Abstract
Background
Acute complete occlusion of a coronary artery results in progressive ischemia, moving from the endocardium to the epicardium (ie, wavefront). Dependent on time to reperfusion and collateral flow, myocardial infarction (MI) will manifest, with transmural MI portending poor prognosis. Late gadolinium enhancement cardiac magnetic resonance imaging can detect MI with high diagnostic accuracy. Primary percutaneous coronary intervention is the preferred reperfusion strategy in patients with ST‐segment–elevation MI with <12 hours of symptom onset. We sought to visualize time‐dependent necrosis in a population with ST‐segment–elevation MI by using late gadolinium enhancement cardiac magnetic resonance imaging (STEMI‐SCAR project).
Methods and Results
ST‐segment–elevation MI patients with single‐vessel disease, complete occlusion with TIMI (Thrombolysis in Myocardial Infarction) score 0, absence of collateral flow (Rentrop score 0), and symptom onset <12 hours were consecutively enrolled. Using late gadolinium enhancement cardiac magnetic resonance imaging, the area at risk and infarct size, myocardial salvage index, transmurality index, and transmurality grade (0–50%, 51–75%, 76–100%) were determined. In total, 164 patients (aged 54±11 years, 80% male) were included. A receiver operating characteristic curve (area under the curve: 0.81) indicating transmural necrosis revealed the best diagnostic cutoff for a symptom‐to‐balloon time of 121 minutes: patients with >121 minutes demonstrated increased infarct size, transmurality index, and transmurality grade (all P<0.01) and decreased myocardial salvage index (P<0.001) versus patients with symptom‐to‐balloon times ≤121 minutes.
Conclusions
In MI with no residual antegrade and no collateral flow, immediate reperfusion is vital. A symptom‐to‐balloon time of >121 minutes causes a high grade of transmural necrosis. In this pure ST‐segment–elevation MI population, time to reperfusion to salvage myocardium was less than suggested by current guidelines.
Keywords: cardiac magnetic resonance imaging, coronary artery disease, ST‐segment–elevation myocardial infarction, necrosis
Subject Categories: Magnetic Resonance Imaging (MRI), Stenosis, Myocardial Infarction
Clinical Perspective
What Is New?
In patients with first‐time acute ST‐segment–elevation myocardial infarction presenting with complete occlusion of the culprit coronary artery and no coronary collaterals, cardiac magnetic resonance imaging visualized time‐dependent transmural infarction with a cutoff of 121 minutes, which seems much lower than reported for viable myocardium.
What Are the Clinical Implications?
This study highlights the therapeutic priority of reducing ischemic times of patients presenting with ST‐segment–elevation myocardial infarction during emergency care because in this pure ST‐segment–elevation myocardial infarction population, time to reperfusion to salvage myocardium was less than suggested by current guidelines.
Introduction
Ischemic heart disease is the most common cause of death worldwide.1 Despite major advances in both interventional medical treatment/secondary prevention, mortality and morbidity in patients with ST‐segment–elevation myocardial infarction (STEMI) remain substantial. Mortality is affected by various factors including Killip class, local emergency logistics, number of diseased coronary vessels, left ventricular (LV) ejection fraction, and time to treatment.1 In cases of acute and complete coronary occlusion, besides area at risk (AAR), protecting collateral flow, and ischemic preconditioning, time to reperfusion is the most important factor in the treatment of STEMI patients. The wavefront concept of myocardial infarction (MI) states that myocardial necrosis is a time‐dependent ischemic process beginning from the endocardial layers and moving up to the epicardium without essential lateral extension2: A prolonged occlusion of the infarct‐related artery (IRA) will result in a high amount of myocardial necrosis and a low amount of myocardial salvage. To limit myocardial necrosis of the myocardium supplied by the IRA, the AAR, blood flow to the IRA has to be restored as soon as possible.3 Without protecting collateral flow, sustained ischemia will result in progressive transmural expansion of the myocardial necrosis with the loss of myocardial salvage, portending adverse prognosis.4, 5, 6 Thus, current guidelines recommend primary percutaneous coronary intervention (PCI) as the preferred reperfusion strategy in patients with STEMI within 12 hours of symptom onset, provided it can be performed within 120 minutes from STEMI diagnosis (the time at which the ECG of a patient with ischemic symptoms is interpreted as presenting ST‐segment elevation).1 However, despite major advances in treatment and emergency medical care, a substantial number of STEMI patients have transmural infarction with adverse prognosis.7, 8, 9
Late gadolinium enhancement cardiac magnetic resonance imaging (LGE‐CMR) can determine ischemic scar with high diagnostic accuracy compared with histology.10, 11, 12
By visualizing the ischemic wavefront process, the AAR, infarct size (IS), and myocardial salvage are determined.11, 13, 14 Most CMR studies assessing the extent of MI in STEMI patients are performed in patients with a symptom‐to‐balloon time of >180 minutes.15 Furthermore, most studies also included initial TIMI (Thrombolysis in Myocardial Infarction) antegrade flow ≥1 and patients with visible retrograde collateral flow (Rentrop grade ≥1).15 However, both of the latter conditions mitigate the time‐dependent progress of the ischemic wavefront after total occlusion of a coronary artery.
We sought to investigate the time‐dependent ischemic wavefront process in a “pure” STEMI population lacking the mentioned limitations by using a dedicated LGE‐CMR protocol in STEMI patients with the following characteristics: (1) single‐vessel disease only, (2) complete absence of antegrade flow (TIMI 0), (3) complete absence of visible retrograde collateral flow (Rentrop grade 0), and (4) presentation within 12 hours of symptom onset.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Patient Population
Patients admitted with first‐time STEMI, no preinfarction angina, and no prior cardiac history treated with primary PCI at one of the 4 institutions (Stuttgart and Tübingen, Germany; Innsbruck, Austria; Bern, Switzerland) were eligible for study participation if they had STEMI according to the European Society of Cardiology/American College of Cardiology committee redefinition16 and had onset of symptoms <12 hours.
Patients with (1) initial antegrade flow of TIMI >0, (2) retrograde collateral flow of Rentrop >0, (3) multivessel disease (presence of >50% stenosis in >1 large epicardial coronary artery according to coronary angiography during primary PCI), (4) contraindications for CMR, (5) presence of silent MI in remote myocardium, or (6) poor CMR image quality were excluded (Figure 1. At each participating site, the respective institutional review board approved the research protocol, and informed consent was obtained from the participants. Since analyses were performed in STEMI patients, visualizing myocardial scar by CMR, the project was called STEMI‐SCAR.
Figure 1.
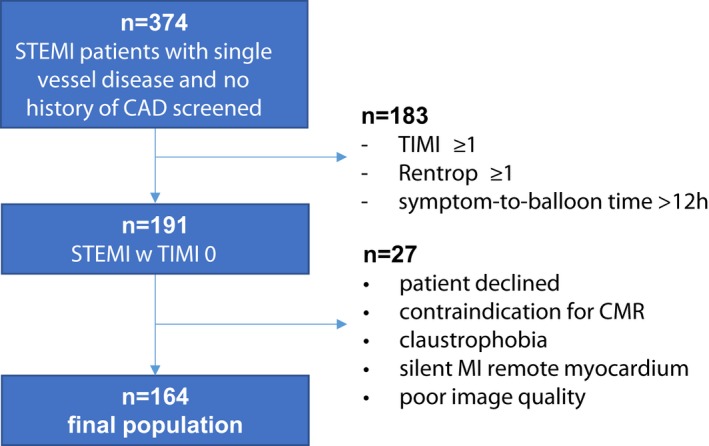
Flowchart demonstrating the study population. CAD indicates coronary arterial disease; CMR, cardiac magnetic resonance; MI, myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Primary PCI and Angiographic Analysis
Primary PCI was performed by experienced interventional cardiologists. Pre‐ and post‐PCI coronary angiographies were performed, with the same projections permitting offline evaluation of (1) TIMI flow before and after PCI (range: 0–3), (2) the Rentrop collateral grade (range: 0–3), and (3) the myocardial blush grade (range: 0–3) at the final angiogram by 2 independent interventional cardiologists (S.G., T.S.). Symptom‐to‐balloon times (ie, time from onset of symptoms to first balloon inflation) were precisely reported for each patient.
CMR Protocol
ECG‐gated CMR was performed in breath hold using a 1.5‐T Magnetom Symphony, Magnetom Sonata, Magnetom Avanto, or Magnetom Aera (Siemens Healthcare) in line with Society for Cardiovascular Magnetic Resonance recommendations.12 Both cine and LGE short‐axis CMR images were prescribed every 10 mm (slice thickness: 6 mm) from base to apex. Cine CMR was performed using a steady‐state free‐precession sequence. LGE images were acquired on average 10 minutes after contrast administration, using segmented inversion recovery gradient echo constantly adjusting inversion time. The contrast dose (gadodiamide, gadopentetate dimeglumine, or gadobutrol) was 0.15 mmol/kg.
CMR Analysis
Image postprocessing was performed using dedicated software (Argus [Siemens] and Mass [Medis], respectively). Cine and contrast images were evaluated by experienced observers (A.M., S.B., A.S., S.G.), as described elsewhere,17 and any differences were resolved by consensus. In brief, endocardial and epicardial borders were outlined on the short‐axis cine images. Volumes and LV ejection fractions were derived by summation of epicardial and endocardial contours. LV mass was calculated by subtracting endocardial from epicardial volume at end‐diastole and multiplying by 1.05 g/cm3. AAR was drawn in LGE images indicating MI, as previously described.11 AAR, IS, and microvascular obstruction (MVO) were assessed using the Mass analysis software, and the results were expressed as percentage of myocardial LV mass. LGE was defined of an image intensity level ≥2 SD above the mean of remote myocardium.18 MVO was defined as a persisting area of hypoenhancement within regions of LGE.19 Furthermore, myocardial salvage index (MSI), equal to (AAR−IS)/AAR×100, and transmurality index (100−MSI) were derived. In addition, infarct transmurality was graded visually by quarters: <25%, 25% to 50%, 51% to 75%, and 76% to 100% indicating transmural infarction.8, 20 Analyses were made blinded to clinical and angiographic parameters.
Statistical Analysis
Absolute numbers and percentages were computed to describe the patient population. Categorical variables were expressed as counts and percentages. Continuous variables were expressed as mean±SD or median (interquartile range), as appropriate. Variables were tested for normal distribution applying the D'Agostino–Pearson normality test. Comparisons between groups were made using the Student t test for normally distributed variables and the Mann‐Whitney U test for nonnormal distribution. Dichotomous variables were compared using the Fisher exact test. P<0.05 (2‐tailed) was considered significant. The Youden index was used to depict the optimal cutoff value from the receiver operating characteristic curve. Statistical analyses were performed using GraphPad software or SPSS 22.0 (IBM Corp).
Results
Patient Characteristics
Of the 374 screened patients, 164 were included in the final study cohort (Figure 1. Patients were aged 53.7±10.8 years and predominantly male (80%); median symptom‐to‐balloon time was 133 minutes (Table 1. The left anterior descending artery was the most frequent IRA. TIMI 3 flow after PCI was achieved in >90% of patients.
Table 1.
Baseline Characteristics
| Entire Group | ≤121 min | >121 min | P Value | |
|---|---|---|---|---|
| (n=164) | (n=64) | (n=100) | ||
| Age, y | 53.7±10.8 | 53.8±10.0 | 53.5±11.3 | 0.70 |
| Sex (female) | 33 (20) | 16 (25) | 17 (17) | 0.22 |
| Symptom‐to‐balloon, min | 133 (103–196) | 95 (77–110) | 180 (137–249) | <0.001a |
| CAD risk factors | ||||
| Diabetes mellitus | 17 (10) | 5 (8) | 12 (12) | 0.48 |
| Hypertension | 74 (45) | 33 (52) | 41 (41) | 0.31 |
| Smokingb | 48 (29) | 14 (22) | 34 (34) | 0.11 |
| Hyperlipidemia | 70 (43) | 34 (53) | 36 (36) | 0.04a |
| Family history of CVD | 64 (39) | 28 (44) | 36 (36) | 0.33 |
| BMI, kg/m2 | 27±4.5 | 27.2±3.8 | 26.8±4.9 | 0.40 |
| Hemodynamics | ||||
| Systolic BP, mm Hg | 130±26 | 130±27 | 130±26 | 0.79 |
| Diastolic BP, mm Hg | 81±16 | 83±16 | 80±16 | 0.13 |
| Medication | ||||
| Statins | 28 (17) | 9 (14) | 19 (19) | 0.52 |
| β‐Blockers | 33 (20) | 14 (22) | 19 (19) | 0.69 |
| Aspirin | 28 (17) | 10 (16) | 18 (18) | 0.83 |
| Clopidogrel | 7 (4) | 4 (6) | 3 (3) | 0.43 |
| ARB | 31 (19) | 10 (16) | 21 (21) | 0.42 |
| Biomarkers | ||||
| CK | 2668 (1431–4950) | 2210 (1227–3509) | 2912 (1606–5467) | 0.02a |
| Coronary angiography | ||||
| LAD | 100 (61) | 34 (53) | 66 (66) | 0.10 |
| RCA | 39 (24) | 20 (31) | 19 (19) | 0.09 |
| RCX | 25 (15) | 10 (16) | 15 (15) | 1.0 |
| DES | 144 (88) | 54 (84) | 90 (90) | 0.33 |
| Number of stents | 1.3±0.6 | 1.3±0.6 | 1.3±0.6 | 0.85 |
| Femoral approach | 139 (85) | 54 (84) | 85 (85) | 0.98 |
| TIMI 1 after PCI | 1 (1) | ··· | 1 (1) | ··· |
| TIMI 2 after PCI | 14 (9) | 5 (8) | 9 (9) | 1.0 |
| TIMI 3 after PCI | 149 (91) | 59 (92) | 90 (90) | 0.78 |
| MBG 0–1 | 23 (14) | 10 (16) | 13 (13) | 0.65 |
| MBG 2 | 49 (30) | 20 (31) | 29 (29) | 0.86 |
| MBG 3 | 92 (56) | 34 (53) | 58 (58) | 0.63 |
Values are n (%), mean±SD, or median (interquartile range). ARB indicates angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CK, creatine kinase; CVD, cardiovascular disease; DES, drug‐eluting stent; LAD, left anterior descending; MBG, myocardial blush grade; PCI, percutaneous coronary intervention; RCA, right coronary artery; RCX, right circumflex artery; TIMI, Thrombolysis in Myocardial Infarction
Statistically significant P‐values (P<0.05).
Current smokers.
CMR Characteristics
CMR was performed after a median of 4 days after STEMI. LV ejection fraction was moderately reduced (50±10%). Patients demonstrated a low MSI (32.4±18.2) and, consequently, a high transmurality index (67.6±18.2), which increased further by prolonged symptom‐to‐balloon time (Figure 2. Among the 12 patients (7%) demonstrating a transmurality grade <50%, 3 patients (2%) showed no myocardial scar at all. Among those, the mean symptom‐to‐balloon time was 56±15 minutes, whereas the mean symptom‐to‐balloon time for all 12 patients demonstrating a transmurality grade <50% was 77±29 minutes. Conversely, the majority of patients (n=137; 84%) showed a high transmurality grade (76–100%) for the AAR. Moreover, 64% of the patients demonstrated MVO, indicating severe myocardial injury (Table 2.
Figure 2.
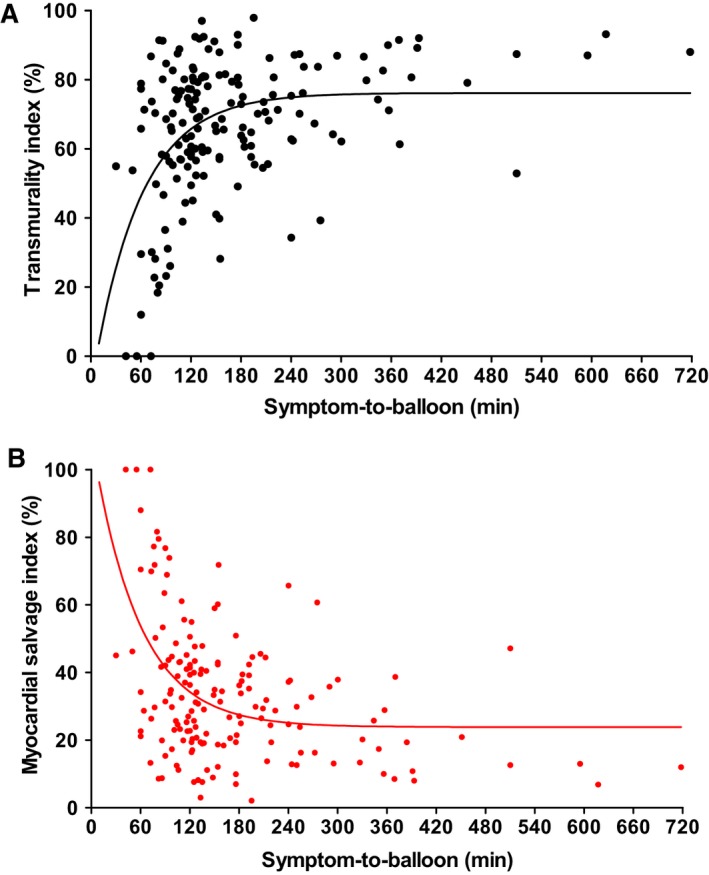
Transmurality index and myocardial salvage index: time‐dependent parameters. A, Transmurality index increased, with longer symptom‐to‐balloon time (minutes) mirroring the ischemic wavefront. B, In contrast, the myocardial salvage index decreased, with longer symptom‐to‐balloon time (minutes) indicating progressive loss of myocardial salvage.
Table 2.
CMR characteristics
| Entire Group | ≤121 min | >121 min | P Value | |
|---|---|---|---|---|
| (n=164) | (n=64) | (n=100) | ||
| Days to CMR | 4 (2–6) | 4 (2–5) | 4 (2–8) | 0.82 |
| LVEF, % | 50±10 | 51±10 | 49±10 | 0.24 |
| LVEDV, mL | 159±48 | 157±45 | 160±51 | 0.56 |
| LVESV, mL | 84±39 | 79±34 | 88±42 | 0.15 |
| LVSV, mL | 78±21 | 80±21 | 77±20 | 0.50 |
| LV mass, g | 130.7±31.8 | 130.1±35.5 | 131.9±30.2 | 0.75 |
| AAR mass, g | 48.4±25.8 | 44.6±27.8 | 50.8±25.1 | 0.10 |
| Area at risk, % | 36.9±17.7 | 34.0±19.4 | 38.4±16.7 | 0.17 |
| Infarct mass, g | 33.0±22.0 | 27.1±23.2 | 37.1±20.5 | 0.001a |
| Infarct size, % | 26.0±16.0 | 20.8±16.9 | 29.2±14.7 | 0.003a |
| MSI, % | 32.4±18.2 | 39.4±20.0 | 27.7±14.0 | <0.001a |
| Transmurality index, % | 67.6±18.2 | 60.6±20.0 | 72.3±14.0 | <0.001a |
| Transmurality | ||||
| 0–50% | 12 (7) | 12 (19) | ··· | ··· |
| 51–75% | 15 (9) | 11 (17) | 4 (4) | 0.01a |
| 76–100% | 137 (84) | 41 (64) | 96 (96) | <0.001a |
| MVO | ||||
| Patients | 105 (64) | 34 (53) | 71 (71) | 0.03a |
| Mass, g | 3.9±6.2 | 3.4±6.5 | 4.3±6.0 | 0.04a |
| % LV mass | 3.2±4.5 | 2.8±4.7 | 3.4±4.4 | 0.09 |
| % Infarct | 9.2±10.9 | 9.1±12.8 | 9.3±9.5 | 0.20 |
Values are n (%), mean±SD, or median (interquartile range). AAR indicates area at risk; CMR, cardiac magnetic resonance; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVSV, left ventricular stroke volume; MSI, myocardial salvage index; MVO, microvascular obstruction.
Statistically significant P‐values (P<0.05).
Symptom‐to‐Balloon Time Cutoff for Imminent Transmural Myocardial Necrosis
The receiver operating characteristic curve (area under the curve: 0.81) indicating transmural myocardial necrosis revealed the best diagnostic cutoff with a symptom‐to‐balloon time of 121 minutes (Figure 3.
Figure 3.
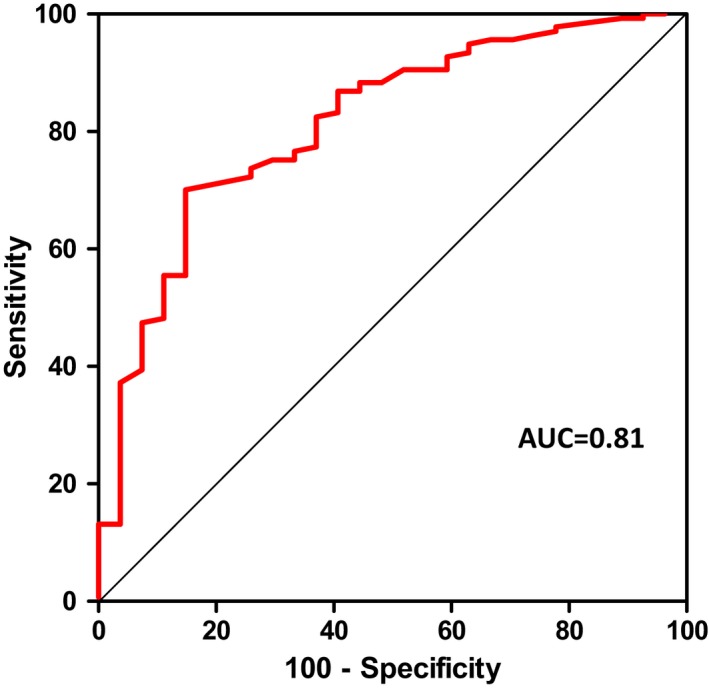
ROC curve: cutoff of 121 minutes. ROC curve (AUC: 0.81; 95% CI, 0.73–0.90; P<0.0001) indicating transmural myocardial necrosis revealed the best diagnostic cutoff for a symptom‐to‐balloon time of 121 minutes. AUC indicates area under the curve; ROC, Receiver operating characteristic.
Dichotomous interpretation identified 64 patients with a symptom‐to‐balloon time of ≤121 minutes (median: 95minutes) and 100 with a symptom‐to‐balloon time of >121 minutes (median 180 minutes; P<0.001). Beside the symptom‐to‐balloon time, hyperlipidemia, and the serum creatine kinase level (P=0.02), both groups showed similar baseline characteristics (Table 1. However, CMR in patients with a symptom‐to‐balloon time >121 minutes revealed a larger infarct mass and IS, a higher grade of transmural infarction (ie, a higher transmurality index), and a lower MSI compared with patients with a symptom‐to‐balloon time ≤121 minutes (all P≤0.01; Table 2. Consequently, in patients with a symptom‐to‐balloon time >121 minutes, no patient showed a transmurality grade of <50% compared with 12 (19%) patients in the group with a symptom‐to‐balloon time of ≤121 minutes (Figure 4. The relationship between symptom‐to‐balloon time and the occurrence of transmural necrosis is illustrated in Figure 5. Plausibly, MVO was more frequent in patients with a symptom‐to‐balloon time >121 minutes (P=0.03; Figure 6.
Figure 4.
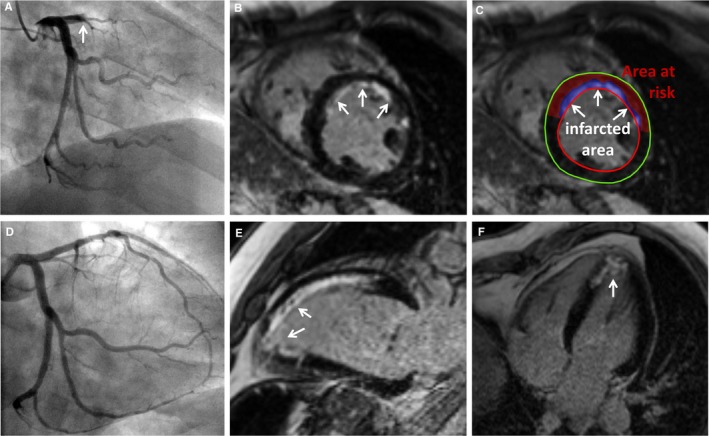
Symptom‐to‐balloon time of 106 minutes: subendocardial necrosis. A 46‐year‐old man presenting with chest pain and ST‐segment elevation in the anterior leads. Coronary angiography revealed TIMI 0 occlusion of the LAD (A, arrow); primary PCI with stent implant was performed, resulting in TIMI 3 flow after PCI (D). Symptom‐to‐balloon time was 106 minutes. CMR revealed a preserved global left ventricular ejection fraction (56%) with mild hypokinesia in the anteroseptal and apical wall. Late gadolinium enhancement CMR images revealed subendocardial necrosis (arrows) in the anteroseptal wall (B, C, E) and the apex (E, F), consistent with the region supplied by the infarct‐related artery (LAD). CMR indicates cardiac magnetic resonance; LAD, left anterior descending; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Figure 5.
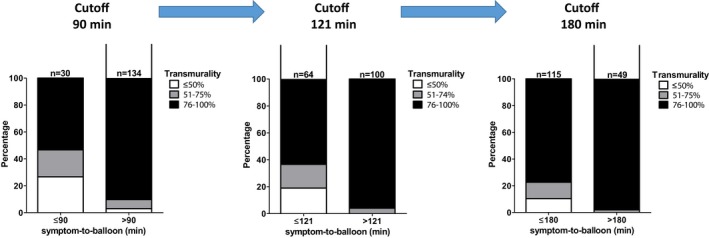
Grade of transmurality at different symptom‐to‐balloon times. The left bar (cutoff: 90‐minute symptom‐to‐balloon time) demonstrates a significant percentage of subendocardial MI at ≤90 minutes of symptom‐to‐balloon time. The middle bar (cutoff: 121 minutes) demonstrates a smaller but still substantial percentage of subendocardial MI ≤121 minutes. The right bar (cutoff: 180 minutes) shows further reduction in the percentage of subendocardial MI ≤180 minutes. MI indicates myocardial infarction.
Figure 6.
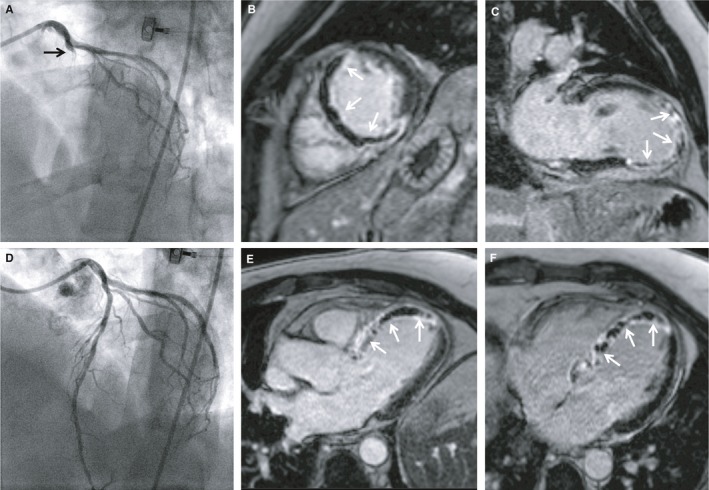
Symptom‐to‐balloon time of 141 minutes: transmural necrosis and extensive MVO. A 48‐year‐old man presenting with chest pain and ST‐segment elevation in the anterior leads. Coronary angiography revealed TIMI 0 occlusion of the LAD (A); primary PCI with stent implantation was performed, resulting in TIMI 3 flow after PCI (D). Symptom‐to‐balloon time was 141 minutes. CMR revealed impaired left ventricular ejection fraction (38%) with akinesia in the anteroseptal, septal, and apical wall. Late gadolinium enhancement CMR images revealed extensive transmural necrosis with concomitant MVO (arrows) in the anteroseptal and septal wall (B, C, E) and the apex (E, F), consistent with the region supplied by the infarct‐related artery (large LAD). CMR indicates cardiac magnetic resonance; LAD, left anterior descending; MVO, microvascular obstruction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Discussion
This study is the first to evaluate the time‐dependent progress of myocardial necrosis in a relatively pure model of STEMI in patients with acute total occlusion of the IRA with completely abolished antegrade flow (TIMI 0) and without mitigation by visible retrograde collateral flow (Rentrop 0). Current guidelines recommend primary PCI as the preferred reperfusion strategy in STEMI patients within 12 hours of symptom onset (class 1 indication), and an absolute time from STEMI diagnosis to mechanical reperfusion within 120 minutes.1 However, in our more strictly defined STEMI cohort, a symptom‐to‐balloon time >121 minutes caused a high grade of transmural infarction (and low MSI), underscoring the paramount importance of the symptom‐to‐balloon time in the wavefront of myocardial ischemia in patients not protected by a certain extent of residual myocardial blood flow from ante‐ or retrograde.
Patient Characteristics
Consistent with previous findings from STEMI registries, patients were relatively young and predominantly male1, 21; median symptom‐to‐balloon time was 133 minutes, which was much shorter than in other STEMI trials.15 TIMI 3 after PCI could be achieved in >90% of the patients, similar to previous STEMI studies.22
CMR Characteristics
LV ejection fraction was moderately reduced (50±10%), in line with previous data.23, 24 Overall, patients demonstrated low MSI (32±18%) and a consistently high transmurality index (68±18%), which proceeds at sustained occlusion (longer symptom‐to‐balloon time; Figure 2. Time dependency of transmurality is further illustrated in the small subset of patients with <50% transmurality: Of these, mean symptom‐to‐balloon time increased from 56±15 minutes in the 3 patients (2%) with no necrosis to 77±29 minutes for all 12 patients (7%), demonstrating a degree of transmurality <50%. In this relatively pure model of STEMI, these remarkably short time intervals underscore the need for urgent restoration of flow in the IRA to prevent transmural wavefront expansion. However, even in this patient cohort with relatively short symptom‐to‐balloon times, the vast majority (n=137; 84%) had a high transmurality grade (76–100%). Consequently, the current recommendation of reperfusion in STEMI patients presenting within 12 hours of symptom onset and 120 minutes from the STEMI diagnosis to primary PCI 1 may apply only to a mixed all‐comers STEMI population with partly residual antegrade‐ and retrograde collateral flow slowing the ischemic wavefront to a certain extent.
Symptom‐to‐Balloon Time of 121 Minutes: The Cutoff for Imminent Transmural Necrosis
Receiver operating characteristic curve analysis (area under the curve: 0.81) indicating transmural myocardial necrosis revealed the best diagnostic cutoff for a symptom‐to‐balloon time of 121 minutes (Figure 3. CMR revealed larger infarct mass and IS, higher grade of transmural infarction, and increased transmurality index and lower MSI in the subgroup with a symptom‐to‐balloon time >121 minutes versus patients with a symptom‐to‐balloon time ≤121 minutes (all P≤0.01; Table 2. Although previous studies support the finding that late PCI resulted in larger IS and smaller MSI,23, 25 it is important to note that the IS is rather determined by the IRA; for example, in the AAR of an occluded left circumflex artery, IS may be rather small despite a high transmurality index. Conversely, rapid recanalization of an occluded large left anterior descending artery (with a large AAR) may reveal a relatively large IS despite a relatively low transmurality index and high MSI, respectively.
A meta‐analysis of 2632 STEMI patients demonstrated that IS measured by CMR or single‐photon emission computed tomography early after PCI was strongly associated with 1‐year hospitalization for heart failure and all‐cause mortality,9 underscoring the high prognostic value of a distinct evaluation of the IS in STEMI patients. In addition, LGE‐CMR–derived MSI is considered a more sensitive measure than IS alone because it normalizes the IS to the AAR, which shows tremendous interindividual variation.26
Beek et al demonstrated that the transmural extent is relevant in predicting myocardial functional recovery: The likelihood of complete functional recovery of segments without LGE was 3.8, 11.1, and 50 times higher than that of segments with 26% to 50%, 51% to 75%, and >75% LGE, respectively (P<0.001).27 Other studies demonstrated a significant association with the extent of ischemic LGE and mortality in patients with coronary arterial disease.7, 8, 9
The link between symptom‐to‐balloon time and the occurrence of transmural necrosis can be viewed in Figure 5. After a 180‐minute cutoff of symptom‐to‐balloon time, most patients had irreversible transmural necrosis. Corroborating this result, a pooled analysis revealed that IS was large when the first balloon inflation was performed >180 minutes from symptom onset, with little impact of further delays beyond 180 minutes, suggesting a kind of plateau (or “flat” part) of the transmurality index and MSI curves.25 However, few patients demonstrate substantial myocardial salvage in stages at >180 minutes of symptom‐to‐balloon time, suggesting some other potential mechanisms to salvage myocardium (Figure 2.
Successful recanalization of the IRA per se does not guarantee the normalization of myocardial perfusion which is impaired in 30% to 75% of patients due to MVO of various extents.28 MVO refers to the inability to reperfuse the intramyocardial microcirculation in an ischemic region despite restoring flow in the epicardial vessel and is accurately displayed by LGE‐CMR.11 These patients have a higher prevalence of early postinfarct complications, adverse LV remodeling, recurrent hospitalizations for heart failure, and mortality.15, 24, 29, 30, 31 The high prevalence of MVO in our population (64%) is similar to those in other reports showing rates of MVO up to 60% in patients receiving primary PCI for acute STEMI (Table 2.24, 32 MVO was more common in patients with longer symptom‐to‐balloon time (Figure 6), which is consistent with previous data in STEMI patients.33, 34
Aside from major logistic advancements (eg, preannounced STEMI, direct handoff from ambulances to catheterization laboratories), improved patient awareness may play a crucial role in the reduction of the symptom‐to‐balloon time. A prospective multicenter study of STEMI patients revealed time intervals from onset of symptoms to first medical contact far beyond the symptom‐to‐balloon time that seemed to be desirable in STEMI patients as suggested by this study.35
Limitations
Although the wavefront concept of myocardial ischemia is well accepted, there might be some lateral extent of MI, leading to a certain inaccuracy in the definition of the AAR (and its components). However, these potential inaccuracies might be negligible.14
Myocardial blush grade did not differ significantly between patients with a symptom‐to‐balloon time >121 versus ≤121 minutes, but CMR‐derived MVO was more common in patients with a symptom‐to‐balloon time >121 minutes (P=0.03). Symptom‐to‐balloon time does not necessarily imply time to reperfusion because in some cases TIMI 3 flow cannot be achieved or, very rarely, the myocardial blush grade is 0/1. Nevertheless, neither TIMI flow after PCI nor myocardial blush grade was significantly different between groups (Table 1.
T2‐weighted images were not consistently performed. Those images were used in some studies to delineate the AAR.36, 37 However, image quality is often impaired and prone to artifacts, and T2‐weighted images are problematic on many levels.11, 38 Moreover, post‐MI edema in patients is not stable but follows a bimodal pattern that affects CMR estimates of the AAR by T2‐weighted images.39 Furthermore, Kim et al clearly demonstrated that T2‐weighted CMR does not depict the AAR.14
Although coronary angiography is a commonly used method for evaluation of collateral circulation, and visible retrograde collateral flow was systematically excluded from the present study, smaller, invisible, but functionally present and beneficial collateral vessels may have been missed.40, 41
CMR mapping sequences were not performed. However, despite the promising results of T1 mapping in STEMI for measuring IS without the use of contrast agent, studies have been small and from single centers.42, 43 Larger multicenter studies are required to investigate the clinical value of mapping techniques before they can be more widely adopted.
Conclusions
In MI not mitigated by either residual antegrade flow through incomplete coronary occlusion or visible retrograde collateral flow, immediate reperfusion is vital. A symptom‐to‐balloon time >121 minutes causes a high grade of transmural necrosis. In this pure STEMI population, time to reperfusion to salvage myocardium was less than suggested by current guidelines.
Sources of Funding
This work was funded in part by the Robert Bosch Foundation: KKF 13‐2, KKF 15‐5, and KKF 770. This project was supported by the Deutsche Forschungsgemeinschaft (Klinische Forschungsgruppe; KFO‐274: “Platelets‐Molecular Mechanisms and Translational Implications”) and by the Deutsche Forschungsgemeinschaft (German Research Foundation; no. 374031971–TRR 240).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012429 DOI: 10.1161/JAHA.119.012429.)
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 2. Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–644. [PubMed] [Google Scholar]
- 3. Seiler C, Kirkeeide RL, Gould KL. Measurement from arteriograms of regional myocardial bed size distal to any point in the coronary vascular tree for assessing anatomic area at risk. J Am Coll Cardiol. 1993;21:783–797. [DOI] [PubMed] [Google Scholar]
- 4. Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293:979–986. [DOI] [PubMed] [Google Scholar]
- 5. Eitel I, Desch S, de Waha S, Fuernau G, Gutberlet M, Schuler G, Thiele H. Long‐term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038–2045. [DOI] [PubMed] [Google Scholar]
- 6. Bodi V, Sanchis J, Nunez J, Mainar L, Lopez‐Lereu MP, Monmeneu JV, Rumiz E, Chaustre F, Trapero I, Husser O, Forteza MJ, Chorro FJ, Llacer A. Prognostic value of a comprehensive cardiac magnetic resonance assessment soon after a first ST‐segment elevation myocardial infarction. JACC Cardiovasc Imaging. 2009;2:835–842. [DOI] [PubMed] [Google Scholar]
- 7. Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ. Comparison of myocardial infarct size assessed with contrast‐enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. [DOI] [PubMed] [Google Scholar]
- 8. Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed‐enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. [DOI] [PubMed] [Google Scholar]
- 9. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben‐Yehuda O. Relationship between infarct size and outcomes following primary PCI: patient‐level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67:1674–1683. [DOI] [PubMed] [Google Scholar]
- 10. Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. [DOI] [PubMed] [Google Scholar]
- 11. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST‐segment‐elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137:1949–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortiz‐Pérez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. [DOI] [PubMed] [Google Scholar]
- 14. Kim HW, Van Assche L, Jennings RB, Wince WB, Jensen CJ, Rehwald WG, Wendell DC, Bhatti L, Spatz DM, Parker MA, Jenista ER, Klem I, Crowley AL, Chen EL, Judd RM, Kim RJ. Relationship of T2‐weighted MRI myocardial hyperintensity and the ischemic area‐at‐risk. Circ Res. 2015;117:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben‐Yehuda O, Jenkins P, Thiele H, Stone GW. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 2017;38:3502–3510. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction , Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 17. Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast‐enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. [DOI] [PubMed] [Google Scholar]
- 18. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 19. Wu KC, Kim RJ, Bluemke DA, Rochitte CE, Zerhouni EA, Becker LC, Lima JA. Quantification and time course of microvascular obstruction by contrast‐enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756–1764. [DOI] [PubMed] [Google Scholar]
- 20. Yokota H, Heidary S, Katikireddy CK, Nguyen P, Pauly JM, McConnell MV, Yang PC. Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khera S, Kolte D, Gupta T, Subramanian KS, Khanna N, Aronow WS, Ahn C, Timmermans RJ, Cooper HA, Fonarow GC, Frishman WH, Panza JA, Bhatt DL. Temporal trends and sex differences in revascularization and outcomes of ST‐segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol. 2015;66:1961–1972. [DOI] [PubMed] [Google Scholar]
- 22. Kosmidou I, Redfors B, Selker HP, Thiele H, Patel MR, Udelson JE, Magnus Ohman E, Eitel I, Granger CB, Maehara A, Kirtane A, Généreux P, Jenkins PL, Ben‐Yehuda O, Mintz GS, Stone GW. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: results from an individual patient‐level pooled analysis of 10 randomized trials. Eur Heart J. 2017;38:1656–1663. [DOI] [PubMed] [Google Scholar]
- 23. Stiermaier T, Eitel I, de Waha S, Pöss J, Fuernau G, Thiele H, Desch S. Myocardial salvage after primary percutaneous coronary intervention in patients with ST‐elevation myocardial infarction presenting early versus late after symptom onset. Int J Cardiovasc Imaging. 2017;33:1571–1579. [DOI] [PubMed] [Google Scholar]
- 24. van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930–939. [DOI] [PubMed] [Google Scholar]
- 25. Stone GW, Dixon SR, Grines CL, Cox DA, Webb JG, Brodie BR, Griffin JJ, Martin JL, Fahy M, Mehran R, Miller TD, Gibbons RJ, O'Neill WW. Predictors of infarct size after primary coronary angioplasty in acute myocardial infarction from pooled analysis from four contemporary trials. Am J Cardiol. 2007;100:1370–1375. [DOI] [PubMed] [Google Scholar]
- 26. Bøtker HE, Kaltoft AK, Pedersen SF, Kim WY. Measuring myocardial salvage. Cardiovasc Res. 2012;94:266–275. [DOI] [PubMed] [Google Scholar]
- 27. Beek AM, Kühl HP, Bondarenko O, Twisk JW, Hofman MB, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast‐enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895–901. [DOI] [PubMed] [Google Scholar]
- 28. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no‐reflow in humans. J Am Coll Cardiol. 2009;54:281–292. [DOI] [PubMed] [Google Scholar]
- 29. Wu KC, Zerhouni EA, Judd RM, Lugo‐Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 97:765–772. [DOI] [PubMed] [Google Scholar]
- 30. Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodeling and long‐term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. [DOI] [PubMed] [Google Scholar]
- 31. de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long‐term outcome after ST‐elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–2668. [DOI] [PubMed] [Google Scholar]
- 32. Feistritzer HJ, Reinstadler SJ, Klug G, Reindl M, Wöhrer S, Brenner C, Mayr A, Mair J, Metzler B. Multimarker approach for the prediction of microvascular obstruction after acute ST‐segment elevation myocardial infarction: a prospective, observational study. BMC Cardiovasc Disord. 2016;16:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tarantini G, Cacciavillani L, Corbetti F, Ramondo A, Marra MP, Bacchiega E, Napodano M, Bilato C, Razzolini R, Iliceto S. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast‐enhanced magnetic resonance. J Am Coll Cardiol. 2005;46:1229–1235. [DOI] [PubMed] [Google Scholar]
- 34. Francone M, Bucciarelli‐Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F, Passariello R, Bogaert J, Agati L. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST‐segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:2145–2153. [DOI] [PubMed] [Google Scholar]
- 35. Scholz KH, Maier SK, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ahlersmann D, Keating FK, Jacobshagen C, Moehlis H, Hilgers R, Maier LS. Reduction in treatment times through formalized data feedback: results from a prospective multicenter study of ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2012;5:848–857. [DOI] [PubMed] [Google Scholar]
- 36. Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hadamitzky M, Langhans B, Hausleiter J, Sonne C, Kastrati A, Martinoff S, Schömig A, Ibrahim T. The assessment of area at risk and myocardial salvage after coronary revascularization in acute myocardial infarction: comparison between CMR and SPECT. JACC Cardiovasc Imaging. 2013;6:358–369. [DOI] [PubMed] [Google Scholar]
- 38. Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular MR imaging: T2‐weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265:12–22. [DOI] [PubMed] [Google Scholar]
- 39. Fernández‐Jiménez R, Barreiro‐Pérez M, Martin‐García A, Sánchez‐González J, Agüero J, Galán‐Arriola C, García‐Prieto J, Díaz‐Pelaez E, Vara P, Martinez I, Zamarro I, Garde B, Sanz J, Fuster V, Sánchez PL, Ibanez B. Dynamic edematous response of the human heart to myocardial infarction: implications for assessing myocardial area at risk and salvage. Circulation. 2017;136:1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seiler C, Engler R, Berner L, Stoller M, Meier P, Steck H, Traupe T. Prognostic relevance of coronary collateral function: confounded or causal relationship? Heart. 2013;99:1408–1414. [DOI] [PubMed] [Google Scholar]
- 41. Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–2220. [DOI] [PubMed] [Google Scholar]
- 42. Bulluck H, Hammond‐Haley M, Fontana M, Knight DS, Sirker A, Herrey AS, Manisty C, Kellman P, Moon JC, Hausenloy DJ. Quantification of both the area‐at‐risk and acute myocardial infarct size in ST‐segment elevation myocardial infarction using T1‐mapping. J Cardiovasc Magn Reson. 2017;19:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu D, Borlotti A, Viliani D, Jerosch‐Herold M, Alkhalil M, De Maria GL, Fahrni G, Dawkins S, Wijesurendra R, Francis J, Ferreira V, Piechnik S, Robson MD, Banning A, Choudhury R, Neubauer S, Channon K, Kharbanda R, Dall'Armellina E. CMR native T1 mapping allows differentiation of reversible versus irreversible myocardial damage in ST‐segment‐elevation myocardial infarction: an OxAMI Study (Oxford Acute Myocardial Infarction). Circ Cardiovasc Imaging. 2017;10:e005986. [DOI] [PMC free article] [PubMed] [Google Scholar]


