Abstract
Despite its promising therapeutic potential, nanoparticle-mediated magnetic hyperthermia is currently limited to treatment of localized and relatively accessible cancer tumors because the required therapeutic temperatures above 40 °C can only be achieved by direct intratumoral injection of conventional iron oxide nanoparticles. To realize the true potential of magnetic hyperthermia for cancer treatment, there is an unmet need for nanoparticles with high heating capacity that can efficiently accumulate at tumor sites following systemic administration and generate desirable intratumoral temperatures upon exposure to an alternating magnetic field (AMF). Although there have been many attempts to develop the desired nanoparticles, reported animal studies reveal the challenges associated with reaching therapeutically relevant intratumoral temperatures following systemic administration at clinically relevant doses. Therefore, we developed efficient magnetic nanoclusters with enhanced heating efficiency for systemically delivered magnetic hyperthermia that are composed of cobalt- and manganese-doped, hexagon-shaped iron oxide nanoparticles (CoMn-IONP) encapsulated in biocompatible PEG-PCL (poly(ethylene glycol)-b-poly(ɛ-caprolactone))-based nanocarriers. Animal studies validated that the developed nanoclusters are non-toxic, efficiently accumulate in ovarian cancer tumors following a single intravenous injection, and elevate intratumoral temperature up to 44 °C upon exposure to safe and tolerable AMF. Moreover, the obtained results confirmed the efficiency of the nanoclusters to generate the required intratumoral temperature after repeated injections and demonstrated that nanoclusters-mediated magnetic hyperthermia significantly inhibits cancer growth. In summary, this nanoplatform is a milestone in the development of systemically delivered magnetic hyperthermia for treatment of cancer tumors that are difficult to access for intratumoral injection.
Keywords: magnetic hyperthermia, magnetic nanoparticles, nanoclusters, systemic delivery
Graphical Abstract
Magnetic hyperthermia is a highly promising therapeutic modality for treatment of various cancers.1,2 It is based on the concept that magnetic nanoparticles delivered to tumors can generate heat after exposure to a non-invasive external alternating magnetic field (AMF) and consequently induce cell death.2,3 In general, cancer cells heated to temperatures between 42 and 46 °C for 30 min or longer undergo apoptosis, whereas temperatures above 46 °C (thermoablation) result in cancer cell necrosis.3,4 In contrast to thermoablation, hyperthermia-induced apoptosis is a preferable strategy for tumor eradication without causing severe side effects to healthy tissues. It has also been validated that magnetic hyperthermia at moderate temperatures increases the susceptibility of cancer cells to chemotherapy, radiation and immunotherapy.1,2,5,6,7,8
Clinical trials confirmed that external AMF can remotely generate therapeutic temperatures (≥ 42 °C) in deep-lying cancer tumors (e.g., prostate cancer) containing iron oxide nanoparticles, without causing severe side effects to healthy tissue.9,10,11 Despite this success, magnetic hyperthermia is currently limited to treatment of localized and relatively accessible tumors because the required therapeutic temperatures (≥ 42 °C) can only be achieved by direct intratumoral injection of conventional iron oxide nanoparticles.12,13 Intratumoral delivery of magnetic nanoparticles, however, is challenging for non-localized tumors with regional metastases. It is also invasive and success is dependent on the skill and experience of the operator. For example, the delivery of iron oxide nanoparticles to cancer tumors was performed intraoperatively in patients with cervical carcinoma.11 In contrast, intravenous administration is minimally invasive and can potentially deliver magnetic nanoparticles to poorly defined, non-localized cancer tumors of various shapes and sizes, including small metastatic tumor growths.
Previous reports suggest that the moderate heating efficiency of iron oxide nanoparticles combined with their relatively low tumor accumulation determine their limited application in systemically delivered magnetic hyperthermia.12,13 Several preclinical studies have revealed the challenges associated with elevating intratumoral temperatures above 38 °C after intravenous injection of iron oxide nanoparticles at clinically relevant doses.14,15,16 To generate desired intratumoral temperatures, Huang et al. reported that mice require intravenous injection with conventional nanoparticles at an extremely high dose of 1700 mg of iron (Fe) per kg of body weight.12 Importantly, the recommended intravenous dosage of the FDA-approved iron oxide nanoparticle, Ferumoxytol, is 510 mg, which corresponds to 8.5 mg of iron per kilogram of body weight for a 60 kg patient.17,18
Many attempts have been made to develop efficient nanoparticles capable of generating sufficient heat at low concentrations in order to overcome the limitations of conventional iron oxide nanoparticles associated with their relatively low heating efficiency.4,14,19,20,21,22,23,24,25,26 A literature review, however, revealed that the heating efficiency of the developed nanoparticles was predominantly evaluated following intratumoral injections and the number of reports demonstrating the intratumoral heating efficiency of systemically administered nanoparticles is sparse.4,14,20,21 One of the few available reports demonstrated that intravenously injected nanoclusters, composed of multiple iron oxide nanoparticles, were able to elevate the temperature of the tumor to ~38–39 °C.14 In a separate study, Xie et al. reported that cancer-targeted iron oxide nanoparticles co-doped with zinc and manganese elevated the temperature of tumors to ~40 °C following a single intravenous injection of a relatively high dose (30 mg Fe/kg).19 To further increase the average temperature of the tumor to ~42.8 °C, five repeated intravenous injections of the developed nanoparticles were required, resulting in a total dose of ~150 mg of iron per kg of body weight.19
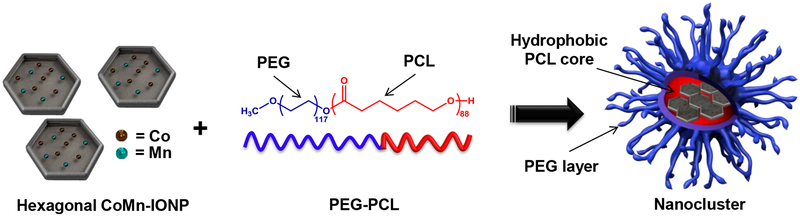
To the best of our knowledge, there are no published studies demonstrating nanoparticles capable of increasing intratumoral temperatures over 42 °C following a single intravenous injection at a clinically relevant dose. Therefore, we hypothesized that magnetic nanoclusters consisting of hexagon-shaped cobalt- and manganese-doped iron oxide nanoparticles (CoMn-IONP, Figure 1) can efficiently accumulate in cancer tumors following a single intravenous injection at a low dose (6.0 mg Fe/kg), elevate intratumoral temperature to 44 °C upon exposure to AMF, and significantly inhibit tumor growth.
Figure 1.
Schematic illustration of the nanoclusters for magnetic hyperthermia. The nanoclusters are prepared by encapsulation of hexagon-shaped cobalt- and manganese-doped iron oxide nanoparticles (CoMn-IONP) into the hydrophobic core of PEG-PCL-based nanocarriers.
RESULTS AND DISCUSSION
Development and characterization of nanoclusters with enhanced heating efficiency
The literature suggests that nanoparticles with non-spherical shapes (e.g., cubical, hexagonal) demonstrate superior heating efficiency in comparison to their spherical counterparts of the same size and composition.25,27 However, non-spherical nanoparticles have not been adequately explored yet for systemically delivered magnetic hyperthermia. In an attempt to develop systemically delivered intratumoral nanoheaters with high heating efficiency, we, therefore, synthesized hexagon-shaped iron oxide nanoparticles (IONP, Figure 1) coated with oleic acid. According to the literature, nanoparticles of hexagonal shape demonstrate enhanced heating performance due to their optimal surface magnetic anisotropy.27 To further enhance their heating efficiency, hexagonal IONP were doped with cobalt (Co) and manganese (Mn). Incorporating certain metals into iron oxide nanoparticles at a specific ratio can enhance their magnetic properties and improve the heating performance of magnetic nanoparticles.20,23,26,28
Bauer et al. previously suggested that heating efficiency of spherical iron oxide nanoparticles could potentially be enhanced by changing both the shape and composition of nanoparticles.29 It was confirmed that iron oxide nanoparticles with both cubical shape and Zn doping exhibited significantly higher heating efficiency than Zn-doped spherical nanoparticles and non-doped cubical nanoparticles alone.29
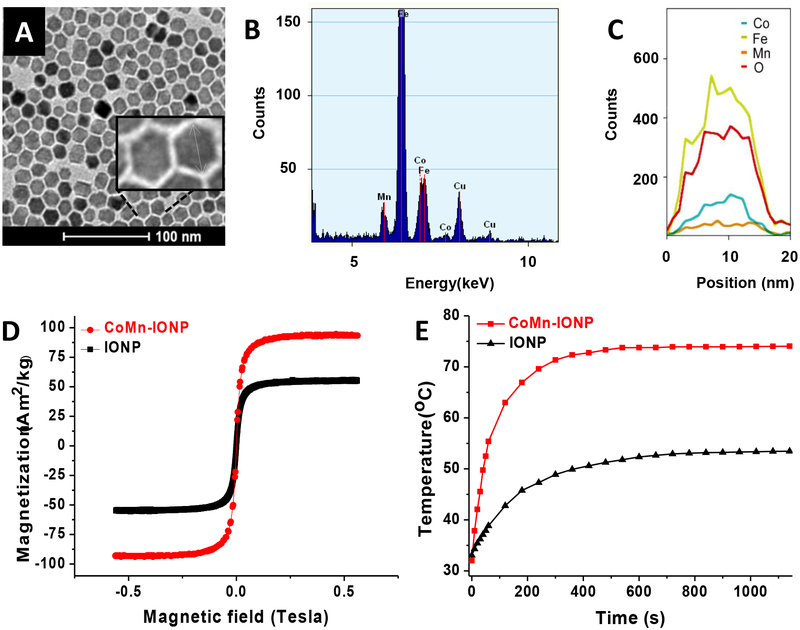
TEM imaging revealed that synthesized CoMn-IONP display an irregular hexagonal shape with an average size (width) of 14.80 ± 3.52 nm (Figure 2A). The size of CoMn-IONP was measured as the longest distance between two opposite vertices of the irregular hexagon (Figure 2A, inset). The EDX (energy dispersive x-ray spectroscopy) spectrum of CoMn-IONP reveals the presence of Fe, Co, and Mn within the magnetic nanoparticles (Figure 2B). EDX spectroscopy analysis of multiple nanoparticles further confirmed that each CoMn-IONP contains 8.14 ± 0.98% Co and 5.3 ± 0.82% Mn (Figure S1).
Figure 2.
(A) Representative TEM image of hexagonal iron oxide nanoparticles doped with Co and Mn (CoMn-IONP). (B) Representative EDX spectrum of CoMn-IONP demonstrating the presence of Co, Mn, and Fe in CoMn-IONPs. (C) Co, Fe, Mn and oxygen (O) EDX line scanning profile of a single CoMn-IONP confirms that Co and Mn are distributed throughout the nanoparticle. (D) Magnetization curves of CoMn-IONP (red) and spherical IONP (black) at room temperature. The magnetization values were normalized by the total weight of nanoparticles. (E) Heating profiles of CoMn-IONP and IONP dispersed in THF (1 mg Fe/mL) and subjected to AMF (420 kHz, 26.9 kA/m).
Finally, the EDX line scanning profile verified that Fe, Co, and Mn are distributed through the entire nanoparticle (Figure 2C). In addition, EDX mapped images of CoMn-IONP (Figure S2) confirmed that Co, Mn and Fe elements are distributed throughout the nanoparticle.
In order to make a valid comparison and estimate the efficiency of the developed nanoheaters in an unbiased manner, commercially available spherical IONP30 with a similar size (14.91 ± 1.12 nm) and surface coating (oleic acid) were used as a reference sample in all of the studies (Figure S3). The magnetization curves of CoMn-IONP and IONP nanoparticles were recorded using a custom-built vibrating magnetometer at room temperature (Figure 2D). Our measurements verified that the saturation magnetization (Ms) of the developed CoMn-IONP (93 Am2/kg) is 1.7 times higher than that of the spherical IONP (54 Am2/kg) tested under identical conditions (Figure 2D). The saturation magnetization was normalized by the total weight of nanoparticles. The obtained Ms value of 93 Am2/kg (93 emu/g) for the developed 15 nm CoMn-IONP is in good agreement with previous reports.28 For example, Lee et al. reported the Ms value of 110 emu/g (measured at 300 K) for 15 nm core-shell CoFe2O4@MnFe2O4 nanoparticles.28 As shown in Figure 2D, almost no coercivity is observed for either CoMn-IONP nanoparticles or IONP, reaffirming their superparamagnetic properties at room temperature.26 Of note, a specific absorption rate (SAR), as a measure of nanoparticle heating efficiency, is highly dependent on, and proportional to, the saturation magnetization.31,32 It is also known that SAR values depend on the size and shape of nanoparticles as well as on AMF parameters (frequency (f) and magnetic field strength (H)) during measurement.25 Consequently, a comparison of SAR values of nanoparticles with different shapes or sizes will naturally be biased toward a certain type of nanoparticle. According to the literature, however, this strategy is widely used to evaluate the effect of shape or size on nanoparticles heating efficiency.25,26,29,33,34 Therefore, we compare SAR values of spherical IONP and hexagonal-shaped CoMn-IONP with similar size (IONP: 14.91 ± 1.12 nm vs CoMn-IONP: 14.80 ± 3.52 nm) and surface coating upon exposure to AMF with the same parameters (f = 420 kHz; H = 26.9 kA/m, H × f = 11.3 × 109 A m−1 s−1). Based on the obtained heating profiles of CoMn-IONP and IONP in tetrahydrofuran (THF) (Figure 2E), it was further calculated that CoMn-IONP have a specific absorption rate 3.6 times larger than that of IONP with values of 1718.0 and 475.3 W/g, respectively, under the same experimental conditions (f = 420 kHz; H = 26.9 kA/m). In addition, oleic acid-coated IONP with an average diameter of 13.97 ± 3.63 nm (Figure S4A) were prepared in our lab under the same experimental conditions used for CoMn-IONP synthesis and their heating efficiency was evaluated (Figure S4B). The obtained results revealed that our IONP with a SAR value of 586.2 W/g (f = 420 kHz; H = 26.9 kA/m) demonstrate slightly higher heating efficiency than commercially available IONP (475.3 W/g) and significantly lower heating efficiency than CoMn-IONP (1718.0 W/g).
To minimize non-selective heating of healthy tissue in patients by AMF alone, Hergt et al. proposed that the product of the magnetic field strength and the frequency (H × f) should be ≤ 5 × 109 A m−1 s−1.35 Therefore, the heating profiles of spherical and hexagonal-shaped nanoparticles were recorded (Figure S5A) upon exposure to AFM with the suggested field−frequency product of 4.5 × 109 A m−1 s−1 (f = 420 kHz; H = 10.6 kA/m). It was calculated that the SAR value of CoMn-IONP (897.9 W/g) is 6.2 and 2.6 times higher than that of commercially available IONP (145.9 W/g) and IONP prepared in our lab (348.5 W/g) under the same experimental conditions (f = 420 kHz; H = 10.6 kA/m), respectively.
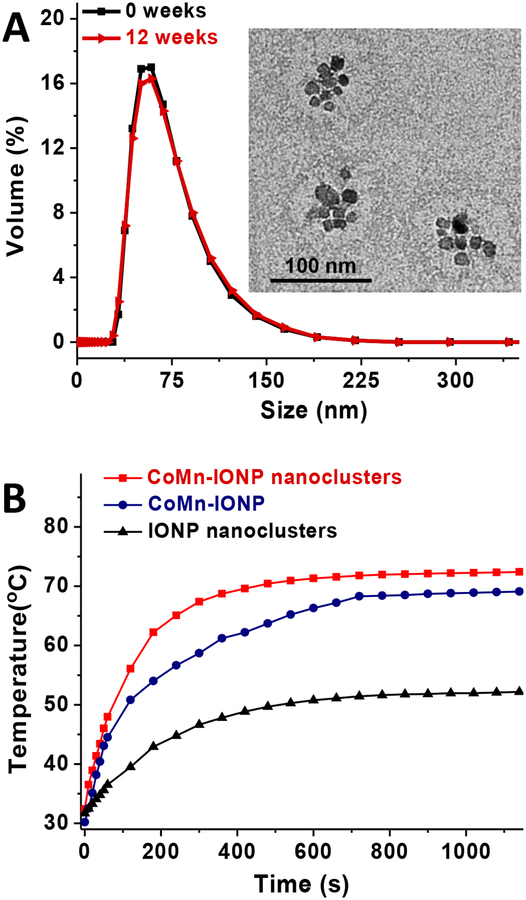
To transform hydrophobic, oleic acid-coated, CoMn-IONP into a water-soluble nanoplatform, the constructs were loaded into the hydrophobic interior of a PEG-PCL-based polymeric nanoparticle (Figure 1). PEG-PCL-based delivery vehicles were selected for the developed nanoheaters based on previous reports confirming their safety and biocompatibility.36,37 Most importantly, systemic administration of PEG-PCL-based polymeric nanoparticles was reported to efficiently deliver hydrophobic payloads to subcutaneous and orthotopic cancer tumors while preventing their leaching into systemic circulation.36,37 Furthermore, PEG-PCL-based carriers are simple to prepare and characterized by a uniform size distribution, extended shelf life, and batch-to-batch reproducibility.36,37 All of these features are critical requirements for clinical translation of nanomedicines. The CoMn-IONP nanoclusters were prepared by using our previously developed solvent evaporation approach for transformation of hydrophobic entities into water-soluble PEG-PCL-based polymeric nanoplatforms.37 Our previous reports confirm that this highly reproducible approach results in PEG-PCL-based polymeric nanoparticles with monodispersed size distribution as indicated by a polydispersity index (PDI) of ≤ 0.1.36,37 Of note, PDI values of ≤ 0.1 specify unimodal distribution of nanoparticles. Briefly, this approach is based on solubilizing hydrophobic entities (e.g., oleic acid-coated CoMn-IONP) and non-water soluble PEG−PCL copolymer in THF followed by the addition of water to produce an emulsion. After evaporation of THF, the amphiphilic PEG-PCL molecules, which consist of hydrophilic 5 kDa poly(ethylene glycol) (PEG) and hydrophobic 10 kDa poly(ε-caprolactone) (PCL) blocks, self-assemble in aqueous solution to form nanoparticles with a hydrophobic PCL core suitable for loading multiple hexagon-shaped CoMn-IONP (Figure 1). TEM images reveal that CoMn-IONP form clusters within a single PEG-PCL nanoparticle (Figure 3A, inset). The final nanoclusters have a hydrodynamic size of 78.67 ± 0.49 nm (Figure 3A) with a monodispersed size distribution (PDI = 0.113), and a slightly negative charge (−2.64 ± 0.34 mV). The obtained size of nanoclusters was achieved by dissolving the appropriate concentrations of CoMn-IONP (1.33 mg Fe/mL) and PEG–PCL (1.67 mg/mL) in 6 mL of THF. The nanoclusters are characterized by an extended shelf life, which further indicates their translational potential: no noticeable change in nanoparticle size distribution and surface charge was detected over the tested 12-week period (Figure 3A).
Figure 3.
(A) Size distribution of CoMn-IONP nanoclusters tested by DLS before and after storage for 12 weeks at room temperature. Inset: TEM image of CoMn-IONP nanoclusters. (B) Heating profiles of CoMn-IONP nanoclusters, individual CoMn-IONP and IONP nanoclusters in water (1 mg Fe/mL) subjected to AMF (420 kHz, 26.9 kA/m).
As a control, we used the same approach to prepare nanoclusters of commercially available, spherical IONP with similar parameters (size: 89.52 ± 1.17 nm (Figure S3B, C); PDI: 0.104; surface charge: −5.40 ± 0.83 mV). We demonstrated that the developed CoMn-IONP nanoclusters in aqueous solution have a significantly higher heating efficiency upon exposure to AMF (f = 420 kHz; H = 26.9 kA/m) than the nanoclusters of spherical IONP (IONP nanoclusters). Figure 3B demonstrates that CoMn-IONP nanoclusters increased the solution temperature by 40 °C within 10 min (red curve), while the temperature increase mediated by nanoclusters of spherical IONP (black curve) was only 20 °C under identical conditions. It was calculated that the SAR value (f = 420 kHz; H = 26.9 kA/m) of CoMn-IONP nanoclusters (1237.0 W/g) in aqueous solution is 3.2 times higher than that of IONP nanoclusters (390.4 W/g). Previous reports suggest that clustering of magnetic nanoparticles produces strong magnetic dipole-dipole attractions between the nanoparticles that result in enhanced magnetic anisotropy at small cluster sizes, and helps to delay the relaxation time of magnetic moment, thereby maximizing their heating efficiency.14,24,31,38 We also confirmed that CoMn-IONP nanoclusters exhibit enhanced heating efficiency when compared to their individual nanoparticles (Figure 3B, navy curve). The SAR values (f = 420 kHz; H = 26.9 kA/m) of individual CoMn-IONP and IONP dispersed in aqueous solution are only 997.2 W/g and 364.5 W/g, respectively. To obtain water-dispersed individual nanoparticles, a previously reported ligand exchange method was employed.39 Briefly, dispersions of oleic acid-coated CoMn-IONP and IONP in THF were mixed with a solution of 2,3-Dimercaptosuccinic acid (DMSA) dissolved in DMSO and stirred for 24 h to allow the ligand exchange. Afterward, the particles were precipitated by centrifugation and re-dispersed in water. The TEM imaging revealed that individual nanoparticles with an average size of 18.94 ± 3.91 nm were obtained (Figure S6A). The size distribution of the prepared individual nanoparticles in aqueous solution was measured by DLS and validated by comparison to water-dispersed individual nanoparticles with 15 nm iron oxide core that were obtained from Ocean Nanotech (Figure S6B).40
Of note, the measured SAR values (f = 420 kHz; H = 26.9 kA/m) of the individual oleic acid coated CoMn-IONP (1718.0 W/g) and IONP (475.3 W/g) are higher than the SAR values (f = 420 kHz; H = 26.9 kA/m) of the same CoMn-IONP (997.2 W/g) and IONP (364.5 W/g) transferred to water through oleic acid substitution with DMSA. This discrepancy could be related to the fact that the heating efficiencies of oleic acid and DMSA-coated nanoparticles were measured in different media, displaying different viscosities: THF (lower viscosity, 0.48 cP at 25 °C) and water (higher viscosity, 0.89 cP at 25 °C). The previous studies revealed a decrease in the SAR values with increasing solvent viscosity.33,41 Therefore, it is important to compare the heating efficiency of nanoparticles measured in the same medium. Our data confirmed that the SAR values (f = 420 kHz; H = 26.9 kA/m) of water-dispersed nanoclusters of CoMn-IONP (1237.0 W/g) and spherical IONP (390.4 W/g) are higher than SAR values (f = 420 kHz; H = 26.9 kA/m) of individual CoMn-IONP (997.2 W/g) and IONP (364.5 W/g) dispersed in water. Of note, a large value of SAR is crucial for the use of nanoparticles for systemically delivered magnetic hyperthermia because it allows a smaller dose of nanoparticles to be injected to achieve the desirable intratumoral temperature.
To validate the observed trend in the SAR values of nanoclusters and individual nanoparticles, we also employed oleic acid-coated IONP synthesized in our lab for use in preparation of corresponding nanoclusters (size: 80.10 ± 0.25 nm (Figure S4C); PDI: 0.180; surface charge: −2.48 ± 0.19 mV) and individual, water-dispersed IONP. The obtained data (Figure S4D) suggest the prepared nanoclusters (SAR: 493.8 W/g) demonstrate higher heating efficiency than their individual nanoparticles dispersed in aqueous solution (SAR: 391.3 W/g) under the same experimental conditions (f = 420 kHz; H = 26.9 kA/m). It was also confirmed that the SAR value of these nanoclusters (493.8 W/g) is 2.5 times lower than that of CoMn-IONP nanoclusters (1237.0 W/g). Finally, the SAR value (f = 420 kHz; H = 26.9 kA/m) of the individual oleic acid coated IONP (586.2 W/g) measured in THF is higher than the SAR value (f = 420 kHz; H = 26.9 kA/m) of the same DMSA-coated IONP (391.3 W/g) dispersed in water.
In addition, the heating efficiency of all nanoclusters was evaluated upon exposure to AFM with the field−frequency product of 4.5 × 109 (f = 420 kHz; H = 10.6 kA/m, Figure S5B). The SAR value of CoMn-IONP nanoclusters (732.4 W/g) is 11.8 and 3.3 times higher than that of nanoclusters of commercially available IONP (61.9 W/g) and IONP prepared in our lab (224.3 W/g), respectively.
In vitro characterization of the developed nanoclusters
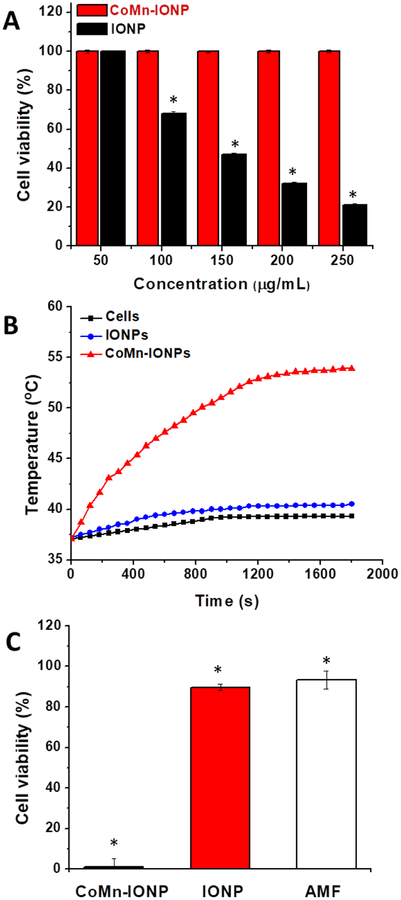
In vitro studies revealed that the developed CoMn-IONP nanoclusters are non-toxic at high concentrations (~1000 μg/mL), efficiently deliver their payload into ovarian cancer cells and exhibit significant heating and therapeutic efficacy. ICP-MS measurements indicated that 14.04 ± 0.02 pg of iron was delivered to each cell as a result of incubation of ES-2 human ovarian cancer cells with CoMn-IONP nanoclusters at an iron concentration of 100 μg/mL for 24 h. The detected cellular internalization efficiency of the developed non-targeted CoMn-IONP nanoclusters is comparable with that of the previously reported iron oxide nanoparticles equipped with cancer-targeted peptides.42 Despite their high internalization efficiency, the nanoclusters do not compromise the viability of cancer cells at iron concentrations up to 250 μg/mL (Figure 4A), and cell viability only decreased by 10–25% at significantly high concentrations (300–1000 μg/mL) (Figure S7A).
Figure 4.
(A) Viability of ES-2 cells incubated for 24 h with different concentrations of CoMn-IONP and IONP nanoclusters (50–250 μg Fe/mL). *p < 0.05 when compared with non-treated cells. (B) Representative heating profiles of ES-2 cells incubated with media only (cells only), CoMn-IONP and IONP nanoclusters (50 μg Fe/mL) for 24 h, and subjected to AMF (420 kHz, 26.9 kA/m). (C) The viability of ES-2 cells incubated with CoMn-IONP nanoclusters, IONP nanoclusters (50 μg Fe/mL), and medium (AMF) for 24 h and exposed to AMF (420 kHz, 26.9 kA/m) for 30 min. *p < 0.05 when compared with untreated cells.
It was also validated that the developed CoMn-IONP nanoclusters are not toxic to non-malignant, human embryonic kidney cells (HEK-293) at iron concentrations up to 300 μg/mL (Figure S8A). Finally, in vitro micronucleus assay (formation of micronuclei) was performed to evaluate the genotoxicity of CoMn-IONP nanoclusters. Compared to the negative control, CoMn-IONP nanoclusters did not induce the formation of more micronuclei (Figure S8B), suggesting no genotoxicity observed at the tested concentration.
In contrast to CoMn-IONP nanoclusters, nanoclusters of commercially available IONP considerably reduced cancer cell viability at concentrations above 50 μg/mL (Figure 4A). Of note, the developed hexagon-shaped CoMn-IONP and commercially available IONP have similar size and surface coating. Moreover, the prepared nanoclusters of CoMn-IONP and commercially available IONP are characterized by similar hydrodynamic size and surface charge. Therefore, we speculate that the observed discrepancy in toxicity profiles of the tested nanoclusters could be due to discrepancies in methods of nanoparticle preparation and purification used in our and Ocean NanoTech laboratories. This speculation can be further supported by the fact that the nanoclusters of IONP (prepared in our lab) do not compromise the viability of ES-2 ovarian cancer cells and human kidney cells (HEK-293) at iron concentrations up to 300 μg/mL (Figure S8C, D).
To evaluate and compare the heating efficiencies of both CoMn-IONP and IONP nanoclusters, ES-2 cells were incubated with nanoclusters at a non-toxic concentration of 50 μg/mL for 24 h, and formed cell pellets were exposed to AMF at a magnetic field frequency of 420 kHz and strength of 26.9 kA/m. The recorded temperature profiles demonstrated that CoMn-IONP nanoclusters increased cellular temperature by 17 °C upon exposure to AMF, reaching 46 °C inside the cell pellets within ~8 min (Figure 4B). In comparison, the IONP nanoclusters were only capable of elevating cellular temperature by 3 °C under identical experimental conditions (Figure 4B).
To evaluate anticancer effect of nanocluster-mediated magnetic hyperthermia, ES-2 cells were incubated for 24 h with CoMn-IONP, and IONP nanoclusters (50 μg Fe/mL), and cell culture media (AMF) and the cells pellets (maintained in 0.1 mL of cell culture media) were exposed to AMF (420 kHz, 26.9 kA/m) for 30 min. Then, the cells were resuspended in media, seeded in 96-well plates at a density of 104 cells/well and were cultured for an additional 48 h. Cell viability measurements further demonstrated that magnetic hyperthermia mediated by CoMn-IONP nanoclusters at a concentration of 50 μg/mL reduced the viability of cancer cells by ~99%, while treatment mediated by IONP nanoclusters under the same experimental conditions was less efficient, resulting in only ~10% reduction of cellular viability (Figure 4C). Of note, the nanoclusters at the tested concentration of 50 μg/mL (Figure 4A) or the applied AMF alone (Figure 4C) did not result in a significant reduction in cell viability. It was also confirmed that the heating and therapeutic efficiencies of magnetic hyperthermia depend on the concentration of the CoMn-IONP nanoclusters (Figure S7B, C). For example, CoMn-IONP nanoclusters incubated with ES-2 cells at a non-toxic Fe concentration of 200 μg/mL can further elevate the cellular temperature by 23 °C and consequently reduced cell viability by ~100 % (Figure S7B, C). We also showed that the desired temperature increase could be achieved by varying AMF strength (Figure S7D), providing the possibility for controlling therapeutic temperature during treatment. Finally, the enhanced heating potency and therapeutic efficiency were also validated for the treatment of the platinum-resistant A2780 human ovarian cancer line (Figure S9).
In vivo evaluation of the developed nanoclusters
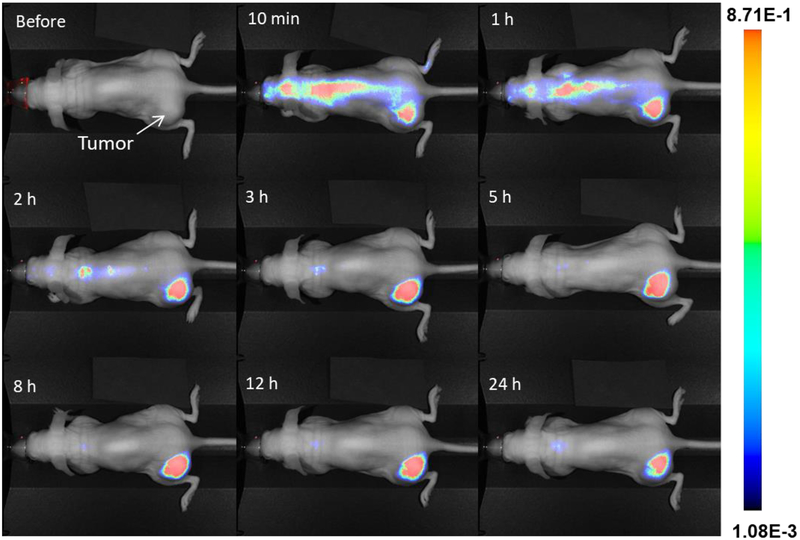
In vivo studies revealed that the CoMn-IONP nanoclusters intravenously (i.v.) injected into nude mice bearing subcutaneous ovarian cancer xenografts of ES-2 cells efficiently accumulated in the tumors. The NIR fluorescence signal generated by the dye (SiNc), encapsulated into the CoMn-IONP nanoclusters, was detected in the tumor as early as ~10 min following intravenous administration and reached its maximum intensity at ~5 h with a gradual decrease at 24 h post-injection (Figure 5 and Figure S10).
Figure 5.
Representative NIR fluorescence images of a live anesthetized mouse with an ES-2 subcutaneous tumor at various time points after i.v. injection of CoMn-IONP nanoclusters loaded with a hydrophobic NIR dye (silicon 2,3-naphthalocyanine bis(trihexylsilyloxide), SiNc).
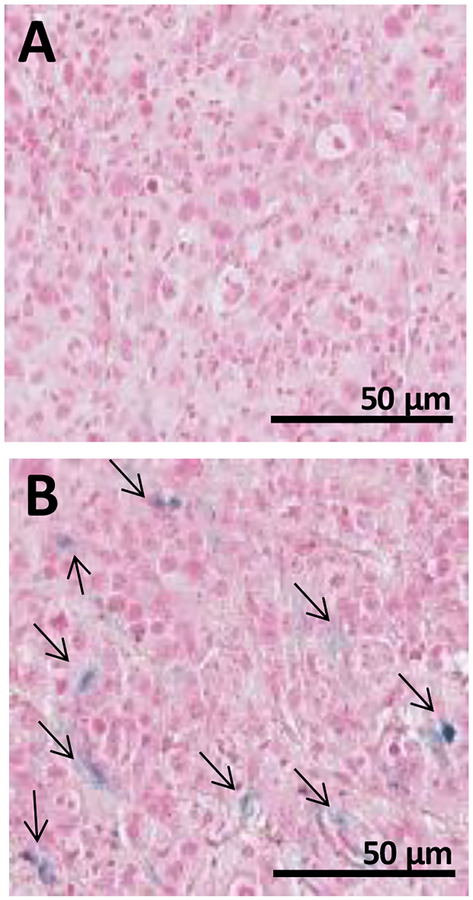
Microscopic analysis of tumor slices, collected and stained with Prussian blue for Fe detection at 12 h post-injection, further confirmed accumulation and penetration of CoMn-IONP nanoclusters in the tumors following systemic administration (Figure 6).
Figure 6.
Prussian blue staining of tumor slices harvested from mice 12 h after i.v. injection with (A) 5% Dextrose and (B) CoMn-IONP nanoclusters (6 mg Fe/kg). Black arrows indicate Prussian blue staining of iron. Scale bar = 50 μm.
Finally, by quantifying the amount of Fe in cancer tissue with ICP-MS, it was determined that 3.5% (26 μg of Fe per gram of tissue) of the injected dose accumulated in the tumor at 12 h post-injection. This value is relatively high based on a Chan et al. review of 117 papers, which reported that a mere 0.7% (median) of the administered nanoparticle dose is found to be delivered to solid tumors.43 In a separate study, Hayashi et al. also reported that the concentration of folate receptor-targeted iron oxide nanoclusters in the tumor was only 1.3 μg Fe per gram of tissue (24 nmol Fe/g) at 24 h post i.v. injection.14 The detected nanocluster concentration was capable of elevating the temperature of the tumor to ~38–39 °C upon exposure to AMF.14
The above-described results suggested that the systemically injected CoMn-IONP nanoclusters efficiently accumulated in the tumors via passive targeting (EPR effect). It is believed that long-circulating nanoparticles leak preferentially into cancer tissue through fenestrated tumor blood vessels and are then retained in the tumor bed due to reduced lymphatic drainage.44 Our nanoclusters’ hydrodynamic size (78.67 nm, Figure 3A) is optimal for minimization of rapid clearance by the kidneys (<10 nm), identification by macrophages (<100 nm), and increased accumulation in cancer tumors through a leaky tumor vasculature (<200 nm).45,46,47 In addition, the PEG-PCL co-polymer introduces a hydrophilic, non-charged PEG layer to the nanocluster surface (Figure 1), which provides slightly negative zeta potential of the nanoparticles (−2.64 mV).37 The PEG layer and neutral/slightly negative surface charge significantly reduce non-specific interaction with plasma proteins, qualities which increase nanoparticle circulation in blood and enhance accumulation in cancer tumors.45,48,49 Finally, our previous reports confirmed that PEG-PCL-based polymeric nanoparticles prevent leaching of hydrophobic payloads into the systemic circulation and efficiently accumulate in subcutaneous and metastatic ovarian cancer tumors after i.v. injections via passive targeting.36 Notably, there is considerable debate in the literature surrounding the existence of the EPR phenomenon in human tumors. For example, Danhier indicated that there is great tumor heterogeneity among and even within separate tumors in patients with regard to the extent of vascular disarray and permeability (e.g., “EPR”ness).50 Leaders in the field including Mark Davis have noted from their clinical data that this EPR phenomenon does occur in human tumors,51 however, it is not as universally present as previously hypothesized.52 The over-enthusiasm for EPR effect, and subsequent backlash on nanoparticle technologies, should not diminish its utility for the subset of patients in which it may exist. There are studies considering companion imaging agents to stratify patient populations where exploiting the EPR phenomenon would be effective.52 Such a patient pre-selection would lead to improved response rates and facilitate the clinical translation of the proposed nanocluster-based magnetic hyperthermia.
By quantifying the amount of Fe in various organs, it was revealed that 6.2 % and 19.5% of the injected dose were found in the spleen and liver at 12 h post injection, suggesting that the developed nanoclusters of CoMn-IONP are cleared from the body by these organs.
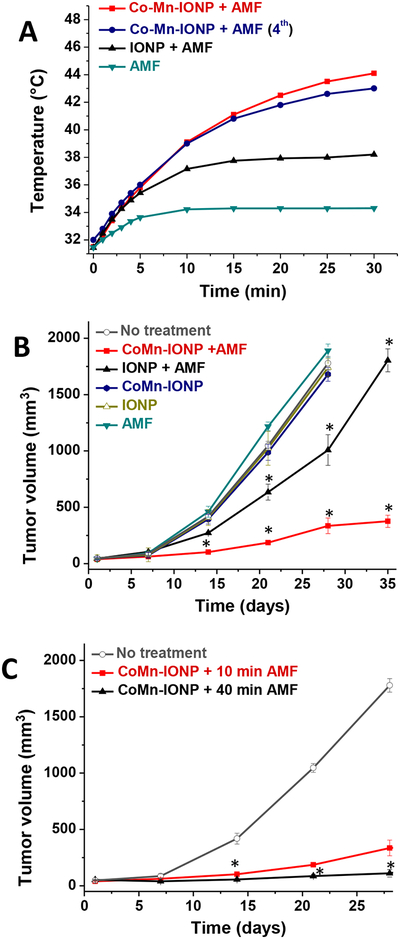
Next, we demonstrated that 12 h after i.v. injection of the CoMn-IONP nanoclusters, the tumors were heated to 44 °C upon exposure to AMF (Figure 7A, red curve). In contrast, the intratumoral temperature measured by a fiber optic probe in mice treated with the same dose of nanoclusters of spherical IONP from Ocean NanoTech reached only 38.5 °C (Figure 7B, black curve). The nanoclusters of IONP from our lab demonstrated slightly better heating efficiency and increased intratumoral temperature to 40.5 °C under the same experimental conditions (Figure S11). The obtained results are in agreement with a previous report demonstrating that i.v. injection of 100 nm clusters consisting of spherical superparamagnetic IONP elevated the temperature of a subcutaneous tumor to ~39 °C upon exposure to AMF.14
Figure 7.
(A) Representative intratumoral temperature profiles during AMF (420 kHz, 26.9 kA/m) exposure of mice injected with a single dose of 5% dextrose (AMF), CoMn-IONP nanoclusters (6 mg Fe/kg) and IONP nanoclusters (6 mg Fe/kg). The navy curve shows the intratumoral temperature during the 4th cycle in a mouse that was treated with CoMn-IONP nanoclusters (6 mg Fe/kg) and AMF once a week for 4 weeks. (B) Tumor growth profiles of mice with ES-2 xenografts after 4 cycles of the following treatments: (i) no treatment; (ii) CoMn-IONP + AMF, mice injected with CoMn-IONP nanoclusters (6 mg Fe/kg) and exposed to AMF for 30 min; (iii) IONP + AMF, mice injected i.v. with IONP nanoclusters (6 mg Fe/kg) and exposed to AMF for 30 min; (iv) CoMn-IONP, mice injected i.v. with CoMn-IONP nanoclusters (6 mg Fe/kg); (v) IONP, mice injected with IONP nanoclusters (6 mg Fe/kg); and (vi) AMF, mice injected with 5% dextrose and exposed to AMF. *p < 0.05 when compared with non-treated animals. (C) Tumor growth profiles of mice with ES-2 xenografts after the following treatments: (i) no treatment; (ii) CoMn-IONP + 10 min AMF, mice injected with CoMn-IONP nanoclusters (6 mg Fe/kg) and exposed to AMF for 10 min when intratumoral temperatures reached 42 °C (total exposure time to AMF 30 min) once a week during four weeks (4 cycles); (iii) CoMn-IONP + 40 min AMF, mice injected with CoMn-IONP nanoclusters (6 mg Fe/kg) and exposed once to AMF for 40 min when intratumoral temperatures reached 42 °C (total exposure time AMF 60 min). *p < 0.05 when compared with CoMn-IONP + 10 min AMF.
Because several cycles of hyperthermia need to be performed during a treatment regimen, we also validated the efficiency of CoMn-IONP nanoclusters to generate the required intratumoral temperature during a 4th weekly treatment (Figure 7A, navy curve). In addition, it was detected that untreated mice exposed to AMF showed little elevation (~2 °C) in tumor temperature (Figure 7A, dark cyan curve).
Of note, Figure 7A shows that the intratumoral temperature prior to AMF exposure (0 h) is ~32 °C. It is presumably related to the fact that mice were maintained under isoflurane anesthesia during exposure to AMF and general anesthesia causes a loss in thermoregulation. The loss of temperature occurs in small rodents due to their high body surface area to volume ratio, and their reliance upon locomotor activity for heat generation.53 A significant number of reports focused on evaluation of anticancer effects of nanoparticle-mediated magnetic hyperthermia in mice revealed that initial temperature of subcutaneous cancer tumors was in the range of 28 – 33 °C.5,12,14,21,33,54 Our previous reports also confirmed that initial temperature of subcutaneous cancer tumors measured in nude mice that were anesthetized and placed on a heating pad (37 °C) was in the range of 31 – 34 °C.36,37
Finally, in vivo studies demonstrated that magnetic hyperthermia mediated by CoMn-IONP nanoclusters significantly inhibited the growth of tumors. Mice bearing subcutaneous xenografts of ovarian ES-2 cancer cells were injected i.v. with CoMn-IONP nanoclusters at a dose of 6 mg Fe/kg, and the whole mouse was exposed to AMF at 12 h post-injection. When intratumoral temperatures reached 42 °C, AMF-induced heating was continued for 10 min (total exposure time to AMF 30 min), and the treatment procedure was repeated once a week for four weeks. Thirty-five days after initiation of treatment, the tumor volume of mice treated with hyperthermia mediated by CoMn-IONP nanoclusters (Figure 7B, red curve) was 5 and 3 times smaller than that of mice treated with hyperthermia generated by nanoclusters of spherical IONP from Ocean Nanotech (Figure 7B, black curve) and nanoclusters of IONP prepared in our lab (Figure S11B, black curve), respectively.
The obtained results also confirmed that cancer growth was not affected by AMF alone (Figure 7B, dark cyan curve) or non-activated nanoclusters (Figure 7B, navy and olive curves). We also demonstrated that the therapeutic efficacy of CoMn-IONP nanocluster-mediated hyperthermia significantly increases with AMF exposure time. The volume of tumor 28 days after treatment with one cycle of magnetic hyperthermia for 40 min was 3 times smaller than that of tumor exposed to 4 cycles of hyperthermia for 10 min (Figure 7C).
It is obvious that the employed mouse with a subcutaneous cancer xenograft is not an ideal model. However, this model was widely used in the previous reports to evaluate the potential of nanoparticles for systemically delivered magnetic hyperthermia.12,14,19 By using this model we were able to prove our hypothesis that the developed nanoclusters efficiently accumulate in cancer tissue after a single intravenous injection at a clinically relevant dose and increase intratumoral temperatures over 42 °C upon exposure to safe AMF. Most importantly, this model allowed monitoring intratumoral temperature in a minimally invasive way during hyperthermia treatment.
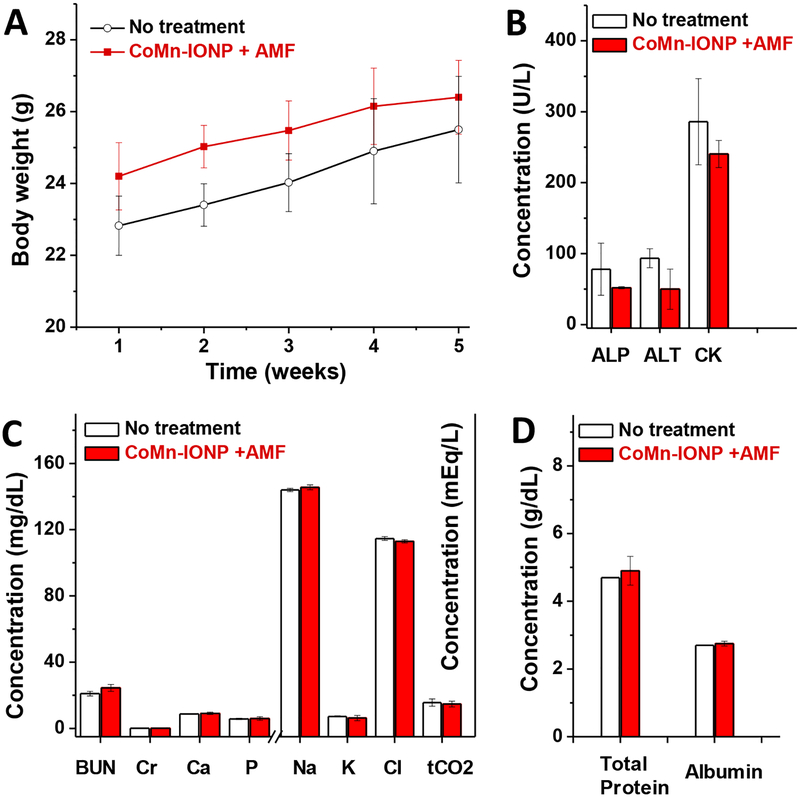
Despite the high intratumoral temperature (44 °C) and strong anticancer effect (Figure 7B), mice did not lose body weight (Figure 8A) or display any signs of toxicity (e.g., appearance, death) after 4 cycles of nanocluster-mediated hyperthermia.
Figure 8.
(A) Changes in body weights of untreated mice and mice treated with 4 cycles of CoMn-IONP nanocluster-mediated hyperthermia. (B-D) Blood levels of biomarkers (alkaline phosphatase (ALP), aminotransferase (ALT), creatine kinase (CK), blood urea nitrogen (BUN) and creatinine (Cr)), blood electrolytes, and proteins in non- and hyperthermia-treated mice.
To evaluate the effect of the treatment on acute liver, renal and muscle toxicity, we measured the concentrations of surrogate biomarkers in the blood for liver function (ALP and ALT), muscle function (CK) (Figure 8B), and kidney function (BUN and Cr) (Figure 8C). In addition, the serum levels of blood electrolytes (Figure 8C) and proteins (Figure 8D) were evaluated as an indicator of major organ toxicity.55 The measured serum levels of blood electrolytes, proteins and surrogate biomarkers in the mice treated with 4 cycles of magnetic hyperthermia were not different than those of non-treated mice. These results show that repeated injections of CoMn-IONP nanoclusters (6 mg Fe/kg) and exposure to the employed AMF (420 kHz; 26.9 kA/m) did not cause any acute toxicity.
The developed nanoclusters are doped with Co, and therefore it is expected that significant leaching of Co ions from these nanoclusters following systemic injection could result in toxic side effects. To address this concern, potential leaching of Co ions following the incubation of CoMn-IONP nanoclusters in complete culture medium at 37 °C for 7 days was evaluated. The obtained results revealed only negligible leaching of Co (< 0.03%) from the developed nanoclusters under these experimental conditions. The translational potential of the developed nanoclusters might also be supported by the results of a recent clinical trial that demonstrated no side effects in people who voluntarily ingested ~ 1.0 mg Co/day (0.080–0.19 mg Co/kg × day) of a commercially available cobalt supplement over a 3-mo period. This study concluded that peak cobalt whole blood concentrations ranging between 9.4 and 117 μg/L were not associated with clinically significant changes in basic hematologic and clinical variables.56
It is a known fact that AMF with specific parameters (frequency (f) and magnetic field strength (H)) can cause excessive non-selective Joule heating of healthy tissue due to the generation of eddy currents.57 To minimize undesired side effects of AMF on healthy tissue during magnetic hyperthermia, Hergt et al. suggested that focused AMF has to be applied to the targeted area of the body and the field−frequency product (H × f) should be ≤ 5 × 109 A m−1 s−1.35 For example, AMF with a field−frequency product up to 1.8 × 109 A m−1 s−1 (f = 100 kHz; H = 18 kA/m) was safely applied to the human brain in multiple 60 min sessions.1,10,58 Notably, the maximum allowed frequency and strength of AMF that can be considered safe and tolerable is not completely agreed upon by the magnetic hyperthermia community, and the main requirement is to minimize non-specific heating to tissue due to eddy currents generated by AMF alone.31,59 Currently, several groups work on the development of different strategies to decrease eddy current heating during magnetic hyperthermia57 and, therefore the suggested upper limit for the product of frequency and field strength may be reconsidered in the future. Consequently, clinical safety studies will be needed for each developed magnetic hyperthermia regimen achieved with the specific AMF system. Although the product of frequency and field strength (f = 420 kHz; H = 26.9 kA/m, H × f = 11.3 × 109 A m−1 s−1) used in the current studies was 2.3 times higher than the previously suggested upper limit (≤ 5 × 109 A m−1 s−1), both in vitro and in vivo studies validated the safety and tolerability of the selected AMF parameters. The recorded temperature profiles suggest that AMF alone only increases intratumoral and rectal temperatures by 2.3 °C° and 1.5 °C, respectively (Figure 7A, dark cyan curve and Figure S12). In a clinical trial, median rectal temperature of prostate cancer patients was 39.8 °C during magnetic hyperthermia treatment and the applied AMF was tolerable without significant side effects.9 The obtained results also confirmed that AMF alone did not significantly affect cell viability in vitro (Figure 4C) or tumor growth in animal studies (Figure 7B). It was further documented that after whole-body exposure to the selected AMF for four sessions (30 min each), mice displayed no signs of discomfort or toxicity. Finally, it was revealed that the employed AMF did not cause any major organ toxicity (Figure 8B–D).
Of note, the current report demonstrates proof of concept that PEG-PCL-based nanoclusters of magnetic nanoparticles with high heating efficiency can increase intratumoral temperatures over 42 °C following a single intraveneous injection at a clinically relevant dose and upon exposure to safe AMF. However, the developed nanoclusters could potentially be optimized in order to provide similar or even higher heating efficiency in vivo upon exposure to AFM with the previously suggested parameters (H × f = 5 × 109 A m−1 s−1). For example, one strategy might be to increase the nanoclusters accumulation and retention in cancer tumors by modifying their surface with cancer targeting moieties. The previous reports by Chen et al. concluded that various targeted nanoparticles outperformed non-targeted nanoparticles, affording ≥2 times higher delivery efficiency to tumors.43,60,61 It was also revealed that accumulation in the RES organs (e.g., liver) was significantly lower for the targeted nanoparticles.61 The non-targeted nanoclusters reported herein show significantly higher accumulation in tumors after intraveneous injection (3.5% ID (injected dose)) relative to that reported by Chen et al. (< 1% ID). If the targeting moieties could provide ~2 times higher delivery of these nanoclusters to tumors while limiting their accumulation in healthy tissue, their ability to generate high intratumoral temperature upon exposure to AMF with low parameters will be substantially improved. Another strategy could be to further increase the SAR of the current CoMn-IONP that are clustered in the hydrophobic interior of a PEG-PCL-based delivery vehicle. It could be achieved by modifying CoMn-IONP core size, shape, composition, etc. Moreover, hydrophobic nanoparticles with a superior SAR that will be developed in the future could also be encapsulated into the reported PEG-PCL-based nanoclusters.
CONCLUSIONS
In summary, we have designed and synthesized efficient hexagon-shaped iron oxide nanoparticles doped with Co and Mn and confirmed that they exhibit significantly higher saturation magnetization and heating efficiency in comparison with conventional spherical iron oxide nanoparticles. The developed nanoparticles clustered inside a PEG-PCL-based nanocarrier were found to be safe and efficiently accumulated in cancer tumors following intraveneous administration. Moreover, we demonstrated that systemically delivered nanoclusters elevate the intratumoral temperature up to 44 °C in the presence of a safe AMF, and the required temperatures could be achieved after repeated injections of the nanoclusters. Finally, animal studies validated that the nanocluster-mediated hyperthermia efficiently inhibited the growth of subcutaneous ovarian tumors. These nanoparticles, and their capability to achieve therapeutically relevant temperatures in tumors following a single intraveneous injection at low dose, will allow the scientific community to exploit the full potential of magnetic hyperthermia for treatment of cancer, either alone or in combination with other therapies including radiation, chemotherapy, or immunotherapy. To further advance this technology, it will be important in future studies to evaluate the therapeutic efficacy of the developed nanoclusters in orthotopic animal models with deep-seated primary and metastatic tumors. In addition, to minimize non-specific heating of healthy tissues due to eddy currents, there is a need to optimize current, or develop new, AMF systems that can deliver the focused AMF to a small part of the body where the cancer tumor is located.
EXPERIMENTAL SECTION
Materials
Iron (III) acetylacetonate ((Fe(acac)3) was purchased from ACROS Organics (Fair Lawn, NJ). Oleylamine was purchased from Sigma-Aldrich (St. Louis, MO). Oleic acid, manganese (II) chloride tetrahydrate (MnCl2·4H2O), and cobalt (II) chloride hexahydrate (CoCl2·6H2O) were obtained from Alfa Aesar (Ward Hill, MA). Oleic acid-coated iron oxide nanoparticles (IONP, 15 nm) dispersed in tetrahydrofuran (THF) and iron oxide nanoparticles with carboxylic acid were obtained from Ocean NanoTech (San Diego, CA). Trioctyl ether and n-octyl ether were purchased from Tokyo chemical industry Co. (Tokyo, Japan). m-PEG–PCL (methoxy poly(ethylene glycol)-b-poly(ε-caprolactone), MW: 5k-10k) was obtained from Advanced Polymer Materials Inc. (Montreal, Canada). SiNc (silicon 2,3-naphthalocyanine bis(trihexylsilyloxide)) was purchased from Sigma-Aldrich (Milwaukee, WI). All other chemicals and supplies were obtained from VWR International (Radnor, PA).
Synthesis of cobalt- and manganese-doped iron oxide nanoparticles
Iron oxide nanoparticles doped with cobalt and manganese (CoMn-IONP) were synthesized by a modified thermal decomposition method.28,62,63 First, cobalt (II) chloride hexahydrate (CoCl2·6H2O, 3.25 mmol) and iron (III) acetylacetone (Fe(acac)3, 5.00 mmol) were added to a solution containing oleic acid (2.0 mL), oleylamine (2.0 mL) and trioctyl ether (20 mL). The obtained reaction mixture was placed in a 250 mL three-neck round-bottom flask and heated at 300 °C under nitrogen flow and vigorous stirring. After 1 h, the mixture was cooled to room temperature and the product was precipitated with ethanol (30 mL), followed by centrifugation at 7000 rpm for 30 min. The obtained precipitate was re-dispersed in hexane (10 mL), and the purification process was repeated three times to produce black cobalt ferrite (CoFe2O4) nanoparticles. In the next step, manganese chloride (MnCl2·4H2O, 3.25 mmol) and Fe(acac)3 (5.00 mmol) were placed in a 250 mL three-neck round-bottom flask containing oleic acid (2.0 mL), oleylamine (2.0 mL) and trioctyl ether (20 mL). After addition of freshly made CoFe2O4 nanoparticles suspended in 10 mL of hexane (8 mg/mL), the reaction mixture was heated at 360 °C for 1 h under stirring and nitrogen flow. Subsequently, the resulting reaction mixture was cooled to room temperature, ethanol (30 mL) was added, followed by centrifugation at 7000 rpm for 30 min, and the obtained precipitate was re-dispersed in hexane (10 mL). The purification procedure was repeated three times. The obtained CoMn-IONP were dried at 70 °C for 12 h.
Nanoparticle characterization
The shape and size of the synthesized CoMn-IONP and IONP were examined using an FEI Tecnai™ Spirit transmission electron microscopy (TEM) system. The chemical composition and distribution of elements (Fe, Co, Mn) in a single CoMn-IONP was evaluated by energy dispersive x-ray spectroscopy (EDX) using an FEI 80–300 kV Titan. The magnetization curves of tested nanoparticles were determined with a homemade vibrating magnetometer at room temperature.42 The heating efficiency of nanoparticles in the presence of an alternating magnetic field generated by an induction heating system (MSI Automation, Wichita, KS) was evaluated, and the corresponding specific absorption rates (SAR) were calculated according to previously reported procedures.42 Detailed characterization procedures are provided in Supporting Information.
Nanocluster preparation
Nanoclusters loaded with either CoMn-IONP or IONP were prepared by a solvent evaporation method.36,37,64 Briefly, CoMn-IONP or IONP suspended in 1 mL of THF (8 mg Fe/mL) were added to 5 mL of THF solution containing m-PEG–PCL (2 mg/mL) followed by constant stirring for 20 min. Next, 6 mL of 5% dextrose solution in water was added to the reaction mixture and stirred for 20 min. THF was removed by using a rotovap. To remove any non-encapsulated, hydrophobic CoMn-IONP and non-soluble PEG-PCL molecules, the prepared aqueous solution was centrifuged at 1,000 g for 5 min and filtered through a 0.2 μm cellulose acetate filter (cellulose acetate, VWR International, Radnor, PA). Of note, the employed methoxy PEG–PCL diblock copolymer consists of hydrophilic 5 kDa PEG and hydrophobic 10 kDa PCL blocks. Due to the high molecular weight of the hydrophobic PCL block, the copolymer has negligible water solubility and therefore free PEG-PCL molecules cannot be dissolved in the final aqueous solution of nanoclusters. The certificate of analysis provided by the manufacturer and the corresponding experiment in our lab validated that the employed PEG-PCL is insoluble in water.
It is possible, however, that some PEG–PCL molecules could self-assemble into empty nanoparticles (without CoMn-IONP) during the preparation of nanoclusters. To remove any empty PEG-PCL nanoparticles, the filtered solution was placed on a strong magnet (SuperMag Separator, Ocean NanoTech) for 6 h and PEG-PCL nanoparticles loaded with magnetic CoMn-IONP (nanoclusters) were separated and re-dispersed in 5% dextrose. The DLS spectra of CoMn-IONP nanoclusters and separately prepared empty PEG-PCL nanoparticles suggest that empty PEG-PCL nanoparticles were not present in the final nanocluster solution (Figure S13). High-performance liquid chromatography (HPLC) was also used to verify that THF (solvent) was not present in the final nanocluster solution (Figure S14).
Nanocluster characterization
The hydrodynamic diameter, zeta potential, and polydispersity index (PDI) of the obtained nanoclusters were evaluated by dynamic light scattering (DLS, ZetaSizer NanoSeries, Malvern, UK).36,37,64 The morphology and size of the nanoclusters were determined by transmission electron microscopy (TEM). The iron content in the obtained nanoclusters was determined using a colorimetric ferrozine-based assay according to our previously reported procedure.42 The heating efficiency of the obtained nanoclusters in the presence of AMF was measured as described above and in Supporting Information.
In vitro studies
A2780/CDDP human ovarian carcinoma and ES-2 human ovarian clear cell carcinoma cell lines were purchased from Developmental Therapeutics Core (Northwestern University, IL) and ATCC (Manassas, VA) and cultured in RPMI 1640 medium supplemented with fetal bovine serum (10%) and penicillin-streptomycin (1%).
To evaluate the cytotoxicity of the developed nanoclusters, the employed cancer cells were seeded in 96-well plates at a density of 10×103 cells/well and allowed to grow for 24 h. Culture medium was removed, and the cells were treated for 24 h with 100 μL of cell culture medium containing different concentrations (50–1000 μg Fe/mL) of CoMn-IONP and IONP nanoclusters. After treatment, cell viability was evaluated using a modified Calcein AM assay according to a previously published protocol.65
To evaluate cellular internalization efficiency, cancer cells seeded in T-25 cell culture flasks were incubated with 6 mL of media containing CoMn-IONP nanoclusters (100 μg Fe/mL) for 24 h. Afterward, cells were collected, counted, rinsed with DPBS in pre-cleaned Sarstedt digestion tubes, and 106 cells were digested with 150 μL of 75% HNO3 (trace metal grade, Fisher Scientific, Fair Lawn, NJ) by incubating for 2 h at 90 °C. Finally, 850 μL of 1% HNO3 was added to each tube, and the amount of Fe in cancer cells was quantified by inductively coupled plasma mass spectrometry (ICP-MS) at the Oregon Health & Science University (OHSU) Elemental Analysis Core.
Evaluation of the heating efficiency of nanoclusters in vitro was accomplished according to our previously published protocol.42 Briefly, after incubation with different non-toxic concentrations of CoMn-IONP nanoclusters (50, 100, 150, 200 μg Fe/mL) and IONP nanoclusters (50 μg Fe/mL) for 24 h, cancer cells were washed with DPBS, detached by 0.25% trypsin/EDTA and resuspended in cell culture media prior to counting. After counting, a portion of the cell suspension containing 5×106 cells were centrifuged at 1000 rpm for 5 min to form the cell pellet. The pellets were maintained in a constant volume of 0.1 mL of cell culture media in a 0.5 mL microcentrifuge tube. Samples were then placed in the center of a 6-turn copper coil (inner diameter: 40 mm) and exposed to AMF (420 kHz, 26.9 kA/m) for 30 min. The temperature changes were measured by placing a fiber optic probe (Neoptix Inc., QC, Canada) inside the cell pellets. The water jacket inside the coil was maintained at ~37 °C, and the samples were allowed to equilibrate to this temperature before exposure to AMF. Controls were carried out with non-treated cells exposed to AMF alone and with cells exposed to no field.
To evaluate the anticancer effect of nanocluster-mediated magnetic hyperthermia, cancer cells were incubated with CoMn-IONP nanoclusters (50–200 μg Fe/mL) and IONP nanoclusters (50 μg Fe/mL) for 24 h and the formed cell pellets were exposed to AMF (420 kHz, 26.9 kA/m) for 30 min as described above. The cell pellets were maintained during exposure to AMF in a constant volume of 0.1 mL of cell culture media in a 0.5 mL microcentrifuge tube. Then, the cells were resuspended in media and seeded in 96-well plates at a density of 10×103 cells/well and cultured for an additional 48 h. Finally, cell viability was assessed using a Calcein AM assay.
In vivo studies
All animal studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Oregon State University and Oregon Health and Science University.
Development of a subcutaneous xenograft mouse model of human ovarian cancer
Three million ES-2 cells re-suspended in 50 μL of RPMI 1460 medium, along with 50 μL of Matrigel, were injected subcutaneously into the flank of 5-week-old female nude mice purchased from Charles River Laboratories (Wilmington, MA, USA). The tumors were measured by a caliper every other day, and animal experiments started when tumors reached 50 mm3.
Evaluation of nanoclusters biodistribution
Five mice bearing subcutaneous xenografts of ES-2 ovarian cancer cells were injected via tail vein with 150 μL of CoMn-IONP nanoclusters at a dose of 6 mg Fe/kg. The nanoclusters distribution in the mouse body after injection was monitored using the Pearl® Impulse Small Animal Imaging System at various time points after injection. For this purpose, nanoclusters were loaded with the near-infrared (NIR) fluorescent dye SiNc (see Supporting Information). White light and fluorescence images of each mouse were recorded and overlaid by the imaging system.
To confirm the accumulation of CoMn-IONP nanoclusters in tumors following i.v. injection, tissue samples dissected at 12 h after administration were stained with Prussian blue (iron) according to a previously published procedure (see Supporting Information).66
To quantify the amount of nanoclusters accumulated in tumors, the collected tissues were weighed and digested with 200 mL of 70% HNO3 for 2 h at 90 °C. The iron (Fe) concentrations in the tested samples were determined using ICP-MS.
Evaluation of the therapeutic efficiency of nanoclusters-mediated hyperthermia
Mice bearing subcutaneous ES-2 xenografts were randomly divided into six groups with five animals per group. To evaluate the therapeutic efficacy of magnetic hyperthermia mediated by the developed nanoclusters, mice in groups 1 and 2 were injected intravenously (i.v.) with CoMn-IONP nanoclusters or IONP nanoclusters (5% dextrose solution), respectively, at a dose of 6 mg Fe/kg. Twelve hours following injection, animals were anesthetized using isoflurane in an induction chamber and maintained under anesthesia during treatment by isoflurane administration via a face mask. A fiber optic temperature probe was inserted into the center of the tumor following our previously reported procedure.67 Afterward, each mouse was centered inside the induction coil, and temperature measurements were taken during animal exposure to AMF (420 kHz, 26.9 kA/m). When the intratumoral temperature reached 42 °C, AMF-induced heating was continued for 10 min (total exposure time to AMF 30 min). Each group was treated once per week for 4 weeks with appropriate formulations. The tumor size in each group was measured every seven days for 5 weeks and compared to the tumor size of mice injected once per week for 4 weeks with 5% dextrose (group 3), CoMn-IONP nanoclusters (group 4), and IONP nanoclusters (group 5) without AMF exposure. To evaluate whether AMF alone increases the tumor temperature and demonstrates any anticancer effect, mice injected with 5% dextrose (group 6) were exposed to AMF once per week for 4 weeks as described above. To evaluate the influence of AFM exposure time on the therapeutic efficacy of CoMn-IONP nanoclusters-mediated hyperthermia, five mice were injected i.v. with CoMn-IONP nanoclusters at a dose of 6 mg Fe/kg. Twelve hours following injection, each mouse was centered inside the induction coil and exposed to AMF (420 kHz, 26.9 kA/m). When the intratumoral temperature reached 42 °C, AMF-induced heating was continued for 40 min (total exposure time to AMF 60 min).
To assess potential side effects caused by nanocluster-mediated hyperthermia, blood and organs were collected post-euthanasia and examined at the Oregon State University veterinary diagnostic laboratory.
Statistical analysis
The data were analyzed using descriptive statistics and presented as mean value ± standard deviation (SD) from 3–5 separate measurements. Comparisons among groups were executed using independent samples Student’s t-tests. Differences between groups were considered statistically significant at p < 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH/NBIB (1R15EB020351-01A1), the College of Pharmacy at Oregon State University (OSU), Najran University, and National Center for Advancing Translational Sciences of NIH (KL2 TR002370). The funding sources had no involvement in the collection, analysis, and interpretation of the data or in the decision to submit the article for publication. Electron microscopy was performed using the Multiscale Microscopy Core (MMC) at Oregon Health & Science University with technical support from the (OHSU)-FEI Living Lab and the Center for Spatial Systems Biomedicine (OCSSB). ICP-MS measurements were performed by M. Ralle at the OHSU Elemental Analysis Core with partial support from NIH core grant S10RR025512.
Footnotes
Supporting Information
Additional experimental details on synthesis and characterization of the developed nanoparticles are given. Figures S1–S14 provide more details on characterization of IONP in solution and studies of CoMn-IONP and IONP in vitro and in vivo.
REFERENCES
- (1).Luo S; Wang LF; Ding WJ; Zhou MJ; Jin HK; Su SF; Ouyang WW Clinical Trials of Magnetic Induction Hyperthermia for Treatment of Tumours. OA Cancer 2014, 2, 2. [Google Scholar]
- (2).Torres-Lugo M; Rinaldi C Thermal Potentiation of Chemotherapy by Magnetic Nanoparticles. Nanomedicine 2013, 8, 1689–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Deatsch AE; Evans BA Heating Efficiency Efficiency In Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater 2014, 354, 163–172. [Google Scholar]
- (4).Kumar CS; Mohammad F Magnetic Nanomaterials for Hyperthermia-Based Therapy and Controlled Drug Delivery. Adv. Drug Delivery Rev 2011, 63, 789–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sato I; Umemura M; Mitsudo K; Fukumura H; Kim JH; Hoshino Y; Nakashima H; Kioi M; Nakakaji R; Sato M; Fujita T; Yokoyama U; Okumura S; Oshiro H; Eguchi H; Tohnai I; Ishikawa Y Simultaneous Hyperthermia-Chemotherapy with Controlled Drug Delivery Using Single-Drug Nanoparticles. Sci. Rep 2016, 6, 24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shetake NG; Balla MMS; Kumar A; Pandey BN Magnetic Hyperthermia Therapy: An Emerging Modality of Cancer Treatment in Combination with Radiotherapy. J. Radiat. Cancer Res 2016, 7, 13–17. [Google Scholar]
- (7).Toraya-Brown S; Sheen MR; Zhang P; Chen L; Baird JR; Demidenko E; Turk MJ; Hoopes PJ; Conejo-Garcia JR; Fiering S Local Hyperthermia Treatment of Tumors Induces CD8(+) T Cell-Mediated Resistance Against Distal and Secondary Tumors. Nanomedicine 2014, 10, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Moy AJ; Tunnell JW Combinatorial Immunotherapy and Nanoparticle Mediated Hyperthermia. Adv. Drug Delivery Rev 2017, 114, 175–183. [DOI] [PubMed] [Google Scholar]
- (9).Johannsen M; Gneveckow U; Taymoorian K; Thiesen B; Waldofner N; Scholz R; Jung K; Jordan A; Wust P; Loening SA Morbidity and Quality of Life During Thermotherapy Using Magnetic Nanoparticles in Locally Recurrent Prostate Cancer: Results of a Prospective Phase I Trial. Int. J. Hyperthermia 2007, 23, 315–323. [DOI] [PubMed] [Google Scholar]
- (10).Maier-Hauff K; Rothe R; Scholz R; Gneveckow U; Wust P; Thiesen B; Feussner A; von Deimling A; Waldoefner N; Felix R; Jordan A Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neuro-Oncol 2007, 81, 53–60. [DOI] [PubMed] [Google Scholar]
- (11).Wust P; Gneveckow U; Johannsen M; Bohmer D; Henkel T; Kahmann F; Sehouli J; Felix R; Ricke J; Jordan A Magnetic Nanoparticles for Interstitial Thermotherapy--Feasibility, Tolerance and Achieved Temperatures. Int. J. Hyperthermia 2006, 22, 673–685. [DOI] [PubMed] [Google Scholar]
- (12).Huang HS; Hainfeld JF Intravenous Magnetic Nanoparticle Cancer Hyperthermia. Int. J. Nanomed 2013, 8, 2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hilger I In Vivo Applications of Magnetic Nanoparticle Hyperthermia. Int. J. Hyperthermia 2013, 29, 828–834. [DOI] [PubMed] [Google Scholar]
- (14).Hayashi K; Nakamura M; Sakamoto W; Yogo T; Miki H; Ozaki S; Abe M; Matsumoto T; Ishimura K Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pham HN; Pham TH; Nguyen DT; Phan QT; Le TTH; Ha PT; Do HM; Hoang TMN; Nguyen XP Magnetic Inductive Heating of Organs of Mouse Models Treated by Copolymer Coated Fe3O4 Nanoparticles. Adv. Nat. Sci.: Nanosci. Nanotechnol 2017, 8, 025013. [Google Scholar]
- (16).Kalber TL; Ordidge KL; Southern P; Loebinger MR; Kyrtatos PG; Pankhurst QA; Lythgoe MF; Janes SM Hyperthermia Treatment of Tumors by Mesenchymal Stem Cell-Delivered Superparamagnetic Iron Oxide Nanoparticles. Int. J. Nanomed 2016, 11, 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).RxList https://www.rxlist.com/feraheme-drug.htm#description (accessed March 19, 2019).
- (18).Nguyen KL; Yoshida T; Han F; Ayad I; Reemtsen BL; Salusky IB; Satou GM; Hu P; Finn JP MRI with Ferumoxytol: A Single Center Experience of Safety Across the Age Spectrum. J. Magn. Reson. Imaging 2017, 45, 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xie J; Yan C; Yan Y; Chen L; Song L; Zang F; An Y; Teng G; Gu N; Zhang Y Multi-Modal Mn-Zn Ferrite Nanocrystals for Magnetically-Induced Cancer Targeted Hyperthermia: A Comparison of Passive and Active Targeting Effects. Nanoscale 2016, 8, 16902–16915. [DOI] [PubMed] [Google Scholar]
- (20).Xie J; Zhang Y; Yan C; Song L; Wen S; Zang F; Chen G; Ding Q; Yan C; Gu N High-Performance Pegylated Mn-Zn Ferrite Nanocrystals as a Passive-Targeted Agent for Magnetically Induced Cancer Theranostics. Biomaterials 2014, 35, 9126–9136. [DOI] [PubMed] [Google Scholar]
- (21).Cho M; Cervadoro A; Ramirez MR; Stigliano C; Brazdeikis A; Colvin VL; Civera P; Key J; Decuzzi P Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials 2017, 7, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lartigue L; Hugounenq P; Alloyeau D; Clarke SP; Levy M; Bacri JC; Bazzi R; Brougham DF; Wilhelm C; Gazeau F Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [DOI] [PubMed] [Google Scholar]
- (23).He S; Zhang H; Liu Y; Sun F; Yu X; Li X; Zhang L; Wang L; Mao K; Wang G; Lin Y; Han Z; Sabirianov R; Zeng H Maximizing Specific Loss Power for Magnetic Hyperthermia by Hard-Soft Mixed Ferrites. Small 2018, e1800135. [DOI] [PubMed] [Google Scholar]
- (24).Qu Y; Li J; Ren J; Leng J; Lin C; Shi D Enhanced Magnetic Fluid Hyperthermia by Micellar Magnetic Nanoclusters Composed of MnXZn1-XFe2O4 Nanoparticles for Induced Tumor Cell Apoptosis. ACS Appl. Mater. Interfaces 2014, 6, 16867–16879. [DOI] [PubMed] [Google Scholar]
- (25).Guardia P; Di Corato R; Lartigue L; Wilhelm C; Espinosa A; Garcia-Hernandez M; Gazeau F; Manna L; Pellegrino T Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [DOI] [PubMed] [Google Scholar]
- (26).Noh SH; Na W; Jang JT; Lee JH; Lee EJ; Moon SH; Lim Y; Shin JS; Cheon J Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett 2012, 12, 3716–3721. [DOI] [PubMed] [Google Scholar]
- (27).Wang H; Shrestha TB; Basel MT; Pyle M; Toledo Y; Konecny A; Thapa P; Ikenberry M; Hohn KL; Chikan V; Troyer DL; Bossmann SH Hexagonal Magnetite Nanoprisms: Preparation, Characterization and Cellular Uptake. J. Mater. Chem. B 2015, 3, 4647–4653. [DOI] [PubMed] [Google Scholar]
- (28).Lee JH; Jang JT; Choi JS; Moon SH; Noh SH; Kim JW; Kim JG; Kim IS; Park KI; Cheon J Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol 2011, 6, 418–422. [DOI] [PubMed] [Google Scholar]
- (29).Bauer LM; Situ SF; Griswold MA; Samia AC High-Performance Iron Oxide Nanoparticles for Magnetic Particle Imaging - Guided Hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [DOI] [PubMed] [Google Scholar]
- (30).Ocean NanoTech https://www.oceannanotech.com/products-type/iron-oxide-nanoparticles-5-30nm/iron-oxide-in-organic-solvents/sor-15.html (accessed March 19, 2019).
- (31).Obaidat IM; Issa B; Haik Y Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tong S; Quinto CA; Zhang L; Mohindra P; Bao G Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [DOI] [PubMed] [Google Scholar]
- (33).Jang JT; Lee J; Seon J; Ju E; Kim M; Kim YI; Kim MG; Takemura Y; Arbab AS; Kang KW; Park KH; Paek SH; Bae S Giant Magnetic Heat Induction of Magnesium-Doped Gamma-Fe2O3 Superparamagnetic Nanoparticles for Completely Killing Tumors. Adv. Mater 2018, 30, 1704362. [DOI] [PubMed] [Google Scholar]
- (34).Di Corato R; Espinosa A; Lartigue L; Tharaud M; Chat S; Pellegrino T; Menager C; Gazeau F; Wilhelm C Magnetic Hyperthermia Efficiency in the Cellular Environment for Different Nanoparticle Designs. Biomaterials 2014, 35, 6400–6411. [DOI] [PubMed] [Google Scholar]
- (35).Hergt R; Dutz S Magnetic Particle Hyperthermia - Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater 2007, 311, 187–192. [Google Scholar]
- (36).Li X; Schumann C; Albarqi HA; Lee CJ; Alani AWG; Bracha S; Milovancev M; Taratula O; Taratula O A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy. Theranostics 2018, 8, 767–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Taratula O; Doddapaneni BS; Schumann C; Li X; Bracha S; Milovancev M; Alani A; Taratula O Naphthalocyanine-Based Biodegradable Polymeric Nanoparticles for Image-Guided Combinatorial Phototherapy. Chem. Mater 2015, 27, 6155–6165. [Google Scholar]
- (38).Martinez-Boubeta C; Simeonidis K; Makridis A; Angelakeris M; Iglesias O; Guardia P; Cabot A; Yedra L; Estradé S; Peiró F Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep 2013, 3, 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Salas G; Casado C; Teran FJ; Miranda R; Serna CJ; Morales MP Controlled Synthesis of Uniform Magnetite Nanocrystals with High-Quality Properties for Biomedical Applications. J. Mater. Chem 2010, 22, 21065–21075. [Google Scholar]
- (40).Ocean NanoTech https://www.oceannanotech.com/products-type/iron-oxide-nanoparticles-5-30nm/functionalized-iron-oxide-nanoparticles/carboxyl-iron-oxide-nanoparticles/carboxyl-iron-oxide-nanoparticles-534.html (accessed March 19, 2019).
- (41).Pineiro-Redondo Y; Banobre-Lopez M; Pardinas-Blanco I; Goya G; Lopez-Quintela MA; Rivas J The Influence of Colloidal Parameters on the Specific Power Absorption of PAA-Coated Magnetite Nanoparticles. Nanoscale Res. Lett 2011, 6, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Taratula O; Dani RK; Schumann C; Xu H; Wang A; Song H; Dhagat P; Taratula O Multifunctional Nanomedicine Platform for Concurrent Delivery of Chemotherapeutic Drugs and Mild Hyperthermia to Ovarian Cancer Cells. Int. J. Pharm 2013, 458, 169–180. [DOI] [PubMed] [Google Scholar]
- (43).Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater 2016, 1, 16014. [Google Scholar]
- (44).Maeda H; Nakamura H; Fang J The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging In Vivo. Adv. Drug Delivery Rev 2013, 65, 71–79. [DOI] [PubMed] [Google Scholar]
- (45).Alexis F; Pridgen E; Molnar LK; Farokhzad OC Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Longmire M; Choyke PL; Kobayashi H Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Maruyama K Intracellular Targeting Delivery of Liposomal Drugs to Solid Tumors Based on EPR Effects. Adv. Drug Delivery Rev 2011, 63, 161–169. [DOI] [PubMed] [Google Scholar]
- (48).Duan X; Li Y Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [DOI] [PubMed] [Google Scholar]
- (49).Petros RA; DeSimone JM Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discovery 2010, 9, 615–627. [DOI] [PubMed] [Google Scholar]
- (50).Danhier F To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Controlled Release 2016, 244, 108–121. [DOI] [PubMed] [Google Scholar]
- (51).Clark AJ; Wiley DT; Zuckerman JE; Webster P; Chao J; Lin J; Yen Y; Davis ME CRLX101 Nanoparticles Localize in Human Tumors and Not in Adjacent, Nonneoplastic Tissue after Intravenous Dosing. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Golombek SK; May JN; Theek B; Appold L; Drude N; Kiessling F; Lammers T Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Delivery Rev 2018, 130, 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kersemans V; Gilchrist S; Allen PD; Beech JS; Kinchesh P; Vojnovic B; Smart SC A Resistive Heating System for Homeothermic Maintenance in Small Animals. Magn. Reson. Imaging 2015, 33, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kolosnjaj-Tabi J; Di Corato R; Lartigue L; Marangon I; Guardia P; Silva AK; Luciani N; Clement O; Flaud P; Singh JV, Decuzzi P; Pellegrino T; Wilhelm C; Gazeau F Heat-Generating Iron Oxide Nanocubes: Subtle “Destructurators” of the Tumoral Microenvironment. ACS Nano 2014, 8, 4268–4283. [DOI] [PubMed] [Google Scholar]
- (55).Shah VM; Nguyen DX; Alfatease A; Bracha S; Alani AW Characterization of Pegylated and Non-Pegylated Liposomal Formulation for the Delivery of Hypoxia Activated Vinblastine-N-Oxide for the Treatment of Solid Tumors. J. Controlled Release 2017, 253, 37–45. [DOI] [PubMed] [Google Scholar]
- (56).Tvermoes BE; Unice KM; Paustenbach DJ; Finley BL; Otani JM; Galbraith DA Effects and Blood Concentrations of Cobalt after Ingestion of 1 mg/d by Human Volunteers for 90 d. Am. J. Clin. Nutr 2014, 99, 632–646. [DOI] [PubMed] [Google Scholar]
- (57).Stigliano RV; Shubitidze F; Petryk JD; Shoshiashvili L; Petryk AA; Hoopes PJ Mitigation of Eddy Current Heating During Magnetic Nanoparticle Hyperthermia Therapy. Int. J. Hyperthermia 2016, 32, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Mahmoudi K; Bouras A; Bozec D; Ivkov R; Hadjipanayis C Magnetic Hyperthermia Therapy for the Treatment of Glioblastoma: A Review of the Therapy’s History, Efficacy and Application in Humans. Int. J. Hyperthermia 2018, 34, 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Ortega D; Pankhurst QA Magnetic Hyperthermia In Nanoscience: Volume 1: Nanostructures through Chemistry; O’Brien P, Ed.; Royal Society of Chemistry: Cambridge, 2013; pp 60–88. [Google Scholar]
- (60).Sykes EA; Chen J; Zheng G; Chan WC Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [DOI] [PubMed] [Google Scholar]
- (61).Dai Q; Wilhelm S; Ding D; Syed AM; Sindhwani S; Zhang Y; Chen YY; MacMillan P; Chan WCW Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [DOI] [PubMed] [Google Scholar]
- (62).Sun S; Zeng H Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc 2002, 124, 8204–8205. [DOI] [PubMed] [Google Scholar]
- (63).Sun S; Zeng H; Robinson DB; Raoux S; Rice PM; Wang SX; Li G Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc 2004, 126, 273–279. [DOI] [PubMed] [Google Scholar]
- (64).Duong T; Li X; Yang B; Schumann C; Alabrqi HA; Taratula O; Taratula O Phototheranostic Nanoplatform Based on a Single Cyanine Dye for Image-Guided Combinatorial Phototherapy. Nanomedicine 2016, 13, 955–963. [DOI] [PubMed] [Google Scholar]
- (65).Taratula O; Schumann C; Naleway MA; Pang AJ; Chon KJ A Multifunctional Theranostic Platform Based on Phthalocyanine-Loaded Dendrimer for Image-Guided Drug Delivery and Photodynamic Therapy. Mol. Pharmaceutics 2013, 10, 3946–3958. [DOI] [PubMed] [Google Scholar]
- (66).Sharkey J; Lewis PJS; Barrow M; Alwahsh SM; Noble J; Livingstone E; Lennen RJ; Jansen MA; Carrion JG; Liptrott N Functionalized Superparamagnetic Iron Oxide Nanoparticles Provide Highly Efficient Iron-Labeling in Macrophages for Magnetic Resonance–Based Detection In Vivo. Cytotherapy 2017, 19, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Taratula O; Schumann C; Duong T; Taylor KL Dendrimer-Encapsulated Naphthalocyanine as a Single Agent-Based Theranostic Nanoplatform for Near-Infrared Fluorescence Imaging and Combinatorial Anticancer Phototherapy. Nanoscale 2015, 7, 3888–3902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.