Abstract
Knowledge of upper limb activity in the natural environment is critical for evaluating the effectiveness of rehabilitation services. Wearable sensors allow efficient collection of these data, and have the potential to be less burdensome than self-report measures of activity. Sensors can capture many different variables of activity and daily performance, many of which could be useful in identifying deviation from typical movement behavior and/or measuring outcomes from rehabilitation interventions. While it has potential, sensor measurement is just emerging and there is a lack of consensus on which variables of daily performance are valid, sensitive, specific, and useful. We propose that symmetry of full-day upper limb movement is a key variable. We describe here that symmetry is valid, robustly observed within a narrow range across the lifespan in typical development, and shows evidence of being different in populations with neuromotor impairment. Key next steps include the determination of sensitivity, specificity, minimal detectable change, and minimal clinically important change/difference. This information is needed to determine whether an individual belongs to the typical or atypical group, whether or not change has occurred, and whether or not that change is beneficial.
Keywords: wearable sensor, symmetry, upper limb activity, neurologic, pediatric
Use of wearable-sensors to collect upper limb activity in daily life
Knowledge of upper limb activity in the natural environment is critical for evaluating the effectiveness of rehabilitation services. People often seek out rehabilitation because they want to be able to function better in their daily lives. The World Health Organization International Classification of Functioning, Disability and Health model describes three levels of measurement: body structures and function, activity (task execution), and participation (involvement in life situations).1 The activity level is subdivided into capacity, i.e. an individual’s ability to execute a task or an action in a structured setting, and performance, i.e. what an individual does in his or her own current, unstructured environment.1 Clinicians and researchers often assess functional capacity over short periods of time and/or in structured clinical or laboratory environments. This common practice, while efficient, does not assess a person’s functional performance across time in the unstructured, natural environment where they live. Unless we measure upper limb activity in the natural environment, we have no way of determining if the rehabilitation services provided have achieved the intended goal of improving performance in daily life. There is a growing consensus in the field that knowledge of upper limb movement activity in the natural environment is critical for evaluating the effectiveness of neurorehabilitation services for people across the lifespan, and that assessment tools are lacking.2–5
Wearable sensors for tracking human physical activity are a relatively recent technological advancement, allowing for efficient collection of upper limb activity data in daily life. Currently, there are research (e.g., Actigraph, APDM), commercial (e.g., Fitbit), repurposed (e.g., Gulf Coast), and custom-built devices available. While comparing and contrasting different devices is beyond the scope of this paper, all types of wearable sensors offer a unique opportunity for measuring movement as they can record full days of activity in the natural environment. The specific sensors inside the devices typically consist of an accelerometer and sometimes gyroscopes or other types of sensors. The devices are placed on the wrists, and currently are about the size of a large wrist watch. The devices do not obstruct movement and allow people to go about their typical activities. For example, Opal sensors record tri-axial accelerometer, gyroscope, and magnetometer data at 20–128 Hz.6 Actigraph sensors (wGT3X-BT) record tri-axial accelerometer data at 30–100 Hz.7 Accelerometers measure acceleration (m/s2), gyroscopes measure angular velocity (rad/s), and magnetometers measure the magnetic field (gauss/tesla). How these signals are analyzed to measure movement depends on the analyses performed. With modern computing and software capabilities, it is relatively simple to download data from one or more days. And while this paper focuses on upper limb activity, it should also be noted that wearable sensors are also being used to measure other types of movement, including infant leg movements,8,9 gait,10 and wheelchair propulsion.11
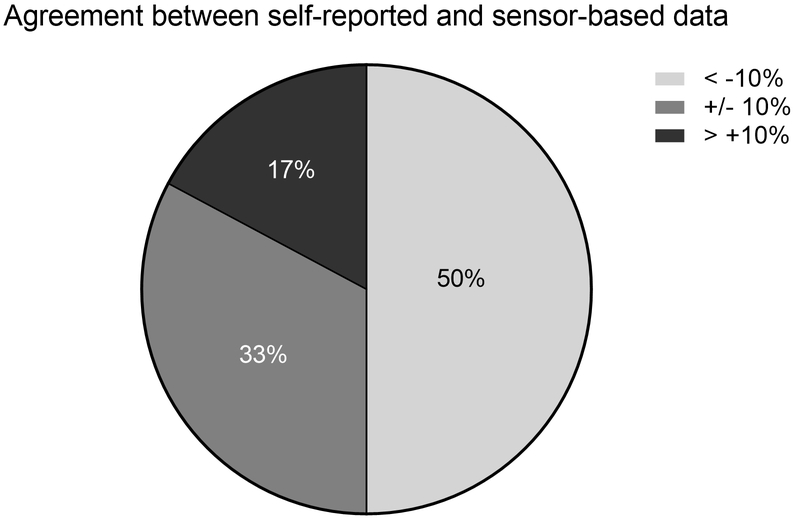
When considering deploying wearable sensors, we note that self-report questionnaires or activity logs can also be used to collect upper limb activity data in daily life. Wearable sensors allow for more efficient collection of these data, and have the potential to be less burdensome than the self-report measures.12–14 Previous studies comparing self-report vs. sensor-based measures of physical activity reveal that the different ways of measuring do not yield equivalent results.15 This appears to hold true for upper limb performance data as well, where measured values from wearable sensors and activity questionnaires or logs are not interchangeable. Conceptually, two different constructs are being measured and these two constructs may not be identical: the amount of upper limb activity a person says they do vs. the amount of upper limb activity that is measured at the wrist. Figure 1 shows that upper limb performance quantified by self-report often does not match performance quantified by wearable sensors.16 One third of a sample (total n = 64 persons with upper limb paresis ≥ 6 months post stroke) of persons with upper limb paresis post stroke had values from self-report that were within ± 10% of sensor values. The other two-thirds either over-reported (17%) or under-reported (50%) compared to sensor values. To add to the challenge of measurement, individual participants were often in one category (under, accurate, or over) at one time point and a different category at a later time point.16 While self-report measures provide important information about a person’s perception of their own activity, sensor-based measures are necessary if the outcome of interest is quantification of movement as a measure of performance across the day in the natural environment.
Figure 1.
Conceptual issues surrounding sensor-based measures. Pie chart showing that performance quantified by self-report often does not match performance quantified by wearable sensors (data from Waddell et al., 201816). The data indicate that 50% of the sample under-reported and 17% over-reported upper limb activity compared to the sensor values. Only 33% were considered accurate, with self-report and sensor values within 10% of each other.
Wearable sensors can capture numerous different variables of upper limb activity, many of which could be useful in identifying deviation from typical movement behavior and/or measuring outcomes from rehabilitation interventions. Table 1 provides a list of many different variables that have been developed thus far. Each variable uses a different calculation from the sensor data, where some are derived from sensors on one limb (top of Table 1) while others are calculated using the sensors on both limbs (bottom of Table 1). Each variable quantifies a specific feature of upper limb performance over the recording period. No single sensor variable likely capture the whole construct of upper limb performance.
Table 1.
Wearable sensor variables of upper limb activity
| Variable | Brief conceptual description | Source data for variable | Population/Sensor |
|---|---|---|---|
| Variables calculated using a single arm | |||
| Movement bout | Identification of start and stop of arm movement bout, regardless of direction | Resultant tri-axial acceleration above threshold and resultant tri-axial angular velocity | Infants with typical development and at risk for developmental disability. Sensor = Opal17 |
| Bout duration | Duration of a bout | Resultant tri-axial acceleration above threshold and resultant tri-axial angular velocity | Infants with typical development. Sensor = Opal17 |
| Bout average acceleration | Average acceleration magnitude of a bout | Resultant tri-axial acceleration | Infants with typical development. Sensor = Opal17 |
| Bout peak acceleration | Peak acceleration magnitude of a bout | Resultant tri-axial acceleration | Infants with typical development. Sensor = Opal17 |
| Acceleration area | Intensity of arm movement activity, measured by area under the total acceleration curve | Resultant tri-axial acceleration | Infants with typical development. Sensor = Opal17 |
| Duration of upper extremity activity | Duration of arm movement (in hours or %) that occurred during the wearing period | Vector magnitude of tri-axial activity counts of acceleration per second greater than or equal to 2 | Adults: nondisabled. Children with hemiparesis. Sensor = Actigraph GT3X+4,18 |
| Median and maximum acceleration magnitude | Representative value of acceleration magnitude over the entire monitoring period | Resultant tri-axial acceleration median and maximum accelerations | Adults: nondisabled and with stroke < 30 days or > 6 months. Sensor = Actigraph GT3X+19,20 |
| Acceleration variability | Magnitude of fluctuation of acceleration values from the mean acceleration over the entire monitoring period | Resultant tri-axial acceleration spread of accelerations around the mean acceleration | Adults: nondisabled and with stroke < 30 days or > 6 months. Sensor = Actigraph GT3X+19,20 |
| Variables calculated using both arms | |||
| Bilateral magnitude | Summed intensity of activity across both arms, calculated for each second of activity | Sum of the smoothed vector magnitude of tri-axial activity counts of acceleration per second of the nondominant and dominant upper extremities | Adults: nondisabled and with chronic stroke. Sensor = Actigraph GT3X+21,22 |
| Magnitude ratio | Contribution of each arm to activity, calculated for each second of activity | Ratio of the magnitude of paretic upper extremity resultant acceleration to the magnitude of the nonparetic upper extremity resultant acceleration | Adults: nondisabled and with stroke > 6 mos. Sensor = Actigraph GT3X+19,21,22 |
| Use ratio (also called activity ratio) | Ratio of the total movement duration of one arm compared to the other arm, across a day | Vector magnitude of tri-axial activity counts of acceleration per second greater than or equal to 2 | Adults: nondisabled and with subacute or chronic stroke. Children with hemiparesis. Sensors = Computer Science and Applications Inc. model 7164, Manufacturing Technologies Inc, Actigraph GT3X+, Actigraph GTIM.4,18,23–25 |
| Jerk asymmetry | Ratio of average jerk magnitude between one arm and the other. Higher jerk represents less smooth motion | Tri-axial resultant acceleration was converted into activity counts, where 1 activity count = 0.017 g, then magnitude of the differential of the acceleration vector | Adults with chronic stroke. Sensor = custom26 |
| Acceleration magnitude asymmetry | Ratio of average acceleration magnitude between one arm and the other | Tri-axial resultant acceleration was converted into activity counts, where 1 activity count = 0.017 g, then magnitude of the acceleration vector | Adults with chronic stroke. Sensor = custom26 |
| Laterality index | Ratio of amount of activity between one arm and the other, from fully bimanual to fully unimanual activity | Tri-axial resultant acceleration was converted into activity counts, where 1 activity count = 0.017 g, then (counts on the affected side - non-affected side)/ (counts on the affected side + non-affected side) | Adults with chronic stroke. Sensor = custom26 |
| Percentage of contribution of each arm | Percentage contribution of one arm to overall arm activity | Vector magnitude of tri-axial resultant acceleration, dominant/anatomical arm divided by the total vector magnitude across both arms, and any time points where the vector magnitude across both arms was equal to 0 (no activity) were removed from the dataset | Adults: two nondisabled and two upper limb prosthetic users. Sensor = Actigraph GT3X+27 |
| Variation Ratio | Ratio of acceleration variability (see above) between one arm and the other | Ratio of the standard deviation of acceleration on the paretic upper extremity to the standard deviation on nonparetic upper extremity | Adults: nondisabled and with stroke < 30 days or > 6 months. Sensor = Actigraph GT3X+19,20 |
Of the numerous variables available, many sensor-derived variables are highly variable within and across participants. This is because human movement is variable within and across participants, and the sensor-derived variables are capturing normal human movement variability.17,22 As a simple example, when neurologically-intact adults are asked to reach as fast as possible, the wide range of peak velocity values shows how some people can move quite rapidly and others do not (see control data in Wagner et al., 200628 and Lang et al., 200629). Thus, some variables that can be computed from sensors reflect highly varying features of human movement and other variables reflect more tightly constrained features of human movement.
While sensors capture the natural variability in human movement, another source of variability in sensor data (or any form of measurement) is measurement error. The sensors themselves reliably capture movement every time the sensor is moved, as indicated by manufacturer testing. When the sensor is place on humans and variables are calculated, each variable will, of course, have its own measurement characteristics, and these psychometric values may differ between typical and atypical populations. Reliability and validity of variables for upper limb activity in daily life is reasonably well established, as can be seen in Table 1 in Uswattte et al., 2000,30 Table 1 in Hayward et al., 2015,31 Table 3 in Lang et al., 2013,32 and in recent systematic reviews.2,3 Psychometric properties are beginning to be established in other populations. For example, video data confirms number of limb movements measured by wearable sensors in infants with typical development and at risk for developmental disability across samples of 20 seconds of video data of spontaneous movement in the supine position.9,17
High variability is problematic when the purpose of the variable is to accurately identify deviation from typical and measure change over time. A useful variable would be one that is highly reliable and narrowly observed within the typical population across the lifespan. A variable with these characteristics would support accurate identification of deviation from typical and has the potential to be sensitive to change. While sensor variables hold enormous potential for capturing real-world upper limb performance, there is a lack of consensus on which variables are valid, sensitive, specific, and useful(for review see Hayward et al., 201531). As computing power grows and sensors shrink, new variables will emerge that may make quantification better at the same time as they make achieving consensus more challenging.
The purpose of this special communication is to propose that symmetry of upper limb activity during daily life is a key variable, and one that could be useful in research and clinical practice now. We make the argument that symmetry is valid, robustly observed within a narrow range across the lifespan in typical development, and shows evidence of being different in populations with neuromotor impairment. Our aims in this article are 1) to provide clinicians and scientists with a summary of current evidence that symmetry of movement is a key variable for quantifying upper limb movement activity in natural environments; and 2) to improve future research by providing recommendations for next steps for development of symmetry of upper limb movement as an assessment tool and outcome measure.
Variables of symmetry in adults
Symmetry of movement can be conceptualized in different ways. Here, we operationally define symmetry to express the idea that the right and left limbs are equally selected and active during daily activities. Symmetry, as used here, is not intended to express simultaneous and/or matching actions of the limbs.
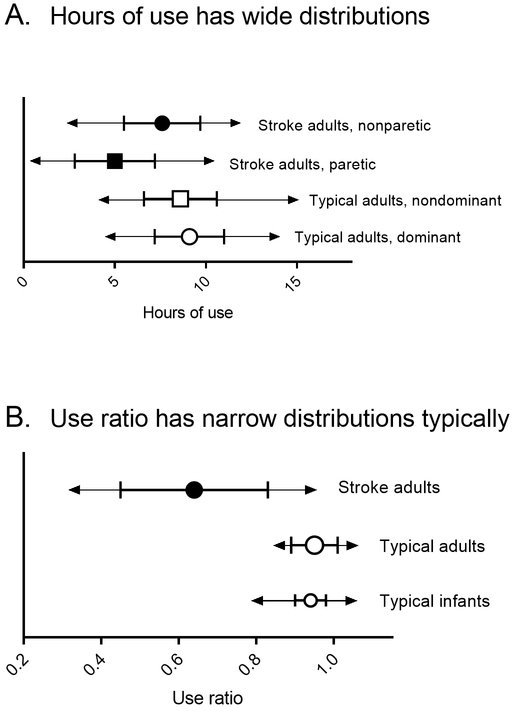
The idea of measuring symmetry of upper limb movement with wearable sensors originated with Uswatte and colleagues as a means to test the efficacy of constraint-induced movement therapy.23,24,30 In observing human movement and analyzing sensor data, they recognized that calculating the ratio of the movement of the paretic limb compared to the non-paretic limb provided a cleaner measure of activity than looking at variables from a single limb, such as hours of movement or total magnitude of accelerations. Thus, the simplest and most studied variable of upper limb symmetry is the use ratio, also sometimes referred to as the activity ratio. The use ratio equals the hours of activity of the non-dominant limb divided by the hours of activity of the dominant limb, and is usually calculated from sensor data spanning 24 hours or more.18 Values close to 1.0 indicate that the limbs are active for similar amounts of time throughout the day, while values less than 1.0 indicate that the dominant limb is more active than the non-dominant limb. The use ratio is reliable, with strong face, construct, and concurrent validity, as demonstrated by many groups (see Hayward et al., 2015 Table 1 for review31). The use ratio is highly repeatable in community-dwelling adults (Lang, unpublished data) and typically developing children.33 The striking factor about this symmetry variable is its narrow range in the typical population.18 Figure 2 shows examples of widely vs. narrowly distributed variables. Figure 2A shows the distribution of hours of use, a widely distributed variable in typical, neurologically-intact, community-dwelling adults (open symbols) and in community-dwelling persons with stroke (closed symbols). Figure 2B, in contrast, shows the distribution of the use ratio, a narrowly distributed variable in typical adults (larger, open symbols). Indeed, the mean use ratio is 0.95 ± 0.06,18 indicating symmetry of upper limb activity throughout the wearing period. The use ratio is independent of age, such that young adults have the same values as older adults.18 The narrow distribution of the use ratio in the typical population makes it relatively easy to differentiate between typical and a population of persons with stroke, who do not have a narrow distribution (filled symbols).
Figure 2.
Examples of sensor variables with wide vs. narrow distributions. Symbols represent sample means, with error bars at ± 1 SD and arrows at the minimum and maximum values observed. 2A: Hours of use is a variable with a wide distribution in typical adult populations (open symbols, data from Bailey et al., 2013 and 201518,21) and a wide distribution in persons with stroke (closed symbols, data from Bailey et al., 201521). 2B: The use ratio, however, is a variable with a narrow distribution in typical adult and infant populations (open symbols, infant data from Trujillo-Priego et al., 201717). As is characteristic of infant data, the range from minimum to maximum is a bit wider than in adults. The wide distribution of use ratio values in the stroke population (closed symbols) includes people who have normal daily activity (the most mildly affected) and some who have almost no daily activity (the most severely affected).
The use ratio is one, but not the only, variable of upper limb symmetry that can be derived from wearable sensors (Table 1, bottom). For example, the magnitude ratio also captures the activity in one limb vs. the other, but instead does it on a second-by-second basis.21,22 In this case, values of 0 indicate both limbs have the same magnitude of acceleration at that instant in time. Values less than 0 indicate larger accelerations in the dominant limb and values greater than one indicate larger accelerations in the nondominant limb. Like the use ratio, the mean of magnitude ratio values, compiled across a day or more also has a narrow distribution, with a mean of −0.1 ± 0.3.21 Other examples of symmetry variables under development include jerk asymmetry and acceleration magnitude asymmetry,26 percentage of contribution of each arm,27 and variation ratio.19 Early data from these more complex symmetry variables suggest that, like the use ratio, they will have a narrow range of distribution in the typical adult population. Thus regardless of the specific way symmetry is quantified, across different types of sensors and different analysis approaches, there appears to be a narrow range of symmetry in the typical adult population. This narrow range permits ready discrimination of atypical values. The narrow range also opens up the possibility to detect how atypical values might progress towards typical values over time or with specific rehabilitation interventions.
Variables of symmetry earlier in the lifespan
Just because there is a narrow range of symmetry in the typical adult population, one cannot assume that infants and children show the same upper limb behaviors. After all, infants and children are in the early stages of acquiring upper limb motor skills to accomplish functional tasks. While it is well-established that adults use their dominant and non-dominant arms in different ways to complete functional tasks, infants and young children are just learning. Infants do not show right or left dominance in arm reaching, an early behavior that is clearly goal directed. Instead, they show a shifting preference for using one arm or the other.34 Whether they reach with one arm or two, for example, is influenced by the size and shape of the object.35
Despite difference in arm movement behaviors from adults, infants demonstrate narrowly distributed use ratio values, just like adults. Sensor data from a sample of infants (n= 22 infants observed over 73 visits, age 38–203 days17,36) were re-examined to determine symmetry values. Since hand dominance is not yet established in this population, use ratio values were calculated as the ratio of hours of use of the less active side divided by the more active side. Infants had a mean ratio of 0.94 (SD =0.04). Figure 2B shows that the distribution of typical infant use ratio values (smaller open symbols) is strikingly similar to the distribution of typical adult values. Emerging data suggests similar use ratio values from the end of infancy to adulthood.33
Asymmetry shows evidence of being different in populations with neuromotor impairment
Symmetry is a variable that has been shown to be different in adults with stroke. On average, adults with stroke demonstrate less symmetrical arm use across the day and more between-participant variability compared to typical adults (Figure 2B).21,31 As can be seen in Figure 2B, there is a great deal of variability in the use ratio values in persons with stroke. Some individuals are very asymmetrical (values as low as 0.3), while others are near normal. This mirrors the clinical presentation of persons that are very severely affected all the way to persons that are very mildly affected. Note how one can readily identify abnormal use ratio values in Figure 2B, while it is much harder to identify abnormal hours of use values in Figure 2A.
Asymmetrical arm use across the day in the natural environment has also been documented in children with hemiparesis due to cerebral palsy.4 The children in this sample used their less-affected limbs more than the more-affected limbs, as indicated by lower than normal use ratios and an intensity of movement ratio (another method to capture symmetry, reflecting the magnitude of accelerations in one limb vs the other). Additionally, and not surprisingly, the hours of use for this pediatric population was much higher than hours of use in adults. Thus, the symmetry values helped to distinguish from typical but not the other variables. These data provide preliminary support for the value of symmetry variables in children.
Multiple sensor-based variables of upper limb movement, including use ratio, are sensitive to change in adults with upper limb hemiparesis post-stroke.19,20,23 Persons undergoing inpatient rehabilitation therapy showed large changes in use ratio and other symmetry variables from admission to discharge, with discharge values moving closer to typical population values.20 Sensors worn within therapy sessions can track changes from session to session that mirror the capacity measure changes across those same sessions.19 Interestingly, the use ratio and other symmetry values are different during training in a structured environment (capacity) vs. within the free-living environment (performance), supporting the concern that capacity and performance are not interchangeable. In other words, the symmetry variables are indicating that what someone is able to do in an assessment may or may not be what they typically do. The mismatch between capacity and performance based on sensor data can be seen in persons with stroke in the inpatient rehabilitation setting shortly after stroke,37 at home in the first year after stroke,38 and even after active engagement in high high-repetition, individualized and progressive task-specific training.39 As the goal of rehabilitation is to improve how people function in their daily lives, it is becoming clear that measuring their capacity alone is not sufficient, we need to measure performance.
Moving from adults to children, researchers in infant neurological rehabilitation/habilitation have recently been developing capacity measures of asymmetrical upper limb use in infants. The Hand Assessment for Infants was developed to evaluate asymmetries between upper limbs in goal-directed unimanual and bimanual upper limb actions in infants with asymmetrical brain injury aged 3 to 12 months.40 The Grasp and Reach Assessment of Brisbane was developed to evaluate asymmetries between upper limbs in emerging reach and grasp behavior in infants with asymmetrical brain injury aged 14 to 18 weeks.41 These examples, despite being measures of functional capacity in a structured setting, support the importance and relevance of symmetry as a variable that is important for functional performance in daily life. How well the clinical assessment asymmetry data will relate to full-day sensor asymmetry data is unknown, and the opportunity exists to compare the two methods. Relationships between full-day upper extremity movement variables and developmental status on standardized tests of infant development are just starting to be explored, and, to date, have been observed in infants with typical development using the Bayley Scales of Infant Development.36
There is an important consideration in regard to measuring differences in asymmetry in populations across the lifespan. All of the examples of group differences published to date are from populations in which there is a known clinical presentation of asymmetry. While many other populations often present with asymmetrical movements, sometimes influenced by tremor or rigidity (e.g., Parkinson’s disease, brain injury, multiple sclerosis), these asymmetries may be more subtle. How these more subtle asymmetries are reflected in full-day wearable sensor measures is currently unknown. Based on the sensors abilities to detect subtle asymmetries in active children (see figure 5C in Hayward et. al, 201531), less-obvious asymmetries in other neurological populations are likely to be detectable but perhaps less quickly and may be more difficult to interpret. Further, there are populations such as autism spectrum disorder, where an increase in symmetry of movement might be hypothesized in the presence of stereotypical, rhythmic behaviors. While full-day wearable sensor symmetry data has the potential to provide valuable measures of performance in these populations, different variables other than use ratio will likely be needed to create useful assessment tools and outcome measures. Alternative approaches to measuring symmetry as well as variables other than symmetry can be explored.
Limitations
While wearable sensors have great potential for assessing performance in the natural environment, there are two key disadvantages to consider. First, wearable sensors collect more general, as opposed to specific, measures of physical activity. Wearable sensors measure movement using accelerometers, and sometimes also gyroscopes or other sensors. They are measuring all movements above the threshold of each device or analysis algorithm, not solely movements we consider functional or purposeful. For example, passive movements of the limbs cannot be differentiated from active movements, and passive movements could occur when an adult or infant is being assisted with dressing. While using these signals to classify movement vs. no movement is fairly straightforward, identifying or classifying specific orientations/postures or types of body movements is not straightforward (for review see Preece et al., 200942). Classification of specific body movements is an area of active investigation, but to date researchers have only succeeded in identifying a few selected, specific upper limb movements in constrained situations22,43 or in a single participant over 30 minutes in an unconstrained environment.44 And second, most sensors are currently worn on the wrist, reflecting activity of the joints proximal to the wrist but not measuring finger movements. Wearable systems for measuring finger movements along with wrist movements are being developed.45
Summary and next steps
There is much ongoing work in the area of wearable sensors for rehabilitation assessment, as depicted in conference presentations and funded work. As sensors become smaller with more advanced technology for computation and communication, the opportunities for measuring performance in daily life will expand. While the future potential for useful sensor information is enormous, the goal of this paper was to provide a summary of current evidence that symmetry of movement is a key variable for quantifying upper limb movement activity in natural environments. Symmetry is a useful variable at this point in time, and we advocate that the community collectively works together to move this variable forward. From a clinical perspective, using wearable sensors to calculate the ratio of use of the nondominant arm to use of the dominant arm across a full day in the natural environment (or less to more active arm in infants) is a straightforward calculation. Although thresholds for identifying movement vs. no movement may be different between specific types of sensors and/or sensitive to sensor placement, the symmetry variable would not be affected as long as procedures (same placement on each side of the body, same sensors) were consistent across repeated assessments and participants.
While the adult wearable sensor research is a bit ahead of pediatric research, more studies in both populations are sorely needed. The adult literature would suggest that capacity will likely not reflect performance in pediatric populations, however adult and infant capacity assessment is different. Adults will typically follow instructions to attempt certain activities requested of them, while infants do not! As a result, infant capacity and performance assessment may be more closely related than in adults. Nonetheless, sensor-based asymmetry measures could be used to efficiently assess capacity and performance in this population. Key next steps for research and clinical use of sensor-based asymmetry measures are the determination of the psychometric properties of sensitivity, specificity, minimal detectable change, and minimal clinically important change/difference. This information is needed to determine whether an individual belongs to the typical or atypical group, whether or not change has occurred, and whether or not that change is beneficial. This psychometric research has not been considered historically ‘exciting’ from a funding perspective, increasing the challenge to execute the work. This research will require large data sets from both cross-sectional and longitudinal designs in infant, children, and adult populations. Ideally, these psychometric values will be calculated in one or a few samples and then validated in additional, independent samples. Efforts to obtain and analyze these data may require large research consortiums, since collecting data from one or a few lab groups will likely not be sufficient.
Acknowledgements.
Thank you to the American Physical Therapy Association Section on Research Traveling Fellow Award for supporting Dr. Smith’s visit to Dr. Lang’s laboratory during the conceptualization and execution of this special communication. Portions of the work were supported by NIH R01HD068290 and Bill & Melinda Gates Foundation OPP1119189.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The contents of this special communication have not been presented.
The authors declare no conflicts of interest.
References
- 1.World Health Organization. International Classification of Functioning, Disability and Health (ICF). http://www.who.int/classifications/icf/en/. Accessed 09/26/18
- 2.Lemmens RJ, Timmermans AA, Janssen-Potten YJ, Smeets RJ, Seelen HA. Valid and reliable instruments for arm-hand assessment at ICF activity level in persons with hemiplegia: a systematic review. BMC Neurol. 2012;12(1):21. doi: 10.1186/1471-2377-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noorkõiv M, Rodgers H, Price CI. Accelerometer measurement of upper extremity movement after stroke: a systematic review of clinical studies. J Neuroeng Rehabil. 2014;11(1):144–11. doi: 10.1186/1743-0003-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokal B, Uswatte G, Vogtle L, Byrom E, Barman J. Everyday movement and use of the arms: Relationship in children with hemiparesis differs from adults. J Pediatr Rehabil Med. 2015;8(3):197–206. doi: 10.3233/PRM-150334 [DOI] [PubMed] [Google Scholar]
- 5.Uswatte G, Taub E, Griffin A, Vogtle L, Rowe J, Barman J. The Pediatric Motor Activity Log-Revised: Assessing real-world arm use in children with cerebral palsy. Rehabil Psychol. 2012;57(2):149–158. doi: 10.1037/a0028516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.APDM Wearable Sensors. https://www.apdm.com/wearable-sensors/. Accessed 09/26/18.
- 7.Activity Monitor wGT3X-BT I ActiGraph. https://www.actigraphcorp.com/actigraphwgt3x-bt/. Accessed 09/26/18.
- 8.Trujillo-Priego IA, Smith BA. Kinematic characteristics of infant leg movements produced across a full day. JRATE. 2017;4:1–10. doi: 10.1177/2055668317717461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BA, Trujillo-Priego IA, Lane CJ, Finley JM, Horak FB. Daily Quantity of Infant Leg Movement: Wearable Sensor Algorithm and Relationship to Walking Onset. Sensors. 2015;15(8):19006–19020. doi: 10.3390/s150819006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao W, Liu T, Zheng R, Feng H. Gait Analysis Using Wearable Sensors. Sensors. 2012;12(2):2255–2283. doi: 10.3390/s120202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kressler J, Koeplin-Day J, Muendle B, Rosby B, Santo E, Domingo A. Accuracy and precision of consumer-level activity monitors for stroke detection during wheelchair propulsion and arm ergometry. Jan Y-K, ed. PLoS ONE. 2018;13(2):e0191556–15. doi: 10.1371/journal.pone.0191556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires * Commentary. Br J Sports Med. 2003;37(3):197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamo KB, Prince SA, Tricco AC, Connor Gorber S, Tremblay M. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: A systematic review. Int J Pediatr Obes. 2009;4(1):2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]
- 14.Brett KE, Wilson S, Ferraro ZM, Adamo KB. Self-report Pregnancy Physical Activity Questionnaire overestimates physical activity. Can J Public Health. 2015;106(5):1–7. doi: 10.17269/cjph.106.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince SA, Adamo KB, Hamel M, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(56):1–24.doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddell KJ, Lang CE. Comparison of Self-Report Versus Sensor-Based Methods for Measuring the Amount of Upper Limb Activity Outside the Clinic. Arch Phys Med Rehabil. 2018;99(9):1913–1916. doi: 10.1016/j.apmr.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trujillo-Priego IA, Lane CJ, Vanderbilt D, et al. Development of a Wearable Sensor Algorithm to Detect the Quantity and Kinematic Characteristics of Infant Arm Movement Bouts Produced across a Full Day in the Natural Environment. Technologies. 2017;5(3):1–16. doi: 10.3390/technologies5030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey RR, Lang CE. Upper-limb activity in adults: Referent values using accelerometry. J Rehabil Res Dev. 2013;50(9):1213–1222 doi: 10.1682/JRRD.2012.12.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbin MA, Bailey RR, Lang CE. Validity of Body-Worn Sensor Acceleration Metrics to Index Upper Extremity Function in Hemiparetic Stroke. J Neurol Phys Ther. 2015;39:111–118. doi: 10.1097/NPT.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbin MA, Waddell KJ, Lang CE. Acceleration metrics are responsive to change in upper extremity function of stroke survivors. Arch Phys Med Rehabil. 2015;96(5):854–861. doi: 10.1016/j.apmr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey RR, Klaesner JW, Lang CE. Quantifying Real-World Upper-Limb Activity in Nondisabled Adults and Adults With Chronic Stroke. Neurorehabil Neural Repair. 2015;29(10):969–978. doi: 10.1177/1545968315583720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey RR, Klaesner JW, Lang CE. An Accelerometry-Based Methodology for Assessment of Real-World Bilateral Upper Extremity Activity. Zadpoor AA, ed. PLoS ONE. 2014;9(7):e103135–e103139. doi: 10.1371/journal.pone.0103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory Monitoring of Arm Movement Using Accelerometry: An Objective Measure of Upper-Extremity Rehabilitation in Persons With Chronic Stroke. Arch Phys Med Rehabil. 2005;86(7):1498–1501. doi: 10.1016/j.apmr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of Accelerometry for Monitoring Real-World Arm Activity in Patients With Subacute Stroke: Evidence From the Extremity Constraint-Induced Therapy Evaluation Trial. Arch Phys Med Rehabil. 2006;87(10):1340–1345. doi: 10.1016/j.apmr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Lang CE, Waddell KJ, Klaesner JW, Bland MD. A Method for Quantifying Upper Limb Performance in Daily Life Using Accelerometers. JoVE. 2017;(122):1–8. doi: 10.3791/55673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lucena DS, Stoller O, Rowe JB, Chan V, Reinkensmeyer DJ. Wearable sensing for rehabilitation after stroke: Bimanual jerk asymmetry encodes unique information about the variability of upper extremity recovery. IEEE Int Conf Rehabil Robot. 2017:1603–1608. doi: 10.1109/IC0RR.2017.8009477. [DOI] [PubMed] [Google Scholar]
- 27.Chadwell A, Kenney L, Granat M, Thies S, Head JS, Galpin A. Visualisation of upper limb activity using spirals: A new approach to the assessment of daily prosthesis usage. Prosthet Orthot Int. 2018;42(1):37–44. doi: 10.1177/0309364617706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner JM, Lang CE, Sahrmann SA, et al. Relationships between Sensorimotor Impairments and Reaching Deficits in Acute Hemiparesis. Neurorehabil Neural Repair. 2006;20(3):406–416. doi: 10.1177/1545968306286957. [DOI] [PubMed] [Google Scholar]
- 29.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of Grasp versus Reach in People with Hemiparesis Poststroke. Neurorehabil Neural Repair. 2006;20(4):444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 30.Uswatte G, Miltner WH, Foo B, Varma M, Moran S, Taub E. Objective measurement of functional upper-extremity movement using accelerometer recordings transformed with a threshold filter. Stroke. 2000;31(3):662–667. [DOI] [PubMed] [Google Scholar]
- 31.Hayward KS, Eng JJ, Boyd LA, Lakhani B, Bernhardt J, Lang CE. Exploring the Role of Accelerometers in the Measurement of Real World Upper-Limb Use After Stroke. Brain Impair. 2015;17(01):16–33. doi: 10.1017/BrImp.2015.21. [DOI] [Google Scholar]
- 32.Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier RL. Assessment of upper extremity impairment, function, and activity after stroke: foundations for clinical decision making. J Hand Ther. 2013;26(2):104–115. doi: 10.1016/j.jht.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyt Drazen C, Nguyen A, Everett E, et al. Using Accelerometry to Detect Upper Extremity Motor Deficits and Delays in Early Childhood. American Occupational Therapy Association Annual Conference, Philadelphia, PA; 2017. [Google Scholar]
- 34.Corbetta D, Thelen E. Lateral biases and fluctuations in infants’ spontaneous arm movements and reaching. Dev Psychobiol. 1999;34:237–255. [PubMed] [Google Scholar]
- 35.Corbetta D, Snapp-Childs W. Seeing and touching: the role of sensory-motor experience on the development of infant reaching. Inf Behav Dev. 2009;32(1):44–58. [DOI] [PubMed] [Google Scholar]
- 36.Shida-Tokeshi J, Lane CJ, Trujillo-Priego IA, et al. Relationships between full-day arm movement characteristics and developmental status in infants with typical development as they learn to reach: An observational study. Gates Open Res. 2018;2:17. doi: 10.12688/gatesopenres.12813.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand D, Eng JJ. Disparity Between Functional Recovery and Daily Use of the Upper and Lower Extremities During Subacute Stroke Rehabilitation. Neurorehabil Neural Repair. 2012;26(1):76–84. doi: 10.1177/1545968311408918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rand D, Eng JJ. Predicting Daily Use of the Affected Upper Extremity 1 Year after Stroke. J Stroke Cerebrovasc Dis. 2015;24(2):274–283. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 39.Waddell KJ, Strube MJ, Bailey RR, et al. Does Task-Specific Training Improve Upper Limb Performance in Daily Life Poststroke? Neurorehabil Neural Repair. 2017;31(3):290–300. doi: 10.1177/1545968316680493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krumlinde-Sundholm L, Ek L, Sicola E, et al. Development of the Hand Assessment for Infants: evidence of internal scale validity. Dev Med Child Neurol. 2017;59(12):1276–1283. doi: 10.1111/dmcn.13585. [DOI] [PubMed] [Google Scholar]
- 41.Perez M, Ziviani J, Guzzetta A, et al. Development, and construct validity and internal consistency of the Grasp and Reach Assessment of Brisbane (GRAB) for infants with asymmetric brain injury. Inf Behav Dev. 2016;45(Pt A):110–123. doi: 10.1016/j.infbeh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Preece SJ, Goulermas JY, Kenney LPJ, Howard D, Meijer K, Crompton R. Activity identification using body-mounted sensors—a review of classification techniques. Physiol Meas. 2009;30(4):R1–R33. doi: 10.1088/0967-3334/30/4/R01. [DOI] [PubMed] [Google Scholar]
- 43.Biswas D, Corda D, Baldus G, et al. Recognition of elementary arm movements using orientation of a tri-axial accelerometer located near the wrist. Physiol Meas. 2014;35(9):1751–1768. doi: 10.1088/0967-3334/35/9/1751 [DOI] [PubMed] [Google Scholar]
- 44.Lemmens RJM, Janssen-Potten YJM, Timmermans AAA, Smeets RJEM, Seelen HAM. Recognizing Complex Upper Extremity Activities Using Body Worn Sensors. Chen H-CI, ed. PLoS ONE. 2015;10(3):e0118642–20. doi: 10.1371/journal.pone.0118642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe JB, Friedman N, Bachman M, Reinkensmeyer DJ. The Manumeter: A non-obtrusive wearable device for monitoring spontaneous use of the wrist and fingers. IEEE Int Conf Rehabil Robot; 2013. doi: 10.1109/IC0RR.2013.6650397. [DOI] [PMC free article] [PubMed] [Google Scholar]