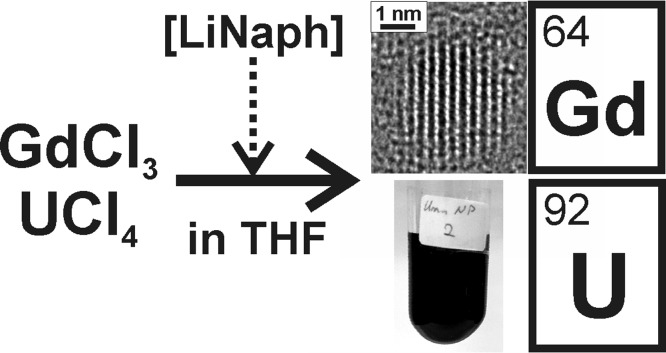
Abstract
Gadolinium (Gd0) and uranium (U0) nanoparticles are prepared via lithium naphthalenide ([LiNaph])-driven reduction in tetrahydrofuran (THF) using GdCl3 and UCl4, respectively, as low-cost starting materials. The as-prepared Gd0 and U0 suspensions are colloidally stable and contain metal nanoparticles with diameters of 2.5 ± 0.7 nm (Gd0) and 2.0 ± 0.5 nm (U0). Whereas THF suspensions are chemically stable under inert conditions (Ar and vacuum), nanoparticulate powder samples show high reactivity in contact with, for example, oxygen, moisture, alcohols, or halogens. Such small and highly reactive Gd0 and U0 nanoparticles are first prepared via a dependable liquid-phase synthesis and stand as representatives for further nanosized lanthanides and actinides.
Introduction
The access to metal nanoparticles is the more challenging the lower the electrochemical potential of the metal, and the smaller the size of the particles.1 Both—strong base character and small size—result in a tremendous increase of the nanoparticle reactivity. In this context, iron is an illustrative example: whereas the corrosion of bulk iron occurs on a timescale of several months and years, nanosized iron is pyrophoric and shows immediate ignition in contact with air.2 This enormous increase in reactivity is related to the significantly higher surface area and the absence of any passivation layer.1 In terms of standard electrode potential, however, bulk iron (E0bulk(Fe0/Fe3+) = −0.81 V) still has a moderate base character.3 The realization of nanosized lanthanide and actinide metals is another magnitude of challenge that is addressed here with gadolinium (E0(Gd0/Gd3+) = −2.28 V)3 and uranium (E0bulk(U0/U4+) = −2.23 V)3 nanoparticles as representative examples. Although the standard electrode potentials (taken for alkaline conditions) account for high oxygen affinity and high chemical reactivity of gadolinium and uranium, they only refer to the bulk metals. Nanosized Gd0 and U0 are of course much more reactive as implied by the standard electrode potential of the bulk metals.
Nanoparticles of highly reactive base metals are yet most often made via gas-phase techniques or by the decomposition of elaborate metal–organic precursors, partially involving harsh and less reproducible conditions.4−6 In view of Gd0 and U0, only few publications are available and involve evaporation methods, γ-ray irradiation, and pulsed laser ablation.7−9 These methods only result in nonuniform, heavily aggregated, and partially oxidized particles. In this concern, it needs to be noted that the most nanoparticles designated “gadolinium” or “gadolinium-based” contain only Gd3+ (e.g., Gd2O3).10−14 The only option for the liquid-phase synthesis of Gd0 nanoparticles dates back to Tsai and Dye and the use of crown ether-stabilized alkalides as powerful reducing agents.15 This strategy was developed further by Wagner et al., who studied the magnetic properties in detail.16 Via electron microscopy, however, they could only identify 10–15 nm sized Gd2O3 nanoparticles. Recently, they also prepared Gd@Au core–shell nanoparticles comprising a dense gold shell to protect the Gd0 core from oxidation.17 In regard to U0—to the best of our knowledge—not any liquid-phase synthesis of nanoparticles has been reported yet.
Taken together, straightforward liquid-phase synthesis of Gd0 and U0 nanoparticles is lacking. Dependable and comparably simple synthesis is even more relevant because Gd0 and U0 stand as representatives for further lanthanide and actinide metals. Owing to this concern, we present here the synthesis of Gd0 and U0 nanoparticles with diameters <5 nm as colloidally stable suspensions in tetrahydrofuran (THF) or toluene.
Results and Discussion
General Aspects
Several requirements are essential for preparing high-quality nanoparticles of highly reactive base metals such as lanthanide and actinide metals. First of all, a powerful reducing agent is essentially required. Here, hydrogen and hydrides (e.g., [BH4]− and [BEt3H]−) as well as elemental metals (e.g., Na0 and Mg0) have been suggested.18 Aiming at metals with a standard electrode potential below −2.0 V, however, hydrides are not powerful enough. Elemental metals, on the other hand, are difficult to handle and need to be dissolved, for instance, in liquid ammonia.19 Alkaline metal naphthalenides represent an alternative option that was already widely used for establishing main-group element–element bonding,20,21 as so-called activated Rieke metals in alternative to Grignard reagents in organic synthesis,22,23 or for obtaining main-group-element nanoparticles such as boron and silicon.24−26 In view of nanosized transition metals (e.g., Ti0, Mo0, W0, Re0, Fe0, and Zn0), we also have good experience with lithium and sodium naphthalenides ([LiNaph] and [NaNaph]).27,28
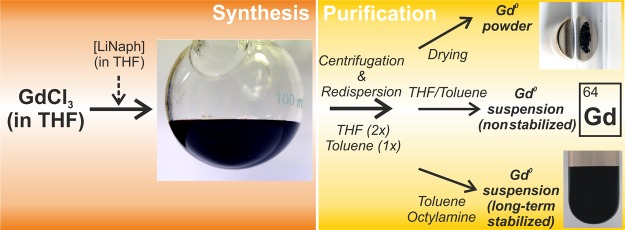
In fact, [MNaph] (M: Li and Na) is ideal for the synthesis of nanoparticles because the injection of a homogenously dissolved reducing agent is optimal for controlling particle nucleation and particle growth. Hence, the high reducing power causes very fast formation of the elemental metal, which is insoluble in THF. Consequently, high oversaturation occurs, which—in accordance with the model given by LaMer and Dinegar29—results in very small particles. Moreover, the deep green [MNaph] solutions are chemically stable for weeks (under argon) and easy to portion with a syringe (in contrast to solid sodium metal), and they can be handled at ambient pressure and temperature (in contrast to liquid ammonia) (Figure 1). Finally, the concentration and the reducing power of the intensely colored [MNaph] solutions can be easily determined via the Lambert–Beer law.
Figure 1.
Scheme illustrating the synthesis of Gd0 nanoparticles including purification and realization of magnetic powder samples as well as of long-term stable octylamine-modified suspensions.
Beside the reducing agent, the use of low-cost metal chlorides (i.e., GdCl3 and UCl4) as the starting materials and of THF as a standard solvent are specific benefits of the synthesis approach applied here (Figure 1). Thus, elaborate precursors (e.g., carbonyls and organometallic compounds) that need advanced multistep preparation are not needed. All in all, straightforward synthesis of Gd0 and U0 nanoparticles as well as of other nanosized lanthanide and actinide metals can be highly relevant in view of their properties, including magnetism (e.g., Sm–Co magnets),30 catalysis (e.g., Pt5Ln),31 superconductivity (e.g., heavy fermion actinide- and lanthanide-based materials),32 metallic glasses (Ce–Al alloys),33 or nanodispersed nuclear fuels.34−36
Gd0 Nanoparticles
Gd0 nanoparticles were made via the [LiNaph]-driven reduction of GdCl3 in THF. The immediate formation of a deep black suspension upon the addition of the greenish [LiNaph] into the colorless GdCl3 solution indicates the formation of Gd0 nanoparticles (Figure 1). Subsequent to the reaction, side products were removed by repeated centrifugation and redispersion. Thus, LiCl was removed via dissolution in THF; naphthalene was removed by dissolution in toluene (Figure 1). After careful purification, the as-prepared Gd0 nanoparticles can be either dried in vacuum to obtain blackish powder samples or, in alternative, suspended in THF or toluene to obtain suspensions that are colloidally stable for several hours (i.e., not showing agglomeration and/or sedimentation). For long-term stabilization, a certain amount of octylamine can be added (i.e., 38 mL of toluene with 2 mL of octylamine). Such alkylamines (especially oleylamine) are well-known stabilizers for all kinds of metal nanoparticles.37 In difference to the literature, however, alkylamines are not required here to control the particle nucleation and only optionally added after the formation of the nanoparticles.
Particle size and particle size distribution of the as-prepared Gd0 nanoparticles were evaluated by transmission electron microscopy (TEM) (Figure 2). Overview images show uniform nonagglomerated nanoparticles with a narrow size distribution (Figure 2a,b). Statistical evaluation of 100 nanoparticles results in a mean diameter of 2.5 ± 0.7 nm. High-resolution TEM (HRTEM) confirms the uniform spherical shape of the Gd0 nanoparticles (Figure 2c). Moreover, HRTEM indicates the crystallinity of the as-prepared nanoparticles and clearly shows lattice fringes. Fast Fourier transformation (FFT) analysis of the nanoparticle ensembles confirms an excellent coincidence with the diffraction pattern of hexagonal bulk Gd0 (lattice parameters: a = 3.62 and c = 5.82 Å) (Figure 2d).38 Single particles are monocrystalline, as demonstrated by the FFT analysis of a particle on the HRTEM image (Figure 2e), which is also in good agreement with the calculated diffraction pattern of hexagonal bulk Gd0 (space group: P63/mmc) in the [210] zone axis (Figure 2f).
Figure 2.
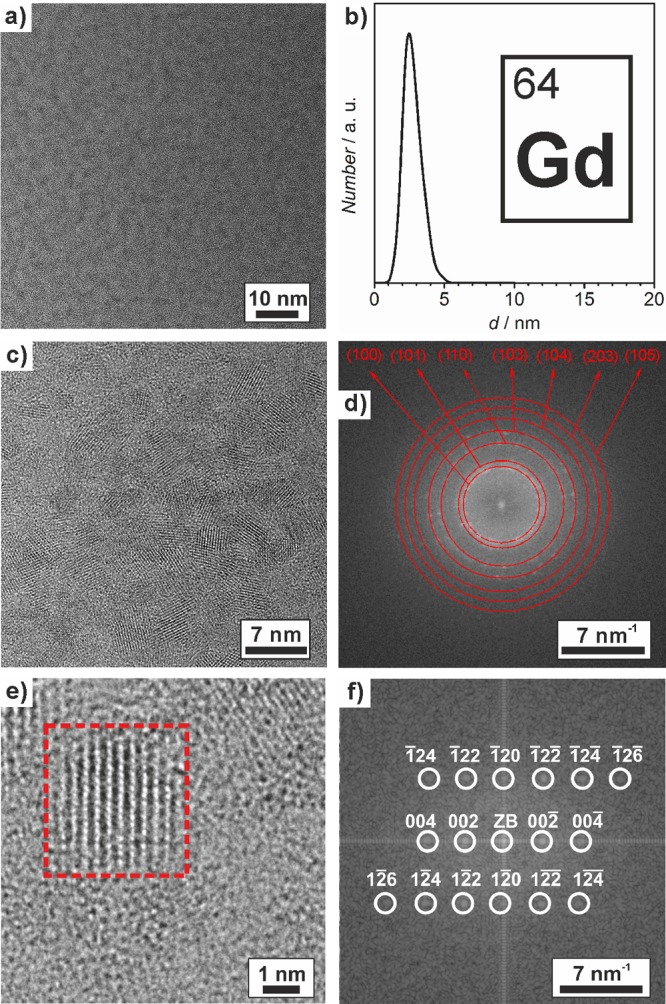
Electron microscopy of the as-prepared Gd0 nanoparticles: (a) TEM overview image, (b) size distribution based on statistical evaluation of ≥100 nanoparticles on TEM images, (c) HRTEM image, (d) FFT analysis of a particle ensemble, (e) HRTEM image of a single particle, and (f) FFT analysis of the red marked area with calculated diffraction pattern of hexagonal bulk Gd0 (a = 3.62 and c = 5.82 Å in the [210] zone axis; ZB indicates the zero-order beam).
Because of the high reactivity of the Gd0 nanoparticles, not only the chemical synthesis but also the sample handling (e.g., purification procedure, centrifugation, and transfer into the electron microscope) and all analytical characterizations require strict inert conditions and the exclusion of all traces of oxygen and moisture. Thus, the transfer of samples into the electron microscope needs to be performed via suitable vacuum and inert gas transfer modules (see the Supporting Information). Here, it turned out as essential that the resting time of the sample grid with the deposited Gd0 nanoparticles in the transfer module is as short as possible (<30 min) to avoid oxygen contamination and formation of Gd2O3 (Supporting Information: Figure S1).
The high reactivity of the as-prepared Gd0 nanoparticles can be demonstrated by two illustrative examples. On the one hand, they even react with the carbon layer of the Lacey carbon copper grid under high-energy-electron bombardment in the electron microscope. On the other hand, octylamine-stabilized nanoparticles that were centrifuged and sintered as powder samples (900 °C) in vacuum, according to X-ray powder diffraction, show reaction to GdN, with octylamine being the only available nitrogen source (Supporting Information: Figure S2). The absence of impurities such as Gd2O3, GdCl3, or LiCl, moreover, confirms the purity of the as-prepared nanoparticles.
U0 Nanoparticles
U0 nanoparticles were prepared similarly to Gd0 via [LiNaph]-driven reduction of UCl4 in THF. Side products of the reaction were again removed by repeated centrifugation in THF (i.e., removal of LiCl) and toluene (i.e., removal of naphthalene). The as-prepared U0 nanoparticles were either dried to blackish powder samples or redispersed in THF or toluene and result in colloidally highly stable suspensions upon the addition of a certain amount of octylamine. According to TEM analysis, the as-prepared U0 nanoparticles exhibit a facetted shape and a narrow size distribution with an average particle diameter of 2.0 ± 0.5 nm (Figure 3a,b). The similar size of Gd0 and U0 can be attributed to the very fast reduction that results in a high supersaturation of insoluble elemental metal in THF. This situation—according to the LaMer model—favors very fast nucleation and the formation of small particles.29
Figure 3.
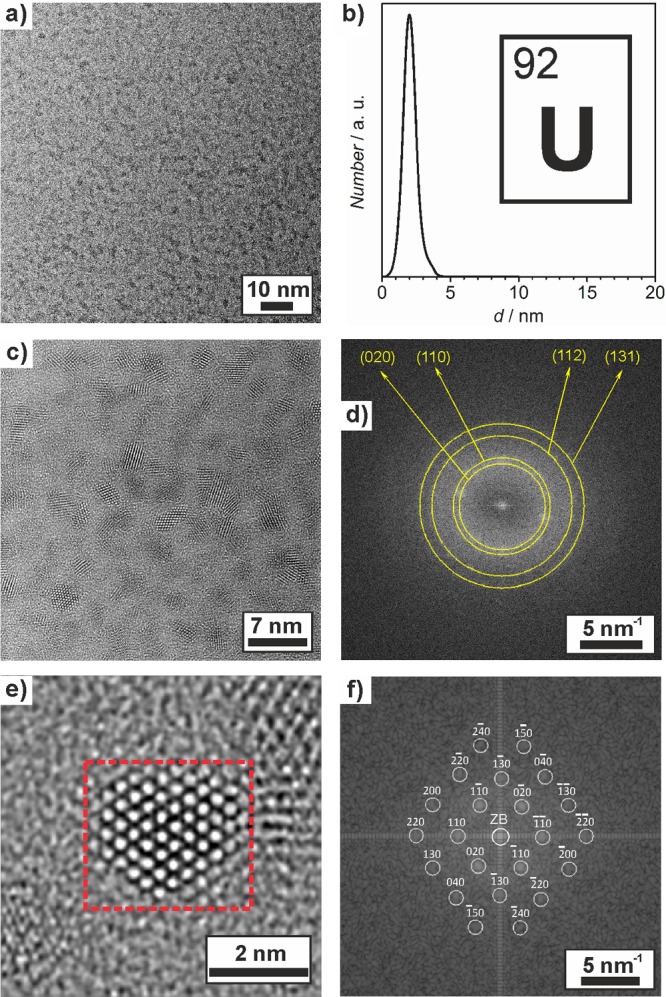
Electron microscopy of the as-prepared U0 nanoparticles: (a) TEM overview image, (b) size distribution based on statistical evaluation of ≥100 nanoparticles on TEM images, (c) HRTEM image, (d) FFT analysis of a particle ensemble, (e) HRTEM image of a single particle, and (f) FFT analysis of the red marked area with calculated diffraction pattern of orthorhombic bulk α-U0 (a = 2.85, b = 5.87, and c = 4.96 Å along the [001] zone axis; ZB indicates the zero-order beam).
The as-prepared U0 nanoparticles also turned out as crystalline. Thus, HRTEM indicates lattice fringes (Figure 3c,e), and the FFT analysis of the nanoparticle ensembles shows excellent coincidence with the diffraction pattern of orthorhombic bulk α-U0 (lattice parameters: a = 2.85, b = 5.87, and c = 4.96 Å) (Figure 3d).39 Single particles are monocrystalline as demonstrated by the good agreement between the FFT of the particle on the HRTEM image (Figure 3e) and the calculated diffraction pattern of bulk orthorhombic α-U0 (space group: Cmcm) in the [001] zone axis (Figure 3f).
To illustrate the high reactivity of the as-prepared U0 nanoparticles, we have evaluated several reactions. Whereas the dark black suspensions of the U0 nanoparticles in toluene are chemically highly stable under argon, decolorization under the formation of brownish UO2 suspensions occurred in the presence of air on a timescale of few minutes (Figure 4). Powder samples of the U0 nanoparticles react with air, humidity, and acids (e.g., aqueous hydrochloric acid) as well as neat bromine or iodine under immediate ignition. In a more controlled manner, iodine was slowly added to the THF suspensions of the U0 nanoparticles to form bluish solutions, from which dark blue single crystals were obtained after 2–3 days. Lattice parameter determinations of these single crystals proved their identity as UI3 × 4THF.40 This reaction also confirms the reactivity of the as-prepared U0 nanoparticles.
Figure 4.
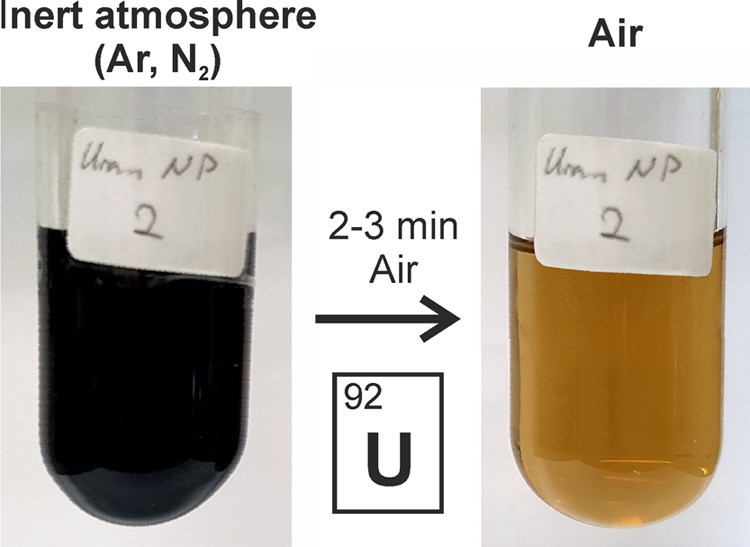
Sensitivity and reactivity of the as-prepared U0 nanoparticles when in contact with air (2–3 min).
Conclusions
Gadolinium (Gd0) and uranium (U0) nanoparticles are prepared via lithium naphthalenide ([LiNaph])-driven reduction of GdCl3 and UCl4, respectively, in THF. Because of the powerful reducing agent, particle nucleation and particle size are well-controlled, which results in uniform nanoparticles with a narrow size distribution with average diameters of 2.5 ± 0.7 nm (Gd0) and 2.0 ± 0.5 nm (U0). Specific surface-active agents are not needed to control the particle size. Such a liquid-phase synthesis is shown here for the first time. U0 nanoparticles are made via the liquid phase for the first time in general. Subsequent to suitable purification, the Gd0 and U0 nanoparticles are available as powder samples, suspensions in THF or toluene, or long-term-stabilized suspensions upon the addition of octylamine. The nanoparticles are chemically stable in suspension under inert conditions. However, they are highly reactive in the presence of oxygen and moisture of other oxidizing agents, where they react with immediate ignition.
The straightforward, highly dependable liquid-phase synthesis of Gd0 and U0 nanoparticles offers an access to all additional lanthanide and actinide metal nanoparticles. Using THF as the solvent and simple metal chlorides (i.e., GdCl3 and UCl4) as the starting materials is an additional benefit of the synthesis strategy. Beside fundamental research and establishing a new, reliable liquid-phase synthesis strategy for Gd0 and U0, the synthesis of further nanosized lanthanide/actinide metals as well as their use as reactive intermediates for obtaining nanosized lanthanide/actinide compounds (e.g., alloys, intermetallics, sulfides, and nitrides) can become highly relevant. This also includes a bunch of material properties ranging from magnetism, catalysis, and superconductivity to nuclear applications.
Experimental Section
Starting Materials
THF (99%, Seulberger) and toluene (99%, Seulberger) were refluxed and freshly distilled from sodium and benzophenone. Octylamine (Aldrich, 99%) was refluxed and distilled in a first step from CaH2 and thereafter, in a second step, from NaH and degassed by three freeze–pump–thaw cycles. Gadolinium(III) chloride (99.99%, Sigma-Aldrich), lithium (99%, Alfa Aesar), and naphthalene (99%, Alfa Aesar) were used as received. Highly pure single-crystalline uranium(IV) chloride was prepared according to the literature by the chemical transport of UCl4 prepared in situ from UO2 and AlCl3.41
Lithium naphthalenide ([LiNaph]) was prepared by dissolving 28 mg of Li (4.0 mmol) and 600 mg of naphthalene (4.7 mmol) in 10 mL of THF over a period of 24 h.
Synthesis of Gd0 Nanoparticles
Gadolinium(III) chloride (703 mg, 2.67 mmol) was dissolved in 20 mL of THF. Thereafter, a solution containing 56 mg of lithium (8.0 mmol) and 1200 mg of naphthalene (9.4 mmol) in 20 mL of THF was added with vigorous stirring. The formation of the Gd0 nanoparticles can be followed by the naked eye based on the immediate change from a colorless solution to a dark black suspension. The Gd0 nanoparticles were separated by centrifugation and washed twice by redispersion and centrifugation in/from 20 mL of THF and once in/from toluene. Finally, the nanoparticles were centrifuged and dried in vacuum to obtain powder samples. In alternative, the Gd0 nanoparticles can be redispersed in 38 mL of THF and 2 mL of octylamine to obtain long-term-stabilized suspensions.
Synthesis of U0 Nanoparticles
U0 nanoparticles were prepared similarly to the Gd0 nanoparticles. Accordingly, 380 mg of UCl4 (1.0 mmol) was dissolved in 15 mL of THF. Thereafter, a solution containing 28 mg of lithium (4.0 mmol) and 600 mg of naphthalene (4.7 mmol) in 10 mL of THF was injected with vigorous stirring. Again, a deep black suspension was obtained immediately. Separation, washing, and formation of powder samples and suspensions were performed as described above.
Sample Handling
Because of the sensitivity and reactivity of the as-prepared Gd0 and U0 nanoparticles, strict sample handling with inert conditions (i.e., Ar and vacuum) is required, ranging from synthesis to sample transfer and all analytical studies (see the Supporting Information).
Safety Advice
Although the as-prepared Gd0 and U0 nanoparticles are chemically highly stable in THF or toluene suspensions under inert conditions (Ar), they are highly reactive in the presence of all kinds of oxidizing agents including air, humidity, halogens, and acids. Even alcohols (e.g., hexanol and octanol) lead to heavy evolution of hydrogen. Powder samples are highly pyrophoric and can cause immediate ignition.
Working with radioactive materials may demand special radiation protection depending on your local legislation.
More information regarding the applied analytical techniques can be found in the Supporting Information.
Acknowledgments
C.S., R.P., D.G., and C.F. are grateful to the Deutsche Forschungsgemeinschaft (DFG) for funding of personnel (NanoMet: FE911/11-1, GE 841/29-1) and TEM equipment (INST 121384/33-1 FUGG). Moreover, C.S. acknowledges the Karlsruhe Graduate School of Optics and Photonics (KSOP) for scholarship. F.K. thanks the DFG for his Heisenberg Professorship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01442.
Data regarding the analytical techniques, practical handling of the highly reactive base metal nanoparticles and their transfer from synthesis to electron microscopy, and thermal behavior and formation of GdN (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ozin G. A.; Arsenault A.; Cademartiri L.. Nanochemistry: A Chemical Approach to Nanomaterials; RSC Publishing: London, 2005. [Google Scholar]
- Huber D. L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- Bard A. J.; Parsons R.; Jordan J.. Standard Potentials in Aqueous Solutions; Dekker: New York, 1985. [Google Scholar]
- Janiak C.Metal nanoparticle synthesis in ionic liquids. Ionic Liquids (ILs) in Organometallic Catalysis; Topics in Organometallic Chemistry; Springer, 2015; Vol. 51, pp 17–53. [Google Scholar]
- Zeng H.; Du X.-W.; Singh S. C.; Kulinich S. A.; Yang S.; He J.; Cai W. Nanomaterials via laser ablation/irradiation in liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. 10.1002/adfm.201102295. [DOI] [Google Scholar]
- Mariotti D.; Sankaran R. M. Microplasmas for nanomaterials synthesis. J. Phys. D: Appl. Phys. 2010, 43, 323001. 10.1088/0022-3727/43/32/323001. [DOI] [Google Scholar]
- Aruna I.; Mehta B. R.; Malhotra L. K.; Shivaprasad S. M. Stability and hydrogenation of “bare” gadolinium nanoparticles. Adv. Funct. Mater. 2005, 15, 131–137. 10.1002/adfm.200400097. [DOI] [Google Scholar]
- Nenoff T. M.; Ferriera S. R.; Huang J.; Hanson D. J. Formation of uranium based nanoparticles via gamma-irradiation. J. Nucl. Mater. 2013, 442, 162–167. 10.1016/j.jnucmat.2013.08.027. [DOI] [Google Scholar]
- Trelenberg T. W.; Glade S. C.; Tobin J. G.; Hamza A. V. The production and oxidation of uranium nanoparticles produced via pulsed laser ablation. Surf. Sci. 2006, 600, 2338–2348. 10.1016/j.susc.2006.03.028. [DOI] [Google Scholar]
- Dufort S.; Le Duc G.; Salomé M.; Bentivegna V.; Sancey L.; Bräuer-Krisch E.; Requardt H.; Lux F.; Coll J.-L.; Perriat P.; Roux S.; Tillement O. The high radiosensitizing efficiency of a trace of gadolinium-based nanoparticles in tumors. Sci. Rep. 2016, 6, 29678. 10.1038/srep29678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detappe A.; Thomas E.; Tibbitt M. W.; Kunjachan S.; Zavidij O.; Parnandi N.; Reznichenko E.; Lux F.; Tillement O.; Berbeco R. Ultrasmall silica-based bismuth gadolinium nanoparticles for dual magnetic resonance-computed tomography image guided radiation therapy. Nano Lett. 2017, 17, 1733–1740. 10.1021/acs.nanolett.6b05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-T.; Padmanabhan P.; Gulyás B. Z. Gadolinium(III) based nanoparticles for T1-weighted magnetic resonance imaging probes. RSC Adv. 2016, 6, 60945–60966. 10.1039/c6ra07782j. [DOI] [Google Scholar]
- Shetty A. N.; Pautler R.; Ghaghada K.; Rendon D.; Gao H.; Starosolski Z.; Bhavane R.; Patel C.; Annapragada A.; Yallampalli C.; Lee W. A liposomal Gd contrast agent does not cross the mouse placental barrier. Sci. Rep. 2016, 6, 27863. 10.1038/srep27863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. C.; Kurland B. F.; Liu C.-L.; Ho R. J. Y.; Ruddell A. Tumor-induced lymph node alterations detected by MRI lymphography using gadolinium nanoparticles. Sci. Rep. 2015, 5, 15641. 10.1038/srep15641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K. L.; Dye J. L. Nanoscale metal particles by homogeneous reduction with alkalides or electrides. J. Am. Chem. Soc. 1991, 113, 1650–1652. 10.1021/ja00005a031. [DOI] [Google Scholar]
- Nelson J. A.; Bennett L. H.; Wagner M. J. Solution synthesis of gadolinium nanoparticles. J. Am. Chem. Soc. 2002, 124, 2979–2983. 10.1021/ja0122703. [DOI] [PubMed] [Google Scholar]
- Yan C.; Wagner M. J. Air- and water-stable gold coated gadolinium metal nanocrystals. Nano Lett. 2013, 13, 2611–2614. 10.1021/nl400720n. [DOI] [PubMed] [Google Scholar]
- Connelly N. G.; Geiger W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 1996, 96, 877–910. 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]
- Schöttle C.; Bockstaller P.; Gerthsen D.; Feldmann C. Tungsten nanoparticles from liquid-ammonia-based synthesis. Chem. Commun. 2014, 50, 4547–4550. 10.1039/c3cc49854a. [DOI] [PubMed] [Google Scholar]
- Fischer R. C.; Power P. P. π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. 10.1021/cr100133q. [DOI] [PubMed] [Google Scholar]
- Braunstein P.; Oro L. A.; Raithby P. R.. Metal Clusters in Chemistry; Wiley-VCH: Weinheim, 2008. [Google Scholar]
- Rieke R. D. Preparation of organometallic compounds from highly reactive metal powders. Science 1989, 246, 1260–1264. 10.1126/science.246.4935.1260. [DOI] [PubMed] [Google Scholar]
- Garza-Rodríguez L. A.; Kharisov B. I.; Kharisova O. V. Overview on the synthesis of activated micro- and nanostructurized Rieke metals: history and present state. Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 2009, 39, 270–290. 10.1080/15533170902918825. [DOI] [Google Scholar]
- Pickering A. L.; Mitterbauer C.; Browning N. D.; Kauzlarich S. M.; Power P. P. Room temperature synthesis of surface-functionalized boron nanoparticles. Chem. Commun. 2007, 580–582. 10.1039/b614363f. [DOI] [PubMed] [Google Scholar]
- Chiu H. W.; Chervin C. N.; Kauzlarich S. M. Phase changes in Ge nanoparticles. Chem. Mater. 2005, 17, 4858–4864. 10.1021/cm050674e. [DOI] [Google Scholar]
- Baldwin R. K.; Pettigrew K. A.; Ratai E.; Augustine M. P.; Kauzlarich S. M. Solution reduction synthesis of surface stabilized silicon nanoparticles. Chem. Commun. 2002, 1822–1823. 10.1039/b205301b. [DOI] [PubMed] [Google Scholar]
- Schöttle C.; Bockstaller P.; Popescu R.; Gerthsen D.; Feldmann C. Sodium-naphthalenide-driven synthesis of base-metal nanoparticles and follow-up reactions. Angew. Chem., Int. Ed. 2015, 54, 9866–9870. 10.1002/anie.201503269. [DOI] [PubMed] [Google Scholar]
- Schöttle C.; Doronkin D. E.; Popescu R.; Gerthsen D.; Grunwaldt J.-D.; Feldmann C. Ti0 nanoparticles via lithium-naphthalenide-driven reduction. Chem. Commun. 2016, 52, 6316–6319. 10.1039/c6cc01957a. [DOI] [PubMed] [Google Scholar]
- LaMer V. K.; Dinegar R. H. Theory, Production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. 10.1021/ja01167a001. [DOI] [Google Scholar]
- Frey N. A.; Peng S.; Cheng K.; Sun S. Magnetic Nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. 10.1039/b815548h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero-Escribano M.; Malacrida P.; Hansen M. H.; Vej-Hansen U. G.; Velazquez-Palenzuela A.; Tripkovic V.; Schiotz J.; Rossmeisl J.; Stephens I. E. L.; Chorkendorff I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76. 10.1126/science.aad8892. [DOI] [PubMed] [Google Scholar]
- Aynajian P.; da Silva N. E. H.; Gyenis A.; Baumbach R. E.; Thompson J. D.; Fisk Z.; Bauer E. D.; Yazdani A. Visualizing heavy fermions emerging in a quantum critical Kondo lattice. Nature 2012, 486, 201–206. 10.1038/nature11204. [DOI] [PubMed] [Google Scholar]
- Wu M.; Tse J. S.; Wang S. Y.; Wang C. Z.; Jiang J. Z. Origin of pressure-induced crystallization of Ce75Al25 metallic glass. Nat. Commun. 2015, 6, 6493. 10.1038/ncomms7493. [DOI] [PubMed] [Google Scholar]
- Hickam S.; Burns P. C.. Oxo clusters of 5f elements. Recent Development in Clusters of Rare Earths and Actinides: Chemistry and Materials; Structure and Bonding; Springer, 2017; Vol. 173, pp 121–153. [Google Scholar]
- Tyrpekl V.; Cologna M.; Vigier J.-F.; Cambriani A.; De Weerd W.; Somers J. Preparation of bulk-nanostructured UO2 pellets using high-pressure spark plasma sintering for LWR fuel safety assessment. J. Am. Ceram. Soc. 2017, 100, 1269–1274. 10.1111/jace.14647. [DOI] [Google Scholar]
- Wu H.; Yang Y.; Cao Y. C. Synthesis of colloidal uranium-dioxide nanocrystals. J. Am. Chem. Soc. 2006, 128, 16522–16523. 10.1021/ja067940p. [DOI] [PubMed] [Google Scholar]
- Mourdikoudis S.; Liz-Marzán L. M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. 10.1021/cm4000476. [DOI] [Google Scholar]
- Oudet X. Comparison between crystal structure and magnetic properties of rare earth and 3d transition metal compounds. J. Magn. Magn. Mater. 1985, 47–48, 397–399. 10.1016/0304-8853(85)90449-4. [DOI] [Google Scholar]
- Eeles W. T.; Sutton A. L. X-ray determination of the atomic positions in α-uranium at 22 °C and 600 °C. Acta Crystallogr. 1963, 16, 575. 10.1107/s0365110x63001511. [DOI] [Google Scholar]
- Avens L. R.; Bott S. G.; Clark D. L.; Sattelberger A. P.; Watkin J. G.; Zwick B. D. A Convenient Entry into Trivalent actinide chemistry: synthesis and characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An = U, Np, Pu). Inorg. Chem. 1994, 33, 2248–2256. 10.1021/ic00088a030. [DOI] [Google Scholar]
- Rudel S. S.; Kraus F. Facile syntheses of pure uranium halides: UCl4, UBr4 and UI4. Dalton Trans. 2017, 46, 5835–5842. 10.1039/c7dt00726d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.