement with the observed expression difference between the two soybean varieties described above (Fig 2D), expression of GmOLEO1 in both the OE lines and WT increased continuously as the seeds developed and reached its highest levels at 40 DAF. This gene expression result was further verified by comparative Western blot analysis of GmOLEO1 protein between WT and the OE lines using an antibody against GmOLEO1. A higher expression of GmOLEO1 was observed in the three OE lines than in the WT at 25 and 40 DAF (Fig 3C).
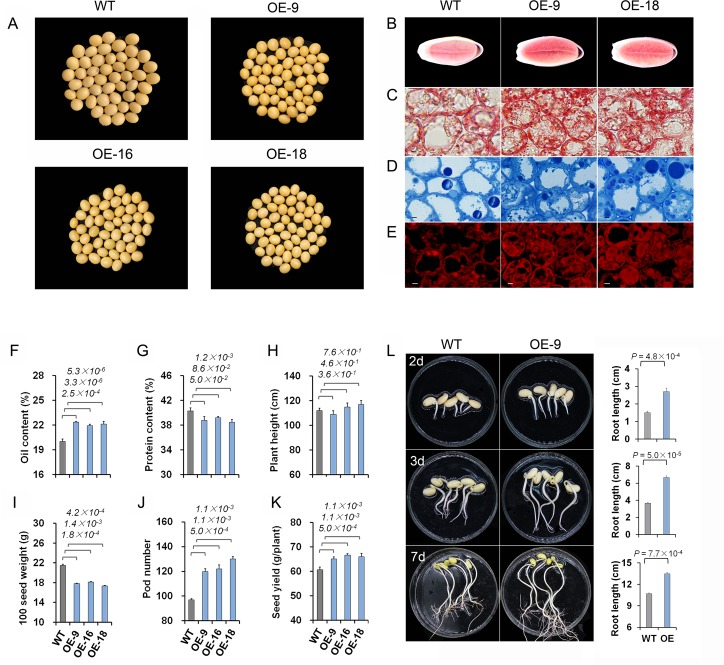
Compared with WT, mature seeds from OE lines had shinier surfaces with more yellowish colors and smaller sizes (Fig 4A). The oil contents of the seeds of the three OE lines were 22.35%, 21.91%, and 22.14%, respectively, which were all significantly higher (an absolute average increase of 2.12%, a relative increase of 10.6%, P = 4.6 × 10−6) than that in WT seeds (20.01%) (Fig 4F). Not surprisingly, the increase in oil content in the OE lines resulted in a significant decrease (P = 0.006) in protein content (Fig 4G). To further verify the oil increase in the OE seeds, we conducted a series of microscopy analyses of developing OE seeds (OE-9 and OE-18) at 25 DAF, where sharp increases in the expression of GmOLEO1 and oleosin were observed (Fig 4C–4F). Microscopy analyses of cross-sections from developing seeds stained with Oil Red O showed that OE seeds have markedly stronger Oil Red O staining than WT seeds, indicating that OE seeds contain a higher level of neutral lipid accumulation than WT seeds (Fig 4B). A further examination of the seed cells using an optical microscope (Nikon, Eclipse Ci, Japan) showed that more OBs were deposited in the two OE lines (OE-9 and OE-18) than in WT (Fig 4C), and a consistent result was found via staining with toluidine blue O (Fig 4D). These observations were further verified by a comparative analysis of Nile Red staining of accumulated oil between OE and WT seeds using a confocal microscope (Nikon, C2, Japan) (Fig 4E). These results visibly illustrated that OE plants overexpressing GmOLEO1 contained higher levels of oil accumulation in seed cells than WT.
Fig 4. Characterization of transgenic soybeans overexpressing GmOLEO1.
A, Seed appearance comparison between WT and OE-9, OE-16, OE-18. T3 seeds showed a shinier seed surface and smaller seed size than WT. B-E, Microscope-based visualization of lipid and OB accumulation in soybean seeds. B, Cross sections of soybean seeds at 25 DAF showing lipid accumulation as stained with Oil Red O. Two transgenic soybean seeds, OE-9 and OE-18, showed a higher level of TAG accumulation than WT seeds. C and D, Comparison of the abundance of oil bodies (OBs) stained with Oil Red O (C) and alkaline toluidine blue O (D) between the OE lines and WT as visualized using an optical microscope. E, Comparison of the abundance of OBs stained with Nile Red between the OE lines and WT as visualized with a confocal microscope, Bar = 10 μm. F-K, Comparison of seed traits (F and G) and yield-related phenotypes between the OE and WT seeds, including plant height (H), 100-seed weight (I), pod number (J) and seed yield (K) per plant. L, Comparison of seed germination rate and root length between WT and OE-9 at 2, 3 and 7 days postgermination. OE-9 seeds showed faster seed germination than WT seeds. Error bars indicate SD (n = 5). Statistical significance was determined by ANOVA.
In addition, we observed that overexpression of GmOLEO1 has pleiotropic effects on other agronomic traits. The phenotypic evaluation indicated that the overexpression resulted in a significant decrease in 100-seed weight in the OE lines compared with that in WT (Fig 4I). However, a significant increase (P < 0.01) in pod number per plant and a slight increase in plant height in the OE lines compared with WT lines (Fig 4H and 4J) were observed, which led to an increase (P = 0.017) in seed yield per OE plant compared with WT plants (Fig 4K, S4 Table). We also compared the seed germination between two lines. We found that seed germination and root growth were faster in the OE lines than in WT (Fig 4L). These results indicate that GmOLEO1 is involved in oil accumulation in soybean seeds with pleiotropic effects on yield-related traits, and no yield penalty was found in the current preliminary study.
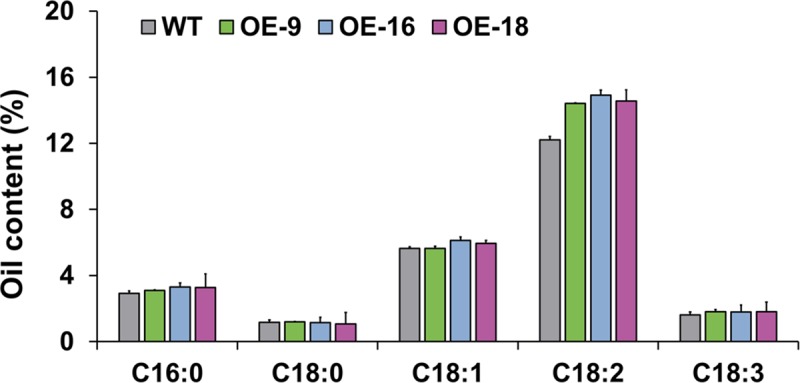
In light of the role of GmOLEO1 in oil accumulation, we further measured and compared the fatty acids between WT and OE seeds to test whether GmOLEO1 affected FA composition (Fig 5). Compared with the WT, OE seeds contained a higher average total FA content of 12.7% (P = 3.2× 10−4). Further analysis of five important oil components (TAGs) indicated that two polyunsaturated oil components, linoleic acid (18:2) and linolenic acid (18:3), were significantly increased by 14.4% and 14.9% (P = 1.2× 10−4 and 9.7× 10−5, n = 3), respectively, in the OE seeds compared with WT, while no significant changes in the contents of palmitic acid (16:0), stearic acid (18:0) and oleic acid (18:1) were observed between the OE seeds and WT (Fig 5, S5 Table). This result indicates that the overexpression of GmOLEO1 also led to increased accumulation of polyunsaturated FAs.
Fig 5. Comparison of fatty acid composition between OE and WT seeds.
Last, we compared the OBs of OE and WT seeds using transmission electron microscopy. At 25 and 40 DAF, the OBs of WT seeds showed typically spherical and ovoid structures and were distributed mostly between protein bodies at the periphery of the cells (Fig 6). In contrast, OE seed cells contained apparently smaller OBs than those of WT (Fig 6).
Fig 6. Microscope analyses of OBs in WT and OE seeds.
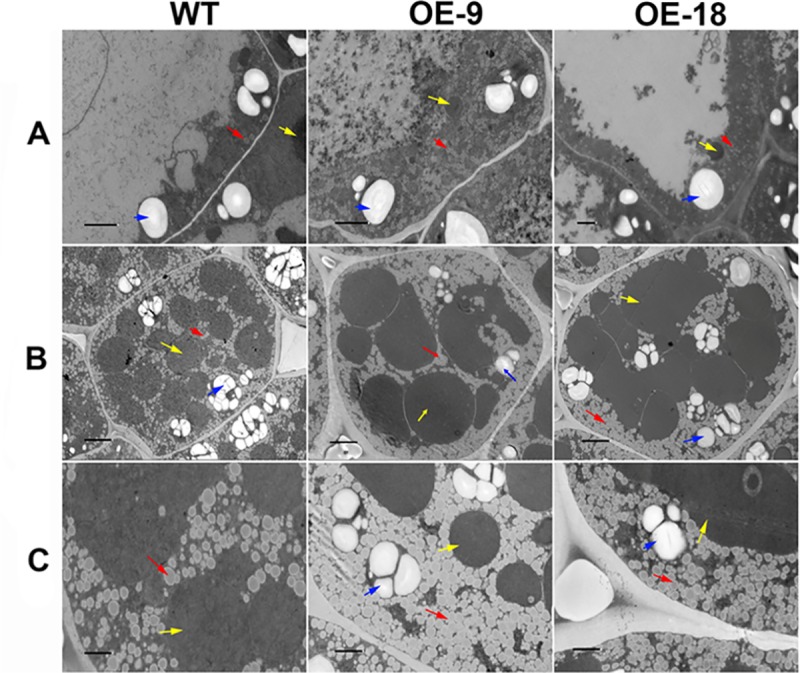
A, OBs in WT and OE seeds at 25 DAF, scale bars = 3 μm. B, Distribution of OBs in a whole seed cell at 40 DAF, scale bars = 5 μm. C, Increased magnification of OB distribution in a seed cell at 40 DAF, scale bars = 2 μm. The seed cells in OE-9 and OE-18 appear to contain smaller OBs than those in WT. Red arrows, oil body; yellow arrows, protein body; blue arrows, starch granules.
Overexpression of GmOLEO1 affected the expression of genes related and unrelated to oil synthesis
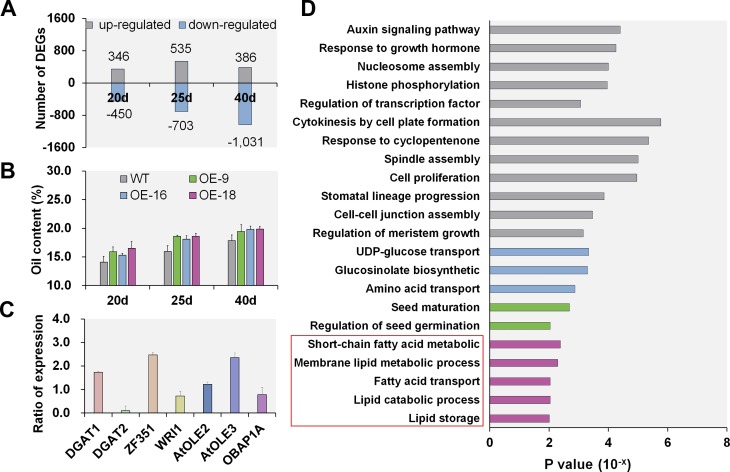
To better understand the molecular mechanism by which GmOLEO1 increased oil accumulation in soybean seeds, we compared the transcriptomes of OE and WT seeds at three seed development stages (20, 25 and 40 DAF) using RNA-seq analysis. In total, 796, 1238, and 1417 differentially expressed genes (DEGs) were identified by comparing OE with WT seeds at 20, 25, and 40 DAF, respectively (Fig 7A, S6–S8 Tables). The RNA-seq result was validated by qPCR analyses of 16 randomly selected genes (S9 Table, R2 = 0.84). We observed a trend toward increasing numbers of DEGs as DAF increased (Fig 7B). This increasing trend in the number of DEGs is consistent with the pattern of oil content increase in seeds as DAF increases (Fig 7B). This result indicated that overexpression of GmOLEO1 resulted in significant changes in the transcriptomes in the developing OE seeds, and the changes became more dramatic as the seeds developed. To understand the biological processes in which GmOLEO1 participates, we performed Gene Ontology (GO) enrichment analysis for these DEGs. In addition to the enrichment of GO terms associated with the regulatory pathways essential for plant growth and development, such as seed development and germination, amino acid and sucrose metabolism, and response to growth hormone, we found that GO terms associated with linoleic acid metabolism, fatty acid transport, lipid metabolism and storage were also significantly enriched for these DEGs (Fig 7D).
Fig 7. Comparative transcriptomic analysis of developing seeds between the OE lines and WT at different seed development stages.
A, Histograms showing the differentially expressed genes (DEGs) in OE seeds relative to WT seeds at 20, 25 and 40 DAF, respectively. B, Comparison of oil content accumulation between the OE and WT seeds at 20, 25 and 40 DAF (P < 0.01). C, Expression analysis of the putative genes involved in plant oil biosynthesis in OE-9 seeds at 40 DAF. Error bars are SD. The Y-axis represents the ratio of expression of a gene in the OE lines relative to WT, D, GO enrichment analysis for the DEGs between the OE and WT seeds at 40 DAF. Purple, green, and blue columns represent the enriched GO terms associated with oil metabolism pathway, seed germination, and protein metabolism pathway, respectively.
The RNA-seq results were further verified by the increased expression of several known genes participating in TAG biosynthesis in OE seeds as shown by qPCR, such as diacylglycerol acyltransferase (DGAT1) [18], wrinkled 1 (WRI1) [29], zinc-finger protein (GmZF351) [17], two Arabidopsis OLEO orthologs (AtOLE2 and AtOLE3) [30], and oil body associated protein 1 (OBAP1A) [31] (Fig 7C), indicating that the expression of these genes may be affected by GmOLEO1 overexpression. These results indicated that overexpression of GmOLEO1 promoted the expression of TAG biosynthesis-related genes and led to the enhancement of TAG biosynthesis.
GmOLEO1 underwent artificial selection for increased seed oil accumulation
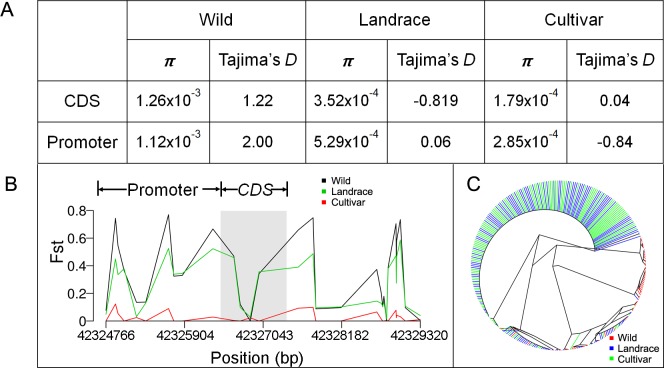
Because higher expression of GmOLEO1 in cultivated soybean than in wild soybean was observed, we hypothesized that the variations in its promoter region were under selection. Statistical analyses were performed using a large population of 302 soybean accessions [24]. We first evaluated Fst for different comparisons, including wild soybean vs. cultivar, wild soybean vs. landrace, and cultivar vs. landrace. The results showed that the Fst between wild vs. cultivated soybean is considerably higher than that between cultivar vs. landrace, especially in the promoter region (Fig 8A and 8B). The nucleotide diversity (π) analysis showed that π was higher in wild soybean than cultivated soybean in the promoter region and the coding region (Fig 8A). Tajima's D in the promoter region was 2.00, 0.06 and -0.84 for wild, landrace and cultivar, respectively, while Tajima's D in the coding region was 1.22, -0.819, and 0.04 for wild, landrace and cultivar, respectively (Fig 8A), implying that positive selection had occurred in the promoter region. A phylogenetic analysis using variants in the promoter and coding regions identified three clusters, corresponding to wild soybean, landrace, and cultivar (Fig 8C). Taken together, these results indicated that the promoter region was subjected to artificial selection during domestication.
Fig 8. Diversity and evolutionary analyses of GmOLEO1 between wild, landrace and cultivated soybeans.
A, Gene diversity (π) and Tajima's D of GmOLEO1 in the three groups. B, Fst in the promoter and coding sequence (CDS) of GmOLEO1 between the three groups. C, A phylogenetic tree comprising the three groups constructed using the SNPs in the promoter and CDS region of GmOLEO1.
Discussion
It is known that seed oil content has been subjected to artificial selection targeting higher oil content [25]. This finding was further validated by our study, in which a significant difference in seed oil content between cultivated and wild soybeans was observed (Fig 1D). Unlike other domestication traits in soybean, such as stem growth habit [32] and pod shattering [33], soybean oil content is highly complex; it is regulated by many genes of small effect and is easily influenced by various environmental factors [1]. Our GWAS study across multiple environments allowed us to identify a new environmentally stable QTL, GqOil20, and an underlying candidate gene, GmOLEO1 that is capable of increasing seed oil content in soybean. Notably, the GmOLEO1 locus was previously identified as a possible candidate for an eQTL associated with seed oil accumulation [34], and it is physically close to other oil-related QTLs previously identified by linkage mapping [21–23]. GmOLEO1 may have been identified in this study because the corresponding alleles were fixed with respect to oil variation during domestication. Given the complexity of oil metabolism, the observed phenotypic variation (23.7%) could be due to the combined effects of GmOLEO1 and other genes at this locus. Other oleosin genes, regardless of sequence variation, with increased expression at the gene or protein level [35–36] during seed filling/maturation may also substantially affect oil accumulation. Nevertheless, our study further functionally verified that human-selected GmOLEO1 might be involved in seed oil accumulation, possibly by indirectly affecting oil biosynthesis via efficient feedback.
Our study and previous studies have indicated that the improvement of seed oil content in soybean during domestication was achieved by artificial selection of multiple major genes, and some of those genes may not be directly involved in oil biosynthesis, such as B1 [37] and GmZF351 [17]. In contrast to previous studies that identified oil-related genes using a reverse genetic approach, GmOLEO1 was pinpointed in an artificially selected locus, GqOil20, using an integrated strategy of high-density genetic mapping and genomics. Haplotype and expression analyses of GmOLEO1 between cultivated and wild soybean in our study provided additional evidence of selection at the GmOLEO1 locus. This artificially imposed selection pressure on the expression of GmOLEO1 could be an important factor affecting the observed difference in oil accumulation in soybean, because the overexpression of Hap2 of GmOLEO1 resulted in enhanced oil accumulation in transgenic soybean (Figs 1G and 4F). Whether other genes in this block have functions associated with oil accumulation requires further determination.
Seed yield and quality represent two of the most important traits in soybean improvement. Breeding soybeans with high oil stability across environments while maintaining protein content and yield has been difficult due to the complex genetic architecture of oil regulation. In our study, GmOLEO1 was functionally identified as a candidate for the environmentally stable QTL GqOil20. Overexpression of GmOLEO1 significantly elevated oil content and the percentage of polyunsaturated FAs without detriment to the overall plant performance, especially yield, in our preliminary study, making GmOLEO1 a promising candidate gene for use in breeding high-oil soybeans with improved levels of healthy polyunsaturated FAs. Although Hap2 (Williams 82-type) is not the most favored haplotype for increasing oil accumulation, enhanced seed oil accumulation was observed in this study, indicating that the GmOLEO1 allele in Hap2 enhanced oil accumulation independent of the amino acid substitution (Ala265Pro). One possible reason for this finding is that the substitution, which does not change hydropathy, may not affect the secondary structure of oleosins in OBs. The strong correlation between Hap1 and high expression levels of GmOLEO1 alleles (Fig 1) suggests the importance of unique variation (Hap1) in the promoter region in enhancing the expression of GmOLEO1. The discovery of the molecular function DNA marker, Indel P237167, from the unique variation in the promoter of GmOLEO1 will facilitate marker-assisted selection (MAS) in soybean high-oil breeding programs.
The importance of oleosins in lipid accumulation and oil body formation in seed plants has been gradually recognized over the past three decades, and it has demonstrated an important role in the maintenance of OBs and preventing them from coalescence [9]. The Arabidopsis genome contains 17 oleosin genes [38], of which AtOLE1 has been reported to be involved in lipid biosynthesis [39]. In soybean, 13 putative oleosin genes were found in the G. max reference genome, while only GmOLEO1 colocalized with the associated SNP (AX-93661332) in GqOil20 in our study (S10 Table). The overexpression of GmOLEO1 showed consistent results, as observed in AtOLE1, revealing conserved functions between GmOLEO1 and AtOLE1 in increasing oil accumulation. Despite being rarely studied in other species, the high similarity in amino acid sequence and structural domains (Fig 2A and 2B) suggests that GmOLEO1-like proteins from the Faboideae, Brassicaceae, and grass clades might have a conserved function in determining OB size but lineage-specific roles [7]. For example, expression of GmOLEO1 correlated with oil content in our study, and OE seeds with increased oil content contained smaller OBs; conversely, the expression of oleosin genes was independent of oil content in maize, and a high-oil maize strain contained larger, more spherical OBs than did low-oil maize [40]. The conserved role of OLEOs from various plant species in enhancing oil accumulation suggests that GmOLEO1 orthologs have considerable potential for oil improvement in other oil-producing crops.
It has been demonstrated that oleosins have important functions in OB formation, stabilization, and transgenic addition of oleosin increased oil content in Arabidopsis, Brassica and yeast [10, 31, 41–42], but oleosins’ role in increasing oil accumulation in soybean seeds has rarely been reported previously. In addition to these potentially cross-species functions in determining the size of OBs and affecting oil accumulation, our study showed that increased oleosins resulted in apparent reductions in OB size and increases in OB number in seed cells (Fig 6) and increased seed oil contents (Fig 2), in agreement with a study in which suppression of OLEO1 resulted in larger OBs and reduced total lipid levels in seeds [9–10]. Oil accumulation was gradually regulated as the seeds matured, possibly due to a gradual increase in the expression of GmOLEO1, which was concomitant with the enhanced TAG metabolism during seed maturation identified by RNA-Seq (Fig 7). Thus, it is logical that a positive correlation between oil content and GmOLEO1 expression was observed in our study (Fig 1G). A similar correlation has also been observed in Brassica napus, where the expression levels of OLEO/oleosin in high-oil genotypes were considerably higher than those in low-oil seeds [41]. The higher expression levels of GmOLEO1 in high-oil soybean varieties might be attributed to the unique variation present in the promoter of Hap1. The presence of putative seed-maturation-related cis-elements (abscisic acid (ABA) response and seed regulation, Fig 1F) in the promoter region of GmOLEO1 may be responsible for its exclusive expression during seed maturation.
In addition to stabilizing the structures of lipid droplets (LDs), oleosins also serve other functions, including enzymatic and signaling roles. Some of these proteins are ubiquitous in cells with and without LDs, thus exerting broader functions in seeds and other organs [43]. In peanut, oleosin3 (OLE3) was shown to exhibit bifunctional activities and was phosphorylated by STYK (AhSTYK) to regulate MGAT and PLA2 activity; it could be involved in the biosynthesis and mobilization of TAGs during seed maturation and germination [44]. However, a recent report showed that the bifunctional enzymic motifs are present in only peanut oleosins and not in those of other plants [7]; thus, another possibility is that oil accumulation increases as a result of GmOLEO1 overexpression, which might lead to efficient feedback by producing smaller OBs [45]. The detailed mechanisms underlying the regulation of gene expression by GmOLE1 must be deciphered in future work.
The level of oleosin itself is regulated during seed development and germination. When seeds germinate, oleosin degradation occurs prior to OB degradation. A recent study revealed that the ubiquitin binding protein PUX10 and division cycle 48 homolog A (CDC48A) are core components of an LD-associated ERAD-like degradation machinery, which facilitates the dislocation of oleosins from LDs [46–47]. In our study, faster seed germination of OE lines might be associated with higher levels of some oleosin-degradation proteins (e.g., PUX10 and CDC48A) [46–47], but this hypothesis remains to be experimentally determined.
Based on the results of our preliminary study and a previous finding [9], we proposed that the biosynthesis of TAGs was enhanced in the OE lines, possibly because of the affected TAG metabolic pathway, as a result of increased expression of GmOLEO1/oleosins. Smaller OBs gradually accumulated as the newly produced TAGs reached the minimum size that could be completely covered by the increased number of oleosin proteins, given that oleosins serve as surfactant to prevent OBs from coalescence [10, 48]. Thus, increased oleosin production in OE seeds, which resulted in reduced size but increased turnover of OBs during seed maturation, could be a more efficient way to use the limited intracellular space than larger OBs, leading to increased total oil content in OE seeds.
Material and methods
Plant materials and field experiments
The association panel for GWAS consisted of a diverse collection of 219 soybean accessions (including 195 landraces and 24 elite varieties) originating from 26 provinces across six different agroecological regions in China, ranging from latitudes 53 to 24°N and longitudes 134 to 97°E [49]. Field experiments were performed in the 2009, 2011, 2012, 2013 and 2014 growing seasons at four different geographic locations as previously described [50]. Briefly, soybean plants were examined under field conditions at the following experimental stations: Jiangpu Experimental Station of Nanjing Agricultural University (32.1°N 118.4°E), Nanjing, in 2009 (designated as Environment 1, E1); Maozhuang Experimental Station (34.8°N 113.6°E) of Henan Agricultural University Zhengzhou, in 2009 (E2) and 2011 (E3); the Fangcheng Experimental Farm (33.2°N 112.9°E) of Henan Agricultural University in 2012 (E4), and Yuanyang Experimental Station of Henan Academy of Agricultural Sciences, Zhengzhou, in 2013 (E5) and in 2014 (E6). A randomized block design was used for all field trials. In all environments, each accession was planted in a three-row plot, with each row 200 cm long and 50-cm row spacing.
Phenotyping and genotyping
Mature soybean seeds were harvested and air-dried, and fully filled seeds were used for oil content measurement. Measurement of soybean oil, protein, and FA components was conducted using a near infrared spectrophotometer (NIR) seed analyzer (DA7200, Perten Instruments, Huddinge, Sweden) as previously described [51]. This association panel was genotyped using the NJAU 355K SoySNP array as previously described [26], and a total of 292,035 high-quality SNPs were used for association mapping.
Phenotyping analysis
Phenotypic data for soybean seed oil across different environments were subjected to an ANOVA using the PROC GLM (general linear model) mixed model of SAS version 9.2 (SAS Institute, 2002). The linear statistical model includes the effects of genotype, environment and the environment × genotype interaction. The BLUP for each line was calculated with PROC MIXED in SAS (SAS Institute, 2002) and used as the phenotypic input for the subsequent GWAS. The violin plot was drawn using the R package vioplot [52]. The heritability of oil content was calculated using h2 = Vg/ (Vg+Ve), where Vg and Ve represent genetic and environmental variation, and each term was extracted from the ANOVA results.
Genome-wide association study
GWAS was conducted using the compressed mixed linear model with TASSEL 5.0 [53, 54] using SNP with minor allele frequency greater than 0.05, and the threshold was determined with Bonferroni threshold of ≤ 4.95 × 10−6 (P = 1/n) [55], where n is the SNP number used in GWAS. The population structure and the relatedness were described previously [26]. The Manhattan plot was drown using the R package qqman [56]. The LD heat map was plotted using the LDheatmap R package [57].
Quantitative real-time PCR
Expression of the candidate gene was examined in different soybean tissues, including roots, shoots, leaves, flowers, pods, and developing seeds at different developmental stages (10, 20, 25, 30 and 40 days after flowering). Total RNA was isolated from the tissues using the RNAsimple Total RNA Kit (TaKaRa, Japan), and 1 μg of RNA was treated with 10 units of RNase-free DNase I (TaKaRa) prior to cDNA synthesis. The first strand of cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, USA) following the manufacturer's instructions. Gene expression was determined using the Bio-Rad CFX96 Touch Real-Time PCR System (Bio-Rad, California, USA). The PCRs contained 5 μL of the first-strand cDNA, 0.5 μL of 10 μmol L−1 gene-specific primers (S11 Table), and 10 μL of Real-Time PCR SYBR Mix (PC3302; Aidlab). The PCR conditions were as follows: 94°C for 3 min and 40 cycles at 94°C for 15 s and 60°C for 15 s. The soybean tubulin gene (GenBank: AY907703.1) was amplified as an internal reference, and a negative control reaction was performed using water instead of cDNA. Three biological replicates per sample were used, and each reaction was performed in triplicate.
Dual luciferase assay
In the protoplast transient expression experiments, the dual luciferase assay vector pGreenII 0800-LUC was used to analyze the activity of the different promoters. This vector contains a firefly luciferase (LUC) reporter gene that can be driven by the target promoter and a Renilla luciferase (REN) reporter gene driven by 35S. The purified DNA fragment of the target promoter was fused with the LUC reporter gene in the vector digested with HindIII and SalI enzymes to construct the recombinant vector. The vector pGreenII 0800-LUC without promoter insertion before the LUC reporter gene was used as a control. The recombinant vector and the control were individually transformed into Arabidopsis protoplasts via PEG-calcium transfection. The isolation of Arabidopsis protoplasts and protoplast culture were performed according to standard protocols [58]. The ratio of LUC and REN activity (LUC/REN) was used to reflect the activity of the target promoter. The LUC/REN value was determined using the dual luciferase reporter assay system (Promega, USA).
Vector construction and generation of transgenic plants
The complete coding sequence of GmOLEO1 was amplified from the cDNA of Williams 82 by regular PCR using gene-specific primers (S11 Table). The PCR product was subcloned into the pMD-19 T vector (TaKaRa, Japan) for sequence verification. The verified GmOLEO1 sequence was then cloned into the dicotyledon expression vector pCAMBIA3300, which contains a selection marker gene, phosphinothricin acetyltransferase (bar), using the ClonExpress Entry One Step Cloning Kit. The resulting recombinant pCAMBIA3300-GmOLEO1 construct was transformed into Williams 82 via the Agrobacterium tumefaciens-mediated soybean cotyledon node transformation system as previously described [59]. Extraction of genomic DNA from the leaves of PPT-resistant plants and nontransformed plants was performed using the cetyltrimethylammonium bromide (CTAB) method [60]. Transformants were verified by leaf-painting assay with herbicide phosphinothricin (PPT), PCR analysis for the presence of introduced GmOLEO1 and bar (482 bp), and LibertyLink strip detection for the expression of the bar gene using the QuickStix Kit (EnviroLogix Inc., ME, USA) were considered positive transgenics for further analysis. For LibertyLink strip detection, a total of 100 mg leaf tissue was collected and ground completely in the bottom of a conically tapered 1.5 ml tube by pestle rotation, followed by adding 0.5 mL of extraction buffer and a strip into the tube. After ten minutes, strips containing only the control line were negative for PAT protein expression, while those with two lines (control line and test line) were positive for PAT protein expression [61].
Subcellular localization and colocalization assay
The full-length GmOLEO1 cDNA was amplified and cloned into the pBWA (V)HS-osgfp vector to obtain the pBWA(V)HS-osgfp-35S::GmOLEO1-GFP construct under control of the cauliflower mosaic virus (CaMV) 35S promoter (Biorun Co., Ltd). The binary vector 35S::GmOLEO1-GFP was transiently coexpressed in the leaves of Nicotiana benthamiana via agro-infiltration. Then, the tobacco leaf epidermal cells agro-infiltrated with the GmOLEO1-GFP construct were stained with Nile Red, a lipophilic dye used to visualize OBs [4]. Fresh leaves were placed in a solution containing Nile Red stock (100 mg/mL dimethyl sulfoxide) diluted 100× with 1×PBS for 10 min and washed with PBS twice for 30 s each time. Fluorescence signals were detected using a confocal laser scanning microscope (Nikon C2-ER, Japan) 2–3 days after infiltration. GFP, mKate and Nile Red were excited at 488, 561 and 559 nm, and their emission was detected at 510 to 540, 580 to 620 and 570 to 670 nm, respectively. All of these fluorescence experiments were independently repeated at least three times.
Western blot analysis for GmOLEO1
Immunogenicity peptides of GmOLEO1 protein were predicted by bioinformatics analysis. The sequences of the peptides were as follows: MAELHYQQQHQYPHR and KDYGQQQISGVQAS. The peptides were commercially synthesized and purified (Wuhan GeneCreate Biological Engineering Co., Ltd, China). Two male Japanese White rabbits were used for the immune procedure. Next, a polyclonal antibody of GmOLEO1 protein was separated and purified for immunoblot analysis. Proteins of fresh soybean seed were extracted by Triton X-100 lysate (0.5%). Then, 30 μg of protein extracts mingling with 2× SDS-PAGE sample loading buffer (Solarbio, Beijing, China) were loaded and subjected to SDS-PAGE. Afterward, protein bands were transferred onto polyvinylidene fluoride (PVF) membranes (Solarbio, Beijing, China). The membranes were blocked with 5% skimmed milk powder solution for 2 h at room temperature, followed by incubation with a polyclonal antibody against GmOLEO1 diluted to 1:10000 in phosphate-buffered saline overnight at 4°C. Finally, the blot was detected with horseradish peroxidase (HRP)-conjugated goat-anti-rabbit secondary antibody (Santa Cruz Biotechnology, USA) for another 1 h. The protein bands were visualized using a chemiluminescence system (Pierce, Rockford, Illinois, USA).
RNA-seq analysis of transgenic soybean
Transcriptomes were compared between pooled OE seeds at 20, 25, and 40 DAF, respectively, with the WT seeds at the corresponding developmental stage. For each time point, two developing seeds from each of the three OE lines (OE-9, -16 and -18) were collected and pooled as one biological replicate, and three biological replicates were used per sample. Library construction was performed as previously described [62]. The library was sequenced with the Illumina HiSeq 2500 analyzer at Biomarker Technologies (Beijing, China), producing 200-bp paired-end reads. An average of 6.47 gigabases of clean data per sample was generated. Differential gene expression was determined using the DESeq R package [52]. A gene with an adjusted P < 0.05 and a fold change (FC) >1.5 were defined as DEGs. Enrichment analysis of Gene Ontology of biological pathways (GOBPs) was performed using the GOseq R packages [63] to compute P values that indicate the significance of each GOBP being represented by the genes. GOBPs with P < 0.01 were identified as enriched biological processes.
Microscopy analysis
Fresh immature soybean seeds harvested at 25 and 40 DAF were fixed in FAA fixation solution for at least 24 h. The main experimental steps for Oil Red O staining are as follows: cutting the whole sample into small blocks, removing excess water with tissue paper, immersing the small tissue blocks in Oil Red O (Servicebio, G1016, Wuhan, China) solution and incubating at 37°C for 60 min. Excess staining solution was removed by rinsing with tap water. The stained tissue blocks were immersed in 75% ethanol for 30 min or until no fading occurred; then, they were preserved in 4% paraformaldehyde and kept in the dark. Photos were taken using a digital camera (Canon 7D). The fixed tissue samples were embedded with OCT compound (Sakura, Japan). Frozen sections (8–10 μm) were obtained with Cryostat Microtome (Thermo, CRYOSTAR NX50, USA) and mounted on a prechilled glass slide. The frozen sections were stained with 0.1% Nile Red (Servicebio, G1073, Wuhan, China) and Oil Red O (Servicebio, G1016, Wuhan, China). Image observation for Nile red staining was performed using a Nikon confocal scanning microscope (Nikon, C2, Japan). The excitation wavelength was 488 nm, the emission wavelengths were 593–654 nm, and the OBs were imaged at 800× magnification. Oil Red O staining was imaged using an optical microscope (Nikon, Eclipse Ci, Japan) at 800× magnification.
Tissues (1×3 mm3 in size) of developing soybean seeds were fixed in 2.5% glutaraldehyde buffered with 0.1 M phosphate buffer (pH 7.2) for 12 h. Postfixation was subsequently conducted in 1% osmic acid in 0.1 M phosphate buffer (pH 7.2) for 5 h. The blocks were then washed, dehydrated through an ethanol series of 30–100%, and embedded in EMbed 812 media. The samples were cut into 1 μm slices using an ultramicrotome (Leica UC7, Germany), stained with alkaline toluidine blue O solution (Servicebio, G1032, Wuhan, China), and then imaged (800×) using an optical microscope (Nikon, Eclipse Ci, Japan). For transmission electron microscopy (TEM), the samples were cut into 60 nm slices using an ultramicrotome (Leica UC7, Germany) and then separately stained with uranyl acetate and lead citrate for 15 min. The slice samples were photographed under a TEM (HT7700, Hitachi, Japan).
Gene resequencing and haplotype analysis of GmOLEO1
The 2.3-kb genomic region spanning from 1,500 bp upstream from the translation start codon (ATG) to the 3’-untranslated region (UTR) of GmOLEO1 was sequenced and analyzed. Haplotype analysis was performed by resequencing this region in 20 high-oil, 20 low-oil and 10 moderate-oil accessions. All primers (S11 Table) used in this study were designed using the Primer 3 online tool (http://frodo.wi.mit.edu/primer3/). All sequences were verified manually, and all observed polymorphisms were reverified by resequencing of another amplicon. All the verified sequences were aligned using ClustalX version 1.83 [64]. The polymorphism data were analyzed using DnaSP version 4.10 [65] to identify sequence variation. Prediction of cis-elements in the promoter region was carried out using the online web tool PlantCARE [66].
Fatty acid component analysis
FA components in soybean seeds were analyzed as previously described [17]. Briefly, 10 mg fine powder of soybean seeds was used for FA isolation. FAs were extracted with 1 mL of extraction buffer (2.5% [v/v] H2SO4 in CH3OH) at 85°C for 1 h. The supernatant (500 μL) was mixed with 300 μL of hexane and 600 μL of 0.9% (w/v) NaCl. FA methyl esters were redissolved in 200 μL of ethyl acetate and analyzed immediately with a gas chromatography system (GC-2014; Shimadzu, Beijing, China). Peaks corresponding to each FA species were identified by comparison to a FA methyl ester analytical standard (Supelco, Poole, UK). Concentrations of FA species were normalized against the internal control heptadecanoic acid (Sigma-Aldrich, USA). Five biological replicates per line were analyzed in this experiment.
Germination test
The seeds were surface-sterilized with chlorine gas for 4 h prior to germination in darkness in Petri dishes (90 mm in diameter) on two sheets of filter paper moistened with deionized water (15 seeds per Petri dish). Germination tests were carried out in an incubator (MGC-400B, YIHENG, Shanghai, China) equipped at 25°C with 75% humidity. The filter paper was replaced once a day, and germinated seeds with healthy roots were counted. Root length was measured using a ruler at 2, 3 and 7 days postgermination. Three replicates per treatment were performed.
Gene diversity analysis
The published whole genome sequencing data were used for gene diversity analysis [25]. VCFtools was used to estimate gene diversity (Fst, nucleotide diversity (π) and Tajima’s D) [67]. SNPRelate combined with APE was used to construct the phylogenetic tree [68, 69].
Supporting information
A, Oil content variation in all 219 accessions across six environments. B, Quantile-quantile plot of the GWAS results under a general linear model (GLM) and mixed linear model (MLM, Q+K). C, Phenotypic distribution of the oil trait across six environments. E1-E6 denote the oil content in the corresponding environments.
(TIF)
The x-axis shows the 20 soybean chromosomes, and the y-axis shows the significance expressed as a −log10P value. The quantile-quantile plot corresponding to the GWAS (MLM, Q+K, P < 4.95 × 10−6) result in each environment is given beside the Manhattan plot.
(TIF)
The dotted line represents the 95% tails for the empirical distribution of FST statistics.
(TIF)
(A-C) Identification of positive transgenic plants by leaf-painting assay (A), polymerase chain reaction (PCR) verification (B) and strip detection for the presence of the selective bar gene (C).
(TIF)
The indicated scale is the log2 value of the normalized level of gene expression.
(TIFF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Wenhua Zhang from Nanjing Agricultural University for his critical reading of this manuscript. Three anonymous reviewers are thanked for their critical and highly valued comments.
Data Availability
The RNA-seq data we mentioned in our manuscript has been uploaded to NCBI (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA551573), (BioProject: PRJNA551573), (SRA Study: SRP212336).
Funding Statement
DZ is the recipient of Ministry of Science and Technology of China http://www.most.gov.cn/eng/ (grant number 2016YFD0100500), key scientific and technological project of Henan Province (192102110023), and the Henan agricultural university science and technology innovation fund (KJCX2019C02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Clemente TE, Cahoon EB. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009;151(3):1030–40. 10.1104/pp.109.146282 000271430500010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman KD, Ohlrogge JB. Compartmentation of triacylglycerol accumulation in plants. J Biol Chem. 2012;287(4):2288–94. 10.1074/jbc.R111.290072 000300292300004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang AHC. Oil bodies and oleosins in seeds. Annu Rev Plant Phys. 1992; 43:177–200. 10.1146/annurev.pp.43.060192.001141 A1992HW51800007 [DOI] [Google Scholar]
- 4.Huang CY, Chung CI, Lin YC, Hsing YIC, Huang AHC. Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol. 2009;150(3):1192–203. 10.1104/pp.109.138123 000268696800008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CY, Huang AHC. Unique motifs and length of hairpin in oleosin target the cytosolic side of endoplasmic reticulum and budding lipid droplet. Plant Physiol. 2017;174(4):2248–60. 10.1104/pp.17.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parthibane V, Rajakumari S, Venkateshwari V, Iyappan R, Rajasekharan R. Oleosin is bifunctional enzyme that has both monoacylglycerol acyltransferase and phospholipase activities. J Bio Chem. 2012;287(3):1946 10.1074/jbc.M111.309955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming-Der Huang, Anthony H C Huang. Bioinformatics reveal five lineages of oleosins and the mechanism of lineage evolution related to structure/function from green algae to seed plants. Plant Physiol. 2015, 169(1):453–70. 10.1104/pp.15.00634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu JW, Chao HB, Wang H, Li YH, Li DR, Xiang J, et al. Identification of the relationship between oil body morphology and oil content by microstructure comparison combining with QTL analysis in Brassica napus. Front Plant Sci. 2017;7 ARTN 1989. 10.3389/fpls.2016.01989 000391331000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siloto RMP, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell. 2006;18(8):1961–74. 10.1105/tpc.106.041269 000239703000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MA, Herman EM. Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant. 2008;1(6):910–24. 10.1093/mp/ssn049 000262858000004 [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Sun Y, Su W, Yang J, Liu X, Wang Y, et al. Species-specific size expansion and molecular evolution of the oleosins in angiosperms. Gene. 2012;509(2):247–57. 10.1016/j.gene.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 12.Eskandari M, Cober ER, Rajcan I. Genetic control of soybean seed oil: II. QTL and genes that increase oil concentration without decreasing protein or with increased seed yield. Theor Appl Genet. 2013;126(6):1677–87. 10.1007/s00122-013-2083-z 000319477900022 [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Li S, Wang Z, Chang F, Kong J, Gai J, et al. Identification of major quantitative trait loci for seed oil content in soybeans by combining linkage and genome-wide association mapping. Frontiers in plant science. 2017; 8:1222 Epub 2017/07/28. 10.3389/fpls.2017.01222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YH, Reif JC, Hong HL, Li HH, Liu ZX, Ma YS, et al. Genome-wide association mapping of QTL underlying seed oil and protein contents of a diverse panel of soybean accessions. Plant Sci. 2018; 266:95–101. 10.1016/j.plantsci.2017.04.013 000423003300011 [DOI] [PubMed] [Google Scholar]
- 15.Van K, McHale LK. Meta-analyses of QTLs associated with protein and oil contents and compositions in soybean [Glycine max (L.) Merr.] seed. Int J Mol Sci. 2017;18(6). ARTN 1180. 10.3390/ijms18061180 000404581500079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lardizabal K, Effertz R, Levering C, Mai J, Pedroso MC, Jury T, et al. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008;148(1):89–96. 10.1104/pp.108.123042 000258947600009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li QT, Lu X, Song QX, Chen HW, Wei W, Tao JJ, et al. Selection for a zinc-finger protein contributes to seed oil increase during soybean domestication. Plant Physiol. 2017;173(4):2208–24. 10.1104/pp.16.01610 000402054300020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roesler K, Shen B, Bermudez E, Li CJ, Hunt J, Damude HG, et al. An improved variant of soybean type 1 diacylglycerol acyltransferase increases the oil content and decreases the soluble carbohydrate content of soybeans. Plant Physiol. 2016;171(2):878–93. 10.1104/pp.16.00315 000380699200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Li X, Zhang Z, Wang K. Characterization of genetic diversity and structures in natural Glycine tomentella populations on the southeast islands of China. Genet Resour Crop Evol. 2018; 66(1):47–59. 10.1007/s10722-018-0694-6 [DOI] [Google Scholar]
- 20.Wang XL, Wurmser C, Pausch H, Jung S, Reinhardt F, Tetens J, et al. Identification and dissection of four major QTL affecting milk fat content in the german Holstein-Friesian population. PloS One. 2012;7(7). 10.1371/journal.pone.0040711 000306362400093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HW, Zhao TJ, Wang YF, Yu DY, Chen SY, Zhou RB, et al. Genetic structure composed of additive QTL, epistatic QTL pairs and collective unmapped minor QTL conferring oil content and fatty acid components of soybeans. Euphytica. 2011;182(1):117–32. 10.1007/s10681-011-0524-9 000296011200011 [DOI] [Google Scholar]
- 22.Priolli RHG, Campos JB, Stabellini NS, Pinheiro JB, Vello NA. Association mapping of oil content and fatty acid components in soybean. Euphytica. 2015;203(1):83–96. 10.1007/s10681-014-1264-4 [DOI] [Google Scholar]
- 23.Reinprecht Y, Poysa VW, Yu KF, Rajcan I, Ablett GR, Pauls KP. Seed and agronomic QTL in low linolenic acid, lipoxygenase-free soybean (Glycine max (L.) Merrill) germplasm. Genome. 2006;49(12):1510–27. 10.1139/g06-112 000245549600002 [DOI] [PubMed] [Google Scholar]
- 24.Bandillo N, Jarquin D, Song QJ, Nelson R, Cregan P, Specht J, et al. A population structure and genome-wide association analysis on the USDA soybean germplasm collection. Plant Genome. 2015; 8(3):13 10.3835/plantgenome2015.04.0024 0003673 90800010 [DOI] [PubMed] [Google Scholar]
- 25.Zhou ZK, Jiang Y, Wang Z, Gou ZH, Lyu J, Li WY, et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat Biotechnol. 2015;33(4):408–U125. 10.1038/nbt.3096 000352348500028 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Chu SS, Zhang HR, Zhu Y, Cheng H, Yu DY. Development and application of a novel genome-wide SNP array reveals domestication history in soybean. Sci Rep-Uk. 2016;6 10.1038/srep20728 000369615600001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WS, Tzen JTC, Kridl JC, Radke SE, Huang AHC. Maize oleosin is correctly targeted to seed oil bodies in Brassica-Napus transformed with the maize oleosin gene. P Natl Acad Sci USA. 1991;88(14):6181–5. 10.1073/pnas.88.14.6181 A1991FW76100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng P, Vadnais DA, Zhang Z, Polacco JC. Refined glufosinate selection in Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill]. Plant Cell Rep. 2004;22(7):478–82. 10.1007/s00299-003-0712-8 000188457400006 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Zheng YH, Dong ZM, Meng FF, Sun XM, Fan XH, et al. Soybean (Glycine max) WRINKLED1 transcription factor, GmWRI1a, positively regulates seed oil accumulation. Mol Genet Genomics. 2018;293(2):401–15. 10.1007/s00438-017-1393-2 000427630400009 [DOI] [PubMed] [Google Scholar]
- 30.Deruyffelaere C, Bouchez I, Morin H, Guillot A, Miquel M, Froissard M, et al. Ubiquitin-mediated proteasomal degradation of oleosins is involved in oil body mobilization during post-germinative seedling growth in Arabidopsis. Plant Cell Physiol. 2015;56(7):1374–87. 10.1093/pcp/pcv056 000359648900013 [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Ribera I, La Paz JL, Repiso C, Garcia N, Miquel M, Hernandez ML, et al. The evolutionary conserved oil body associated protein OBAP1 participates in the regulation of oil body size. Plant Physiol. 2014; 164(3):1237–49. 10.1104/pp.113.233221 000332475000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian ZX, Wang XB, Lee R, Li YH, Specht JE, Nelson RL, et al. Artificial selection for determinate growth habit in soybean. P Natl Acad Sci USA. 2010; 107(19):8563–8. 10.1073/pnas.1000088107 000277591200015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Yang X, Liu J, Wang BH, Liu BL, Wang YZ. Pod shattering resistance associated with domestication is mediated by a NAC gene in soybean. Nat Commun. 2014;5 10.1038/ncomms4352 000332669600007 [DOI] [PubMed] [Google Scholar]
- 34.Bolon YT, Hyten DL, Orf JH, Vance CP, Muehlbauer GJ. eQTL networks reveal complex genetic architecture in the immature soybean seed. Plant Genome-Us. 2014;7(1). 10.3835/plantgenome2013.08.0027 000338833800004 [DOI] [Google Scholar]
- 35.Tzen Jason T. C., Yiu-Kay Lai2, Kwai-Lan Chan2, and Anthony H. C. Huang*. Oleosin isoforms of high and low molecular weights are present in the oil bodies of diverse seed species. Plant Physiol. 1990, 94(3):1282–1289. 10.1104/pp.94.3.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severin AJ; Woody JL; Bolon YT; Joseph B; Diers BW; Farmer AD; Muehlbauer GJ; Nelson RT; Grant D; Specht JE; Graham MA; Cannon SB; May GD; Vance CP; Shoemaker RC. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. Bmc Plant Biol. 2010, 10(1):160 10.1186/1471-2229-10-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JH, Hao Q, Bai LL, Xu J, Yin WB, Song LY, et al. Overexpression of the soybean transcription factor GmDof4 significantly enhances the lipid content of Chlorella ellipsoidea. Biotechnol Biofuels. 2014;7 10.1186/s13068-014-0128-4 000341391200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HU, Hsieh K, Ratnayake C, Huang AHC. A novel group of oleosins is present inside the pollen of Arabidopsis. J Biol Chem. 2002;277(25):22677–84. 10.1074/jbc.M109298200 000176313600075 [DOI] [PubMed] [Google Scholar]
- 39.Zhai ZY, Liu H, Shanklin J. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell. 2017;29(4):871–89. 10.1105/tpc.17.00019 000400746600024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ting JTL, Lee K, Ratnayake C, Platt KA, Balsamo RA, Huang AHC. Oleosin genes in maize kernels having diverse oil contents are constitutively expressed independent of oil contents. Planta. 1996;199(1):158–65. 10.1007/bf00196892 [DOI] [PubMed] [Google Scholar]
- 41.Hu ZY, Wang XF, Zhan GM, Liu GH, Hua W, Wang HZ. Unusually large oilbodies are highly correlated with lower oil content in Brassica napus. Plant Cell Rep. 2009;28(4):541–9. 10.1007/s00299-008-0654-2 000264622400001 [DOI] [PubMed] [Google Scholar]
- 42.Ting J. T. L., Balsamo R. A., Ratnayake C., & Huang A. H. C. (1997). Oleosin of plant seed oil bodies is correctly targeted to the lipid bodies in transformed yeast. J Bio Chem. 272(6), 3699–706. 10.1074/jbc.272.6.3699 [DOI] [PubMed] [Google Scholar]
- 43.Huang AHC. Plant lipid droplets and their associated proteins: potential for rapid advances. Plant Physiol. 2018;176(3):1894–918. 10.1104/pp.17.01677 PubMed 000426848300008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parthibane V, Iyappan R, Vijayakumar A, Venkateshwari V, Rajasekharan R. Serine/threonine/tyrosine protein kinase phosphorylates oleosin, a regulator of lipid metabolic functions. Plant Physiol. 2012;159(1):95–104. 10.1104/pp.112.197194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miquel M, Trigui G, d'Andrea S, Kelemen Z, Baud S, Berger A, et al. Specialization of oleosins in oil body dynamics during seed development in Arabidopsis seeds. Plant Physiol. 2014;164(4):1866–78. 10.1104/pp.113.233262 000334342800028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deruyffelaere C, Bouchez I, Morin H, Guillot A, Miquel M, Froissard M, et al. Ubiquitin-mediated proteasomal degradation of oleosins is involved in oil body mobilization during post-germinative seedling growth in Arabidopsis. Plant Cell Physiol. 2015;56(7):1374–87. Epub 2015/04/25. 10.1093/pcp/pcv056 [DOI] [PubMed] [Google Scholar]
- 47.Deruyffelaere C, Purkrtova Z, Bouchez I, Collet B, Cacas JL, Chardot T, et al. PUX10 is a CDC48A adaptor protein that regulates the extraction of ubiquitinated oleosins from seed lipid droplets in Arabidopsis. Plant Cell. 2018;30(9):2116–36. 10.1105/tpc.18.00275 000446563700014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leprince O, van Aelst AC, Pritchard HW, Murphy DJ. Oleosins prevent oil-body coalescence during seed imbibition as suggested by a low-temperature scanning electron microscope study of desiccation-tolerant and -sensitive oilseeds. Planta. 1998;204(1): 109–19. 10.1007/s004250050236 [DOI] [Google Scholar]
- 49.Wang Y, Gai J. Study on the ecological regions of soybean in China. II. Ecological environment and representative varieties. J Appl Ecol. 2002;13(1):71 10.1006/jfls.2001.0409 [DOI] [PubMed] [Google Scholar]
- 50.Zhang D, Lü H, Chu S, Zhang H, Zhang H, Yang Y, et al. The genetic architecture of water-soluble protein content and its genetic relationship to total protein content in soybean. Sci Rep. 2017;7(5053):5053 10.1038/s41598-017-04685-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D, Kan G, Hu Z, Cheng H, Zhang Y, Wang Q, et al. Use of single nucleotide polymorphisms and haplotypes to identify genomic regions associated with protein content and water-soluble protein content in soybean. Theor Appl Genet. 2014;127(9): 1905–15. 10.1007/s00122-014-2348-1 [DOI] [PubMed] [Google Scholar]
- 52.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10). 10.1186/gb-2010-11-10-r106 000287378900008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–5. 10.1093/bioinformatics/btm308 000250673800021 [DOI] [PubMed] [Google Scholar]
- 54.Zhang ZW, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, Gore MA, et al. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42(4):355–U118. 10.1038/ng.546 000276150500017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang N, Lu YL, Yang XH, Huang J, Zhou Y, Ali F, et al. Genome wide association studies using a new nonparametric model reveal the genetic architecture of 17 agronomic traits in an enlarged maize association panel. Plos Genet. 2014;10(9). 10.1371/journal.pgen.1004573 000343009600010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. 2014:1–2. biorXiv 10.1101/005165 [DOI] [Google Scholar]
- 57.Shin J-H, Blay S, McNeney B, Graham J. LDheatmap: An R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J Stat Soft. 2006; 16:Code Snippet 3. http://stat.sfu.ca/statgen/research/ldheatmap.html. [Google Scholar]
- 58.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–72. Epub 2007/06/23. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- 59.Song ZY, Tian JL, Fu WZ, Li L, Lu LH, Zhou L, et al. Screening Chinese soybean genotypes for Agrobacterium-mediated genetic transformation suitability. J Zhejiang Univ-Sc B. 2013;14(4):289–98. 10.1631/jzus.B1200278 000317607900004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keim P, Diers BW, Olson TC, Shoemaker RC. Rflp mapping in soybean—association between marker loci and variation in quantitative traits. Genetics. 1990;126(3):735–42. 10.1007/BF00056365 A1990EF29100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao L, Ding XN, Li K, Liao WL, Zhong YK, Ren R, et al. Characterization of Soybean mosaic virus resistance derived from inverted repeat-SMV-HC-Pro genes in multiple soybean cultivars. Theor Appl Genet. 2015;128(8):1489–505. 10.1007/s00122-015-2522-0 000356778300004 [DOI] [PubMed] [Google Scholar]
- 62.Zhang D, Zhang H, Chu S, Li H, Chi Y, Triebwasser-Freese D, et al. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol Biol. 2017;93(1):137–50. 10.1007/s11103-016-0552-x [DOI] [PubMed] [Google Scholar]
- 63.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2). 10.1186/gb-2010-11-2-r14 000276434300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–82. 10.1093/nar/25.24.4876 000071498400004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19(18):2496–7. 10.1093/bioinformatics/btg359 [DOI] [PubMed] [Google Scholar]
- 66.Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7. 10.1093/nar/30.1.325 000173077100087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8. 10.1093/bioinformatics/btr330 000292778700023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20(2):289–90. 10.1093/bioinformatics/btg412 000188389700026 [DOI] [PubMed] [Google Scholar]
- 69.Zheng XW, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28(24):3326–8. 10.1093/bioinformatics/bts606 000312105300026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Oil content variation in all 219 accessions across six environments. B, Quantile-quantile plot of the GWAS results under a general linear model (GLM) and mixed linear model (MLM, Q+K). C, Phenotypic distribution of the oil trait across six environments. E1-E6 denote the oil content in the corresponding environments.
(TIF)
The x-axis shows the 20 soybean chromosomes, and the y-axis shows the significance expressed as a −log10P value. The quantile-quantile plot corresponding to the GWAS (MLM, Q+K, P < 4.95 × 10−6) result in each environment is given beside the Manhattan plot.
(TIF)
The dotted line represents the 95% tails for the empirical distribution of FST statistics.
(TIF)
(A-C) Identification of positive transgenic plants by leaf-painting assay (A), polymerase chain reaction (PCR) verification (B) and strip detection for the presence of the selective bar gene (C).
(TIF)
The indicated scale is the log2 value of the normalized level of gene expression.
(TIFF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The RNA-seq data we mentioned in our manuscript has been uploaded to NCBI (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA551573), (BioProject: PRJNA551573), (SRA Study: SRP212336).