Abstract
Background
Only 50% of atrial fibrillation (AF) patients recommended for oral anticoagulation (OAC) use these medications, and less than half of them adhere to OAC. In a cohort of Medicare beneficiaries newly diagnosed with AF, we identified groups of patients with similar trajectories of OAC use and adherence, and evaluated patient characteristics affecting group membership.
Methods and Results
We selected continuously enrolled Medicare Part D beneficiaries with first AF diagnosis in 2014 to 2015 (n=34 898). We calculated the proportion of days covered with OAC over the first 12 months after diagnosis and identified OAC adherence trajectories using group‐based trajectory models. We constructed multinomial logistic regression models to evaluate how demographics, system‐level factors, and clinical characteristics were associated with group membership. We identified 4 trajectories of OAC adherence: patients who never used OAC (43.8%), late OAC initiators (7.6%), early OAC discontinuers (8.9%), and continuously adherent patients (40.1%). Predictors such as sex, black race, residence in the South, or HAS‐BLED score were associated with not only OAC use, but also the timing of initiation and the likelihood of discontinuation. For example, HAS‐BLED score ≥4 was associated with a higher likelihood of not using OAC (odds ratio 1.35; 95% CI, 1.14–1.62), of late initiation (1.55; 95% CI, 1.11–2.05), and of early discontinuation (odds ratio 1.35; 95% CI, 1.01–1.84).
Conclusions
We identified 4 distinct trajectories of OAC adherence after first AF diagnosis, with <45% of newly diagnosed AF patients belonging to the trajectory group characterized by continuous OAC adherence. Trajectories were associated not only with demographic and clinical characteristics but also with regional factors.
Keywords: adherence, anticoagulation, atrial fibrillation
Subject Categories: Atrial Fibrillation, Quality and Outcomes
Clinical Perspective
What Is New?
Among patients newly diagnosed with atrial fibrillation, we identified 4 trajectories of adherence to oral anticoagulation (OAC): patients who never used OAC (43.8%), late OAC initiators (7.6%), early OAC discontinuers (8.9%), and continuously adherent patients (40.1%).
Important predictors such as sex, black race, or HAS‐BLED score were associated not only with OAC use, but also with the timing of initiation and the likelihood of discontinuation.
Membership in adherence trajectories was associated not only with demographic and clinical characteristics but also with regional factors.
What Are the Clinical Implications?
With <45% of newly diagnosed atrial fibrillation patients adhering to OAC, underuse and suboptimal adherence to OAC remain a significant clinical challenge, even after the approval of direct oral anticoagulants.
Suboptimal use and adherence to OAC is not only a product of intermittent gaps in therapy, but also of lack of OAC initiation, of late initiation, and of discontinuation of therapy among initiators.
Given the major potential impact associated with stroke prevention, interventions designed to improve OAC use and adherence that address each of these underlying reasons are warranted.
Atrial fibrillation (AF) is associated with a 5‐fold increase in stroke risk and is the most common cause of ischemic stroke in the elderly.1, 2, 3 Oral anticoagulation (OAC) reduces the risk of stroke associated with AF by 60%4; yet, only half of AF patients recommended for OAC actually receive these medications, and less than half of them adhere to OAC over time.5, 6, 7, 8, 9, 10, 11, 12, 13 Before 2010, one of the main reasons for OAC underuse was the sole availability of warfarin for stroke prevention in AF.5, 14, 15 Warfarin has multiple limitations, including a narrow therapeutic index, requirement for routine blood monitoring, significant interactions with diet and other medications used for AF, and a nonnegligible risk of intracranial bleeding.5, 16, 17 However, recent evidence suggests that underuse and lack of adherence to OAC have barely improved after the approval of direct oral anticoagulants (DOACs),18, 19, 20, 21, 22 even though these agents have a more stable pharmacokinetic profile than warfarin, lower risk of intracranial bleeding, and do not require routine blood monitoring.23, 24, 25, 26
Previous research evaluating what patient characteristics affect OAC use and adherence found that risk factors for stroke, such as age >75 years, hypertension, or a history of stroke, increase use and adherence to OAC.12, 21, 22, 27, 28, 29 However, most prior studies used the proportion of days covered (PDC) with OAC as the single measure of adherence,28, 29 and did not examine the longitudinal pattern of OAC use—that is, the timing of OAC initiation after the first AF diagnosis, adherence to OAC, and rates of treatment discontinuation. Evaluating adherence to OAC longitudinally is important because continuous adherence to OAC is crucial in stroke prevention,5, 14 and because patients with similar measures of PDC over a certain time period can have strong differences in the underlying patterns of adherence,30, 31 which can affect the incidence of stroke. For instance, the PDC measure would categorize similarly a patient who was intermittently adherent to OAC for over 1 year and a patient who was fully adherent to OAC over a few months and then discontinued OAC. Although both patients would have the same PDC, their thromboembolic risk over time would differ significantly, and so would the reasons for suboptimal OAC use and adherence.
In order to fully understand how patient demographics, social determinants, and clinical characteristics are related to the longitudinal pattern of OAC use and adherence after first AF diagnosis, we distinguished groups of patients with similar adherence patterns of OAC use using group‐based trajectory models, and we estimated how certain patient characteristics and system‐level factors are associated with trajectory group membership.30, 32
Methods
Data Source and Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because Medicare claims data were obtained under a Data User Agreement that does not allow data sharing. Using 2013 to 2016 medical and pharmacy claims from a 5% random sample of Medicare beneficiaries, we included patients who were newly diagnosed with AF between January 1, 2014 and December 31, 2015 (Figure 1). We used the Centers for Medicare and Medicaid Chronic Condition Warehouse indicator of AF,33 which traces the first AF diagnosis back to January 1999. Index date was defined as the first diagnosis of AF. We excluded patients who had a diagnosis of valvular disease in the year before the index date (definition of valvular disease in Table S1). Because the objective of the study was to describe patterns of OAC adherence in the first year after AF diagnosis, we excluded patients who died within 360 days of diagnosis, or who were not continuously enrolled in Stand‐Alone Prescription Drug Plans. The final sample included 34 898 patients. All individuals were followed for 360 days after first AF diagnosis. This study was deemed exempt by the Institutional Review Board at the University of Pittsburgh.
Figure 1.
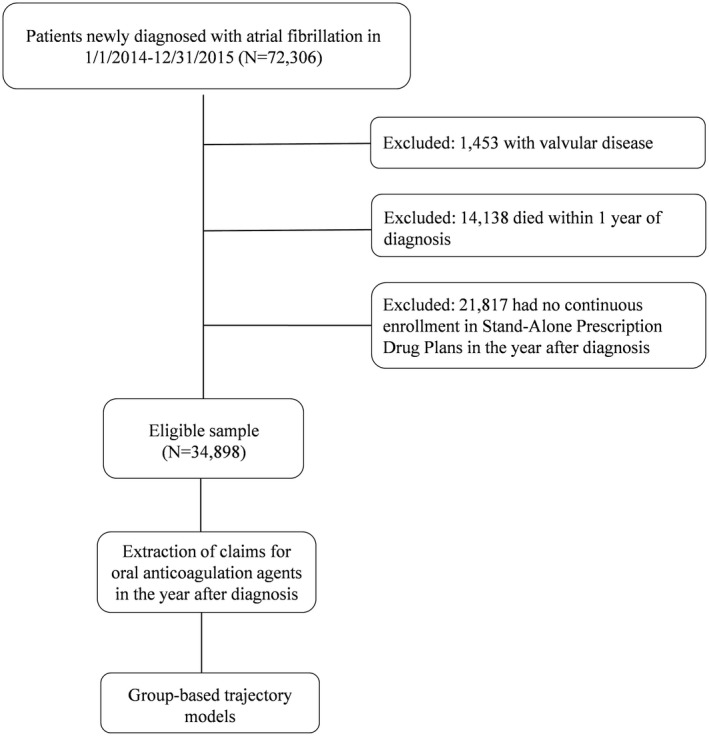
Overview of the study sample selection and analysis. Using a 5% random sample of Medicare part D beneficiaries, we selected patients newly diagnosed with atrial fibrillation in 2014 to 2015. After excluding those with valvular disease, who died within 1 year of diagnosis or who had no continuous enrollment in Stand‐Alone Prescription Drug plans, the sample included 34 898 patients. We extracted their prescriptions filled for oral anticoagulants in the 12 months after atrial fibrillation diagnosis and used group‐based trajectory models to identify groups of patients with similar adherence patterns.
Covariates
Covariates included patient demographics, social determinants, and clinical characteristics, and were all defined on index date. Demographics included age, sex, and race as indicated in the Medicare Master Beneficiary Summary file. Social determinants included eligibility for Medicaid coverage, low‐income subsidy receipt, region of residence (Northeast, Midwest, West, Southeast, or Southwest), socioeconomic score, measured at the zip code level, and index of dissimilarity, measured at the Metropolitan Statistical Area level.34 Data from American Community Survey Data35 obtained from the US Census Bureau were linked to Medicare claims using the zip code, and the socioeconomic score was calculated using a factor analysis approach that identified key census variables and combined them using z‐scores into a meaningful score that represents socioeconomic status.36 The index of dissimilarity, which measures the fraction of blacks (or whites) who would have to move from their neighborhoods to other neighborhoods to achieve perfect integration, was also calculated on the basis of American Community Survey data and a previously defined formula.37, 38
Clinical characteristics included CHAD2S2‐VASc score, HAS‐BLED score, history of acute myocardial infarction (AMI), Alzheimer disease or dementia, chronic kidney disease, heart failure, diabetes mellitus, hypertension, stroke or transient ischemic attack, recent bleeding, and recent use of antiplatelet agents and of NSAIDs. CHA2DS2‐VASc and HAS‐BLED scores are validated tools that predict the risk of ischemic stroke in AF and the risk of bleeding on OAC, respectively.39, 40 Because claims data do not contain international normalized ratio levels, we calculated the HAS‐BLED score as the sum of all factors except international normalized ratio, as done previously in the literature.19, 41, 42, 43, 44 In defining each of the factors included in CHAD2S2‐VASc and HAS‐BLED scores, we used Centers for Medicare and Medicaid Chronic Condition Warehouse definitions when available.33 When not available, we used 12 months of claims data before AF diagnosis and published definitions of covariates (Data S1 and Table S1).19, 20, 43, 45, 46, 47, 48
Outcomes
Our primary outcome was the PDC with OAC, and was measured at each 30‐day interval after first AF diagnosis. To define PDC with OAC, we extracted all prescriptions for OACs, including warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban filled after the first AF diagnosis, and arrayed them chronologically. Using the date of fill and the days of supply, we created a supply diary for each patient. We then calculated PDC with OAC for each 30‐day interval as the ratio of the number of days covered with OAC in each 30‐day interval (numerator) and 30 (denominator).
Statistical Analysis
We used group‐based trajectory models to identify patient groups with similar adherence patterns. Group‐based trajectory models assume that the population is composed of heterogeneous groups, each with a distinct trajectory. These models are preferred over methods such as latent class analysis for modeling trajectories that do not vary monotonically, as can be the case for drug adherence (patients can be intermittently adherent to drugs).49 To implement group‐based trajectory models, we first transformed the PDC with OAC using the arcsine transformation so that it followed a censored normal distribution, which is one of the assumptions of this model.49 The time variable was months since first AF diagnosis (1–12). We used the most flexible functional form of time, allowing up to a fifth‐order polynomial, as previously done in the literature.30 Group‐based trajectory analyses were performed using PROC TRAJ (https://www.andrew.cmu.edu/user/bjones/) in SAS statistical software. The final model was selected using Nagin's criteria.50 Models with a lower number of groups were favored in order to allow for a robust estimation of the effect of patient characteristics on group membership using multinomial logistic regression. The output of the final model included the estimated average trajectory for each group, and the estimated probabilities of membership in each trajectory group for each patient. Using these estimated probabilities, patients were assigned to the trajectory group for which membership probability was highest, and the assigned groups were used in analyses to determine how patient characteristics were associated with trajectory membership. We compared patient characteristics across trajectory groups using χ2 tests. We further constructed a multinomial logistic regression model regressing trajectory group against the independent variables listed above. A stepwise selection procedure was used to select covariates to be included in the models using P value for entry 0.3 and P value for removal 0.1.
Results
Study Cohort Characteristics
The mean age of the study sample was 75.4 years (SD 10.0), 55.5% were female, and 87.3% were white. Among the 34 898 study participants, annual PDC was 40.0% (SD 39.3%), and 21 213 (60.8%) filled at least 1 prescription for OAC in the year after AF diagnosis, including 10 383 who filled at least 1 prescription for warfarin, and 12 579 who filled at least 1 prescription for DOACs. Among the 21 213 patients who filled at least 1 OAC prescription, the mean annual PDC was 65.8% (SD 29.0%). Table S2 compares selected characteristics between patients included in the study and those who were excluded because they died within 12 months of AF diagnosis. Excluded patients were generally older and more likely to have CHA2DS2‐VASc ≥5.
Adherence Trajectories
Group‐based trajectory analyses identified 4 distinct trajectories of OAC adherence: patients who never used OAC (“non‐users,” group 1, 43.8%), patients who initiated OAC in months 3 to 8 post AF diagnosis (“late initiators,” group 2, 7.6%), patients who initiated OAC early after AF diagnosis but who discontinued treatment in months 3 to 8 (“early discontinuers,” group 3, 8.6%), and patients who were continuously adherent to OAC (“continuously adherent patients,” group 4, 40.1%) (Figure 2). This 4‐group model met all of Nagin's criteria (average posterior probability >70%, narrow CIs for estimated probability, and odds of correct classification >5 for all 4 groups, Table S3). The model diagnostics indicate that the 4 group model performs exceptionally well for this sample.
Figure 2.

Trajectories of adherence to oral anticoagulation in the first year after atrial fibrillation diagnosis among Medicare beneficiaries. The x‐axis represents time in months since the first diagnosis of atrial fibrillation. The y‐axis represents the proportion of days covered with oral anticoagulation in each month. The proportions in the legends represent the estimated proportion of participants in each trajectory group among all study participants. Dashed lines represent 95% CIs. AF indicates atrial fibrillation.
Association Between Patient Characteristics and Group Trajectory Membership
Unadjusted results
Table 1 compares the observed characteristics of patients in each of the 4 trajectory groups. Approximately half of the study participants were 75 years or older, and the proportion of patients older than 75 was higher for the nonusers group (54.0%), followed by the continuously adherent group (51.5%), the late initiator group (50.0%), and finally the early discontinuers group (49.7%), with P<0.001. Female sex was most prevalent in the nonusers (57.6%) and late initiators (57.0%) groups than in the continuously adherent (53.9%) and early discontinuers (50.9%) groups. The proportion of black patients and of those eligible for Medicaid and for low‐income subsidy was significantly lower in the continuously adherent group than in the remainder of the groups. The proportion of patients with CHA2DS2‐VASc score ≥5, HAS‐BLED ≥4, Alzheimer disease or other dementia, and chronic kidney disease was highest in the nonusers group.
Table 1.
Baseline Patient Characteristics of Medicare Beneficiaries With New Atrial Fibrillation Diagnosis, by Oral Anticoagulation Trajectory Group
| Variable, n (%) | Nonusers (Group 1, n=15 273) | Late Initiators (Group 2, n=2639) | Early Discontinuers (Group 3, n=3010) | Continuously Adherent Patients (Group 4, n=13 976) | P Value |
|---|---|---|---|---|---|
| Initiation of OAC | |||||
| Filled ≥1 Rx for warfarina | 632 (4.1) | 1118 (42.4) | 1461 (48.5) | 7172 (51.3) | <0.001 |
| Filled ≥1 Rx for DOACsa | 979 (6.4) | 1716 (65.0) | 1781 (59.2) | 8103 (58.0) | <0.001 |
| Demographics | |||||
| Age (y) | <0.001 | ||||
| <65 | 1330 (8.7) | 231 (8.8) | 231 (7.7) | 1029 (7.4) | |
| 65–74 | 5692 (37.3) | 1089 (41.3) | 1283 (42.6) | 5753 (41.2) | |
| ≥75 | 8251 (54.0) | 1319 (50.0) | 1496 (49.7) | 7194 (51.5) | |
| Female sex | 8801 (57.6) | 1505 (57.0) | 1533 (50.9) | 7537 (53.9) | <0.001 |
| Race | <0.001 | ||||
| White | 13 069 (85.6) | 2288 (86.7) | 2596 (86.2) | 12 507 (89.5) | |
| Black | 1222 (8.0) | 221 (8.4) | 255 (8.5) | 774 (5.5) | |
| Hispanic | 281 (1.8) | 39 (1.5) | 42 (1.4) | 161 (1.2) | |
| Other | 701 (4.6) | 91 (3.4) | 117 (3.9) | 534 (3.8) | |
| Social determinants | |||||
| Eligibility for Medicaid | 4319 (28.3) | 653 (24.7) | 704 (23.4) | 2958 (21.2) | <0.001 |
| Eligibility for low‐income subsidy | 4960 (32.5) | 769 (29.1) | 832 (27.6) | 3465 (24.8) | <0.001 |
| Quartiles Socioeconomic Scoreb | <0.001 | ||||
| Q1 | 3866 (26.1) | 675 (26.6) | 763 (26.2) | 3145 (23.2) | |
| Q2 | 3639 (24.6) | 679 (26.7) | 690 (23.7) | 3389 (25.0) | |
| Q3 | 3511 (23.7) | 585 (23.0) | 730 (25.1) | 3488 (25.7) | |
| Q4 | 3779 (25.5) | 600 (23.6) | 724 (24.9) | 3556 (26.2) | |
| Quartiles index of dissimilarityc | 0.1871 | ||||
| Q1 | 3816 (25.0) | 660 (25.0) | 797 (26.6) | 3404 (24.4) | |
| Q2 | 3672 (24.1) | 635 (24.1) | 665 (22.2) | 3378 (24.2) | |
| Q3 | 3797 (24.9) | 648 (24.6) | 727 (24.2) | 3429 (24.6) | |
| Q4 | 3971 (26.0) | 692 (26.3) | 812 (27.1) | 3754 (26.9) | |
| Region | <0.001 | ||||
| Midwest | 3449 (22.6) | 595 (22.6) | 742 (24.7) | 3716 (26.6) | |
| Northeast | 3504 (23.0) | 623 (23.6) | 640 (21.3) | 3492 (25.0) | |
| Southeast | 4436 (29.1) | 824 (31.3) | 886 (29.5) | 3736 (26.8) | |
| Southwest | 1500 (9.8) | 261 (9.9) | 283 (9.4) | 1122 (8.0) | |
| West | 2364 (15.5) | 333 (12.6) | 451 (15.0) | 1892 (13.6) | |
| Clinical characteristics | |||||
| CHA2DS2‐VASc score | <0.001 | ||||
| 0–2 | 2439 (16.0) | 451 (17.1) | 454 (15.1) | 2264 (16.2) | |
| 3–4 | 5387 (35.3) | 942 (35.7) | 1140 (37.9) | 5530 (39.6) | |
| ≥5 | 7447 (48.8) | 1246 (47.2) | 1416 (47.0) | 6182 (44.2) | |
| HAS‐BLED scored | <0.001 | ||||
| 0–1 | 1498 (9.8) | 314 (11.9) | 264 (8.8) | 1599 (11.4) | |
| 2–3 | 9341 (61.2) | 1589 (60.2) | 1993 (66.2) | 9325 (66.7) | |
| ≥4 | 4434 (29.0) | 736 (27.9) | 753 (25.0) | 3052 (21.8) | |
| AMI | 1303 (8.5) | 208 (7.9) | 242 (8.0) | 837 (6.0) | <0.001 |
| Alzheimer disease or dementia | 3111 (20.4) | 342 (13.0) | 379 (12.6) | 1522 (10.9) | <0.001 |
| Chronic kidney disease | 5763 (37.7) | 966 (36.6) | 1060 (35.2) | 4306 (30.8) | <0.001 |
| Heart failure | 6625 (43.4) | 1153 (43.7) | 1366 (45.4) | 5674 (40.6) | <0.001 |
| Diabetes mellitus | 6457 (42.3) | 1195 (45.3) | 1393 (46.3) | 5869 (42.0) | <0.001 |
| Hypertension | 13 481 (88.3) | 2253 (85.4) | 2680 (89.0) | 12 104 (86.6) | <0.001 |
| Stroke or TIA | 3202 (21.0) | 558 (21.1) | 571 (19.0) | 2800 (20.0) | 0.032 |
| Recent bleeding | 3033 (19.9) | 490 (18.6) | 550 (18.3) | 2084 (14.9) | <0.001 |
| Recent antiplatelet use | 2353 (15.4) | 344 (13.0) | 391 (13.0) | 1584 (11.3) | <0.001 |
| Recent NSAID use | 2115 (13.8) | 361 (13.7) | 427 (14.2) | 1725 (12.3) | <0.001 |
AMI indicates acute myocardial infarction; DOACs, direct oral anticoagulants; INR, international normalized ratio; OAC, oral anticoagulation; Q, quartile; Rx, prescription; TIA, transient ischemic attack.
Indicates whether patients filled at least 1 prescription for the respective type of oral anticoagulant during the first 360 days after first atrial fibrillation diagnosis.
The socioeconomic score was calculated using zip‐code level American Community Survey census data and a factor analysis approach that identifies key census variables and combines them using z‐scores into a meaningful score that represents socioeconomic status.36
The index of dissimilarity is a measure of segregation, and it measures the fraction of blacks (or whites) who would have to move from their neighborhoods to other neighborhoods to achieve perfect integration.
Because Medicare claims data do not contain information on INR levels, we calculated the HAS‐BLED score as the sum of all factors except labile INR.
Adjusted results
Table 2 shows the results of multivariable multinomial logistic regression models. Specifically, it presents the odds ratio (OR) for each covariate selected in the stepwise selection procedure of belonging to a given adherence trajectory group compared with belonging to the continuously adherent trajectory group. The reference trajectory group for all ORs listed in the text below is the continuously adherent trajectory group; this is often omitted from the writing for the sake of simplicity.
Table 2.
Estimated Odds Ratios for the Association Between Patient Characteristics and Trajectory Group Membership
| Variable | Reference Group | Odds Ratio of Group Membership (95% CI) | |||
|---|---|---|---|---|---|
| Nonusers vs Continuously Adherent Patients (Group 1 [n=15 273] vs Group 4 [n=13 976]) | Late Initiators vs Continuously Adherent Patients (Group 2 [n=2639] vs Group 4 [n=13 976]) | Early Discontinuers vs Continuously Adherent Patients (Group 3 [n=3010] vs Group 4 [n=13 976]) | |||
| Age (y) | 65–74 | <65 | 0.85 (0.76, 0.94)a | 0.85 (0.73, 1.07) | 1.05 (0.85, 1.23) |
| >74 | 0.85 (0.77, 0.98)a | 0.85 (0.69, 1.04) | 0.95 (0.73, 1.10) | ||
| Sex | Female | Male | 1.15 (1.09, 1.22)a | 1.15 (1.06, 1.30)a | 0.85 (0.78, 0.94)a |
| Race | Black | White | 1.35 (1.17, 1.44) a | 1.35 (1.14, 1.59) a | 1.45 (1.25, 1.72) a |
| Hispanic | 1.25 (0.99, 1.49) | 1.05 (0.73, 1.55) | 1.05 (0.74, 1.51) | ||
| Other | 1.15 (1.05, 1.34)a | 0.95 (0.73, 1.18) | 0.95 (0.79, 1.21) | ||
| Socioeconomic status | Low‐income subsidy | No low‐income subsidy | 1.15 (1.11, 1.25)a | 1.05 (0.96, 1.20) | 1.05 (0.95, 1.17) |
| Region | Midwest | Northeast | 0.95 (0.88, 1.01) | 0.95 (0.81, 1.04) | 1.15 (1.00, 1.26) |
| Southeast | 1.15 (1.08, 1.23)a | 1.25 (1.09, 1.37)a | 1.25 (1.13, 1.42)a | ||
| Southwest | 1.35 (1.21, 1.47)a | 1.35 (1.13, 1.56)a | 1.35 (1.17, 1.62)a | ||
| West | 1.25 (1.15, 1.35) a | 1.05 (0.88, 1.18) | 1.35 (1.18, 1.55) a | ||
| CHA2DS2‐VASc Score | 3–4 | 0–2 | 0.75 (0.71, 0.86)a | 0.85 (0.71, 1.01) | 0.95 (0.81, 1.12) |
| ≥5 | 0.85 (0.74, 0.98)a | 0.85 (0.67, 1.12) | 1.15 (0.87, 1.41) | ||
| HAS‐BLED Score | 2–3 | 0–1 | 1.15 (0.99, 1.29) | 1.15 (0.88, 1.39) | 1.35 (1.03, 1.64)a |
| ≥4 | 1.35 (1.14, 1.62)a | 1.55 (1.11, 2.05)a | 1.35 (1.01, 1.84)a | ||
| History of | AMI | No history of the disease | 1.25 (1.15, 1.40)a | 1.25 (1.05, 1.48)a | 1.25 (1.06, 1.46)a |
| Alzheimer disease or dementia | 1.95 (1.82, 2.10)a | 1.15 (1.00, 1.32) | 1.15 (1.02, 1.33)a | ||
| Chronic kidney disease | 1.15 (1.09, 1.24)a | 1.15 (0.98, 1.23) | 1.05 (0.94, 1.17) | ||
| Heart failure | 0.95 (0.86, 0.97)a | 1.05 (0.93, 1.15) | 1.05 (0.94, 1.15) | ||
| Diabetes mellitus | 0.85 (0.82, 0.93)a | 1.05 (0.98, 1.21) | 1.05 (0.95, 1.16) | ||
| Hypertension | 1.05 (0.89, 1.14) | 0.75 (0.61, 0.93)a | 0.97 (0.79, 1.20) | ||
| Stroke or TIA | 0.75 (0.68, 0.80)a | 0.85 (0.73, 0.98)a | 0.75 (0.65, 0.87)a | ||
| Recent bleeding | 1.25 (1.14, 1.32)a | 1.05 (0.96, 1.24) | 1.15 (1.03, 1.32)a | ||
| Recent antiplatelet use | 1.25 (1.17, 1.37)a | 0.95 (0.84, 1.12) | 1.05 (0.89, 1.18) | ||
Results from a multinomial logistic regression model whose outcome was trajectory group (group 4 set as reference) and predictors included all covariates listed in Table 1. Stepwise procedure was used to select predictors, using P value for entry=0.3 and P value for removal=0.1. The reference for each selected covariate is presented on the first column of the table. AMI indicates acute myocardial infarction; TIA, transient ischemic attack.
Indicates statistically significant results.
Demographic Characteristics
Age >65 years was associated with a lower likelihood of not using OAC (OR of not using OAC versus continuous adherence 0.85; 95% CI, 0.76–0.97 for 65–74 years, and 0.85; 95% CI, 0.77–0.98 for ≥75, both compared with <65). Female sex was associated with higher odds of not using OAC (OR 1.15; 95% CI 1.09–1.22), and of late initiation (OR 1.15; 95% CI, 1.06–1.30), but with lower odds of early discontinuation (OR 0.85; 95% CI, 0.78–0.94). Additionally, black race was associated with a higher likelihood of not using OAC (OR 1.35; 95% CI, 1.17–1.44), of late initiation (OR 1.35; 95% CI, 1.14–1.59), and of early discontinuation (OR 1.45; 95% CI, 1.25–1.72), compared with white race.
Social Determinants
Eligibility for low‐income subsidy was associated with higher odds of not using OAC (OR 1.15; 95% CI, 1.11–1.25). Region of residence was also significantly associated with trajectory group membership: Compared with residence in the Northeast, residence in the Southeast or the Southwest increased the likelihood of not using OAC (OR 1.15; 95%, CI 1.08–1.23 for Southeast; 1.35; 95% CI, 1.21–1.47 for Southwest), of late initiation (OR 1.25; 95% CI, 1.09–1.37 for Southeast; 1.35; 95% CI 1.13–1.56 for Southwest), and of early discontinuation (OR 1.25; 95% CI, 1.13–1.43 for Southeast; 1.35; 95% CI, 1.17–1.62 for Southwest).
Clinical Characteristics
Higher risk of stroke, as measured by CHA2DS2‐VASc score, was associated with lower odds of not using OAC (OR 0.75; 95% CI, 0.71–0.69 for CHA2DS2‐VASc 3–4, and 0.85; 95% CI, 0.74–0.98 for CHA2DS2‐VASc ≥5, both compared with CHA2DS2‐VASc ≤2). However, CHA2DS2‐VASc was not significantly associated with early discontinuation or late initiation. In contrast, higher risk of bleeding, as measured by HAS‐BLED score ≥4, was associated with a higher likelihood of not using OAC (OR 1.35; 95% CI, 1.14–1.62), of late initiation (OR 1.55; 95% CI, 1.11–2.05), and of early discontinuation (OR 1.35; 95% CI, 1.01–1.84), compared with HAS‐BLED ≤1.
A history of AMI was associated with a higher likelihood of not using OAC (OR 1.25; 95% CI, 1.15–1.40), of late initiation (OR 1.25; 95% CI, 1.05–1.48), and of early discontinuation (1.25; 95% CI, 1.06–1.46). In addition, Alzheimer disease or other dementia, chronic kidney disease, a history of recent bleeding and antiplatelet use were all associated with increased odds of not using OAC. The magnitude of the association was particularly strong for Alzheimer disease or other dementia (OR 1.95; 95% CI, 1.82–2.10). Additionally, Alzheimer disease or other dementia was also associated with an increased likelihood of early discontinuation (OR 1.15; 95% CI, 1.02–1.33). Finally, a history of stroke or transient ischemic attack decreased the likelihood of not using OAC (OR 0.75; 95% CI, 0.68–0.80), of late initiation (OR 0.85, 95% CI, 0.73–0.98), and of early discontinuation (OR 0.75; 95% CI, 0.65–0.87).
Discussion
To our knowledge, our study is the first to use a nationally representative sample of Medicare beneficiaries to study longitudinal patterns of OAC adherence after first AF diagnosis. Our study yielded 3 main findings: First, we identified 4 main trajectories of OAC adherence in the first year after AF diagnosis. Only 44% of patients were continuously adherent to therapy in the first year, with 40% never initiating therapy, 9% discontinuing early, and 8% initiating late. Second, in addition to clinical characteristics, demographics, socioeconomic factors, and region of residence were important predictors of membership into adherence trajectories. Third, important predictors such as sex, race, region, or risk of bleeding not only affected the odds of OAC use, but were also associated with the timing of OAC initiation and the likelihood of discontinuation.
Our estimates for the rates of OAC initiation are in line with a prior body of literature that showed that only around 50% to 60% of newly diagnosed AF patients initiate OAC in the United States.19, 20, 21, 22 In addition, our findings for the association between patient characteristics and membership in adherence trajectory groups are consistent with a prior study from the Veterans Health Administration that found that older age, male sex, white race, and higher CHA2DS2‐VASc score all increased the odds of OAC adherence.28 Our results are consistent as well with prior observations that Medicare patients with AF living in the Southern United States are less likely to initiate OAC.20
Nevertheless, our study is an important contribution to the existing literature because instead of capturing adherence using a single measure of PDC, it leveraged advanced models to identify longitudinal patterns of OAC adherence over time. In doing so, we demonstrated that some important predictors of OAC use such as sex, black race, region of residence, HAS‐BLED score, and a history of stroke or transient ischemic attack not only affect the initiation of OAC, but also the timing of the initiation and the likelihood of discontinuation. This is important because, in our study, late initiators and early discontinuers accounted for a nonnegligible fraction of newly diagnosed AF patients, and because continuous adherence to OAC is crucial in stroke prevention.5, 14 In fact, recent evidence has shown that thromboembolic risk is significantly higher in the first month after AF diagnosis than subsequently,51 and prior research associated a gap in OAC therapy of 1 to 3 months with 96% increased risk of stroke in AF patients at high risk of stroke, which would characterize the majority of our study sample.28
Because our findings demonstrate that suboptimal OAC adherence is not only a product of intermittent gaps in therapy, but also a product of lack of OAC initiation, of late initiation, and of discontinuation of therapy among initiators, interventions designed to improve OAC use and adherence should address each of these underlying reasons. Given the lack of success of most prior interventions attempting to mitigate OAC underuse and suboptimal adherence, and because of the cost‐saving potential of OAC in the prevention of stroke, the implementation of payment models that incentivize OAC use has recently been proposed as one of the strategies most likely to mitigate OAC underuse.52, 53 In fact, the Pharmacy Quality Alliance recently endorsed the inclusion of adherence to DOACs in the calculation of Medicare star ratings.54 This measure, however, would unlikely mitigate the lack of initiation of OAC or suboptimal adherence to warfarin therapy. The development of more comprehensive quality measures that not only capture adherence among patients using OAC, but also the proportion of nontreated patients, would be more powerful in mitigating OAC underuse. For example, a quality measure to be included in calculations of payments to both payers and providers could reflect the proportion of patients who have ≥80% PDC with OAC among all AF patients with CHA2DS2‐VASC ≥2 and no contraindications for OAC therapy.
Additionally, our study explored the association between OAC adherence and a comprehensive list of patient characteristics, which included factors not captured in prior research, such as socioeconomic score, measures of segregation, and region of residence. We found that adherence trajectory group membership was impacted by receipt of subsidies and region of residence, but not by socioeconomic score or measures of segregation. The lack of significant association between adherence and measures of segregation is interesting, because of the strong impact of black race on trajectory group membership.
Not surprisingly, patients with risk factors for stroke were more likely to initiate and adhere to OAC. However, we observed that patients with a history of AMI were less likely to initiate OAC, which is consistent with prior literature.28 Patients with a history of AMI may be less likely to use OAC because of the perceived risk of bleeding with concurrent anticoagulant and antiplatelet therapy. Although we captured prescription claims for antiplatelets, claims data do not contain information on prescriptions filled over‐the‐counter, including aspirin. In light of the recent evidence suggesting that the risk of stroke post‐AMI may be higher than originally thought,55 future research should evaluate patterns of OAC and antiplatelet use in AF patients with a history of AMI, particularly in the first months after AMI.
Our study is subject to several limitations. First, our analyses did not explore reasons behind OAC discontinuation or the consequences of discontinuation. With group‐based trajectory models, it was not possible to model whether having a bleeding event after OAC initiation increased the odds of discontinuation. This could have certainly been the case, since we observed that 23.8% of the patients who discontinue OAC treatment had a bleeding event around the time of discontinuation. In future analyses, we intend to simultaneously model OAC adherence trajectories and outcomes events, using advanced techniques such as joint latent class mixed models. Second, claims data do not contain certain pieces of information about prescriptions that are relevant for adherence measurement. For example, they do not contain information on whether patients take the medications they fill, and they cannot differentiate whether behaviors such as discontinuation reflect prescriber or patient decision making. Additionally, claims data do not capture prescriptions paid with cash, and thus our estimates could underestimate the proportion of OAC initiators, particularly because of the possibility to purchase warfarin through $4 generic programs. In addition, we have no information on free samples, and some individuals who appear as late initiators may in fact be continuous adherers who start therapy using free samples for a few weeks’ worth supply and thus not show up in claims until later. Third, we did not limit the analyses to patients with CHA2DS2‐VASc ≥2, who are recommended for OAC under the American College of Cardiology/American Heart Association/Heart Rhythm Society,56 because patients with CHA2DS2‐VASc <2 represented only 4% of the study sample. Fourth, in our analyses, we grouped warfarin and DOAC use together. Whereas adherence patterns to DOACs and warfarin may vary, it is reasonable to group them for the study of suboptimal OAC use and adherence, given that the differences in the comparative effectiveness of these agents are of a considerably smaller magnitude than the difference in stroke risk with and without OAC.4, 57, 58 Finally, our results are not generalizable to patients who died soon after AF diagnosis or to those enrolled in Medicare Advantage prescription drug plans or intermittently enrolled in stand‐alone plans, because they were excluded from analyses, since group‐based trajectory models cannot handle data not missing at random. Excluded patients were generally older and more likely to have CHA2DS2‐VASc ≥5. As a result, our included sample is overrepresentative of healthier and younger patients.
In conclusion, applying group‐based trajectory models to Medicare claims data on newly diagnosed AF patients, we described 4 trajectories of adherence to OAC, and observed that <45% of newly diagnosed AF patients belonged to the trajectory group characterized by continuous OAC adherence. Trajectories of OAC adherence were associated not only with demographics and clinical characteristics, but also with regional factors. Some predictors of OAC use were also associated with the timing of the OAC initiation and the likelihood of discontinuation.
Sources of Funding
This work was funded by the National Heart, Lung, and Blood Institute (grant number K01HL142847).
Disclosures
Saba has received research support from Boston Scientific. Hernandez has received consulting fees from Pfizer. The remaining authors have no disclosures to report. This work represents the opinions of the authors alone and does not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Supporting information
Data S1. Supplemental methods.
Table S1. Diagnosis Codes Used in the Definition of Covariates
Table S2. Comparison of Selected Baseline Characteristics Between the Study Participants and Beneficiaries Excluded From the Study Because of Death Within 12 Months of Atrial Fibrillation Diagnosis
Table S3. Group‐Based Trajectory Model Diagnostics
(J Am Heart Assoc. 2019;8:e011427 DOI: 10.1161/JAHA.118.011427.)
This article was handled independently by Jason H. Wasfy, MD, MPhil, as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Hart RG, Halperin JL. Atrial fibrillation and stroke: concepts and controversies. Stroke. 2001;32:803–808. [DOI] [PubMed] [Google Scholar]
- 2. Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, Albert CM. Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA. 2011;305:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Members ATF , Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines ECfP , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Reviewers D , Vardas P, Al‐Attar N, Alfieri O, Angelini A, Blömstrom‐Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbüchel H, Heldal M, Kristensen SD, Le Heuzey J‐Y, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FWA. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 5. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e634. [DOI] [PubMed] [Google Scholar]
- 6. Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice‐level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, Kothawala P, Emons M. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med. 2010;123:446–453. [DOI] [PubMed] [Google Scholar]
- 8. Lang K, Bozkaya D, Patel AA, Macomson B, Nelson W, Owens G, Mody S, Schein J, Menzin J. Anticoagulant use for the prevention of stroke in patients with atrial fibrillation: findings from a multi‐payer analysis. BMC Health Serv Res. 2014;14:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fireman B, Bartlett J, Selby J. Can disease management reduce health care costs by improving quality? Health Aff. 2004;23:63–75. [DOI] [PubMed] [Google Scholar]
- 10. Kakkar AK, Mueller I, Bassand J‐P, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GYH, Mantovani LG, Verheugt FWA, Jamal W, Misselwitz F, Rushton‐Smith S, Turpie AGG. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J. 2012;163:13–19.e11. [DOI] [PubMed] [Google Scholar]
- 11. Bahri O, Roca F, Lechani T, Druesne L, Jouanny P, Serot JM, Boulanger E, Puisieux F, Chassagne P. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc. 2015;63:71–76. [DOI] [PubMed] [Google Scholar]
- 12. Patel AA, Lennert B, Macomson B, Nelson WW, Owens GM, Mody SH, Schein J. Anticoagulant use for prevention of stroke in a commercial population with atrial fibrillation. Am Health Drug Benefits. 2012;5:291–298. [PMC free article] [PubMed] [Google Scholar]
- 13. Whittle J, Wickenheiser L, Venditti LN. Is warfarin underused in the treatment of elderly persons with atrial fibrillation? Arch Intern Med. 1997;157:441–445. [PubMed] [Google Scholar]
- 14. Reynolds MR, Shah J, Essebag V, Olshansky B, Friedman PA, Hadjis T, Lemery R, Bahnson TD, Cannom DS, Josephson ME, Zimetbaum P. Patterns and predictors of warfarin use in patients with new‐onset atrial fibrillation from the FRACTAL registry. Am J Cardiol. 2006;97:538–543. [DOI] [PubMed] [Google Scholar]
- 15. Ogilvie IM, Welner SA, Cowell W, Lip GY. Characterization of the proportion of untreated and antiplatelet therapy treated patients with atrial fibrillation. Am J Cardiol. 2011;108:151–161. [DOI] [PubMed] [Google Scholar]
- 16. Gattellari M, Worthington J, Zwar N, Middleton S. Barriers to the use of anticoagulation for nonvalvular atrial fibrillation: a representative survey of Australian family physicians. Stroke. 2008;39:227–230. [DOI] [PubMed] [Google Scholar]
- 17. Lane DA, Lip GY. Barriers to anticoagulation in patients with atrial fibrillation: changing physician‐related factors. Stroke. 2008;39:7–9. [DOI] [PubMed] [Google Scholar]
- 18. Alamneh EA, Chalmers L, Bereznicki LR. Suboptimal use of oral anticoagulants in atrial fibrillation: has the introduction of direct oral anticoagulants improved prescribing practices? Am J Cardiovasc Drugs. 2016;16:183–200. [DOI] [PubMed] [Google Scholar]
- 19. Hernandez I, Zhang Y, Saba S. Comparison of the effectiveness and safety of apixaban, dabigatran, rivaroxaban and warfarin in newly diagnosed atrial fibrillation. Am J Cardiol. 2017;120:1813–1819. [DOI] [PubMed] [Google Scholar]
- 20. Hernandez I, Saba S, Zhang Y. Geographic variation in the use of oral anticoagulation in stroke prevention in atrial fibrillation. Stroke. 2017;48:2289–2291. [DOI] [PubMed] [Google Scholar]
- 21. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
- 22. Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR Pinnacle Registry. JAMA Cardiol. 2016;1:55–62. [DOI] [PubMed] [Google Scholar]
- 23. Food and Drug Administration . Pradaxa prescribing information. 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022512s028lbl.pdf. Accessed January 12, 2016.
- 24. Food and Drug Administration . Prescribing information for Xarelto. 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022406s012lbl.pdf. Accessed April 26, 2017.
- 25. Food and Drug Administration . Prescribing information for Eliquis. 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/202155s011lbl.pdf. Accessed April 26, 2017.
- 26. Food and Drug Administration . Prescribing information for Savaysa. 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316s002lbl.pdf. Accessed January 12, 2016.
- 27. Baik SH, Hernandez I, Zhang Y. Evaluating the choice of novel oral anticoagulants in Medicare. J Manag Care Spec Pharm. 2016;22: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borne RT, O'Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, Masoudi FA, Hess PL, Maddox TM, Ho PM. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the Veterans Health Administration. BMC Cardiovasc Disord. 2017;17:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown JD, Shewale AR, Talbert JC. Adherence to rivaroxaban, dabigatran, and apixaban for stroke prevention in incident, treatment‐naive nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2016;22:1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo‐Ciganic WH, Donohue JM, Jones BL, Perera S, Thorpe JM, Thorpe CT, Marcum ZA, Gellad WF. Trajectories of diabetes medication adherence and hospitalization risk: a retrospective cohort study in a large state Medicaid program. J Gen Intern Med. 2016;31:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gellad WF, Thorpe CT, Steiner JF, Voils CI. The myths of medication adherence. Pharmacoepidemiol Drug Saf. 2017;26:1437–1441. [DOI] [PubMed] [Google Scholar]
- 32. Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Center for Medicare and Medicaid Services Chronic Conditions Data Warehouse . 27 chronic condition algorithm. 2018. Available at: https://www.ccwdata.org/web/guest/condition-categories. Accessed October 30, 2018.
- 34. Meigs JB, Nathan DM, Cupples LA, Wilson PW, Singer DE. Tracking of glycated hemoglobin in the original cohort of the Framingham Heart Study. J Clin Epidemiol. 1996;49:411–417. [DOI] [PubMed] [Google Scholar]
- 35. United States Census Bureau . American Community Survey. 2014. Available at: https://www.census.gov/programs-surveys/acs/. Accessed October 29, 2018.
- 36. Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349:1350–1359. [DOI] [PubMed] [Google Scholar]
- 37. Duncan OD, Duncan B. A methodological analysis of segregation indexes. Am Sociol Rev. 1955;20:210–217. [Google Scholar]
- 38. Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67:281–315. [Google Scholar]
- 39. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 40. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 41. Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48:2142–2149. [DOI] [PubMed] [Google Scholar]
- 42. Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med. 2014;127:1075–1082.e1071. [DOI] [PubMed] [Google Scholar]
- 43. Hernandez I, Zhang Y, Brooks MM, Chin PK, Saba S. Anticoagulation use and clinical outcomes after major bleeding on dabigatran or warfarin in atrial fibrillation. Stroke. 2017;48:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hernandez I, Zhang Y. Comparing stroke and bleeding with rivaroxaban and dabigatran in atrial fibrillation: analysis of the US Medicare Part D data. Am J Cardiovasc Drug. 2017;17:37–47. DOI: 10.1007/s40256-016-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gray M, Saba S, Zhang Y, Hernandez I. Outcomes of atrial fibrillation patients newly recommended for oral anticoagulation under the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline. J Am Heart Assoc. 2018;7:e007881 DOI: 10.1161/JAHA.117.007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hernandez I, Zhang Y, Saba S. Effectiveness and safety of direct oral anticoagulants and warfarin, stratified by stroke risk in patients with atrial fibrillation. Am J Cardiol. 2018;122:69–75. [DOI] [PubMed] [Google Scholar]
- 49. Nagin DS, Odgers CL. Group‐based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 50. Nagin D. Group‐Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 51. Bassand JP, Virdone S, Goldhaber SZ, Camm AJ, Fitzmaurice DA, Fox KAA, Goto S, Haas S, Hacke W, Kayani G, Mantovani LG, Misselwitz F, Pieper KS, Turpie AGG, van Eickels M, Verheugt FWA, Kakkar AK. Early risks of death, stroke/systemic embolism, and major bleeding in patients with newly diagnosed atrial fibrillation. Circulation. 2019;139:787–798. [DOI] [PubMed] [Google Scholar]
- 52. Peterson ED, Pokorney SD. New treatment options fail to close the anticoagulation gap in atrial fibrillation. J Am Coll Cardiol. 2017;69:2485–2487. [DOI] [PubMed] [Google Scholar]
- 53. Hess PL, Mirro MJ, Diener HC, Eikelboom JW, Al‐Khatib SM, Hylek EM, Bosworth HB, Gersh BJ, Singer DE, Flaker G, Mega JL, Peterson ED, Rumsfeld JS, Steinberg BA, Kakkar AK, Califf RM, Granger CB. Addressing barriers to optimal oral anticoagulation use and persistence among patients with atrial fibrillation: proceedings, Washington, DC, December 3–4, 2012. Am Heart J. 2014;168:239–247.e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crivera C, Nelson WW, Bookhart B, Martin S, Germain G, Laliberte F, Schein J, Lefebvre P. Pharmacy quality alliance measure: adherence to non‐warfarin oral anticoagulant medications. Curr Med Res Opin. 2015;31:1889–1895. [DOI] [PubMed] [Google Scholar]
- 55. Merkler AE, Diaz I, Murthy SB, Wu X, Gialdini G, Navi BB, Yaghi S, Weinsaft JW, Okin PM, Safford MM, Iadecola C, Kamel H. Duration of heightened stroke risk after acute myocardial infarction. Stroke. 2018;49:Abstract 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 57. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 58. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Diagnosis Codes Used in the Definition of Covariates
Table S2. Comparison of Selected Baseline Characteristics Between the Study Participants and Beneficiaries Excluded From the Study Because of Death Within 12 Months of Atrial Fibrillation Diagnosis
Table S3. Group‐Based Trajectory Model Diagnostics


