SUMMARY
Genetically wired neural mechanisms inhibit mating between species because even naive animals rarely mate with other species. These mechanisms can evolve through changes in expression or function of key genes in sensory pathways or central circuits. Gr32a is a gustatory chemoreceptor that, in D. melanogaster, is essential to inhibit interspecies courtship and sense quinine. Similar to D. melanogaster, we find that D. simulans Gr32a is expressed in foreleg tarsi, sensorimotor appendages that inhibit interspecies courtship, and it is required to sense quinine. Nevertheless, Gr32a is not required to inhibit interspecies mating by D. simulans males. However, and similar to its function in D. melanogaster, Ppk25, a member of the Pickpocket family, promotes conspecific courtship in D. simulans. Together, we have identified distinct evolutionary mechanisms underlying chemosensory control of taste and courtship in closely related Drosophila species.
Graphical Abstract
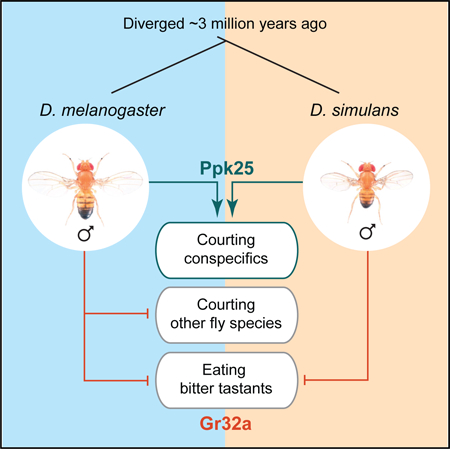
In Brief
Mechanisms that inhibit interspecies mating are critical to reproductive isolation of species. Ahmed et al. show that Gr32a, a chemoreceptor that inhibits interspecies courtship by D. melanogaster males, does not inhibit this behavior in the closely related D. simulans, indicating rapid evolution of peripheral sensory mechanisms that preclude interspecies breeding.
INTRODUCTION
A species can be defined as a set of organisms that share a gene pool and breed with one another (Darwin, 1860; Dobzhansky, 1937; Mayr, 1988). The lack of interspecies breeding preserves advantages conferred by species-specific allele combinations (Mayr, 1988; Mayr and Dobzhansky, 1945; Orr, 2005; Orr et al., 2004), and mechanisms that preclude interbreeding must evolve rapidly to facilitate reproductive isolation between closely related species (Coyne and Orr, 1989; Mendelson, 2003). Individuals from closely related species rarely attempt to mate, suggesting that neural pathways underlying behavioral barriers to interbreeding must also evolve rapidly. How such neural pathways evolve is poorly understood.
Drosophilids provide a facile model for studies on how neural pathways have evolved. There are ~1,500 drosophilid species, many of which co-exist in overlapping habitats (Jezovit et al., 2017; Markow, 2015). They engage in species-typical stereotyped courtship rituals, and many genetic and neural pathways that regulate courtship of D. melanogaster are well defined (Bastock and Manning, 1955; Clowney et al., 2015; Demir and Dickson, 2005; Gill, 1963; Greenspan and Ferveur, 2000; Hall, 1978, 1994; Hotta and Benzer, 1976; Kallman et al., 2015; Kohatsu et al., 2011; Lin et al., 2016; Manoli et al., 2005; Pavlou and Goodwin, 2013; Ryner et al., 1996; Spieth, 1952; Thistle et al., 2012; Tootoonian et al., 2012). We previously demonstrated that sensory neurons expressing the gustatory chemoreceptor Gr32a are necessary to suppress interspecies courtship by D. melanogaster males (Fan et al., 2013). In addition, Gr32a is required to recognize cuticular hydrocarbons on non-melanogaster drosophilids and to inhibit interspecies mating. Strikingly, Gr32a is also necessary to inhibit courtship displays toward the closely related D. simulans, which last shared an ancestor with D. melanogaster ~3 million to 5 million years ago (mya) (David et al., 2007; Tamura et al., 2004). D. simulans and D. melanogaster co-exist globally (reviewed in Jezovit et al., 2017) and are very similar in behavior and appearance (Sturtevant, 1919, 1920). Here we have examined how the Gr32a chemosensory pathway has evolved to inhibit interspecies courtship in D. simulans.
RESULTS
The Chemosensory Pathway that Inhibits Interspecies Courtship Is Conserved
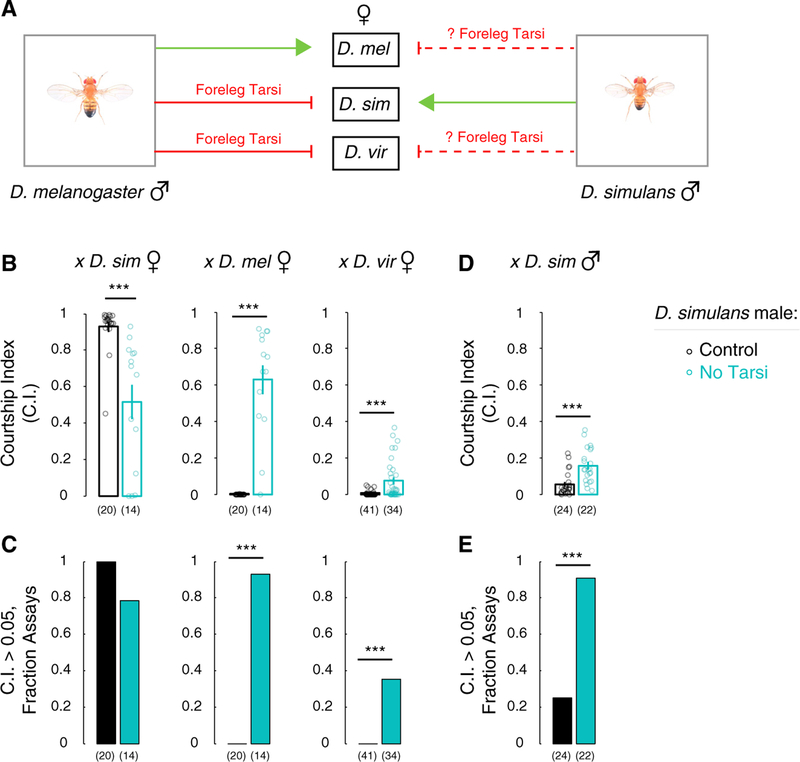
D. melanogaster males tap potential mates with their foreleg tarsi very early in courtship. This tapping restricts courtship to conspecifics because males lacking foreleg tarsi court conspecifics as well as other drosophilid species (Figure 1A) (Fan et al., 2013; Manning, 1959; Spieth, 1952). Similarly, D. simulans males also tap potential mates with foreleg tarsi (Manning, 1959; Spieth, 1952), and surgical extirpation of these tarsi enables D. simulans males to court D. melanogaster females (Fan et al., 2013; Manning, 1959; Spieth, 1952). We found that tarsiless D. simulans males also courted D. virilis females, a distantly related drosophilid (shared last common ancestor ~40 mya), and conspecific males (Figures 1B–1E and S1A). Tarsiless D. simulans males, like their D. melanogaster counterparts (Fan et al., 2013), also courted conspecific females (Figures 1B and 1C). Such conspecific courtship was performed by the tarsiless males at reduced intensity, likely because of reduced effectiveness in pursuing females or from loss of tarsal neurons that promote courtship. However, loss of tarsi did not lead to overall reduction in locomotor activity during conspecific courtship (Figure S1I); tarsiless males did show a small increase in locomotor activity when paired with D. melanogaster females (Figure S1J), most likely because they persisted in courting the females despite being rejected. Regardless, tarsiless D. simulans males, similar to their D. melanogaster counterparts, courted other species.
Figure 1. D. simulans Male Foreleg Tarsi Inhibit Courtship of Other Species and Are Not Essential for Courtship of Conspecific Females.
(A) We tested whether, similar to D. melanogaster males, foreleg tarsi also inhibited interspecies courtship by D. simulans males.
(B) D. simulans males lacking foreleg tarsi court conspecific, D. melanogaster, and D. virilis females.
(C) D. simulans males lacking foreleg tarsi are more likely to show intense courtship toward D. melanogaster and D. virilis females.
(D) D. simulans males lacking foreleg tarsi show more courtship toward conspecific males.
(E) D. simulans males lacking foreleg tarsi are more likely to show intense courtship toward conspecific males.
Mean ± SEM. CI, fraction time spent courting target fly. Each circle denotes CI of one male. n = 14–41 per cohort. ***p < 0.001. See also Figure S1.
The hydrocarbon 7-tricosene is enriched on the cuticle of D. simulans females and depleted on the cuticle of D. melanogaster females, and it serves as an aphrodisiac and repellent, respectively, for D. simulans and D. melanogaster males (Billeter et al., 2009; Coyne et al., 1994; Everaerts et al., 2010; Fan et al., 2013; Ferveur, 2005; Jallon, 1984; Lacaille et al., 2007; Wang et al., 2011). Accordingly, wild-type (WT) D. simulans courted D. yakuba females, whose cuticle is enriched in 7-tricosene, albeit with lower intensity compared with conspecific females (p < 0.001, n = 20–22 males/cohort; see Figures S1B and 1B). Tarsectomy of males did not further increase courtship toward D. yakuba females (Figure S1B), suggesting that multiple pathways exist in D. simulans to inhibit interspecies courtship. Nevertheless, severing foreleg tarsi of D. simulans males disinhibits courtship toward other species without abolishing courtship with conspecific females.
Gr32a Expression Is Conserved in D. simulans Foreleg Tarsi
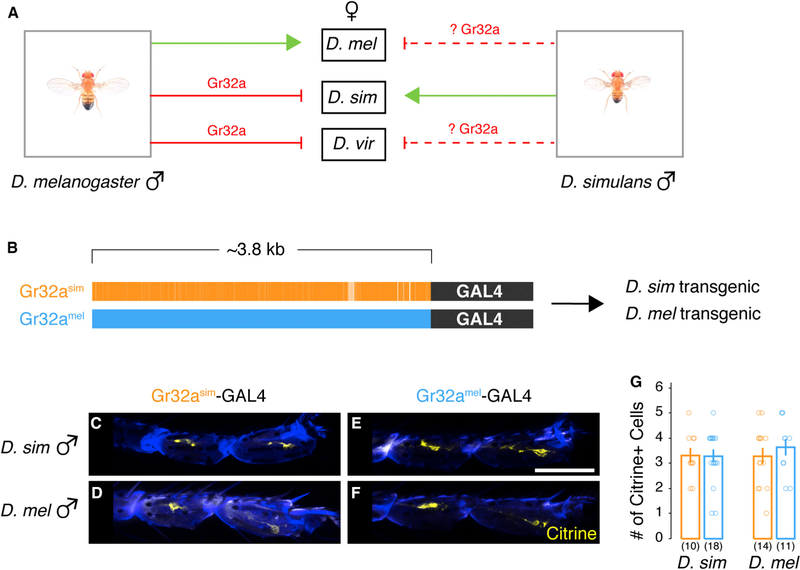
Gr32a is expressed in sensory neurons in distal foreleg tarsi of D. melanogaster (Koganezawa et al., 2010; Miyamoto and Amrein, 2008; Moon et al., 2009; Scott et al., 2001; Thistle et al., 2012; Thorne et al., 2004), and it is required to detect contact-dependent cues on other species and to inhibit interspecies courtship (Fan et al., 2013) (Figure 2A). The genome of D. simulans encodes an ortholog of Gr32a (Drosophila 12 Genomes Consortium et al., 2007) (with four coding exons in both species and 97.8% identity in the encoded protein; Data S1), and we wondered whether this gene is expressed in foreleg tarsi of this species. The ~3.8 kb of D. melanogaster genomic DNA 5′ of the start codon is sufficient to drive reporter expression in subsets of neurons in chemosensory organs known to express Gr32a (Scott et al., 2001; Wang et al., 2004). Similar stretches of genomic DNA are also sufficient to drive reporter expression of other Grs (Weiss et al., 2011), indicating a conserved regulatory logic of expression for this gene family in D. melanogaster. We subcloned ~3.8 kb of genomic DNA upstream of the D. simulans Gr32a start codon and used it to drive GAL4 expression (Gr32asim-GAL4) in transgenic D. simulans and D. melanogaster flies (Figure 2B). Transgene expression was visualized via the fluorescent reporter citrine (Inagaki et al., 2014) (Figures 2C and 2D). We observed citrine expression in three or four neurons in T4–T5 tarsal segments of D. simulans and D. melanogaster, demonstrating that regulatory sequences in the D. simulans Gr32a locus drive reporter expression in foreleg tarsi of both species (Figures 2C, 2D, and 2G). Moreover, the projections of Gr32a sensory neurons in the subesophageal zone (SEZ) appeared similar between the two species (Figures S1K and S1L), indicative of a shared peripheral expression pattern (Wang et al., 2004).
Figure 2. A Regulatory Region in the Gr32a Locus Is Functionally Conserved.
(A) We sought to determine whether, similar to D. melanogaster, Gr32a was expressed in D. simulans foreleg tarsi.
(B) Schematic of transgenic constructs using a DNA sequence 5´ of Gr32a start codon from D. simulans (orange) and D. melanogaster (blue) to drive GAL4 expression. Sequence identity in this region between the two species is noted by solid orange color.
(C–F) Gr32asim-GAL4 (C and D) and Gr32amel-GAL4 (E and F) each drive comparable citrine expression in distal tarsal segments T4 and T5 in both D. simulans (C and E) and D. melanogaster (D and F) male forelegs.
(G) Quantification of data shown in histological panels (C–F).
Mean ± SEM. Each circle denotes the number of citrine+ cells per male foreleg tarsi per genotype. n = 11–18 per genotype. Scale bar, 50 µm. See also Table S1 and Figure S1.
We next tested whether the ~3.8 kb regulatory DNA sequence from these two species drives expression in the same tarsal neurons. We generated D. melanogaster flies harboring GAL4 under control of conspecific ~3.8 kb DNA sequence 5′ of Gr32a such that this transgene (Gr32amel-GAL4) was inserted into the same landing site that we had used for Gr32asim-GAL4 (Figures 2B, 2D, and 2F). Importantly, Gr32amel-GAL4 regulated reporter expression in D. melanogaster foreleg tarsi, as described previously for other GAL4 alleles of Gr32a (Fan et al., 2013; Miyamoto and Amrein, 2008; Moon et al., 2009; Scott et al., 2001). In D. melanogaster flies bearing both Gr32amel-GAL4 and Gr32asim-GAL4, we observed a similar number of citrine+ foreleg tarsal neurons compared with flies bearing these GAL4 drivers individually (Figure S1C). Together, these data are consistent with the notion that the upstream regulatory region of Gr32a in the two species is functionally conserved and sufficient to drive expression in the same foreleg tarsi neurons of D. melanogaster.
We next tested whether the ~3.8 kb genomic DNA 5′ of D. melanogaster Gr32a start codon would drive expression in foreleg tarsal neurons of D. simulans. We inserted Gr32amel-GAL4 into the landing site we used to generate D. simulans flies bearing Gr32asim-GAL4 (Figures 2B and 2E). We observed reporter expression in three or four neurons restricted to T4–T5 tarsal segments of D. simulans in a pattern mirroring that observed in D. simulans bearing Gr32asim-GAL4 (Figures 2C, 2E, and 2G). Given that all GAL4 and UAS transgenes we built in D. simulans were inserted into a single landing site that afforded us reliable and non-leaky expression, we could not directly test whether the same neurons were labeled by Gr32asim-GAL4 and Gr32amel-GAL4 in this species. Nevertheless, our findings strongly suggest that similar cis and trans regulatory features regulate Gr32a expression in foreleg tarsi of the two species.
We find that the ~3.8 kb of regulatory genomic DNA is conserved in multiple insects (mean nucleotide conservation phyloP score = 1.4; see Figures S1D–S1F). Coding exons for another gene (D. melanogaster CG6201) contribute to this sequence similarity, but some of the most conserved blocks of sequence are intergenic regions (Figure S1E). Overall, nucleotide substitutions have occurred in this region at 42.5% the rate of 4-fold degenerate sites in protein-coding exons, slower than expected under a neutral model of DNA evolution (p < 1 × 10−5; see STAR Methods for details; Figure S1F). Within D. melanogaster and D. simulans, >95% of the DNA sequence is identical across this ~3.8 kb region. To examine sequence differences at single-nucleotide resolution, we tested each position for a faster or slower rate of DNA substitutions in D. melanogaster than expected, given the rate in D. simulans and 25 other insects. We also conducted the comparable test for D. simulans. This analysis revealed that few bases in the ~3.8 kb region are evolving faster than expected (>99% bases with phyloP score > −2; Figures S1G and S1H). Because the ~3.8 kb region is highly conserved and D. melanogaster and D. simulans diverged from a common ancestor only recently, it was difficult to detect whether this stretch of DNA is evolving slower than expected subsequent to speciation from this shared ancestor. Together, our findings show that this ~3.8 kb region is conserved in sequence and function in D. melanogaster and D. simulans such that it is sufficient to drive expression in neurons of foreleg tarsi.
Gr32a and Gr33a Are Not Essential to Inhibit Interspecies Courtship in D. simulans Males
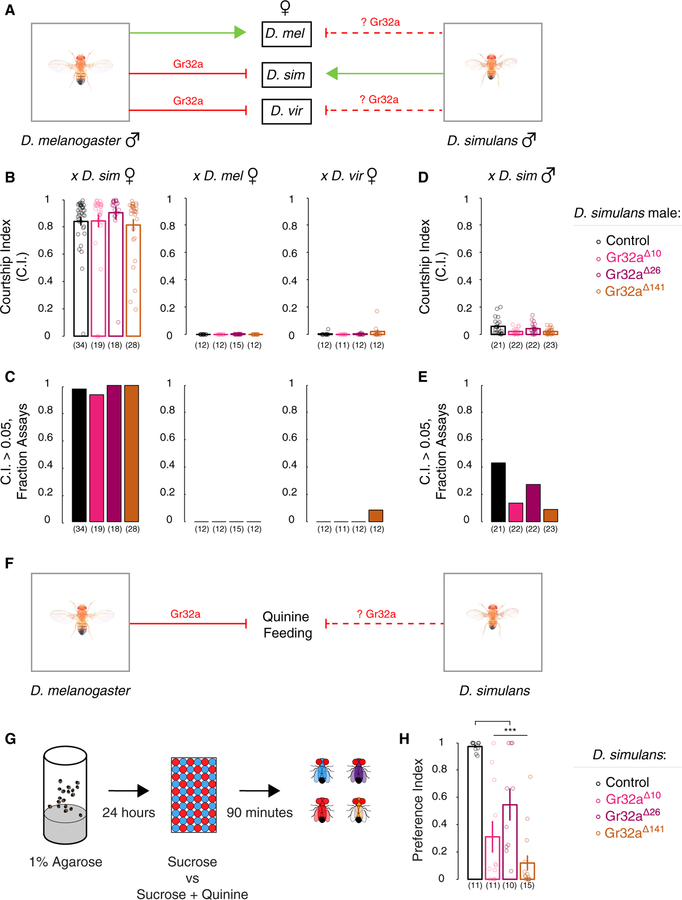
We tested whether Gr32a was essential to inhibit interspecies courtship in D. simulans males (Figure 3A). We targeted distinct sequences in the first coding exon of D. simulans Gr32a to generate three different mutant alleles via the CRISPR/Cas9 system (Figures S2A–S2C). Two of the alleles (Gr32a∆10 and Gr32a∆26) are predicted to lead to 10 and 26 bp deletions in the first coding exon that result in a frameshift and premature stop codon; these likely encode a non-functional Gr32a chemoreceptor protein (Figures S2C and S2D). The third allele (Gr32a∆141) has a 141 bp deletion that is predicted to eliminate 47 amino acids from the predicted N-terminal intracellular domain of this chemoreceptor (Figures S2B–S2D, S2F, and S2G). We next tested D. simulans males homozygous mutant for these Gr32a alleles for courtship displays toward conspecifics and members of other species. We observed that each of the three mutants courted conspecific females similar to WT controls (Figures 3B and 3C). Moreover, these mutants did not increase courtship toward conspecific males or D. melanogaster, D. yakuba, or D. virilis females (Figures 3B–3E and S2E). Our findings indicate a divergence in behavioral function of Gr32a between D. simulans and D. melanogaster, a conclusion consistent with previous sequence analyses showing that bitter-sensing Grs such as Gr32a may be evolving rapidly (Gardiner et al., 2009; McBride et al., 2007). In summary, Gr32a mutant D. simulans males do not show elevated courtship toward other species, a finding in sharp contrast to Gr32a-null D. melanogaster males, which court other species avidly (Fan et al., 2013).
Figure 3. Gr32a Is Not Required to Inhibit Interspecies Courtship but Is Essential for Quinine Sensing in D. simulans.
(A) We tested whether, similar to D. melanogaster males, Gr32a inhibits interspecies courtship by D. simulans males.
(B and C) WT and Gr32a mutant D. simulans males court conspecific but not D. melanogaster or D. virilis females.
(D and E) WT and Gr32a mutant D. simulans males show similar low levels of courtship toward conspecific males.
(F) We tested whether, similar to D. melanogaster, Gr32a inhibits feeding on quinine-containing food in D. simulans.
(G) Schematic of feeding assay for starved D. simulans given choice of colored food containing sucrose or sucrose and quinine. Flies with blue, red, purple, or no food dye colored abdomens were enumerated after exposure to food for 90 min.
(H) Significant decrease in preference by Gr32a mutant D. simulans for food containing only sucrose.
Mean ± SEM. In (B)–(E), each circle denotes CI of one male, and n = 11–34 per genotype. In (G) and (H), preference index = {(# flies that ate sucrose-only food + 0.5*(purple flies)}/(number of flies that ate). Each circle denotes the preference index for one experiment. For each experiment, 106 ± 6 D. simulans of each genotype were used. n = 11–15 experiments/genotype. ***p < 0.001. See Tables S1–S3 and Figures S2 and S3.
Gr33a is co-expressed with Gr32a in foreleg tarsi in D. melanogaster, and it is required to inhibit intermale but not interspecies courtship in males of this species (Fan et al., 2013; Moon et al., 2009). Gr33a is also encoded in the D. simulans genome (Drosophila 12 Genomes Consortium et al., 2007), and we wondered if this chemoreceptor had evolved to inhibit interspecies courtship in this species. Using CRISPR/Cas9, we generated two mutant alleles of Gr33a, one with a 10 bp deletion (Gr33a∆10) that leads to a frameshift and premature stop codon and the other encompassing an in-frame deletion (96 bp, Gr33a∆96) (Figures S3A–S3D). Male D. simulans mutant for each of these alleles courted conspecific females similar to WT controls and did not increase courtship toward conspecific males or D. melanogaster, D. yakuba, or D. virilis females (Figures S3E–S3H). Together, our results indicate that chemosensory receptor-mediated inhibition of courtship toward reproductively futile targets (conspecific males and members of other species) has diverged between the closely related D. melanogaster and D. simulans.
Both Gr32a and Gr33a Are Required in D. simulans to Detect Quinine
In D. melanogaster, Gr32a and Gr33a are also essential for a behavioral aversion to quinine, a bitter tastant (Lee et al., 2010; Moon et al., 2009). Chemoreceptors can evolve to facilitate food sensing in different ecological niches (Baldwin et al., 2014; Jordt and Julius, 2002; Prieto-Godino et al., 2017; Wisotsky et al., 2011). Given the divergence of behavioral function of Gr32a between D. melanogaster and D. simulans, we wondered if Gr32a and Gr33a were required in D. simulans for a response to quinine (Figures 3F and S3I). We tested this in a feeding preference assay in which starved flies were offered a choice between food containing a low concentration of sugar (1 mM sucrose) or a high concentration of sugar (5 mM sucrose) spiked with quinine (0.5 mM) (Montell, 2009; Moon et al., 2009; Tanimura et al., 1982) (Figure 3G). WT D. simulans preferred feeding on the low concentration of sugar, whereas flies mutant for either Gr32a or Gr33a showed reduced preference for feeding on sugar alone (Figures 3H and S3J). Although all mutant lines showed a loss of preference for feeding on sugar alone, there was some variability in the phenotypes observed for the different alleles. Such variability likely resulted from subtle differences in the assay conditions or genetic background; consistent with this notion, there was no statistical difference in behavior between flies bearing the largest and smallest deletions for both genes. It is possible that all D. simulans Gr32a and Gr33a mutations we have generated disrupt sensing quinine but not chemosensory cues from other species, a notion that could be tested when deficiencies spanning Gr32a and Gr33a become available in this species. Our present findings show that quinine sensing via Gr32a and Gr33a is conserved between D. melanogaster and D. simulans.
Ppk25 Promotes Conspecific Courtship in D. simulans Males
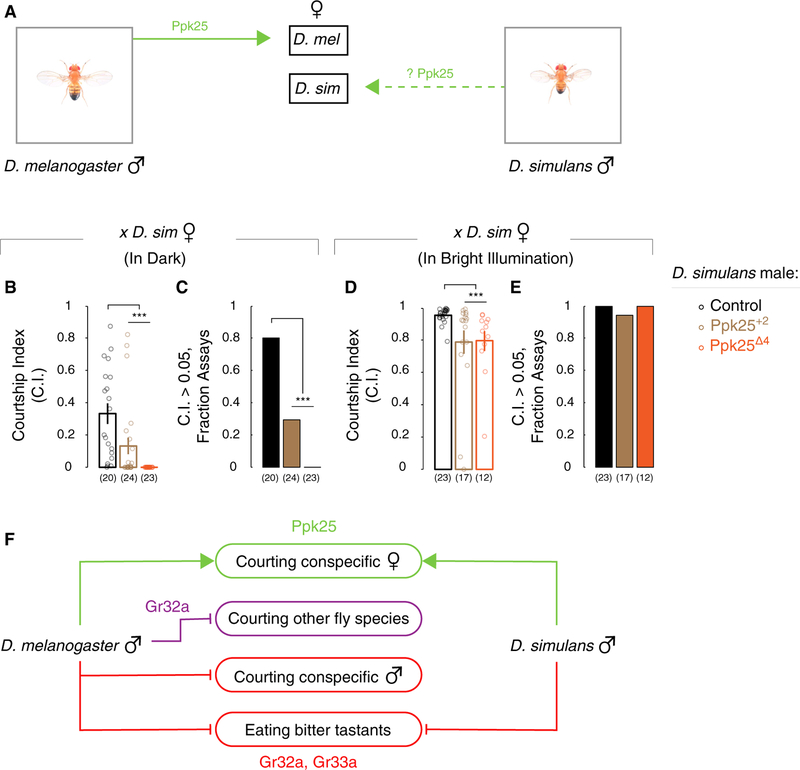
Our findings show that chemosensory receptor mechanisms that inhibit courtship of reproductively futile targets in D. melanogaster are not used in D. simulans. We wondered whether genetic loci that promote courtship had also differentiated between these two species. Many loci promote courtship of D. melanogaster males toward conspecific females (reviewed in Dickson, 2008; Yamamoto and Koganezawa, 2013). We chose to test the function of the Ppk25 pickpocket ion channel subunit that is expressed in foreleg tarsi chemosensory neurons and appears to exclusively promote courtship in D. melanogaster (Figure 4A) (Clowney et al., 2015; Kallman et al., 2015; Lin et al., 2005; Starostina et al., 2012; Vijayan et al., 2014). We generated two alleles of Ppk25 in D. simulans via CRISPR/Cas9, a 2 bp insertion and a 4 bp deletion in the first coding exon, that are predicted to lead to frameshifts and premature stop codons and are likely to be null mutations (Figures S4A–S4D). D. melanogaster Ppk25 is required for male courtship in the dark (Boll and Noll, 2002; Jezovit et al., 2017; Kohatsu and Yamamoto, 2015; Krstic et al., 2009; Lin et al., 2005; Spieth, 1974). Unlike D. melanogaster, D. simulans males court conspecific females vigorously only under bright illumination (Grossfield, 1971; Jezovit et al., 2017) (Figures S4E and S4F). Furthermore, this requirement for bright illumination in D. simulans overrides courtship disinhibition following tarsectomy (Figure S4G). We tested whether Ppk25 modulated courtship by D. simulans males in bright light or dark conditions. D. simulans males mutant for Ppk25 showed reduced courtship of conspecific females in the dark (Figures 4B and 4C). These mutants also showed subtle, but significant, reduction in courtship under bright illumination, suggesting a more stringent requirement for Ppk25 in courtship in this species (Figures 4D and 4E). D. simulans males mutant for Ppk25 did not display elevated courtship to other drosophilids (Figures S4H and S4I), indicating that it does not function in this species to inhibit interspecies courtship. In fact, we found that compared with WT males, Ppk25 mutant D. simulans showed reduced courtship of D. yakuba females (Figures S4H and S4I). In summary, Ppk25 functions in both D. melanogaster and D. simulans to promote WT courtship displays.
Figure 4. Ppk25 Promotes Conspecific Courtship by D. simulans Males.
(A) We tested whether, similar to D. melanogaster, Ppk25 promotes conspecific courtship by D. simulans males.
(B–D) Ppk25 mutant D. simulans males show decreased courtship index (C.I.) in dark (B) and bright illumination (D) and a reduction in high levels of C.I. in dark (C) but not bright illumination (E) toward conspecific females.
(E) No difference between WT and Ppk25 mutant D. simulans males in percentage assays with high levels of courtship of conspecific females.
(F) Summary of the roles of Gr32a, Gr33a, and Ppk25 in D. melanogaster and D. simulans.
Mean ± SEM. Each circle denotes CI for one male. n = 12–24 per genotype. ***p < 0.001. See Tables S1 and S3 and Figure S4.
DISCUSSION
Changes in morphological or other traits across evolution continue to be vigorously investigated (Carroll, 2008). We have examined whether the Gr32a+ chemosensory pathway that inhibits interspecies courtship in D. melanogaster functions similarly in D. simulans. We find that although D. simulans Gr32a is expressed in foreleg tarsi, similar to its counterpart in D. melanogaster, it is not required to inhibit interspecies courtship. It is possible that Gr32a neurons in foreleg tarsi still function to inhibit this behavior, a notion we attempted to address experimentally by inactivating Gr32a+ neurons. However, it was technically challenging to generate the requisite reagents required (Kir2.1, tetanus toxin light chain, shibirets) (Luo et al., 2008) in this species, despite numerous attempts. D. simulans males sense aversive cues on the cuticle of D. melanogaster females (Billeter et al., 2009; Coyne et al., 1994; Ferveur, 2005; Jallon, 1984). Given that Gr32a is not essential for this function, what chemoreceptors might be used to detect such repellents in D. simulans? It is possible that in this species, Gr32a and Gr33a function redundantly to inhibit interspecies courtship, a hypothesis difficult to test directly because these loci are only 1 Mb apart in the genome. Regardless, our findings still demonstrate a divergence in the function of Gr32a between D. melanogaster and D. simulans. The gustatory and ionotropic chemoreceptor families contain many members, and our results are also consistent with the idea that a different chemoreceptor(s) functions to inhibit interspecies courtship by D. simulans males (Joseph and Carlson, 2015). Although changes in centrally located courtship circuits may confer species-specific pheromonal responses (Seeholzer et al., 2018), our results show that there is divergence in chemoreceptor-mediated suppression of interspecific courtship between D. melanogaster and D. simulans (Figure 4F). In other words, our findings show that these closely related species use distinct peripheral chemosensory pathways to suppress interspecific courtship.
The divergence in chemoreceptor-mediated suppression of courtship between D. melanogaster and D. simulans does not reflect a global reorganization of molecular pathways that regulate courtship (Figure 4F). We find that similar to its role in D. melanogaster, Ppk25 is required to promote courtship toward conspecific females in D. simulans. Ppk25 is required to sense 7,11-heptacosadiene, an aphrodisiac cue, in D. melanogaster (Kallman et al., 2015; Starostina et al., 2012); however, 7,11-heptacosadiene is an aversive cue for D. simulans males (Billeter et al., 2009), so it will be interesting to understand how Ppk25 functions in both species to promote conspecific courtship. Although 7,11-heptacosadiene serves as a cuticular attractant to D. melanogaster males, elimination of all cuticular pheromones in D. melanogaster females does not eliminate courtship by D. melanogaster males, and in fact, it disinhibits courtship by D. simulans males (Billeter et al., 2009; Coyne et al., 1994; Savarit et al., 1999). Thus cuticular attractants are not essential for courtship and anti-aphrodisiacs may guide avoidance of courtship with reproductively futile targets such as individuals of other species; together with our previous findings (Fan et al., 2013), our results show that Gr32a is essential for detection of such aversive compounds by D. melanogaster but not D. simulans males.
Both Gr32a and Gr33a are required for avoidance of quinine in D. melanogaster and D. simulans. Thus, the chemosensory functions of Gr32a and Gr33a in avoiding quinine and inhibiting courtship of reproductively futile targets are evolutionarily dissociable (Figure 4F). The same behavioral trait (tapping) and sensorimotor appendage (foreleg) inhibit courting of reproductively dead-end targets in D. melanogaster and D. simulans, but our studies show that the molecular mechanisms that preclude such courtship have diverged between these species. Previous work from our and other labs shows that different genetic pathways control distinct quantitative aspects of behavioral subroutines (Ding et al., 2016; Greenwood et al., 2013; Weber et al., 2013; Xu et al., 2012). Together, these findings demonstrate that modifications in genetic pathways can be used to gate a behavior or to implement quantitative changes in that behavior. We also find that although chemoreceptor mechanisms inhibiting interspecies courtship have differentiated between closely related species, a chemosensory pathway promoting courtship appears to have a similar positive valence in both species. It will be interesting to determine whether these courtship-promoting and courtship-inhibiting pathways evolve in a similar pattern across other drosophilid species. Alternatively (Jacob, 1977; Luo, 2015), our findings may reflect the idiosyncratic nature of selective forces that exploit mutations in apparently random pathways to effect evolutionary change. It should be possible to distinguish between these alternatives by studying mechanisms that regulate courtship in additional drosophilid species.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nirao Shah (nirao@stanford.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
D. simulans (14021-0251.001), w501 D. simulans (14021-0251.195), D. yakuba (14021-0261.00), and D. virilis (15010-1051.00) were obtained from the Drosophila Species Stock Center at the University of California, San Diego. WT D. melanogaster were in the Canton-S background. D. melanogaster UAS-ReaChR::Citrine.VK05 was obtained from the Bloomington Drosophila Stock Center (#53749). Transgenic and CRISPR-mediated mutant flies were generated as described below.
METHOD DETAILS
Generating D. simulans Gr32a, Gr33a, or Ppk25 mutants
CRISPR guides were chosen from a list generated by flyCRISPR Optimal Target Finder (flycrispr.molbio.wisc.edu/tools). We targeted exon 1 of D. simulans Gr32a and Ppk25, and exon 2 of Gr33a. CRISPR oligos were annealed and ligated to plasmid pDCC6 {Addgene # 59985, (Gokcezade et al., 2014)} following restriction digest with BbsI. Sequences used to synthesize CRISPR oligos are provided in Table S1. Plasmids were injected at 100 ng/uL concentrations for each of 2 – 3 plasmids targeting a single gene. Animals were screened for mutations by PCR followed by 15% non-denaturing PAGE (Zhu et al., 2014) or directly by sequencing. Please see Table S3 for details on results of CRISPR injections for D. simulans. All CRISPR-generated mutant strains were backcrossed at least 5 times to WT D. simulans before testing for behavior in order to minimize effects of off-target mutations on phenotypes under study. Subsequent to this out-crossing to WT D. simulans, we mated heterozygous flies to obtain homozygous stocks for each allele. Given the absence of balancers in D. simulans, we verified genotypes at each generation by PCR analysis to generate homozygous stocks.
Generating D. simulans and D. melanogaster transgenic animals
To make Gr32a-GAL4 lines, we amplified the ~3.8 kb region upstream of the Gr32a start codon from D. simulans or D. melanogaster (primer sequences provided in Table S1) and subcloned it into pENTR/TOPO plasmid followed by Gateway-mediated subcloning into pBPGw. We then phiC31-integrated each DNA construct into Chr III landing sites for each species, sim986 for D. simulans and attp2 for D. melanogaster (Groth et al., 2004; Knapp et al., 2015; Pfeiffer et al., 2010; Stern et al., 2017). pJFRC2(10xUAS-ReaChR::Citrine) plasmid (Inagaki et al., 2014) was provided by David Anderson, and it was used to generate the Citrine reporter in D. simulans using the landing site described above. Embryo injections were performed by Rainbow Transgenics (Camarillo, CA) or BestGene (Chino Hills, CA).
Molecular analysis of Gr32a, Gr33a, and Ppk25 mutations in D. simulans
RNA was isolated from 10 WT or mutant D. simulans males (Trizol, ThermoFisher) and converted to cDNA using SuperScript III First-Strand Synthesis (Invitrogen, ThermoFisher). RT-PCR was performed using primers based on coding sequence (Table S1) that spanned exon-intron junctions of the respective locus (Gr32a, Gr33a, or Ppk25) to avoid amplifying products from genomic DNA. Use of these primers did not generate detectable product in no-RT controls. We subcloned and sequenced RT-PCR products from flies mutant for each allele of Gr32a, Gr33a, and Ppk25; we also directly sequenced RT-PCR products from flies mutant for each allele of Gr32a (except Gr32a∆26), Gr33a, and Ppk25. RNA isolation and the subsequent RT-PCR and sequencing were performed on 2–3 independent cohorts of WT and mutant flies. Sequence reads of subclones obtained from these RT-PCR studies and their alignment to the corresponding WT allele confirmed the presence of the expected mutation for each fly stock.
Histology
Tarsi were dissected in ice-cold PBS, fixed in fresh 4% paraformaldehyde at 22°C, washed 3x in PBT, and then mounted as described before (Fan et al., 2013). Samples were imaged using a Zeiss LSM700 (Z stacks) and processed in ImageJ.
Courtship assays
All courtship assays were performed at zeitgeber time 6–10 at 22°C, illuminated by a fluorescent ring lamp (22W) suspended 4 cm above the courtship chamber and recorded with a Sony camcorder (HDR-XR550V) (Fan et al., 2013). Experiments performed under dark conditions were illuminated by red LEDs and recorded as above in a dark room. Virgin flies were collected at eclosion and light entrained (12 hours L/D, 25C) for 5–7 days prior to testing. Experimental males were kept in isolation and tested with flies that were group-housed (~20 flies per vial) by species and sex. Foreleg tarsi were surgically removed at eclosion and males were tested as described above. We used w501 D. simulans as targets in male-male assays to distinguish them by eye color from test males. Behavioral assays were scored blind to genotype, using the MATLAB software ScoreVideo (Wu et al., 2009). We scored courtship as the period of time male flies spent chasing the stimulus fly, performing unilateral wing extension (courtship song), licking, abdominal bending (attempted copulation), or copulation. Courtship Index (CI) was calculated as the time spent by the male performing these behaviors, divided by the total assay time (15 minutes).
Taste assay
Preference assays were performed as described previously (Moon et al., 2009). 60-well plates were prepared the day prior to experimentation and kept at 4°C. Dyes were diluted from stock solutions (Brilliant blue FCF and Sulforhodamine B, 12.5 mg/ml each) and resuspended in agarose, to which sucrose or sucrose spiked with quinine-HCl were subsequently added. Final concentrations were: agarose (1%), Brilliant blue FCF (0.125 mg/mL; Wako Pure Chemical), Sulforhodamine B (0.125 mg/mL; SigmaAldrich), sucrose (1 mM; JT Baker), and sucrose (5 mM) spiked with quinine (0.5 mM; SigmaAldrich). Substrate with sucrose or sucrose spiked with quinine were randomly colored blue or red and counterbalanced for all experiments. 3–4 day old male and female flies were flipped into fresh food for 2 days at 12-hour light/dark cycle at 25°C. Flies were then food deprived by flipping them into vials containing 1% agarose and placed in the dark for 24 hours. Flies were then briefly anesthetized with CO2 and loaded onto the 60-well plates (zeitgeber time 2–3), which were placed in the dark at 25°C for 90 min. Abdomens were scored as blue, red, purple (mixed eating), or no food coloring blind to genotype and color condition. A Preference Index was calculated for each 60-well plate as follows: (NB + 0.5*NP)/(NB + NR + 0.5*NP) or (NR + 0.5*NP)/(NB + NR + NP) where NB, NR, and NP = total # flies with blue, red, and purple abdomens, respectively. Each genotype was tested ≥ 6 times.
Tests for Non-Neutral Evolution
Alignments of genomes from 27 insect species (23 drosophilids, housefly, mosquito, honeybee, and beetle) were generated for coordinates (dm6: chr2L:11,110,412-11,114,209) encompassing the D. melanogaster Gr32a ~3.8 kb regulatory sequence, and this alignment was subsequently downloaded from the Table Browser (UCSC Genome Browser, 2015 update) (Blanchette et al., 2004; Karolchik et al., 2004; Rosenbloom et al., 2015). PhyloP scores were computed for this region for three main tests: 1) a basewise ‘‘all-branches’’ test for conserved or accelerated evolution in all species compared to a neutral model (one test per nucleotide), 2) a whole-region ‘‘all-branches’’ test for conserved evolution in all species compared to a neutral model (one test for the whole region), and 3) a basewise ‘‘subtree’’ test for conserved or accelerated evolution in the designated species (D. melanogaster or D. simulans) compared to the other species (one test per nucleotide for each designate species) (Pollard et al., 2010). PhyloP scores are negative log10 P values of a likelihood ratio test comparing two evolutionary models (alternate versus neutral or subtree versus subtree complement). Scores near ‘‘0’’ indicate the expected rate of evolution, while large scores indicate conservation (phyloP score > 2) or acceleration (phyloP score < −2). PhyloP scores were tallied across coding sequence, introns, UTRs, and intergenic regions (Siepel et al., 2005). The phylogenetic model for neutral evolution was based on 4-fold degenerate sites in the 27-species genomic alignment and also downloaded from the UCSC Genome Browser. PhyloP scores and R code are made available for reproducible workflow at https://github.com/aavilaherrera/flymating (Allaire et al., 2017; R Core Team, 2017; Xie, 2016) (https://cran.r-project.org/doc/FAQ/R-FAQ.html#Citing-R). This code uses bedtools and bedops (Neph et al., 2012; Quinlan and Hall, 2010).
Hydrophobicity plot
Hydrophobicity scores were generated with ProtScale (Artimo et al., 2012) using D. melanogaster or D. simulans Gr32a amino acid sequences as input. We used the Kyte and Doolittle hydrophobicity scale with a window size of 19 amino acids and uniform weights across all residues. The seven transmembrane domains were identified using HMMTOP (Tusnády and Simon, 1998, 2001) to predict the topology of Gr32a for both D. melanogaster and D. simulans.
QUANTIFICATION AND STATISTICAL ANALYSIS
We used Fisher’s exact test to analyze categorical data (e.g., percent assays with CI > 0.05) and we used the Bonferroni correction for multiple group comparisons as necessary. For other comparisons, we first tested whether data were normally distributed using a Lillefors’ goodness-of-fit test using MATLAB. Data for Figure S1B were analyzed with a Student’s t test; data for all other figure panels were tested with a non-parametric test (Kolmogorov-Smirnov test for two groups or Kruskal-Wallis test). A Tukey’s post hoc test following multiple group comparisons was used to determine which groups differed significantly.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and
Recombinant Proteins | ||
| Quinine-HCl | SigmaAldrich | CAS: 6119-47-7 |
| Sucrose | JT Baker | CAS: 57-50-1 |
| Brilliant blue FCF | Wako Pure Chemical | CAS: 3844-45-9 |
| Sulforhodamine | SigmaAldrich | CAS: 3520-42-1 |
|
Critical Commercial Assays | ||
| SuperScript III First-Strand Synthesis | Invitrogen, ThermoFisher | Cat # 18080051 |
|
Experimental Models: Organisms/Strains | ||
| D. simulans: wildtype | Drosophila Species Stock Center (University of California, San Diego) | 14021-0251.001 |
| D. simulans: w501 | Drosophila Species Stock Center (University of California, San Diego) | 14021-0251.195 |
| D. yakuba: wildtype | Drosophila Species Stock Center (University of California, San Diego) | 14021-0261.00 |
| D. virilis: wildtype | Drosophila Species Stock Center (University of California, San Diego) | 15010-1051.00 |
| D. melanogaster: Canton-S | Bloomington Drosophila Stock Center | RRID:BDSC_64349, |
| D. simulans: Gr32a∆10 | This paper | N/A |
| D. simulans: Gr32a∆26 | This paper | N/A |
| D. simulans: Gr32a∆141 | This paper | N/A |
| D. simulans: Gr33a∆10 | This paper | N/A |
| D. simulans: Gr33a∆96 | This paper | N/A |
| D. simulans: Ppk25+2 | This paper | N/A |
| D. simulans: Ppk25∆4 | This paper | N/A |
| D. simulans: UAS-ReaChR::Citrine.sim986 | This paper | N/A |
| D. melanogaster: UAS-ReaChR::Citrine.VK05 | Bloomington Drosophila Stock Center | RRID:BDSC_53749 |
| D. simulans: Gr32asim-GAL4.sim986 | This paper | N/A |
| D. simulans: Gr32amel-GAL4.sim986 | This paper | N/A |
| D. melanogaster: Gr32asim-GAL4.attP2 | This paper | N/A |
| D. melanogaster: Gr32amel-GAL4.attP2 | This paper | N/A |
|
Oligonucleotides | ||
| Primers: Amplifying Gr32a Regulatory Region | See Table S1 | N/A |
| CRISPR oligos: Targeting D. simulans Gr32a | See Table S1 | N/A |
| CRISPR oligos: Targeting D. simulans Gr33a | See Table S1 | N/A |
| CRISPR oligos: Targeting D. simulans Ppk25 | See Table S1 | N/A |
| Primers: RT-PCR of D. simulans Gr32a, Gr33a, Ppk25, and tubulin | See Table S1 | N/A |
|
Recombinant DNA | ||
| pJFRC2(UAS-ReaChR::Citrine) | Inagaki et al., 2014 | N/A |
| pBPGw | Pfeiffer et al., 2008 | RRID: Addgene_17574 |
| pDCC6 | Gokcezade et al., 2014 | RRID: Addgene_59985 |
|
Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/index.html; RRID: SCR_003070 |
| MATLAB | MathWorks | https://www.mathworks.com/products.html; RRID: SCR_001622 |
| ProtScale: Kyte and Doolittle hydrophobicity scale | Artimo et al., 2012 | https://web.expasy.org/protscale/ |
| HMMTOP | Tusnády and Simon, 1998, 2001 | http://www.enzim.hu/hmmtop/ |
| UCSC Genome Browser, 2015 update | Blanchette et al., 2004; Karolchik et al., 2004; Rosenbloom et al., 2015 | https://genome.ucsc.edu/cgi-bin/hgTracks?db=dm6&position=chr2L%3A11110412-11114209; RRID:SCR_005780 |
| phyloP | Pollard et al., 2010; Siepel et al., 2005 | https://github.com/CshlSiepelLab/phast; http://compgen.cshl.edu/phast/background.php |
| bedops | Neph et al., 2012 | https://bedops.readthedocs.io/; RRID:SCR_012865 |
| bedtools | Quinlan and Hall, 2010 | https://github.com/arq5x/bedtools2; RRID:SCR_006646 |
| R | Comprehensive R Archive Network (CRAN) | https://cran.r-project.org/; RRID:SCR_003005 |
| R, Python and shell script code | See STAR Methods: Tests for non-neutral evolution | https://github.com/aavilaherrera/flymating |
| CRAN: tidyverse | Comprehensive R Archive Network (CRAN) | https://cran.r-project.org/package=tidyverse |
| CRAN: rmarkdown | Comprehensive R Archive Network (CRAN) | https://cran.r-project.org/package=rmarkdown |
| CRAN: knitr | Comprehensive R Archive Network (CRAN) | https://cran.r-project.org/package=knitr |
| CRAN: kableExtra | Comprehensive R Archive Network (CRAN) | https://cran.r-project.org/package=kableExtra |
Highlights.
Gr32a and Gr33a do not inhibit interspecies or intermale mating by male D. simulans
Gr32a and Gr33a inhibit feeding of bitter tastants by D. simulans
Ppk25 promotes mating with conspecific females by male D. simulans
Pathways that promote or inhibit mating have evolved differentially in D. simulans
ACKNOWLEDGMENTS
We thank Drs. Thomas Clandinin, Pu Fan, Liqun Luo, Devanand Manoli, and Z. Yan Wang and members of the Shah lab for helpful comments during the course of this work or on the manuscript and Dr. Mala Murthy for graciously enabling completion of this project by O.M.A. in her laboratory. O.M.A. dedicates this work to the memory of Robert I. Mozia. We thank Dr. David Anderson for sharing the pJFRC2[UAS-ReaChR::Citrine] plasmid. This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under contract DE-AC52-07NA27344 (A.A.-H.). It was funded by the National Science Foundation Graduate Research Fellowships Program (O.M.A.), the NIH (grant R35NS097212 to G.W.D.), the Human Frontier Science Program Postdoctoral Fellowship (S.P.), the Gladstone Institutes (A.A.-H., K.S.P.), funds from the Department of Psychiatry and Behavioral Sciences at Stanford, Career Awards in Biomedical Sciences from the Burroughs Wellcome Fund, the Ellison Medical Foundation, the McKnight Foundation for Neuroscience, and the Sloan Foundation (N.M.S.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.04.104.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Allaire JJ, Cheng J, Xie Y, McPherson J, Chang W, Allen J, Wickham H, Atkins A, Hyndman R, and Arslan R (2017). rmarkdown: Dynamic Documents for R https://cran.r-project.org/web/packages/rmarkdown/index.html.
- Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, et al. (2012). ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res 40, W597–W603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MW, Toda Y, Nakagita T, O’Connell MJ, Klasing KC, Misaka T, Edwards SV, and Liberles SD (2014). Sensory biology. Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science 345, 929–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock M, and Manning A (1955). The courtship of Drosophila melanogaster. Behaviour 8, 85–111. [Google Scholar]
- Billeter J-C, Atallah J, Krupp JJ, Millar JG, and Levine JD (2009). Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991. [DOI] [PubMed] [Google Scholar]
- Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AF, Roskin KM, Baertsch R, Rosenbloom K, Clawson H, Green ED, et al. (2004). Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res 14, 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, and Noll M (2002). The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129, 5667–5681. [DOI] [PubMed] [Google Scholar]
- Carroll SB (2008). Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36. [DOI] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, and Ruta V (2015). Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, and Orr HA (1989). Patterns of speciation in Drosophila. Evolution 43, 362–381. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Crittenden AP, and Mah K (1994). Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265, 1461–1464. [DOI] [PubMed] [Google Scholar]
- Darwin C (1860). On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life (Appleton) [PMC free article] [PubMed]
- David JR, Lemeunier F, Tsacas L, and Yassin A (2007). The historical discovery of the nine species in the Drosophila melanogaster species subgroup. Genetics 177, 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, and Dickson BJ (2005). Fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794. [DOI] [PubMed] [Google Scholar]
- Dickson BJ (2008). Wired for sex: the neurobiology of Drosophila mating decisions. Science 322, 904–909. [DOI] [PubMed] [Google Scholar]
- Ding Y, Berrocal A, Morita T, Longden KD, and Stern DL (2016). Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature 536, 329–332. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T (1937). Genetic nature of species differences. Am. Nat 71, 404–420. [Google Scholar]
- Drosophila 12 Genomes Consortium; Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, et al. (2007). Evolution of genes and genomes on the Drosophila phylogeny. Nature 450, 203–218. [DOI] [PubMed] [Google Scholar]
- Everaerts C, Farine J-P, Cobb M, and Ferveur J-F (2010). Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5, e9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J, Renslo A, Baker BS, and Shah NM (2013). Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J-F (2005). Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet 35, 279–295. [DOI] [PubMed] [Google Scholar]
- Gardiner A, Butlin RK, Jordan WC, and Ritchie MG (2009). Sites of evolutionary divergence differ between olfactory and gustatory receptors of Drosophila. Biol. Lett 5, 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KS (1963). A mutation causing abnormal courtship and mating behavior in males of Drosophila melanogaster. Am. Zool 3, 507. [Google Scholar]
- Gokcezade J, Sienski G, and Duchek P (2014). Efficient CRISPR/Cas9 plasmids for rapid and versatile genome editing in Drosophila. G3 (Bethesda) 4, 2279–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ, and Ferveur JF (2000). Courtship in Drosophila. Annu. Rev. Genet 34, 205–232. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Yoshida K, and Peichel CL (2013). Genetic and neural modularity underlie the evolution of schooling behavior in threespine sticklebacks. Curr. Biol 23, 1884–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossfield J (1971). Geographic distribution and light-dependent behavior in Drosophila. Proc. Natl. Acad. Sci. U S A 68, 2669–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, and Calos MP (2004). Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166, 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC (1978). Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav. Genet 8, 125–141. [DOI] [PubMed] [Google Scholar]
- Hall JC (1994). The mating of a fly. Science 264, 1702–1714. [DOI] [PubMed] [Google Scholar]
- Hotta Y, and Benzer S (1976). Courtship in Drosophila mosaics: sex-specific foci for sequential action patterns. Proc. Natl. Acad. Sci. U S A 73, 4154–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, and Anderson DJ (2014). Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F (1977). Evolution and tinkering. Science 196, 1161–1166. [DOI] [PubMed] [Google Scholar]
- Jallon JM (1984). A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet 14, 441–478. [DOI] [PubMed] [Google Scholar]
- Jezovit JA, Levine JD, and Schneider J (2017). Phylogeny, environment and sexual communication across the Drosophila genus. J. Exp. Biol 220, 42–52. [DOI] [PubMed] [Google Scholar]
- Jordt S-E, and Julius D (2002). Molecular basis for species-specific sensitivity to ‘‘hot’’ chili peppers. Cell 108, 421–430. [DOI] [PubMed] [Google Scholar]
- Joseph RM, and Carlson JR (2015). Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet 31, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman BR, Kim H, and Scott K (2015). Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolchik D, Hinrichs AS, Furey TS, Roskin KM, Sugnet CW, Haussler D, and Kent WJ (2004). The UCSC Table Browser data retrieval tool. Nucleic Acids Res 32, D493–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J-M, Chung P, and Simpson JH (2015). Generating customized transgene landing sites and multi-transgene arrays in Drosophila using phiC31 integrase. Genetics 199, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganezawa M, Haba D, Matsuo T, and Yamamoto D (2010). The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol 20, 1–8. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, and Yamamoto D (2015). Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat. Commun 6, 6457. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, and Yamamoto D (2011). Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508. [DOI] [PubMed] [Google Scholar]
- Krstic D, Boll W, and Noll M (2009). Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE 4, e4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, Manière G, Marion-Poll F, Ozaki M, Francke W, Cobb M, et al. (2007). An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2, e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SH, and Montell C (2010). Avoiding DEET through insect gustatory receptors. Neuron 67, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Mann KJ, Starostina E, Kinser RD, and Pikielny CW (2005). A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc. Natl. Acad. Sci. U S A 102, 12831–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-H, Cao D-S, Sethi S, Zeng Z, Chin JSR, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su C-Y, and Wang JW (2016). Hormonal modulation of pheromone detection enhances male courtship success. Neuron 90, 1272–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L (2015). Principles of Neurobiology (Garland Science)
- Luo L, Callaway EM, and Svoboda K (2008). Genetic dissection of neural circuits. Neuron 57, 634–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A (1959). The sexual isolation between Drosophila melanogaster and Drosophila simulans. Anim. Behav 7, 60–65. [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, and Baker BS (2005). Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400. [DOI] [PubMed] [Google Scholar]
- Markow TA (2015). The secret lives of Drosophila flies. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E (1988). The why and how of species. Biol. Philos 3, 431–441. [Google Scholar]
- Mayr E, and Dobzhansky T (1945). Experiments on sexual isolation in Drosophila: IV. Modification of the degree of isolation between Drosophila pseudoobscura and Drosophila persimilis and sexual preferences in Drosophila prosoltans. Proc. Natl. Acad. Sci. U S A 31, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CS, Arguello JR, and O’Meara BC (2007). Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics 177, 1395–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson TC (2003). Sexual isolation evolves faster than hybrid inviability in a diverse and sexually dimorphic genus of fish (Percidae: Etheostoma). Evolution 57, 317–327. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, and Amrein H (2008). Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci 11, 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C (2009). A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol 19, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, and Montell C (2009). A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol 19, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neph S, Kuehn MS, Reynolds AP, Haugen E, Thurman RE, Johnson AK, Rynes E, Maurano MT, Vierstra J, Thomas S, et al. (2012). BEDOPS: high-performance genomic feature operations. Bioinformatics 28, 1919–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA (2005). The genetic basis of reproductive isolation: insights from Drosophila. Proc. Natl. Acad. Sci. U S A 102 (Suppl 1), 6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA, Masly JP, and Presgraves DC (2004). Speciation genes. Curr. Opin. Genet. Dev 14, 675–679. [DOI] [PubMed] [Google Scholar]
- Pavlou HJ, and Goodwin SF (2013). Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr. Opin. Neurobiol 23, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Hubisz MJ, Rosenbloom KR, and Siepel A (2010). Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Godino LL, Rytz R, Cruchet S, Bargeton B, Abuin L, Silbering AF, Ruta V, Dal Peraro M, and Benton R (2017). Evolution of acid-sensing olfactory circuits in drosophilids. Neuron 93, 661–676.e6. [DOI] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom KR, Armstrong J, Barber GP, Casper J, Clawson H, Die-khans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M, et al. (2015). The UCSC Genome Browser database: 2015 update. Nucleic Acids Res 43, D670–D681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, and Wasserman SA (1996). Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089. [DOI] [PubMed] [Google Scholar]
- Savarit F, Sureau G, Cobb M, and Ferveur JF (1999). Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl. Acad. Sci. U S A 96, 9015–9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R Jr., Cravchik A, Morozov P, Rzhetsky A, Zuker C, and Axel R (2001). A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673. [DOI] [PubMed] [Google Scholar]
- Seeholzer LF, Seppo M, Stern DL, and Ruta V (2018). Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, et al. (2005). Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15, 1034–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth HT (1952). Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist 99, 395–474. [Google Scholar]
- Spieth HT (1974). Courtship behavior in Drosophila. Annu. Rev. Entomol 19, 385–405. [DOI] [PubMed] [Google Scholar]
- Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, and Pikielny CW (2012). A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J. Neurosci 32, 4665–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL, Crocker J, Ding Y, Frankel N, Kappes G, Kim E, Kuzmickas R, Lemire A, Mast JD, and Picard S (2017). Genetic and transgenic reagents for Drosophila simulans, D. mauritiana, D. yakuba, D. santomea, and D. virilis. G3 (Bethesda) 7, 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A (1919). A new species closely resembling Drosphila melanogaster. Psyche (Stuttg.) 26, 153–155. [Google Scholar]
- Sturtevant AH (1920). Genetic studies on Drosophila simulans. I. Introduction. Hybrids with Drosophila melanogaster. Genetics 5, 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Subramanian S, and Kumar S (2004). Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol. Biol. Evol 21, 36–44. [DOI] [PubMed] [Google Scholar]
- Tanimura T, Isono K, Takamura T, and Shimada I (1982). Genetic dimorphism in the taste sensitivity to trehalose inDrosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol 147, 433–437. [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing (R Foundation for Statistical Computing; ). [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, and Scott K (2012). Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, and Amrein H (2004). Taste perception and coding in Drosophila. Curr. Biol 14, 1065–1079. [DOI] [PubMed] [Google Scholar]
- Tootoonian S, Coen P, Kawai R, and Murthy M (2012). Neural representations of courtship song in the Drosophila brain. J. Neurosci 32, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnády GE, and Simon I (1998). Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol 283, 489–506. [DOI] [PubMed] [Google Scholar]
- Tusnády GE, and Simon I (2001). The HMMTOP transmembrane topology prediction server. Bioinformatics 17, 849–850. [DOI] [PubMed] [Google Scholar]
- Vijayan V, Thistle R, Liu T, Starostina E, and Pikielny CW (2014). Drosophila pheromone-sensing neurons expressing the ppk25 ion channel subunit stimulate male courtship and female receptivity. PLoS Genet 10, e1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, and Scott K (2004). Taste representations in the Drosophila brain. Cell 117, 981–991. [DOI] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter J-C, Miyamoto T, Amrein H, Levine JD, and Anderson DJ (2011). Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci 14, 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JN, Peterson BK, and Hoekstra HE (2013). Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature 493, 402–405. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, and Carlson JR (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisotsky Z, Medina A, Freeman E, and Dahanukar A (2011). Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat. Neurosci 14, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, and Shah NM (2009). Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y (2016). knitr: a general-purpose package for dynamic report generation in R https://cran.r-project.org/web/packages/knitr/index.html.
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, and Shah NM (2012). Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, and Koganezawa M (2013). Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci 14, 681–692. [DOI] [PubMed] [Google Scholar]
- Zhu X, Xu Y, Yu S, Lu L, Ding M, Cheng J, Song G, Gao X, Yao L, Fan D, et al. (2014). An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep 4, 6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.