Abstract
Purpose
Blood flow in the optic nerve head (ONH) is known to be reduced in eyes with advanced glaucoma. However, experimental results from non-human primates suggest an initial increase in ONH blood flow at the earliest stages of damage. This study assesses flow and pulsatile hemodynamics across a range of severities to test the hypothesis that this also occurs in human glaucoma.
Methods
Laser speckle flowgraphy was used to measure average mean blur rate (MBRave) within ONH tissue (a correlate of capillary blood flow) and the pulsatile waveform in 93 eyes with functional loss and 74 glaucoma suspect/fellow eyes without functional loss. These were compared against results from 92 healthy control eyes. Parameters produced by the instrument's software were age-corrected, then compared between groups using generalized estimating equation models.
Results
The mean MBRave in the control eyes was 12.5 units. In glaucoma suspect/fellow eyes, the mean was 16.4 units, higher with P < 0.0001. In eyes with functional loss, the mean was 13.8 units, lower than eyes without functional loss with P < 0.0001, although still higher than control eyes with P = 0.0096. Analysis of the pulsatile waveform suggested that the deceleration in flow as it approaches its maximum across the cardiac cycle was delayed in glaucoma.
Conclusions
Blood flow within ONH capillaries was higher in glaucoma suspect eyes than in healthy controls. It was less elevated in eyes that had developed functional loss. The mechanisms causing these changes and their relation to concurrent changes in pulsatile hemodynamics remain under investigation.
Keywords: blood flow, optic nerve head, laser speckle flowgraphy, glaucoma, pulse cycle
It has long been known that ocular blood flow is compromised in eyes with primary open-angle glaucoma (POAG),1 but it remains unclear whether these changes are a primary insult, a consequence of optic neuropathy, or a combination of the two. The majority of studies have shown significantly reduced ocular blood flow in eyes with manifest glaucoma.1–3 However, there are also reports showing little or no change in blood flow.4–6 Intriguingly, while overall blood flow in the retina is lower in glaucomatous eyes than in normal eyes, during the early stage of the disease blood flow has been reported to be inversely correlated with retinal nerve fiber layer thickness (RNFLT), that is, the thinner the RNFLT, the higher the blood flow.7 At later stages, it was postulated that this inverse correlation eventually “burns out” with a continuing decrease in blood flow accompanying further RNFL thinning. The discrepancies among these studies might therefore be attributable to the subjects being at different stages of the disease.7
Within the optic nerve head (ONH), substantial variations in the change in blood flow have been found between individuals in non-human primate models of experimental glaucoma. In a study by Quigley et al.,8 while most eyes with chronic IOP elevation showed decreased blood flow in ONH, a third of the eyes showed a slight increase, resulting in an overall lack of correlation between blood flow and severity of damage. In that study, blood flow was measured at a late stage of the disease, up to 20 months after moderate IOP elevation.
In a recent study using a similar experimental model in our lab, ONH blood flow was measured using laser speckle flowgraphy (LSFG).9 Raw data from the LSFG is reported as the mean blur rate (MBR), which has been shown to be linearly correlated with capillary blood flow,10,11 allowing a more direct assessment of blood flow than is possible when relying on the density of perfused vessels measured by optical coherence tomography angiography (OCTA). Eyes were followed longitudinally, with weekly LSFG scans from baseline measurements prior to the initial IOP insult until up to 60% loss of RNFLT had occurred. Prior to approximately 10% RNFLT loss, the ONH blood flow increased by 9% (±10%) compared to baseline, causing an inverse relation with RNFLT similar to that reported for blood flow in the major retinal arteries.7 However, after that point, blood flow then steadily declined to 40% of its baseline level.9 An initial increase in ONH blood flow could occur as a consequence of autoregulation disruption.12 Since most clinical studies used cross-sectional designs and measured blood flow at different stages of the disease, any increase in blood flow at the earliest stage of glaucomatous pathophysiology could be obscured by a decrease in blood flow during later stages of the disease.
LSFG analyzes blood flow based on averaging a series of pulsatile blood flow waves over several cardiac cycles across a period of 4 seconds. As such, it is able to parameterize and hence quantify aspects of the pulsatile waveform, potentially revealing insights into pathophysiological changes in the response across the cardiac cycle. Though the physiological specifications of these parameters have not been systemically investigated, multiple studies have shown they can be used to reveal hemodynamic differences in glaucoma.10,13–15 This suggests that LSFG may provide a way to assess and quantify changes in both ONH blood flow and its pulsatile variation in vivo in human glaucoma.
In this current study, we aimed to determine whether our previous observations in an experimental model of glaucoma translated to clinical studies of human patients and to further elucidate the role that changes in ONH blood flow may have in the pathophysiology of glaucoma. We used the LSFG technique to measure ONH blood flow (using MBR) and parameters of the pulsatile waveform in cohorts comprising eyes with glaucomatous visual field loss, glaucoma suspect/fellow eyes that have not yet developed detectable visual field loss, and healthy controls. We aimed to test the hypothesis that ONH blood flow is increased at the earliest stages of pathophysiology, prior to a subsequent decrease, in human glaucoma and to test whether corresponding changes are observed in pulsatile hemodynamics.
Methods
Subjects
Blood flow measurements were compared between a cohort with glaucoma/glaucoma suspects and those from a published normative database,16 supplemented by a smaller cohort of normal subjects tested at Devers Eye Institute (Portland, OR, USA) to detect any differences that might occur due to subtle inconsistencies between instruments or testing protocols. Thus, data were used from the following three independent cohorts. All research adhered to the tenets of the Declaration of Helsinki.
Portland Progression Project (P3) Cohort
One hundred sixty-seven eyes of 88 subjects with open-angle glaucoma or suspected glaucoma, as determined by the subject's clinician, were tested at Devers Eye Institute. These cross-sectional data were collected in subjects enrolled in the ongoing longitudinal P3 study.17–19 Subjects underwent a set of functional and structural diagnostic tests once every 6 months, including standard automated perimetry (SAP) (HFAII; Carl Zeiss Meditec Inc., Dublin, CA, USA), 24-2 test pattern, and SITA Standard test strategy; optical coherence tomography (Spectralis OCT2; Heidelberg Engineering, Heidelberg, Germany); and systemic blood pressure measurement using an arm cuff. Subjects were excluded if they had significant visual field loss due to causes other than glaucoma or if they had systemic hypertension (based on the same criteria as in the Wien controls, below). If LSFG data were available for more than one time point, the most recent test was used. Both eyes were tested, even if only one had extant glaucomatous loss, on the basis that this implies that the fellow eye could be considered as a “glaucoma suspect” that is at increased risk of developing glaucomatous loss in the future.20
Wien Controls
Seventy-seven eyes of 77 healthy white subjects of European descent were tested at the Medical University of Vienna. This data set was previously published in its entirety by Luft et al.16 All subjects were free of ocular disease or abnormality (as judged by the study investigators) and systemic hypertension (systolic pressure ≥160 mm Hg and/or diastolic pressure ≥100 mm Hg). Data collected under pupil dilation were used for the current study to maximize consistency with data collection in the P3 cohort. Eyes were imaged three times under mydriasis, and the average parameter values over those three images were used for the current study. Of the 80 eyes in the original study, there were three eyes for which only two of the three obtained images were of sufficient quality, and these three eyes were excluded from the current study.
Portland Controls
Fifteen eyes of 15 healthy white subjects were tested at Devers Eye Institute. Subjects were recruited from a list of 42 participants who volunteered for a previous study conducted within the Devers Eye Institute. These participants had all received a comprehensive eye examination and were regarded as having normal vision and healthy eyes. Eyes were not dilated for LSFG testing in this cohort, although it should be noted that the effect of pupil dilation in the Wien controls was minimal.16
The P3 cohort was then subdivided into 74 eyes without functional loss, defined as a glaucoma hemifield test (GHT)21 of either within normal limits or abnormally high sensitivity on that test date and 93 eyes with existing functional loss, defined as other categories of GHT.
Laser Speckle Flowgraphy
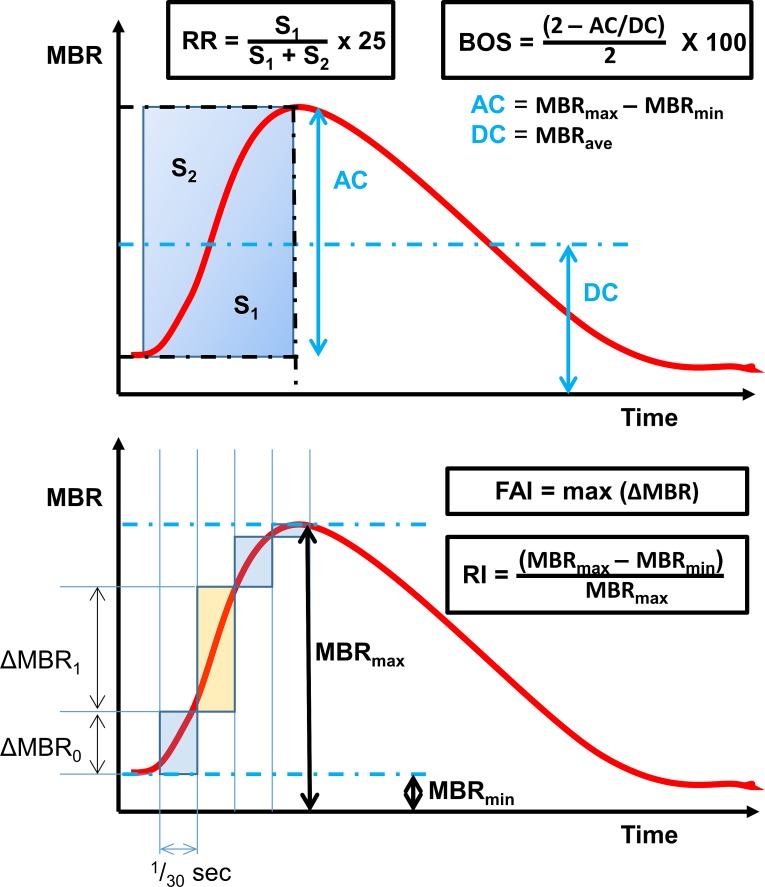
Measurements were performed using an LSFG instrument (LSFG-NAVI, Softcare Co., Ltd., Fukuoka, Japan) to obtain parameters of ONH blood flow and pulsatile hemodynamics. The LSFG technique used to measure blood flow has been described in detail in previous studies.9,10 In brief, a fundus camera equipped within the LSFG device was focused on a 750 × 360 pixel area (approximately 6 × 3.8 mm)10 centered on the ONH. An 830-nm laser generates a speckle pattern due to random interference of scattered light from the illuminated tissue area. The speckle pattern is continuously imaged by a charge-coupled device at a frequency of 30 frames per second for a period of 4 seconds. The MBR of the speckle contrast within the images is computed by the manufacturer's analysis software (LSFG Analysis; Softcare Co., Japan). MBR at a given pixel is defined based on the intensity at that pixel in that frame, at each of the eight surrounding pixels in that frame, and at each of those same nine pixels in the preceding and following frames. Specifically, MBR = (M/D)2, where M/26 is the mean intensity across the 26 pairs of pixels (the pixel of interest and each of those 26 other pixels in turn), and D/26 is the mean difference within these pairs of pixels.22 As a ratio, MBR is reported in arbitrary units. MBR varies temporally and spatially according to the velocity of blood cell movement and correlates well with blood flow within the ONH.10,11 From a composite map of the 4-second recording of the MBR, the area corresponding to all large blood vessels within the ONH disc is masked, such that the resulting MBR values represent the capillary blood flow within the tissue. The analysis software then generates the average pulsatile waveform of MBR for a single cardiac cycle (Fig. 1). The software proceeds to generate a number of parameters from the shape of this pulsatile waveform.16 These parameters include the following:
Figure 1.
Definition of some of the parameterizations of pulsatile waveform produced by the LSFG instrument software, based on the average pulse cycle within a 4-second window. MBR, mean blur rate; BOS, blowout score; RR, rising rate; FAI, flow acceleration index; RI, resistivity index.
Average mean blur rate (MBRave) of the tissue, defined as the mean MBR of the waveform, representing a surrogate measure of mean capillary blood flow;
Skew, an index of the skewness of the pulsatile waveform, such that a higher value indicates that MBR decreases more rapidly from its peak;
Blowout score (BOS), a measure of the proportion of blood flow that is maintained in the vessel between heartbeats, calculated from the average MBR and the difference of the maximum and minimum MBR of the waveform (Fig. 1);
Blowout time (BOT), the proportion of the pulsatile waveform in which MBR is closer to its maximum than its minimum;
Rising rate (RR), defined as the integral of the waveform during the time period in which MBR is increasing, expressed as a proportion of the theoretical maximum, multiplied by 25 (Fig. 1);
Falling rate (FR), the equivalent integral of the waveform during the time period in which MBR is decreasing, expressed as a proportion of the theoretical maximum, multiplied by 25;
Flow acceleration index (FAI), an index of momentary power in one frame (1/30 seconds), defined as the maximum amount of change in MBR between two frames (see Fig. 1);
Acceleration time index (ATI), the time taken for the waveform to reach its peak as a proportion of the length of the waveform; and
Resistivity index (RI), defined as the difference between the maximum MBR and the minimum MBR divided by the maximum MBR (see Fig. 1).
Analysis
Many of the LSFG parameters change with normal aging.16 Therefore, age-corrected parameters were created, adjusting each of the listed LSFG parameters to their equivalent value for a patient of age 60 years, based on linear regression against age within the Wien control eyes, under the null hypothesis that age-corrected LSFG parameters should not differ between cohorts and hence the same age correction is appropriate for all cohorts.
For the P3 cohort, each age-corrected LSFG parameter was plotted against mean deviation (MD) from SAP and compared against normative limits based on the control eyes in the Wien and Portland control cohorts combined. The values of these age-corrected parameters were formally compared between three groups of eyes: the combined set of control eyes from both cohorts; P3 eyes without existing functional loss on SAP on the day of testing; and P3 eyes with existing functional loss. These comparisons were performed using generalized estimating equation (GEE) models23 to account for intereye correlations within the P3 cohort. A subanalysis was also performed, comparing the two eyes of subjects with unilateral functional loss, that is, one eye with functional loss using the same definition as before compared with a fellow eye without functional loss.
Results
Demographic information for the P3 cohort, split into without versus with functional loss, is shown in Table 1. The average age in the combined control eyes was 47.7 years (standard deviation (SD) 16.6; mean 48.4 years for the Wien control eyes and 44.3 years for the Portland control eyes), which was significantly younger than the average for the P3 eyes (P < 0.0001), underscoring the need to age-correct all LSFG parameters prior to further analysis. The mean systemic blood pressure in the Wien control eyes was 128/81, and their mean IOP was 12.8 mm Hg.16
Table 1.
Demographic Information for Eyes in the P3 Cohort, Split According to the Presence of Significant Functional Loss (According to the Glaucoma Hemifield Test) on the Day of Testing
| Parameter |
Mean (SD) |
|
| P3 Eyes Without Functional Loss, n = 74 |
P3 Eyes With Functional Loss, n = 93 |
|
| Age, y | 69.1 (8.7) | 72.3 (8.4) |
| IOP, mm Hg | 17.6 (3.5) | 15.3 (4.3) |
| Systolic blood pressure, mm Hg | 127 (18) | 126 (17) |
| Diastolic blood pressure, mm Hg | 76 (11) | 74 (10) |
| MD from automated perimetry, dB | +0.72 (1.11) | −4.18 (5.00) |
| RNFLT, μm | 93.6 (9.2) | 74.5 (17.7) |
Means and standard deviations are reported per eye, not per subject. SD, standard deviation.
Without age correction, the average value of MBRave in the control eyes was 13.3 units (SD 3.0). The average value in the P3 eyes without functional loss was 15.9 units (SD 3.7), which was significantly higher than the control eyes, with P < 0.0001 (GEE regression). The average value among P3 eyes with functional loss was 13.1 units (SD 3.5), not significantly different from the control eyes (P = 0.8246).
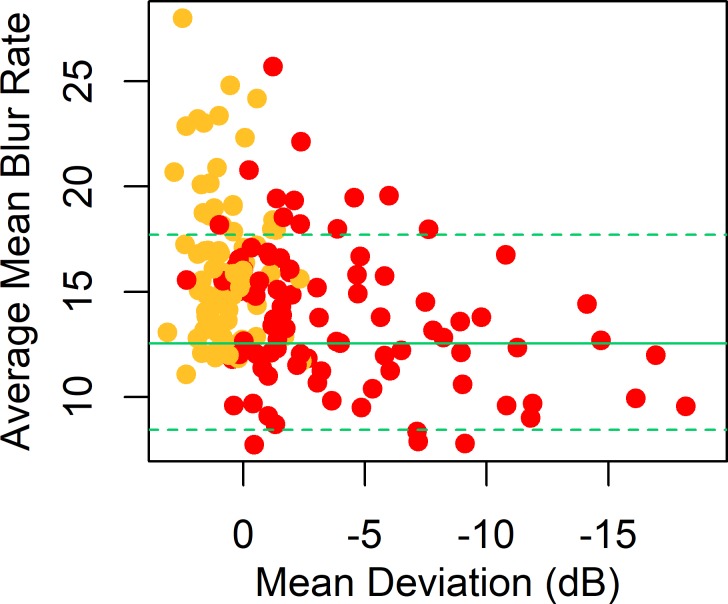
In the Wien control eyes, MBRave decreased by 0.058 units per year.16 After age-correcting the data to the equivalent value for age 60 years, the average MBRave among the control eyes (the combined Wien and Portland cohorts) was 12.5 units (SD 2.8), representing mean capillary blood flow within the ONH tissue. Figure 2 shows a plot of the age-corrected MBRave against MD from perimetry in the P3 cohort, together with the 5th and 95th percentiles among the control eyes. The average value of MBRave, among P3 eyes without functional loss was 16.4 units (SD 3.6). This was significantly higher than the control eyes, with P < 0.0001 (GEE regression).
Figure 2.
MBRave in arbitrary units, plotted against MD from SAP, for eyes in the P3 cohort. MBRave is corrected to the equivalent value at age 60 years. Plotting symbols are red for eyes with existing functional loss according to the Glaucoma Hemifield Test and yellow for eyes without existing functional loss. Green horizontal lines show the mean (solid line) and 5th and 95th percentiles (dashed lines) among the control eyes.
Among P3 eyes with functional loss, the mean value of MBRave was 13.8 units (SD 3.4), which was still significantly higher than the control eyes, with P = 0.0096, but significantly lower than the P3 eyes without functional loss, with P < 0.0001. In this group, MBRave decreased significantly with MD; correlation coefficient 0.321, P < 0.0001 (GEE linear regression).
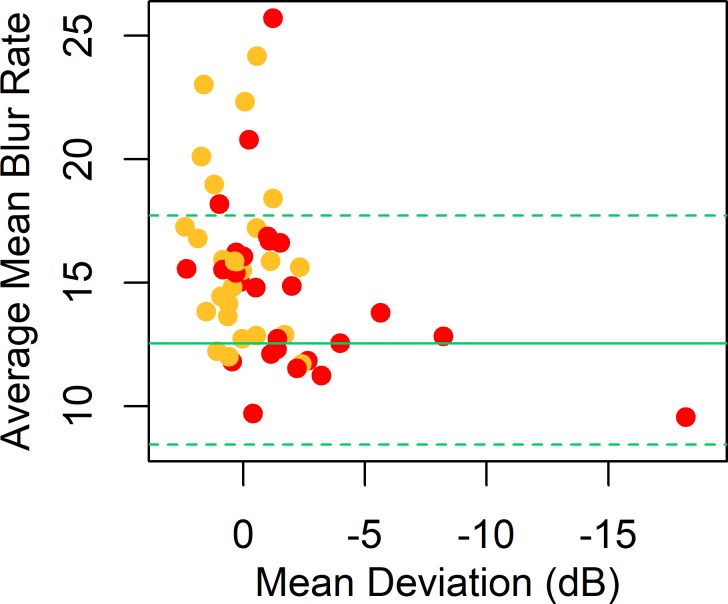
When the analysis was restricted to the 52 eyes of 26 subjects with unilateral functional loss, results were similar, as seen in Figure 3. The average value of MBRave among the eyes without functional loss (average MD +0.21 decibels [dB], SD 1.26 dB) was 16.1 units (SD 3.4), still significantly higher than the control eyes, with P < 0.0001. The average among eyes with functional loss (average MD −1.11dB, SD 3.96 dB) was 14.6 units (SD 3.5), significantly higher than the control eyes, with P = 0.0038, but lower than the eyes without functional loss, with P = 0.0003.
Figure 3.
MBRave in arbitrary units, plotted against MD from SAP, for eyes in the P3 cohort with unilateral visual field loss. MBRave is corrected to the equivalent value at age 60 years. Plotting symbols are red for eyes with existing functional loss according to the Glaucoma Hemifield Test and yellow for fellow eyes without existing functional loss. Green horizontal lines show the mean (solid line) and 5th and 95th percentiles (dashed lines) among the control eyes.
The Portland control eyes actually had slightly higher age-adjusted MBRave than the Wien control eyes (14.6 vs. 12.1, P = 0.0114). However, the P3 eyes without functional loss still had significantly higher MBRave than the Portland controls, with P = 0.0479, despite the greatly reduced sample size (15 control eyes instead of 92 when the control groups were combined).
Thirty-five of the 74 P3 eyes without functional loss were receiving at least one topical antiglaucoma medication according to self-report on the day of testing. Treated eyes had slightly lower MBRave than untreated eyes, 15.6 vs. 17.1, but this difference was not statistically significant (P = 0.1050). Similarly, among P3 eyes with functional loss, the 60 treated eyes had slightly lower MBRave than the 33 untreated eyes, 13.7 vs. 14.2, but again this difference was not statistically significant (P = 0.4593).
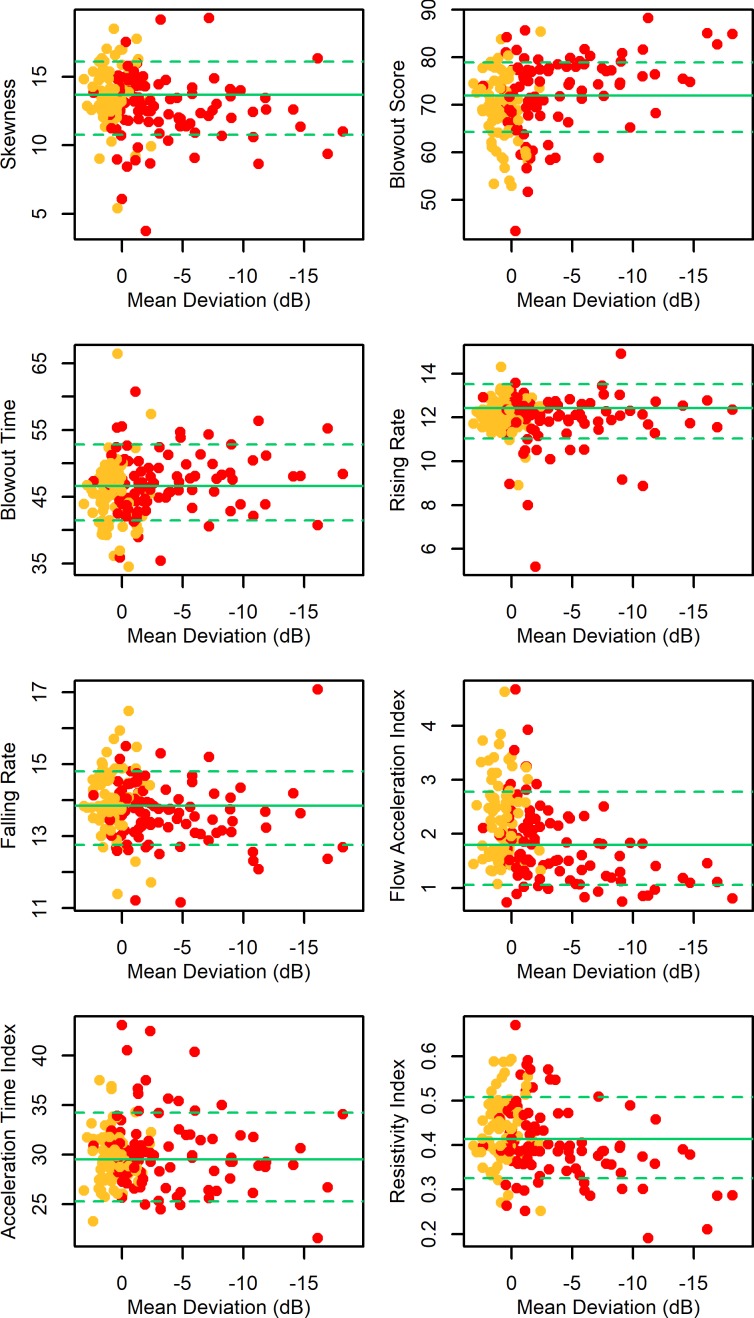
In order to further explore ONH capillary blood flow alterations beyond the apparent increase in basal flow rate of early-stage glaucoma and suspect/fellow eyes, we examined the parameterizations of the pulsatile waveform derived by the LSFG analysis software. Each parameter was age-corrected as before and then compared between groups. The mean within each group and comparisons against the control eyes are shown in Table 2, and each parameter is plotted against MD in Figure 4. In addition to increased MBRave, the P3 eyes without functional loss showed decreased BOS, indicating that a greater proportion of the blood is expelled from the capillaries with each cycle; increased FAI, indicating that the MBR accelerated more rapidly toward its peak; and decreased RR, indicating that the pulsatile waveform began to decelerate later, that is, closer in time to its peak.
Table 2.
Comparison of LSFG Parameters Between Control Eyes (Combined Portland and Wien Cohorts) and P3 Eyes Without and With Existing Functional Loss According to the Glaucoma Hemifield Test
| Age-Corrected Parameter |
Mean (SD) in Control Eyes |
Mean (SD) in P3 Eyes Without Functional Loss |
Comparison P3 Eyes Without Functional Loss vs. Control Eyes |
Mean (SD) in P3 Eyes With Functional Loss |
Comparison P3 Eyes With Functional Loss vs. Control Eyes |
| MBRave | 12.5 (2.8) | 16.4 (3.6) | P < 0.0001 | 13.8 (3.4) | P = 0.0096 |
| Skew | 13.7 (1.7) | 13.9 (2.1) | P = 0.6393 | 12.8 (2.4) | P = 0.0033 |
| BOS | 71.9 (4.8) | 69.6 (7.1) | P = 0.0385 | 72.5 (7.8) | P = 0.6019 |
| BOT | 46.6 (3.5) | 45.6 (4.7) | P = 0.1911 | 47.1 (4.3) | P = 0.3746 |
| RR | 12.45 (0.75) | 12.16 (0.74) | P = 0.0223 | 11.90 (1.22) | P = 0.0006 |
| FR | 13.84 (0.68) | 13.99 (0.88) | P = 0.2879 | 13.65 (0.87) | P = 0.0940 |
| FAI | 1.80 (0.57) | 2.30 (0.74) | P < 0.0001 | 1.78 (0.73) | P = 0.8323 |
| ATI | 29.5 (2.8) | 29.7 (2.7) | P = 0.5356 | 30.3 (3.8) | P = 0.1409 |
| RI | 0.415 (0.056) | 0.438 (0.076) | P = 0.0580 | 0.403 (0.086) | P = 0.3362 |
values are from GEE models (accounting for the presence of both eyes in the P3 cohort). Bold P values indicate statistical significance.
Figure 4.
Parameterizations of the averaged pulsatile waveform (as defined in the Methods section) plotted against MD from SAP for eyes in the P3 cohort. All parameters are corrected to the equivalent value at age 60 years. Plotting symbols are red for eyes with existing functional loss according to the Glaucoma Hemifield Test and yellow for eyes without existing functional loss. Green horizontal lines show the mean (solid line) and 5th and 95th percentiles (dashed lines) among the control eyes.
For each comparison that was found to be statistically significant (P < 0.05) in Table 2, further models were formed to determine whether the differences in that parameter could be related to IOP or to mean arterial pressure (MAP). GEE models were used to predict the age-corrected values of each LSFG parameter based on both MD and either IOP or MAP, within the appropriate cohort. Neither IOP nor MAP were found to be a significant predictor in any of those models. In particular, the relation between MBRave and MD was not affected by IOP in either the P3 eyes without functional loss (P = 0.4750) nor in the P3 eyes with functional loss (P = 0.2458). It should be noted, however, that P3 eyes with elevated IOP were likely to be treated, reducing the range of values and limiting the power to detect any effects.
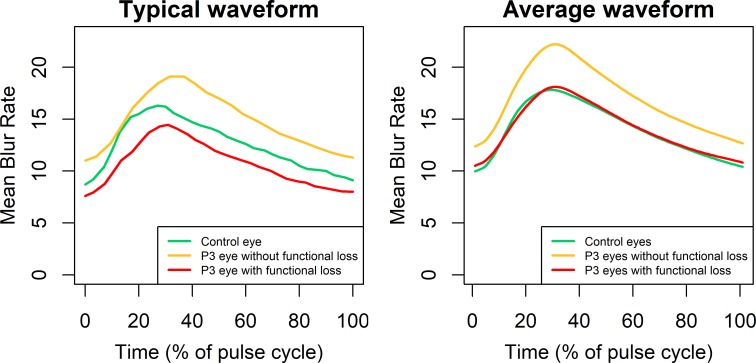
Figure 5 shows, on the left, example pulsatile waveforms for the different study groups. In each case, in order to provide a representative example, the eye was selected to have all of MBRave, RR, and FAI as close as possible to (and all within 1 SD ) the group mean. Age-corrected values for MBRave were 12.4 for the control eye (green, age 62 years); 15.1 for the P3 eye without functional loss (yellow, age 65 years); and 11.8 for the P3 eye with functional loss (red, age 80 years). The values of RR for the three respective eyes were 12.38, 12.23, and 12.45, respectively, and for FAI the values were 1.89, 2.06, and 1.42, respectively. It can be seen that MBRave is consistently higher for the P3 eye without functional loss across the waveform and also that the waveform does not decelerate as quickly as the control eye when approaching its peak, as indicated by a reduced value of RR. In this particular example, the time until the waveform reached its peak was later for the P3 eye without functional loss, giving an increased value of the parameter ATI, but this was not typical of the group. This parameter did not differ significantly between the three groups, as seen in Table 2. The plot on the right of Figure 5 shows averaged waveforms for each group, defined as the mean MBR among eyes in that group at a given percentage of the way through that eye's pulse cycle.
Figure 5.
Pulsatile waveforms for MBR for three representative eyes (left) and averaged across all eyes in each group (right). To ensure that the examples in the left-hand plot are representative, the eyes were chosen to have parameters MBRave, RR, and FAI (as defined in the Methods section) within 1 SD of the mean for that group.
Discussion
Blood flow within the capillaries of the ONH tissue was significantly higher in glaucoma suspect/fellow eyes than in healthy control eyes. In eyes with glaucomatous functional loss, a worse MD was correlated with lower blood flow. This nonmonotonic change in capillary blood flow in cross-sectional human data is consistent with longitudinal changes observed in a non-human primate model of glaucoma, whereby ONH blood flow significantly increased in the earliest stages of damage (<10% loss of RNFLT) but then decreased thereafter.9 The fact that blood flow decreases with damage once beyond the initial stage may explain why an initial increase has not been found by studies focusing on eyes that have already developed incontrovertible glaucomatous damage.2,3,24–27
From the pulsatile waveform analysis, it is seen that during the increase in MBR over the first part of the cardiac cycle, this increase slows markedly in normal eyes as the MBR gradually approaches its maximum, as exemplified by the green line in Figure 5. This is reflected in a high value of the RR, meaning that in the top panel of Figure 1, the area S1 constitutes a large proportion (average 49.8% in the control eyes) of the theoretical maximum given by S1 + S2. However, in the glaucoma suspect/fellow eyes in the P3 cohort without functional loss, the MBR continues to increase rapidly as it approaches its maximum, reflected in significantly lower values of the parameter RR. This trend continues in the eyes with functional loss, which have still lower values of RR.
It is known that autoregulation, occurring primarily at the level of the arteries, is disrupted in glaucoma.1,28 It has been suggested that this could mean that blood flow drops too low when ocular perfusion pressure is reduced by elevated IOP or decreased blood pressure, resulting in the reduced ONH blood flow that has been detected in clinical studies.2,3 However, glial cells, which are reportedly activated in glaucoma,29,30 can contribute to both constriction and dilation of blood vessels.31 It is possible, therefore, that dysfunctional autoregulation could cause an increase in basal ONH blood flow in the ONH in early glaucoma.9 Alternatively, autoregulation that has not yet become dysfunctional may be acting to increase blood flow in response to increased metabolic demand as the ONH and surrounding tissues remodel in response to elevated IOP.32,33
Glaucoma is well known to be influenced by increased IOP, which exerts mechanical load on the ONH tissues. Intriguingly, external mechanical compression of the heart has been reported to cause an increase in expression of nitric oxide, with resultant vasodilation and hence increase in coronary blood flow.34 Suppression of nitric oxide synthesis prevented this vasodilatory effect. An increase in unidirectional shear stress also increased nitric oxide expression in ex vivo Schlemm's canal endothelial cells.35 It is plausible that mechanical compression of the ONH at the earliest stages of glaucoma may be causing a similar increase in nitric oxide expression in activated glial cells,36 resulting in vasodilation in the ONH and hence causing the initial increase in basal blood flow. An elevated presence of nitric oxide has been reported in the ONH of glaucomatous eyes.37 Nitric oxide is being investigated for its potential ability to increase outflow through the trabecular meshwork in glaucomatous eyes,38 and it is therefore important to learn whether this would influence blood flow in the ONH.
MBRave is related to the velocity of blood flow, not just to its volume. An explanation, therefore, for changes in the pulsatile waveform could be a reduction in the ability of vessels to expand in response to moments of high flow during the pulsatile cycle. It has been shown that coronary arterioles vasodilate with increasing pulse pressure,39 reducing resistance and hence allowing increased flow. It seems reasonable to assume that similar pulsatile changes in vessel diameter occur in the ONH, even if those changes are too small to be easily visible. A reduction in vessel elasticity, and hence in pulsatile accommodation, would mean that the blood would have to pass through these vessels at higher velocity. Several mechanisms for such a reduction seem plausible. There is evidence that ONH tissues stiffen with aging40 and in glaucoma41 and that retinal arteries are also stiffer in late glaucoma than in healthy eyes.42 This stiffening may therefore prove to be the link connecting the mechanical and vascular theories of glaucoma.1 Alternatively, it could be caused by inflammatory mediators associated with the structural remodeling of the lamina cribrosa and prelaminar tissues in the ONH that is known to be induced by elevated IOP.32,33 There is also evidence of regulation at the level of capillaries mediated by pericytes, contractile cells that wrap around the capillary walls,43–45 and results from animal models suggest that pericytes may be compromised46,47 and/or lost48 in glaucoma. Such an increase in maximal blood flow velocity would result in the changes in the pulsatile waveform observed in this study, including an increase in FAI and a decrease in RR. This study does not allow us to spatially localize the observed changes in the pulsatile waveform. They could originate in the capillary bed, but they could also result from upstream changes in the retinal arteries. It is also possible that the changes in the pulsatile waveform are systemic rather than restricted to the ONH vessels,49 implying that the hemodynamics of an individual would influence susceptibility to glaucoma. Further studies are needed to resolve this issue.
Intuitively, it seems reasonable to hypothesize the presence of causative links between the changes in pulsatile waveform and the changes in MBRave. However, these remain speculative. An increase in blood flow caused by autoregulation could result in the retinal arterioles approaching their maximum flow capacity, which could manifest as alterations of pulsatile flow dynamics. Conversely, an increase in the maximal velocity across the cardiac cycle would be consistent with results showing that the proportion of oxygen extracted from the blood is lower in glaucomatous eyes.50 It can then be hypothesized that the metabolic demand, combined with reduced oxygen supply and presumably reduced carbon dioxide extraction, would cause the autoregulatory mechanisms to increase the overall blood flow into the ONH. The changes in ONH blood flow in glaucoma could even create a vicious cycle involving a combination of both altered autoregulation and altered capillary regulation.
In 48 eyes with open-angle glaucoma, Takeshima et al.13 found that BOS increased and RI decreased following trabeculectomy, with no accompanying change in average MBR. Similarly, in our study, as seen in Table 2, BOS was significantly higher in normal control eyes than in the P3 eyes without functional loss, with P = 0.039, while RI was lower in the normal control eyes, although with P = 0.058. This may suggest a mechanism for the protective effect of trabeculectomy on the ONH and that the early changes in hemodynamics could be reversible. However, this could also be evidence that those parameters are influenced directly by IOP, which was higher in our P3 eyes than in the control eyes (albeit not measured at exactly the same time as imaging was conducted). This potential caveat with our results could only be resolved by performing LSFG scans at different IOPs in the same eye. It should also be noted that the higher IOP in our P3 eyes without functional loss would seem unlikely to cause the observed increase in capillary blood flow in those eyes, since a 10- to 15-mm Hg elevation in IOP has been shown to have no significant effect on blood flow in a non-human primate model of glaucoma51 nor on perfused ONH vessel density in human subjects measured by OCTA.52
In 61 eyes with normal tension glaucoma, Shiga et al.15 found lower skew and higher ATI compared with 21 control eyes. Similarly, we found lower skew in the eyes with functional loss than in the control eyes (P = 0.003; see Table 2). However, we found no evidence of any change in skew at the earlier stage of the disease. Notably, even the mild glaucoma eyes in their study had an average MD of −3.7 dB, making them more similar to the P3 eyes with functional loss in our study. Shiga et al. have also reported reduced MBRave in a cohort of eyes with preperimetric glaucoma compared with age-matched normal eyes,53 but again, that cohort had a worse average MD (−0.4 dB) than the P3 eyes without functional loss in the present study (+0.7 dB). They were required to have a documented significant RNFL defect, suggesting that most of the eyes with preperimetric glaucoma in the Shiga et al. study may have had a slightly more advanced stage of early glaucoma than did our glaucoma suspect/fellow eye group. It is also important to note that the glaucoma eyes in those studies had normal tension glaucoma, as is more prevalent in a Japanese population; it is possible that there may be important differences in blood flow and hemodynamics between normal tension glaucoma and high tension glaucoma.54
The control eyes in this study were sourced from two separate populations. The two groups had significantly different MBRave, suggesting possible subtle unwritten differences in population and/or protocol. In particular, the Wien control eyes were tested under pupil dilation, while the Portland control eyes were undilated. It should be noted, however, that there was no difference in MBRave in the Wien eyes between dilated versus undilated states.16 However, even when we used only the control eyes tested in Portland, which had higher MBRave than those tested in Vienna, the MBRave was still significantly higher in the P3 eyes without functional loss (P = 0.048) tested using the same instrument, with the higher P value compared with the primary results likely explained by the reduced sample size. A further caveat with the control eyes is that they were significantly younger, on average, than the P3 cohort (P < 0.001). All LSFG parameters were age-corrected, but this correction necessarily assumes that the change with age remains linear even when extrapolating beyond the oldest control eye (79 years) to the oldest eye in the P3 cohort (90 years).
No significant differences in MBRave were found between treated versus untreated eyes in this cohort. Some topical medications have been reported to affect ONH blood flow,55 and it may be that our study was not adequately powered to detect such effects given that both the specific antiglaucoma medication and dosage were variable. Perhaps more importantly, though, the results of this subanalysis mean that the primary finding of the study, of increased MBRave in the P3 eyes without functional loss, is not caused by topical antiglaucoma medications since the difference from normal was actually slightly greater among the untreated P3 eyes.
The main criterion for inclusion in the P3 cohort was glaucoma or suspected glaucoma in at least one eye, as determined by the subject's clinician. It is therefore possible that a subset of the P3 eyes without functional loss will in fact never go on to develop any glaucomatous functional loss, especially if the eye is only included because it is the fellow eye of an individual with unilateral glaucoma. However, the presence of these eyes actually strengthens our conclusions; significant differences were found between the P3 eyes without functional loss and the control eyes despite the fact that there may have been some normal eyes within the glaucoma suspect/fellow eye group.
Another possible reason for the increased ONH blood flow observed in this study could be that if there has been a loss of prelaminar tissue, then the laser beam could be penetrating deeper into the ONH tissue. However, it has been shown that deeper ONH tissues have lower blood flow than do more anterior tissues.56 Therefore, while we cannot discount this possibility, it seems unlikely to be driving our results. A further potential caveat is that caffeine has been shown to increase blood vessel resistance within the ONH, decreasing flow.57 However, the control subjects in the Wien cohort were explicitly instructed to abstain from caffeine for 12 hours prior to testing,16 and thus this factor is unlikely to explain the Wien cohort having lower flow than the P3 cohort.
A more major caveat with our study is its cross-sectional nature. It is therefore impossible for us to know whether the P3 eyes without functional loss had previously experienced an increase in MBRave, as would happen if it were part of the disease process, or if they had in fact always had high MBRave. Either of these possibilities would have both mechanistic and diagnostic consequences, and thus it is important to now distinguish between the two. A longitudinal study to address this issue is underway.
A consequence of the nonmonotonic relation between severity and blood flow is that the parameter MBRave is unlikely to be useful for the diagnostic detection of disease since a value within normal limits would reappear after considerable loss of axons. This may explain why vessel density measured using OCTA has been reported as having better discriminatory power between eyes with glaucoma versus controls than do LSFG parameters.58 Other LSFG parameters may prove more useful for discrimination than MBRave. Changes in LSFG parameters may also prove useful for monitoring disease progression, and this will also be able to be assessed using the longitudinal data currently being collected.
In conclusion, we found evidence in vivo of increased capillary blood flow in the ONH in the earliest stages of human glaucoma. The blood flow appears to subsequently decrease as damage becomes more severe. Longitudinal studies are underway to confirm these changes and to learn more about their mechanistic role in glaucomatous pathophysiology.
Acknowledgments
Supported by NIH R01-EY020922 (SKG), NIH R01-EY019939 (LW), and unrestricted research support from The Legacy Good Samaritan Foundation, Portland, Oregon. The sponsors/funding organizations had no role in the design or conduct of this research.
Disclosure: S.K. Gardiner, None; G. Cull, None; B. Fortune, None; L. Wang, None
References
- 1.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retinal Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 2.Hafez AS, Bizzarro RL, Lesk MR. Evaluation of optic nerve head and peripapillary retinal blood flow in glaucoma patients, ocular hypertensives, and normal subjects. Am J Ophthalmol. 2003;136:1022–1031. doi: 10.1016/s0002-9394(03)00632-9. [DOI] [PubMed] [Google Scholar]
- 3.Piltz-Seymour JR, Grunwald JE, Hariprasad SM, Dupont J. Optic nerve blood flow is diminished in eyes of primary open-angle glaucoma suspects. Am J Ophthalmol. 2001;132:63–69. doi: 10.1016/s0002-9394(01)00871-6. [DOI] [PubMed] [Google Scholar]
- 4.Bossuyt J, Vandekerckhove G, De Backer TL, et al. Vascular dysregulation in normal-tension glaucoma is not affected by structure and function of the microcirculation or macrocirculation at rest: a case-control study. Medicine. 2015;94:e425. doi: 10.1097/MD.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollo G, van den Berg TJ, Greve EL. Scanning laser Doppler flowmetry in glaucoma. Int Ophthalmol. 1996;20:63–70. doi: 10.1007/BF00212948. [DOI] [PubMed] [Google Scholar]
- 6.Samsudin A, Isaacs N, Tai M-LS, Ramli N, Mimiwati Z, Choo MM. Ocular perfusion pressure and ophthalmic artery flow in patients with normal tension glaucoma. BMC Ophthalmol. 2016;16:39. doi: 10.1186/s12886-016-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berisha F, Feke GT, Hirose T, McMeel JW, Pasquale LR. Retinal blood flow and nerve fiber layer measurements in early-stage open-angle glaucoma. Am J Ophthalmol. 2008;146:466–472. doi: 10.1016/j.ajo.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, Hohman RM, Sanchez R, Addicks EM. Optic nerve head blood flow in chronic experimental glaucoma. Arch Ophthalmol. 1985;103:956–962. doi: 10.1001/archopht.1985.01050070082035. [DOI] [PubMed] [Google Scholar]
- 9.Cull G, Burgoyne CF, Fortune B, Wang L. Longitudinal hemodynamic changes within the optic nerve head in experimental glaucoma. Invest Ophthalmol Vis Sci. 2013;54:4271–4277. doi: 10.1167/iovs.13-12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama T. Basic technology and clinical applications of the updated model of laser speckle flowgraphy to ocular diseases. Photonics. 2014;1:220. [Google Scholar]
- 11.Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci. 2012;53:8303–8309. doi: 10.1167/iovs.12-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Cull G, Burgoyne CF, Thompson S, Fortune B. Longitudinal alterations in the dynamic autoregulation of optic nerve head blood flow revealed in experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3509–3516. doi: 10.1167/iovs.14-14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshima S, Higashide T, Kimura M, et al. Effects of trabeculectomy on waveform changes of laser speckle flowgraphy in open angle glaucoma. Invest Ophthalmol Vis Sci. 2019;60:677–684. doi: 10.1167/iovs.18-25694. [DOI] [PubMed] [Google Scholar]
- 14.Bhatti MS, Tang TB, Laude A. Effects of water drinking test on ocular blood flow waveform parameters: a laser speckle flowgraphy study. PLoS One. 2017;12:e0181512. doi: 10.1371/journal.pone.0181512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiga Y, Omodaka K, Kunikata H, et al. Waveform analysis of ocular blood flow and the early detection of normal tension glaucoma. Invest Ophthalmol Vis Sci. 2013;54:7699–7706. doi: 10.1167/iovs.13-12930. [DOI] [PubMed] [Google Scholar]
- 16.Luft N, Wozniak PA, Aschinger GC, et al. Ocular blood flow measurements in healthy white subjects using laser speckle flowgraphy. PLoS One. 2016;11:e0168190. doi: 10.1371/journal.pone.0168190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardiner SK, Johnson CA, Demirel S. Factors predicting the rate of functional progression in early and suspected glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3598–3604. doi: 10.1167/iovs.11-9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner SK, Fortune B, Demirel S. Localized changes in retinal nerve fiber layer thickness as a predictor of localized functional change in glaucoma. Am J Ophthalmol. 2016;170:75–82. doi: 10.1016/j.ajo.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardiner SK, Mansberger SL, Demirel S. Detection of functional change using cluster trend analysis in glaucoma. Invest Ophthalmol Vis Sci. 2017;58:BIO180–BIO190. doi: 10.1167/iovs.17-21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohnell H, Heijl A, Brenner L, Anderson H, Bengtsson B. Structural and functional progression in the early manifest glaucoma trial. Ophthalmology. 2016;123:1173–1180. doi: 10.1016/j.ophtha.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asman P, Heijl A. Glaucoma Hemifield test. Automated visual field evaluation. Arch Ophthalmol. 1992;110:812–819. doi: 10.1001/archopht.1992.01080180084033. [DOI] [PubMed] [Google Scholar]
- 22.Konishi N, Tokimoto Y, Kohra K, Fujii H. New laser speckle flowgraphy system using CCD camera. Opt Rev. 2002;9:163–169. [Google Scholar]
- 23.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.Michelson G, Langhans MJ, Groh MJ. Perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open angle glaucoma. J Glaucoma. 1996;5:91–98. [PubMed] [Google Scholar]
- 25.Gottanka J, Kuhlmann A, Scholz M, Johnson DH, Lutjen-Drecoll E. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2005;46:4170–4181. doi: 10.1167/iovs.05-0289. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011;52:840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson DR. Introductory comments on blood flow autoregulation in the optic nerve head and vascular risk factors in glaucoma. Surv Ophthalmol. 1999;43(suppl 1):S5–S9. doi: 10.1016/s0039-6257(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 29.Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6:303–313. [PubMed] [Google Scholar]
- 30.Wang JW, Chen SD, Zhang XL, Jonas JB. Retinal microglia in glaucoma. J Glaucoma. 2016;25:459–465. doi: 10.1097/IJG.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 31.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts MD, Grau V, Grimm J, et al. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Reynaud J, Lockwood H, et al. The connective tissue phenotype of glaucomatous cupping in the monkey eye—clinical and research implications. Prog Retin Eye Res. 2017;59:1–52. doi: 10.1016/j.preteyeres.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D, Huang A, Kaley G. Mechanical compression elicits NO-dependent increases in coronary flow. Am J Physiol Heart Circ Physiol. 2004;287:H2454–H2460. doi: 10.1152/ajpheart.00364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashpole NE, Overby DR, Ethier CR, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm's canal cells. Invest Ophthalmol Vis Sci. 2014;55:8067–8076. doi: 10.1167/iovs.14-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Neufeld AH. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 2000;30:178–186. doi: 10.1002/(sici)1098-1136(200004)30:2<178::aid-glia7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- 38.Aliancy J, Stamer WD, Wirostko B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 2017;6:221–232. doi: 10.1007/s40123-017-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto M, VanBavel E, Giezeman MJ, Spaan JA. Vasodilatory effect of pulsatile pressure on coronary resistance vessels. Circ Res. 1996;79:1039–1045. doi: 10.1161/01.res.79.5.1039. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, McNally S, Kilpatrick JI, Jarvis SP, O'Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthal. 2018;63:56–74. doi: 10.1016/j.survophthal.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Quigley H, Arora K, Idrees S, et al. Biomechanical responses of lamina cribrosa to intraocular pressure change assessed by optical coherence tomography in glaucoma eyes. Invest Ophthalmol Vis Sci. 2017;58:2566–2577. doi: 10.1167/iovs.16-21321. [DOI] [PubMed] [Google Scholar]
- 42.Oettli A, Gugleta K, Kochkorov A, Katamay R, Flammer J, Orgul S. Rigidity of retinal vessel in untreated eyes of normal tension primary open-angle glaucoma patients. J Glaucoma. 2011;20:303–306. doi: 10.1097/IJG.0b013e3181e666a1. [DOI] [PubMed] [Google Scholar]
- 43.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Trost A, Lange S, Schroedl F, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci. 2016;10:20. doi: 10.3389/fncel.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson DR. Glaucoma, capillaries and pericytes. 1. Blood flow regulation. Ophthalmologica. 1996;210:257–262. doi: 10.1159/000310722. [DOI] [PubMed] [Google Scholar]
- 46.Brooks DE, Samuelson DA, Gelatt KN. Ultrastructural changes in laminar optic nerve capillaries of beagles with primary open-angle glaucoma. Am J Vet Res. 1989;50:929–935. [PubMed] [Google Scholar]
- 47.Alarcon-Martinez L, Vargas JLC, Belforte N, Villafranca-Baughman D, Polo AD. AB011. Live imaging of retinal pericytes: evidence for early calcium uptake, capillary constriction and vascular dysregulation in ocular hypertension glaucoma. Ann Eye Sci. 2018. 3.
- 48.Trost A, Motloch K, Bruckner D, et al. Time-dependent retinal ganglion cell loss, microglial activation and blood-retina-barrier tightness in an acute model of ocular hypertension. Exp Eye Res. 2015;136:59–71. doi: 10.1016/j.exer.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27:94–101. doi: 10.1097/ICU.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasan SM, Hammer M, Meller D. Correlation of the retinal parapapillary perfusion and the retinal vessel oxygen saturation in glaucoma patients. Invest Ophthalmol Vis Sci. 2019;60:1309–1315. doi: 10.1167/iovs.18-26099. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Burgoyne CF, Cull G, Thompson S, Fortune B. Static blood flow autoregulation in the optic nerve head in normal and experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55:873–880. doi: 10.1167/iovs.13-13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Jonas JB, Wang Q, et al. Optical coherence tomography angiography vessel density changes after acute intraocular pressure elevation. Sci Rep. 2018;8:6024. doi: 10.1038/s41598-018-24520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiga Y, Kunikata H, Aizawa N, et al. Optic nerve head blood flow, as measured by laser speckle flowgraphy, is significantly reduced in preperimetric glaucoma. Curr Eye Res. 2016;41:1447–1453. doi: 10.3109/02713683.2015.1127974. [DOI] [PubMed] [Google Scholar]
- 54.Fan N, Wang P, Tang L, Liu X. Ocular blood flow and normal tension glaucoma. Biomed Res Int. 2015;2015:308505–308505. doi: 10.1155/2015/308505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiyama T, Shibata M, Kojima S, Ikeda T. Optic nerve head blood flow in glaucoma. In: Kubena T, editor. The Mystery of Glaucoma. New York, NY: IntechOpen;; 2011. pp. 207–218. [Google Scholar]
- 56.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 57.Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T. Effects of caffeine on microcirculation of the human ocular fundus. Jpn J Ophthalmol. 2002;46:170–176. doi: 10.1016/s0021-5155(01)00498-1. [DOI] [PubMed] [Google Scholar]
- 58.Takeyama A, Ishida K, Anraku A, Ishida M, Tomita G. Comparison of optical coherence tomography angiography and laser speckle flowgraphy for the diagnosis of normal-tension glaucoma. J Ophthalmol. 2018;2018:9. doi: 10.1155/2018/1751857. [DOI] [PMC free article] [PubMed] [Google Scholar]