Abstract
Respiratory syncytial virus (RSV) is one of the most common causes of acute respiratory tract infections among children. 1%–2% of RSV infections require hospitalization. In addition to the respiratory system, cardiovascular system may be also affected by the RSV infection. A 7-year-old, previously healthy, female patient presenting with respiratory difficulties was admitted to the paediatric intensive care unit. The patient was intubated and connected to a mechanical ventilator because of acute respiratory failure. Her tracheal aspirate was studied for viral multiplex polymerase chain reaction (PCR), and RSV positivity was detected. Her echocardiogram revealed left ventricular dysfunction. She was put on fluid restriction, intravenous furosemide, and inotropic support. Her cranial magnetic resonance examination showed the signs of acute haemorrhagic encephalopathy. She underwent five sessions of therapeutic plasma exchange with fresh frozen plasma. She was extubated on the 18th day of admission and provided with respiratory support with high-flow oxygen therapy thereafter. On the 23rd day, when her clinical status remained stable, she was transferred to the paediatrics ward. An RSV infection should be considered in cases with acute necrotising encephalitis and myocarditis.
Keywords: Encephalitis, intensive care, respiratory syncytial virus
Introduction
Respiratory syncytial virus (RSV) is a Pneumovirus that belongs to the Pneumovirinae subfamily of the Paramyxoviridae family. Humans are the only source of RSV. Transmission occurs when contaminated secretions reach the nasopharyngeal mucosa, ocular mucosa, and less commonly, oral mucosa. Viral replication usually lasts 3–8 days. However, it may be prolonged by up to 3–4 weeks in infants and immunocompromised persons. The incubation period lasts 2–8 days, but most commonly 4–6 days. The RSV incidence peaks in winter months (1). In addition to the respiratory system, the cardiovascular and central nervous system may also be affected by the RSV infection. It may rarely cause severe extrapulmonary manifestations such as cerebellitis, encephalitis, fatal myocarditis, hepatitis and Reye-like syndrome (2). Herein, by reporting the case of a 7-year-old female patient, we aimed to emphasise the importance of considering an RSV infection in cases with acute necrotising encephalitis and myocarditis.
Case Presentation
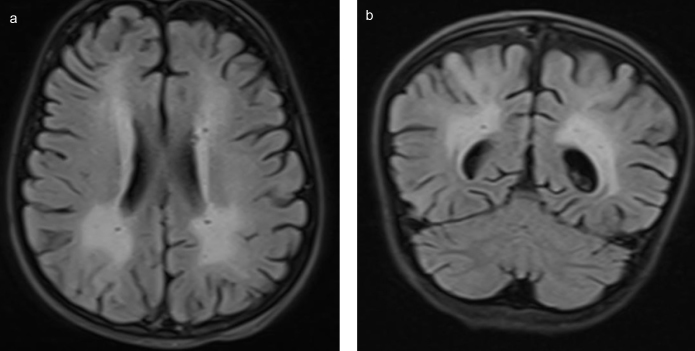
A 7-year-old girl admitted to the paediatric emergency department for respiratory difficulties had started to show the signs of fever, vomiting and cough 3 days before; she had been treated with oral antibiotics in another medical centre, but her symptoms had not abated. Her past medical and family history were non-remarkable. On physical examination, her body temperature was 38.4°C; the heart rate, 170/min; blood pressure, 110/60 mmHg; respiratory rate, 40/min; and oxygen saturation, 80%, while breathing 8L min−1 oxygen via a reservoir (non-rebreathing) mask. Her general condition was poor; she was conscious but agitated. She had tachypnoea and dyspnoea, and nasal flaring. She had diffuse rales over the basal segments of the lungs. She also had tachycardia, but no additional heart sounds or murmurs. Her liver was palpable 2 cm below the lower costal margin. The patient was transferred to the paediatric intensive care unit where she was intubated for hypoxic respiratory failure and provided respiratory support with a mechanical ventilator. She was also administered midazolam infusion (0.1 mg−1 kg−1 h−1) for sedation, fentanyl infusion (1 mcg−1 kg−1 h−1) for analgesia, and rocuronium infusion (1 mg−1 kg−1 h−1) for neuromuscular blockade. Her blood count revealed a white blood cell count of 41.550 mm3; the haemoglobin level of 12.1 g dL−1; haematocrit, 38.3%; thrombocyte count of 315.000 mm3; aspartate aminotransferase, 94 U L−1; alanine aminotransferase, 60 U L−1; urea, 40.6 mg dL−1; lactate dehydrogenase, 560 U L−1; albumin, 2.9 g dL−1; INR, 1.47; procalcitonin, 16.5 ng mL−1; C-reactive protein, 1.1 mg dL−1; sedimentation rate, 43 mm hour−1; calcium, 8.3 mg dL−1; troponin I, 0.77 ng mL−1 (N<0.026); creatine kinase, 385 U L−1 (N, 29–168); creatine kinase-MB, 79 IU L−1 (N:<25); pro-brain natriuretic peptide (BNP), 1526 pg mL−1 (N, <100). Antinuclear antibodies and anti-double stranded DNA were negative; the serum lactate, pyruvate, complement 3, complement 4, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, and thyroid function tests were normal. A lumbar puncture revealed a normal CSF biochemistry analysis, no cells, no CSF culture proliferation, and a negative CSF herpes simplex type 1,2 PCR and cytomegalovirus PCR, and oligoclonal band. The patient was started on ceftriaxone, clindamycin and oseltamivir treatments. A chest X-ray and arterial blood gas analysis were consistent with acute respiratory distress syndrome. She was put on high positive end expiratory pressure with a mechanical ventilator. An echocardiographic examination revealed left ventricular dysfunction (ejection fraction [EF], 35.7%, fractional shortening [FS], 16.7%), second-degree mitral regurgitation and first-degree tricuspid regurgitation. Her intravenous fluid intake was restricted to 1000 cc/m2, and dobutamine infusion (10 mcg kg−1 min−1) and furosemide 1 mg kg−1 day−1 were initiated. Her treatment was switched to meropenem, caspofungin and vancomycin owing to persistent fever and progressive infiltration on the chest X-ray. Her blood and urine cultures were negative. On the 5th day of the follow-up, a repeat echocardiogram was taken, which showed an EF of 44%. Therefore, dobutamine was stopped, and milrinone was commenced at a dose of 0.5 mcg kg−1 min−1. In a cranial magnetic resonance imaging examination, the axial and coronal fluid attenuated inversion recovery images show a widespread T2A hyperintense pathologic signal in bilateral periventricular white matter (Figure 1a, b), and haemorrhagic signals in bilateral lentiform nucleus, caudate nucleus were detected (Figure 2).
Figure 1.
a,b. (a) In fluid attenuated inversion recovery images, an increased signal can be noted in the left insular cortex, left temporal lobe, hypothalamus, hippocampus, and amygdala. (b) Computed tomography images demonstrate hypoattenuation in same locations
Figure 2.
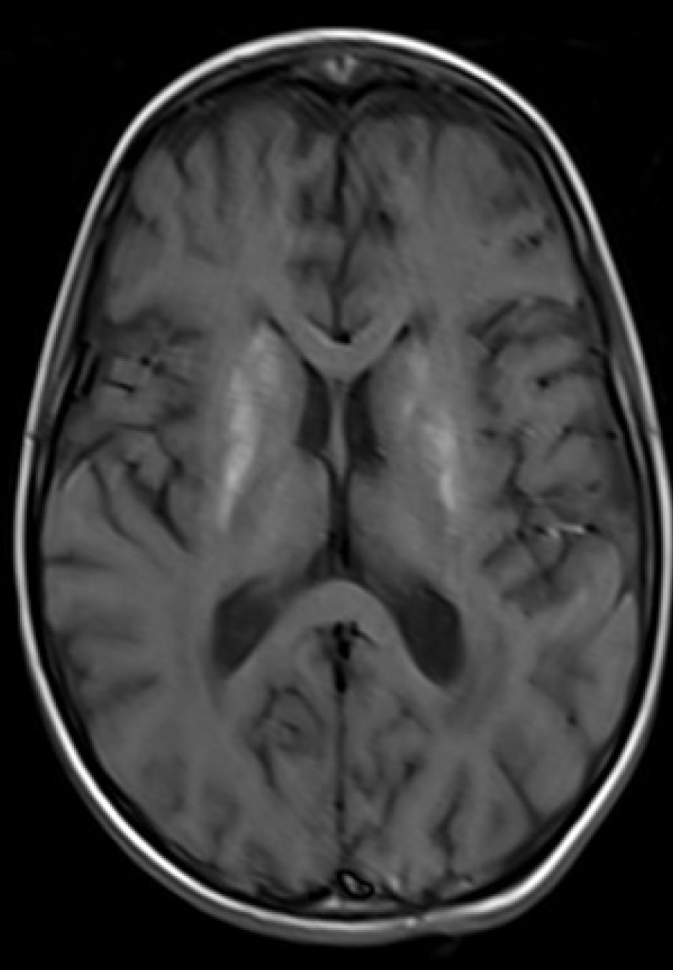
T1A and SWI secans demonsrates haemorrhagic signals in bilateral lentiform nucleus, caudate nucleus
Viral multiplex PCR was studied in tracheal aspirate, which showed the RSV positivity but negative results for other viral serology. The patient was applied five sessions of therapeutic plasma exchange (TPE) with fresh frozen plasma. On the 14th day of the follow-up, she developed hypertension, for which a calcium channel blocker was started, but no clinical response could be obtained; thus, esmolol was started at a loading dose of 500 mcg kg−1 followed by 50 mcg −1 kg hour−1 starting infusion dose, which was gradually increased to 200 mcg kg−1 hour−1. Serum norepinephrine, epinephrine, metanephrine and normetanephrine levels studied for hypertension aetiology were within normal limits. The renal ultrasonography (USG) revealed bilateral kidney size at the upper limit; parenchymal echoes increased at Grade 1–2; the renal artery and vein Doppler examination were normal. A thoracic USG revealed bilateral pleural effusion of 2 cm at both lungs’ basal parts. She was provided human albumin support and packed red blood cell transfusion. As she could not tolerate enteral trophic feeding, she was fed with total parenteral nutrition. She was extubated on the 18th day of admission and provided with respiratory support with high-flow nasal cannula; the latter was stopped 5 days later. The patient showed a significant improvement in neurological symptoms. As the clinical condition remained stable, the patient was transferred to the paediatric regular ward.
Informed written consent was obtained from the parents for publication.
Discussion
Respiratory syncytial virus is one of the common causes of acute respiratory tract infections among children. 1%–2% percent of RSV infections require hospitalisation. In addition to the respiratory system, the cardiovascular system may also be affected by RSV infections. It has been shown that various rhythm disorders occur in relation to an RSV infection, which include bradyarrhythmias, atrioventricular blocks of different degrees, multifocal atrial tachycardia, supraventricular tachycardia, atrial flutter, ventricular fibrillation and ventricular tachycardia (3). Cardiac troponin elevation was observed in 35%–54% of infants with an RSV infection. Recently, RSV was isolated from the myocardia of patients with myocarditis using the PCR method (4). Treatment of patients with myocarditis ranges from administering fluid boluses to inotropic support. Our patient had elevated troponin, pro-BNP, and CK-MB levels, and he had a reduced EF on echocardiographic examination. Inotropic support was provided with dopamine and milrinone infusions.
Compared to non-RSV viral infections, these complications are not more common in RSV bronchiolitis (5). The neurological complications of RSV bronchiolitis include seizures and acute encephalopathy. An RSV infection may cause febrile convulsions in children aged 3 months-5 years, particularly those younger than 2 years (6). Ng et al. (7) reported that 1.8% of children admitted to hospital for RSV bronchiolitis exhibited symptoms of acute encephalopathy.
Acute necrotizing encephalopathy is a rare form of encephalopathy that affects healthy children aged 5 months to 11 years, which has specific imaging findings and which may be rapidly progressive and even fatal. Its mortality rate is 30%, and 15% of survivors have been reported to suffer from severe neurological sequela (8). The pathogenesis of RSV-associated encephalitis is unclear. Possible hypotheses include the entry of RSV into the central nervous system haematologically via the blood-brain barrier or its action as a result of its invasion through the release of some neurotoxins (9). In patients unresponsive to other treatments TPE may offer a treatment alternative. Despite the absence of randomised studies, there are reports of cases successfully treated with TPE (10). We also carried out five sessions of plasmapheresis for our patient and discontinued the treatment due to clinical improvement.
Conclusion
By reporting this case, we aim to stress the importance of considering an RSV infection in the differential diagnosis of acute necrotising encephalitis and myocarditis.
Footnotes
Informed Consent: Written informed consent was obtained from patients’ parents who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.E.; Design – S.E., K.Y.; Supervision – S.E., S.K.; Resources – S.E., K.Y.; Materials – S.K.; Data Collection and/or Processing – K.Y.; Analysis and/or Interpretation – S.E., S.K.; Literature Search – K.Y., S.K.; Writing Manuscript – S.E., S.K.; Critical Review – S.E., S.K., K.Y.; Other – S.E., S.K., K.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th edition. Elk Grove Village (IL): American Academy of Pediatrics; 2015. pp. 667–76. [Google Scholar]
- 2.Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection: A systematic review. Crit Care. 2006;10:R107. doi: 10.1186/cc4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito S, Salice P, Bosis S, Ghiglia S, Tremolati E. Altered cardiac rhythm in infants with bronchiolitis and respiratory syncytial virus infection. BMC Infect Dis. 2010;10:305. doi: 10.1186/1471-2334-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R. Detection of viruses in myocardial tissues by polymerase chain reaction. Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–72. doi: 10.1016/S0735-1097(03)00648-X. [DOI] [PubMed] [Google Scholar]
- 5.Meissner HC, Hall CB. Respiratory syncytial virus. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, editors. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 7th edition. Philadelphia: Elsevier Saunders; 2014. pp. 2407–34. [Google Scholar]
- 6.Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol. 2006;35:165–72. doi: 10.1016/j.pediatrneurol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Ng YT, Cox C, Atkins J, Butler IJ. Encephalopathy associated with respiratory syncytial virus bronchiolitis. J Child Neurol. 2001;16:105–8. doi: 10.2310/7010.2001.6913. [DOI] [PubMed] [Google Scholar]
- 8.Marco EJ, Anderson JE, Neilson DE, Strober JB. Acute necrotizing encephalopathy in 3 brothers. Pediatrics. 2010;125:e693–8. doi: 10.1542/peds.2009-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park A, Suh SI, Son GR, Lee YH, Seo HS, Eun BL, et al. Respiratory syncytial virus-related encephalitis: Magnetic resonance imaging findings with diffusion-weighted study. Neuroradiology. 2014;56:163–8. doi: 10.1007/s00234-013-1305-z. [DOI] [PubMed] [Google Scholar]
- 10.Al-Maskari N, Mohsin J, Al-Maani A, Al-Macki N, Al-İsmaili Atypical presentations of respiratory syncytial virus infection. Sultan Qaboos University Med J. 2016;16:86–91. doi: 10.18295/squmj.2016.16.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]