Abstract
Neuromuscular blocking agents can be used for purposes such as eliminating ventilator-patient dyssynchrony, facilitating gas exchange by reducing intra-abdominal pressure and improving chest wall compliance, reducing risk of lung barotrauma, decreasing contribution of muscles to oxygen consumption by preventing shivering and limiting elevations in intracranial pressure caused by airway stimulation in patients supported with mechanical ventilation in intensive care units. Adult Respiratory Distress Syndrome (ARDS), status asthmaticus, increased intracranial pressure and therapeutic hypothermia following ventricular fibrillation–associated cardiac arrest are some of clinical conditions that can be sustained by neuromuscular blockade. Appropriate indication and clinical practice have gained importance considering side effects such as ICU-acquired weakness, masking seizure activity and longer durations of hospital and ICU stays. We mainly aimed to review the current literature regarding neuromuscular blockade in up-to-date clinical conditions such as improving oxygenation in early ARDS and preventing shivering in the therapeutic hypothermia along with summarising the clinical practice in adult ICU in this report.
Keywords: Intensive care unit, mechanical ventilation, neuromuscular blocking agents
Introduction
This review has been prepared upon inspiration from the latest clinical guideline published in 2016 that had updated two reviews on the long-term use of neuromuscular blocking (NMB) agents in adult intensive care unit patients published in 1995 and 2002 as well as examination of other recent reviews and research. The main aim of this review was to summarise NMB use practice in adult intensive care units as well as compiling information on the current use of NMBs in intensive care units, such as preventing shivering in therapeutic hypothermia occurring after cardiopulmonary resuscitation and improving oxygenation during adult respiratory distress syndrome (ARDS) (1–3).
Neuromuscular blocking agents are non-sedative, non-amnestic and non-analgesic drugs that cause paralysis of skeletal muscles via the inhibition of signal transduction in neuromuscular junction (Table 1). In intensive care, along with preparation for intubation, these agents can be used to eliminate patient-ventilator dyssynchrony in patients supported with mechanical ventilation, thus reducing intra-abdominal pressure, facilitating gas exchange by improving chest wall compliance, reducing the risk of lung barotrauma, decreasing the contribution of muscles to oxygen consumption by preventing shivering and limiting elevations in intracranial pressure (ICP) caused by airway stimulation in patients with mechanical ventilation (4). ARDS, status asthmaticus, increased ICP and therapeutic hypothermia following ventricular fibrillation-associated cardiac arrest are some of clinical conditions where intensive care physicians prefer to use NMB (5–7).
Table 1.
Neuromuscular blocking agents
| Class | Start of effect (min) | End of effect (min) | Dose (bolus) (mg kg−1) | Dose (infusion) (mcg kg−1 min−1) | Elimination | Side effect | |
|---|---|---|---|---|---|---|---|
| Pancuronium | Long effect | 2–3 | 60–100 | 0.05–0.1 | 0.8–1.7 | 45%–70% renal, 15% hepatic | Vagal blockade, sympathetic stimulation |
| Vecuronium | Moderate effect | 3–4 | 20–35 | 0.08–0.1 | 0.8–1.7 | 10%–50% renal, 35%–50% hepatic | Vagal blockade in high dose |
| Rocuronium | Moderate effect | 1–2 | 20–35 (60–80 with high induction dose) | 0.6–1 (1–1.2 for high induction) | 8–12 | 33% renal, <75% hepatic | Vagal blockade in high dose |
| Atracurium | Moderate effect | 3–5 | 20–35 | 0.4–0.5 | 5–20 | 5%–10% renal, Hoffman elimination | Histamine release, minimal ganglionic blockade |
| Cisatracurium | Moderate effect | 2–3 | 30–60 | 0.1–0.2 | 1–3 | 5%–10% renal, Hoffman elimination | None |
| Succinylcholine | Short effect | <1 | 5–10 | 1 (higher doses in children) | Mostly no infusion use | Plasma cholinesterase | Minimal histamine release, muscarinic stimulation (bradycardia) |
The selection of appropriate patients and appropriate duration is very important since these agents have high risks, such as intensive care unit-acquired weakness (ICU-AW), elongated mechanical ventilation duration, awareness during paralysis, deep vein thrombosis, cornea abrasions and anaphylaxis, especially during long-term use (4). The use of NMB in intensive care units has slightly decreased due to some hesitations and discussions on this topic. In the 1980s, a study that investigated the practice in 34 different intensive care units showed that NMB was applied to 90% of patients supported with mechanical ventilation, whereas in an international observational study in 2005, this ratio was detected as 13% (8, 9). The use of NMB for appropriate indications, in the appropriate time and for an appropriate duration reduces hesitations on the side effects while enabling the advantage of its important benefits, such as improving oxygenation.
Indications of the NMB Agents in Intensive Care
1. ARDS
Mechanical ventilation can be considered as a type of organ replacement therapy in ARDS that can be described as non-cardiogenic pulmonary oedema accompanying severe hypoxaemia. The main objective in the treatment of patients with ARDS in need of mechanical ventilation is minimising ventilator-associated pulmonary injury while treating the main cause of respiratory failure. In this strategy named as ‘protective lung ventilation’, low tidal volume (VT) (6 mL kg−1 of predicted body weight) and low plateau pressures (28–30 cm H2O) are used (10). In a study published in 2005 where nine previous randomised studies including 3562 data of patients with ARDS were analysed, the strong correlation between compliance of the respiratory system and functional lung size (VT) during disease has been considered as the starting point, and a very strong statistical relationship has been detected between survival and ‘driving pressure’ (ΔP) that can be described as normalisation of VT to functional lung size or that can be derived by subtraction of positive end-expiratory pressure (PEEP) from plateau pressure. Changes in VT and PEEP were independently in correlation with survival only if it could cause a decrease in ΔP (11).
Adult respiratory distress syndrome has been categorised into three groups as mild, moderate and severe considering PaO2/FiO2 ratio for determination of prognosis and the most appropriate treatment strategy according to the latest Berlin criteria (Table 2) (12). Especially in moderate and severe ARDS cases, NMB can be necessary for application of special treatment options, such as prone position, and putting protective ventilation strategies accurately into practice since these agents improve chest wall compliance and reduce ΔP while eliminating patient-ventilator dyssynchrony and decreasing hyperinflation. It eases lung recruitment. Some studies showed their reducing effect on inflammatory mediator release (5, 13). They can regulate oxygenation in patients with ARDS through these effects.
Table 2.
ARDS Berlin criteria
| Timing | New or worsening respiratory symptoms within 1 week of a known clinical insult | ||
| Chest imaging | Bilateral opacities (not fully explained by effusion, lobar/lung collapse or nodules) | ||
| Oedema aetiology | Respiratory failure not fully explained by cardiac failure or fluid overload (hydrostatic oedema should be rejected by objective methods, such as echocardiography) | ||
| Oxygenation | Mild | Moderate | Severe |
| 200<PaO2/FiO2 ≤300 (with PEEP or CPAP ≥5 cm H2O) | 100<PaO2/FiO2 ≤200 (with PEEP or CPAP ≥5 cm H2O) | PaO2/FiO2 ≤100 (PEEP or CPAP ≥5 cm H2O) | |
In the literature, there are three multicentre studies on the effect of NMB use on oxygenation in ARDS (14–16). The early start and 48-h application of cisatracurium infusion in adult patients with ARDS who receive mechanical ventilation support with low VT have been shown to provide better oxygenation than those in controls in all three studies. In a later meta-analysis with 431 patients of these three studies, 48-h application of cisatracurium infusion has been shown to reduce 28 day mortality, barotrauma risk and ICU-AW (17).
In a retrospective study that included 5183 patients supported with mechanical ventilation for >12 h, it has been stated that NMB infusion was performed at least 1 day in 13% of cases, and more NMB infusion was applied to patients with ARDS. The average mechanical ventilation and intensive care unit durations were longer, whereas mortality was much higher in 549 patients who used NMB (9). In this retrospectively designed study, the application of NMB in patients whose oxygenation could not be managed with other methods might have contributed to this result.
In conclusion, continuous NMB infusion is recommended in patients with early ARDS with PaO2/FiO2 <150 (1).
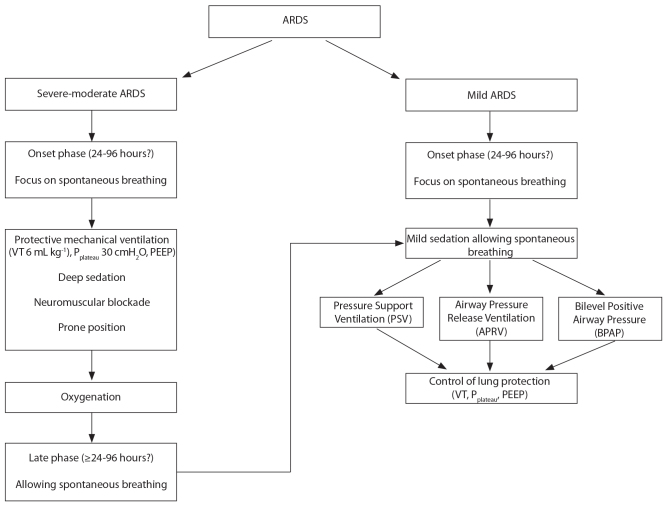
Sedation and neuromuscular blockage algorithm in ARDS can be seen in Figure 1.
Figure 1.
Sedation and neuromuscular blockade algorithm in ARDS
ARDS: adult respiratory distress syndrome; VT: tidal volume; PEEP: positive end-expiratory pressure; Pplat: plateau pressure
2. Status asthmaticus
In four retrospective studies that analysed 863 patients with status asthmaticus supported by mechanical ventilation, the use of NMB has been associated with ICU-AW and longer mechanical ventilation use (18–21). This positive relationship can be due to frequently used corticosteroids in patients with status asthmaticus. Although there are no studies with controls in the literature, clinical experience and information obtained from case studies show that neuromuscular blockade can fix oxygenation and severe dynamic hyperinflation-associated haemodynamic problems in patients with status asthmaticus who suffer from hypoxaemia even though 100% oxygen was supplied (refractory hypoxaemia) (19–21). Thus, the benefits of using NMB can be more than their harm in cases of deep sedation that cannot be cured, life-threatening hypoxaemia and dynamic hyperinflation.
3. Increased ICP
Neuromuscular blocking agents can be used in elevated ICP cases that cannot be prevented by sedation (22). In an observatory study, NMBs were shown to cause reduce coughing during tracheal aspiration and related ICP and cerebral perfusion pressure changes in 18 neurosurgery patients with a Glasgow Coma Scale <7 (23). Furthermore, in accordance with another study including 71 patients when patients with severe head trauma who were supported by mechanical ventilation were divided into three groups as taking only opioid, NMB together with opioid and taking neither, intracranial hypertension due to endotracheal aspiration was found to be significantly lower in patients taking both NMB and opioid than in other two groups (24). While these two studies focused on physiological changes rather than clinical findings, other two studies that investigated the clinical results of NMB use in patients with intracranial hypertension obtained negative results. While in one of these studies on 514 patients with traumatic brain injury with a Glasgow Coma Scale score <8, the risk of pneumonia and the duration in intensive care unit stay were increased as the use of neuromuscular blockade time increased. The other study could not define the difference in mortality and hospitalisation duration in patients with traumatic brain injury using and not using NMB. The results of these two retrospective studies cannot be accepted as reliable enough due to large patient spectrum with mild, moderate and severe ICP increase (25, 26).
Therapeutic hypothermia
In two studies published in 2002, for the first time, 12–24-h application of mild hypothermia (32–34°C) to unconscious patients with cardiac arrest due to ventricular fibrillation outside hospital was shown to improve neurological results (27, 28). Randomised studies and meta-analyses performed in the following years have also supported this evidence (7, 29–32). NMBs used in these studies can be neuroprotective since they inhibit shivering and related oxygen consumption increase and shorten the time to reach the target temperature. On the other hand, the possibility of masking seizures in these patients can significantly affect the results. Limited information regarding this topic belongs to a prospective observatory study including 111 patients. Among these patients, 18 patients who received NMB at least for 24 h showed higher survival rate than the remaining 93 patients (33). Similar result has been obtained from the reanalysis of the same study (34). Limited patient cohort and patients not being fully randomised reduce the reliability of this study.
Since hypothermia-associated variation may occur in train-of-four response during therapeutic hypothermia application, peripheral nerve stimulator (PNS) monitorisation may not provide reliable information (35). Thus, NMB dosage should be evaluated according to clinical parameters, such as the loss of shivering, absence of spontaneous breathing trigger on ventilation and PNS monitorisation.
There is no definite protocol regarding the selection of NMBs, sedatives and analgesics to be used during therapeutic hypothermia. In a review containing 44 studies investigating the use of sedative, analgesic and NMB during therapeutic hypothermia in 68 different intensive care units, most of which were from Europe, major differences have been detected between protocols. Among these intensive care centres, 54 used NMB routinely to prevent shivering, whereas 8 used NMB for treatment after the occurrence of shivering. Pancuronium and cisatracurium are the most commonly used agents, respectively (36).
Therapy suggestions
Analgesia/sedation
Pain is a disturbing situation that causes delay in wound healing, has immunosupressive and catabolic effects and reduces life quality (37, 38). Meanwhile, awareness can cause posttraumatic stress disorder, panic attack, anxiety and problems in concentration and sleep (39). Intensive care unit patients taking NMB should definitely use sedative and analgesics if necessary simultaneously (1). Benzodiazepines and propofol are the most commonly used agents for sedation. It can be necessary to decrease doses due to cumulative effects. Barbiturates and ketamine are also agents that can be used for sedative purposes in intensive care units. Dexmedetomidine is not appropriate for deep sedation necessary during NMB use (40).
It can be hard to evaluate pain and awareness during NMB agent application. Although sympathetic activation signs, such as tachycardia, hypertension and tears, can be used for this purpose, these symptoms are not reliable enough since they can also be observed during haemodynamic dysfunction (41). In a survey study on 11 intensive care unit patients taking mechanical ventilation support and NMB, disruption of reality and time perception, weird dreams, fear and sense of death have been defined although sedation and analgesics were applied to all and five, respectively (42).
Monitorisation techniques developed via multiple parameters obtained from electroencephalography (EEG), such as bispectral index (BIS), E–entropy and cerebral state index are used for evaluation of the depth of anaesthesia and sedation. BIS parameter, which is obtained from the evaluation of EEG data out of 100 in a wide patient cohort under general anaesthesia, values between 40 and 60 are considered as sufficient depth of anaesthesia (43). In the Cochrane review that evaluated studies on the effect of BIS monitorisation on intraoperative awareness, this monitorisation was shown to inhibit awareness significantly when compared with clinical symptoms and anaesthesia gas concentration follow-up (44). In another multicentre study including 5713 patients with general anaesthesia, the superiority of BIS over end tidal anaesthetic gas concentration monitorisation was not detected (45).
Consistent data could also not be obtained from studies on BIS monitorisation of intensive care unit patients (46, 47). In a study performed on three awake volunteers, a significant decrease was also detected in BIS levels when NMB was applied without sedatives or opioids (48). There are also studies showing the decreasing effect on BIS scores of NMB application in sedated patients (49, 50). The variability in patient responses makes BIS scores, which are affected by forehead muscle tonus, abnormal electroencephalographic activity and electrical and mechanical interference, unreliable especially in intensive care unit patients using NMB (43).
There are studies that show that pause in use of sedation during daytime in intensive care unit patients to whom continuous sedative agents are applied causes reduction in mechanical ventilation and duration of intensive care and hospitalisation (51, 52). When the necessity of sedation to NMB using patients is considered, the same situation will also be true for neuromuscular blockade. These pauses can be used for evaluation of the contribution of blockade to therapy and patient’s awareness and analgesia sufficiency. In addition, there are implications as to the reduction of ICU-AW (1, 53).
Neuromuscular Weakness and Neuropathy (ICU-AW)
ICU-AW is a clinical situation that has conflicts on the reasons for occurrence and thus subjected to different classifications, such as critic illness polyneuropathy, critic illness myopathy or critic illness neuromyopathy, due to differences in electrophysiological test results (54–56). Microcirculation abnormalities, protein malnutrition, systemic inflammation, NMB use and long-term immobilisation can play role in occurrence (55, 56). It is uncertain among which of immobilisation, corticosteroids and NMB is the main result of weakness in intensive care patients and which is the factor increasing this weakness (1, 54, 57).
Neuromuscular blocking agents have been considered as a cause in ICU-AW for multiple cases (54, 58, 59). In most of the studies published on this topic, duration and doses of simultaneously used corticosteroids, NMB and sedative agents in intensive care patients could not be standardised adequately (54–56). In a multicentre double-blind ACURASYS study conducted by Papazian et al. (16) that included 340 patients with ARDS, 48-h cisatracurium infusion did not increase ICU-AW. In a retrospective cohort study where 10-year patient data were considered, acute myopathy ratios in patients with asthma in need of ventilation support were evaluated (60). While the average NMB infusion duration was 3.1±2.3 days, myopathy incidence was calculated as 30% and 10% in the NMB applied group and controls, respectively, and myopathy ratio was shown to increase gradually everyday where NMBs were used. These results may be indicative of a relationship between short-term NMB infusion and low ICU-AW risk.
There are studies that show that ICU-AW occurs rarely in intensive care unit patients whose blood glucose level is kept between 80 and 110 mg dL−1 via intensive insulin treatment (61). Hypoglycaemia attacks can be frequently observed due to intense insulin therapy. Thus, although in perspectives, blood glucose level is suggested to be kept <180 mg dL−1 in intensive care patients, lower levels (100–150 mg dL−1) can be beneficial in specific patient groups. Literature on the ideal blood glucose level range of intensive care unit patients under NMB treatment are insufficient (1).
Physiotherapy
Application of physiotherapy can be of great benefit to patients taking NMB infusion since long-term immobilisation has several side effects, such as disturbed gastrointestinal mobility, venous thromboembolism and muscle weakness (62, 63).
NMB agents in intensive care emergency protocols
In a study investigating NMB use in 566 patients treated outside emergency services and operating rooms in case of urgent intubation need, neuromuscular blockade was shown to decrease hypoxaemia, aspiration, traumatic intubation, oesophagus intubation and endobronchial intubation rates (64).
Short start time (30–60 s) and succinylcholine (1–1.5 mg kg−1) enable airway control with low aspiration risk. There are studies showing that rocuronium establishes succinylcholine-like intubation conditions when applied 1.2 mg kg−1 (65). In a study on intensive care patients, 0.6 mg kg−1 rocuronium was shown to be no different from succinylcholine with regard to intubation conditions, oxygen desaturation rate and severity and unsuccessful intubation frequency (66). Succinylcholine use should be avoided in case of muscle weakness, long-term immobility, massive trauma causing muscle damage, severe intra-abdominal infection, kidney failure, accompanying acidosis, severe hypovolaemia and burns due to its extracellular potassium increasing effect (65, 67–69). While in previous years rocuronium use was not appropriate in patients who needed difficult intubation or difficult mask ventilation due to long-term effect, sugammadex, which has recently been included into clinical use, overcomes this drawback (70).
Eye damage
Neuromuscular blocker use in intensive care patients increases the risk of cornea damage. Incomplete closure of the eyelids and disappearance of the blinking reflex may cause dryness in the cornea, ulceration and infection (71). Ocular surface diseases, such as conjunctivitis, keratitis and corneal erosion, are observed in 20%–60% of patients under deep sedation or NMB. Methods, such as petroleum-based ointment, polyacrylamide gels or full closure of the eyelids, can be used to avoid corneal damage (72–75). In a study on patients under mechanical ventilation support taking NMB or propofol, closing one eye passively while applying artificial tear ointment was shown to be more effective in the inhibition of keratitis (76).
NMB use in obese intensive care patients
When NMB dose is calculated in intensive care patients with a body mass index >30 kg m−2, the ideal body weight should be considered rather than the actual body weight (1).
Conclusion
Neuromuscular blocking agents, after considering benefits/loss, can treat oxygenation in intensive care patients under mechanical ventilation support when used for appropriate durations. NMB use can be considered in cases of early ARDS with PaO2/FiO2 <150, status asthmaticus with refractory hypoxaemia that cannot be treated with deep sedation, ICP increases that cannot be inhibited by sedation and therapeutic hypothermia applications. Side effects, such as ICU-AW, masking of epileptic seizures and prolongation of duration in intensive care units and hospitalisation, should be considered. Simultaneous sedation must definitely be performed, and pain control and analgesia must be applied if necessary. Since ocular surface damage risk increases, appropriate eye protection should not be overlooked.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.Ö.; Design – B.T.; Supervision – A.Ö., S.T.; Resources – B.T.; Materials – S.T., A.Ö., B.T.; Data Collection and/or Processing – B.T.; Analysis and/or Interpretation – B.T.; Literature Search – B.T.; Writing Manuscript – B.T.; Critical Review – S.T., A.Ö.; Other – B.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079–103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro BA, Warren J, Egol AB, Greenbaum DM, Jacobi J, Nasraway SA, et al. Practice parameters for sustained neuromuscular blockade in the adult critically ill patient: An executive summary. Society of Critical Care Medicine. Crit Care Med. 1995;23:1601–5. doi: 10.1097/00003246-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Murray MJ, Cowen J, DeBlock H, Erstad B, Gray AW, Jr, Tescher AN, et al. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists, American College of Chest Physicians: Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142–56. doi: 10.1097/00003246-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Warr J, Thiboutat Z, Rose L, Mehta S, Burry LD. Current therapeutic uses, pharmacology, and clinical considerations of neuromuscular blocking agents for critically ill adults. Ann Pharmacother. 2011;9:1116–26. doi: 10.1345/aph.1Q004. [DOI] [PubMed] [Google Scholar]
- 5.Raoof S, Goulet K, Esan A, Hess DR, Sessler CN. Severe hypoxemic respiratory failure: part 2--nonventilatory strategies. Chest. 2010;137:1437–48. doi: 10.1378/chest.09-2416. [DOI] [PubMed] [Google Scholar]
- 6.Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, Park PK, et al. Acute Respiratory Distress Syndrome Network. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008;36:1083–8. doi: 10.1097/CCM.0B013E3181653895. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, Buist M. Induced hypothermia in critical care medicine: A review. Crit Care Med. 2003;31:2041–51. doi: 10.1097/01.CCM.0000069731.18472.61. [DOI] [PubMed] [Google Scholar]
- 8.Merriman HM. The techniques used to sedate ventilated patients. A survey of methods used in 34 ICUs in Great Britain. Intensive Care Med. 1981;7:217–24. doi: 10.1007/BF01702623. [DOI] [PubMed] [Google Scholar]
- 9.Arroliga AC, Frutos-Vivar F, Hall J, Esteban A, Apeztequia C, Soto L, et al. International Mechanical Ventilation Study Group: Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005;128:496–506. doi: 10.1378/chest.128.2.496. [DOI] [PubMed] [Google Scholar]
- 10.Bourenne J, Hraiech S, Roch A, Gainnier M, Papazian L, Forel JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 2017;5:291. doi: 10.21037/atm.2017.07.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 12.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Bennett S, Hurford WE. When should sedation or neuromuscu-lar blockade be used during mechanical ventilation? Respir Care. 2011;56:168–76. doi: 10.4187/respcare.01095. [DOI] [PubMed] [Google Scholar]
- 14.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–57. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 15.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–9. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 16.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 17.Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: A systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43. doi: 10.1186/cc12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behbehani NA, Al-Mane F, D’Yachkova Y, Pare P, FitzGerald JM. Myopathy following mechani-cal ventilation for acute severe asthma: the roleof muscle relaxants and corticosteroids. Chest. 1999;115:1627–31. doi: 10.1378/chest.115.6.1627. [DOI] [PubMed] [Google Scholar]
- 19.Adnet F, Dhissi G, Borron SW, Galinski M, Rayeh F, Cupa M, et al. Complication profiles of adult asthmatics requiring paralysis during mechanical ventilation. Intensive Care Med. 2001;27:1729–36. doi: 10.1007/s00134-001-1112-6. [DOI] [PubMed] [Google Scholar]
- 20.Kesler SM, Sprenkle MD, David WS, Leatherman JW. Severe weakness complicating status asthmaticus despite minimal duration of neuromuscular paralysis. Intensive Care Med. 2009;35:157–60. doi: 10.1007/s00134-008-1267-5. [DOI] [PubMed] [Google Scholar]
- 21.Leatherman JW, Fluegel WL, David WS, Davies SF, Iber C. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med. 1996;153:1686–90. doi: 10.1164/ajrccm.153.5.8630621. [DOI] [PubMed] [Google Scholar]
- 22.Ohlinger MJ, Rhoney DH. Neuromuscular blocking agents in the neurosurgical intensive care unit. Surg Neurol. 1998;49:217–21. doi: 10.1016/S0090-3019(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 23.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr ME, Sereika SM, Orndoff P, Weber B, Rudy EB, Marion D, et al. Effect of neuromuscular blockers and opiates on the cerebrovascular response to endotracheal suctioning in adults with severe head injuries. Am J Crit Care. 1998;7:205–17. [PubMed] [Google Scholar]
- 25.Hsiang JK, Chesnut RM, Crisp CB, Klauber MR, Blunt BA, Marshall LF. Early, routine paralysis for intracranial pressure control in severe head injury: Is it necessary? Crit Care Med. 1994;22:1471–6. doi: 10.1097/00003246-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Juul N, Morris GF, Marshall SB, Marshall LF. Neuromuscular blocking agents in neurointensive care. Acta Neurochir Suppl. 2000;76:467–70. doi: 10.1007/978-3-7091-6346-7_97. [DOI] [PubMed] [Google Scholar]
- 27.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 28.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 29.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006;70:369–80. doi: 10.1016/j.resuscitation.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Holzer M, Behringer W. Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22:711–28. doi: 10.1016/j.bpa.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Schneider A, Böttiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg. 2009;108:971–9. doi: 10.1213/ane.0b013e318193ca99. [DOI] [PubMed] [Google Scholar]
- 32.Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandín B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: A systematic review. Anesth Analg. 2010;110:1328–35. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 33.Salciccioli JD, Cocchi MN, Rittenberger JC, Peberdy MA, Ornato JP, Abella BS, et al. Continuous neuromuscular blockade is associated with decreased mortality in postcardiac arrest patients. Resuscitation. 2013;84:1728–33. doi: 10.1016/j.resuscitation.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salciccioli J, Donnino M. Reply to letter: Continuous neuromuscular blockade is associated with decreased mortality in post-cardiac arrest patients–problems with the data. Resuscitation. 2014;85:e3. doi: 10.1016/j.resuscitation.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Mueller SW, Winn R, Macht M, Fish DN, Kiser TH, MacLaren R. Neuromuscular blockade resistance during therapeutic hypothermia. Ann Pharmacother. 2011;45:e15. doi: 10.1345/aph.1P547. [DOI] [PubMed] [Google Scholar]
- 36.Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandín B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest:A systematic review. Anesth Analg. 2010;110:1328–35. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 37.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 38.Mohrien KM, Jones GM, MacDermott JR, Murphy CV. Remifentanil, ketamine, and fospropofol: a review of alterative continuous infusion agents for sedation in the critically ill. Crit Care Nurs Q. 2014;37:137–51. doi: 10.1097/CNQ.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery S. ECNP consensus meeting, March 5–6, 1999, Nice. Post traumatic stress disorder: guidelines for investigating efficacy of pharmacological intervention. ECNP and ECST. Eur Neuropsychopharmacol. 2000;10:297–303. doi: 10.1016/s0924-977x(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg SB, Vender J. The use of neuromuscular blocking agents in the ICU: where are we now? Crit Care Med. 2013;41:1332–44. doi: 10.1097/CCM.0b013e31828ce07c. [DOI] [PubMed] [Google Scholar]
- 41.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Ballard N, Robley L, Barrett D, Fraser D, Mendoza I. Patients’ recollections of therapeutic paralysis in the intensive care unit. Am J Crit Care. 2006;15:86–94. [PubMed] [Google Scholar]
- 43.Kelley SD. Monitoring Consciousness Using the Bispectral Index (BIS) During Anesthesia. 2nd ed. Boulder, CO: Covidien; 2012. [Accessed March 18, 2017]. Available from: http://www.covidien.com/imageServer.aspx/doc252087.pdf?contentID=77508&contenttype=application/pdf. [Google Scholar]
- 44.Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007:CD003843. doi: 10.1002/14651858.CD003843.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, et al. BAG-RECALL Research Group. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 46.Haenggi M, Ypparila-Wolters H, Bieri C, Steiner C, Takala J, Korhonen I, et al. Entropy and bispectral index for assessment of sedation, analgesia and the effects of unpleasant stimuli in critically ill patients: An observational study. Crit Care. 2008;12:R119. doi: 10.1186/cc7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arbour R, Waterhouse J, Seckel MA, Bucher L. Correlation between the Sedation-Agitation Scale and the Bispectral Index in ventilated patients in the intensive care unit. Heart Lung. 2009;38:336–45. doi: 10.1016/j.hrtlng.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Messner M, Beese U, Romstöck J, Dinkel M, Tschaikowsky K. The bispectral index declines during neuromuscular block in fully awake persons. Anesth Analg. 2003;97:488–91. doi: 10.1213/01.ANE.0000072741.78244.C0. [DOI] [PubMed] [Google Scholar]
- 49.Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Inoue S, Kawaguchi M, Sasaoka N, Hirai K, Furuya H. Effects of neuromuscular block on systemic and cerebral hemodynamics and bispectral index during moderate or deep sedation in critically ill patients. Intensive Care Med. 2006;32:391–7. doi: 10.1007/s00134-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 51.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 52.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 53.Smetana KS, Roe NA, Doepker BA, Jones GM. Review of Continuous Infusion Neuromuscular Blocking Agents in the Adult Intensive Care Unit. Crit Care Nurs Q. 2017;40:323–43. doi: 10.1097/CNQ.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 54.Puthucheary Z, Rawal J, Ratnayake G, Harridge S, Montgomery H, Hart N. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: Time to rethink the evidence? Am J Respir Crit Care Med. 2012;185:911–7. doi: 10.1164/rccm.201107-1320OE. [DOI] [PubMed] [Google Scholar]
- 55.Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(Suppl 10):S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 56.Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ. Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med. 1998;24:801–7. doi: 10.1007/s001340050669. [DOI] [PubMed] [Google Scholar]
- 57.deBacker J, Hart N, Fan E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest. 2017;151:697–706. doi: 10.1016/j.chest.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 58.MacFarlane IA, Rosenthal FD. Severe myopathy after status asthmati-cus. Lancet. 1977;2:615. doi: 10.1016/s0140-6736(77)91471-4. [DOI] [PubMed] [Google Scholar]
- 59.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 60.Behbehani NA, Al-Mane F, D’Yachkova Y, Pare P, FitzGerald JM. Myopathy following mechani-cal ventilation for acute severe asthma: the roleof muscle relaxants and corticosteroids. Chest. 1999;115:1627–31. doi: 10.1378/chest.115.6.1627. [DOI] [PubMed] [Google Scholar]
- 61.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: Benefit versus harm. Diabetes. 2006;55:3151–9. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 62.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 63.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilcox SR, Bittner EA, Elmer J, Seigel TA, Nguyen NT, Dhillon A, et al. Neuromuscular blocking agent administration for emergent tracheal intubation is associated with decreased prevalence of procedure-related complications. Crit Care Med. 2012;40:1808–13. doi: 10.1097/CCM.0b013e31824e0e67. [DOI] [PubMed] [Google Scholar]
- 65.Perry J, Lee J, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;2:CD002788. doi: 10.1002/14651858.CD002788.pub2. [DOI] [PubMed] [Google Scholar]
- 66.Marsch SC, Steiner L, Bucher L, Pargger H, Schumann M, Aebi T, et al. Succinylcholine versus rocuronium for rapid sequence intubation in intensive care: A prospective, randomized trial. Crit Care. 2011;15:R199–R208. doi: 10.1186/cc10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds SF, Heffner J. Airway management of the critically ill patient: Rapid-sequence intubation. Chest. 2005;127:1397–412. doi: 10.1016/S0012-3692(15)34494-9. [DOI] [PubMed] [Google Scholar]
- 68.El-Orbany M, Connolly LA. Rapid sequence induction and intubation: Current controversy. Anesth Analg. 2010;110:1318–25. doi: 10.1213/ANE.0b013e3181d5ae47. [DOI] [PubMed] [Google Scholar]
- 69.Booij LH. Is succinylcholine appropriate or obsolete in the intensive care unit? Crit Care. 2001;5:245–6. doi: 10.1186/cc1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wołoszczuk-Gębicka B, Zawadzka-Głos L, Lenarczyk J, Sitkowska BD, Rzewnicka I. Two cases of the “cannot ventilate, cannot intubate” scenario in children in view of recent recommendations. Anaesthesiol Intensive Ther. 2014;46:88–91. doi: 10.5603/AIT.2014.0017. [DOI] [PubMed] [Google Scholar]
- 71.Honiden S, Siegel MD. Analytic reviews: Managing the agitated patient in the ICU: Sedation, analgesia, and neuromuscular blockade. J Intensive Care Med. 2010;25:187–204. doi: 10.1177/0885066610366923. [DOI] [PubMed] [Google Scholar]
- 72.Ezra DG, Lewis G, Healy M, Coombes A. Preventing exposure keratopathy in the critically ill: A prospective study comparing eye care regimes. Br J Ophthalmol. 2005;89:1068–9. doi: 10.1136/bjo.2004.062406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg JB, Eisen LA. Eye care in the intensive care unit: Narrative review and meta-analysis. Crit Care Med. 2008;36:3151–5. doi: 10.1097/CCM.0b013e31818f0ee7. [DOI] [PubMed] [Google Scholar]
- 74.Sivasankar S, Jasper S, Simon S, Jacob P, John G, Raju R. Eye care in ICU. Indian J Crit Care Med. 2006;10:11–4. doi: 10.4103/0972-5229.24683. [DOI] [Google Scholar]
- 75.Sorce LR, Hamilton SM, Gauvreau K, Mets MB, Hunter DG, Rahmani B, et al. Preventing corneal abrasions in critically ill children receiving neuromuscular blockade: A randomized, controlled trial. Pediatr Crit Care Med. 2009;10:171–5. doi: 10.1097/PCC.0b013e3181956ccf. [DOI] [PubMed] [Google Scholar]
- 76.Lenart SB, Garrity JA. Eye care for patients receiving neuromuscular blocking agents or propofol during mechanical ventilation. Am J Crit Care. 2000;9:188–91. [PubMed] [Google Scholar]