Abstract
Recent evidence shows how patients’ unique genetic makeup can affect disease outcomes and the increasing availability of targeted treatments promises a future in health care, whereby treatments will be tailored to individual needs. This article reports on the topics discussed at the 13th Annual Texas Conference on Health Disparities, organized by the Texas Center for Health Disparities at the University of North Texas Health Science Center; the meeting focused on the theme, “Diversity in the Era of Precision Medicine” and was held during June 2018 in Fort Worth, Texas. The primary focus of this conference, which brought together clinical and basic scientists, was on the inclusion of diversity in precision medicine to bridge the gap in health disparities. Here, we present the highlights of the conference that include the potential application of precision medicine at the population level, the effects of precision medicine and direct-to-consumer testing on health disparities, genetic basis of health disparities, pharmacogenomics, and strategies to enhance participation of under-represented populations in precision medicine. Furthermore, we conclude with recommendations for future implementation, including how to mitigate disparities in genomics services and enhance participation of diverse groups in clinical trials.
Keywords: Precision Medicine, Health Disparities, Genetic Testing, Genomics, Cancer
Introduction
Precision medicine has been likened to a kind of disruptive innovation, a treatment approach that stands to challenge the conventional “average patient” norm to provide a more individualized approach to treatment, taking into consideration the genetic makeup, environment and lifestyle of individual patients.1 The rapid development of cost-effective, next-generation genome sequencing and the myriad of “omics” fields that have emerged since the culmination of the Human Genome Project are providing the technology and data required to discover novel therapeutic targets and potential clinical implementation of precision medicine. This has also led to the understanding of biological mechanisms that contribute to differing disease outcomes in certain ethnic groups, a genomic health disparity. This terminology is distinct and independent from other determinants of social disadvantage such as socio-economic status, rural, disabled, racial, ethnic and sexual minorities who are burdened with poorer disease outcomes due to unequal access to health care initiatives.2
The existing gap in health outcomes should not widen into greater disparity, especially in the age of precision medicine. This motivates an urgent call to action for an inclusive approach to genomic, clinical and medical research, enabling participation from all racial, ethnic and economic strata for the benefits of precision medicine to be fully realized.
The overarching theme, “Diversity in the Era of Precision Medicine,” set the tone for the 13th Annual Texas Conference on Health Disparities, organized by the Texas Center for Health Disparities at the University of North Texas Health Science Center, a designated Specialized Center of Excellence in Minority Health and Health Disparities by the National Institutes of Health. This article highlights topics discussed at the conference.
Enhancing Participation of Diverse and Under-represented Populations in Precision Medicine
Participation of individuals of non-European ancestry is under-represented in the genome-wide association studies (GWAS).3 The clinical consequence of the dominant presence of one ancestry could be ineffective for minorities and may lead to misinterpretation and propagate undiagnosed causes.4 Translation of research findings at the population-level requires consideration of diversity at all levels of study design. However, increasing the participation of the under-represented population in genomic studies is challenging and requires a long-term effort. Diversity should be kept in the forefront in designing and implementing the study from the beginning to the end. It is important to build a trusting relationship at the community level to enhance participation of minority groups. Particularly, the institution engaged in recruitment needs to be trustworthy to the specific community. Differences in health behaviors exist among populations; therefore, a single approach may not work in each community. The community outreach team needs to organize focus group discussions to learn about barriers and concerns of the stakeholders in a genomic study so that the recruitment process is tailored accordingly. Besides focus group discussions, further engagement with the community is warranted. Creating a community advisory board with community members might be helpful. A number of ethnicity-specific barriers for minority research participation has been reported.5 For African Americans, mistrust is one of the major reasons for lack of participation. The African American population often perceived that the research would benefit Whites or the research institute instead of them.6 Inconvenience, hidden cost of participation, concern about the misuse of research data, lack of understanding the consent form and research materials, language barrier, low perceived risk of disease, and fear of discrimination are some of the shared barriers African Americans, Native Americans, Latinos, Asian Americans, and Pacific Islanders have for participating in research efforts.5 The research enrollment approaches need to incorporate strategies to address or eliminate these barriers.
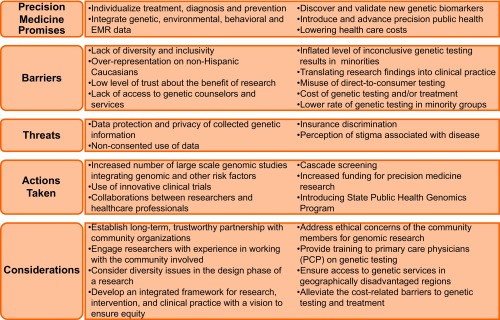
Several actions were taken recently to bring the promises of precision medicine into reality. However, it is crucial to acknowledge the hindrance to the equitable access of precision medicine discoveries for every group and sub-groups of a population. Therefore, we recommended certain changes in precision medicine research approaches, so that any discoveries in precision medicine become scalable at the population level (Figure 1).
Figure 1. Precision medicine: Promises, challenges, and recommendations.
Barriers Contributing to Disparity in Access to Genetic Testing Services
Patient-level barriers contributing to under-utilization of genetic testing services among underserved populations include lower exposure and knowledge about available tests, geographic and language barriers, location (rural vs urban), socioeconomic factors such as education, insurance and income and perceptions of discrimination and stigma. Women with private insurance were more likely to receive genetic testing as compared with women on Medicaid.7
Provider-level barriers include clinician’s lack of time, knowledge, skills, interpretation and screening tools to determine risk along with racial disparities in physician referral and access.8 Additionally, health care system level barriers include: testing services lacking at many hospitals; lack of access to and discussions with genetic counsellors; lack of cancer genetic experts and health care providers; and lack of physician referral. Furthermore, the absence of integrated systems to facilitate identification and referral of patients with high risk and loss of referred patients to follow-up also lead to incomplete care being provided to these patients.7,9
Will Precision Medicine and Direct-to-Consumer Testing Increase Health Disparities?
The boom in genome sequencing has led to many companies offering information on risk prediction for inherited conditions and response to certain drugs directly to individuals, without the intermediary of a health care professional. While this pre-emptive direct-to-consumer (DTC) testing gives consumers access to their genetic data, thus aiding them in taking precautionary action, regulatory authorities such as the CDC and USFDA have taken up a cautionary stance against this. The concerns are many and mostly stem from the genomic disparity or lower access to genetic testing services by all racial and ethnic groups.
One of the main concerns of DTC testing is that individuals may misinterpret the extent of their predisposition to a certain disease, as this information does not come with genetic counselling. Data from 2016 suggest that 81% of participants in GWAS came from European ancestry, an improvement from 2009 data where 96% of participants in GWAS were European.3 However, African, Hispanic and Latin American, and Pacific Islanders are still under-represented in GWAS. Inclusion of underrepresented groups is imperative as certain genetic variants identified in European populations might be false-positives in minority populations.3 About 40% of the variants in certain genes reported by DTC tests are false positives, illustrating the importance of confirming these findings in a clinical laboratory.10 Another key issue to consider is gene variants of unknown significance (VUS) whose clinical significance is ambiguous. Studies using multiple-gene sequencing have reported higher rates of VUS prevalence in minority populations, such as Asians and Blacks, as compared with non-Hispanic Whites.11 Non-inclusion of racial minorities in genetic studies therefore limits our understanding of the variation in these populations, further widening the racial disparity in VUS.11 Little research is available to demonstrate that results from DTC testing leads to behavioral changes or improved patient outcomes.
The large-scale data collected by DTC companies also brings up the issue of privacy and data protection. By giving DNA samples, individuals are at risk of potentially exposing personally identifiable and sensitive information. While federal laws, such as the Genetic Information Nondiscrimination Act (GINA), are in place to safeguard consumers from potential negative repercussions, individuals remain vulnerable to data sharing by third-party organizations.
Currently, various government-sponsored precision medicine initiatives are underway (eg, the Million Veteran Program and the UK Biobank12,13). These are observational cohort studies, whose aims are to create mega-biobanks, by combining questionnaires with electronic medical record data. These will improve understanding of the genetic and lifestyle determinants of health and disease.12,13 In 2015, the US government passed the 21st Century Cures Act, a legislation designed to modernize the drug development process and empower the NIH by funding various cutting-edge research projects focused on accelerating biomedical innovations. One of those investments is the “All of Us” research program formerly called the “Precision Medicine Initiative.”14 This initiative aims to collect longitudinal clinical, biological, socio-behavioral and environmental data from a million individuals, who reflect the diversity makeup of the United States. Participants will have access to their genetic information; this program assures rigorous standards of data security, privacy and confidentiality.14 While this study provides an extensive database that can be used by scientists to address issues relating to the genetic determinants of health disparities in the United States, global efforts for precision medicine in underrepresented communities are underway.3 Other examples include precision medicine efforts funded by the UK Wellcome Trust and the NIH-funded Human Heredity and Health in Africa (H3Africa) initiative.
Mitigation of Disparities in Genomic Services: A Proposal for an Integrated Framework
The importance of utilizing the evidence-based genomic applications in health care, early screening, and disease prevention is mounting. Though a number of genome projects are showing promise in improving health through translation of genetic services in clinical settings, achieving equity of genomic testing is still questionable.15 Besides the lack of participation of non-European descents in genomic studies, there are numerous barriers for certain population groups in accessing genetic services. Genetic testing and genetic counseling services are mostly located in urban settings and the distribution of medical genetic workforce (physician medical geneticists and genetic counselors, for example) are unevenly distributed in the United States.16 Also, funding to support genetic services from both the private and public sectors are insufficient, leading to economic disparity.17 In addition to mistrust in the health care system, racial and ethnic minorities also lack participation in genomic testing due to limited recommendations from primary care physicians, socioeconomic status, risk perception, and psychological and attitudinal factors towards the genetic services.18 It is critical to note that the benefits of genetic services may not be fully recognized unless the disparities in genomic testing are substantially reduced.
A growing body of evidence suggests that screening for hereditary cancer syndromes can improve population health. However, integration of genomic services into population-level chronic disease prevention program is yet to be attained. Also, the existing framework for genomic services may widen the disparity in access to genomic services. There is an utmost need for an integrated framework for research, intervention, and practice in clinical settings that will mitigate disparities in genomic services. Senier et al advocated organizational change using “sensemaking and sensegiving strategies” to translate novel scientific discoveries into practice in organizations with professionals of the diverse backgrounds.19 The Michigan Genomics Program of the Michigan Department of Health and Human Services (DHHS) brought organizational change using these strategies to adopt recent genomic discoveries into practice.19 To address inequalities in accessing genomic services, integration of “fundamental cause theory” and “implementation science framework” may help to bring changes at three broad levels: institutional level, health system level, and interpersonal level that includes both the patients and providers.20 This will address geographic maldistribution of health care services, inequalities in power to influence health care policy, and inequalities in demographic and socioeconomic factors in accessing genomic services.
Public health genomics (PHG) programs, developed by the state health agencies, play a major role in advancing precision medicine. Therefore, adopting implementation strategies in PHG programs is instrumental to mitigate disparities in genomic services. To identify high-risk individuals who lack access to genomic services, it is imperative to modify PHG programs based on demographic characteristics at a granular level, identify potential local partners with expertise in genetic services, collaborate with local institutions to reach to the medically underserved communities, and access readily available resources such as CDC’s Office of Public Health Genomics map.21
Genetic Basis of Health Disparities
Prostate cancer is often cited as a classic health disparity example because men of African descent have the highest incidence and mortality rates.22 Many factors, including genetic and epigenetic differences among the various ethnic groups, have been implicated. Therefore, it is essential that the underlying biological mechanisms contributing to these racial disparities are unraveled to aid management, especially in African American (AA) populations. For example, Kaiso is a transcriptional repressor, encoded by the ZBTB33 gene in humans. It is abundantly expressed in prostate, breast, colon and pancreatic cancers.23 Among AAs, increased expression has been shown to contribute to increased cancer aggressiveness and poor overall survival.24 Kaiso promotes cancer metastases through direct regulation of epithelial-mesenchymal transition (EMT) genes and several other tumor-suppressor micro RNAs.25 In breast cancer, overexpression of Kaiso in infiltrating ductal carcinomas (IDCs) was associated with loss of E-cadherin and increased cell migration and invasiveness. Depletion of Kaiso resulted in increased cell adhesion and development of small-sized primary tumors in in vivo and in vitro studies.23,24 Thus, Kaiso could be a potential target for prostate cancer treatment in this group. Meanwhile, TMPSSR2: ERG gene fusion is a biomarker frequently used for clinical management of prostate cancer. TMPSSR2: ERG fusions are relatively rare in AA men even though they have the highest incidence rate of the disease. This underscores the need for a robust biomarker for prostate cancer in AA men. As such, Kaiso has been proposed as an actionable biomarker for prostate cancer management in African American men.
Pharmacogenomics
Pharmacogenomics focuses on the identification of variants in the genome, especially polymorphisms in drug targets, drug metabolizing enzymes and drug transporters that affect drug pharmacokinetics or pharmacodynamics.26 The aim of pharmacogenomics is to tailor the choice of a drug, based on a variation in an individual’s genetic composition, which contributes to variability in drug response. These polymorphisms oftentimes are present to different extents in different races and consequently, individuals can be characterized as ultra-rapid metabolizers (UM), extensive metabolizers (EM), intermediate metabolizers (IM) and poor metabolizers (PM). The reader is referred to specific case studies of Warfarin,27 Clopidogrel,28 and Tamoxifen29 for more detail. An example of how racial categories found their way into drug development can be seen in the case of the drug BiDil, an anti-hypertensive exclusively for African Americans, whose approval was based on post-hoc analysis.30 The approval of BiDil was heralded as setting an example for bridging the health disparity gap by race-based therapeutics. However, the FDA also noted the use of race as a proxy, until the biological basis explaining the different drug response could be identified.
Precision Medicine to Precision Public Health: Potentials of Precision Medicine in Disease Prevention at the Population-Level
One of the broader goals of precision medicine is to develop evidence-based treatment and prevention strategies tailored to distinct groups in the population.31 Such targeted prevention schemes are based on common genomic traits, socio-economic characteristics, geographical location, demographics, health behaviors and lifestyle factors. Similarly, traditional public health programs are focused on disease prevention at the population level, tailored to the aforementioned factors, except genomic information. This is where the purposes of precision medicine and public health intersect. Recent advancements in genetic epidemiology have generated useful information, through genetic and genomic testing, on a vast number of individuals that unlocked the route to genetic screening of diseases at the population level. Beside genomics, precision medicine initiatives (such as the NIH-funded research project “All of Us”) also collect data on environmental characteristics, lifestyle factors such as diet, exercise, and other health behavior practices, and electronic medical records.14 Integration of genomic data with environmental and lifestyle data is critical for both prevention and treatment of certain diseases. Thus, precision medicine offers a unique opportunity of incorporating genomic markers, in addition to environmental and lifestyle factors, to identify vulnerable groups at the population level.
A number of studies reported that the susceptibility of diseases largely varies based on one’s ethnic identity, gender, geographic location, health behavior and lifestyle, occupation, genetic traits, and many other factors.31-34 Consequently, one prevention strategy does not fit all. Lack of effectiveness of some intervention strategies for certain sub-groups may also support the need for tailored interventions.35 This is one of the motivations for binding genomic studies to public health. Integration of genomic technologies and public health initiatives already show success in preventing diabetes and cancers for certain groups.33,34 Together, precision medicine and public health may introduce the era of precision public health that will optimize prevention and treatment strategies for specific segments of the population. Precision public health will identify those population strata that would benefit most from an intervention as well as identify strata for which the intervention is not optimal.32 In short, it will deliver the right intervention to the right population groups.
Besides optimum allocation of resources, precision public health would be instrumental to discovering and validating new genetic markers of health and diseases. The million person cohort proposed in the “All of Us” precision medicine initiative unwrapped a unique opportunity to take genomic studies to the next level where genomic, environmental, and lifestyle factors are all taken into account together to determine likelihoods of developing a disease.31 Precision public health would allow hypothesis testing, validation, and long-term monitoring of population health for the phenotype-genetic variant association of diseases. However, one of the major prerequisites of ensuring such benefit is to allow equal participation of all subpopulations in the genomic studies, which is still a challenge.3,4 For example, vaccination programs are yet to be integrated into precision medicine. Such integration would benefit the subpopulations who are susceptible to certain diseases that are preventable by vaccination. Furthermore, precision medicine coupled with genomic studies may assess vaccine efficacy as well as immune responses in tailoring the right vaccine to the right person.
Conclusion
At the end of the conference, participants concluded that inequitable, access to genetic services, minorities distrust of the health care system, physicians’ limited knowledge of genetic data, and belief systems were impediments to precision medicine. A plethora of efforts spanning different sectors of the health care industry could help bridge the gap in health disparities in the context of precision medicine. Efforts to bridge this gap were underscored by conference presenters and included: a) integration of genomic sciences and public health interventions; b) increasing participation of minority groups in genomic research; c) the application of available scientific data for novel therapeutic targets in underrepresented racial groups; d) increased community engagement using trusted leaders; e) physicians’ education; and f) increased access to genetic testing and counseling services. Current efforts such as the “All of Us” research initiative were lauded. However, to attain the maximum benefits of precision medicine, more actions are required to enhance diversity in genomic research.
Acknowledgments
The authors acknowledge the administrative support from Rosalba Zamaguey and Patricia Baker. Research reported in this publication was supported by awards from the National Institutes of Health (U54MD006882; P20MD006882; S21MD012472). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- 1. Larry Jameson J, Longo DL Precision medicine—personalized, problematic, and promising. Obstet Gynecol Surv. 2015;70(10):612- 614. https://doi.org/ 10.1097/01. ogx.0000472121.21647.38 [DOI]
- 2. West KM, Blacksher E, Burke W. Genomics, health disparities, and missed opportunities for the nation’s research agenda. JAMA. 2017;317(18):1831-1832. 10.1001/jama.2017.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161-164. 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hindorff LA, Bonham VL, Brody LC, et al. . Prioritizing diversity in human genomics research. Nat Rev Genet. 2018;19(3):175-185. 10.1038/nrg.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16-e31. 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. BeLue R, Taylor-Richardson KD, Lin J, Rivera AT, Grandison D. African Americans and participation in clinical trials: differences in beliefs and attitudes by gender. Contemp Clin Trials. 2006;27(6):498-505. 10.1016/j.cct.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Cragun D, Weidner A, Lewis C, et al. . Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497-2505. 10.1002/cncr.30621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powell KP, Cogswell WA, Christianson CA, et al. . Primary care physicians’ awareness, experience and opinions of direct-to-consumer genetic testing. J Genet Couns. 2012;21(1):113-126. 10.1007/s10897-011-9390-9 10.1007/s10897-011-9390-9 [DOI] [PubMed] [Google Scholar]
- 9. Mays D, Sharff ME, DeMarco TA, et al. . Outcomes of a systems-level intervention offering breast cancer risk assessments to low-income underserved women. Fam Cancer. 2012;11(3):493-502. 10.1007/s10689-012-9541-7 10.1007/s10689-012-9541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tandy-Connor S, Guiltinan J, Krempely K, et al. . False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet Med. 2018;20(12):1515-1521. 10.1038/gim.2018.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caswell-Jin JL, Gupta T, Hall E, et al. . Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20(2):234-239. 10.1038/gim.2017.96 10.1038/gim.2017.96 [DOI] [PubMed] [Google Scholar]
- 12. Gaziano JM, Concato J, Brophy M, et al. . Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 13. Allen N, Sudlow C, Downey P, et al. . UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1(3):123-126. https://doi.org/ 10.1016/j hlpt.2012.07.003 [DOI]
- 14. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795. 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senier L, Kearney M, Orne J. Using public-private partnerships to mitigate disparities in access to genetic services: lessons from Wisconsin. Adv Med Sociol. 2015;16:269-305. 10.1108/S1057-629020150000016010 10.1108/S1057-629020150000016010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooksey JA, Forte G, Benkendorf J, Blitzer MG. The state of the medical geneticist workforce: findings of the 2003 survey of American Board of Medical Genetics certified geneticists. Genet Med. 2005;7(6):439-443. 10.1097/01.GIM.0000172416.35285.9F [DOI] [PubMed] [Google Scholar]
- 17. Graf MD, Needham DF, Teed N, Brown T. Genetic testing insurance coverage trends: a review of publicly available policies from the largest US payers. Per Med. 2013;10(3):235-243. 10.2217/pme.13.9 [DOI] [PubMed] [Google Scholar]
- 18. Armstrong J, Toscano M, Kotchko N, et al. . Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT study. JAMA Oncol. 2015;1(9):1251-1260. 10.1001/jamaoncol.2015.3048 [DOI] [PubMed] [Google Scholar]
- 19. Senier L, Smollin L, Lee R, Nicoll L, Shields M, Tan C. Navigating the evidentiary turn in public health: sensemaking strategies to integrate genomics into state-level chronic disease prevention programs. Soc Sci Med. 2018;211:207-215. https://doi.org/ 10.1016/j socscimed.2018.06.026 PMID:29960172 [DOI] [PMC free article] [PubMed]
- 20. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-1736. 10.1001/jama.293.14.1729 [DOI] [PubMed] [Google Scholar]
- 21. Senier L, Tan C, Smollin L, Lee R. Understanding the potential of state-based public health genomics programs to mitigate disparities in access to clinical genetic services. Genet Med. 2019;21(2):373-381. 10.1038/s41436-018-0056-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noone A, Howlader N, Krapcho M, et al. . SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 23. Jones J, Wang H, Karanam B, et al. . Nuclear localization of Kaiso promotes the poorly differentiated phenotype and EMT in infiltrating ductal carcinomas. Clin Exp Metastasis. 2014;31(5):497-510. 10.1007/s10585-014-9644-7 10.1007/s10585-014-9644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones J, Wang H, Zhou J, et al. . Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am J Pathol. 2012;181(5):1836-1846. 10.1016/j.ajpath.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abisoye-Ogunniyan A, Lin H, Ghebremedhin A, et al. Transcriptional repressor Kaiso promotes epithelial to mesenchymal transition and metastasis in prostate cancer through direct regulation of miR-200c. Cancer Lett. 2018;431:1-10. https://doi.org/ 10.1016/j. canlet.2018.04.044 PMID:29751044 [DOI] [PMC free article] [PubMed]
- 26. Ma Q, Lu AYH. Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol Rev. 2011;63(2):437-459. 10.1124/pr.110.003533 [DOI] [PubMed] [Google Scholar]
- 27. Wadelius M, Chen LY, Downes K, et al. . Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5(4):262-270. 10.1038/sj.tpj.6500313 [DOI] [PubMed] [Google Scholar]
- 28. Scott SA, Sangkuhl K, Stein CM, et al. ; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317-323. 10.1038/clpt.2013.105 10.1038/clpt.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goetz MP, Sangkuhl K, Guchelaar HJ, et al. . Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 2018;103(5):770-777. 10.1002/cpt.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SS. Pharmacogenomics and the challenge of health disparities. Public Health Genomics. 2009;12(3):170-179. 10.1159/000189630 [DOI] [PubMed] [Google Scholar]
- 31. Precision Medicine Initiative Working Group The Precision Medicine Initiative Cohort Program: Building the Foundation for 21st Century Medicine: Report to the Advisory Committee to the Director. National Institutes of Health; 17 September 2015.
- 32. Arnett DK, Claas SA. Precision Medicine, Genomics, and Public Health. Diabetes Care. 2016;39(11):1870-1873. 10.2337/dc16-1763 [DOI] [PubMed] [Google Scholar]
- 33. Levy-Lahad E, Lahad A, King MC. Precision medicine meets public health: population screening for BRCA1 and BRCA2. J Natl Cancer Inst. 2014;107(1):420. 10.1093/jnci/dju420 [DOI] [PubMed] [Google Scholar]
- 34. Ley SH, Ardisson Korat AV, Sun Q, et al. . Contribution of the Nurses’ Health Studies to Uncovering Risk Factors for Type 2 Diabetes: Diet, Lifestyle, Biomarkers, and Genetics. Am J Public Health. 2016;106(9):1624-1630. 10.2105/AJPH.2016.303314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Ijzendoorn MH, Bakermans-Kranenburg MJ. Genetic differential susceptibility on trial: meta-analytic support from randomized controlled experiments. Dev Psychopathol. 2015;27(1):151-162. 10.1017/S0954579414001369 10.1017/S0954579414001369 [DOI] [PubMed] [Google Scholar]