Abstract
Background
Premature coronary artery disease (CAD) is common in patients with coarctation of aorta (COA), but there are limited data about any direct relationship (or lack thereof) between COA and CAD. We hypothesized that atherosclerotic cardiovascular disease risk factors, rather than COA diagnosis, was the primary determinant of CAD occurrence in patients with COA.
Methods and Results
This is a retrospective study of 654 COA patients and a control group of 876 patients with valvular pulmonic stenosis and tetralogy of Fallot to determine prevalence and independent risk factors for CAD. There was no evidence of a difference in the unadjusted CAD prevalence between the COA and control groups (7.8% versus 6.3%, P=0.247), but premature CAD was more common in COA patients (4.4% versus 1.8%, P=0.002). In the analysis of a propensity‐matched cohort of 126 COA and 126 control patients, there was no evidence of a difference in overall CAD prevalence (6.3% versus 5.6% versus P=0.742) and premature CAD prevalence (4.8% versus 3.2%, P=0.518). The multivariable risk factors for CAD were hypertension (odds ratio [OR] 2.14; 95% CI 1.36–3.38), hyperlipidemia (OR 3.33; 95% CI 2.02–5.47), diabetes mellitus (OR 1.98; 95% CI 1.31–3.61), male sex (OR 2.05; 95% CI 1.33–3.17), and older age per year (OR 1.06; 95% CI 1.04–1.07).
Conclusions
After adjusting for atherosclerotic cardiovascular disease risk factors, we did not find evidence of a difference in CAD risk between the patients with COA and other patients with congenital heart disease.
Keywords: cardiovascular disease, coarctation, coronary artery disease, mortality, risk modification
Subject Categories: Congenital Heart Disease
Clinical Perspective
What Is New?
After adjusting for atherosclerotic cardiovascular heart disease risk factors, patients with coarctation of aorta had similar risk for coronary artery disease compared with other patients with congenital heart disease.
What Are the Clinical Implications?
The risk of coronary artery disease in coarctation of aorta patients is attributable to atherosclerotic cardiovascular disease risk factors, therefore premature coronary artery disease is not an inevitable complication of coarctation of aorta diagnosis but a preventable morbidity.
The goal should therefore be to identify and aggressively treat modifiable coronary artery disease risk factors in patients with coarctation of aorta.
Introduction
Coarctation of the aorta (COA) accounts for 5% to 8% of congenital heart diseases.1, 2, 3 The timing of presentation is variable, ranging from newborn to adulthood, and the presentation timing is often related to the severity of the obstruction and the presence of other associated structural heart diseases.1, 2 Previous studies have shown that patients with COA are at risk for cardiovascular mortality because of premature coronary artery disease (CAD) even after successful surgical or transcatheter intervention.2, 4, 5
Although several studies have reported an association between COA and CAD,2, 4, 5, 6, 7, 8 there is only one study that compared CAD prevalence between patients with COA and a matched cohort of patients with ventricular septal defect.9 This study did not show an independent association between COA diagnosis and CAD.9 As the prevalence of acquired heart disease continues to increase in the congenital heart disease population because of aging,10 it becomes imperative to resolve the uncertainty of risk of CAD in patients with COA, as this will potentially change how these patients are managed. Based on the previous report by Roifman et al9 and our clinical observations, we hypothesized that the presence of atherosclerotic cardiovascular disease (ASCVD) risk factors, rather than the diagnosis of COA, was the primary determinant of CAD occurrence in patients with COA. To test this hypothesis, we designed a study with the following specific aims: (1) compared the prevalence of CAD between COA patients and other patients with congenital heart disease; (2) determine the independent risk factors for CAD in COA patients.
Methods
Patient Selection and Data Collection
This is a retrospective review of patients (aged ≥18 years) with a COA who received care at Mayo Clinic Rochester, MN from January 1, 1995 through December 31, 2017. We selected a control group of patients with diagnoses of valvular pulmonic stenosis or tetralogy of Fallot who received care at Mayo Clinic within the same time period. The rationale for using patients with tetralogy of Fallot or valvular pulmonic stenosis as the control group was because there is no known association with CAD and also because we had a well characterized cohort with extensive clinical follow‐up. The Mayo Clinic institutional review board approved this study and waived informed consent for patients who provided research authorization. Data, analytic methods, and study materials will be made available to other researchers on request.
The electronic health records were extensively reviewed in these patients. Clinical data such as ASCVD risk factors, laboratory data, comorbidities, medications and imaging data at the time of initial presentation were collected as the baseline variables. Images and reports of invasive coronary angiogram and cardiac computed tomography (CT) angiogram were reviewed to determine CAD diagnosis.
Study Design and End Points
The primary outcome was prevalence of overall CAD and premature CAD. CAD diagnosis was defined as acute coronary syndrome (ST‐segment–elevation myocardial infarction, non–ST‐–elevation myocardial infarction, or unstable angina), history of coronary revascularization (coronary artery bypass grafting [CABG] or percutaneous coronary intervention) or >50% stenosis in any vessel on invasive coronary or CT angiogram. Premature CAD was defined as CAD diagnosis before the age of 55 or 65 years in men and women, respectively.11 The patients with CAD diagnosis at the time of initial presentation and the patients diagnosed with CAD during follow‐up (incident cases) were combined to determine CAD prevalence in each study group. The secondary outcome was CAD risk factors. Hemodynamically significant residual coarctation was defined as uncorrected peak velocity of >2.5 m/s at the aortic isthmus.12
Two sets of analyses were performed to test the hypothesis about the association between COA and CAD. First, CAD prevalence was compared between the COA and the control groups (unadjusted prevalence) and between propensity‐matched cohorts of COA and the control groups (adjusted prevalence). Second, multivariable analyses of ASCVD risk factors were performed using a combined cohort of both the COA and control groups. Exploratory analysis was performed to determine the performance of the American College of Cardiology ASCVD risk calculator13 in predicting incident CAD in the subset of COA patients without CAD at the beginning of the study.14 The performance of the calculator was assessed by calculating the proportion of these patients who would have qualified for statin therapy based on a 10‐year ASCVD risk score calculated with variables obtained at the time of initial presentation. Based on the guidelines for management of stable ischemic heart disease,11 we defined guideline directed medical therapy as the use of at least two of these medications: antiplatelet therapy, beta‐blocker therapy or angiotensin converting enzyme inhibitor/angiotensin receptor blocker therapy.
Statistical Analysis
Data were presented as mean±SD, median (interquartile range) or counts (%), and between‐group comparisons were performed using t test, Wilcoxon test, chi square test, and Fisher exact test as appropriate. To adjust for differences in the baseline clinical characteristics of both groups, propensity matching was performed using logistics regression to determine the probability of having similar ASCVD risk profile as the case group (COA) adjusting for the following variable: age, male sex, hypertension, hyperlipidemia and diabetes mellitus. Based on the probability estimate for each COA patient we then selected a control patient with a probability estimate within one standard deviation for each particular. Based on these parameters, we were able to identify a suitable “match” for 126 of the COA patients.
Multivariable logistic regression models were used to determine the independent predictors of CAD, and the variables used in the models were chosen a priori on the basis of known ASCVD risk factors.11 COA diagnosis, history of COA repair, and presence of hemodynamically significant residual coarctation gradient (uncorrected peak velocity >2.5 m/s at the aortic isthmus) were also incorporated in the model. A separate multivariable logistic regression models was constructed to determine the predictors of premature CAD. To avoid over‐fitting of the model for premature CAD because of lower event rate, we assessed only the variables that were significant in the model for overall CAD. The adjusted risk from these models were expressed as odds ratio (OR) and 95% CI. All statistical analyses were performed with JMP software (version 13.0; SAS Institute Inc, Cary, NC) and P<0.05 was considered statistically significant.
Results
Baseline Clinical and Echocardiographic Data
A total of 654 patients and 876 patient‐controls met the inclusion criteria for the COA group and the control group, respectively. Of the 654 COA patients, 598 (92%) had prior COA repair, and the mean age at the time of initial repair was 10±4 years. A total of 104 (16%) patients had hemodynamically significant residual coarctation at the time of initial presentation. The control group consisted of 616 (70%) patients with tetralogy of Fallot and 260 (30%) patients with valvular pulmonic stenosis. In comparison with the control group, the patients in the COA group were younger at the time of initial presentation, 38±14 versus 36±16 years, P=0.005. Other significant differences in the clinical, echocardiographic, and exercise data of the COA and control groups at the time of initial presentation are shown in Table 1.
Table 1.
Clinical, Echocardiographic, and Exercise Data
| COA (n=654) | Control (n=876) | P Value | |
|---|---|---|---|
| Age, y | 36±16 | 38±14 | 0.005 |
| Men | 373 (57%) | 391 (45%) | <0.001 |
| Body mass index, kg/m2 | 29±5 | 26±6 | <0.001 |
| Body surface area, m2 | 2.9±0.2 | 1.9±0.3 | 0.476 |
| Comorbidities | |||
| Atrial fibrillation | 70 (11%) | 165 (19%) | <0.001 |
| Atrial flutter/tachycardia | 20 (3%) | 128 (15%) | <0.001 |
| Hypertension | 374 (57%) | 221 (25%) | <0.001 |
| Hyperlipidemia | 205 (31%) | 213 (24%) | 0.006 |
| Current or prior smoker | 152 (23%) | 166 (19%) | 0.041 |
| Diabetes mellitus | 70 (11%) | 106 (12%) | 0.340 |
| Sleep apnea | 96 (15%) | 211 (24%) | <0.001 |
| Stroke | 48 (7%) | 41 (5%) | 0.048 |
| Peripheral arterial disease | 31 (5%) | 13 (2%) | 0.002 |
| Laboratory tests | |||
| Hemoglobin, g/dL | 13.8±1.9 | 14.0±1.8 | 0.103 |
| Creatinine, mg/dL | 0.95±0.27 | 0.99±0.42 | 0.095 |
| NT‐proBNP, pg/mL | 233 (95–634) | 199 (169–246) | 0.065 |
| Medications | |||
| Loop diuretics | 115 (18%) | 116 (14%) | 0.090 |
| Beta blockers | 238 (37%) | 186 (21%) | 0.002 |
| Calcium channel blockers | 62 (10%) | 91 (10%) | 0.327 |
| RAAS antagonist | 171 (26%) | 69 (11%) | <0.001 |
| Statins | 186 (29%) | 212 (24%) | 0.391 |
| Aspirin | 137 (21%) | 212 (24%) | 0.103 |
| Right ventricle | |||
| ≥Moderate RV systolic dysfunctiona | 4 (1%) | 179 (22%) | <0.001 |
| Tricuspid regurgitation velocity, m/s | 2.5±0.4 | 3.1±0.8 | <0.001 |
| TAPSE, cm | 23±3 | 18±4 | <0.001 |
| FAC, % | 48±8 | 40±10 | <0.001 |
| RV s′, cm/s | 0.11±0.02 | 0.10±0.06 | 0.577 |
| Left ventricle | |||
| LV ejection fraction, % | 62±7 | 59±9 | <0.001 |
| Medial E/e′ | 11±5 | 10±5 | 0.006 |
| Lateral E/e′ | 9±5 | 7±3 | 0.022 |
| LV mass index, g/m2 | 110±38 | 91±33 | <0.001 |
| Relative wall thickness | 0.42±0.06 | 0.41±0.08 | 0.473 |
| LV stroke volume index, mL/m2 | 53±8 | 58±14 | 0.071 |
| LV cardiac index, L/min per m2 | 3.7±0.2 | 3.9±0.3 | 0.104 |
| CPET | |||
| Peak VO2, mL/kg per minute | 26.2±10.4 | 22.5±7.6 | <0.001 |
| Peak VO2, % predicted | 70±19 | 65±18 | 0.009 |
| VE/VCO2 nadir | 27±5 | 28±6 | 0.467 |
COA indicates coarctation of aorta; CPET, cardiopulmonary exercise test; E/e′, ratio of mitral inflow early filling velocity to tissue Doppler early velocity; FAC, fractional area change; LV, left ventricle; RAAS, renin angiotensin aldosterone system; RV, right ventricle; s′, tissue Doppler systolic velocity; TAPSE, tricuspid annular plane systolic excursion; VE/VCO2, ventilator equivalent for carbon dioxide; VO2, oxygen consumption.
The assessment of RV systolic dysfunction based on qualitative assessment.
Prevalence of CAD in COA
Of the 654 patients in the COA group, 18 (2.8%) had CAD at the time of initial presentation, and among these patients, 12 had a history of acute coronary syndrome, 13 had prior coronary revascularization (CABG [n=7], and percutaneous coronary intervention [n=6]), and 5 had significant (>50%) stenosis on invasive coronary or CT angiogram without revascularization. These 654 patients were followed for 7±3 years, and during this period there were 33 incident cases of CAD. The incident cases were diagnosed using invasive coronary angiogram (n=21) and CT angiogram (n=12). The indications for angiograms were non‐ST–elevation myocardial infarction (n=4), unstable angina (n=9), abnormal stress test (n=6), left ventricular dysfunction (n=4), and preoperative coronary angiogram (n=10). Of these 33 incident cases, 4 (12%) underwent percutaneous coronary intervention; 7 (21%) had concomitant CABG during aortic valve replacement and/or COA surgery; and 22 (67%) received guideline directed medical therapy alone. The overall CAD prevalence in the COA group was 7.8% (51 of 654), and the prevalence of premature CAD was 4.4% (29 of 654). There was no evidence of a difference in CAD prevalence between patients with versus without history of COA repair, or between patients with versus without hemodynamically significant residual lesions. Among these 51 patients, 20 (39%) had single‐vessel disease while 31 (61%) had multi‐vessel disease (involvement of ≥2 vessels).
Of the 876 patients in the control group, 17 (1.9%) had CAD at the time of initial presentation, and among these patients, 11 had a history of acute coronary syndrome, 9 had prior coronary revascularization (CABG [n=5], and percutaneous coronary intervention [n=4]), and 8 had significant stenosis on invasive coronary or CT angiogram without revascularization. These 876 patients were followed for 8±4 years during which there were 38 incident cases of CAD. These incident cases were diagnosed using invasive coronary angiogram (n=24) and CT angiogram (n=14). The indications for angiograms were non‐ST–elevation myocardial infarction (n=4), unstable angina (n=5), abnormal stress test (n=9), left ventricular dysfunction (n=8), and preoperative coronary angiogram (n=12). Of these 38 incident cases, 4 (11%) underwent percutaneous coronary intervention; 6 (16%) underwent concomitant CABG during pulmonary valve replacement; and 28 (74%) received guideline directed medical therapy alone. The overall CAD prevalence in the control group was 6.3% (55 of 876), and the prevalence of premature CAD was 1.8% (16 of 876). Among these 55 patients, 26 (47%) had single‐vessel disease while 29 (53%) had multi‐vessel disease.
Table 2 shows a comparison of ASCVD risk factors in patients with CAD in both groups. There was no evidence of a difference in CAD prevalence (unadjusted) between the COA group and control group, 7.9% versus 6.3%, P=0.247. However, premature CAD was more common in COA patients compared with the control group, 4.4% versus 1.8%, P=0.002.
Table 2.
ASCVD Risk Factors
| COA (n=51) | Control (n=55) | P Value | |
|---|---|---|---|
| Age at CAD diagnosis, y | 51±12 | 55±13 | 0.113 |
| Men | 42 (82%) | 38 (69%) | 0.118 |
| Body mass index, kg/m2 | 31±5 | 27±6 | 0.015 |
| Hypertension | 44 (86%) | 31 (56%) | <0.001 |
| Hyperlipidemia | 31 (61%) | 25 (45%) | 0.021 |
| Current or prior smoker | 20 (39%) | 22 (42%) | 0.372 |
| Diabetes mellitus | 19 (37%) | 17 (31%) | 0.491 |
| Family h/o CAD | 6 (12%) | 7 (13%) | 0.893 |
ASCVD indicates atherosclerotic cardiovascular disease; CAD, coronary artery disease; COA, coarctation of aorta; h/o, history of.
Risk Factors for CAD
In a combined cohort of both COA and control groups (n=1530), the prevalence of overall CAD and premature CAD were 6.9% and 2.9%, respectively. The multivariable risk factors for CAD were hypertension (OR 2.14; 95% CI 1.36–3.38; P=0.001), hyperlipidemia (OR 3.33; 95% CI 2.02–5.47; P<0.001), diabetes mellitus (OR 1.98; 95% CI 1.31–3.61; P=0.002), male sex (OR 2.05; 95% CI 1.33–3.17; P=0.001), and older age per year (OR 1.06; 95% CI 1.04–1.07; P<0.001), (Figure A). COA diagnosis was not an independent risk factor for overall CAD (OR 0.92; 95% CI 0.59–1.42; P=0.721) or premature CAD (OR 1.81; 95% CI 0.48–3.06; P=0.288), (Figure A and B). Table 3 shows a propensity‐matched cohort of 126 COA patients and 126 patients in the control group. There was no difference in the prevalence of overall CAD (6.3% versus 5.6% versus P=0.742) and premature CAD (4.8% versus 3.2%, P=0.518) between the COA and control groups.
Figure 1.
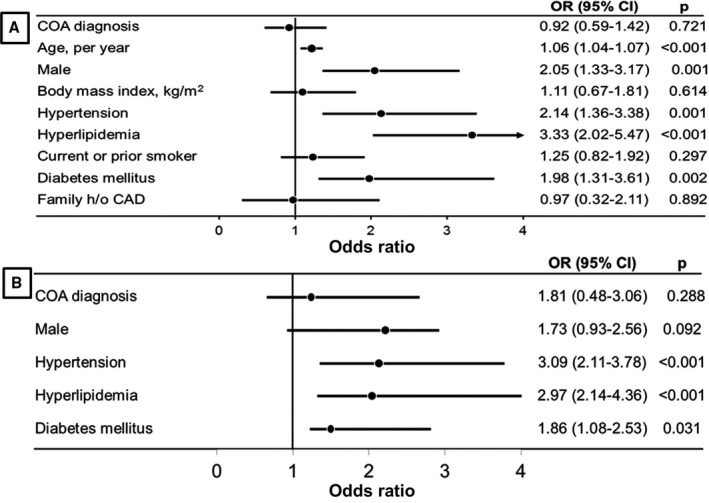
Forest plot showing multivariable risk factors for CAD (A) and premature CAD (B). CAD indicates coronary artery disease; COA, coarctation of aorta; h/o, history of; OR, odds ratio.
Table 3.
Propensity‐Matched Cohort
| COA (n=126) | Control (n=126) | P Value | |
|---|---|---|---|
| CAD | 8 (6.3%) | 7 (5.6) | 0.742 |
| Premature CAD | 6 (4.8%) | 4 (3.2%) | 0.518 |
| Age, y | 41±8 | 41±7 | 0.611 |
| Men | 66 (52%) | 66 (52%) | 0.999 |
| Body mass index, kg/m2 | 29±3 | 28±3 | 0.841 |
| Hypertension | 69 (55%) | 69 (55%) | 0.999 |
| Hyperlipidemia | 37 (29%) | 37 (29%) | 0.999 |
| Current or prior smoker | 28 (22%) | 24 (19%) | 0.387 |
| Diabetes mellitus | 11 (9%) | 11 (9%) | 0.999 |
CAD indicates coronary artery disease; COA, coarctation of aorta.
Of the 636 COA patients without CAD at the time of initial presentation, we had the necessary clinical variables for ASCVD risk calculation in 413 (65%) patients. Only 58 (14%) of these 413 patients would have qualified for statin therapy based on the risk prediction model. Of these 413 patients, there were 23 incident cases of CAD, and only 4 of the 23 patients would have qualified for statin therapy. The proportion of patients who qualified for statin therapy who developed CAD (4 of 23, 18%) was not different from those that did not develop CAD (54 of 390, 14%), P=0.634.
Discussion
Prevalence of CAD in COA
In this retrospective study of 654 patients with COA, we report an overall CAD prevalence of 7.8% and premature CAD prevalence of 4.4%. In a control group of patients with valvular pulmonic stenosis and tetralogy of Fallot, there was an overall CAD prevalence of 6.3% and premature CAD prevalence of 1.8%. There was no significant difference in overall CAD prevalence between patients with COA and the control group, although premature CAD appears to be more common in patients with COA. Several studies have reported reduced long‐term survival after COA repair, and premature CAD has been proposed as the underlying mechanism for early mortality in this population.7, 8, 15 In a longitudinal study of outcomes of 646 patients who underwent COA repair at Mayo Clinic before 1981, there were 87 late deaths at a mean age of 38 years, and 32 of these patients died from CAD‐related complications.7 In a different study of 274 patients who underwent COA repair before 1976, there were 45 late deaths at a mean age of 34 years, and CAD was also identified as the most common cause of death.8 Cokkinos et al studied 203 patients who underwent COA repair before 1979, and reported 66 late deaths of which 11% were attributable to premature CAD.15 These early studies became the foundation of the clinical paradigm of an association between COA and premature CAD. While these 3 studies and several other subsequent studies clearly demonstrated a high incidence of premature CAD and early mortality in COA patients, all the studies also reported high prevalence of ASVCD risk factors such as hypertension, hyperlipidemia, and male sex in these patients.5, 6, 7, 8
To the best of our knowledge, the only study that explored a direct relationship (or lack thereof) between COA and premature CAD was a retrospective study comparing CAD prevalence and risk factors between COA patients and patients with ventricular septal defect using data from the Quebec Congenital Heart Disease database.9 In that study, there was no difference in CAD prevalence among the COA patients (4.9%) and the patients with ventricular septal defect (3.5%) after adjustment for between‐group differences in ASCVD risk factors.9 These results are consistent with our current study which did not show any difference in the adjusted CAD prevalence between patients with COA and a control group of other congenital heart disease patients. The CAD prevalence of 7.8% in the current study is somewhat higher than the 4.9% reported by Roifman et al,9 but this is likely because of a higher prevalence of ASVCD risk factors in the current study.
Risk Factors for CAD in COA
There was no difference in the adjusted prevalence of overall CAD and premature CAD in the current study. Similar to the study by Roifman et al, ASCVD risk factors (such as hypertension, hyperlipidemia, diabetes mellitus, and male sex), and not COA diagnosis, were the predictors of overall CAD and premature CAD. The result of the current study contrasts with the early longitudinal studies that showed a disproportionately high prevalence of CAD in COA patients.7, 8, 15 While these studies also showed high prevalence of ASCVD risk factors in COA patients, none of the studies had a control group, and hence no rigorous analyses to determine if the observed CAD risk was truly because of COA diagnosis or because of the associated ASCVD risk factors. It is also important to highlight that all these studies were based on patients followed before the 1980s; at a time when medical therapy for ASCVD risk factor modification may not have been universally adopted.
Clinical Implications and Future Directions
A recent population‐based study using the Nationwide Inpatient Sample database showed that in comparison with the general population, the patients with COA had elective coronary revascularization and myocardial infarction at a much younger age than expected.16 There is no debate about incidence and clinical implications of premature CAD in the COA population. The primary message of this current study is that the excess burden of premature CAD in this population is not because of COA diagnosis (a non‐modifiable risk) but rather because of ASCVD risk factors, which are modifiable. The results of our study, and the prior study by Roifman et al, support a paradigm shift in the current approach for the management of COA patients. Premature CAD should no longer be viewed as an inevitable complication of COA diagnosis but as a preventable morbidity in this population.
The importance of hypertension in the pathogenies of ASCVD is well established, and the recent practice guidelines recommend a lower threshold for initiating anti‐hypertensive therapy because of the incremental mortality associated with even “low grade” hypertension.17, 18 Patients with COA have underlying vasculopathy and endothelial dysfunction which accounts for high prevalence of hypertension at rest and during exercise in these patients. In a recent study from our group, we reported that hypertensive response to exercise occurred in 19% of COA patients even in the setting of normal resting blood pressure and no residual aortic obstruction.12 In comparison with the COA patients with normal resting and exercise blood pressure, those with hypertensive response to exercise had more cardiovascular adverse events. The current guidelines for the management of adults with congenital heart disease consider the screening of exercise‐induced hypertension as a II‐b recommendation.19 Perhaps these new findings should prompt further investigations to determine if a more aggressive approach to management of hypertension in the COA population is warranted if the goal is to decrease the risk of premature CAD (a preventable complication).
Another clinical implication of the current study is the management of hyperlipidemia in COA patients. Based on the ASCVD risk calculator endorsed by the American College of Cardiology,14 86% of the patients including 82% of those that developed CAD within 10 years would not have qualified for statin therapy at the time of initial presentation. Since older age is a critical metric used in this calculator, and most COA patients develop CAD at a much younger age, and perhaps the ASCVD risk calculator may not capture the risk profile of this unique population. There is a need for further studies to delineate the pathobiology responsible for hyperlipidemia in COA patients, and also to develop new clinical risk indices that will provide optimal risk prediction to guide prophylactic therapy in these patients.
Limitations
This is a retrospective single center study and is therefore prone to referral and ascertainment bias. Although we performed rigorous analyses with propensity matching and logistic regression, the results of the study may be influenced by some other confounders not controlled for in our statistical models. We did not have data about patients’ adherence to medical therapy and how this could have influenced the observed outcomes. These limitations could affect generalizability of our results. Additionally, our analyses and results could have been influenced by small sample size, hence under‐powering the study to detect a statistical a significant difference.
Conclusions
The current study shows that the adjusted prevalence of overall CAD and premature CAD in patients with COA was 6.3% and 4.8%, respectively, and this was not significantly different from other congenital heart disease patients. After adjusting for atherosclerotic cardiovascular disease risk factors, we did not find evidence of a difference in CAD risk between the patients with COA and other patients with congenital heart disease.
Sources of Funding
Dr Egbe is supported by National Heart, Lung, and Blood Institute grant K23 HL141448‐01.
Disclosures
None.
Acknowledgments
We thank Michelle Herberts for her contribution during the data abstraction phase of the study.
(J Am Heart Assoc. 2019;8:e012056 DOI: 10.1161/JAHA.119.012056.)
References
- 1. Rosenthal E. Coarctation of the aorta from fetus to adult: curable condition or life long disease process? Heart. 2005;91:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krieger EV, Stout K. The adult with repaired coarctation of the aorta. Heart. 2010;96:1676–1681. [DOI] [PubMed] [Google Scholar]
- 3. Egbe A, Uppu S, Stroustrup A, Lee S, Ho D, Srivastava S. Incidences and sociodemographics of specific congenital heart diseases in the United States of America: an evaluation of hospital discharge diagnoses. Pediatr Cardiol. 2014;35:975–982. [DOI] [PubMed] [Google Scholar]
- 4. Brown ML, Burkhart HM, Connolly HM, Dearani JA, Hagler DJ, Schaff HV. Late outcomes of reintervention on the descending aorta after repair of aortic coarctation. Circulation. 2010;122:S81–S84. [DOI] [PubMed] [Google Scholar]
- 5. Brown ML, Burkhart HM, Connolly HM, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. [DOI] [PubMed] [Google Scholar]
- 6. Presbitero P, Demarie D, Villani M, Perinetto EA, Riva G, Orzan F, Bobbio M, Morea M, Brusca A. Long term results (15‐30 years) of surgical repair of aortic coarctation. Br Heart J. 1987;57:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long‐term follow‐up and prediction of outcome after surgical correction. Circulation. 1989;80:840–845. [DOI] [PubMed] [Google Scholar]
- 8. Toro‐Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long‐term follow‐up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89:541–547. [DOI] [PubMed] [Google Scholar]
- 9. Roifman I, Therrien J, Ionescu‐Ittu R, Pilote L, Guo L, Kotowycz MA, Martucci G, Marelli AJ. Coarctation of the aorta and coronary artery disease: fact or fiction? Circulation. 2012;126:16–21. [DOI] [PubMed] [Google Scholar]
- 10. Bokma JP, Winter MM, Kuijpers JM, Jongbloed MR, Duijnhouwer AL, Hoendermis ES, Sieswerda GT, Post MC, Mulder BJM, Bouma BJ. Role of acquired cardiovascular disease in tetralogy of Fallot patients >50 years of age. J Am Coll Cardiol. 2017;69:2465–2466. [DOI] [PubMed] [Google Scholar]
- 11. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV; American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions and Society of Thoracic Surgeons . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 12. Yogeswaran V, Connolly HM, Al‐Otaibi M, Ammash NM, Warnes CA, Said SM, Egbe AC. Prognostic role of hypertensive response to exercise in patients with repaired coarctation of aorta. Can J Cardiol. 2018;34:676–682. [DOI] [PubMed] [Google Scholar]
- 13. ACC/AHA . ASCVD Risk Estimator. Available at: http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/. Accessed November 11, 2018.
- 14. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cokkinos DV, Leachman RD, Cooley DA. Increased mortality rate from coronary artery disease following operation for coarctation of the aorta at a late age. J Thorac Cardiovasc Surg. 1979;77:315–318. [PubMed] [Google Scholar]
- 16. Pickard SS, Gauvreau K, Gurvitz M, Gagne JJ, Opotowsky AR, Jenkins KJ, Prakash A. A national population‐based study of adults with coronary artery disease and coarctation of the aorta. Am J Cardiol. 2018;122:2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 18. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 19. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e81–e192. [DOI] [PubMed] [Google Scholar]


