Abstract

Metal oxide nanoparticles (NPs) stabilized by porous carbon materials (PCMs) are very promising for catalysis. In this work, monodispersed small and stable copper oxide (CuO) NPs were prepared with an average size of 10–20 nm without using any capping agent and then these NPs were encapsulated into porous carbon. The chemical and structural properties of the CuO/PCM material were characterized by powder X-ray diffraction, electron microscopy, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, and nitrogen sorption. The obtained CuO/PCM nanocatalytic system has been used for the synthesis of N-arylamides from the reaction of aldoximes and aryl halides. Generally, copper(II) salt was used for the preparation of amides from aldoximes using some ligands and bases, but harsh reaction condition, stoichiometric amount of metal, and lack of recyclability limit their applications in industry. An alternative method is the use of heterogeneous catalysts. More importantly, these heterogeneous catalysts could be easily recycled and reused, showing potential application in organic synthesis.
1. Introduction
Synthesis of N-arylamides has attracted wide attention because of their pharmaceutical, biological, and material importance.1−9 Practically, N-arylamides are the basic precursor for the synthesis of highly biologically active compounds and drugs.10−13 Therefore, the synthesis of amide skeleton has become one of the most challenging tasks to the organic chemists. The conventional methods, for the synthesis of amides, involve coupling of activated carboxylic acid derivatives, viz., acid chloride, anhydride, active esters, and so forth with a variety of amines. However, these methods generate futile wastes, and as an upshot, unconsciously serious environmental problems arise.14 Several alternative graceful methods along with the use of carbonyl compounds (ketones and aldehydes) or carbonyl derivatives (oximes) have been developed for the synthesis of amides.15 The acid-catalyzed rearrangement of ketoximes, known as Beckmann rearrangement,16−21 constitutes a straightforward synthetic way to generate N-substituted amides (RCONHR′). Following the same reaction conditions, aldoximes (RCH=NOH) give the corresponding nitriles (RCN),22 while transition-metal catalysts can be able to convert them into corresponding primary amides.23−29 Usually, the conversion of aldoximes into primary amides is successfully catalyzed by a number of expensive transition metals such as Pd,30 Au/Ag,31 Ir,32 Ru,33 and Rh.34,35 However, less expensive, environment-friendly nature of copper has captured the significant attention. In addition, homogeneous copper catalysts have been used successfully for a variety of organic reactions.36−45 Furthermore, the catalyst systems need to be developed further because of the possibility of metal contamination in the final product, lack of recyclability of the metal catalyst, use of additives, and expensive ligand system. Copper oxide (CuO) nanoparticles (NPs) can be applied in catalysis, gas sensing, and photocatalysis.46−48 The catalytic properties of NPs are related with their size and surface area. Therefore, the development of methodologies for the preparation of small CuO NPs has importance for catalytic applications. Strongly interacting capping agents were required for the synthesis of small CuO NPs.49−51 High-temperature calcination is required to eliminate these capping agents, so the synthesis of small surface clean CuO NPs still remains a challenge.
However, a large number of copper-catalyzed conversion of aldoximes to primary amides are available in the literature,27,52−57 but one-pot synthesis of secondary amides from aldoximes is less explored. Rather, a number of procedures58−60 are reported counting the transformation of ketoximes to N-arylamides. Now, in the case of oximes of unsymmetrical ketones, mixtures of regioisomeric products were formed, which further limited the scope of these reactions. As a result, the development of resourceful, economic, and environment-friendly catalytic system constitutes a motivating area for the one-pot synthesis of secondary amides from aldoximes. Very recently, Panda and co-workers61 reported homogeneous copper sulfate-catalyzed synthesis of N-arylamides from aldoximes. To the best of our knowledge, here we report for the first time that the heterogeneous CuO/PCM nanocomposite catalyzed the one-pot synthesis of N-arylamides from aldoximes and aryl halides (Scheme 1). In the present work, we have prepared monodispersed small CuO NPs. To show their relevance in catalysis, we have used these CuO NPs for the synthesis of N-arylamides from aldoximes. After identifying the significant role of CuO in the above reaction, supported CuO NPs on porous carbon were prepared as the heterogeneous catalyst. The CuO/PCM catalyst shows the catalytic activity similar to that of well-defined CuO NPs.
Scheme 1. Context of the Copper-Catalyzed Amidation of Aryl Halides.

2. Results and Discussion
The CuO/PCM nanocatalytic system was readily prepared by mixing a porous carbon material (PCM) with the recently prepared suspension of CuO NPs (Scheme 2). The latter CuO NPs were obtained within 1 h from copper acetate, sodium hydroxide, ethanol, and a catalytic amount of acetic acid under refluxing conditions. Our heterogeneous catalyst was prepared by mixing the suspension of CuO dropwise to the solution of glucose, and then the mixture was heated at 120–150 °C for 1 h. Finally, the black product was obtained and heated in an alumina boat inside a tube furnace at 850 °C under N2 atmosphere. After slow cooling to room temperature, the resulting dried product was obtained (yield = 50–60%). Copper loading was determined by atomic absorption spectroscopy (AAS), and it was about 8.0 wt %.
Scheme 2. Synthesis of the CuO/PCM Nanocatalyst.
2.1. Characterization of CuO/PCM
Recently, considerable interests have been focused on metal oxide NPs supported on the PCM as heterogeneous catalysts by virtue of their versatility in chemical compositions and structural architectures. Figure 1 shows the typical X-ray diffraction (XRD) patterns of the CuO/PCM nanocatalyst. The diffraction peaks located at about 2θ = 23.6 and 43.2° may be attributed to the presence of amorphous (002) and graphitic (100) carbons.62 On the other hand, the characteristic peaks at 2θ = 32.44, 35.46, 38.59, 48.69, 53.36, 61.47, 66.18, and 68° observed in both Figure 1a,b are ascribed due to the (110), (002), (111), (202), (020), (113), (311), and (113) facets of the monoclinic crystalline CuO in the CuO/PCM sample.
Figure 1.

Powder XRD patterns of (a) fresh CuO/PCM catalyst and (b) spent CuO/PCM catalyst (after 10 cycles).
The distribution of CuO NPs on the carbon support as well as particles sizes was investigated by transmission electron microscopy (TEM) analysis. Figure 2a,b shows the representative images, and the particle size distribution histogram for CuO/PCM was given in the inset. The images reveal homogeneous distribution of the NPs on the carbon support. The histogram shows that CuO NP sizes are in the 10–20 nm range. The average particle size was estimated from the particle distribution histogram, and the obtained value is 14 nm. The selected area electron diffraction (SAED) pattern (Figure 2c) of the CuO/PCM catalyst indicates the crystalline structure of the CuO NPs.
Figure 2.
TEM images of the CuO/PCM nanocatalyst (a,b), particle size distribution of CuO/PCM (given in the inset) and SAED pattern of the CuO/PCM nanocatalyst (c).
The morphology of the catalyst was studied by scanning electron microscopy (SEM, Figure 3) instrument. Figure 3a shows the typical SEM image of the CuO suspension which showed uniform spherical CuO NPs in the range of ∼20 nm. The SEM image of the CuO/PCM nanocomposite has been shown in Figure 3b, in which CuO NPs are well-dispersed in the range of 10–20 nm. The energy-dispersive analysis of X-ray (EDAX) pattern also showed the presence of C and Cu in the sample (Figure 3c). Elemental mapping, obtained from SEM, indicates the presence of C and Cu and the uniform dispersion of CuO NPs on the surface of the carbon support (Figure 3d,e).
Figure 3.

SEM images of suspended CuO NPs (a) and the CuO/PCM nanocomposite (b), EDAX image of the CuO/PCM nanocomposite (c), and elemental mapping of the CuO/PCM nanocomposite (d,e).
Raman spectroscopy is a powerful tool to characterize the carbon material. Figure 4 represents the Raman spectra of fresh and recycled CuO/PCM nanocomposite. The carbon support exhibited two prominent peaks at 1586 cm–1 (G band) arising from the sp2 carbon atoms, whereas the peak at 1345 cm–1 indicates the D band. This D band is normally associated with structural defects and amorphous phases. Therefore, the intensity ratio of the D versus G band (i.e., ID/IG obtained from Figure 4a) is usually used as a measure for the disorder of the carbon structure and was found to be ∼0.87. The CuO/PCM material has ID/IG value less than 1 which means this carbon material has less defective structure.
Figure 4.
Raman spectra of (a) fresh CuO/PCM catalyst and (b) spent CuO/PCM catalyst (after 10 cycles).
The nitrogen adsorption–desorption isotherms of the CuO/PCM catalyst are shown in Figure 5. The isotherm is a typical type IV isotherm. The Brunauer–Emmett–Teller (BET) surface area of the catalyst was determined to be 84.9 m2 g–1. The Barrett–Joyner–Halenda (BJH) pore size distribution plot is given in the inset of Figure 5. This material has various pores, and according to the BJH plot calculated from the N2 desorption isotherm, the sample has mesoscale pores ranging from 2 to 5.5 nm.
Figure 5.

Nitrogen adsorption–desorption isotherms of the catalyst.
2.2. Catalytic Activity
We have investigated the catalytic behaviors of CuO, CuO/AC (activated carbon), and CuO/PCM for the synthesis of N-arylamides from aldoximes and aryl halides under a reported reaction condition.61 To realize our scheme, benzaldoxime (1a) and iodobenzene (2a) were chosen as model substrates to assess the propensity of arylation of aryl halides. All these catalysts exhibited comparable activity (Table 1). There is a possibility of homogeneous copper salt contamination in the final product, lack of isolation, and reusability of the metal salt, which restricts its application in the above reaction. However, we found that the CuO and CuO/AC catalysts partially deactivated after repeated use (Figure 6a). During five recycling tests, the yield of the desired product decreased. The decrease, in the case of CuO, may be due to the agglomeration of the CuO NPs. A significant decrease was observed when we used CuO/AC, and CuO was obtained in the reaction mixture. This suggests the dissolution of CuO/AC in the reaction solution probably because of the low interaction between AC and CuO NPs. We also examined the stability of the CuO/PCM catalyst. Compared to CuO and CuO/AC, CuO/PCM was much more stable during recycling (Figure 6b). No significant decrease in the yield of the amides was observed over repeated use. After five recycles, the yield of amides was sustained at 72%. Therefore, CuO/PCM is a robust catalyst for the synthesis of amides from aldoximes.
Table 1. Catalytic Behavior of Some Catalysts for the Synthesis of N-Arylamides from Aldoximes and Aryl Halides61,a.
| entry | catalyst | yield (%) |
|---|---|---|
| 1 | CuCl2 | 52 |
| 2 | CuO | 60 |
| 3 | CuO/AC | 61 |
| 4 | CuO/PCM | 76 |
Reaction conditions: iodobenzene (100 mg), benzaldoxime (4 equiv), copper catalyst (10 mol %), and K2CO3 (5 equiv) in o-xylene (1 mL) were heated at 130 °C for 12 h.
Figure 6.
Recycling of (a) CuO and CuO/AC and (b) CuO/PCM catalysts for the synthesis of N-arylamides from benzaldoxime and iodobenzene. Reaction conditions: iodobenzene (100 mg), benzaldoxime (4 equiv), copper catalyst (10 mol %), and K2CO3 (5 equiv) in o-xylene (1 mL) were heated at 130 °C for 12 h.
Initially, we performed a series of experiments, with a variation of different reaction parameters such as solvent, base, temperature, catalyst loading, and so forth. The results are summarized in Table 2. Among dimethylformamide (DMF), N-methylpyrrolidone (NMP), toluene, CH3CN, p-xylene, and water, used for this experiment, toluene was found to be the best solvent (Table 2, entry 2). Now, we optimize the bases used for this reaction. Among the various bases, K2CO3 gave a higher product yield (Table 2, entry 2). Although NaHCO3 shows almost parallel activity to K2CO3 (Table 2, entry 8), we have chosen K2CO3 as an optimized base for this reaction. As the reaction did not proceed at room temperature (Table 2, entry 9), all the reactions were performed under refluxing conditions. Also, we have studied the activity of the support PCM in this coupling reaction, and no product was found (Table 2, entry 14). The experimental results revealed that the best optimized reaction condition in terms of yield was obtained using benzaldoxime (3 mmol), iodobenzene (1 mmol), and K2CO3 (4 mmol) in the presence of the CuO/PCM catalyst (6 mg, 0.25 mol %) in toluene under refluxed conditions for 16 h (Table 2, entry 2).
Table 2. Optimization of Reaction Conditionsa.
| entry | solvent | base | yield (%)b |
|---|---|---|---|
| 1 | p-xylene | K2CO3 | 65 |
| 2 | toluene | K2CO3 | 88 |
| 3c | DMF | K2CO3 | 52 |
| 4d | CH3CN | K2CO3 | 9 |
| 5 | toluene | Cs2CO3 | 60 |
| 6 | toluene | K3PO4 | 55 |
| 7 | toluene | KF | 10 |
| 8 | toluene | NaHCO3 | 82 |
| 9e | toluene | K2CO3 | 0 |
| 10 | NMP | K2CO3 | 56 |
| 11 | water | K2CO3 | 5 |
| 12 | toluene | 0 | |
| 13f | toluene | K2CO3 | 86 |
| 14g | toluene | K2CO3 | 0 |
Reaction conditions: 1a (3 mmol), 2a (1 mmol), catalyst (0.25 mol %), base (4 mmol), 16 h, under air and reflux conditions.
Isolated yield.
Reaction conducted at 140 °C.
Reaction conducted at 70 °C.
Reaction conducted at room temperature.
Catalyst used 0.5 mol %.
PCM as the catalyst.
To probe the generality of the reaction conditions, different kinds of aldoximes were coupled with aryl/heteroaryl halides to furnish a library of N-aryl/-heteroaryl amides. The results are summarized in Table 3. Both electron-donating and electron-withdrawing groups attached to the phenyl ring of aryl iodide were well-tolerated in this procedure giving uniform product yields. p-Nitroiodobenzene reacts with benzaldoxime to furnish 72% product yield (3d, Table 3). The reaction of 2-iodopyridine with benzaldoxime is a good example, as it provided N-(pyridine-2-yl)benzamide (3e, Table 3) as the main product. This protocol is also successful for the utilization of bromobenzene as the aryl halide partner giving good yield under optimized reaction conditions (3b, 3e, 3j, 3k, Table 3). The coupling of 4-chlorobenzaldoxime is highly chemoselective, as the nucleophilic attack occurs only at the N atom, leaving the −Cl group of the aromatic ring unaffected (3f, 3g, Table 3). The nitro-substituted aldoxime also reacts under similar reaction conditions. These results clearly indicate that both electron-donating (−MeO, −Me, −OH) and electron-withdrawing (−Cl, −NO2) groups attached to the aldoxime part are well-tolerated under the same reaction conditions. 4-Hydroxybenzaldoxime undergoes this reaction to give the corresponding anilide product in excellent yield (3h, Table 3). The coupling reaction of 4-bromoacetophenone and benzaldoxime exclusively provided N-(4-acetylphenyl)benzamide as the final product (3k, Table 3).
Table 3. Reaction of Aryl Halides with Aromatic Aldoximesa.
Reaction conditions: oxime (3.0 mmol), aryl halide (1.0 mmol), K2CO3 (4.0 mmol), CuO/PCM catalyst (0.25 mol %), toluene (3 mL), reflux condition, 16 h, under air.
Isolated yield.
Turnover frequency
(TOF) given in parentheses. 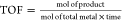 .
.
In the case of aliphatic aldoximes, this protocol reveals equal effectiveness giving the linear N-arylated amides. Acetaldoximes underwent coupling with aryl halides smoothly to produce the corresponding product in superior yield (Table 4).
Table 4. Reaction of Aryl Halides with Aliphatic Aldoximesa.
Reaction conditions: oxime (3.5 mmol), aryl halide (1.0 mmol), K2CO3 (4.0 mmol), CuO/PCM catalyst (0.2 mol %), toluene (3 mL), reflux condition, 20 h, under air.
Isolated yield. TOF given in parentheses.
Next, the scope of our methodology was inspected through the cross-coupling of several heterocyclic aldoximes with aryl halides. The results are summarized in Table 5. Aldoximes containing thiophene and furan moiety react with 2-bromopyridine to form N-heteroarylated amides in good yields (7b and 7e, Table 5).
Table 5. Reaction of Aryl Halides with Heterocyclic Aldoximesa.
Reaction conditions: oxime (4.0 mmol), aryl halide (1.0 mmol), K2CO3 (4.0 mmol), CuO/PCM catalyst (0.2 mol %), toluene (3 mL), reflux condition, 18 h, under air.
Isolated yield. TOF given in parentheses.
To realize the mechanism of this CuO/PCM-catalyzed synthesis of N-arylamides from aldoximes, a detailed investigation was carried out. To check whether the reaction proceeds through the radical mechanism, the coupling reaction of benzaldoxime and iodobenzene was performed in the presence of 1 equiv TEMPO, and it was found that the yield of the reaction remained the same. Therefore, the radical mechanism for the reaction is unlikely. Probable reaction mechanism for the one-pot synthesis of amides catalyzed by CuO/PCM under heterogeneous conditions may follow the established mechanism (Scheme 3).7,11,16,18,63
Scheme 3. Plausible Reaction Mechanism.

To establish the proposed mechanism, we have studied some more reactions using the CuO/PCM catalyst in the coupling of benzaldoxime and iodobenzene (Scheme 4). First, we have performed the coupling reaction under optimized conditions but using dry solvent and molecular sieve (Scheme 4, reaction a). After the reaction, we found the superior yield of the desired secondary amide. Generally, dehydration and rehydration steps are the proposed mechanism for the conversion of oxime to amide. Here, we have performed the reaction under anhydrous condition, so water may not be acting as a nucleophile for the rehydration step, but still secondary amides were obtained. This indicates that another molecule of oxime acts as a nucleophile and initiates the rehydration step.64,65 In this pathway, one molecule of nitrile will be produced, which may interact with the metal oxide and form a metal-bound nitrile intermediate to continue the catalytic reaction. When the reaction was carried out between benzamide (1 mmol) (instead of benzaldoxime) and iodobenzene (1 mmol) under air using K2CO3 as the base and toluene as the solvent (Scheme 4, reaction b), the secondary amide (N-phenylbenzamide, 95%) was obtained which means primary amide is an intermediate during this coupling reaction. This can be established by another reaction, when we have studied the reaction of benzaldoxime (3 mmol) without using aryl halide under similar reaction conditions (Scheme 4, reaction c). In this case, we have found only benzamide (85%) instead of the secondary amide. Therefore, in conclusion, we can propose that in our case the oxime molecule acts as a nucleophile for the rehydration step and the conversion of aldoxime to secondary amide occurs through the primary amide intermediate.
Scheme 4. CuO/PCM-Catalyzed Amide Synthesis Using Different Reaction Parameters under Reflux Conditions.
2.3. Heterogeneity Test
An essential aim of the heterogeneous catalyst is its life span for industrial applications. Heterogeneous nature and the metal leaching of this catalyst were examined using “hot filtration test”66 for the synthesis of N-arylamides from benzaldoxime and iodobenzene.
2.3.1. Hot Filtration Test
After 6 h of the reaction, the CuO/PCM material was separated by filtration and 35% yield was observed by gas chromatography (GC) analysis. The filtrate was again refluxed for another 10 h. After 16 h, from the GC analysis we can conclude that there was no considerable conversion taking place in the filtrate after separation of the catalyst (Figure 7). This result confirms that the reaction relatively did not progress upon the separation of the catalyst. Further, CuO could not be detected by the AAS measurement of the filtrate or in case if it was detected, it was found below the detection limit (0.003 mg/L). However, the negative result of the hot filtration test should not be considered the actual heterogeneity of the active catalysis species. This is because the insoluble solid support carbon can act as a reservoir as well as a scavenger for the leached CuO. In many conditions, the leached metal can be redeposited on the insoluble solid support during the hot filtration test. Therefore, in this situation, the catalytic pathway would be explained by a “release–capture” mechanism.67,68
Figure 7.

Heterogeneity test of the CuO/PCM catalyst in the synthesis of N-arylamides from benzaldoxime and iodobenzene.
2.4. Reusability Test
The recyclability of the catalyst is important for the catalysis reaction. The reusability test also confirms the heterogeneous nature of the catalyst. To examine the reusability of the CuO/PCM material, the catalyst was separated by filtration and washed. The subsequently catalytic runs were repeated with the addition of reactants under optimum reaction conditions, and the nature of the final product as well as yield was recorded. Figure 8 illustrates the recycling ability of the catalyst for the N-arylamide synthesis from benzaldoxime and iodobenzene during 10 cycles. However, the activity of the catalyst decreased slightly from the third run. The TEM image (Supporting Information, Figure S1) of the recycled catalyst after the fifth run showed a small increase in the size of CuO NPs due to agglomeration. This slight loss in the catalytic activity may be due to the increase in size of the CuO NPs. XRD, Raman spectra, and SEM (Supporting Information, Figure S1) of the recycled catalyst were quite similar to that of the fresh catalyst. This indicates the stability and heterogeneous nature of the CuO/PCM catalyst.
Figure 8.

Recycling results of the CuO/PCM nanocatalytic system in N-arylamide synthesis.
3. Conclusions
In conclusion, we have described a mild and efficient procedure for the synthesis and characterization of the CuO/PCM catalyst. Most importantly, this CuO NP catalyst proved its enormous activity for the synthesis of N-arylamides from several aromatic, aliphatic, and heterocyclic aldoximes. Diversely substituted aldoximes were coupled smoothly to produce moderate-to-good yields. To the best of our knowledge, we are not aware of any report demonstrating the synthesis of N-arylamides from aldoximes using our as-synthesized CuO NP catalyst. In a broader context, this protocol will provide a platform for the design of sustainable C–N cross-coupling reactions for organic synthesis. Efforts to develop the efficacy of our new catalyst systems to other cross-coupling reactions are in progress and will be reported in due course.
4. Experimental Section
4.1. Materials
Copper acetate monohydrate (Merck, 99%), acetic acid (Merck, >99%), sodium hydroxide (NaOH) pellets (Alfa Aesar, 98%), and d(+)-glucose (Merck, >99%) were used without further purification. Different aldehydes, aryl halides, and solvents were purchased from Sigma-Aldrich or Merck and used without further purification. Aldoximes were prepared using the literature method reported elsewhere.
4.2. Synthesis of Colloidal CuO NPs
Colloidal CuO NPs were prepared following a reported method.69 In this method, 0.3 g of copper acetate and 0.2 mL of acetic acid were dissolved in 50 mL of ethanol. The solution was refluxed, followed by the addition of 0.3 g NaOH. The reaction mixture was continuously refluxed for 1 h. The colloidal solution of CuO was centrifuged at 5500 rpm for 1 h, followed by filtration to yield CuO NP suspension.
4.3. Synthesis of the CuO/PCM Nanocatalyst
The synthesis procedure of the CuO/PCM catalyst is given in Scheme 2. The colloidal CuO NP solution was added dropwise into the glucose solution (2 g in 100 mL H2O) under vigorous stirring at 120 °C for 1 h. Then the reaction mixture was stirred for another 1 h at temperature 150 °C, followed by cooling to room temperature. The deep black colored product was obtained and ground in mortar. One gram of this black product was then heat-treated inside a tube furnace at 850 °C for 6 h at a rate of 6 °C/min under N2 atmosphere, followed by slow cooling to room temperature. The yield of the CuO/PCM catalyst was 50–60%.
4.4. Characterizations
Powder XRD patterns were recorded on a Bruker D8 ADVANCE X-ray diffractometer with Cu Kα source using a radiation wavelength of 1.5418 Å. Diffraction patterns in the 10°–80° region were recorded at a rate of 0.4° (2θ) per min. The field emission SEM (FESEM) images were recorded by Carl Zeiss SUPRA 55VP FESEM. TEM images were recorded on a UHR-FEG TEM system (JEOL, model JEM 2100 F) using a 200 kV electron source. An Acton SpectraPro SP-2500 Raman spectrometer was employed to analyze the nanostructures. N2 adsorption–desorption isotherms were obtained on a Micromeritics Gemini VII surface area analyzer and reported by the BJH analysis. Each sample was degassed at 300 °C for 3 h. The specific surface area was determined according to the BET method. The products were purified by column chromatography using silica gel (60–120 mesh). 1H and 13C NMR spectra were recorded on a Bruker AMX-400 NMR spectrophotometer (400 MHz for 1H NMR and 100 MHz for 13C NMR) using CDCl3 as the solvent. The 1H chemical shifts are reported in ppm relative to tetramethylsilane.
4.5. Synthetic Procedure of N-Arylamides from Aldoximes and Aryl Halides
To a mixture of aldoxime (3 mmol) and aryl halide (1 mmol) in toluene (3.0 mL), the CuO/PCM catalyst (0.25 mol %) and K2CO3 (4 mmol) were added, and the mixture was refluxed for 16 h under air. Then, the reaction mixture was cooled to room temperature and extracted with ethyl acetate. The organic layer was separated, washed with brine and dried over anhydrous Na2SO4, and concentrated under reduced pressure. Finally, the crude product was purified by column chromatography over silica gel with petroleum ether/ethyl acetate (85:15) as the eluent to furnish pure N-arylamides.
4.6. General Procedure for the “Hot Filtration” Test
To a mixture of benzaldoxime (3 mmol) and aryl iodide (1 mmol) in toluene (3.0 mL), the CuO/PCM catalyst (0.25 mol %) and K2CO3 (4 mmol) were added, and the mixture was refluxed under air. After 6 h of the reaction time, the catalyst was separated from the reaction mixture under hot condition. The yield of the product was determined by GC. Then, the filtrate was again refluxed for another 10 h, and finally, after 16 h the yield of the desired product was determined by using GC.
In a mixture of benzaldoxime (3 mmol) and aryl iodide (1 mmol) in toluene (3.0 mL), the CuO/PCM catalyst (0.25 mol %), and K2CO3 (4 mmol) were added, and the mixture was refluxed under air. To measure the amount of the leached CuO, the catalyst was separated from the reaction mixture under hot condition after 6 h of the reaction time. Then the filtrate was concentrated under reduced pressure. The HNO3 solution [5 mL 5% (in aq)] was added to the crude material and stirred. After that, this solution was taken in a 10 mL volumetric flask, and the volume was made up to the mark. Finally, this solution was studied using the AAS measurement to calculate the amount of leached CuO.
4.7. General Procedure for the Recycling Test
After 16 h of the reaction, the reaction mixture was cooled to room temperature and extracted with ethyl acetate. The catalyst was separated, and the organic layer was used for the determination of the yield of the product. The catalyst was washed three times with toluene, ethyl acetate, and water and dried. Finally, the catalyst was reused for the next run under optimized reaction conditions.
Acknowledgments
S.M.I. acknowledges Department of Science and Technology (DST-SERB, reference no. EMR/2016/004956), New Delhi, Govt. of India and Council of Scientific and Industrial Research (CSIR, sanction no. 02(0284)/16/EMR-II dated 06/12/2016), New Delhi, Govt. of India, for financial support. M.M.I. acknowledges UGC, New Delhi for Maulana Azad National Fellowship and thanks Sk. Shamim Islam for doing Raman spectroscopic analysis. M.H. expresses sincere thanks to UGC, New Delhi, for providing necessary Senior Research Fellowship. The authors gratefully acknowledge DST and UGC for providing grant to the Department of Chemistry, University of Kalyani under FIST, PURSE, and SAP programme. A.S.R. acknowledges Royal Society, UK and DST-SERB, India for his Newton-Bhabha International Postdoctoral Fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01028.
Mechanical experiment, 1H and 13C NMR spectral data of the compounds, TEM and SEM images and elemental mapping of the recycled CuO/PCM material, and 1H and 13C NMR spectra of all products (PDF)
Author Present Address
∥ Aston University, European Bioenergy Research Institute, Aston Triangle B4 7ET, Birmingham, United Kingdom.
The authors declare no competing financial interest.
Supplementary Material
References
- Negwer M.Organic-Chemical Drugs and Their Synonyms: (An International Survey), 7th ed.; Akademie Verlag: Berlin, 1994. [Google Scholar]
- Buckingham J. B.Dictionary of Natural Products; Chapman and Hall: London, UK, 1994; Vol. 1. [Google Scholar]
- Humphrey J. M.; Chamberlin A. R. Chemical Synthesis of Natural Product Peptides: Coupling Methods for the Incorporation of Noncoded Amino Acids into Peptides. Chem. Rev. 1997, 97, 2243–2266. 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- Carey J. S.; Laffan D.; Thomson C.; Williams M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- Cupido T.; Tulla-Puche J.; Spengler J.; Albericio F. The synthesis of naturally occurring peptides and their analogs. Curr. Opin. Drug Discovery Dev. 2007, 10, 768–783. [PubMed] [Google Scholar]
- Mintzer M. A.; Simanek E. E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- Valeur E.; Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. 10.1039/b701677h. [DOI] [PubMed] [Google Scholar]
- Anastas P.; Eghbali N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]
- Allen C. L.; Williams J. M. J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. 10.1039/c0cs00196a. [DOI] [PubMed] [Google Scholar]
- Halder M.; Islam M. M.; Ansari Z.; Ahammed S.; Sen K.; Islam S. M. Biogenic nano-CuO-catalyzed facile C–N cross-coupling reactions: scope and mechanism. ACS Sustainable Chem. Eng. 2017, 5, 648–657. 10.1021/acssuschemeng.6b02013. [DOI] [Google Scholar]
- Bebbington A.; Brimblecombe R. W.; Shakeshaft D. The central and peripheral activity of acetylenic amines related to oxotremorine. Br. J. Pharmacol. Chemother. 1966, 26, 56–67. 10.1111/j.1476-5381.1966.tb01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B.; Demont E.; Dingwall C.; Dunsdon R.; Faller A.; Hawkins J.; Hussain I.; MacPherson D.; Maile G.; Matico R. BACE-1 inhibitors Part 1: Identification of novel hydroxy ethylamines (HEAs). Bioorg. Med. Chem. Lett. 2008, 18, 1011. 10.1016/j.bmcl.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Demont H. E.; Faller A.; MacPherson D. T.; Milner P. H.; Naylor A.; Redshaw S.; Stanway S. J.; Vesey R. D.; Walter D. S.. Hydroxyethylamine derivatives for the treatment of alzheimer’s disease. PCT Int. Appl. WO/2004/050619, 2004.
- Constable D. J. C.; Dunn P. J.; Hayler J. D.; Humphrey G. R.; Leazer J. L.; Linderman R. J.; Lorenz K.; Manley J.; Pearlman B. A.; Wells A.; Zaks A.; Zhang T. Y. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. 10.1039/b703488c. [DOI] [Google Scholar]
- Islam M. M.; Halder M.; Roy A. S.; Chatterjee S.; Bhaumik A.; Islam S. M. Copper(II) incorporated functionalized polystyrene catalyzed N-arylation of amides under solvent free condition with broad substrate scope. RSC Adv. 2016, 6, 109692–109701. 10.1039/c6ra24459a. [DOI] [Google Scholar]
- Beckmann E. Zur Kenntniss der Isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 1886, 19, 988–993. 10.1002/cber.188601901222. [DOI] [Google Scholar]
- Chandrasekhar S.Comprehensive Organic Synthesis; Knochel P., Molander G. A., Eds.; Elsevier: Amsterdam, 2014; Vol. 7, pp 770–800 and references cited therein. [Google Scholar]
- Iranpoor N.; Firouzabadi H.; Aghapour G. A rapid and facile conversion of primary amides and aldoximes to nitriles and ketoximes to amides with triphenylphosphine and N-chlorosuccinimide. Synth. Commun. 2002, 32, 2535–2541. 10.1081/scc-120005936. [DOI] [Google Scholar]
- Iranpoor N.; Firouzabadi H.; Jamalian A.; Tamami M. Silphos [PCl3-n(SiO2)n], a Heterogeneous Phosphine Reagent Mediated the Conversion of Oximes to Nitriles and Amides or Carbonyl Compounds. Lett. Org. Chem. 2006, 3, 267–270. 10.2174/157017806776114504. [DOI] [Google Scholar]
- Zolfigol M. A.; Salehi P. Comment on paper entitled: “Silica Sulfate as a recyclable and efficient catalyst for Beckmann rearrangement under microwave irradiation” [Journal of Molecular Catalysis A: Chemical 250 (2006) 100–103]. J. Mol. Catal. A: Chem. 2006, 256, 346–347. 10.1016/j.molcata.2006.06.010. [DOI] [Google Scholar]
- Kaur N.; Sharma P.; Kishore D. Application of different catalysts in Beckmann Rearrangement. J. Chem. Pharm. Res. 2012, 4, 1938–1946. [Google Scholar]
- Smith M. B.; March J.. March’s Advanced Organic Chemistry; Wiley: New York, 2007; Vol. 6. [Google Scholar]
- Gawley R. E.The Beckmann reactions: Rearrangements, elimination-additions, fragmentations, and rearrangement-cyclization. Organic Reactions; John Wiley & Sons, Inc., 1988; Vol. 35, pp 1–420. [Google Scholar]
- Maruoka K.; Yamamoto H.. Comprehensive Organic Synthesis; Trost B. M., Fleming I., Eds.; Pergamon: Oxford, UK, 1991; Vol. 6, pp 763–765. [Google Scholar]
- Clayden J.; Greeves N.; Warren S.; Wothers P.. Organic Chemistry, 1st ed.; Oxford University Press: Oxford, UK, 2001; pp 997–1000. [Google Scholar]
- Crochet P.; Cadierno V. Catalytic synthesis of amides via aldoximes rearrangement. Chem. Commun. 2015, 51, 2495–2505. 10.1039/c4cc08684h. [DOI] [PubMed] [Google Scholar]; and the references cited therein from 12 to 25
- Ganguly N. C.; Roy S.; Mondal P. An efficient copper(II)-catalyzed direct access to primary amides from aldehydes under neat conditions. Tetrahedron Lett. 2012, 53, 1413–1416. 10.1016/j.tetlet.2012.01.029. [DOI] [Google Scholar]
- Allen C. L.; Davulcu S.; Williams J. M. J. Catalytic Acylation of Amines with Aldehydes or Aldoximes. Org. Lett. 2010, 12, 5096–5099. 10.1021/ol101978h. [DOI] [PubMed] [Google Scholar]; , and references cited therein
- García-Álvarez R.; Díaz-Álvarez A. E.; Borge J.; Crochet P.; Cadierno V. Ruthenium-Catalyzed Rearrangement of Aldoximes to Primary Amides in Water. Organometallics 2012, 31, 6482–6490. 10.1021/om3006917. [DOI] [Google Scholar]
- Ali M. A.; Punniyamurthy T. Palladium-Catalyzed One-Pot Conversion of Aldehydes to Amides. Adv. Synth. Catal. 2010, 352, 288–292. 10.1002/adsc.200900799. [DOI] [Google Scholar]
- Ramón R. S.; Bosson J.; Díez-González S.; Marion N.; Nolan S. P. Au/Ag-Cocatalyzed Aldoximes to Amides Rearrangement under Solvent- and Acid-Free Conditions. J. Org. Chem. 2010, 75, 1197–1202. 10.1021/jo902461a. [DOI] [PubMed] [Google Scholar]
- Owston N. A.; Parker A. J.; Williams J. M. J. Iridium-Catalyzed Conversion of Alcohols into Amides via Oximes. Org. Lett. 2007, 9, 73–75. 10.1021/ol062549u. [DOI] [PubMed] [Google Scholar]
- Owston N. A.; Parker A. J.; Williams J. M. J. Highly Efficient Ruthenium-Catalyzed Oxime to Amide Rearrangement. Org. Lett. 2007, 9, 3599–3601. 10.1021/ol701445n. [DOI] [PubMed] [Google Scholar]
- Fujiwara H.; Ogasawara Y.; Yamaguchi K.; Mizuno N. A One-Pot Synthesis of Primary Amides from Aldoximes or Aldehydes in Water in the Presence of a Supported Rhodium Catalyst. Angew. Chem., Int. Ed. 2007, 46, 5202–5205. 10.1002/anie.200701273. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kim M.; Chang S.; Lee H.-Y. Anhydrous Hydration of Nitriles to Amides using Aldoximes as the Water Source. Org. Lett. 2009, 11, 5598–5601. 10.1021/ol902309z. [DOI] [PubMed] [Google Scholar]
- Kotipalli T.; Kavala V.; Janreddy D.; Bandi V.; Kuo C.-W.; Yao C.-F. Synthesis of 2,3-Disubstituted Quinazolinone Derivatives through Copper Catalyzed C–H Amidation Reactions. Eur. J. Org. Chem. 2016, 1182–1193. 10.1002/ejoc.201501552. [DOI] [Google Scholar]
- Maji B.; Yamamoto H. Copper-Catalyzed Asymmetric Synthesis of Tertiary α-Hydroxy Phosphonic Acid Derivatives with In Situ Generated Nitrosocarbonyl Compounds as the Oxygen Source. Angew. Chem., Int. Ed. 2014, 53, 14472–14475. 10.1002/anie.201408893. [DOI] [PubMed] [Google Scholar]
- Weerasiri K. C.; Gorden A. E. V. Cu(II) 2-Quinoxalinol Salen Catalyzed Oxidation of Propargylic, Benzylic, and Allylic Alcohols Using tert-Butyl Hydroperoxide in Aqueous Solutions. Tetrahedron 2014, 70, 7962–7968. 10.1016/j.tet.2014.08.050. [DOI] [Google Scholar]
- Barsoum D. N.; Okashah N.; Zhang X.; Zhu L. Mechanism of Copper(I)-Catalyzed 5-Iodo-1,2,3-triazole Formation from Azide and Terminal Alkyne. J. Org. Chem. 2015, 80, 9542–9551. 10.1021/acs.joc.5b01536. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Fan X.-M.; Nie M.; Liu H.-W.; Liao D.-H.; Pan X.-D.; Ji Y.-F. Practical Ligand-Free Copper-Catalysed Short-Chain Alkoxylation of Unactivated Aryl Bromides. Eur. J. Org. Chem. 2015, 4744–4755. 10.1002/ejoc.201500500. [DOI] [Google Scholar]
- Yu W.-B.; He Q.-Y.; Ma X.-F.; Shi H.-T.; Wei X. A new copper species based on an azo-compound utilized as a homogeneous catalyst for water oxidation. Dalton Trans. 2015, 44, 351–358. 10.1039/c4dt03097d. [DOI] [PubMed] [Google Scholar]
- Bergbreiter D. E.; Hamilton P. N.; Koshti N. M. Self-Separating Homogeneous Copper (I) Catalysts. J. Am. Chem. Soc. 2007, 129, 10666–10667. 10.1021/ja0741372. [DOI] [PubMed] [Google Scholar]
- Coman S. M.; Parvulescu V. I. Nonprecious Metals Catalyzing Hydroamination and C–N Coupling Reactions. Org. Process Res. Dev. 2015, 19, 1327–1355. 10.1021/acs.oprd.5b00010. [DOI] [Google Scholar]
- Xin X.; Xiang D.; Yang J.; Zhang Q.; Zhou F.; Dong D. Homogeneous and Stereoselective Copper(II)-Catalyzed Monohydration of Methylenemalononitriles to 2-Cyanoacrylamides. J. Org. Chem. 2013, 78, 11956–11961. 10.1021/jo401997v. [DOI] [PubMed] [Google Scholar]
- Xiang S.-K.; Zhang D.-X.; Hu H.; Shi J.-L.; Liao L.-G.; Feng C.; Wang B.-Q.; Zhao K.-Q.; Hu P.; Yang H.; Yu W.-H. Synthesis of N-Arylamides by Copper-Catalyzed Amination of Aryl Halides with Nitriles. Adv. Synth. Catal. 2013, 355, 1495–1499. 10.1002/adsc.201300189. [DOI] [Google Scholar]
- Kuo C.-H.; Huang M. H. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 2010, 5, 106–116. 10.1016/j.nantod.2010.02.001. [DOI] [Google Scholar]
- Zhang Q.; Zhang K.; Xu D.; Yang G.; Huang H.; Nie F.; Liu C.; Yang S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. 10.1016/j.pmatsci.2013.09.003. [DOI] [Google Scholar]
- Salam N.; Kundu S. K.; Roy A. S.; Mondal P.; Roy S.; Bhaumik A.; Islam S. M. Cu-grafted mesoporous organic polymer: a new recyclable nanocatalyst for multi-component, N-arylation and S-arylation reactions. Catal. Sci. Technol. 2013, 3, 3303–3316. 10.1039/c3cy00600j. [DOI] [Google Scholar]
- Yin M.; Wu C.-K.; Lou Y.; Burda C.; Koberstein J. T.; Zhu Y.; O’Brien S. Copper Oxide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. 10.1021/ja050006u. [DOI] [PubMed] [Google Scholar]
- Hua Q.; Cao T.; Gu X.-K.; Lu J.; Jiang Z.; Pan X.; Luo L.; Li W.-X.; Huang W. Crystal-Plane-Controlled Selectivity of Cu2O Catalysts in Propylene Oxidation with Molecular Oxygen. Angew. Chem., Int. Ed. 2014, 53, 4856–4861. 10.1002/anie.201402374. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Shi J.; Peng Q.; Li Y. CuO Quantum-Dot-Sensitized Mesoporous ZnO for Visible-Light Photocatalysis. Chem.—Eur. J. 2013, 19, 4319–4326. 10.1002/chem.201203316. [DOI] [PubMed] [Google Scholar]
- Song Q.; Feng Q.; Yang K. Synthesis of Primary Amides via Copper-Catalyzed Aerobic Decarboxylative Ammoxidation of Phenylacetic Acids and α-Hydroxyphenylacetic Acids with Ammonia in Water. Org. Lett. 2014, 16, 624–627. 10.1021/ol403586c. [DOI] [PubMed] [Google Scholar]
- Zultanski S. L.; Zhao J.; Stahl S. S. Practical Synthesis of Amides via Copper/ABNO-Catalyzed Aerobic Oxidative Coupling of Alcohols and Amines. J. Am. Chem. Soc. 2016, 138, 6416–6419. 10.1021/jacs.6b03931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. C.; Ngiam J. S. Y.; Seayad A. M.; Tuan D. T.; Chai C. L. L.; Chen A. Copper-Catalyzed Oxidative Amidation of Aldehydes with Amine Salts: Synthesis of Primary, Secondary, and Tertiary Amides. J. Org. Chem. 2012, 77, 8007–8015. 10.1021/jo301252c. [DOI] [PubMed] [Google Scholar]
- Sharma S. K.; Bishopp S. D.; Allen C. L.; Lawrence R.; Bamford M. J.; Lapkin A. A.; Plucinski P.; Watson R. J.; Williams J. M. J. Copper-catalyzed rearrangement of oximes into primary amides. Tetrahedron Lett. 2011, 52, 4252–4255. 10.1016/j.tetlet.2011.05.129. [DOI] [Google Scholar]
- Ma X.-Y.; He Y.; Lu T.-T.; Lu M. Conversion of aldoximes into nitriles catalyzed by simple transition metal salt of the fourth period in acetonitrile. Tetrahedron 2013, 69, 2560–2564. 10.1016/j.tet.2013.01.059. [DOI] [Google Scholar]
- Martínez-Asencio A.; Yus M.; Ramón D. J. Copper(II) acetate-catalyzed one-pot conversion of aldehydes into primary amides through a Beckmann-type rearrangement. Tetrahedron 2012, 68, 3948–3951. 10.1016/j.tet.2012.03.085. [DOI] [Google Scholar]
- Arisawa M.; Yamaguchi M. Rhodium-Catalyzed Beckmann Rearrangement. Org. Lett. 2001, 3, 311–312. 10.1021/ol006951z. [DOI] [PubMed] [Google Scholar]
- Ramalingan C.; Park Y.-T. Mercury-Catalyzed Rearrangement of Ketoximes into Amides and Lactams in Acetonitrile. J. Org. Chem. 2007, 72, 4536–4538. 10.1021/jo070297k. [DOI] [PubMed] [Google Scholar]
- Hashimoto M.; Obora Y.; Sakaguchi S.; Ishii Y. Beckmann Rearrangement of Ketoximes to Lactams by Triphosphazene Catalyst. J. Org. Chem. 2008, 73, 2894–2897. 10.1021/jo702277g. [DOI] [PubMed] [Google Scholar]
- Panda N.; Mothkuri R.; Nayak D. K. Copper-Catalyzed Regioselective Synthesis of N-Aryl Amides from Aldoximes and Aryl Halides. Eur. J. Org. Chem. 2014, 1602–1605. 10.1002/ejoc.201301868. [DOI] [Google Scholar]
- Menéndez J. A.; Juárez-Pérez E. J.; Ruisánchez E.; Calvo E. G.; Arenillas A. A microwave-based method for the synthesis of carbon xerogel spheres. Carbon 2012, 50, 3555–3560. 10.1016/j.carbon.2012.03.027. [DOI] [Google Scholar]
- Sarode S. A.; Bhojane J. M.; Nagarkar J. M. An efficient magnetic copper ferrite nanoparticle catalysed ligand and solvent free synthesis of N-aryl amide from aldoximes and iodobenzene. RSC Adv. 2015, 5, 105353–105358. 10.1039/c5ra22777a. [DOI] [Google Scholar]
- Allen C. L.; Lawrence R.; Emmett L.; Williams J. M. J. Mechanistic Studies into Metal-Catalyzed Aldoxime to Amide Rearrangements. Adv. Synth. Catal. 2011, 353, 3262–3268. 10.1002/adsc.201100650. [DOI] [Google Scholar]
- Zhang D.-X.; Xiang S.-K.; Hu H.; Tan W.; Feng C.; Wang B.-Q.; Zhao K.-Q.; Hu P.; Yang H. An improved protocol for synthesis of N-arylamides and benzoxazoles by the copper-catalyzed reaction of aryl halides with nitriles. Tetrahedron 2013, 69, 10022–10029. 10.1016/j.tet.2013.09.059. [DOI] [Google Scholar]
- Karimi B.; Elhamifar D.; Clark J. H.; Hunt A. J. Ordered Mesoporous Organosilica with Ionic-Liquid Framework: An Efficient and Reusable Support for the Palladium-Catalyzed Suzuki–Miyaura Coupling Reaction in Water. Chem.—Eur. J. 2010, 16, 8047–8053. 10.1002/chem.201000538. [DOI] [PubMed] [Google Scholar]
- Jahanshahi R.; Akhlaghinia B. Cu(II)-grafted SBA-15 functionalized S-methylisothiourea aminated epibromohydrin (SBA-15/E-SMTU-CuII): a novel and efficient heterogeneous mesoporous catalyst. New J. Chem. 2017, 41, 7203–7219. 10.1039/c7nj00849j. [DOI] [Google Scholar]
- Karimi B.; Mansouri F.; Mirzaei H. M. Recent Applications of Magnetically Recoverable Nanocatalysts in C-C and C-X Coupling Reactions. ChemCatChem 2015, 7, 1736–1789. 10.1002/cctc.201403057. [DOI] [Google Scholar]
- Pelletier V.; Bhattacharyya S.; Knoke I.; Forohar F.; Bichay M.; Gogotsi Y. Copper Azide Confined Inside Templated Carbon Nanotubes. Adv. Funct. Mater. 2010, 20, 3168–3174. 10.1002/adfm.201000858. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











