Alterations in catecholamine (CA) synthesis and catabolism predispose to or aggravate the course of cardiovascular disorders. In heart failure (HF), excessive norepinephrine, resulting from persistent sympathetic efferent fiber activation, contributes to CA spillover, which is a major prognostic and therapeutic index (1).
Exaggerated epinephrine (EPI) from the chromaffin cells of the adrenal glands (2) is also involved in HF progression. EPI secretion is controlled by β-adrenergic receptors (βARs), which facilitate its release, and α2-adrenergic receptors (α2ARs), which provide negative feedback, preventing unremitted EPI (and norepinephrine) adrenal discharge (1,2). In chromaffin cells of HF rodents, G protein-coupled receptor kinase 2 (GRK2) expression is up-regulated, impairing α2AR-gated EPI secretion via desensitization of these receptors (2). It is unknown whether a similar mechanism is present in humans. Importantly, in ex vivo cultured human pheochromocytoma explants, gallein, a GRK2 inhibitor, prevents uncontrolled CA secretion (3). However, it remains to be determined whether physiologically relevant CA concentrations can up-regulate GRK2 expression in normal human adrenal glands, preventing sympathoinhibitory α2AR effects on EPI (and norepinephrine) release.
To this aim, we used human chromaffin cells isolated from adrenal glands (n = 13) from deceased kidney transplant donors. The local ethics committee of the University “Federico II” reviewed and approved this protocol. All experimental methods were described previously (2). Statistical significance was determined using the Student t test or analysis of variance followed by Bonferroni post hoc correction for multiple comparisons. All data were analyzed using GraphPad Prism version 7 (GraphPad Software, La Jolla, California).
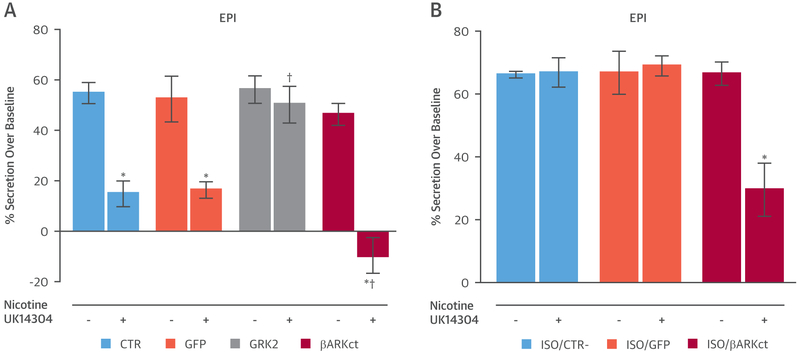
We first increased GRK2 expression and activity via adenovirus (Ad), whereas in separate experiments we expressed βARKct, an inhibitor peptide of GRK2 activation via Gβγ sequestration. Noninfected cells, or Ad-green fluorescent protein (GFP) cells, were used as controls. We stimulated human chromaffin cells with nicotine to induce a marked EPI secretion; this effect was equipotent in all experimental groups (Figure 1A). Interestingly, pre-treating cells with the selective α2AR agonist UK14304 resulted in an abated nicotine-induced EPI release in control (CTR) and AdGFP-infected cells (Figure 1A). However, the α2AR’s sympathoinhibitory effect was markedly blunted in AdGRK2-infected cells, whereas the presence of βARKct potentiated it.
FIGURE 1. In Vitro Modulation of Adrenal G Protein-Coupled Receptor Kinase 2 Activity Regulates Human Chromaffin Cell α2AR Function and Thus Catecholamine Secretion.
Epinephrine (EPI) secretion, measured by enzyme-linked immunosorbent assay, from chromaffin cells isolated from human adrenal glands. (A) Cells were infected with adenovirus-G protein-coupled receptor kinase 2 (AdGRK2) or adenovirus-β-adrenergic receptor Kct (AdβARKct). Noninfected cells (CTR) or adenovirus-green fluorescent protein (AdGFP)-infected cells were used as controls. Cells were stimulated with nicotine (20 μmol/l) for 30 min, following pre-treatment with vehicle or with the α2-adrenergic receptor (α2AR) agonist UK14304 (10 μmol/l) for 30 min (*p < 0.05 vs. CTR + nicotine; †p < 0.05 vs. CTR + nicotine + UK14304; n = 6 per group). (B) Cells were infected with AdβARKct. CTR or AdGFP-infected cells were used as controls. Twenty-four h post-infection, the cells were pre-treated with isoproterenol (ISO) (1 μmol/l) for 12 h. Following ISO stimulation, the media were replaced, and the cells were stimulated with nicotine (20 μmol/l) for 30 min. After ISO, and prior nicotine stimulation, a group of cells was pre-treated with the α2AR agonist UK14304 (10 μmol/l) (*p < 0.05 vs. ISO/CTR + nicotine; n = 6 per group).
Stimulation with CA up-regulates GRK2 in the heart (2). Similar direct evidence in human chromaffin cells is lacking. Therefore, we pre-treated in vitro human chromaffin cells with isoproterenol (1 μmol/l) for 12 h and found a significant rise in GRK2 protein levels (GRK2 levels fold over CTR, CTR 1.00 ± 0.04 vs. isoproterenol 1.88 ± 0.42; p < 0.05 vs. CTR). High GRK2 levels abolished the α2AR-mediated negative feedback on EPI release, in both CTR and GFP-expressing cells (Figure 1B). Importantly, βARKct expression rescued α2AR-mediated inhibition on EPI secretion from these human cells (Figure 1B).
Our study is the first to show that βAR-dependent GRK2 up-regulation accounts for α2AR desensitization in human chromaffin cells. GRK2 elevation abolishes the feedback inhibition operated by α2AR activation on further CA release from adrenal glands.
Our present findings offer a major advancement in our understanding of the pathophysiology of diseases characterized by elevated sympathetic nervous system activity. At the same time, our data suggest that GRK2 blockade could be useful in advanced human HF as well as in other disorders aggravated by the hyperactivity of the central and/or peripheral sympathetic nervous system, namely, hyperthyroidism, hypertension, and pheochromocytoma, all conditions in which excessive circulating CA levels may be pathogenic as well.
Acknowledgments
Please note: This work was supported by National Institutes of Health grants R37 HL061690, RO1 HL088503, P01 HL08806, P01 HL075443, and P01 HK091799 (all to Dr. Koch.) and Italian Ministry of Health-GR-2011-02346878 (to Dr. Rengo). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.FLorea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014;114:1815–26. [DOI] [PubMed] [Google Scholar]
- 2.Lymperopoulos A, Rengo G, Funakoshi H, et al. Adrenal GRK2 upregu-Lation mediates sympathetic overdrive in heart failure. Nat Med 2007;13:315–23. [DOI] [PubMed] [Google Scholar]
- 3.Kamal FA, Mickelsen DM, Wegman KM, et al. Simultaneous adrenal and cardiac g-protein-coupled receptor-Gβγ inhibition halts heart failure progression. J Am Coll Cardiol 2014;63:2549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]