Abstract
Background
The value of glycemic control and preexisting cardiovascular disease in determining the risk of major cardiovascular events (MACE) in type 2 diabetes mellitus is uncertain. Intensive glucose control trials suggest that the 9% lower risk of MACE associated with intensive glycemic control, as compared with conventional glycemic control, is only driven by patients with type 2 diabetes mellitus without cardiovascular disease at baseline.
Methods and Results
We did a meta‐analysis of cardiovascular outcome trials dividing patients with or without preexisting cardiovascular disease; we found that the lower risk of MACE is confined to patients with cardiovascular disease at baseline. Compared with placebo, the use of both glucagon‐like peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors was associated with a significant 14% lower MACE risk in patients with preexisting cardiovascular disease and with a nonsignificant 2% higher MACE risk in those without preexisting cardiovascular disease (P for interaction=0.021). The meta‐regression analysis of all 12 trials demonstrated a significant (P=0.002) association between reductions of glycated hemoglobin in glycated hemoglobin A1C. Accordingly, the reduction of MACE expected if all cardiovascular outcome trials had achieved a 0.9% glycated hemoglobin reduction would have been 33%. Routine clinical care data complement the results of cardiovascular outcome trials but with some differences: the lower risk of MACE with sodium‐glucose cotransporter‐2 inhibitor use is evident in patients with type 2 diabetes mellitus with or without preexisting cardiovascular disease.
Conclusions
Sodium‐glucose cotransporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists should be included in the therapeutic plan of patients with type 2 diabetes mellitus and overt cardiovascular disease, with due attention paid to improvement of glycemic control, which may amplify their benefit on MACE.
Keywords: cardiovascular events, cardiovascular outcome trial, intensive glucose control, type 2 diabetes mellitus
Subject Categories: Metabolism, Vascular Disease
Clinical Perspective
What Is New?
Compared with placebo, the use of newer glucose‐lowering agents, such as glucagon‐like peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors, is associated with a significant 14% lower risk of major cardiovascular events (MACE) in patients with type 2 diabetes mellitus and preexisting cardiovascular disease, and with a nonsignificant 2% higher MACE risk in those without preexisting cardiovascular disease.
What Are the Clinical Implications?
Sodium‐glucose cotransporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists should be included in the therapeutic plan of patients with type 2 diabetes mellitus and overt cardiovascular disease, with due attention paid to improvement of glycemic control, which may amplify their benefit on MACE.
The prognosis of patients with type 2 diabetes mellitus (T2DM) has improved in the past 20 years, with a substantial decrease of mortality1 and also of cardiovascular and renal complications.2 This has occurred in both primary and secondary prevention settings, mainly because of improved management of cardiovascular risk factors. In a real‐world population of 12 544 Danish patients with T2DM without angiographically documented coronary artery disease, for example, a high level of preventive therapy with statins and aspirin may remove the diabetes mellitus–associated increased risk of myocardial and cardiac death for at least a 7‐year period.3 In the TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin) trial population of 13 616 patients with T2DM and established cardiovascular disease (CVD), statin and aspirin users, as compared to nonusers, had improved cardiovascular outcome (25% and 21% reduction of major cardiovascular events, respectively).4
As far as glycemic control of diabetes mellitus is concerned, it did not improve substantially in the past 10 years: the proportion of diabetic patients achieving a hemoglobin A1c (A1C) target <7% is still around 50%.5 The situation may be worse in the real world,6 with only ≈40% of patients in the Health Maintenance Organization population or 30% of patients in the Medicaid population consistently achieving A1C levels <7% over the time period spanning 2007‐2014.
Recently, a significant reduction in the incidence of cardiovascular events has been observed with some newer glucose‐lowering drugs, suggesting the possibility of cardioprotective actions beyond glycemia for some molecules. We assessed the current evidence regarding the relationship among glycemic control, preexisting CVD, and major cardiovascular events (MACE), as revealed by both intensive glucose control trials (IGCTs) and cardiovascular outcome trials (CVOTs) in T2DM. We did a meta‐analysis of CVOTs by dividing patients with or without preexisting CVD; we also assessed, with a meta‐regression analysis of all 12 CVOTs, the relationship between reductions of A1C and risk of MACE.
Methods
We conducted this systematic review and meta‐analysis based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines.7 The PRISMA checklist is provided (Table S1). Neither ethics approval nor patient consent was required for this analysis. The authors are experienced in meta‐analyses.8, 9
Literature Search
Databases for our search included PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and ClinicalTrials.gov. (http://www.clinicaltrials.gov). The last search was performed on January 15, 2019. The search terms used were “dipeptidyl‐peptidase inhibitor,” “saxagliptin,” “alogliptin,” “sitagliptin,” linagliptin,” “glucagon‐like peptide‐1 receptor agonist,” “exenatide,” “lixisenatide,” “liraglutide,” “semaglutide,” “dulaglutide,” “albiglutide,” “sodium‐glucose cotransporter‐2 inhibitor,” “empagliflozin,” “canagliflozin,” “dapagliflozin,” “cardiovascular outcome trials,” “intensive glucose control trials,” “cardiovascular outcome trials,” and “major cardiovascular events.”
Selection Criteria
We included CVOTs if they were randomized controlled trials performed in adults with T2DM, compared add‐on therapy with any dipeptidyl peptidase‐4 (DPP‐4) inhibitor, glucagon‐like peptide‐1 (GLP‐1) receptor agonists, or sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitor with placebo, and had MACE as the primary outcome. We excluded trials if they were completed before the FDA guidance of 2008.10 We included IGCTs if they were randomized controlled trials designed to assess the impact of achieving lower versus higher levels of glycemia on MACE in adults with T2DM and had large size (at least 1000 person‐years of follow‐up in each treatment arm) and a minimum of 2 years median postrandomization follow‐up. Two investigators (D.G., M.I.M.) used a standardized tool to independently abstract all data, and disagreements were resolved by consensus.
Statistical Analyses
Findings are given as both hazard ratios (HRs) with 95% CIs and HR reduction. We did de novo meta‐analyses to assess the effect of both GLP‐1 agonists and SGLT‐2 inhibitors on MACE risk in T2DM patients with or without a history of CVD at baseline. Heterogeneity between studies was assessed by using the Q statistic and I2, which is the proportion of total variance observed between the trials attributed to the differences between trials rather than to sampling error. I2<25% was considered as low in heterogeneity, and a Q statistic P value of <0.10 was considered significant.11 In a conservative way we calculated the summary estimates and 95% CIs for cardiovascular efficacy outcomes using a random‐effects model meta‐analysis. However, we used a fixed‐effects model in the case that heterogeneity was not significant. Publication bias was assessed with the Egger test12; a P value of <0.10 was considered significant.
We also did a meta‐regression analysis including all CVOTs in order to describe the relationship between the differences in achieved A1C at the end of CVOTs and the corresponding HR reduction for MACE. The meta‐regression relates the treatment effect to study‐level covariates while assuming additivity of within‐study and between‐studies components of variance.13 Restricted maximum‐likelihood estimators were used to estimate model parameters. A permutation test (using 1000 reallocations) was used for assessing the true statistical significance of an observed meta‐regression finding. In the meta‐regression model, as recommended by Higgins and Thompson,13 P‐values were calculated by using a Monte Carlo permutation test. This test is implemented in the meta‐reg function of Stata statistical software (Statacorp, College Station, TX) using the option permute. Permutation tests are well established as a means of calculating significance levels. A permutation test may be constructed in the same way as in standard linear regression, that is, by randomly shuffling the rows of the design matrix and reassigning them to the response vector. We used the meta‐regression analysis to calculate the expected decrease of MACE risk at any A1C reduction.
The data that support the findings of this study are available from the corresponding author on reasonable request.
IGCTs and MACE
Intensive glycemic control (IGC) has an imperfect role in reducing the cardiovascular complications associated with T2DM. The CONTROL (Collaborators on Trials of Glucose Lowering) meta‐analysis14 included 4 large IGCTs (UKPDS (United Kingdom Prospective Diabetes Study), ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease), and VADT (Veterans Administration Diabetes Trial))15, 16, 17, 18 with 27 049 patients: IGC led to a mean 0.9% A1C reduction and was associated with a significant 9% reduction of MACE (Table 1), although the reduction was not significant in any single trial. Moreover, compared with less intensive glycemic control, IGC was associated with a clear risk of serious hypoglycemia (HR=2.48, 95% CI 1.91‐3.21).14 There is some evidence favoring a delayed cardiovascular benefit of early IGC, as suggested by the 10‐year follow‐up of UKPDS,19 which enrolled newly diagnosed T2DM patients, most without preexisting CVD. On the other hand, the attainment of IGC in long‐established and poorly controlled T2DM was associated with 22% excess cardiovascular mortality, prompting early termination of the intensive arm of the ACCORD trial.16 This evidence has generated the concept of “residual vascular risk,” the risk of macrovascular event that persists high to very high after IGC despite the attainment of prespecified and near‐to‐normal A1C targets.20, 21
Table 1.
IGCTs, CVOTs, and Risk of MACE in Patients With T2DM
| Trials | ΔA1C (%) | Hazard Ratio for MACE |
|---|---|---|
| IGCTs | −0.90 (−1.30 to −0.50) | 0.91 (0.84 to 0.99) |
| N=27 049 | ||
| CVOTs | −0.42 (−0.53 to −0.30) | 0.92 (0.87 to 0.96) |
| N=120 765 | ||
| CVOTs | −0.90 | 0.67 (0.49 to 0.93) |
| meta‐regression |
CVOTs indicates cardiovascular outcome trials; ΔA1C, change in glycated hemoglobin; IGCTs, intensive glucose control trials; MACE, major cardiovascular events; T2DM, type 2 diabetes mellitus.
CVOTs and MACE
The leading IGCTs that have been the object of the CONTROL meta‐analysis used glucose‐lowering drugs that were on the market when they were planned. In particular, the use of sulfonylureas was as high as 90% in ADVANCE participants, the use of insulin 87% in VADT participants, and the use of thiazolidinediones 55% in ACCORD participants. In 2008 the US Food and Drug Administration issued a guidance to pharmaceutical companies requiring the proof of cardiovascular safety as a necessary condition for the approval of new glucose‐lowering drugs.7 The guidance has led to dedicated CVOTs with the aim of ruling out unacceptable MACE risk (maximum hazard of 1.30, upper 95% CI) of any licensed glucose‐lowering medications.
Twelve CVOTs22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 have been completed and published to date, with the following classes of glucose‐lowering medications: DPP‐4 inhibitors saxagliptin, alogliptin, sitagliptin, and linagliptin; GLP‐1 receptor agonists lixisenatide, liraglutide, semaglutide, once‐weekly exenatide, and albiglutide; and SGLT‐2 inhibitors empagliflozin, canagliflozin, and dapagliflozin (Table 2). The total number of T2D patients evaluated in the 12 CVOTs was 120 765. Table 1 shows the effects of the newer glucose‐lowering medications on the mean A1C reduction at the end of treatment and the risk of MACE. The overall analysis of the 12 CVOTs showed a highly significant (P=0.001) 8% reduction of MACE risk associated with no publication bias (Egger test, P=0.210) and significant heterogeneity (I2=45.8%, P=0.041), indicating the lack of homogeneity in the whole analysis that grouped 3 different classes of glucose‐lowering drugs. Use of DPP‐4 inhibitors in 4 CVOTs (SAVOR‐TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)‐Thrombolysis in Myocardial Infarction (TIMI) 53), EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care), TECOS, CARMELINA (Cardiovascular and Renal Microvascular Outcome Study With Linagliptin)) was associated with a negligible and not significant 1% reduction of MACE risk (HR=0.99, 95% CI 0.94‐1.05), as compared with placebo; in contrast, the use of both SGLT‐2 inhibitors (3 CVOTs: EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients), CANVAS (Canagliflozin Cardiovascular Assessment Study), DECLARE (Dapagliflozin Effect on Cardiovascular Events‐Thrombolysis in Myocardial Infarction 58)) and GLP‐1 agonists (5 CVOTs: ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome), LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results), SUSTAIN‐6 (Trial to Evaluate Cardiovascular and Other Long‐term Outcomes with Semaglutide in Subjects with Type 2 Diabetes), EXSCEL (Exenatide Study of Cardiovascular Event Lowering), HARMONY (Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease) OUTCOME) was associated with 11% (P=0.005) and 12% (P=0.001) MACE risk reductions, respectively. The interaction between subgroups was significant (P=0.021), indicating the differences of the MACE risk reduction obtained with the 3 classes of drugs were not due to chance.
Table 2.
Summary of CVOTs and IGCTs Evaluating the HR of MACE in T2DM patients with Pre‐existing CVD
| Trial/year of publication | Study drug/ Mean follow up (years) | Participants (n) | Participants with prior CVD | Type of prior CVD | HR fo MACE (whole population) and 95% CI | HR for MACE (prior CVD) and 95% CI |
|---|---|---|---|---|---|---|
| CVOTs | ||||||
| DPP‐4i | ||||||
| SAVOR‐TIMI 53 | Saxagliptin | 16 492 | 12,963 | History of CVD | 1.0 | NR |
| 2013 | 2.1 yr | 78.6% | (CHD, CeVD, PVD) | 0.91‐1.10 | ||
| EXAMINE | Alogliptin | 5380 | 5380 | Acute coronary | 0.96 | 0.96 |
| 2013 | 1.5 yr | 100% | syndrome | 0.79‐1.16 | 0.79‐1.16 | |
| TECOS | Sitagliptin | 14,671 | 10,857 | Established CVD | 0.98 | NR |
| 2015 | 74% | (CHD, CeVD, PVD) | 0.89‐1.08 | |||
| CARMELINA | Linagliptin | 6979 | 6979 | History of CVD | 1.02 | 1.02 |
| 2018 | 2.2 yr | 100% | (CHD, CeVD, PVD) | 0.89‐1.17 | 0.89‐1.17 | |
| GLP‐1RAs | ||||||
| ELIXA | Lixisenatide | 6068 | 6068 | Acute coronary | 1.02 | 1.02 |
| 2015 | 2.1 yr | 100% | syndrome | 0.89‐1.17 | 0.89‐1.17 | |
| LEADER | Liraglutide | 9340 | 7598 | CVD or CKD or CV | 0.83 | 1.20 |
| 2016 | 3.8 yr | 81.3% | risk factors | 0.74‐0.93 | 0.86‐1.67 | |
| SUSTAIN‐6 | Semaglutide | 3297 | 2735 | CVD or CKD or CV | 0.74 | 0.72 |
| 2016 | 3.1 yr | 83% | risk factors | 0.58‐0.95 | 0.55‐0.93 | |
| EXSCEL | Exenatide OW | 14,752 | 10 792 | History of CVD | 0.91 | 0.90 |
| 2017 | 3.2 yr | 73.1% | (CHD, CeVD, PVD) | 0.83‐1.00 | 0.82‐1.00 | |
| HARMONY | Albiglutide | 9463 | 9463 | History of CVD | 0.78 | 0.78 |
| 2018 | 1.6 yr | 100% | (CHD, CeVD, PVD) | 0.68‐0.90 | 0.68‐0.90 | |
| SGLT‐2i | ||||||
| EMPA‐REG | Empagliflozin | 7020 | 7020 | History of CVD | 0.86 | 0.86 |
| OUTCOME 2015 | 3.1 yr | 100% | (CHD, CeVD, PVD) | 0.74‐0.99 | 0.74‐0.99 | |
| CANVAS | Canagliflozin | 10 142 | 6656 | CVD or CV | 0.86 | 0.82 |
| 2017 | 2.4 yr | 72.2% | risk factors | 0.76‐0.98 | 0.72‐0.95 | |
| DECLARE | Dapagliflozin | 17,160 | 6974 | Established CVD | 0.93 | 0.90 |
| 2019 | 4.2 yr | 40.6% | 0.84‐1.03 | 0.79‐1.02 | ||
| IGCTs | ||||||
| UKPDS | Sulfonylurea | 3867 | 116 | Previous CVD | 0.80 | 0.98 |
| 1998 | or insulin (10 yr) | 3% | 0.62‐1.04 | 0.64‐1.51 | ||
| ACCORD | Any (A1C <6%) | 10 251 | 3609 | Previous CV events | 0.90 | 1.02 |
| 2008 | 3.5 yr | 35% | 0.78‐1.04 | 0.85‐1.21 | ||
| ADVANCE | Gliclazide + any | 11 140 | 3590 | Previous CVD | 0.94 | 0.99 |
| 2008 | (A1C ≤6.5%) 5.0 yr | 32% | 0.84‐1.06 | 0.85‐1.15 | ||
| VADT | Any (A1C <6%) | 1791 | 723 | Previous CVD | 0.90 | 1.01 |
| 2009 | 5.6 yr | 40% | 0.70‐1‐16 | 0.87‐1‐18 | ||
CVOTs, cardiovascular outcome trials; IGCTs, intensive glucose control trials; HR, hazard ratio; TD2M, type 2 diabetes mellitus; MACE, major cardiovascular events; DPP‐4i, dipeptidyl‐peptidase 4 inhibitors; GLP‐1RAs, glucagon‐like peptide‐1 receptor agonists; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitors; CVD, cardiovascular disease; CDH, coronary heart disease; CKD, chronic kidney disease; CeVD, cerebrovascular disease; CV, cardiovascular; OW, once weekly exenatide; PVD, peripheral artery disease; NR, not reported
GLP‐1 receptor agonists reduce glycemia in T2DM patients by augmenting insulin secretion and suppressing glucagon release via the stimulation of GLP‐1 receptors; they also exert beneficial effects on an array of cardiovascular risk factors (decreased weight and appetite, reduced blood pressure, amelioration of the lipid profile, amelioration of endothelial dysfunction and inflammation) that may improve the poor cardiovascular outlook of the diabetic patient.34 SGLT‐2 inhibitors exert their glucose‐lowering effects by promoting glycosuria, which also results in body‐weight and fat‐mass reductions; moreover, they increase natriuresis, thereby lowering extracellular volume and arterial blood pressure, as well as uricosuria, which may result in better cardiovascular and renal outcomes and rates of mortality.35
CVOTs, IGCTs, and Glycemic Control
All the CVOTs were designed to promote “glycemic equipoise” in order to reduce the influence of different glucose levels between treatment and placebo groups. Even then, some difference between treatment and placebo groups in A1C levels was observed at the end of CVOTs: compared with placebo, the range of A1C reduction in CVOTs was 0.27% to 0.86%. The mean weighted A1C reduction was the least with DPP‐4 inhibitors (0.30%) and the highest with GLP‐1 receptor agonists (0.47%), with intermediate values for SGLT‐2 inhibitors (0.44%). Because of the principle of “glycemic equipoise,” the weighted mean A1C reduction in all 12 CVOTs was more than halved (−0.42%) as compared with that obtained in IGCTs (−0.9%) (Table 1). In spite of this difference in the attained A1C levels at the end of respective treatments, the HR for MACE was almost identical in CVOTs (0.92) and IGCTs (0.91), as were their respective 95% CIs (0.84‐0.99 and 0.87‐0.96). Heterogeneity was moderate and significant in CVOTs (I2=46%, P=0.041) and was null (I2=0%) and nonsignificant (P=0.72) in IGCTs.
This evidence has led to the suggestion that newer glucose‐lowering drugs may have additional beneficial effects on MACE that are not dependent on the amelioration of the glycemic control they produce. Table 1 also shows the extrapolation of MACE risk reduction if the A1C decrease in the CVOTs had equaled that obtained in IGCTs. This was obtained by fitting the desired A1C reduction (0.9%) in the meta‐regression analysis. Figure 1 shows the results of the meta‐regression analysis of all 12 trials demonstrating a significant (P=0.002) association between reductions of A1C and risk of MACE. Accordingly, the reduction of MACE expected if all CVOTs had achieved a 0.9% A1C reduction would have been 33% (expected β=0.67, 95% CI 0.49‐0.93). SUSTAIN‐6 was the only CVOT that succeeded in obtaining an A1C reduction (0.86%) closer to those obtained in IGTs; in fact, the reduction of MACE risk in SUSTAIN‐6 was 26% less than that with placebo (HR 0.74, CI 0.58‐0.95). This evidence suggests that (1) A1C reduction may also play a role in the mediation of benefit on MACE risk observed with the newer glucose‐lowering drugs, in particular GLP‐1 agonists and SGLT‐2 inhibitors, and (2) at the same level of glycemic control, the use of these newer glucose‐lowering drugs is associated with more benefit on MACE risk than the use of older ones.
Figure 1.
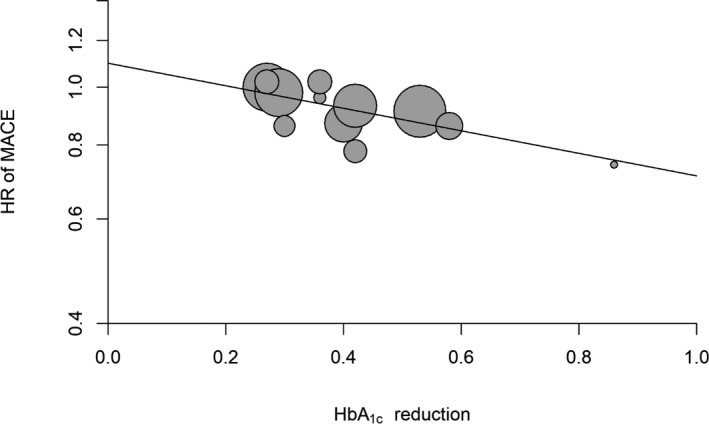
Meta‐regression analysis between reduction of HbA1c and MACE risk in the 12 CVOTs. CVOT indicates cardiovascular outcome trial; HbA1c, glycated hemoglobin; HR, hazard ratio; MACE, major cardiovascular events.
The greater benefit on MACE risk might be due to the beneficial effects of the newer glucose‐lowering drugs, the detrimental effects of the older glucose‐lowering drugs, or both. However, the use of older drugs was fully permitted in the CVOTs just to compensate the different potencies of the added newer drug versus placebo. For example, the use of sulfonylureas in CVOT participants ranged from 31% in CARMELINA to 50% in LEADER, the use of insulin from 23% in TECOS to 60% in HARMONY, and the use of metformin from 54% in CARMELINA to 82% in both DECLARE and TECOS. So, in CVOTs, the newer glucose‐lowering drugs were added to older glucose‐lowering drugs, formerly assessed in IGCTs, without too much emphasis on the glycemic control as being the reason for “glycemic equipoise.” In contrast to IGCTs, this may have reduced the occurrence of hypoglycemia, especially severe hypoglycemia, with a possible better cardiovascular outlook, as happened in the LEADER trial.36 This post hoc analysis has demonstrated an association between the occurrence of severe hypoglycemia and an increased risk of cardiovascular events and mortality in the overall LEADER trial population (irrespective of randomized treatment). It seems that both GLP‐1 agonists and SGLT‐2 inhibitors may allow near normoglycemia to exert its full potential to improve MACE risk in patients with T2DM.
Diabetic Population
In CVOTs, many patients had established CVD, which ranged from 41% in DECLARE to 100% in EXAMINE, ELIXA, EMPA‐REG OUTCOME, and HARMONY (Table 2). The main reason underlying the inclusion of participants with established CVD was to ensure sufficient events in the shortest possible time. This choice has limited generalizability of the results to other diabetic populations, prevented the assessment of primary prevention, as well as the identification of longer‐term safety issues, and the identification of slowly evolving benefits; on the other hand, it has allowed a distinction of the T2DM population in high‐risk patients (with established CVD) and lower‐risk patients (without established CVD), where such distinction was possible.
Table 3 shows the effect of IGCTs and CVOTs on MACE risk divided according to the presence/absence of CVD at baseline. In IGCTs, 8038 participants had CVD at baseline, representing 30% of the whole diabetic population (27 049) in the trials: 3% in UKPDS, 40% in VADT, 35% in ACCORD, and 32% in ADVANCE (Table 2). In this subset of diabetic population the cardiovascular benefit of the IGC was completely absent (HR=1.00, 95% CI 0.91‐1.10), indicating that the reduction of MACE risk observed in the whole population was driven by patients without cardiovascular disease at baseline.37 Although these data diminish previous alerts about the cardiovascular safety of IGC in the subgroup of individuals with preexisting CVD, they also admit that IGC cannot reduce MACE risk in this population.
Table 3.
HR and 95% CI of MACE in Trials Divided According to Presence of CVD at Baseline
| Trials | HR | 95% CI | I2 (%) | P Value Q Test |
|---|---|---|---|---|
| IGCTs | ||||
| All | 0.91 | 0.84 to 0.99 | 0.0 | 0.94 |
| With CVD | 1.00 | 0.91 to 1.10 | 0.0 | 0.47 |
| SGLT‐2i | ||||
| All | 0.89 | 0.83 to 0.96 | 0.0 | 0.55 |
| With CVD | 0.86 | 0.79 to 0.95 | 0.0 | 0.423 |
| Without CVD | 1.00 | 0.87 to 1.16 | 0.0 | 0.900 |
| GLP‐1 RAs | ||||
| All | 0.88 | 0.80 to 0.96 | 58.8 | 0.045 |
| With CVD | 0.86 | 0.80 to 0.92 | 31.7 | 0.231 |
| Without CVD | 1.06 | 0.87 to 1.29 | 0.0 | 0.660 |
CVD indicates cardiovascular disease; GLP‐1 RAs, glucagon‐like peptide‐1 receptor agonists; HR, hazard ratio; IGCTs, intensive glycemic control trials; MACE, major adverse cardiovascular events; SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitor.
In CVOTs the situation is inverted: the lower risk of MACE is confined to patients with CVD at baseline. Figures 2 and 3 show the meta‐analysis of the 5 CVOTs that reported the evaluation of MACE risk as a subanalysis of T2DM people divided according to the presence or absence of CVD at baseline, respectively. In the 3 CVOTs with GLP‐1 receptor agonists (LEADER, SUSTAIN‐6, EXSCEL), the percentage of patients with CVD at baseline was 77%; compared with placebo, treatment with GLP‐1 agonists was associated with a 14% lower risk of MACE (P<0.001) in T2DM patients with preexisting CVD and with a nonsignificant 6% higher risk of MACE (P=0.563) in those without preexisting CVD. In the 2 CVOTs with SGLT‐2 inhibitors (CANVAS, DECLARE), the percentage of patients with CVD at baseline was 66%; compared with placebo, treatment with SGLT‐2 inhibitor was associated with a 14% lower risk of MACE (P=0.002) in T2DM patients with preexisting CVD and with a null effect (P=0.977) in those without preexisting CVD. Two important conclusions emerge from these data: the equal reduction of MACE risk (14%) with both GL‐1 agonists and SGLT‐2 inhibitors and the absence of any heterogeneity in both evaluations (I2=0%), indicating a robust and reproducible effect with no variation among studies.
Figure 2.
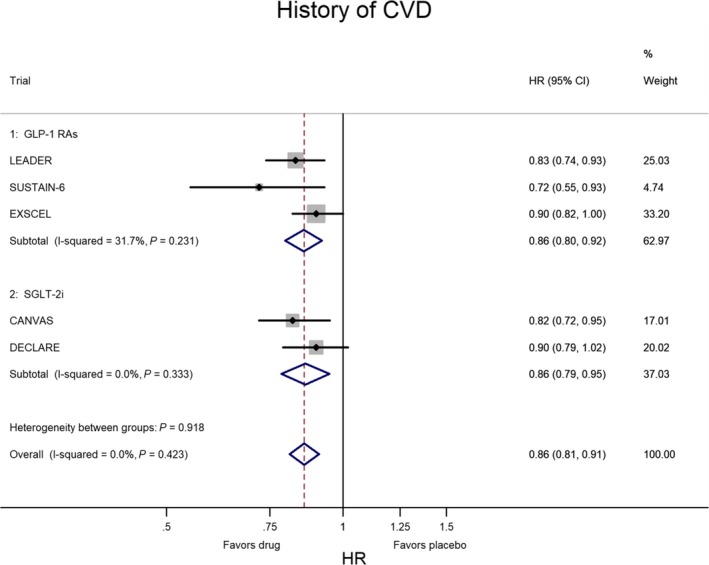
Meta‐analysis of 5 CVOTs (3 with GLP‐1 RAs and 2 with SGLT‐2i) in patients with history of CVD at baseline. The results are highly homogeneous, as heterogeneity was almost nil and not significant. CVD indicates cardiovascular disease; CVOTs, cardiovascular outcome trials; GLP‐1 RAs, glucagon‐like peptide‐1 receptor agonists; HR, hazard ratio; SGLT‐2i, sodium‐glucose cotransporter 2 inhibitor.
Figure 3.
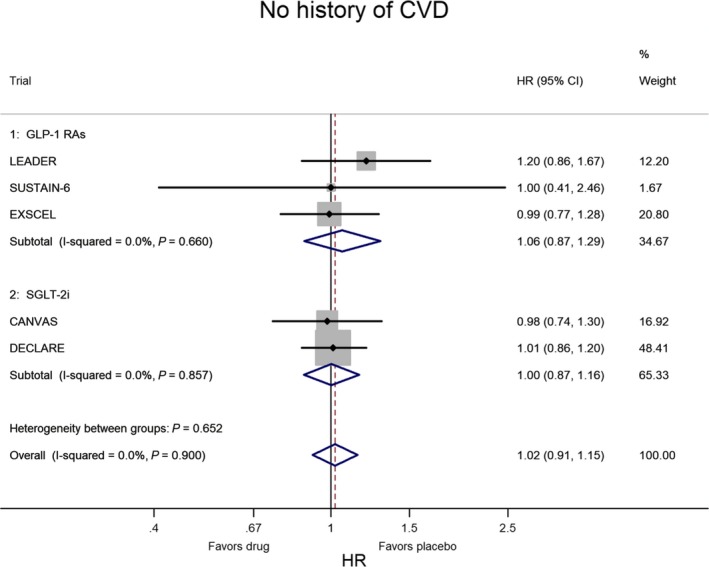
Meta‐analysis of the 5 CVOTs in patients without history of CVD at baseline. The results are highly homogeneous, as heterogeneity was almost nil and not significant. CVD indicates cardiovascular disease; CVOTs, cardiovascular outcome trials; GLP‐1 RAs, glucagon‐like peptide‐1 receptor agonists; HR, hazard ratio; SGLT‐2i, sodium‐glucose cotransporter 2 inhibitor.
Translation Into Routine Clinical Care
Although randomized controlled trials are the gold standard in assessing the effectiveness of medications, the restricted environment of CVOTs limits generalizability. Observational data from large international studies including a broad population of T2DM patients seen in clinical practice are largely consistent with the results observed in CVOTs. In the CVD‐REAL,38 for example, patients with T2DM (13% with preexisting CVD) receiving SGLT‐2 inhibitors had a 51% lower risk of all‐cause mortality compared with a propensity‐matched cohort of patients receiving other oral glucose‐lowering drugs, but the effect on MACE risk was not reported. Among patients with T2DM and established CVD, compared with non‐SGLT2 inhibitors, initiation of therapy with an SGLT‐2 inhibitor was associated with a 33% lower risk of MACE.39 The differences seen between CVOTs and routine clinical care data are the likely consequence of the overestimation of the effectiveness of these medications in the latter setting.
Two recent large‐scale studies assessed the cardiovascular effects of liraglutide as compared with DPP‐4 inhibitors40 and SGLT‐2 inhibitors as compared with sulfonylureas41 in routine clinical practice. Both reported sensitivity analyses according to the presence/absence of CVD at baseline. Data from nationwide registers in Denmark and Sweden (2010‐2016) indicate that 3‐point MACE occurred significantly less frequently when liraglutide was used instead of a DPP‐4 inhibitor (HR 0.90, 95% CI 0.83‐0.98). The HRs were 0.81 (95% CI 0.71‐0.92) for patients with a history of CVD (19%, n=4491) and 0.96 (0.86‐1.06) for patients without such a history (81%, n=18 911), suggesting that patients with CVD might derive the largest absolute benefit from treatment with liraglutide.40 In a retrospective cohort analysis, a total of 125 534 patients were included in the SGLT‐2 inhibitor/sulfonylurea cohort (n=62 767 per exposure group); use of SGLT‐2 inhibitors was associated with a decreased risk of developing cardiovascular events as compared with use of sulfonylureas (HR, 0.50; 0.45‐0.55), with no significant difference in effect when stratifying by baseline CVD presence (HR=0.51, 0.43‐0.60) or absence (HR=0.56, 0.49‐0.64).41 These data confirm that liraglutide may produce the largest absolute cardiovascular benefit in patients with preexisting CVD; on the other hand, they add further emphasis to the disproportional increased cardiovascular benefit in clinical practice, as compared with CVOTs. Confirming previous observational data (CVD‐REAL), most people taking SGLT‐2 inhibitors in routine clinical care are those without CVD, and even in this population the drugs can lower MACE risk. According to 1 large‐scale study with 803 836 patients,42 the general T2DM population has less prevalent CVD (25% to 44%), and patients are slightly older than those included in the CVOTs. Although observational data have important limitations,43 they are thought to be complementary to those from randomized controlled trials in supporting the beneficial cardiovascular effects of both GLP‐1 receptor agonists and SGLT‐2 inhibitors.44
Ideal Patient: T2DM With CVD
T2DM is a complex and heterogeneous disease, with CVD representing its main and often fatal complication. In high‐risk patients with T2DM and CVD, the best possible glycemic control obtained with the older glucose‐lowering medications (different combinations of metformin, sulfonylureas, thiazolidinediones, glinides, and insulin) is unlikely to improve their cardiovascular outlook, although it can improve kidney and eye vascular complications.45 The newer glucose‐lowering drugs, in particular SGLT‐2 inhibitors and GLP‐1 agonists, have demonstrated a consistent effect of reducing MACE risk in both CVOTs and in routine clinical care; therefore, they should be included in the therapeutic plan of these patients, for most in addition to the existing glucose‐lowering therapy. In addition to their benefits on MACE risk, SGLT‐2 inhibitors and, to a lesser extent, GLP‐1 agonists display protective actions on the kidney that may be useful to delay diabetic nephropathy.46 In patients with T2DM and kidney disease, for example, the risk of kidney failure and cardiovascular events was reduced by 30% with canagliflozin as compared to placebo at a median follow‐up of 2.6 years.47 Last, SGLT‐2 inhibitors, but not GLP‐1 receptor agonists, exert a robust and consistent reduction (≈30%) in the risk of heart failure regardless the presence of established atherosclerotic CVD.21 Thus, there are many reasons for adding a newer glucose‐lowering drug in people with T2DM and preexisting CVD: at present, this patient is the ideal, although not the exclusive one, in whom SGLT‐2 inhibitors and GLP‐1 agonists exert their best cardiorenal effects.
In patients with T2DM and no preexisting CVD, the best possible glycemic control can reduce MACE by 9%; this can be obtained with any combination of older glucose‐lowering drugs. In consideration of the cardiorenal protective effect of SGLT‐2 inhibitors on both nephropathy (reduced progression of kidney disease) and heart failure (demonstrated protection in primary prevention),21 their use should also be taken into consideration by clinicians.
Areas of Uncertainty
The evidence for a MACE benefit of both SGLT‐2 inhibitors and GLP‐1 agonists in patients with well‐controlled A1C (<7%) is limited, as the patients with both T2DM and preexisting CVD in most CVOTs (LEADER, SUSTAIN‐6, CANVAS) had a baseline A1C value ≥7%. It has been stated that the cardiovascular benefit of both GLP‐1 agonists and SGLT‐2 inhibitors appears to be unrelated to the direct glucose‐lowering effects of these agents.44 That statement does not take into account the evidence that part of their cardiovascular benefit, at least for MACE, is mediated by amelioration of glycemic control, as herein discussed. Moreover, acknowledging the contributive role for the blood glucose reduction in decreasing the risk of MACE during treatment with GLP‐1 agonists and SGLT‐2 inhibitors may overcome some residual reluctance of conservative specialists, who are still not ready to acknowledge that drugs might work in diabetes mellitus independently of blood glucose.
Conclusions
T2DM and CVD are so close to have merited the nickname of “deadly duo” (more than 1 million results in Google at the input “deadly duo for diabetes”). Because the prevalence of CVD in the T2DM population may range from 24% (real‐world) to 100% (CVOTs),42 at least one fourth of the average T2DM outpatients are possible candidates for the use of the newer glucose‐lowering medications demonstrated to improve the MACE outcome. Liraglutide, semaglutide, and albiglutide for the GLP‐1 agonist class and empagliflozin and canagliflozin for the SGLT‐2 inhibitor class have been demonstrated to significantly reduce the risk of MACE in CVOTs, although this effect is limited to T2DM patients with preexisting CVD.
Sources of Funding
This study was supported in part by Associazione Salute con Stile and by the Department of Advanced Medical and Surgical Sciences, Università della Campania Luigi Vanvitelli, Naples, Italy.
Disclosures
Giugliano reports honoraria for speaking at meetings from Novartis, Sanofi‐Aventis, Lilly, AstraZeneca, and NovoNordisk. Esposito reports honoraria for speaking at meetings from Novartis, Sanofi‐Aventis, Lilly, AstraZeneca, Boehringer Ingelheim, and NovoNordisk. Maiorino reports honoraria for speaking at meetings from Lilly and NovoNordisk. The remaining authors have no disclosures to report.
Supporting information
Table S1. PRISMA Checklist
(J Am Heart Assoc. 2019;8:e012356 DOI: 10.1161/JAHA.119.012356.)
References
- 1. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. [DOI] [PubMed] [Google Scholar]
- 2. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 3. Olesen KKW, Madsen M, Egholm G, Thim T1, Jensen LO, Raungaard B, Bøtker HE, Sørensen HT, Maeng M. Patients with and without diabetes without significant angiographic coronary artery disease have the same risk of myocardial infarction in a real‐world population receiving appropriate prophylactic treatment. Diabetes Care. 2017;40:1103–1110. [DOI] [PubMed] [Google Scholar]
- 4. Pagidipati NJ, Navar AM, Pieper KS, Green JB, Bethel MA, Armstrong PW, Josse RG, McGuire DK, Lokhnygina Y, Cornel JH, Halvorsen S, Strandberg TE, Delibasi T, Holman RR, Peterson ED; TECOS Study Group . Secondary prevention of cardiovascular disease in patients with type 2 diabetes: international insights from the TECOS trial. Circulation. 2017;136:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipska KJ, Yao X, Herrin J, McCoy RG, Ross JS, Steinman MA, Inzucchi SE, Gill TM, Krumholz HM, Shah ND. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40:1469–1478. [DOI] [PubMed] [Google Scholar]
- 7. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 8. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta‐analysis. Diabetes Care. 2012;35:2402–2411. DOI: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon‐like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Care. 2017;40:614–624. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration . Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes[Internet]. Available at: www.fda.gov/downloads/Drugs/Guidance ComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed January 17, 2019.
- 11. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG. Controlling the risk of spurious findings from metaregression. Stat Med. 2004;23:1663–1682. [DOI] [PubMed] [Google Scholar]
- 14. Control Group , Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. [DOI] [PubMed] [Google Scholar]
- 15. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 16. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail‐Beigi F, Grimm RH Jr, Probstfield JL, Simons‐Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ADVANCE Collaborative Group , Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 18. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 19. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 20. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Glycemic control in type 2 diabetes: from medication nonadherence to residual vascular risk. Endocrine. 2018;61:23–27. DOI: 10.1007/s12020-017-1517-9. [DOI] [PubMed] [Google Scholar]
- 21. Giugliano D, Meier JJ, Esposito K. Heart failure and type 2 diabetes: from CVOTs, with hope. Diabetes Obes Metab. 2019;21:1081–1087. [DOI] [PubMed] [Google Scholar]
- 22. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR‐TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 23. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 24. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 25. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK; CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk. The CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 27. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN‐6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 29. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group . Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes Committees and Investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 31. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 32. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 33. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 34. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Circulation. 2017;136:849–870. [DOI] [PubMed] [Google Scholar]
- 35. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 36. Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators . Hypoglycemia, cardiovascular outcomes, and death: the LEADER experience. Diabetes Care. 2018;41:1783–1791. [DOI] [PubMed] [Google Scholar]
- 37. Barer Y, Cohen O, Cukierman‐Yaffe T. Effect of glycaemic control on cardiovascular disease in individuals with type 2 diabetes with pre‐existing cardiovascular disease: a systematic review and meta‐analysis. Diabetes Obes Metab. 2019;21:732–735. [DOI] [PubMed] [Google Scholar]
- 38. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P; CVD‐REAL Investigators and Study Group . Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation. 2017;136:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Udell JA, Yuan Z, Rush T, Sicignano N, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL population‐based cohort study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation. 2018;137:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svanström H, Ueda P, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Pasternak B. Use of liraglutide and risk of major cardiovascular events: a register‐based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. 2019;7:106–114. DOI: 10.1016/S2213-8587(18)30320-6. [DOI] [PubMed] [Google Scholar]
- 41. Dawwas GK, Smith SM, Park H. Cardiovascular outcomes of sodium glucose cotransporter‐2 inhibitors in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:28–36. [DOI] [PubMed] [Google Scholar]
- 42. Birkeland KI, Bodegard J, Norhammar A, Kuiper JG, Georgiado E, Beekman‐Hendriks WL, Thuresson M, Pignot M, Herings RMC, Kooy A. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2018. DOI: 10.1111/dom.13612. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41:6–10. [DOI] [PubMed] [Google Scholar]
- 44. Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, Hayward RA, Craven T, Coleman RL, Chalmers J; Collaborators on Trials of Lowering Glucose (CONTROL) Group . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomized controlled trials. Lancet Diabetes Endocrinol. 2017;5:431–437. [DOI] [PubMed] [Google Scholar]
- 46. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 47. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019. DOI: 10.1056/NEJMoa1811744. [Epub ahead of print]. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA Checklist


