Abstract
Background
While there is some evidence of elevated blood pressure later in life in preterm survivors, data on adult women are still lacking. Thus, we assessed the associations between preterm birth and blood pressure in young adult women.
Methods and Results
We studied 5232 young adult women who volunteered for military service in Sweden between 1990 and 2007. Anthropometric and clinic blood pressure data were collected during the medical examination at the time of conscription. There was a progressive decline in systolic and diastolic blood pressures, as well as in mean arterial pressure, with increasing gestational age. Women born preterm had an adjusted increase in systolic blood pressure of 3.8 mm Hg (95% CI, 2.5–5.1; P<0.0001) and mean arterial pressure of 1.9 mm Hg (95% CI, 0.9–2.8; P=0.0001) compared with young women born at term. Rates of systolic hypertension were also considerably higher in young women born preterm (14.0% versus 8.1%, P<0.0001), as were rates of isolated systolic hypertension. The adjusted relative risk of systolic hypertension in women born preterm was 1.72 (95% CI, 1.26–2.34; P<0.001) that of women born at term or post‐term, but there was no significant difference in the risk of diastolic hypertension (adjusted relative risk, 1.60; 95% CI, 0.49–5.20).
Conclusions
Young adult women born preterm display elevated systolic blood pressure and an increased risk of hypertension compared with peers born at term or post‐term.
Keywords: high blood pressure, hypertension, prematurity, preterm birth, systolic blood pressure, women, young adult
Subject Categories: Cardiovascular Disease, Epidemiology, Pediatrics, Risk Factors, Women
Short abstract
See Editorial Jones et al
Clinical Perspective
What Is New?
Decreasing gestational age was associated with a progressive increase in blood pressure among young adult women.
At ≈18 years of age, women born preterm already displayed an increased risk of systolic hypertension compared with those born at term or post‐term.
What Are the Clinical Implications?
Our observations are compounded by previous studies showing long‐term metabolic and cardiovascular abnormalities in preterm survivors.
Greater recognition of this at‐risk group should lead to early preventative measures to improve patient outcomes, including protective information, closer monitoring, and timely treatment of early signs of hypertension.
The fetal environment is delicately poised and adverse early life events can have long‐term physiological effects.1 There is extensive evidence showing that the time spent by the fetus in utero and the mechanisms affecting gestational length have numerous long‐term effects, which are evident in both childhood and adulthood, and can even affect the subsequent generation.2, 3, 4, 5
While a large number of studies have examined outcomes in childhood, studies extending into adulthood are more scarce. Nonetheless, the adverse outcomes in adulthood associated with preterm birth include reduced insulin sensitivity,3 increased adiposity,4 increased risk of short stature,6 and elevated blood pressure (BP).5, 7
A systematic review and meta‐analysis of 1342 individuals born preterm found that, at a mean age of 17.8 years, they had a moderate increase in systolic BP relative to those born at term7 However, studies on preterm survivors are often confounded by the inclusion of participants born at very low birth weight. Further, there is a paucity of data examining associations between preterm birth and BP specifically in females. Previous large studies focused exclusively on males,8 hindering our ability to draw conclusions on the association between preterm birth and later BP among women. It is not possible to readily extrapolate observations on males to females, as there are recognized sexual dimorphisms regarding predisposition to various noncommunicable diseases (including hypertension) in association with early life events.9 Thus, we aimed to identify whether previous observations of increased BP in young adult males born preterm8 are also present in females.
Methods
Ethics approval was granted by the Uppsala's regional ethical review board. This study was conducted in accordance with approved national and international guidelines. Informed consent was not required and thus not requested from participants, as this is a register‐based study using anonymized data where participants were not contacted.
Data were extracted from the Swedish Conscript Register, which records information on young men and women who are assessed for military service in Sweden. This study covers data on female conscripts born between 1973 and 1988 who were assessed for military service at a mean age of 18 years between 1990 and 2007. Note that during the period covered by this study, conscription was mandatory for all Swedish men but not for women. Thus, all women included in this study had volunteered for military service.
The initial process of conscription consists of an interview and an IQ test, and only participants with acceptable cognitive function and physical history proceeded to physical examination.8 As a result, all women in our cohort had passed the initial screening. Further exclusion criteria from our study included birth outside Scandinavia, having congenital malformations (International Classification of Diseases, Ninth Revision 740–759 and International Classification of Diseases, Tenth Revision Q0–Q99), or being born to a mother younger than 18 years or 45 years or older.
Data from the conscript register were subsequently linked to the Swedish Birth Register, which records information on >99% of all births in Sweden with a low error rate for key parameters.10 Gestational age at birth was estimated from the date of the last menstrual period for the majority of participants, otherwise estimates were based on ultrasound scans. Women were grouped into those born preterm (<37 weeks of gestation) or not (≥37 weeks of gestation). Birth weight data were also extracted and transformed into age‐ and sex‐adjusted Z scores as per Swedish population standards.11
Weight and height were measured at the time of conscription. BP was measured using sphygmomanometers following standardized procedures, as described in detail by Johansson et al.8 In brief, BP was measured in the right upper arm while in the supine position after at least 5 minutes of rest. A second BP measurement was taken if the first measurement was elevated (systolic BP [SBP] ≥135 mm Hg or diastolic BP [DBP] ≥85 mm Hg), with the lowest measurement recorded. Mean arterial pressure (MAP) was subsequently calculated as:
Forms of hypertension were defined as per 2013 guidelines of the European Society of Hypertension and the European Society of Cardiology12: systolic hypertension as SBP ≥140 mm Hg, diastolic hypertension as DBP ≥90 mm Hg, and isolated systolic hypertension as both SBP ≥140 mm Hg and DBP <90 mm Hg.
Statistical Analyses
Analyses were performed in SPSS version 25 (IBM Corp) and SAS version 9.4 (SAS Institute Inc). Outcomes of interest were initially compared between women born preterm and others using 1‐way ANOVA, chi‐square test, or Fisher exact test, as appropriate. All data were then analyzed using linear mixed effects models, adjusting for woman's birth weight Z scores, maternal education (university versus lesser), paternal education (university versus lesser), and maternal identification number as a random factor to account for the correlation of siblings. Size at birth is known to be associated with BP later in life,13, 14 but we incorporated birth weight adjusted for gestational age and sex so that it was comparable across the gestational age range. Maternal and paternal education were used as proxy measures of socioeconomic status among conscripts,15 and were included in our models as parental education and socioeconomic position are associated with long‐term BP in the offspring.16, 17 In addition, models also adjusted for the woman's height where the outcome was BP18 or weight. Generalized linear models were then run to examine the relative risk of hypertension among women born preterm in comparison to those born at term or post‐term, accounting for the same confounding factors. Specifically, we used PROC GENMOD and log‐linked binomial models in SAS.19 Similar models were adopted to examine the same associations among male conscripts, for comparison with the original findings by Johansson et al.8 All tests were 2‐tailed, with statistical significance set at P<0.05 and without adjustment for multiple comparisons.
Results
A total of 6759 women volunteered for the Swedish military service during the study period. From these, 1527 women were excluded (242 born outside Scandinavia, 224 with malformations, 228 with missing anthropometric data, 32 whose mothers were younger than 18 years or 45 years or older at childbirth, 785 with missing BP data, and 16 with missing gestational age information). Thus, we studied 5232 young women at a mean age of 18 years, of whom 299 were born preterm (5.7%). Subsequent adjusted analyses were performed on 4973 women who had additional data on parental education and birth weight (5.7% born preterm).
At the time of conscription, there were no differences in anthropometry between young women born preterm and those who were not (Table). On average, women born preterm had SBP 3.8 mm Hg higher (P<0.0001), DBP 1.1 mm Hg higher (P=0.026), and MAP 2.0 mm Hg higher (P<0.0001) than those born at term or post‐term (Table). Differences in SBP and MAP were unchanged after adjustment for confounders, but the difference in DBP was no longer statistically significant (Table). Nonetheless, there was a progressive increase in SBP, DBP, and MAP with decreasing gestational age (Figure 1).
Table 1.
Anthropometric and Blood Pressure Data From Young Women Who Volunteered for Military Service in Sweden (1990–2007) According to Their Gestational Age at Birth
| Unadjusted Data | Adjusted Data | |||||
|---|---|---|---|---|---|---|
| Preterm | Term or Post‐Term | P Value | Preterm | Term or Post‐Term | P Value | |
| No. | 299 | 4933 | 282 | 4691 | ||
| Anthropometry | ||||||
| Height, cm | 167.3±6.2 | 167.8±5.8 | 0.12 | 167.5 (166.8–168.1) | 167.8 (167.8–168.1) | 0.17 |
| Weight, kg | 64.37±9.08 | 64.66±9.00 | 0.59 | 64.28 (63.36–65.21) | 64.39 (64.15–64.63) | 0.84 |
| BMI, kg/m2 | 22.98±2.80 | 22.95±2.86 | 0.84 | 22.82 (22.49–23.15) | 22.85 (22.76–22.93) | 0.86 |
| Overweight/obesity | 61 (20.4) | 993 (20.1) | 0.85 | – | – | |
| Obesity | 6 (2.0) | 107 (2.2) | 0.91 | – | – | |
| Blood pressure | ||||||
| SBP, mm Hg | 126.2±10.4 | 122.4±11.1 | <0.0001 | 126.1 (124.8–127.4) | 122.4 (122.0–122.7) | <0.0001 |
| DBP, mm Hg | 70.5±8.1 | 69.4±8.2 | 0.026 | 70.4 (69.4–71.4) | 69.5 (69.2–69.7) | 0.06 |
| MAP, mm Hg | 89.1±7.8 | 87.1±8.0 | <0.0001 | 89.0 (88.0–89.9) | 87.1 (86.9–87.3) | 0.0001 |
| Systolic hypertension | 42 (14.0) | 400 (8.1) | <0.001 | – | – | |
| Diastolic hypertension | 4 (1.3) | 31 (0.6) | 0.14 | – | – | |
| Isolated systolic hypertension | 38 (12.7) | 383 (7.8) | 0.002 | – | – | |
Unadjusted data are expressed as mean±SD or number (percentage). Adjusted data are estimated marginal means and 95% CIs adjusted for confounding factors. Diastolic hypertension, ≥90 mm Hg; isolated systolic hypertension, systolic blood pressure (SBP) ≥140 mm Hg and diastolic BP (DBP) <90 mm Hg; overweight/obesity, body mass index (BMI) ≥25 kg/m2; obesity, BMI ≥30 kg/m2; preterm, birth at <37 weeks of gestation; systolic hypertension, SBP ≥140 mm Hg; and term or post‐term, birth at ≥37 weeks of gestation. Adjusted data were analyzed using generalized linear models, accounting for birth weight Z score, maternal education, and paternal education. Where weight or blood pressure was the outcome, models were also adjusted for the woman's height. MAP indicates mean arterial pressure.
Figure 1.
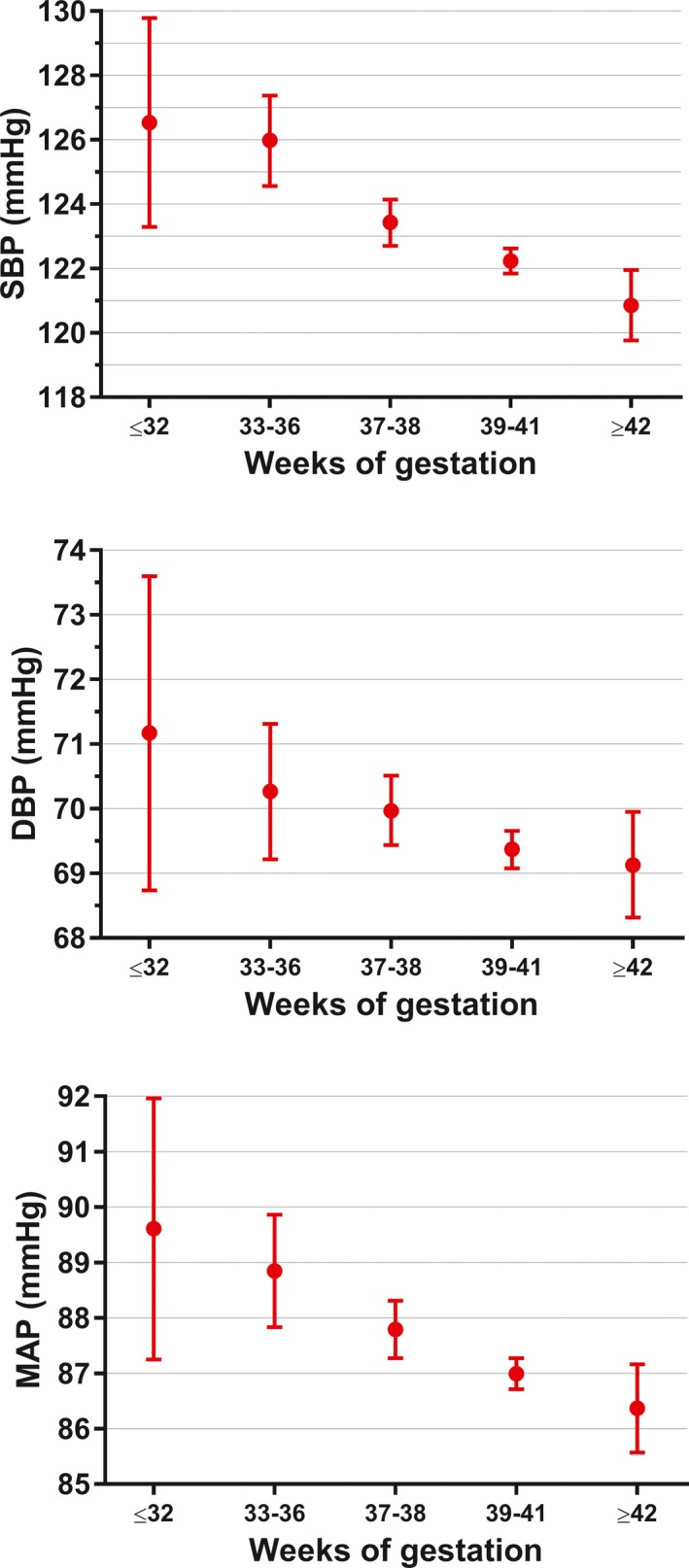
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) according to gestational age among 4973 young Swedish women who volunteered for military service between 1990 and 2007. Data are expressed as estimated means and 95% CIs adjusted for birth weight Z score, height, maternal education, and paternal education.
Rates of systolic hypertension were considerably higher in young women born preterm compared with those born at term or post‐term (14.0% versus 8.1%, P<0.0001), as were rates of isolated systolic hypertension (Table). The adjusted relative risk of systolic hypertension in women born preterm was 1.72 times (95% CI, 1.26–2.34; P<0.001) that of women born at term or post‐term, but there was no difference in the risk of diastolic hypertension (adjusted relative risk, 1.60; 95% CI, 0.49–5.20).
Male Conscripts
For comparison, we also examined the associations between preterm birth and BP among 366,399 male conscripts (5.0% born preterm). As observed among females, there was a progressive decrease in BP as gestational age increased (Figure 2). Thus, young males born preterm had increased BP compared with their peers born at term or post‐term: SBP +1.5 mm Hg (95% CI, 1.4–1.7; P<0.0001), DBP +0.4 mm Hg (95% CI, 0.2–0.5; P<0.0001), and MAP +0.8 mm Hg (95% CI, 0.6–0.9; P<0.0001). In addition, the adjusted relative risk of systolic hypertension was 1.22 (95% CI, 1.19–1.25; P<0.0001) and of diastolic hypertension was 1.29 (95% CI, 1.09–1.52; P<0.0001) among males born preterm.
Figure 2.
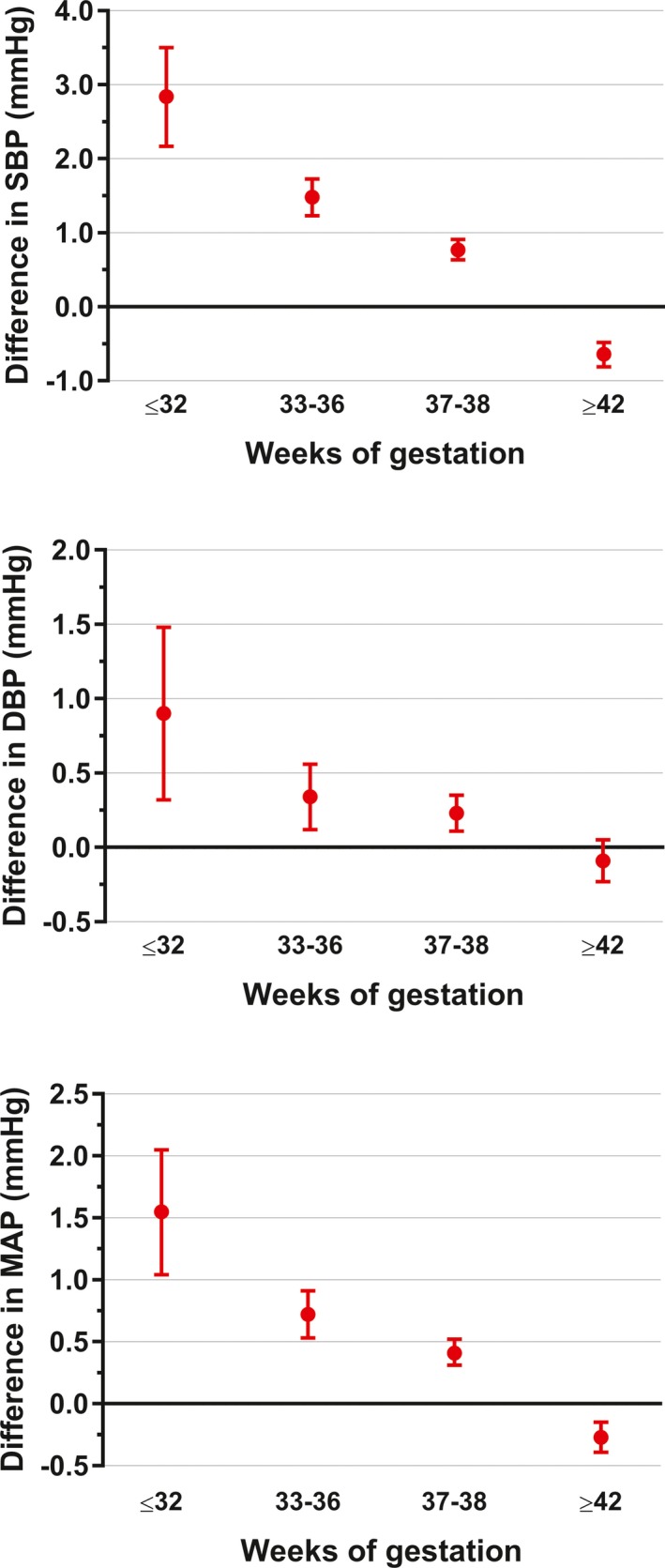
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) according to gestational age among 366 399 men conscripted to military service in Sweden between 1990 and 2007. Data are expressed as the estimated mean differences in comparison to men born at full term (39–41 weeks of gestation) and the respective 95% CIs, adjusted for birth weight Z score, height, maternal education, and paternal education.
Discussion
Our findings show that among young adult women in Sweden, decreasing gestational age was associated with a progressive increase in BP. Thus, at ≈18 years of age, women born preterm already displayed an increased risk of systolic hypertension compared with those born at term or post‐term. A similar pattern was observed among male conscripts, but the observed increase in BP in males born preterm was of a lesser magnitude.
To our knowledge, this study is the first to examine BP in a large cohort of women born preterm. Notably, we observed an adjusted SBP difference of 3.7 mm Hg between women born preterm and those born at term or post‐term, which is remarkably comparable to the observed difference of 3.8 mm Hg in de Jong et al's meta‐analysis7 of 5 high‐quality studies. Our study also corroborates the findings of Johansson et al8 on male Swedish conscripts born preterm who also had an increased risk of systolic hypertension but with no observed effect on DBP. Calculation of their pooled20 unadjusted mean difference in SBP between males born preterm and at term estimates it as 1.2 mm Hg, which is relatively similar to our observed difference among males but 3‐fold lower than the difference we found among females and in de Jong et al's meta‐analysis.7
A number of developmental alterations in utero and in the early neonatal period have been described as the basis for the prehypertensive and hypertensive states diagnosed later in life in preterm survivors. Being born preterm interrupts development at a critical stage, as the final development of systemic processes and structures that regulate BP (including the renin‐angiotensin system and kidney nephrons) are only completed in the late stages of gestation, so that a premature birth results in abnormalities that impair the individual's ability to adequately regulate BP.21 This association has been demonstrated in animal models, where a reduction in nephron numbers alone was associated with hypertension later in life,22, 23, 24 with similar observations reported in humans.25 In addition, arterial wall elastin synthesis peaks towards the end of gestation,26 which is particularly important for the development of the arterial tree that, in turn, has a crucial role in maintaining normotension.27 Other studies have also shown an association between cardiac underdevelopment in animals born preterm and the long‐term development of increased BP.28 Further, Kowalski et al29 recently reported on the relationship between preterm birth and increased aortic wave reflection, which was associated with elevated central aortic systolic pressure in adolescents.
Study Limitations
It should be noted that our study cohort included only women who passed the initial conscription screening in Sweden for acceptable physical history and cognitive function. Since they have also volunteered for military conscription, it is likely that they were healthier than the general population of young adult women in Sweden of similar age. As a result, it is possible that the nature and magnitude of the associations observed here between preterm birth and later BP could differ in the general population. We were also unable to account for other socioeconomic and lifestyle factors, as well as whether the woman was a twin or singleton, which could have affected the accuracy of our parameter estimates. In addition, gestational age was estimated based on the date of the last menstrual period for the majority of participants in our study, which is less precise than estimates based on ultrasound scans.30
Conclusions
Our findings of increased SBP and a greater risk of hypertension in young women born preterm corroborates previous findings in men. These observations are compounded by previous studies showing long‐term metabolic and cardiovascular abnormalities in preterm survivors.3, 4, 5 Greater recognition of this at‐risk group should lead to early preventative measures to improve patient outcomes, including protective information, closer monitoring, and timely treatment of early signs of hypertension.31, 32
Disclosures
None.
Acknowledgments
We are grateful to Dr John Gibbins (University of Auckland) for editorial revision and thorough feedback on a previous version of this article.
(J Am Heart Assoc. 2019;8:e012274 DOI: 10.1161/JAHA.119.012274.)
Data Availability Statement
Data used in this study were obtained from the Swedish Medical Birth Register and the Military Service Conscription Register, and cannot be made publicly available. However, these data can be accessed upon request to the Swedish National Board of Health and Welfare, pending approval by the appropriate ethics committee.
References
- 1. Barker DJP. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. [DOI] [PubMed] [Google Scholar]
- 2. Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, Cutfield WS. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–2186. [DOI] [PubMed] [Google Scholar]
- 3. Mathai S, Cutfield WS, Derraik JG, Dalziel SR, Harding JE, Robinson E, Biggs J, Jefferies C, Hofman PL. Insulin sensitivity and β‐cell function in adults born preterm and their children. Diabetes. 2012;61:2479–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathai S, Derraik JG, Cutfield WS, Dalziel SR, Harding JE, Biggs J, Jefferies C, Hofman PL. Increased adiposity in adults born preterm and their children. PLoS One. 2013;8:e81840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathai S, Derraik JG, Cutfield WS, Dalziel SR, Harding JE, Biggs JB, Jefferies C, Hofman PL. Blood pressure abnormalities in adults born moderately preterm and their children. Int J Cardiol. 2015;181:152–154. [DOI] [PubMed] [Google Scholar]
- 6. Derraik JG, Lundgren M, Cutfield WS, Ahlsson F. Association between preterm birth and lower adult height in women. Am J Epidemiol. 2017;185:48–53. [DOI] [PubMed] [Google Scholar]
- 7. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation. 2005;112:3430–3436. [DOI] [PubMed] [Google Scholar]
- 9. Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–148. [DOI] [PubMed] [Google Scholar]
- 11. Niklasson A, Ericson A, Fryer J, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr Scand. 1991;80:756–762. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 13. Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. [DOI] [PubMed] [Google Scholar]
- 14. Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. [DOI] [PubMed] [Google Scholar]
- 15. Aaro LE, Flisher AJ, Kaaya S, Onya H, Namisi FS, Wubs A. Parental education as an indicator of socioeconomic status: improving quality of data by requiring consistency across measurement occasions. Scand J Public Health. 2009;37:16–27. [DOI] [PubMed] [Google Scholar]
- 16. Kivimäki M, Smith GD, Elovainio M, Pulkki L, Keltikangas‐Jarvinen L, Talttonen L, Raitakari OT, Viikari JS. Socioeconomic circumstances in childhood and blood pressure in adulthood: the Cardiovascular Risk in Young Finns Study. Ann Epidemiol. 2006;16:737–742. [DOI] [PubMed] [Google Scholar]
- 17. Kivimaki M, Lawlor DA, Smith GD, Keltikangas‐Jarvinen L, Elovainio M, Vahtera J, Pulkki‐Raback L, Taittonen L, Viikari JS, Raitakari OT. Early socioeconomic position and blood pressure in childhood and adulthood: the Cardiovascular Risk in Young Finns Study. Hypertension. 2006;47:39–44. [DOI] [PubMed] [Google Scholar]
- 18. Bourgeois B, Watts K, Thomas DM, Carmichael O, Hu FB, Heo M, Hall JE, Heymsfield SB. Associations between height and blood pressure in the United States population. Medicine. 2017;96:e9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 20. National Collaborating Centre for Mental Health . Methods for calculating means and standard deviations for pooled treatment groups. Post‐traumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. NICE Clinical Guidelines, No. 26. Leicester (UK): Gaskell; 2005:Appendix 10. [PubMed]
- 21. Alexander BT, Intapad S. Preterm birth: a novel risk factor for higher blood pressure in later life. Hypertension. 2012;59:189–190. [DOI] [PubMed] [Google Scholar]
- 22. Brennan KA, Gopalakrishnan GS, Kurlak L, Rhind SM, Kyle CE, Brooks AN, Rae MT, Olson DM, Stephenson T, Symonds ME. Impact of maternal undernutrition and fetal number on glucocorticoid, growth hormone and insulin‐like growth factor receptor mRNA abundance in the ovine fetal kidney. Reproduction. 2005;129:151–159. [DOI] [PubMed] [Google Scholar]
- 23. Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. [DOI] [PubMed] [Google Scholar]
- 26. Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. [DOI] [PubMed] [Google Scholar]
- 27. Sutherland MR, Bertagnolli M, Lukaszewski MA, Huyard F, Yzydorczyk C, Luu TM, Nuyt AM. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014;63:12–18. [DOI] [PubMed] [Google Scholar]
- 28. Mrocki MM, Nguyen VB, Lombardo P, Sutherland MR, Bensley JG, Nitsos I, Allison BJ, Harding R, De Matteo R, Schneider M, Polglase GR, Black MJ. Moderate preterm birth affects right ventricular structure and function and pulmonary artery blood flow in adult sheep. J Physiol. 2018;596:5965–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kowalski RR, Beare R, Mynard JP, Cheong JL, Doyle LW, Smolich JJ, Cheung MM. Increased aortic wave reflection contributes to higher systolic blood pressure in adolescents born preterm. J Hypertens. 2018;36:1514–1523. [DOI] [PubMed] [Google Scholar]
- 30. Savitz DA, Terry JW, Dole N, Thorp JM, Siega‐Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. [DOI] [PubMed] [Google Scholar]
- 31. Gulec S. Early diagnosis saves lives: focus on patients with hypertension. Kidney Int Suppl. 2013;3:332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Volpe M, Gallo G, Tocci G. Is early and fast blood pressure control important in hypertension management? Int J Cardiol. 2018;254:328–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study were obtained from the Swedish Medical Birth Register and the Military Service Conscription Register, and cannot be made publicly available. However, these data can be accessed upon request to the Swedish National Board of Health and Welfare, pending approval by the appropriate ethics committee.


