Abstract
Background
Previous studies have reported an association between the timing of menarche and cardiovascular disease (CVD). However, emerging studies have not examined the timing of menarche in relation to role of estrogen over a lifetime and major adverse cardiac events (MACE).
Methods and Results
A total of 648 women without surgical menopause undergoing coronary angiography for suspected ischemia in the WISE (Women's Ischemia Syndrome Evaluation) study were evaluated at baseline and followed for 6 years (median) to assess major adverse CVD outcomes. MACE was defined as the first occurrence of all‐cause death, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization. Age at menarche was self‐reported and categorized (≤10, 11, 12, 13, 14, ≥15 years) with age 12 as reference. Total estrogen time and supra–total estrogen time were calculated. Cox regression analysis was performed adjusting for CVD risk factors. Baseline age was 57.9 ± 12 years (mean ± SD), body mass index was 29.5 ± 6.5 kg/m2, total estrogen time was 32.2 ± 8.9 years, and supra–total estrogen time was 41.4 ± 8.8 years. MACE occurred in 172 (27%), and its adjusted regression model was J‐shaped. Compared with women with menarche at age 12 years, the adjusted MACE hazard ratio for menarche at ≤10 years was 4.53 (95% CI 2.13‐9.63); and at ≥15 years risk for MACE was 2.58 (95% CI, 1.28‐5.21).
Conclusions
History of early or late menarche was associated with a higher risk for adverse CVD outcomes. These findings highlight age at menarche as a potential screening tool for women at risk of adverse CVD events.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00000554.
Keywords: cardiovascular disease outcomes, estrogen, menarche, risk factors, women
Subject Categories: Women, Cardiovascular Disease, Risk Factors
Clinical Perspective
What Is New?
In the WISE (Women's Ischemia Syndrome Evaluation) study, women who experienced early or later age at menarche had a higher risk of developing major adverse cardiac events, defined as first occurrence of all‐cause death, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization.
This effect persisted after adjustment for traditional cardiovascular risk factors and lifetime total estrogen exposure, supporting the notion that age at menarche may be an independent risk factor for major adverse cardiac events.
What Are the Clinical Implications?
Specific to women, traditional cardiovascular disease risk factors do not fully capture major adverse cardiac event risk assessment, and it is important to further investigate sex‐specific risk factors such as menarche to elucidate the different mechanisms in which cardiovascular disease may occur in women.
Cardiovascular disease (CVD) continues to be the leading cause of mortality in women, causing nearly 1 in every 3 deaths1 and about 400 000 deaths per year in the United States.2 Despite advances in research and increases in clinical awareness, a gap still remains in understanding and effectively recognizing sex‐specific risks of preventable CVD.3 Specifically, women experience less obstructive coronary artery disease yet experience a greater burden of ischemic symptoms and mortality compared with men.4 The limited knowledge of the CVD risk factor profile specific to women contributes to management delays with underdiagnoses and undertreatment, resulting in poorer outcomes.5 Identifying sex‐specific risk factors for adverse CVD events in midlife could result in more timely primary prevention strategies and yield better clinical outcomes.6
Age at menarche, defined as the age at first occurrence of menstruation, heralds the onset of cyclic ovarian function, which includes increased endogenous estradiol secretion and exposure. Menarche is a complex reproductive physiologic milestone that depends on a tightly orchestrated set of neurohormonal changes in the hypothalamus‐pituitary‐ovarian axis that influence further maturation of the female body. Menarche has also been associated with CVD risk factors.7, 8, 9 Early menarche (before age 12 years) has been associated with adiposity, metabolic syndrome, and increased risk of breast cancer, suggested to be due, at least in part, to increased lifetime exposure to estrogen.10 Both early and late age at menarche have been associated with increased risks of coronary heart disease.11 Others have reported that early age at menarche is associated with increased risk of CVD events and all‐cause mortality.12 However, despite preliminary findings, the clinical significance of age at menarche as a sex‐specific finding for major adverse cardiovascular events (MACE) in women represents an important knowledge gap.
To address this knowledge gap, we investigated the association between age at menarche and MACE among women participating in the original cohort of the National Heart Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. We further considered age of onset of menstruation and length of total estrogen exposure to control for the potential influence of reproductive hormones. We also evaluated biomarkers of inflammation to determine if there was an association between age at menarche and MACE.
Methods
Study Participants
The original cohort of the WISE study is a National Heart Lung and Blood Institute–sponsored 4‐center study of 936 women undergoing clinically indicated coronary angiography to further evaluate suspected myocardial ischemia. Additional details regarding the project methodologies are described elsewhere.14 Briefly, women >18 years old underwent a detailed standardized symptom evaluation, risk assessment, and laboratory evaluation at baseline. Exclusion criteria included emergency referral, pregnancy, cardiomyopathy (as defined in the patients’ medical records), New York Heart Association class IV congestive heart failure, acute ischemic syndrome, defined as acute myocardial infarction or unstable angina within 1 month before study entry, coronary revascularization within 6 months before study entry, conditions other than ischemic heart disease likely to be fatal or to require frequent hospitalization within 4 years (such as severe lung, renal, or hepatic disease, or surgically uncorrected significant congenital or valvular heart disease), contraindication to provocative myocardial stress or coronary reactivity testing, malignancy other than skin cancer, and any condition likely to affect study retention (alcoholism, drug abuse, or severe psychiatric illness). All women provided written informed consent in accordance with institutional guidelines, and the project was approved by the institutional review board at each site.
This study cohort included 648 women who reported age at menarche and had both ovaries to obtain the calculation for total estrogen time and supra–total estrogen time. Analyses of the biomarkers were added later during the recruitment period and included 505 women for serum amyloid A (SAA) and C‐reactive protein (CRP), and 471 women for interleukin‐6, and tumor necrosis factor‐α.
Baseline Evaluation
Women underwent a baseline evaluation that included collection of demographic information, age at menarche, reproductive history, exogenous hormone use, and history of other medical conditions from 1998 to 2002.14 These measures also included relevant health behavior information such as smoking history. The WISE reproductive status questionnaire, a validated assessment of menopause status, included a detailed history of menarche, date of last menstrual period, menstrual cycling patterns, parity, gynecological surgeries, hormone therapy, and oral contraceptive use. Exclusion criteria included surgical menopause as determined by the WISE hormone committee caused by abrupt loss of endogenous hormones. Coronary angiography for suspected ischemia was assessed by a core laboratory masked to other patient data as described elsewhere.15 Blood was drawn at baseline after an overnight fast in close temporal proximity to the WISE testing.
Measurement of Inflammatory Markers
Plasma sampled at baseline was frozen at −70°C for measurement of the inflammatory markers. Levels of CRP and SAA were measured by a high‐sensitivity method at a core laboratory using a BN II analyzer (Dade Behring, Deerfield, IL) by previously validated techniques.16 Levels of interleukin‐6 and tumor necrosis factor‐α were measured with commercially available enzyme‐linked immunoabsorbent assay kits (R&D systems, Minneapolis, MN).
Menarche Status and Total Estrogen Time
Menarche history was obtained as part of the self‐reported reproductive questionnaire at the baseline visit. Total estrogen time (TET) and supra–total estrogen time (sTET) were calculated with equations as previously published.17 Briefly, TET included time of estimation of endogenous estrogen exposure during a woman's lifetime. sTET also included time of supra– physiologic levels of endogenous and exogenous hormone exposure such as pregnancy and oral contraceptive use during a woman's lifetime.
Major Adverse Cardiovascular Events
MACE were collected as first occurrence among all‐cause mortality, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization. Patients were contacted by telephone at 3 months, then annually, and asked about adverse CVD events or hospitalizations. All‐cause mortality data were obtained from a search of the National Death Index and confirmed by death certificates, when available.
Data Collection
Statistical Analyses
Women were grouped into categories by age at menarche (≤10, 11, 12, 13, 14, ≥15 years), with age 12 as reference, from the mean age at menarche for US women of 12.4 years.18 Baseline data were reported as means±SDs stratified by age at menarche. All P‐values (with the exception of menopause status and use of hormone replacement therapy) were adjusted for age by general linear model or, where appropriate, logistic models. Skewed variables were reported as medians (interquartile range) by age at menarche. Traditional CVD risk factors such as diabetes mellitus, hyperlipidemia, and smoking history were included in the descriptive analyses. Tests for clinical and demographic variables by age at menarche were chi‐squared tests for categorical variables or nonparametric Kruskal‐Wallis tests for continuous variables. Log transformation was performed where appropriate to reduce the effect of outliers in the distribution of inflammatory biomarkers.
For the analyses, women were examined by age at menarche to the first instance of MACE and all‐cause mortality reported by frequency. For multivariate modeling, Cox proportional‐hazards models were used to test the association of age at menarche with MACE adjusted for baseline demographics and CVD risk factors of age, body mass index, diabetes mellitus, dyslipidemia, hypertension, history of smoking, and log serum amyloid A. CRP was not included in the fully adjusted model because of its collinearity with SAA. Two different adjusted models were run in parallel—the first with TET and second model with sTET. These models were also run using all‐cause mortality only. Models were assessed for proportional‐hazards violations using plots of Schoenfeld residuals with a smoothed curve for the β estimates versus rank‐transformed event times. Age at menarche was treated as a categorical variable to estimate the form of the relationship with relative hazard of MACE as well as all‐cause mortality. A significance level of 0.05 was used for all tests. Analyses were conducted with SAS v9.3 (SAS Institute, Cary, NC).
Results
Baseline demographic data, stratified by age at menarche, are summarized in Table 1. There were 55 women in the ≤10‐years group, 99 women in the 11‐years group, 152 women in the 12‐years group, 161 women in the 13‐years group, 82 women in the 14‐years group, and 99 women in the 15‐years‐or‐older group. The mean (±SD) age in this cohort was 57.9±12 years. Women who entered menarche early (≤10 years old) presented with signs and symptoms of ischemia at an earlier age, 55 years as opposed to women who entered menarche late (≥15 years old), 59 years (P=0.037). Overall, 496 (77%) were postmenopausal at baseline, and there were no age‐adjusted differences among age‐at‐menarche groups (P=0.59). Mean body mass index (±SD) was 29.5±6.5 kg/m2 and not found to be significant after age adjustment (P=0.23). Most (81%) of the women were non‐Hispanic whites. Traditional CVD risk factors were prevalent in this population, with 25% diagnosed with diabetes mellitus, 53% with dyslipidemia, and 57% with hypertension; 51% had ever smoked.
Table 1.
Baseline Characteristics by Age at Menarche Category
| ≤10 y (n=55) | 11 y (n=99) | 12 y (n=152) | 13 y (n=161) | 14 y (n=82) | ≥15 y (n=99) | Overall (n=648) | P Value | |
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD), y | 55 (10.5) | 58 (11.3) | 56 (12.7) | 59 (11.0) | 59 (12.4) | 59 (12.7) | 58 (12.0) | 0.037 |
| BMI, mean (SD), kg/m2 | 31.6 (6.2) | 30.0 (5.4) | 29.8 (6.9) | 28.9 (6.3) | 29.3 (7.0) | 28.8 (6.7) | 29.5 (6.5) | 0.23 |
| White race, n (%) | 42 (76) | 80 (81) | 124 (82) | 134 (83) | 66 (80) | 76 (77) | 522 (81) | 0.74 |
| Ever smoker, n (%) | 31 (56) | 55 (56) | 79 (52) | 89 (55) | 38 (46) | 40 (40) | 332 (51) | 0.16 |
| Hypertension, n (%) | 32 (58) | 67 (68) | 80 (53) | 98 (61) | 41 (51) | 51 (52) | 369 (57) | 0.06 |
| Diabetes mellitus, n (%) | 19 (35) | 31 (31) | 33 (22) | 36 (22) | 35 (30) | 18 (18) | 162 (25) | 0.08 |
| Dyslipidemia, n (%) | 24 (46) | 55 (63) | 69 (49) | 80 (54) | 45 (58) | 43 (48) | 316 (53) | 0.20 |
| Postmenopausal, n (%) | 45 (82) | 76 (77) | 110 (72) | 128 (80) | 60 (73) | 77 (78) | 496 (77) | 0.59 |
| HRT, n (%) | 18 (33) | 33 (34) | 48 (32) | 53 (33) | 25 (31) | 30 (31) | 207 (32) | 0.99 |
| TET, mean (SD), y | 35.0 (6.3) | 33.6 (9.3) | 32.2 (9.4) | 32.2 (8.4) | 30.9 (8.2) | 30.1 (9.5) | 32.2 (8.9) | <0.0001 |
| sTET, mean (SD), y | 43.3 (7.8) | 43.8 (8.2) | 41.3 (8.8) | 41.5 (8.6 | 40.4 (8.6) | 38.6 (9.6) | 41.4 (8.8) | <0.0001 |
| MACE, n (%) | 22 (40) | 27 (27) | 23 (15) | 36 (22) | 19 (23) | 22 (22) | 149 (23) | 0.0009 |
| Death, n (%) | 16 (30) | 25 (25) | 20 (13) | 28 (17) | 15 (18) | 18 (19) | 122 (19) | 0.016 |
BMI indicates body mass index; HRT, hormone replacement therapy; MACE, major adverse cardiovascular events; sTET, supra‐TET; TET, total estrogen time.
During follow‐up at 5.8 years (median) for MACE and 9.2 years (median) for all‐cause death, 172 (27%) women experienced MACE. Early age at menarche (≤10 and 11 years) had the greatest burden of MACE (40% and 27%, respectively), as well as all‐cause mortality (30% and 25%), respectively. After adjustment for age, the earliest age‐at‐menarche group had the highest rates of both MACE and all‐cause mortality.
TET as estimated by our algorithm was 32.2±8.9 years, and sTET was 41.4±8.8 years (Table 1). As expected, mean TET and sTET were longer in women who had entered menarche earlier and shorter in those who entered menarche later (P<0.0001 for both, age‐adjusted). Among postmenopausal women, age‐adjusted endogenous sex hormone levels did not differ across age at menarche categories (P>0.05, all variables, see Table S1).
Inflammatory biomarker levels by age at menarche are summarized in Table 2. Median SAA ranged from 0.45 to 0.74 μg/mL and was significantly different among the groups (P=0.017). Median CRP ranged from 0.26 to 0.51 mg/L and also was significantly different among the groups (P=0.004). For the inflammatory markers SAA and CRP, median ranks were higher in the early age‐of‐menarche groups than in the older age‐at‐menarche groups, particularly the age‐11 and ‐12 groups, compared with those at age 15 and older. Tumor necrosis factor‐α and interleukin‐6 did not differ significantly among groups.
Table 2.
Inflammatory Biomarkers by Age at Menarche
| ≤10 y (n=55) | 11 y (n=99) | 12 y (n=152) | 13 y (n=161) | 14 y (n=82) | ≥15 y (n=99) | Overall (n=648) | P Value | |
|---|---|---|---|---|---|---|---|---|
| SAA, median (IQR)a μg/mL | 0.45 (0.27, 0.89) | 0.74 (0.35, 1.26) | 0.56 (0.35, 1.26) | 0.56 (0.31, 1.08) | 0.56 (0.30, 0.84) | 0.44 (0.27, 0.71) | 0.54 (0.31, 0.98) | 0.017 |
| CRP, median (IQR)a mg/L | 0.39 (0.13, 0.82) | 0.51 (0.19, 0.87) | 0.39 (0.15, 1.06) | 0.29 (0.15, 0.81) | 0.26 (0.11, 0.66) | 0.27 (0.11, 0.58) | 0.35 (0.14, 0.79) | 0.004 |
| IL‐6, median (IQR)b pg/mL | 3.2 (2.1, 5.8) | 3.4 (2.1, 5.8) | 3.1 (1.7, 5.8) | 2.8 (1.6, 6.5) | 3.0 (1.7, 5.7) | 2.4 (1.5, 3.8) | 3.0 (1.8, 5.6) | 0.12 |
| TNF‐α, median (IQR)b pg/mL | 3.7 (2.5, 6.6) | 3.2 (2.0, 5.0) | 3.2 (1.8, 4.7) | 2.9 (2.1, 5.2) | 3.3 (1.9, 4.9) | 3.2 (2.2, 4.6) | 3.2 (2.0, 5.0) | 0.29 |
CRP indicates C‐reactive protein; IL‐6, interleukin‐6; IQR, interquartile range; SAA, serum amyloid A; TNF‐α, tumor necrosis factor‐α.
n=505.
n=471.
Adjusted hazard ratios from a Cox proportional‐hazards model in Figure 1 show the relative hazard of developing MACE by age at menarche including the variable TET. The model demonstrated that both early and late menarche age were associated with higher adjusted hazard rates (HR) of MACE: menarche age ≤10, HR 4.21 (95% CI 1.90‐9.30); age 11, HR 2.27 (95% CI 1.09‐4.75); age 13, HR 1.95 (95% CI 0.97‐3.92); age 14, HR 2.74 (95% CI 1.21‐6.20), and age ≥15, HR 2.52 (95% CI 1.14‐5.57). The highest risk for MACE was found in women with menarche ≤10 years of age (HR 4.21 [95% CI 1.90‐9.30]) compared with women with menarche at age 12. In women with menarche at ≥15 years, there was also an elevated risk for MACE (HR 2.52 [95% CI 1.14‐5.57]). Similarly, the adjusted Cox regression model using sTET shows a similar J‐shaped curve for risk of MACE (see Figure S1). Furthermore, modeling with TET and sTET as well as without TET or sTET did not change the effect size (see Tables S2 and S3) for either CVD or time to death. The association of TET with time to MACE was not found to be different, adjusted for age, body mass index, diabetes mellitus, dyslipidemia, hypertension, smoking status, log serum amyloid A, and age at menarche (P=0.64, HR 0.99, 95% CI 0.97‐1.02). The adjusted association of sTET with time to MACE was also not different (P=0.10, HR 0.98, 95% CI 0.95, 1.01).
Figure 1.
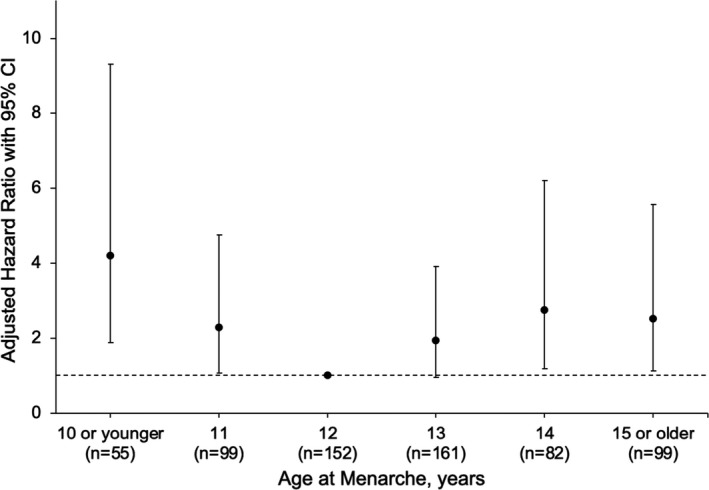
Adjusted relative hazard (with TET) and 95% CI of major adverse cardiac events (MACE) by age at menarche. TET indicates total estrogen time.
The adjusted hazard ratios from a Cox proportional hazards model in Figure 2 show the relative hazard of all‐cause mortality by age at menarche including the variable TET. The model demonstrates similar results to MACE in that both early and late menarche ages were associated with higher adjusted HRs for all‐cause mortality: menarche age ≤10 HR 2.67 (95% CI 1.38‐9.72); age 11, HR 2.62 (95% CI 1.12‐6.11); age 13, HR 1.32 (95% CI 0.58‐3.02); age 14, HR 2.54 (95% CI 0.97‐6.66), age ≥15, HR 2.63 (95% CI 1.11‐6.26). Results using sTET in the model showed no differences from the model with TET (see Figure S2).
Figure 2.
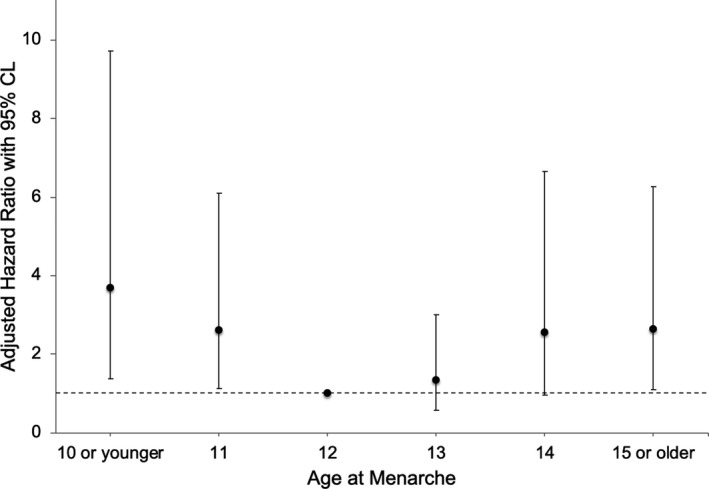
Adjusted relative hazard (with TET) and 95% CI of mortality by age at menarche. TET indicates total estrogen time.
Discussion
We observed that, among women with symptoms and/or signs of ischemic heart disease referred for coronary angiography, both early and late age at menarche predicted higher risk of developing MACE over intermediate‐term follow‐up. This effect persisted after adjustment for multiple CVD risks, estrogen exposure, and inflammatory markers. Overall, the adjusted model illustrated that the relationship of age at menarche to the risk of MACE was J‐shaped, with higher MACE risk observed in those with earlier age at menarche. Additionally, MACE was not associated with TET or sTET, suggesting that effects of estrogen exposure did not account for the differences observed by age at menarche. Inflammatory markers such as SAA and CRP were also different among women by age at menarche. In prior WISE analyses, SAA levels were found to be independently predictive of obstructive coronary artery disease and related MACE, further supporting the notion that state of systemic inflammation may contribute to atherosclerotic plaque destabilization and lead to MACE in women.19 All‐cause mortality with a longer follow‐up also had a J‐shaped relationship after adjustment for the same covariates. This finding further supports the notion that age at menarche may be an independent risk factor for MACE and all‐cause mortality.
Our findings are similar to those from the United Kingdom population‐based Million Women Study that evaluated postmenopausal women without prior CVD. They found that the relation of age at menarche to CVD was U‐shaped, with both early and late menarche being associated with increased risk in that women with early menarche (≤10 years) had a relative risk of 1.25 (95% CI 1.22‐1.31), and those with later menarche (≥17 years) had a relative risk of 1.23 (95% CI 1.16‐1.30).11 Another UK study found that early menarche (<12 years) and early menopause (<47 years) were independently associated with a higher CVD risk later in life (adjusted HRs 1.10 and 1.33, respectively).20 The association between age at menarche and CVD risk has been also found outside of Western populations. In a study on Chinese women, early and later age at menarche and higher CVD risks were similarly present.21 Early age at menarche was associated with increased risk of ischemic heart disease in a Shanghai cohort.22 A Singaporean study found increased CVD mortality risk with decreasing age at menarche among nonsmoking women.23 Early age at menarche was also associated with risk of coronary heart disease in a Korean cohort.24 These international studies suggest that there are specific factors that trigger early menarche, such as stress, that also accelerate onset of CVD.
Findings for association between age at menarche and mortality were varied. In a Japanese study there was a J‐shaped association of all‐cause mortality and age at menarche that was independent of lifestyle and reproductive factors.25 A study on Norwegian women found a similar J‐shaped association with total mortality.26 However, a prospective cohort study in Europe reported lower all‐cause mortality with a later age at menarche.27 The Japan Collaborative Cohort study found that a later age at menarche was associated with a higher mortality due to stroke.28 It is unclear what factors that trigger later menarche may affect risk for mortality from any causes.
Although increased estrogen exposure from earlier age at menarche has been correlated with increased rates of breast cancer29 and later age at menarche with lower bone mineral density,30 we did not find estrogen exposure linked with MACE and all‐cause mortality. In premenopausal women disruption of ovulatory cycling characterized by hypothalamic hypoestrogenemia has been associated with increased risk of obstructive coronary artery disease.31 Despite the notion that exposure to estrogens may be cardioprotective, we did not find this to be the case based on age at menarche.32 Factors linked to earlier menarche include psychosocial stress and overnutrition. Factors linked to later menarche include polycystic ovary syndrome, excessive exercise, and undernutrition; later menarche may be linked to relative hypercortisolism and hypoestrogenism, whereas earlier menarche may be linked to metabolic syndrome. Our study did not demonstrate a significant link between estrogen exposure and MACE. It is possible that some other mechanism, such as the oxidative damage to vessels caused by states of chronic inflammation as mediated by understudied biomarkers, may predispose women to a higher CVD risk.33, 34, 35 Glucocorticoids play a significant role not just in the stress‐induced reproduction response but also in the establishment and maintenance of fertility.36 Hypercortisolemia interferes with estrogen action at the cellular level, so chronic stress may not only lower endogenous estrogen exposure, it may also counteract the benefits of estrogen. States of inflammation have been linked to the pathology of formation and disruption of plaques. Further studies to specifically measure vascular damage and other markers of inflammation may be necessary to delineate possible mechanisms.
The strengths of the present study are many. The original WISE cohort is a longitudinal, prospective, and validated study ongoing since 1998. Reproductive health has been comprehensively assessed both by collecting clinical data and by measuring salient reproductive hormones. It is the first study of menarche and MACE in women that incorporates calculation of lifetime estrogen exposure by means of TET and sTET. The lack of a robust association between estrogen exposure and MACE suggests that the precursors of MACE are multifactorial. CVD risk factors and MACE were assessed comprehensively and prospectively. In contrast to studies that examined links between International Classification of Diseases (ICD) codes or deaths, the WISE repository contained valuable patient data such as blood work, inflammatory biomarkers, and years of hospital admission, data that permitted a more nuanced examination of cytokines and oxidative stress. The WISE prospectively collected clinical data using standardized methods and biochemical data to test again the hypothesis that inflammatory states promote CVD.
One limitation of this study includes the historical study variables used such as age at menarche, pregnancy, and contraceptive use, which were self‐reported and may be subject to recall bias. The WISE sample, which consisted of women who were recruited on the clinical suspicion of myocardial ischemia, may not be representative of a general population of symptomatic women in the United States. Furthermore, the women were mostly white, so the findings may not apply to minority ethnic groups, particularly Hispanic and black populations, who present at an earlier age at menarche and are more likely to present with traditional CVD risk factors.2
Conclusions
Using data from the original WISE prospective cohort, we observed that women with early or later menarche had a relatively higher risk of MACE and all‐cause mortality. These associations were not explained by previously known CVD risk factors or estrogen exposure or supra–physiologic states of estrogen exposure. Inflammatory biomarkers such as SAA have shown a connection with angiographic coronary artery disease and thus could play a role in mediating atherosclerotic plaque destabilization. Women who experience earlier and later menarche may differ in fundamental ways from women whose menarche occurred on time. To date, traditional CVD risk factors do not appear to fully capture MACE risk assessment in women. Future studies should focus on female‐specific risk factors that include menarche and mechanisms by which inflammatory biomarkers increase risk of CVD events in women.
Sources of Funding
This work was supported by contracts from the National Heart, Lung, and Blood Institute nos. N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164, K23‐HL127262‐01A1, K23‐HL125941‐01A1, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1‐RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc, Laurence Harbor, NJ, the Edythe L. Broad, and the Constance Austin Women's Heart Research Fellowships, Cedars‐Sinai Medical Center, Los Angeles, CA, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars‐Sinai Medical Center, Los Angeles, The Society for Women's Health Research (SWHR), Washington, DC, The Linda Joy Pollin Women's Heart Health Program, and the Erika Glazer Women's Heart Health Project, Cedars‐Sinai Medical Center, Los Angeles, CA.
Disclosures
Dr Berga would like to report consultantships from the following: AMAG Pharmaceuticals, Inc Advisory Board Meeting, November 10, 2017, Dallas, TX; Lupin Women's Health Advisory Board, September 23, 2018, Dallas, TX. She would also like to report research funding from the following: Ferring Pharmaceuticals Inc, Site PI, Clinical Trial “LutrePulse OmniPod Study 000070: A Multicenter, Double‐Blind, Randomized, Placebo‐Controlled Study Evaluating Three Doses of Subcutaneous Pulsatile GnRH Administered via OmniPod Pump for Ovulation Induction in Female Subjects with Primary Amenorrhea with Hypogonadotropic Hypogonadism,” 2014‐2018. Dr Stanczyk would like to report consultantships with the following: Agile Therapeutics, Therapeutics MD, Mithra Pharmaceuticals, Dr Reddy's Laboratories. The remaining authors have no disclosures to report.
Supporting information
Table S1. Sex Hormone Levels by Age at Menarche Among Postmenopausal Women
Table S2. Models With TET and sTET for Time to CVD Event
Table S3. Models With TET and sTET for Time to Death
Figure S1. Adjusted relative hazard (with sTET) and 95% CI of major adverse cardiac events (MACE) by age at menarche. sTET indicates supra‐total estrogen time.
Figure S2. Adjusted relative hazard (with sTET) and 95% CI of mortality by age at menarche. sTET indicates supra‐total estrogen time.
(J Am Heart Assoc. 2019;8:e012406 DOI: 10.1161/JAHA.119.012406.)
Data Availability
The data that support the findings of this study are available from the BioLINCC data repository from the WISE data repository.13
References
- 1. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee, Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110:752–757. [DOI] [PubMed] [Google Scholar]
- 3. Robertson RM. Women and cardiovascular disease: the risks of misperception and the need for action. Circulation. 2001;103:2318–2320. [DOI] [PubMed] [Google Scholar]
- 4. Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G; WISE Investigators . Insights from the NHLBI‐Sponsored Women's Ischemia Syndrome Evaluation (WISE) study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender‐optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. [DOI] [PubMed] [Google Scholar]
- 5. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 6. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G; WISE Investigators . Insights from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender‐based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. [DOI] [PubMed] [Google Scholar]
- 7. Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90:2718–2724. [DOI] [PubMed] [Google Scholar]
- 8. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960. [DOI] [PubMed] [Google Scholar]
- 9. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab. 1983;57:82–86. [DOI] [PubMed] [Google Scholar]
- 11. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, Green J, Cairns BJ; Million Women Study Collaborators . Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131:237–244. [DOI] [PubMed] [Google Scholar]
- 12. Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. Int J Epidemiol. 2009;38:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bairey Merz CN. Women's Ischemia Syndrome Evaluation (WISE). Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) 2008. Available at: https://biolincc.nhlbi.nih.gov/studies/wise/?q=WISE.
- 14. Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. [DOI] [PubMed] [Google Scholar]
- 15. Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ; WISE Investigators . Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 16. Rifai N, Joubran R, Yu H, Asmi M, Jouma M. Inflammatory markers in men with angiographically documented coronary heart disease. Clin Chem. 1999;45:1967–1973. [PubMed] [Google Scholar]
- 17. Merz CN, Johnson BD, Berga SL, Braunstein GD, Azziz R, Yang Y, Reis SE, Bittner V, Hodgson TK, Pepine CJ, Sharaf BL, Sopko G, Kelsey SF; Women's Ischemia Syndrome Evaluation Study Group . Total estrogen time and obstructive coronary disease in women: insights from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE). J Womens Health (Larchmt). 2009;18:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Adolesc Health. 2007;40:227–231. [DOI] [PubMed] [Google Scholar]
- 19. Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, Olson MB, Reis SE; National Heart Lung and Blood Institute . Serum amyloid a as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:726–732. [DOI] [PubMed] [Google Scholar]
- 20. Peters SA, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104:1069–1075. [DOI] [PubMed] [Google Scholar]
- 21. Yang L, Li L, Millwood IY, Peters SAE, Chen Y, Guo Y, Bian Z, Chen X, Chen L, Feng S, Lv S, Pang Z, Woodward M, Chen Z; China Kadoorie Biobank Study Collaborative Group . Age at menarche and risk of major cardiovascular diseases: evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int J Cardiol. 2017;227:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallagher LG, Davis LB, Ray RM, Psaty BM, Gao DL, Checkoway H, Thomas DB. Reproductive history and mortality from cardiovascular disease among women textile workers in Shanghai, China. Int J Epidemiol. 2011;40:1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yuan JM, Pereira MA. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: the Singapore Chinese Health Study. Ann Epidemiol. 2012;22:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang HS, Odongua N, Ohrr H, Sull JW, Nam CM. Reproductive risk factors for cardiovascular disease mortality among postmenopausal women in Korea: the Kangwha Cohort Study, 1985–2005. Menopause. 2011;18:1205–1212. [DOI] [PubMed] [Google Scholar]
- 25. Tamakoshi K, Yatsuya H, Tamakoshi A; JACC Study Group . Early age at menarche associated with increased all‐cause mortality. Eur J Epidemiol. 2011;26:771–778. [DOI] [PubMed] [Google Scholar]
- 26. Jacobsen BK, Heuch I, Kvale G. Association of low age at menarche with increased all‐cause mortality: a 37‐year follow‐up of 61,319 Norwegian women. Am J Epidemiol. 2007;166:1431–1437. [DOI] [PubMed] [Google Scholar]
- 27. Merritt MA, Riboli E, Murphy N, Kadi M, Tjønneland A, Olsen A, Overvad K, Dossus L, Dartois L, Clavel‐Chapelon F, Fortner RT, Katzke VA, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Tumino R, Sacerdote C, Panico S, Bueno‐de‐Mesquita HB, Peeters PH, Lund E, Nakamura A, Weiderpass E, Quirós JR, Agudo A, Molina‐Montes E, Larrañaga N, Dorronsoro M, Cirera L, Barricarte A, Olsson Å, Butt S, Idahl A, Lundin E, Wareham NJ, Key TJ, Brennan P, Ferrari P, Wark PA, Norat T, Cross AJ, Gunter MJ. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med. 2015;13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, Tamakoshi A. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006;16:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McPherson K, Steel CM, Dixon JM. Breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tuppurainen M, Kröger H, Saarikoski S, Honkanen R, Alhava E. The effect of gynecological risk factors on lumbar and femoral bone mineral density in peri‐ and postmenopausal women. Maturitas. 1995;21:137–145. [DOI] [PubMed] [Google Scholar]
- 31. Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI‐sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. [DOI] [PubMed] [Google Scholar]
- 32. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang‐Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amrita J, Mahajan M, Bhanwer AJ, Mohan G. Oxidative stress: an effective prognostic tool for an early detection of cardiovascular disease in menopausal women. Biochem Res Int. 2016;2016:6157605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pashkow FJ. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflam. 2011;2011:514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J, Xia S, Kalionis B, Wan W, Sun T. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int. 2014;2014:615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whirledge S, Cidlowski JA. A role for glucocorticoids in stress‐impaired reproduction: beyond the hypothalamus and pituitary. Endocrinology. 2013;154:4450–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sex Hormone Levels by Age at Menarche Among Postmenopausal Women
Table S2. Models With TET and sTET for Time to CVD Event
Table S3. Models With TET and sTET for Time to Death
Figure S1. Adjusted relative hazard (with sTET) and 95% CI of major adverse cardiac events (MACE) by age at menarche. sTET indicates supra‐total estrogen time.
Figure S2. Adjusted relative hazard (with sTET) and 95% CI of mortality by age at menarche. sTET indicates supra‐total estrogen time.
Data Availability Statement
The data that support the findings of this study are available from the BioLINCC data repository from the WISE data repository.13


