Abstract
Background
Vasovagal reflex is the most common form of syncope, but the pathophysiological mechanisms that initiate the reflex are not well understood. We aimed to study supine and early orthostatic levels of the neurohormones involved in control of circulatory homeostasis in relation to the onset of tilt‐induced vasovagal syncope (VVS).
Methods and Results
A total of 827 patients who were investigated for unexplained syncope with head‐up tilt test (HUT) and optional nitroglycerin provocation (Italian protocol) had blood samples collected while supine and after 3‐minutes of HUT. Of these, 173 (20.9%) patients developed VVS during drug‐free HUT, 161 of whom (males 44.7%; age 45±21 years) had complete data. We analyzed levels of epinephrine, norepinephrine, C‐terminal pro–arginine vasopressin, C‐terminal endothelin‐1, and midregional fragments of pro–atrial natriuretic peptide and pro‐adrenomedullin in relation to time from tilt‐up to onset of VVS. We applied a linear regression model adjusted for age and sex. The mean time to syncope was 11±7 minutes. Older age (β=0.13; SE=0.03, P<0.001), higher supine systolic blood pressure (β=0.06; SE=0.03, P=0.02), and higher supine midregional fragment of pro‐adrenomedullin predicted longer time to syncope (β=2.31; SE=0.77, P=0.003), whereas supine levels of other neurohormones were not associated with time to syncope. Among 151 patients who developed VVS later than 3 minutes of HUT, increase in epinephrine (β=−3.24; SE=0.78, P<0.001) and C‐terminal pro–arginine vasopressin (β=−2.07; SE=0.61, P=0.001) at 3 minutes of HUT were related to shorter time to syncope.
Conclusions
Older age, higher blood pressure, and higher level of pro‐adrenomedullin are associated with later onset of VVS during tilt testing, whereas greater increase of tilt‐induced epinephrine and vasopressin release correlate with shorter time to syncope.
Keywords: autonomic function, biomarker, neurocardiology, syncope
Subject Categories: Electrophysiology
Short abstract
See Editorial Williford et al
Clinical Perspective
What Is New?
This study highlights that age, increased supine systolic blood pressure, and higher level of pro‐adrenomedullin are associated with later onset of vasovagal syncope during tilt testing, whereas greater increase of tilt‐induced epinephrine and vasopressin release correlate with shorter time to syncope.
What Are the Clinical Implications?
Characterization of the neuroendocrine profile in vasovagal syncope may assist in the development of effective strategies for syncope prevention in patients experiencing recurrent and unpredicted events.
Introduction
Syncope is a common clinical condition having many possible causes,1, 2 with vasovagal reflex syncope (VVS) being by far the most common. Despite extensive studies, the basis of the VVS reflex remains incompletely understood.1 In this regard the head‐up tilt test (HUT) is routinely used in clinical practice not only for diagnosing VVS susceptibility but also for studying its pathophysiology.1
Currently, it is unclear whether neurohormones that are involved in cardiovascular homeostasis contribute to VVS pathophysiology. Further, it is uncertain whether neurohormones contribute directly to triggering VVS or if they instead reflect a compensatory mechanism caused by hemodynamic stress.3 Defining the neuroendocrine pathophysiology of VVS may determine potential targets for therapy or diagnostic testing.
Previous studies have indicated that resting and orthostatic levels of various neurohormones, such as catecholamines, vasopressin, adrenomedullin, and natriuretic peptides are associated with vasovagal reflex susceptibility.4, 5, 6, 7 Conversely, in the setting of HUT‐induced VVS, a decrease in circulating levels of atrial natriuretic peptide is associated with VVS. Additionally, increased levels of vasopressin and epinephrine have been reported before VVS.6, 8, 9 However, it is unclear whether or not the magnitude of neuroendocrine changes during orthostatic challenge correlates with VVS susceptibility.
Consequently, we studied changes in supine and early orthostatic levels of neurohormones involved in control of circulatory homeostasis with the goal of assessing their relation to the onset of the tilt‐induced VVS reflex. Utilizing the concept that a shorter time to syncope during HUT reflected greater VVS susceptibility,8 we hypothesized that greater neuroendocrine changes during HUT would correlate with a shorter time to syncope.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Patient Population
The present study was conducted from August 27, 2008 through December 31, 2014 as a part of the ongoing SYSTEMA (Syncope Study of Unselected Population in Malmö) project.6, 10 Patients with unexplained syncope were referred to the tertiary syncope unit at Skåne University Hospital in Malmö from primary care and hospitals in southern Sweden. The syncope unit is the only referral center in the catchment area of about 1.5 million citizens. Unexplained syncope was defined as transient loss of consciousness without established diagnosis after the initial evaluation.1, 2 Before the standard diagnostic workup at the syncope unit, which included cardiovascular autonomic testing and HUT according to the Italian protocol,11 additional tests were carried out: resting, exercise, and continuous 24‐hour (Holter) ECG, external event recorder, echocardiography, coronary angiography, brain imaging, and electroencephalography, if appropriate and recommended by the referring physician. Patients with an established diagnosis of cardiac syncope were excluded.
During the study period, 1141 consecutive patients (60.6% women; age 53±22 years) were enrolled. Of these, 827 patients had blood samples collected from an intravenous line obtained both in supine position and after 3 minutes of HUT as per the initial SYSTEMA study protocol.6 Among patients with collected blood samples, 466 (56.3%) were diagnosed with VVS according to the current syncope management guidelines, that is, a positive HUT with a typical pattern of sudden‐onset hypotension and bradycardia accompanied by the reproduction of characteristic symptoms.1, 2 Using a concept previously applied by Kohno and collaborators,8 we identified 173 patients (20.9%) who developed VVS during the drug‐free HUT; of these a total of 161 patients had no missing values and were analyzed. Figure 1 illustrates the flowchart of the study.
Figure 1.
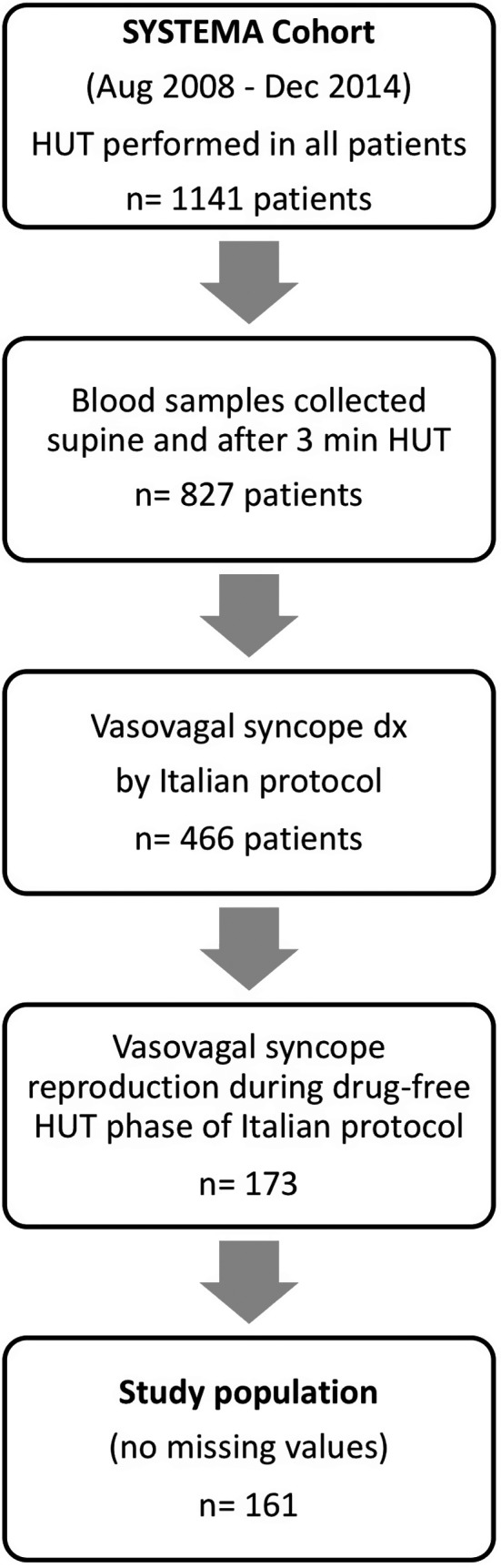
Study design and patient selection process. From the cohort of patients investigated in 2008‐2014 for unexplained syncope with head‐up tilt testing (HUT) according to the Italian protocol,11 ie, a 20‐minute drug‐free HUT phase followed by 15‐minute nitroglycerin‐potentiated phase, 872 had blood samples collected while supine and after 3 minutes of HUT. A total of 173 patients developed and reproduced vasovagal syncope during the drug‐free phase. Of these, 161 had no missing values and were included in the analyses of 6 neurohormones vs time to syncope onset during the drug‐free HUT phase. SYSTEMA indicates Syncope Study of Unselected Population in Malmö.
Examination Protocol and Blood Samples
Patients were instructed to take their regular medications and fast for 2 hours before the test but were allowed to drink water. Data on past medical history and the characteristics of syncope‐related symptoms were collected using a self‐administered questionnaire. The examination included a standard HUT according to the Italian protocol,11 that is, a drug‐free HUT phase of 20 minutes or until syncope occurred, followed by an optional 15 minutes of drug‐potentiated phase with sublingual 400 μg nitroglycerin, if the drug‐free phase was negative. Beat‐to‐beat blood pressure and electrocardiogram were monitored continuously by a validated noninvasive method (Nexfin monitor; BMEYE, Amsterdam, the Netherlands).12 Blood samples were collected from an intravenous line installed on the left arm ≈10 minutes before the test. Blood samples were obtained both in a supine position and after 3 minutes of HUT. The time point of 3 minutes of HUT for the assessment of circulating neurohormones was based on previous physiological studies13 indicating that the thoracic blood volume displacement to the lower body, heart rate, and the total peripheral resistance usually reach a steady state after 3 minutes of active standing or HUT, which implies that the initial neurohormonal responses are fully developed at that time. The first author (P.T.) performed an offline post hoc analysis of HUT records and retrieved the time‐to‐syncope variable for each patient with VVS during the drug‐free HUT.
Neuroendocrine Measurements
The assessment of plasma neurohormone levels has been described previously.6
Briefly, we analyzed baseline and 3‐minute HUT levels of epinephrine, norepinephrine, C‐terminal pro–arginine vasopressin (CT‐proAVP), C‐terminal pro‐endothelin‐1, midregional fragments of pro–atrial natriuretic peptide, and pro‐adrenomedullin (MR‐proADM). The analyzed nonactive peptides (CT‐proAVP, C‐terminal pro‐endothelin‐1, midregional pro– atrial natriuretic peptide, and MR‐proADM) are generated from the pro‐neurohormone molecule in the ratio 1:1 in relation to the active neuropeptide and are more stable than their corresponding biologically active substances. We used high‐performance liquid chromatography with a fluorescence detection method for catecholamines14 and ThermoScientific BRAHMS assays (BRAHMS GmbH, ThermoFisher Scientific, Hennigsdorf, Germany) for measurement of CT‐proAVP, C‐terminal pro‐endothelin‐1, midregional pro–atrial natriuretic peptide, and MR‐proADM, according to the manufacturer's instructions.15, 16
The study protocol was approved by the regional ethical review board in Lund, Sweden (reference no 82/2008). Written informed consent was obtained from all study participants.
Statistical Analysis
The main characteristics of the study population are presented as mean and standard deviation for normally distributed continuous variables, as median and interquartile range for non–normally distributed variables, and percentages for categorical variables.
We applied a linear regression model adjusted for age and sex. We entered time to syncope while in the 70° head‐up position as the dependent variable and neurohormone concentration at baseline, after 3‐minutes HUT, and Δ value (3‐minute HUT minus baseline) as the independent variables. All patients who developed VVS before the 3‐minute time point during HUT were excluded from the orthostatic change analyses because the protocol called for the blood sample to be collected at 3 minutes of upright posture. Neurohormone levels were log‐transformed and standardized (expressed per 1 standard deviation). The normality assumption of residuals for each linear model was checked by histograms and normal quantile‐quantile plots of residuals. The plots showed no violation of the normality assumption. Statistical analyses were carried out using IBM SPSS Statistics version 25 (SPSS Inc, Chicago, IL). All tests were 2‐sided, and P<0.05 was considered significant.
Results
Study Population Characteristics
The main characteristics of the study population are presented in Table 1. The study population included 161 patients with tilt‐induced VVS during drug‐free HUT (56.3% women; age 45±21 years). The mean time to syncope was 11±7 minutes. Ten patients were tilted down due to syncope before the upright blood sample could be collected, that is, within the first 3 minutes of HUT and were, therefore, excluded from the analysis.
Table 1.
Clinical and Hemodynamic Characteristics of the Study Population (n=161)
| Characteristic | |
|---|---|
| Age, y | 45±21 |
| Sex (male), n (%) | 72 (44.7) |
| Body mass index, kg/m2 | 24.7±4.1 |
| Number of syncopal episodes, median (IQR) | 5 (2‐15) |
| Traumatic falls without warning, n (%) | 84 (52.2) |
| Time to syncope, min | 11±7 |
| Mean systolic BP supine, mm Hg | 129.1±20.1 |
| Mean diastolic BP supine, mm Hg | 70.4±8.7 |
| Mean systolic BP standing 3 min, mm Hg | 121.0±21.8 |
| Mean diastolic BP standing 3 min, mm Hg | 73.2±11.8 |
| Mean heart rate supine, bpm | 67.2±10.5 |
| Mean heart rate standing 3 min, bpm | 83.1±16.5 |
| Use of β‐blockers, n (%) | 17 (10.6) |
| Use of calcium channel blockers, n (%) | 12 (7.5) |
| Use of RAAS antagonists, n (%) | 24 (14.9) |
| Use of diuretics, n (%) | 18 (11.2) |
Data are presented as mean±SD unless otherwise indicated. BP indicates blood pressure; IQR, interquartile range; RAAS, renin‐angiotensin‐aldosterone system.
Older age (β=0.13; SE=0.03, P<0.001) and higher supine systolic blood pressure (β=0.06; SE=0.03, P=0.02) but neither sex (β=0.57; SE=1.12, P=0.61) nor higher supine heart rate (β=0.01; SE=0.05, P=0.88) predicted longer time to syncope.
Neurohormones and Time to Syncope
The overall neurohormone plasma concentration levels are shown in Table 2. As can be seen in Table 3, higher baseline supine MR‐proADM predicted longer time to syncope (β=2.31; SE=0.77, P=0.003), whereas other baseline supine neurohormones were not associated with time to syncope. On the other hand, increased levels of epinephrine (β=−2.13; SE=0.59, P<0.001) and CT‐proAVP (β=−1.39; SE=0.68, P=0.043) after 3 minutes of HUT were predictive of shorter time to syncope, whereas higher 3‐minute MR‐proADM (β=1.76; SE=0.82, P=0.035) was associated with prolonged time to syncope.
Table 2.
Plasma Concentrations of Assessed Neurohormones
| Neurohormone | Concentration |
|---|---|
| Epinephrine (0) (nmol/L) | 0.12 (0.12) |
| Epinephrine (3) (nmol/L) | 0.22 (0.28) |
| Norepinephrine (0) (nmol/L) | 1.60 (1.30) |
| Norepinephrine (3) (nmol/L) | 2.75 (1.88) |
| CT‐proAVP (0) (pmol/L) | 6.01 (6.45) |
| CT‐proAVP (3) (pmol/L) | 6.81 (10.5) |
| CT‐proET‐1 (0) (pmol/L) | 48.0 (19.5) |
| CT‐proET‐1 (3) (pmol/L) | 43.6 (22.2) |
| MR‐proANP (0) (pmol/L) | 51.4 (44.0) |
| MR‐proANP (3) (pmol/L) | 51.5 (44.9) |
| MR‐proADM (0) (pmol/L) | 0.47 (0.21) |
| MR‐proADM (3) (pmol/L) | 0.42 (0.23) |
Plasma concentrations of neurohormones are given as median (interquartile range) for supine (0) and 3‐minute HUT (3). CT‐proAVP indicates C‐terminal pro–arginine vasopressin; CT‐proET‐1, C‐terminal pro–endothelin‐1; MR‐proADM, midregional fragment of pro‐adrenomedullin; MR‐proANP, midregional fragment of pro–atrial natriuretic peptide.
Table 3.
Relation Between Neurohormone Level and Time to Syncope in Linear Regression Model Adjusted for Age and Sex
| Neurohormone | Supine (n=161) | P Value | 3‐Minute HUT (n=151) | P Value | Δ Value (n=151) | P Value |
|---|---|---|---|---|---|---|
| Epinephrine | −0.71 (0.59) | 0.23 | −2.13 (0.59)a | <0.001a | −3.24 (0.78)a | <0.001a |
| Norepinephrine | 0.15 (0.65) | 0.82 | 0.97 (0.66) | 0.14 | 2.49 (1.83) | 0.18 |
| CT‐proAVP | 0.46 (0.61) | 0.45 | −1.39 (0.68)a | 0.043a | −2.07 (0.61)a | 0.001a |
| CT‐proET‐1 | 0.96 (0.66) | 0.15 | 0.70 (0.58) | 0.23 | 0.57 (0.84) | 0.50 |
| MR‐proANP | 0.50 (0.77) | 0.52 | −0.14 (0.71) | 0.84 | −0.33 (2.25) | 0.88 |
| MR‐proADM | 2.31 (0.77)a | 0.003a | 1.76 (0.82)a | 0.035a | 1.83 (1.62) | 0.26 |
Data are reported as β‐coefficient and standard error per 1 SD. CT‐proAVP indicates C‐terminal pro–arginine vasopressin; CT‐proET‐1, C‐terminal pro–endothelin‐1; HUT, head‐up tilt test; MR‐proADM, midregional fragment of pro‐adrenomedullin; MR‐proANP, midregional fragment of pro–atrial natriuretic peptide.
P<0.05.
Supine MR‐proADM, Δ (ie, 3‐minute HUT minus supine) epinephrine, and Δ CT‐proAVP concentrations were significantly different across tertiles of the time‐to‐syncope variable (Figures 2, 3 through 4). Accordingly, higher Δ epinephrine (β=−3.24; SE=0.78, P<0.001) and Δ CT‐proAVP (β=−2.07; SE=0.61, P=0.001) concentrations were associated with shorter time to syncope.
Figure 2.
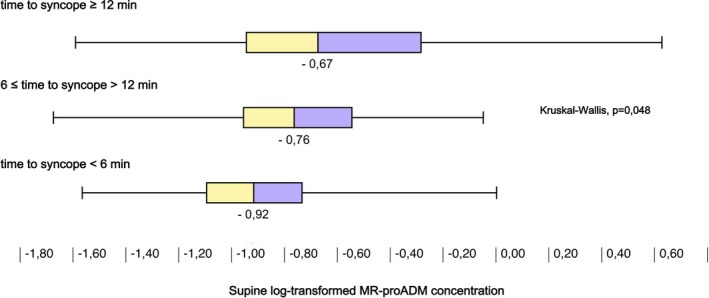
Supine concentration of midregional pro‐adrenomedullin (MR‐proADM) grouped by tertiles of time to syncope during drug‐free head‐up tilt test. Higher levels of circulating MR‐proADM were associated with longer time to vasovagal reflex onset.
Figure 3.
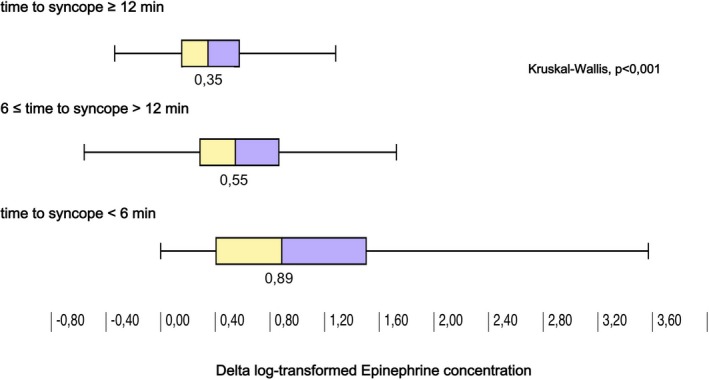
Increase in concentration of epinephrine after 3 minutes of head‐up tilt (HUT) grouped by tertiles of time to syncope during drug‐free HUT. Greater increase in epinephrine was associated with shorter time to vasovagal reflex onset.
Figure 4.
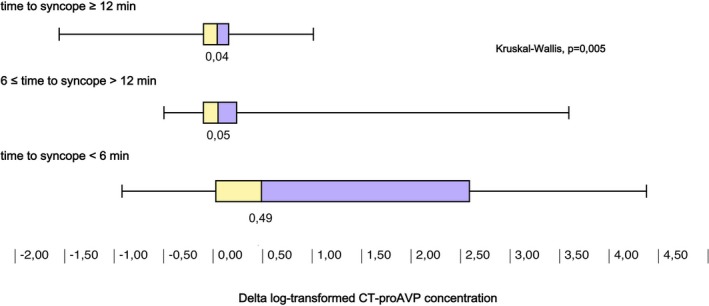
Increase in concentration of C‐terminal pro–arginine vasopressin (CT‐proAVP) after 3 minutes of head‐up tilt test (HUT) grouped by tertiles of time to syncope during drug‐free HUT. Greater increase in CT‐proAVP was associated with shorter time to vasovagal reflex onset.
Discussion
This study examined the hypothesis that greater neuroendocrine changes during a head‐up tilt test would correlate with a shorter time to initiation of the VVS reflex. We observed that a greater presyncopal increase in epinephrine and CT‐pro‐AVP during tilt testing correlated significantly with shorter time to onset of VVS. Further, higher supine MR‐pro‐ADM was associated with a longer time to HUT‐induced VVS, as also were older age and higher systolic blood pressure.
Epinephrine
Previous reports have demonstrated higher orthostatic levels of epinephrine in patients with HUT‐induced VVS compared with HUT‐negative controls.4, 7, 17, 18, 19, 20 However, the exact function of epinephrine in VVS is not completely understood. Does epinephrine act as a direct trigger of VVS or reflect a compensatory mechanism caused by hemodynamic stress? Nilsson et al21 have shown that orthostatic levels of epinephrine are higher in patients with VVS evoked during the drug‐free phase of HUT compared with patients in whom VVS was triggered by addition of nitroglycerin, indicating an association between higher epinephrine levels and increased susceptibility to VVS. However, in that study the relation between the magnitude of epinephrine increase in syncope and the timing of the reflex was not assessed.
Other studies4, 19 have shown that epinephrine begins to rise in patients with HUT‐induced drug‐free VVS before any hemodynamic changes can be observed. This suggests that epinephrine directly affects the vasovagal reflex–triggering mechanism and is not merely a compensatory mechanism. To support this hypothesis, a smaller, more recent study8 examined the relations between time to syncope and catecholamine concentrations and found that higher levels of supine and orthostatic epinephrine, but not norepinephrine, correlate with shorter time to syncope, suggesting a direct role of epinephrine in triggering VVS. Our study, including a much larger population of VVS patients, confirms the latter observation that a higher orthostatic, but not supine, epinephrine level on HUT correlates with shorter time to the onset of VVS reflex.
Vasopressin
Previous studies have shown vasopressin levels to be raised in patients with HUT‐induced VVS compared with HUT‐negative controls.4, 9, 18, 22 Additionally, Nilsson et al21 found that orthostatic CT‐proAVP levels were higher in patients with VVS induced by drug‐free HUT compared with patients in whom HUT required the addition of nitroglycerin to induce VVS; the latter observation suggests increased VVS susceptibility in the former subset of fainters. In terms of pathophysiology, vasopressin is believed to increase the sensitivity of cardiac baroreceptors,23 an effect that is likely to promote the vasovagal reflex. Our study, in which greater CT‐proAVP increase appeared to shorten time to HUT‐induced VVS, supports the concept that AVP plays a role in VVS susceptibility.
Vasopressin is released in response to hyperosmolarity and reduced blood volume and blood pressure, leading to water retention in renal collecting tubes and vasoconstriction.24
Vasopressin acts as a backup system to the renin‐angiotensin and sympathetic systems, and when they are intact, it is not critical for hemodynamic stability.24 If the latter vasopressin function is relevant in VVS, it implies that the vasopressin increase before syncope is a second‐line compensation mechanism caused by hemodynamic instability. However, additionally, vasopressin can modulate its own effects by increasing vagal tone and decreasing sympathetic activity,22, 23, 24 which suggests that it could also have a direct effect on VVS pathophysiology.
Adrenomedullin
Adrenomedullin (ADM) maintains the endothelial barrier function to prevent vascular leakage and edema. Moreover, ADM is released by systemic volume overload, and it has vasodilatory and natriuretic effects.25 Previous, mostly small, studies on ADM levels have reported inconsistent results. Rossler et al26 found that ADM increases in healthy subjects during orthostatic stress. Plasek et al27 reported no significant difference in ADM levels in a small study comparing HUT‐positive patients with a HUT‐negative control group. Hinghofer‐Szalkay et al28 observed no difference in baseline or orthostatic ADM levels in a small study of healthy subjects when presyncope was induced with HUT and lower‐body negative pressure. Hamrefors et al5 observed that in patients >40 years, lower supine MR‐pro‐ADM predicted asystolic VVS on HUT, although orthostatic levels of the biomarker were not assessed. They also reported a linear trend of low ADM levels in asystolic VVS, higher levels in noncardioinhibition VVS, and the highest levels in HUT‐negative subjects, indicating increasing VVS severity with lower ADM levels. Supporting the latter observation,5 the present study shows that higher baseline MR‐proADM during HUT correlates with a longer time to syncope, which suggests a protective effect of ADM against the main VVS reflex triggers: diminishing intravascular volume and reduction of cardiac output.29 Thus, higher habitual levels of ADM may indicate increased resistance against orthostatic volume shift by decreasing vascular wall permeability.
Age
In a study of 208 patients with VVS on HUT, McGavigan and Hood30 found no correlation between age and time to the onset of vasovagal syncope. On the other hand, others have found that a cardioinhibitory response during HUT is more common in younger patients,5, 31 indicating less dramatic vasovagal reflex onset with older age. Our study shows that older age correlates with longer time to syncope and supports this concept. Whether VVS susceptibility depends on aging of neural cardiovascular reflexes with, for instance, slower transmission of neural signals and reduced release of neurotransmitters is a hypothesis warranting further studies.
Systolic Blood Pressure
We observed that higher resting supine systolic blood presure correlates with longer time to syncope. This finding is not at all controversial because it is well known that afferent firing of baroreceptors and consequent sympathetic activation possibly leading to epinephrine spillover and VVS are dependent on the resting blood pressure level—the lower the systolic blood pressure, the higher the baroreceptor afferent activity.32
Limitations
The interpretation of findings in this study is subject to several limitations. First, the concept that earlier triggering of VVS during HUT is a reasonable model of events leading to the initiation of spontaneous VVS is as yet unproven. However, true spontaneous VVS does not lend itself to comparable evaluation. Second, neurohormones were assessed only at baseline and after 3 minutes of HUT, and further changes in levels of neurohormones might have occurred at the time of syncope or after syncope. Third, multiple other neurohormones not included in our studies and currently unmeasured may contribute to VVS. Finally, certain circulating neurohormones represent the “overflow” from sites of action (eg, synapses), and their functional role must be considered in that light.
Conclusions
In conclusion, our findings suggest that susceptibility to HUT‐induced VVS is inversely related to age, higher supine systolic blood pressure, and a higher circulating level of adrenomedullin. During orthostasis, greater increase in epinephrine and vasopressin herald imminent onset of VVS. Together, these observations may assist development of new strategies for VVS prevention in patients experiencing frequent and unpredicted events.
Disclosures
Fedorowski reports personal fees from Cardiome Corp, Thermofisher, and Medtronic. Sutton reports personal fees from Medtronic Inc, and Abbott Laboratories Inc outside the submitted work; performs consultancy for Medtronic Inc; is a member of the Speaker's Bureau of Abbott Laboratories Inc; and is a shareholder in Boston Scientific Inc, Edwards Lifesciences Inc, and AstraZeneca PLC. Benditt is supported in part by a grant from the Dr Earl E. Bakken family in support of Heart‐Brain research. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e012559 DOI: 10.1161/JAHA.119.012559.)
References
- 1. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martin A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group . 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 2. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, Olshansky B, Raj SR, Sandhu RK, Sorajja D, Sun BC, Yancy CW. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e25–e59. [DOI] [PubMed] [Google Scholar]
- 3. Fedorowski A, Sutton R. Understanding vasovagal syncope akin to the philosopher's stone? J Cardiovasc Electrophysiol. 2019;30:297–298. [DOI] [PubMed] [Google Scholar]
- 4. Jardine DL, Melton IC, Crozier IG, Bennett SI, Donald RA, Ikram H. Neurohormonal response to head‐up tilt and its role in vasovagal syncope. Am J Cardiol. 1997;79:1302–1306. [DOI] [PubMed] [Google Scholar]
- 5. Hamrefors V, Nilsson D, Melander O, Sutton R, Low Fedorowski A. Adrenomedullin and endothelin‐1 predict cardioinhibitory response during vasovagal reflex in adults over 40 years of age. Circ Arrhythm Electrophysiol. 2017;10:e005585. [DOI] [PubMed] [Google Scholar]
- 6. Fedorowski A, Burri P, Struck J, Juul‐Moller S, Melander O. Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med. 2013;273:359–367. [DOI] [PubMed] [Google Scholar]
- 7. Benditt DG, Detloff BL, Adkisson WO, Lu F, Sakaguchi S, Schussler S, Austin E, Chen LY. Age‐dependence of relative change in circulating epinephrine and norepinephrine concentrations during tilt‐induced vasovagal syncope. Heart Rhythm. 2012;9:1847–1852. [DOI] [PubMed] [Google Scholar]
- 8. Kohno R, Detloff BLS, Chen LY, Norby FL, Benditt DG. Greater early epinephrine rise with head‐up posture: a marker of increased syncope susceptibility in vasovagal fainters. J Cardiovasc Electrophysiol. 2019;30:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzpatrick A, Williams TR, Ahmed R, Lightman SL, Bloom SR, Sutton R. Echocardiographic end endocrine changes during vasovagal syncope induced by prolonged head‐up tilt. Europace. 1992;2:121–128. [Google Scholar]
- 10. Nilsson D, Sutton R, Tas W, Burri P, Melander O, Fedorowski A. Orthostatic changes in hemodynamics and cardiovascular biomarkers in dysautonomic patients. PLoS One. 2015;10:e0128962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartoletti A, Alboni P, Ammirati F, Brignole M, Del Rosso A, Foglia Manzillo G, Menozzi C, Raviele A, Sutton R. ‘The Italian Protocol’: a simplified head‐up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace. 2000;2:339–342. [DOI] [PubMed] [Google Scholar]
- 12. Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH, Wesseling KR, Blanc S, Wieling W, van Montfrans GA, Settels JJ, Wesseling KH, Westerhof BE. Nexfin noninvasive continuous blood pressure validated against Riva‐Rocci/Korotkoff. Am J Hypertens. 2009;22:378–383. [DOI] [PubMed] [Google Scholar]
- 13. Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. [DOI] [PubMed] [Google Scholar]
- 14. van der Hoorn FA, Boomsma F, Man in ‘t Veld AJ, Schalekamp MA. Determination of catecholamines in human plasma by high‐performance liquid chromatography: comparison between a new method with fluorescence detection and an established method with electrochemical detection. J Chromatogr. 1989;487:17–28. [DOI] [PubMed] [Google Scholar]
- 15. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- 16. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. [DOI] [PubMed] [Google Scholar]
- 17. Sra JS, Murthy V, Natale A, Jazayeri MR, Dhala A, Deshpande S, Sheth M, Akhtar M. Circulatory and catecholamine changes during head‐up tilt testing in neurocardiogenic (vasovagal) syncope. Am J Cardiol. 1994;73:33–37. [DOI] [PubMed] [Google Scholar]
- 18. Roul G, Riehl‐Aleil V, Germain P, Bareiss P. Neurohormonal profile before and after beta‐blockade in patients with neurocardiogenic syncope. Pacing Clin Electrophysiol. 1999;22:1020–1030. [DOI] [PubMed] [Google Scholar]
- 19. Benditt DG, Ermis C, Padanilam B, Samniah N, Sakaguchi S. Catecholamine response during haemodynamically stable upright posture in individuals with and without tilt‐table induced vasovagal syncope. Europace. 2003;5:65–70. [DOI] [PubMed] [Google Scholar]
- 20. Nowak L, Nowak FG, Janko S, Dorwarth U, Hoffmann E, Botzenhardt F. Investigation of various types of neurocardiogenic response to head‐up tilting by extended hemodynamic and neurohumoral monitoring. Pacing Clin Electrophysiol. 2007;30:623–630. [DOI] [PubMed] [Google Scholar]
- 21. Nilsson D, Sutton R, Melander O, Fedorowski A. Spontaneous vs nitroglycerin‐induced vasovagal reflex on head‐up tilt: are there neuroendocrine differences? Heart Rhythm. 2016;13:1674–1678. [DOI] [PubMed] [Google Scholar]
- 22. Theopistou A, Gatzoulis K, Economou E, Sideris S, Hantzos K, Stefanadis C, Toutouzas P. Biochemical changes involved in the mechanism of vasovagal syncope. Am J Cardiol. 2001;88:376–381. [DOI] [PubMed] [Google Scholar]
- 23. Thoren P. Role of cardiac vagal C‐fibers in cardiovascular control. Rev Physiol Biochem Pharmacol. 1979;86:1–94. [DOI] [PubMed] [Google Scholar]
- 24. Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105:599–612: quiz 639–640. [DOI] [PubMed] [Google Scholar]
- 25. Krishnan B, Benditt DG. Neuropeptides and peptide hormones in syncope and orthostatic intolerance. Cardiol J. 2014;21:591–600. [DOI] [PubMed] [Google Scholar]
- 26. Rossler A, Laszlo Z, Haditsch B, Hinghofer‐Szalkay HG. Orthostatic stimuli rapidly change plasma adrenomedullin in humans. Hypertension. 1999;34:1147–1151. [DOI] [PubMed] [Google Scholar]
- 27. Plasek J, Doupal V, Furstova J, Furst T, Safarcik K, Krnacova A, Petejova N, Hrabovska Z, Martinek A, Taborsky M. The role of adrenomedullin and galanin in recurrent vasovagal syncope: a case control study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:162–167. [DOI] [PubMed] [Google Scholar]
- 28. Hinghofer‐Szalkay H, Lackner HK, Rossler A, Narath B, Jantscher A, Goswami N. Hormonal and plasma volume changes after presyncope. Eur J Clin Invest. 2011;41:1180–1185. [DOI] [PubMed] [Google Scholar]
- 29. Jardine DL, Wieling W, Brignole M, Lenders JWM, Sutton R, Stewart J. The pathophysiology of the vasovagal response. Heart Rhythm. 2018;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGavigan AD, Hood S. The influence of sex and age on response to head‐up tilt‐table testing in patients with recurrent syncope. Age Ageing. 2001;30:295–298. [DOI] [PubMed] [Google Scholar]
- 31. Noormand R, Shafiee A, Davoodi G, Tavakoli F, Gheini A, Yaminisharif A, Jalali A, Sadeghian S. Age and the head‐up tilt test outcome in syncope patients. Res Cardiovasc Med. 2015;4:e27871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thrasher TN. Baroreceptors, baroreceptor unloading, and the long‐term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R819–R827. [DOI] [PubMed] [Google Scholar]


